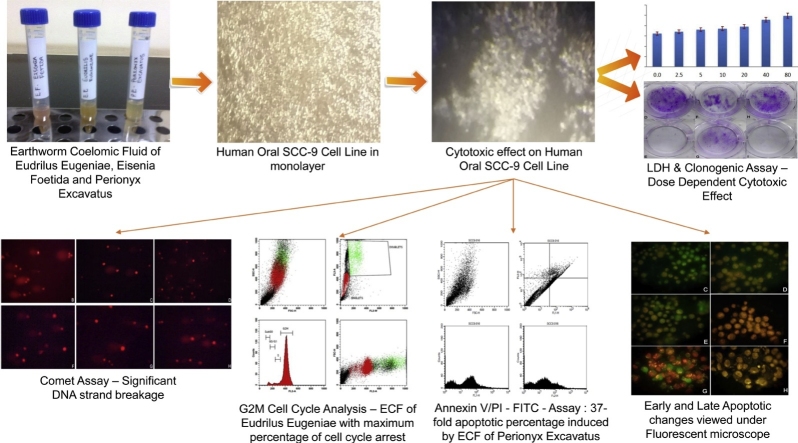
Graphical abstract
Abbreviations: ATCC, American Type Culture Collection; CCD, charged coupled device; DNA, deoxy ribose nucleic acid; DMEM, Dulbecco’s Modified Eagle Medium; ECF, earthworm coelomic fluid; EE, Eudrilus eugeniae; EF, Eisenia foetida; FBS, fetal bovine serum; FITC, fluorescein isothiocyanate; HEPES, 4-2-hydroxyethyl)-1-piperazineethanesulfonic acid; LDH, lactate dehydrogenase; LMPA, low melting point agarose; MEM, Minimal Essential Medium; NAE’s, naturally available extracts; NMA, normal melting agarose; PE, Perionyx excavatus; PS, phosphatidylserine; SCC, squamous cell carcinoma
Keywords: Annexin V – FITC/PI, Apoptosis, Cytotoxicity, Cell cycle analysis, Earthworm coelomic fluid, Eudrilus eugeniae, Eisenia foetida, Perionyx excavatus, SCC-9 cell line
Highlights
-
•
Coelomic fluid of 3 earthworms exhibited cytotoxicity on oral cancer cells.
-
•
Coelomic fluid induced DNA strand breakage in SCC-9 cells.
-
•
Coelomic fluid of Perionyx excavatus showed highest percentage of G2M cycle arrest.
-
•
Coelomic fluid of Eudrilus eugeniae caused a 37-fold rise in apoptosis percentage.
Abstract
The current protocol of cancer management includes surgery, radiotherapy and chemotherapy. However, these modalities have significant adverse effects and affect the quality of life. Further intensification of treatment is hindered as maximal toxicity levels are reached impeding improvement. Hence researchers are in the quest for adjunctive naturally available therapies that can alter tumor proliferation without causing significant adverse reactions. The present study aims to explore the cytotoxic potential of earthworm coelomic fluid (ECF) of Eudrilus eugeniae (EE), Eisenia foetida (EF), and Perionyx excavatus (PE) on oral cancer cell line SCC-9. The effect of ECF on cell cycle analysis and mechanism of cell death have also been investigated. All experiments reported in this paper were performed as 3 replicates per experiment. The results indicated that ECF of EE, EF and PE have potent variable cytotoxic effect on SCC-9 cells demonstrated through LDH, clonogenic and comet assay. An effective cell cycle arrest was observed at the G2M phase of cell cycle with apoptotic induction that was observed through an Annexin V – FITC/PI assay. ECF of EE was found to be superior in its cytotoxic action closely followed by ECF of PE. The present findings provide evidence for the first time that ECF of EE, EF and PE have potent cytotoxic effect on oral cancer cells in vitro. They significantly induce G2M cell cycle arrest and promote apoptosis in SCC-9 cell line. Gene expression studies have been planned to ascertain the pathways of cell death.
1. Introduction
The persistent problem encountering oral cancer treatment has been the inability to prevent tumor cells from endlessly dividing. Few patients with localized disease are suitable for potential curative surgery while the rest may receive combinations of surgery, radiotherapy and chemotherapy [1]. Multimodal treatment is an aggressive strategy that has been adopted in the management of oral cancer, however 40% to 60% of the patients show a recurrence after conventional therapy [2]. Numerous studies have suggested other contributing factors such as neuroendocrine factor dysregulation and systemic illnesses may be involved in accelerating the development and progression of tumorigenesis thereby suppressing the regression of cancers [3]. Few clinical trials have shown improvement in survival outcomes when potent chemotherapeutic compounds are incorporated in conventional treatment regimens [4,5]. These combinations result in systemic toxicity and the administration of several drugs may deteriorate the quality of life.
Naturally available extracts (NAE’s) have been sought after in the adjunctive management of cancer due to their antioxidant and free radical scavenging property. NAE’s possess an inherent mechanism of preventing cancer cells from proliferating [6]. Earthworm extracts which contain proteases are one such NAE’s that have shown to have antiproliferative potential in vitro. Recently we have demonstrated the time and dose dependent antiproliferative effect of Earthworm Coelomic fluid (ECF) on oral cancer cell line KB 3-1 and SCC-9 with significant results [[7], [8], [9]]. However, the cytotoxic potential of ECF on oral cancer cells remains unclear.
The aim of the present study is to evaluate the cytotoxic effect of ECF of Eudrilus eugeniae (EE), Eisenia foetida (EF), and Perionyx excavatus (PE) on oral cancer cell line SCC-9. The present study also attempts to investigate the cell cycle analysis and mechanism of cell death induced by ECF on SCC-9 cell line. Identification of potent biomolecules through anticancer studies may facilitate their usage in drug discovery for adjunctive management of cancer therapies.
2. Methodology
Ethical approval for the study was obtained from the RUAS (Ramaiah University of Applied Sciences), human and animal ethics committee. (No: FDS/EC/2014-16/PhD_03).
2.1. Collection of earthworm coelomic fluid and protein estimation
Mature earthworms weighing 400 g (Age 1–2 years) were obtained from a local vermicomposting unit located in Bangalore. The three species EE, EF and PE were segregated based on their morphological characteristics and validated by a zoologist. The cold shock method of fluid collection was coupled with mechanical agitation method where the petridish containing the earthworms were placed over an ice bath for a period of 15 min followed by 5 min of relaxation at room temperature. Mechanical agitation on a vortex mixer (Eppendorf, India) was performed. The mechanical vibrations induced increased the secretion of coelomic fluid. The modified Bradford protein assay was performed to determine the total protein content of the ECF of EE, EF and PE.
2.2. Cell line used and its maintenance
The human tongue cancer cell line SCC-9 was procured from the American Type Culture Collection (ATCC), (Virginia, USA). The cells were grown in MEM (Sigma-Aldrich, USA) supplemented with 4.5 g/l glucose, 2 mmol/l l-glutamine, 5% fetal bovine serum (growth medium) (Sigma-Aldrich, USA) and 1% penicillin at 37 °C in 5% CO2 incubator. During subculture, cells were trypsinized for detachment until they were 80% confluent.
2.3. Lactate dehydrogenase (LDH) assay
The LDH release assay was performed to assess the cytotoxic potential of ECF. The cultured SCC-9 cells were seeded in a 96 - well culture plate in 200 μl of culture media. Three replicates were prepared for each sample. The SCC-9 cells were treated with the ECF of EE, EF and PE at increasing concentrations of 2.5, 5, 10, 20, 40 and 80 μg/ml for 24 h. The supernatant of the cells was transferred to a 96-well plate. After adding the LDH reaction solution (100 μL) (Sigma-Aldrich, USA) the plate was incubated for 30 min. After incubation the absorbance was read on an ELISA plate reader per minute for 3 min. The following formula was used to calculate the LDH activity: LDH activity (U/L) = (OD/Min) × 16,030.
2.4. Clonogenic assay
The clonogenic assay assesses the reproductive viability of a colony of multiplying cells. The SCC-9 cells were harvested and plated as 1 × 103 cells per 35 mm dish on a 6-well plate in duplicates. The cells were incubated for 24 h in a CO2 incubator at 37 °C followed by incubation with ECF of EE, EF and PE at concentrations of 40 μg/ml and 80 μg/ml. Control dishes were also maintained with saline. After 24 h of treatment, the media was replaced with DMEM with FBS and incubated further at 37 °C for 4 weeks. The media was changed every week and incubated until cells in control plates had formed colonies that were of substantially good size.
