Highlights
-
•
Naringenin and Quercetin decrease ROS and potentiate enzymatic antioxidant production in the hippocampus.
-
•
cART induced marked cytoplasmic shrinkage and several pyknotic nuclei in the dentate gyrus and cornus ammonis region.
-
•
Naringenin and Quercetin attenuates cART-induced upregulation of monoamine oxidase-B expression in neurons.
-
•
Naringenin and Quercetin also ameliorates cART-induced spatial memory impairments.
-
•
Naringenin and Quercetin acted as effective antioxidants in vivo against cART-induced neurotoxicity.
Abbreviations: cART, combination antiretroviral therapy; MAO-B, monoamine oxidase B; TNFα, tumor necrosis factor alpha; CAT, catalase; GSH, reduced glutathione; SOD, superoxide dismutase; MDA, malondialdehyde; TBA, thiobarbituric acid; ELISA, enzyme-linked immunosorbent assay; HCL, hydrochloric acidE; DTA, ethylenediaminetetraacetic acid; ROS, reactive oxygen species; DMSO, dimethyl sulfoxide; Nar, naringenin; Quer, quercetin; cA/N, 24 mg/kg combination antiretroviral therapy + 50 mg Naringenin; CA/Q, 24 mg/kg combination antiretroviral therapy + 50 mg Quercetin
Keywords: Neurodegeneration, cART, Naringenin, Quercetin, Hippocampus, Oxidative stress
Abstract
Introduction
In spite of the multiple benefits of combination antiretroviral therapy (cART) on HIV positive patients, prolonged usage has been reported to exacerbate oxidative stress, and induce neurological and cognitive dysfunction, thus, the need to search for an adjuvant therapy to ameliorate the oxidative and improve treatment adherence with better virological outcome. This study aimed at determining the potential therapeutic effects of Quercetin and Naringenin on cART-induced cyto-architectural, neuro-behavioral and immunohistochemical changes in the hippocampus of the adult Wister rats.
Materials and Methods
The animals were grouped as follows: Control, DMSO, 24 mg/kg cART (Tenovovir 300 mg, Lamivudine 300 mg and Efavirenz 600 mg), 50 mg/kg Naringenin, 50 mg/kg Quercetin, cART + Naringenin, cART + Quercetin were administered orally for 8 weeks. At the end of administration, neurobehavioural test was conducted, animals were euthanized and hippocampus was processed for oxidative stress markers, histology, TNF-α, and Monoamine oxidase-B expression.
Results
At the end of 8 weeks of administration, 24 mg/kg cART decreased superoxide dismutase (SOD), catalase (CAT), reduced glutathione (GSH) and increased Malondialdehyde (MDA). Whereas, 50 mg/kg quercetin, and 50 mg/kg Naringenin decreased the oxidative stress (increased SOD, CAT, GSH, and reduced MDA) induced by cART (reduced SOD, CAT, GSH, and increased MDA). In addition, hematoxylin and eosin stained hippocampus showed that quercetin and naringenin prevented neurodegenerative changes (marked cytoplasmic shrinkage and several pyknotic nuclei in the dentate gyrus and cornus ammonis regions) in cART-treated rats. Furthermore, immunohistochemical studies revealed that quercetin and naringenin attenuates cART-induced upregulation of monoamine oxidase-B (MAO-B) expression. Likewise, from the Morris water maze neurobehavioral studies, naringenin and quercetin also ameliorated cART-induced memory impairments (initial spatial memory, reversal spatial memory and probe tests).
Conclusion
This study shows that Naringenin and Quercetin have a good potential in reversing cART-induced hippocampal disorders in Wistar rats.