Fixing and Staining of Colonies: The media was gently removed from each of the plates by aspiration followed by rinse with 1 ml PBS. The colonies were fixed with 1 ml of 3.7% PFA solution for 15–30 minutes. Staining was done with 1 ml 0.05% (w/v) crystal violet in PBS for 30 min. The excess crystal violet was washed with distilled water and the dishes were allowed to dry.
Colony Counting: Colonies containing more than 50 individual cells were counted using an inverted microscope. Digital images of the colonies were obtained using a CCD Camera (C-Mos, India)
The following formula was used to determine the Plating efficiency and Surviving fraction:
Number of colonies counted
2.5. Comet assay
The comet assay is also known as single cell gel electrophoresis assay. It is a simple method for measuring deoxyribonucleic acid (DNA) strand breaks in eukaryotic cells.
Preparation of base slides: Low melting point agarose (LMPA) 0.5% (250 mg per 50 ml PBS) and 1% normal melting agarose (NMA) (500 mg per 50 ml in Milli Q water) was prepared.
Isolation of cells: The cells were scraped off into the mincing solution using a Teflon scraper to yield approximately 1 × 105 cells/ml. 5–10 μl of the cell suspension was removed and mixed per 75 μl LMPA was processed with addition of equal amount of FBS.
Electrophoresis under pH > 13 alkaline conditions and Neutralization of Microgel Slides: Buffer reservoirs were filled with freshly made Electrophoresis Buffer (pH > 13) and the slides were placed in alkaline buffer for 20 min and the current was adjusted to 300 milliamperes.
Staining and Evaluation of DNA damage: The slides were stained with 80 μl of 1% Ethidium Bromide for 5 min. The slides were drained and kept in cold 100% ethanol for 20 min for dehydration. The slides were air dried and placed in an oven at 50 °C for 30 min. DNA damage was visualized using a fluorescent microscope (Olympus BX60, India). The length of DNA migration and the percentage of migrated DNA was analyzed using Image J software (version 1.47) with open comet plug-in. Generally, 50–100 randomly selected cells were analyzed per sample. The amount of migration per cell and the number of cells with increased tails were compared. Normal saline was maintained as control and hydrogen peroxide (H202) was used as a positive control. The values were analyzed by Dunnet’s multiple comparison test.
2.6. Cell cycle analysis
The cell cycle distribution of the SCC-9 cells was analyzed through a cell cycle analysis. Working solution: A stock solution of 1 mg/ml Propidium iodide - Cat # P4864, (Sigma-Aldrich, USA) was prepared and a working solution of 0.05 mg/ml was obtained. A working solution of 0.05 mg/ml of RNase A - Cat # 109 169, (Bochringer Mannhein, Germany) was also prepared. Procedure: The SCC-9 cells (1 × 106 cells) were cultured in a 6-well plate containing 2 ml of DMEM media with 4-2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer. After a 24-hour incubation period, cells were treated with samples of ECF of EE, EF, PE (concentration of 40 μg/ml and 80 μg/ml) and 1% DMSO as control in sample free DMEM media with HEPES buffer and incubated for 24 h. The SCC-9 cells were collected and pelleted cells at 1500 rpm for 5 min at room temperature, the supernatant was discarded. The pellet was resuspended in 200 μl of 1X PBS and fixed overnight at in 2 ml of 70% ethanol. After overnight fixation, centrifugation was done at 4000 rpm for 10 min at 4 °C. The supernatant was discarded and the pellet was washed two times with 2 ml of cold PBS. Later, the cells were incubated for 15 min at room temperature in 500 μl of sheath fluid containing 0.05 mg/ml PI and 0.05 mg/ml RNaseA. The percentage of cells in various stages of cell cycle in treated and un-treated populations were determined using FACS Caliber (BD Biosciences, San Jose, USA).
2.7. Annexin V – fluorescein isothiocyanate (FITC)/propidium iodide apoptosis assay
To evaluate the shift of phosphatidylserine (PS) from inner leaflets to outer leaflets of the plasma membrane, Annexin V – (FITC)/PI apoptosis detection kit (Thermo Fisher scientific, India) was utilized.
Procedure: Prior to induction of apoptosis, SCC-9 cells were plated as 1 × 106 cells per well in a 6-well plate using Dulbecco’s modified eagle media (DMEM) cell culture. After 18 h, the wells were replaced with new culture media to the original volume. The cells were incubated with 40 and 80 μg/ml concentrations of ECF of EE, EF and PE and incubated for 24 h. A rubber policeman was used to scrape and detach the cells from the dish followed by centrifugation. 500 μl of cell suspension was aliquoted and 10 μl of PI was added followed by 5 μL of Annexin V – FITC. Post incubation, the cells were analyzed by a flow cytometer (BD Biosciences, San Jose, USA).
2.8. Acridine orange/ethidium bromide (AO/EB) dual staining
AO/EB staining is an economic and convenient method to detect apoptosis in tumor cells. SCC-9 cells (25 μl - 1 × 10 5 cells) treated with ECF of EE, EF and PE and untreated control cells were taken separately in micro centrifuge tubes and were stained with dual fluorescent staining solution (5 μl) containing 100 μg/ml AO and 100 μg/ml EB (BD Pharmingen, San Jose, USA) for 2 min followed by gentle mixing. The morphology of apoptotic cells was examined with a fluorescence microscope (Olympus BX60, India).
3. Results
3.1. Collection of earthworm coelomic fluid and Protein Estimation
A final volume of ECF collected for EE, EF and PE was 3.2 ml, 3.0 ml and 2.8 ml. Bradford protein assay recorded the total protein content of EE, EF and PE as 1.34 mg/ml, 1.53 mg/ml and 1.9 mg/ml respectively. Dosing was performed to determine the quantity of ECF required in preparing standard concentrations of 40 μg/ml and 80 μg/ml for the cytotoxic studies.
3.2. Lactate dehydrogenase assay
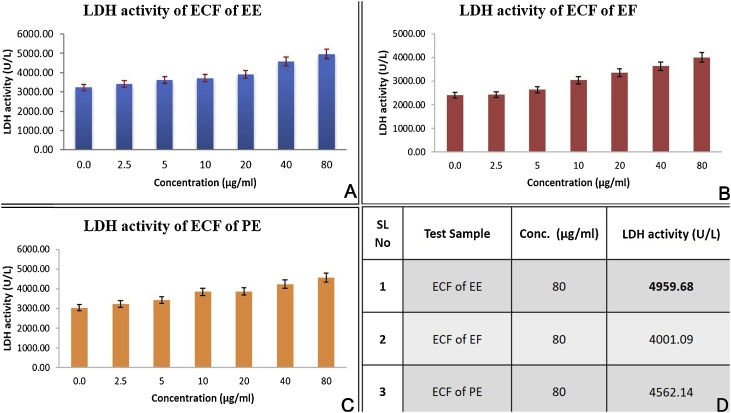
The presence of LDH enzyme in the cell culture medium is an indication of cell membrane damage [10]. The SCC – 9 cells treated with increasing concentrations of samples EE, EF and PE showed an increased release of LDH at higher concentrations compared to untreated cells (Table 1). ECF of EE showed maximum LDH release of 4959.68 IU/L at 80 μg/ml followed by ECF of PE (4562.14) and EF (4001.09 IU/L) (Fig. 1).
Table 1.
Summary of LDH enzyme release activity for samples ECF of EE, EF and PE in SCC-9 cells. ECF of EE induced maximum LDH activity in SCC-9 cells followed by ECF of PE and EF.
| Test Sample | Conc. (μg/ml) | Absorbance | LDH activity (U/L) |
|---|---|---|---|
| ECF of EE | 0.0 | 0.2015 | 3230.05 |
| 2.5 | 0.2135 | 3422.41 | |
| 5 | 0.2251 | 3608.35 | |
| 10 | 0.2315 | 3710.95 | |
| 20 | 0.2437 | 3906.51 | |
| 40 | 0.2849 | 4566.95 | |
| 80 | 0.3094 | 4959.68 | |
| ECF of EF | 0.0 | 0.1496 | 2398.09 |
| 2.5 | 0.1511 | 2422.13 | |
| 5 | 0.1645 | 2636.94 | |
| 10 | 0.1898 | 3042.49 | |
| 20 | 0.2099 | 3364.70 | |
| 40 | 0.2268 | 3635.60 | |
| 80 | 0.2496 | 4001.09 | |
| ECF of PE | 0.0 | 0.1896 | 3039.29 |
| 2.5 | 0.2011 | 3223.63 | |
| 5 | 0.2145 | 3438.44 | |
| 10 | 0.2398 | 3843.99 | |
| 20 | 0.2412 | 3866.44 | |
| 40 | 0.2638 | 4228.71 | |
| 80 | 0.2846 | 4562.14 |
Fig. 1.
Bar graph depicting the dose depending LDH enzyme generation of ECF on SCC-9 cells A. LDH activity of ECF of EE. B. LDH activity of ECF of EF. C. LDH activity of ECF of PE. D. Summary of LDH activity, ECF of EE shows maximum LDH activity of 4959 U/L at 80 μg/ml, followed by ECF of PE and EF.
3.3. Clonogenic assay
Colonies of greater than 50 cells were counted to determine the surviving fraction. (50 cells per colony is the minimum for scoring) [11]. The SCC-9 cells treated with 40 and 80 μg/ml of ECF of EE, EF and PE showed significant inhibition of colony forming capability compared to the controls. Of these, cells treated at 80 μg/ml of ECF of EE and PE showed significant inhibition of colony formation as compared to 40 μg/ml treated cells which showed approximately half the inhibition. ECF of EF showed comparatively lower inhibition with SF of 66.07% and 16.07% at 40 μg/ml and 80 μg/ml (Table 2). ECF of EE was found to be most efficient with SF of 25% and 1.79% at 40 μg/ml and 80 μg/ml respectively. ECF of PE demonstrated a surviving fraction of 32.14% at 40 μg/ml and 0% at 80 μg/ml (Fig. 2).
Table 2.
Clonogenic assay colony count table with plating efficiency and surviving fraction values. ECF of EE was found to be superior to ECF of PE and EF.
| Test Sample | Conc. (μg/ml) | Colony Count | Plating Efficiency | Surviving Fraction |
|---|---|---|---|---|
| Control | 0 | 56 | 1.12 | 100.00 |
| ECF of EE | 40 | 14 | 0.28 | 25.00 |
| 80 | 1 | 0.02 | 1.79 | |
| ECF of EF | 40 | 37 | 0.74 | 66.07 |
| 80 | 9 | 0.18 | 16.07 | |
| ECF od PE | 40 | 18 | 0.36 | 32.14 |
| 80 | 0 | 0 | 0.00 |
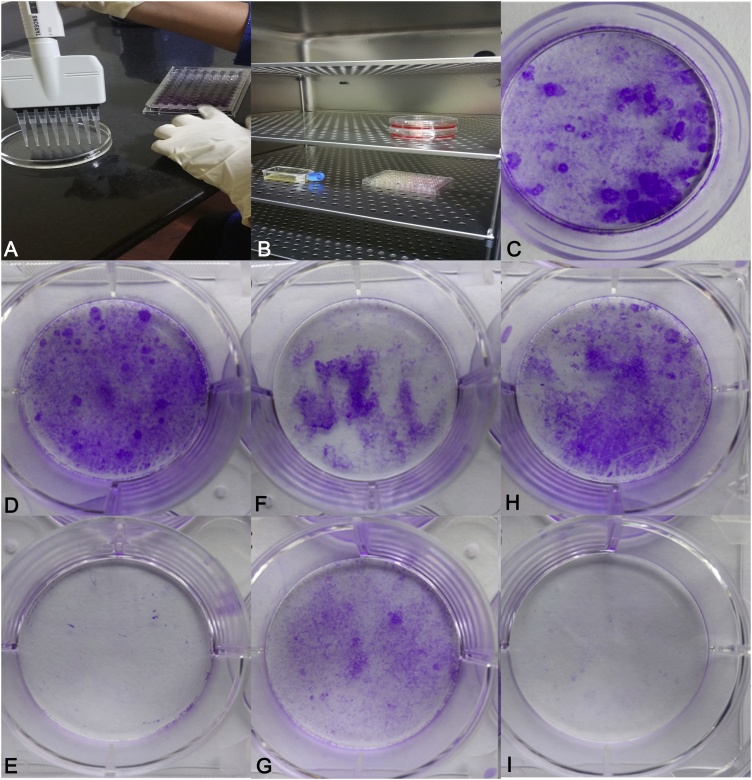
Fig. 2.
Clonogenic assay performed in 35 mm dish, with clones produced by SCC-9 tumor cells. A. Assay procedure and micro pipetting B. Incubation in CO2 incubator at 37 °C. C. Untreated SCC-9 control cells. D. Colonies produced after treatment with ECF of EE – 40 μg/ml. E. Colonies produced after treatment with ECF of EE – 80 μg/ml. F. Colonies produced after treatment with ECF of EF - 40 μg/ml. G. Colonies produced after treatment with ECF of EF – 80 μg/ml. H. Colonies produced after treatment with ECF of PE – 40 μg/ml. I. Colonies produced after treatment with ECF of PE – 80 μg/ml. ECF of EE and PE were most efficient in colony formation inhibition.
3.4. Comet assay
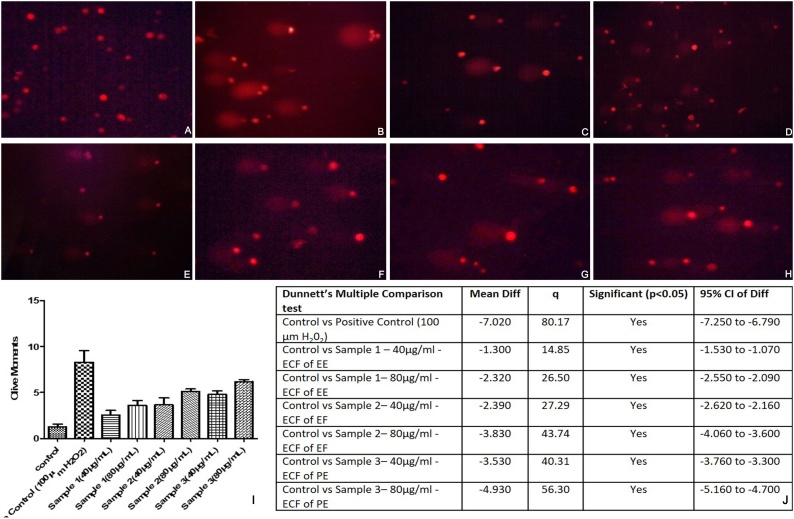
The SCC – 9 cells treated with 40 μg/ml and 80 μg/ml concentrations of ECF of EE, EF and PE showed increased DNA strand breaks and cytotoxicity. The results were statistically significant when compared to controls when analyzed with graph pad prism version 7.04 software (California, USA). The positive control hydrogen peroxide recorded a mean olive moment of 8.28 (Table 3). The fluorescent images obtained indicated ECF of PE to be most potent sample with mean olive moments of 4.79 and 6.19 at 40 μg/ml and 80 μg/ml respectively followed by ECF of EF (3.65 and 5.09 at 40 μg/ml and 80 μg/ml) and EE (2.56 and 3.58 at 40 μg/ml and 80 μg/ml). Dunnet’s multiple comparison test showed significant differences between species (Fig. 3).
Table 3.
Summary of olive moments recorded in test samples. Values are shown as mean ± SD of 3 replicates per experiment.
| Test Samples | Olive Moments (Mean ± SD) |
|---|---|
| Control | 1.26 ± 0.30 |
| Positive Control H2O2 (100 μM) | 8.28 ± 1.25 |
| Sample 1-(40 μg/ml) – ECF of EE | 2.56 ± 0.49 |
| Sample 1-(80 μg/ml) – ECF of EE | 3.58 ± 0.54 |
| Sample 2-(40 μg/ml) – ECF of EF | 3.65 ± 0.76 |
| Sample 2-(80 μg/ml) – ECF of EF | 5.09 ± 0.29 |
| Sample 3-(40 μg/ml) – ECF of PE | 4.79 ± 0.41 |
| Sample 3-(80 μg/ml) – ECF of PE | 6.19 ± 0.23 |
Fig. 3.