1. Background
An average of 37 million people in the world are living with Human Immunodeficiency Virus (HIV) (WHO, 2016). The infection if untreated results in acquired immunodeficiency syndrome (AIDS) which has resulted in the death of over 35 million lives since the epidemic started (UNAIDS, 2017a). Albeit, access to effective combination antiretroviral therapy (cART) has resulted in a significant reduction in AIDS-related morbidity and mortality. More so, UNAIDS targets a decrease from 1 million AIDS-related death in 2016 to 500,000 in 2020, by ensuring that at least 30 million people living with HIV are on cART (UNAIDS, 2017b).
The cART which involves the concurrent use of a combination of three or more antiretroviral drugs to suppress HIV replication (WHO, 2016) has reduced HIV to a manageable disease however, the reports of their adverse effects resulting in organo-toxicities, gross neurological and cognitive dysfunction among HIV infected patients and experimental animals have become a growing concern (Solomon et al., 2017; Adana et al., 2018). Though HIV infects microglial cells leading to severe tissue damage in the central nervous system (CNS); cognitive dysfunction, learning and memory impairments (leading to dementia), and other neurological disorders were observed among patients on cART (Heaton et al., 2011). Whether or not the neurological impairments are associated with cART or the virus itself remains unclear (Solomon et al., 2017). It’s been reported that cART penetrates the blood brain barrier, thus, chronic dependence has a high probability of affecting neurological activities (Robertson et al., 2010). In addition, Anthony et al. (2005) reported that cART causes neuroinflammation in HIV infected patients by inducing microglial reaction especially in the hippocampus which mediates several higher brain functions, such as learning, memory, and spatial coding.
The oxidative stress which is a vital precursor of most diseases is upregulated in continuous and prolonged use of cART (Akay et al., 2014). This may contribute to the myriads of HIV associated neurocognitive disorders (HAND) (Anthony and Bell, 2009; Heaton et al., 2011; Yew et al., 2018). Hence, the upregulation of endogenous antioxidants may be a plausible mechanism to antagonize these drawbacks (Stern et al., 2017).
The use of bioflavonoids in attenuating oxidative stress induced diseases has generated so much interest, as they possess the ability to capture reactive oxygen species (ROS) such as superoxide, hydroxyl and lipid radicals (Robak and Gryglewski, 1988). Flavonoids which are rich phytochemical extracts improve memory, learning and other cognitive functions (Rendeiro et al., 2012). They also protect neurons from neurotoxins by enhancing existing neuronal function, suppressing neuroinflammation and by stimulating neuronal regeneration (Williams and Spencer, 2012) as seen in the treatment of neurodegenerative disorders (Vauzour et al., 2008).
Both Naringenin and Quercetin are major free radical scavengers. Quercetin, one of the most widely distributed flavonoids in fruits and vegetables (eg. red onion, common onion, cranberry, and blueberry) has been reported to inhibit the oxidation of other molecules and hence is classified as an antioxidant (Satyanarayana et al., 2001). Naringenin, commonly found in grape fruits has been reported by Adana et al. (2018) to attenuate EFV/FTC/TDF induced toxicity in rats. These bioflavonoids have also been reported to boost endogenous antioxidants and possess anti-apoptotic properties (Loke et al., 2008; Pan et al., 2010; Sanchez-Gonzalez et al., 2011). The ability of these flavonoids/metabolites to penetrate the blood-brain barrier (BBB) seems to be dependent on their lipophilicity and their interactions with specific efflux transporters (such as p-glycoproteins) expressed in the BBB (Youdim et al., 2004).
Hence, this study is aimed at exploring the therapeutic potential of Naringenin and Quercetin on cART induced Hippocampal disorders.
2. Materials and methods
2.1. Chemicals, reagents and drugs
Quercetin (Cat No: Q4951-100 G), Naringenin (Cat No: W530098-500 G), and Dimethyl sulfoxide (DMSO) (Cat No: 317275-500ML), were purchased from Sigma Aldrich South Africa. While cART a combination of Efavirenz 600 mg, Lamivudine 300 mg, Tenofovir Disoproxil Fumarate 300 mg, manufactured by MACLEODS pharmaceuticals, India was provided by the AIDS Prevention Initiative in Nigeria (APIN) center at Lagos University Teaching Hospital (LUTH).