Florescence images of comets observed for different test samples. A. Comet images for control (saline) on SCC-9 cells. B. Comet images for positive control H2O2 at 100 μM. C. Comet images for ECF of EE – 40 μg/ml. D. Comet images for ECF of EE – 80 μg/ml. E. Comet images for ECF of EF – 40 μg/ml. F. Comet images for ECF of EF – 80 μg/ml. G. Comet images for ECF of PE – 40 μg/ml. H. Comet images for ECF of PE – 80 μg/ml. I. Graphical representation of DNA damage expressed in olive moments in Test samples J. Statistical evaluation of olive moments by Dunnett’s multiple comparison test. ECF of PE showed maximum DNA strand breakage capacity.
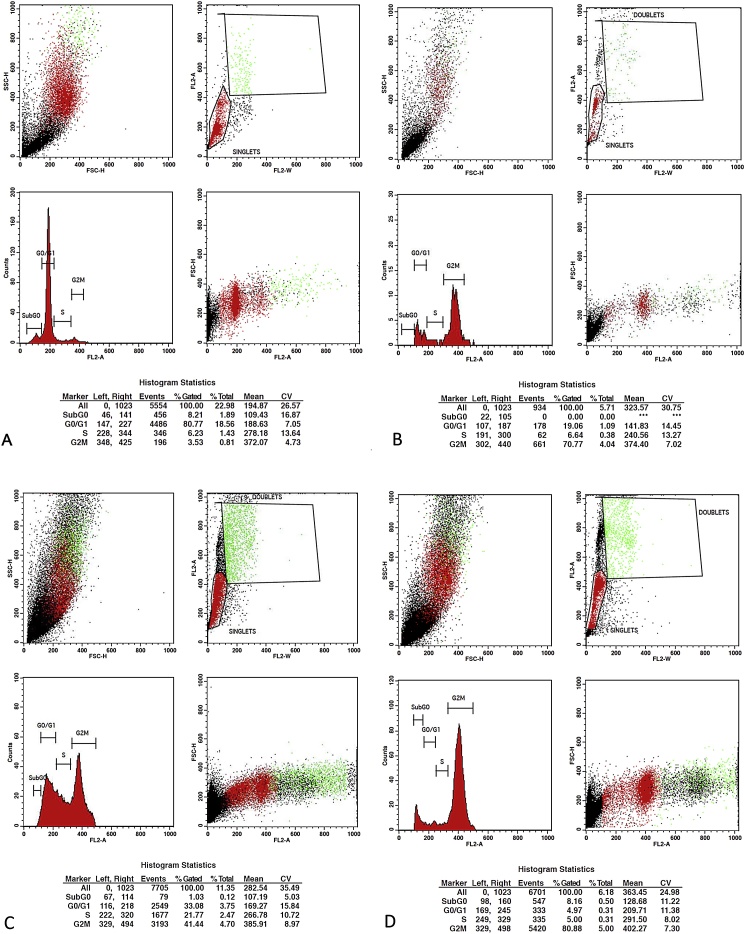
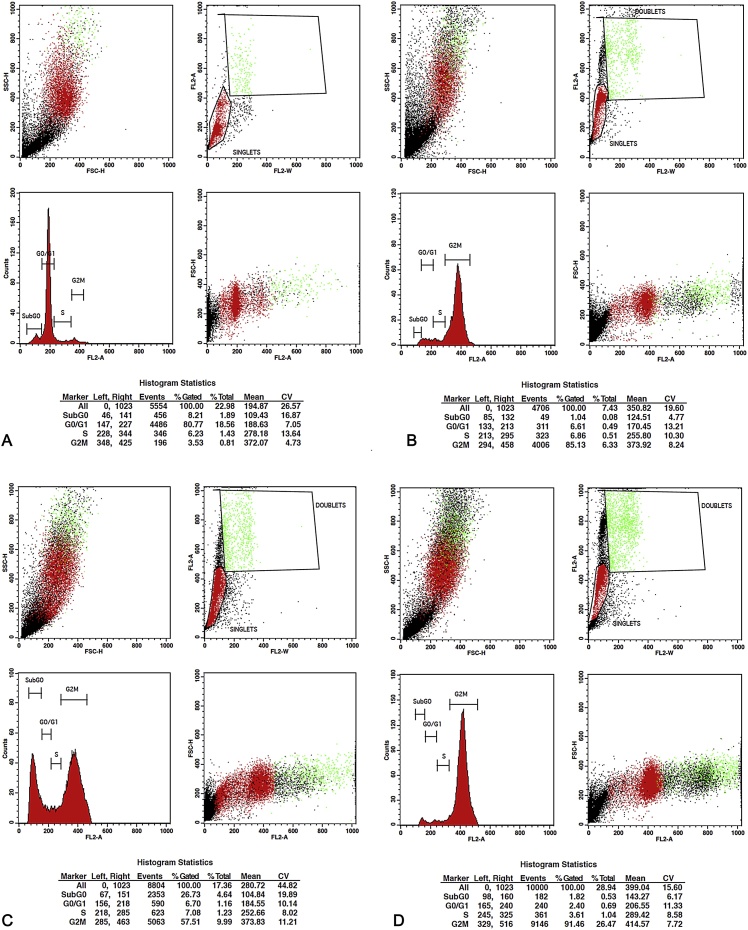
3.5. Cell cycle analysis
The ECF samples of EE, EF and PE showed effective cell cycle arrest. ECF of PE exhibited the highest arrest percentage of 80.88% and 91.46% at concentrations of 40 μg/ml and 80 μg/ml in G2M phase of cell cycle compared to untreated SCC-9 cells (3.53%) (Table 4). ECF of EE displayed a G2M phase cell cycle arrest of 70.77% and 85.13% followed by ECF of EF that recorded a lower arrest percentage of 41.44% and 57.51% respectively at 40 μg/ml and 80 μg/ml concentration (Fig. 4, Fig. 5). Pairwise Kruskal Wallis test comparing the G2M cell cycle arrest between the species at 40 μg/ml showed a statistically significant difference between species EE-EF (p = .004) and PE-EF (p = .004). At 80 μg/ml a statistical significance was observed between EF-PE (p = .001) (Table 5).
Table 4.
Comparison of G2M phase cell cycle arrest of ECF of EE, EF and PE at 40 μg/ml and 80 μg/ml on SCC-9 cells.
| Sl No | Test Sample | G2M Cell Cycle Arrest % | |
|---|---|---|---|
| 1. | Control Cells | – | 3.530 |
| 2. | ECF of EE | 40 μg/ml | 70.77 |
| 80 μg/ml | 85.13 | ||
| 3. | ECF of EF | 40 μg/ml | 41.44 |
| 80 μg/ml | 57.51 | ||
| 4. | ECF of PE | 40 μg/ml | 80.88 |
| 80 μg/ml | 91.46 | ||
Fig. 4.
Flowcytometry images for Cell Cycle Analysis. A. Flow Cytometry plot of SCC-9 cells treated with control. B. Flowcytometry plot for G2M arrest induced by ECF of EE – 40 μg/ml. C. Flowcytometry plot for G2M arrest induced by ECF of EF – 40 μg/ml. D. Flowcytometry plot for G2M arrest induced by ECF of PE – 40 μg/ml. ECF of PE exhibited highest arrest percentage of 80.88% at concentrations of 40 μg/ml, followed by ECF of EE and EF.
Fig. 5.
Flowcytometry images for Cell Cycle Analysis. A. Flow Cytometry plot of SCC-9 cells treated with control. B. Flowcytometry plot for G2M arrest induced by ECF of EE – 80 μg/ml. C. Flowcytometry plot for G2M arrest induced by ECF of EF – 80 μg/ml. D. Flowcytometry plot for G2M arrest induced by ECF of PE – 80 μg/ml. ECF of PE exhibited highest arrest percentage of 91.46% at concentrations of 80 μg/ml, followed by ECF of EE and EF.
Table 5.