2.2. Experimental animals and design
Seventy (70) adult male Wistar rats with weights ranging from 225 to 260 g were used for this study. The animals were acclimatized for three weeks in the Animal House, Department of Anatomy, College of Medicine of the University of Lagos under standard animal housing condition of 24 ± 2 °C and 12/12 h light/dark cycle. The rats had access to standard rat chow and water ad-libitum. All procedures were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996. This research was approved by the Health Research Ethics Committee of the College of Medicine of the University of Lagos with protocol number CMUL/HREC/03/17/113. The animals were randomly divided into seven groups (Control, DMSO, Nar. Quer, cART, cA/N, and cA/Q) of 10 animals each. The control group received 1 ml distilled water; DMSO group received 1% v/v of Dimethyl Sulfoxide, while cART group received 24 mg/kg cART (Tenofovir 300 mg + Lamivudine 300 mg + Efavirenz 600 mg) (WHO, 2016); Nar received 50 mg/kg Naringenin (Lian et al., 2018); Quer received 50 mg/kg Quercetin (Carvalho et al., 2018); Group cA/N received a mixture of 24 mg/kg cART and 50 mg/kg Naringenin; Group cA/Q received a mixture of 24 mg/kg cART and of 50 mg/kg body weight of Quercetin. cART was dissolved in distilled water while the rest of the experimental groups were dissolved in 1% v/v DMSO. Oral route of administration was used for the 56 days of treatment (Adana et al., 2018).
2.3. Animal sacrifice and sample collection
At the end of the experiment, all the animals were euthanized, using urethane (Li et al., 2017). The hippocampus of the rats (n = 5) were dissected on an ice stage and preserved at −80 °C for oxidative stress markers and TNF-α analysis. Other animals (n = 5) for immunohistochemistry of Monoamine oxidase-B (MAO-B) and basic histology (H and E stain) were perfused with 10% neutral buffered formalin, the whole brain was fixed in the same fixative.
2.4. ELISA
For the preparation of oxidative stress markers and TNFα, 0.5 g of the hippocampus was homogenized using tissue homogenizer in 4.5 ml of 0.4 M sodium phosphate buffer (pH 7.0), centrifuge at 3500 rpm for 10 min and the supernatant removed for the assay.
2.4.1. Assessments of total protein, superoxide dismutase (SOD), catalase (CAT) and reduced glutathione (GSH) in hippocampus
Total protein was first determined by Biuret methods. In the Biuret reaction, the cupric ions in the reagent join with the peptide bonds of the protein molecules in an alkaline solution to form a blue-violet coloured complex. Briefly, for each sample, 1.0 ml of biuret reagent (test) and 1.0 ml of blank reagent (reagent blank) were pipetted into test tubes. 0.02 ml (20 μl) of each sample was added to the test and 20 μl water to the blank. A standard test tube was also set up for each batch and contained 20 μl of standard protein 1.0 ml of biuret reagent. This was mixed and allowed to stand at room temperature for 30 min. The instrument was zeroed with the reagent blank solution and the absorbance of the test and the standard was measured at 546 nm wavelength.
Total SOD activity in tissue homogenates was determined following the procedure of Marklund and Marklund (1974) with some modifications. The method is based on the ability of SOD to inhibit the autoxidation of pyrogallol. 970 μL of buffer (100mMTris−HCl, 1 mM EDTA, pH 8.2), 10 μL of homogenates and 20 μL pyrogallol (13 Mm) were mixed. Assay was performed in cuvettes at 25 °C for 5 min and changes in absorbance per minute were recorded using a spectrophotometer at 480 nm.