Pairwise Kruskal Wallis test comparing the G2M cell cycle arrest between the species at 40 μg/ml and 80 μg/ml.
| Samples (at 40 μg/ml) | Test Statistic | Sig. | Adj. Sig |
|---|---|---|---|
| EE-PE | 5.333 | 0.021 | 0.063 |
| EE-EF | 10.500 | 0.001 | 0.004 |
| PE-EF | 10.500 | 0.001 | 0.004 |
| Samples (at 80 μg/ml) | Test Statistic | Sig. | Adj. Sig |
|---|---|---|---|
| EF-EE | 4.667 | 0.125 | 0.374 |
| EF-PE | −11.333 | 0.000 | 0.001 |
| EE-PE | −6.667 | 0.028 | 0.085 |
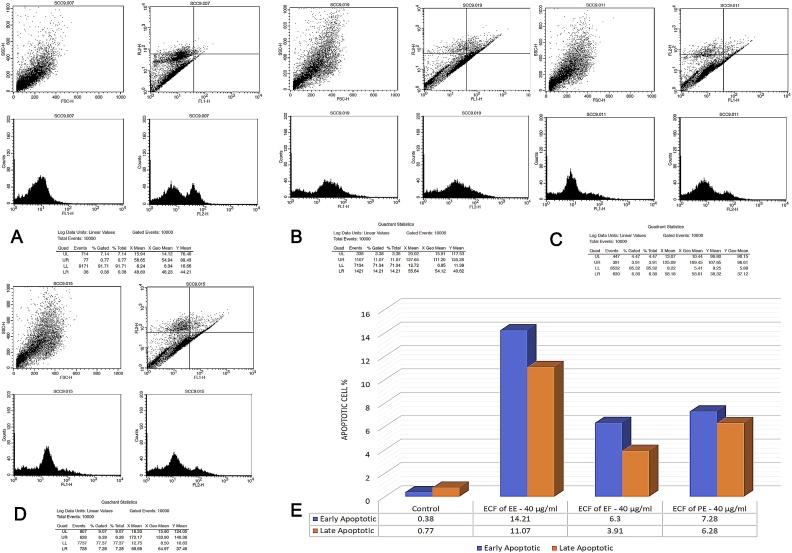
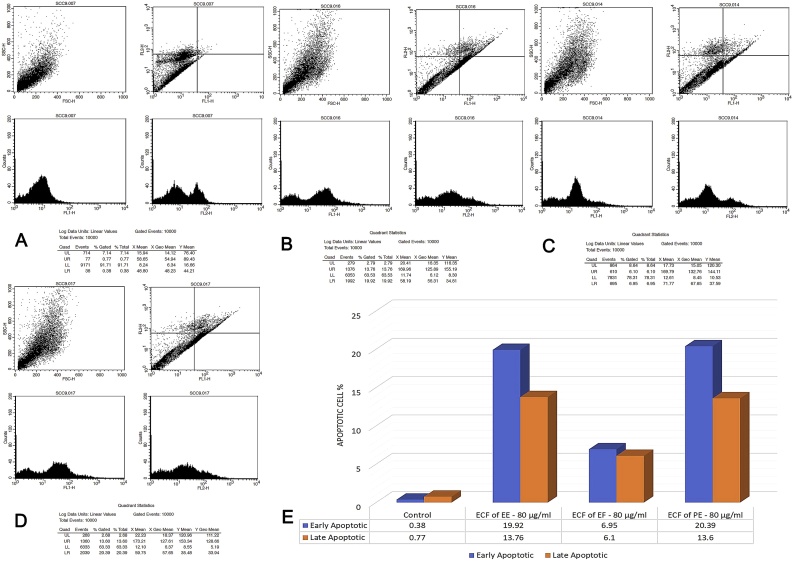
3.6. Annexin V – FITC/PI apoptosis assay
The lower left quadrant displayed the double negative (Annexin V –/ PI –) viable cells. Lower right quadrant displayed the early apoptotic cells (Annexin V +/ PI –). Upper right quadrant displayed the late apoptotic/damaged cells (Annexin V –/ PI +). and the upper left quadrant represented the non-viable double positive (Annexin V +/ PI +) necrotic/dead cells. The ECF of EE produced an early and late apoptotic rate of 14.21% and 11.07% apoptotic cells at 40 μg/ml. At 80 μg/ml early and late apoptotic rate was 19.92% and 13.76% respectively. ECF of EF at 40 μg/ml induced early and late apoptosis in SCC-9 cells with 6.30% and 3.91% cells, at 80 μg/ml early and late apoptotic rate was 6.95% and 6.10% cells respectively. ECF of PE induced early and late apoptotic rate of 7.28% and 6.28% at 40 μg/ml. At 80 μg/ml, 20.39% of early apoptotic cells and 13.60% late apoptotic cells were observed compared to untreated SCC-9 control that showed 0.38% and 0.77% of early and late apoptotic cells (Fig. 6, Fig. 7). Pairwise Kruskal Wallis test revealed a statistically significant difference between EF-EE at 40 μg/ml (p = 0.000). At 80 μg/ml a statistically significant difference was observed between EF-PE (p = 0.000) (Table 6).
Fig. 6.
Flowcytometry plots for Annexin V-FITC/PI assay (at 40 μg/ml). A. Flowcytometry plot for SCC-9 untreated control cells. B. Flowcytometry plot for SCC-9 cells treated with ECF of EE (40 μg/ml). C. Flowcytometry plot for SCC-9 cells treated with ECF of EF (40 μg/ml). D. Flowcytometry plot for SCC-9 cells treated with ECF of PE (40 μg/ml). E. Comparison of apoptotic cell percentage of ECF of EE, EF and PE at 40 μg/ml.
Fig. 7.
Flowcytometry plots for Annexin V-FITC/PI assay (at 80 μg/ml). A. Flowcytometry plot for SCC-9 untreated control cells. B. Flowcytometry plot for SCC-9 cells treated with ECF of EE (80 μg/ml). C. Flowcytometry plot for SCC-9 cells treated with ECF of EF (80 μg/ml). D. Flowcytometry plot for SCC-9 cells treated with ECF of PE (80 μg/ml). E. Comparison of apoptotic cell percentage of ECF of EE, EF and PE at 80 μg/ml.
Table 6.
Pairwise Kruskal Wallis test comparing the early and late apoptosis between the species.
| Samples (Early Apoptois) | Test Statistic | Std Error | Std Test Statistic | Sig. | Adj. Sig |
|---|---|---|---|---|---|
| EF-PE | −6.000 | 3.073 | −1.953 | 0.051 | 0.153 |
| EF-EE | 12.000 | 3.073 | 3.905 | 0.000 | 0.000 |
| PE-EE | 6.000 | 3.073 | 1.953 | 0.051 | 0.153 |
| Samples (Late Apoptois) | Test Statistic | Std Error | Std Test Statistic | Sig. | Adj. Sig |
|---|---|---|---|---|---|
| EF-EE | −6.000 | 3.077 | 1.950 | 0.051 | 0.154 |
| EF-PE | 12.000 | 3.077 | −3.899 | 0.000 | 0.000 |
| EE-PE | 6.000 | 3.077 | −1.950 | 0.051 | 0.154 |
3.7. Acridine orange-ethidium bromide dual staining
SCC-9 cells treated with ECF of EE showed early apoptotic cells at 40 μg/ml concentration, at 80 μg/ml it showed both early and late apoptotic cells. Cells treated with ECF of EF exhibited late apoptotic and necrotic cells at 40 μg/ml concentration, at 80 μg/ml it showed only necrotic cell morphology. Cells treated with ECF of PE displayed early apoptotic, late apoptotic and necrotic cells at 40 μg/ml concentration, at 80 μg/ml it showed only necrotic cell morphology (Fig. 8).