Catalase (CAT) was expressed as moles of hydrogen peroxide (H2O2) consumed/min/mg protein. The reaction mixture (1.5 ml) contained 1.0 ml of 0.01 M pH 7.0 phosphate buffer and 0.4 ml of 2 M H2O2. Assay was performed in cuvettes at 25 °C for 3–5 minutes and changes in absorbance per minute were recorded using a spectrophotometer at 620 nm. (Sinha, 1972).
Reduced glutathione (GSH) was determined by the method described by Ellman (1959). To the homogenate was added 10% Trichloroacetic acid (TCA) (equal volume) and centrifuged. 1.0 ml of supernatant was treated with 0.5 ml of Ellman’s reagent (19.8 mg of 5, 5′-dithiobisnitro benzoic acid (DTNB) in 100 ml of 0.1% sodium nitrate) and 3.0 ml of phosphate buffer (0.2 M, pH 8.0). The absorbance was read at 412 nm.
2.4.2. Determination of Lipid Peroxidation (LPO) through assessment of Malondialdehyde (MDA) in hippocampus
The assay for membrane lipid peroxidation (LPO) was done in accordance with some modifications from Tsikas (2017). The reaction mixture in a total volume of 3.0 ml contained 1.0 ml tissue homogenate, 1.0 ml of TCA (10%), and 1.0 ml TBA (Thiobarbituric acid) (0.67%). All the test tubes were placed in a boiling water bath for a period of 45 min. The tubes were shifted to ice bath and then centrifuged at 2500×g for 10 min. The amount of Malondialdehyde (MDA) formed in each of the samples was assessed by measuring the optical density of the supernatant at 532 nm. The results were expressed as the nmol MDA formed/gram tissue by using a molar extinction coefficient of 1.56 × 105 M−1 cm−1. With the help of formula
2.4.3. Tumor Necrosis Factor Alpha (TNF-α) analysis in hippocampus
TNF-α assay was determined using rat specific Elabscience enzyme immunoassay (ELISA) kit with a catalog No: E-EL-R0019 in accordance with manufacturer`s instructions. Briefly, the cells inoculated in 24-well-plates were treated with 20 μg/ml SEB (24 μl/well) or 24 μl/well PBS for 36 h and centrifuged at 200×g for 5 min. The supernatants were transferred to 96-well-plates (100 μl per well) and incubated at 37 °C for 90 min. Afterward, 100 μl of biotinylated detection antibody was added and incubated at 37 °C for 1 h. The wells were aspirated and washed thrice, and 100 μl of HRP conjugate was added and incubated at 37 °C for 30 min. The wells were subsequently washed five times, and 90 μl substrate solution was added and incubated at 37 °C for 15 min. Then, 50 μl of the stop solution was added. The OD of each well was determined by using a spectrophotometer (Molecular Devices) at 405 nm. The concentration of TNFα was calculated as picogram per milliliter (pg/ml) on the basis of the standard curve.
2.5. Tissue processing for microscopic study
The tissue samples were fixed in 10% neutral buffered formalin and processed for histology and immunohistochemistry via paraffin embedding after which they were sectioned at 4 μm using a Thermo Scientific HM 325 rotatory microtome (CE Bright Company Ltd. Huntington England), cleared in xylene and hydrated in decreasing alcohols, stained with Hematoxylin and Eosin (H&E) and mounted with DPX (Djidja et al., 2017). The histological specimen were captured and examined using the Leica SlidePath Gateway LAN software.