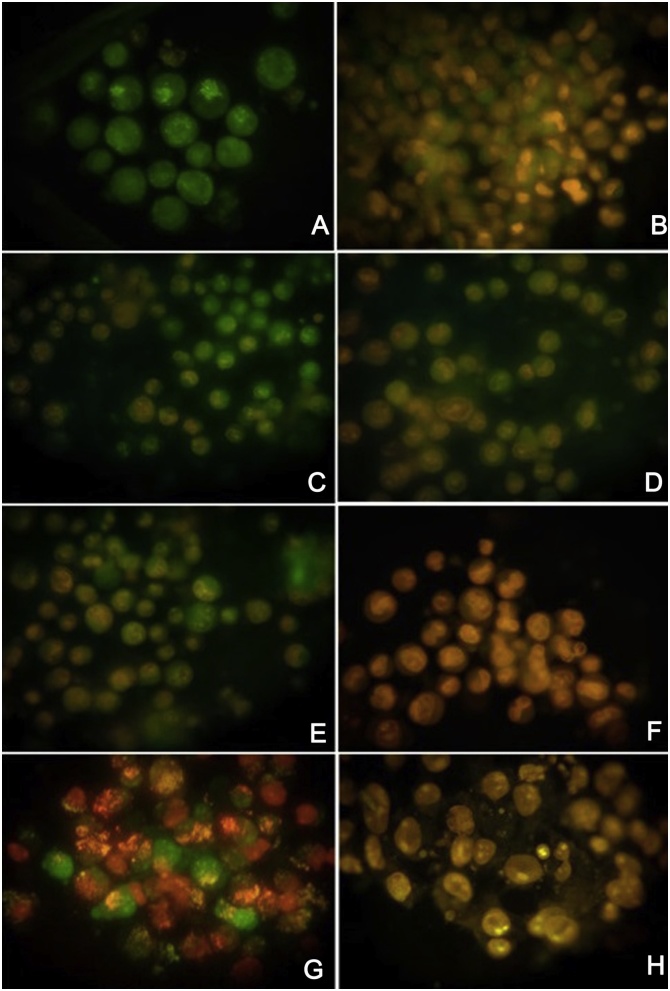
Fig. 8.
Fluorescent images of SCC-9 cells dual staining with AO/EB. A & B. Control cells stained with AO/EB. C. SCC-9 cells treated with ECF of EE at 40 μg/ml has early apoptotic cells. D. SCC-9 cells treated with ECF of EE at 80 μg/ml has early and late apoptotic cells. E. SCC-9 cells treated with ECF of EF at 40 μg/ml has late apoptotic and necrotic cells. F. SCC-9 cells treated with ECF of EF at 80 μg/ml has necrotic cells. G. SCC-9 cells treated with ECF of PE at 40 μg/ml has early apoptotic, late apoptotic and necrotic cells. H. SCC-9 cells treated with ECF of PE at 80 μg/ml has necrotic cells. ECF of EE had more apoptotic cell morphology compared to ECF of EF and PE.
4. Discussion
Oral cancer is documented as the sixth most common cancer globally with approximately 600,000 new cases diagnosed per year. Despite multimodal therapy, the survival rates for oral cancer treated by all modalities of treatment are approximately 40%–60% at 5 years [11,12]. Large randomized studies and meta-analyses have indicated higher response rates to chemoradiotherapy combinations than radiotherapy alone with significant adverse effects [13,14]. Natural extracts are advantageous in this regard as their therapeutic properties can be exploited with minimal adverse effects.
The medicinal benefits of earthworm extracts have been explored by researchers in the past few decades with notable antimicrobial, antipyretic, antiinflammatory, fibrinolytic and antioxidant properties [[15], [16], [17]]. The earthworms possess innate and adaptive immunity properties as a wide range of immunoprotective leukocytes are synthesized and secreted [18,19].
Few studies in the past decade have investigated the anticancer potential of ECF in vitro. Vidya et al. demonstrated significant cytotoxic effect of coelomocytes cell culture of Eudrilus eugeniae on A549 and HCT 116 cell lines in vitro [20]. Dinesh et al. evaluated the cytotoxic potential of coelomic fluid of Eudrilus eugeniae on HeLa cell, colon cancer cells, WBC malignant tumor and brain tumor cells in vitro and procured significant results [21]. Mohamed Jaabir et al. demonstrated the induction of apoptosis in SiHa cells in vitro by the cell free coelomic fluid of Eudrilus eugeniae [22]. The current study has comprehensively evaluated the cytotoxic effect of coelomic fluid of three species of earthworms with known protease activity on oral cancer cells as it is least explored in oral cancer in vitro providing an insight on cell cycle analysis and mechanism of cell death.
The cold shock method of fluid collection was utilized to collect the ECF, this safe method of fluid collection ensured viability of organisms after the procedure. This was followed by mechanical agitation on a vortex that increased the secretion of coelomic fluid. The total protein content of ECF was determined by modified Bradford protein assay, 40 μg/ml and 80 μg/ml were aliquoted for cytotoxic analysis.
The biology of cancer can be studied through cancer cell lines by evaluation of phenotypic and genotypic features resulting from cell divisions [23]. Oncogenic pathways can be demonstrated in cell lines independent of the surrounding environment. For several years testing in cell lines has remained the first step in drug discovery and enables evaluation of several complex therapeutic preparations prior to its use in large scale in vivo [24,25]. Drug testing in cell lines has several advantages over other laboratory models. Cell lines are valuable tools to study mechanism of drug action and drug combinations, screening for sensitivity and resistance, identification of specific protein targets that can be exploited for targeted therapy or drug delivery. Ramirez-Mares et al. conducted an in vitro study using aqueous extracts of Camellia sinensis, Ilex paraguariensis, and Ardisia compressa on HNSCC cell lines (OSCC-3, SCC-61, and SQ-20B) and concluded that Ardisia compressa inhibits HNSCC cell proliferation which makes this aqueous extract a potential source of chemo-preventive agents [26].
A recent study by Periyannan et al. used an in vivo model and showed that NAE syringic acid can attenuate oral carcinogenesis in buccal pouch of hamsters by modulating glycoconjugates and cytokeratin expression [27]. The SCC-9 cell line was employed in the present study which is a human oral squamous carcinoma cell line derived from tongue. This cell line was preferred as carcinoma of tongue is aggressive and has a high propensity for recurrence.
The membrane integrity of SCC-9 cells after exposure to ECF was determined by an enzyme release LDH assay. The LDH enzyme is a soluble cytosolic enzyme present in eukaryotic cells which is released following cell death due to plasma membrane injury. The LDH activity in the test sample is directly proportional to the lysed cells [28]. The assay is accurate and detects low level cellular damage. In the present study the ECF of EE showed maximum LDH activity of 4959.68 IU/L at 80 μg/ml followed by ECF of PE (4562.14) and EF (4001.09 IU/L). ECF of EE was superior in its action of SCC-9 cell lyses as a result of membrane integrity damage.
Colony forming ability of cancer cells enable them to communicate and build tumorigenicity, the inhibition effect of ECF on the colony formation capacity of SCC-9 was analyzed through an in vitro proliferation assay based on the ability of tumor cells to form colonies. The clonogenic assay assesses the cell’s survival by measuring the plating efficiency expressed as the percentage of cells giving rise to colonies seeded at subculture. The proliferative capacity for several cell generations can be measured by the plating efficiency [29]. ECF of EE was found to be most efficient among three species with SF of 25% at 40 μg/ml and only 1.79% at 80 μg/ml. The ECF of EE significantly inhibited colony formation capacity of the SCC-9 cells compared to controls.
DNA damage caused to SCC-9 cells by ECF was assessed by a single cell gel electrophoresis technique that is used for detecting single strand DNA breaks [30]. The comet assay has several advantages compared to other cytotoxicity assays as accurate data is obtained at the cellular level. It can be performed with few cells (<10,000) and is highly sensitive in detecting DNA damage. ECF of PE induced significant DNA breaks recorded as mean olive moments of 4.79 and 6.19 at 40 μg/ml and 80 μg/ml respectively followed by ECF of EF (3.65 and 5.09 at 40 μg/ml and 80 μg/ml) and EE (2.56 and 3.58 at 40 μg/ml and 80 μg/ml). The efficiency of ECF of PE at DNA strand breaking was superior compared to ECF of EE and EF.
The percentage of cells in various stages of cell cycle was evaluated by a cell cycle analysis. Before analysis, the cells are permeabilised and treated with PI that stains DNA quantitatively [31]. PI is also used to remove RNA hence it is used in conjunction with RNase. When stained diploid cells are analyzed through a flowcytometry, fluorescent intensities of a narrow distribution are obtained that are further analyzed [32]. The effect of three samples of ECF was evaluated by cell cycle analysis and flowcytometry which revealed that ECF of PE exhibited highest arrest percentage of 80.88% and 91.46% at concentrations of 40 μg/ml and 80 μg/ml in G2M phase of cell cycle compared to untreated SCC-9 cells (3.53%). ECF of EE showed a G2M phase cell cycle arrest of 70.77% and 85.13% followed by ECF of EF that recorded a lower arrest percentage of 41.44% and 57.51% respectively at 40 μg/ml and 80 μg/ml concentration. Recent studies have shown that cancer cells in the phase of cell cycle arrest are more sensitive to chemotherapeutic drugs. Cell cycle-arrested tumor cells exhibit increased sensitivity towards TNF-related apoptosis-inducing ligand induced apoptosis [33].
Further advanced evaluation of the ECF effect on SCC-9 cells was performed by an Annexin V – FITC/PI apoptotic assay. Annexin V is a useful tool in detecting apoptotic cells and belongs to a recently identified protein family. In the presence of calcium ions, it has affinity for negatively charged PS with deficient binding to sphingomyeline and phosphatidylcholine [34]. The change in PS symmetry can be analyzed by quantifying Annexin V cell membrane binding. These changes can be elicited before changes related to apoptosis that has occurred prior to loss of membrane integrity. With the use of flowcytometry apoptotic cells can be identified by conjugating Annexin V to FITC [35]. The ECF of EE at 40 μg/ml and 80 μg/ml concentration showed a higher apoptotic cell percentage compared to ECF of EF and PE. When compared to control untreated cells ECF of EE showed a 37-fold increase in early apoptotic cell percentage compared to controls. A higher early apoptotic rate of ECF of EE on SCC-9 cells indicates its apoptotic induction effect that is a desired effect on cancer cells.
To view the morphology of the SCC-9 cells after exposure to ECF an AO/EB dual staining was done. AO is a vital dye that stains both live and dead cells. Cell that have lost membrane integrity are stained by ethidium bromide. Cells appearing uniformly green are considered live cells. As a result of chromatin condensation and nuclear fragmentation early apoptotic cells will stain green with bright green dots. Orange stain will be taken up by the late apoptotic cells due to incorporation of ethidium bromide. Unlike necrotic cells, the late apoptotic cells will show fragmented and condensed nuclei. Orange stain is also taken up by the necrotic cells. However, the cells will resemble viable cells with a clear nuclear morphology and no condensed chromatin [[36], [37], [38]]. In the present study SCC-9 cells treated with ECF of EE showed early apoptotic cells at 40 μg/ml concentration, at 80 μg/ml it showed both early and late apoptotic cells. However, ECF of EF and PE had a mixture of early apoptotic, late apoptotic and necrotic cells at 40 μg/ml and 80 μg/ml concentration. ECF of EE demonstrated superior apoptotic induction effect elicited by the morphology of cells viewed. Apoptosis is a preferred mode of cell death compared to necrosis since it will not induce inflammation which may generate more damage.
To summarize the cytotoxic effect of ECF of EE, EF and PE on SCC-9 cells, it was observed that ECF of EE showed superior cytotoxic effect in LDH assay and clonogenic assay. ECF of EE was superior to ECF of PE in Annexin V – FITC/PI apoptosis assay. ECF of PE demonstrated superior DNA strand breaking ability in the comet assay and highest G2M cell cycle arrest percentage. AO/EB dual staining revealed early and late apoptotic cells induced by ECF of EE compared to a mixture of apoptotic and high number of necrotic cells induced by ECF of PE. Identifying the apoptotic pathways induced by ECF in cancer cells and utilizing them as targets would be the next step in this domain of research.
Another interesting finding in this study was exploration of earthworm specie Perionyx excavatus. PE is found in the Indian subcontinent which is a sparsely researched species for its beneficial medicinal effect, its anticancer properties have not been evaluated comprehensively in earlier studies and no study till date has evaluated its coelomic fluid for anticancer properties. The results of the present study demonstrated that ECF of PE had noteworthy anticancer effect on SCC-9 cells determined by high DNA strand breakage values in comet assay and a high G2M cell cycle arrest percentage with a significant apoptotic induction percentage on SCC-9 cell line. The result values were not far behind ECF of EE. The inhibitory effect of ECF of PE on other cancer cell lines is an interesting aspect to investigate. Studies on other cell lines have been planned to ascertain the effect of ECF on different tumor types. Recently investigators have also shown combinations of NAE’s with known anti-cancer drugs like cyclophosmamide to work favorably. Such combinations could counteract the cytotoxicity induced by synthetic anticancer drugs on normal cells. The NAE’s could provide a protective effect against the mutagenicity induced in normal cells by existing anticancer drugs [39,40].
5. Conclusion
The ECF of species EE, EF and PE has shown significant cytotoxic effect on oral cancer cell line SCC-9 with ECF of EE and PE showing optimum effect. The ECF arrested the oral cancer cells at G2M phase of cell cycle and induced apoptotic changes. The ECF of EE, EF and PE demonstrated variability in their cytotoxic effect on oral cancer cells. Information on the mechanisms of anticancer activity exhibited by the ECF on oral cancer cell lines is to be elicited with performance of gene expression and validation studies. ECF could possibly be developed as an adjunctive anticancer agent following isolation of principle component, animal experiments and future clinical trials. The ECF effect on inhibition of early diagnosed oral cancer would be interesting to elicit. Further translational cancer research towards development of anticancer drugs to combat oral cancer is mandated.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors thank Skanda Life Sciences Private Limited (Department for Scientific & Industrial Research recognized laboratory) Bangalore, India for assisting in laboratory procedures.
Contributor Information
Dominic Augustine, Email: dominic2germain@gmail.com.
Roopa S. Rao, Email: drroopasrao1971@gmail.com.
Jayaraman Anbu, Email: anbucologist@gmail.com.
K.N. Chidambara Murthy, Email: kncmurthy@gmail.com.
References
- 1.Huang J., Zhang J., Shi C., Liu L., Wei Y. Survival, recurrence and toxicity of HNSCC in comparison of a radiotherapy combination with cisplatin versus cetuximab: a meta-analysis. BMC Cancer. 2016;16(1):689. doi: 10.1186/s12885-016-2706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Machiels J.P., Lambrecht M., Hanin F.X. Advances in the management of squamous cell carcinoma of the head and neck. F1000Prime Rep. 2014;6(44) doi: 10.12703/P6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lupu M., Caruntu A., Caruntu C. Neuroendocrine factors: the missing link in non-melanoma skin cancer (Review) Oncol. Rep. 2017;38(3):1327–1340. doi: 10.3892/or.2017.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ang K.K., Zhang Q., Rosenthal D.I. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J. Clin. Oncol. 2014;32(27):2940–2950. doi: 10.1200/JCO.2013.53.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burtness B., Goldwasser M.A., Flood W., Mattar B., Forastiere A.A. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern cooperative oncology group study. J. Clin. Oncol. 2005;23(34):8646–8654. doi: 10.1200/JCO.2005.02.4646. [DOI] [PubMed] [Google Scholar]
- 6.Abraham M., Augustine D., Rao R.S., Sowmya S.V., Haragannavar V.C., Nambiar S., Prasad K., Awan K.H., Patil S. Naturally available extracts inhibiting cancer progression: a systematic review. J. Evid. Complement. Altern. Med. 2017;22(October (4)):870–878. doi: 10.1177/2156587217744914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Augustine D., Rao R.S., Anbu J., Chidambara Murthy K.N. Anticancer prospects of earthworm extracts: a systematic review of in vitro and in vivo studies. Phcog. Rev. 2018;12:46–55. [Google Scholar]
- 8.Augustine D., Rao R.S., Anbu J., Chidambara Murthy K.N. In vitro antiproliferative effect of earthworm coelomic fluid of Eudrilus eugeniae, Eisenia foetida, and Perionyx excavatus on squamous cell carcinoma-9 cell line: a pilot study. Phcog. Res. 2017;9(Suppl. S1):61–66. doi: 10.4103/pr.pr_52_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Augustine D., Rao R.S., Jayaraman A., Chidambara Murthy K.N. Anti-proliferative activity of earthworm coelomic fluid using oral squamous carcinoma KB 3-1 cells: an in vitro study with serine protease analysis. Phcog. Mag. 2018;14:528–534. [Google Scholar]
- 10.Chan F.K., Moriwaki K., De Rosa M.J. Detection of necrosis by release of lactate dehydrogenase activity. Methods Mol. Biol. 2013;979:65–70. doi: 10.1007/978-1-62703-290-2_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferlay J., Soerjomataram I., Dikshit R. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 12.Gregoire V., Lefebvre J.L., Licitra L., Felip E., for the EHNS-ESMOESTRO Guidelines Working Group Squamous cell carcinoma of the head and neck: EHNS-ESM–ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2010;21:v184–86. doi: 10.1093/annonc/mdq185. [DOI] [PubMed] [Google Scholar]
- 13.Pignon J.P., le Maitre A., Maillard E. MACH-NC Collaborative Group: meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomized trials and 17,346 patients. Radiother. Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Cooper J.S., Pajak T.F., Forestiere A.A. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 15.Balamurugan M., Parthasarathi K., Cooper E.L., Ranganathan L.S. Earthworm paste (Lampito mauritii, Kinberg) alters inflammatory, oxidative, hematological and serum biochemical indices of inflamed rat. Eur. Rev. Med. Pharmacol. Sci. 2007;11:77–90. [PubMed] [Google Scholar]
- 16.Omar Hossam M., Ibraheim Zedan Z., El-Shimy Nasser A., Ali Rouwaida S. Anti-inflammatory, antipyretic and antioxidant activities of the earthworm’s extract. J. Biol. Earth Sci. 2012;2:10–17. [Google Scholar]
- 17.Verma Yogendra Kumar, Verma Mahendra Kumar. Earthworm—a potential source for stable and potent antimicrobial compounds- isolation and purification study. Int. J. Pharm. Sci. 2012;4:540–543. [Google Scholar]
- 18.Cooper E.L., Balamurugan M., Huang C.Y., Tsao C.R., Heredia J., Tommaseo-Ponzetta M., Paoletti M.G. Earthworms dilong: ancient, inexpensive. Noncontroversial models may help clarify approaches to integrated medicine emphasizing neuroimmune systems. Evid. - Based Complement. Altern. Medicine. 2012;2012:1–11. doi: 10.1155/2012/164152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper E.L., Cossarizza A., Kauschke E., Franceschi C. Cell adhesion and the immune system: a case study using earthworms. Micros Res. Technnol. 1999;44:237–253. doi: 10.1002/(SICI)1097-0029(19990215)44:4<237::AID-JEMT4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 20.Vidya N., Dinesh M.S., Ananda S., Kale Radha D. Cytotoxic Potential of Eudrilus eugeniae coelomcyte culture supernatant against tumor cells. Int. J. Sci. Res. 2016;6(8):202–205. [Google Scholar]
- 21.Dinesh M.S., Sridhar S., Chandana P.G., Pai V., Geetha K.S., Naveen Hegde R. Anticancer potentials of peptides of coelomic fluid of earthworm Eudrilus eugeniae. Biosci. Biotechnol. Res. Asia. 2013;10:601–606. [Google Scholar]
- 22.Mohamed Jaabir M.S., Shamsheerali L., Yasar M., Senthil Kumar S. Evaluation of the cell‑free coelomic fluid of the earthworm Eudrilus euginiae to induce apoptosis in SiHa cell line. J. Pharm. Res. 2011;4:3417–3420. [Google Scholar]
- 23.Raju K.L., Augustine D., Rao R., Sowmya S.V., Haragannavar V., Nambiar S., Prasad K., Awan K., Patil S. Biomarkers in tumorigenesis using cancer cell lines: a systematic review. Asian Pac. J. Cancer Prev. 2017;18(9):2329–2337. doi: 10.22034/APJCP.2017.18.9.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burdall S., Hanby A., Lansdown M., Speirs V. Breast cancer cell lines: friend or foe? Breast Cancer Res. 2003;5:89–95. doi: 10.1186/bcr577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferreira D., Adega F., Chaves R. The importance of cancer cell lines as in vitro models in cancer methylome analysis and anticancer drugs testing. In: Lopez-Camarillo C., Arechaga-Ocampo E., editors. Oncogenomics and Cancer Proteomics — Novel Approaches in Biomarkers Discovery and Therapeutic Targets in Cancer. InTech; 2013. [Google Scholar]
- 26.Ramirez-Mares M.V., Kobayashi H., de Mejia E.G. Inhibitory effect of Camellia sinensis, Ilex paraguariensis and Ardisia compressa tea extracts on the proliferation of human head and neck squamous carcinoma cells. Toxicol. Rep. 2016;3:269–278. doi: 10.1016/j.toxrep.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Periyannan V., Veerasamy V. Syringic acid may attenuate the oral mucosal carcinogenesis via improving cell surface glycoconjugation and modifying cytokeratin expression. Toxicol. Rep. 2018;5:1098–1106. doi: 10.1016/j.toxrep.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith S.M., Wunder M.B., Norris D.A., Shellman Y.G. A simple protocol for using an LDH-based cytotoxicity assay to assess the effects of death and growth inhibition at the same time. PLoS One. 2011;6(11):e26908. doi: 10.1371/journal.pone.0026908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franken N.A., Rodermond H.M., Stap J., Haveman J., van Bree C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006;1:2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 30.Olive Peggy L., Banath Judit P. The comet assay: a method to measure DNA damage in individual cells. Nat. Protoc. 2006;1:23–29. doi: 10.1038/nprot.2006.5. [DOI] [PubMed] [Google Scholar]
- 31.Pozarowski P., Darzynkiewicz Z. Analysis of cell cycle by flow cytometry. In: Schonthal A.H., editor. vol. 281. Humana Press; 2004. (Checkpoint Controls and Cancer. Methods in Molecular Biology). [DOI] [PubMed] [Google Scholar]
- 32.Azimian-Zavareh V., Hossein G., Janzamin E. Effect of lithium chloride and antineoplastic drugs on survival and cell cycle of androgen-dependent prostate cancer LNCap cells. Indian J. Pharmacol. 2012;44(6):714–721. doi: 10.4103/0253-7613.103265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ehrhardt E.D.H., Wachter F., Grunert M., Jeremias I. Cell cycle-arrested tumor cells exhibit increase sensitivity towards TRAIL-induced apoptosis. Cell Death Dis. 2013;4:e661. doi: 10.1038/cddis.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Span L.F., Pennings A.H., Vierwinden G., Boezeman J.B., Raymakers R.A., de Witte T. The dynamic process of apoptosis analyzed by flow cytometry using Annexin-V/propidium iodide and a modified in situ end labeling technique. Cytometry. 2002;47:24–31. [PubMed] [Google Scholar]
- 35.Vermes I., Haanen C., Steffens-Nakken H., Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods. 1995;184(1):39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 36.Smith S.M., Ribble D., Goldstein N.B. Elsevier Inc.; Chennai, India: 2012. A Simple Technique for Quantifying Apoptosis in 96-well Plates PMC (Ed.), Laboratory Methods in Cell Biology (1st Edn) pp. 361–368. [Google Scholar]
- 37.Liu K., Liu P., Liu R., Wu X. Dual AO/EB staining to detect apoptosis in osteosarcoma cells compared with flow cytometry. Med. Sci. Monit. Basic Res. 2015;21:15–20. doi: 10.12659/MSMBR.893327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kasibhatla S. Acridine orange/ethidium bromide (AO/EB) staining to detect apoptosis. CSH Protoc. 2006;1(August (3)) doi: 10.1101/pdb.prot4493. 2006 pii: pdb.prot 4493. [DOI] [PubMed] [Google Scholar]
- 39.Kour J., Ali M.N., Ganaie H.A., Tabassum N. Amelioration of the cyclophosphamide induced genotoxic damage in mice by the ethanolic extract of Equisetum arvense. Toxicol. Rep. 2017;4:226–233. doi: 10.1016/j.toxrep.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yousef M.I., Khalil D.K.A.M., Abdou H.M. Neuro- and nephroprotective effect of grape seed proanthocyanidin extract against carboplatin and thalidomide through modulation of inflammation, tumor suppressor protein p53, neurotransmitters, oxidative stress and histology. Toxicol. Rep. 2018;5:568–578. doi: 10.1016/j.toxrep.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]