2.6. Immunohistochemical (IHC) analysis
Brain tissues were taken from 10% neutral buffered formalin and transferred to 70% ethanol. They were then processed using a graded ethanol series and embedded in paraffin. The paraffin sections were cut into 4 μm-thick slices using a microtome (Microm HM 315 microtome, Walldorf, Germany). The processed and sectioned tissues were dewaxed with 2 changes of xylene and hydrated with decreasing grades of alcohol, and water. The slides were kept in a coplin jar containing a solution of sodium citrate (pH 6.0) before being transferred into the water bath at 95 °C. Tissue sections were washed in wash buffer, blocked with peroxidase and incubated with diluted MAO-B (clone orb157846) (Biorbyte USA) used at 1:200 dilution and incubated for 1 h at room temperature. The secondary antibody UltraVision™ Quanto Detection System HRP - Thermo Fisher Scientific detection kit (USA) was incubated for 25 min at room temperature, DAB and counterstained in hematoxylin, washed in wash buffer, dehydrated, cleared and mounted on DPX. The sections were viewed and photographed using a Leica microscope (DM 750, Germany) and an ICC HD 50 camera. Images were captured at a magnification of x400.
2.6.1. Percentage immunoreactivity
Image analysis and capturing was done using ImageJ 1.51J8 software (NIH, USA). At least six fields of view per slide were randomly selected and captured using a 40X objective. MAO- B expression was determined as percentage of immunostaining (brown) within the field using methods in accordance with the report of Mane et al. (2017).
2.7. Morphometry
The primary aim was to estimate the volume of pyknotic neurons within the hippocampus. Pyknotic neurons were defined as neurons with chromatin condensation, often with a slightly irregular contour. The cytoplasm appears compact, and some the cells gradually lose connections with adjacent cells, and finally, they appear to be floating (Hou et al., 2016). This was done by adapting the methods reported by Howard and Reed (2004) and Akang et al. (2015). Six microscopical fields (at x400) per section, from 4 different sections were randomly chosen for analysis. The fields were sampled as images captured on a Leica DM750 brightfield microscope (Germany) via LAZ software. Volume density of the pyknotic neurons were determined by randomly superimposing a transparent grid comprising 35 test points arranged in a quadratic array. Test points falling on pyknotic neurons were summed over all fields from all sections. The total number of points divided by the total number of points hitting on the hippocampus section multiplied by 100, provided an unbiased estimate of its %volume density/volume fraction.
2.8. Behavioral studies: Morris Water Maze (MWM)
Cognitive evaluation for neurodegenerative behavior was done using Morris Water Maze (MWM) test in accordance with (Dong et al., 2013). The MWM is used to assess learning and recollection ability of rats involving the use of exploratory, navigational, spatial and contextual memory (Vorhees and Williams, 2006; Harricharan et al., 2015). The hidden platform version of MWM is a test of spatial memory which is sensitive to hippocampal damage. It consisted of a circular pool (1 m in diameter and 50 cm in height) of tepid water and a hidden platform was constructed of transparent plastic (11 × 11 cm and a height of 18 cm). Rats (n = 5) were trained for seven days from the 50th day of treatment to use visual cue to locate an escape platform hidden above (1 cm) the surface of the water. The first three days (50th-52nd) were initial spatial training with a visible platform, During each trials, rats were required to swim to find a hidden platform for about 60 s and rats that failed to locate the hidden platform on time were guided towards the platform and were allowed to rest on the platform for about 20 s before being returned to their cage. Rats were trained in the initial spatial learning task for 4 trials per day with 10 min inter-trial intervals for 3 consecutive days for them to be familiar with the pool. During each trial, rats were allowed to swim until they found the hidden platform where they remained for 20 s before being returned to their cages.
Days 53–55 of treatment were reversal training, after the initial spatial learning task, a reversal learning protocol was conducted with some rats. During reversal learning, the hidden platform was moved to the opposite quadrant. Reversal learning entailed 3 additional days with 4 trials per day, again with a visible platform and their swim path time was recorded. On the 56th day of treatment, four trials were conducted using an invisible platform. Twenty-four hours after the final reversal training trial, rats were returned to the pool from a novel drop point with the hidden platform absent for 60 s and their swim path time (latency) away from the initial positions of the platform was recorded. Theoretically, control animals would navigate towards the position of the hidden platform within a shorter time compared to animals that have cognitive impairments.
2.9. Statistical analysis
The values for the results were expressed as mean ± standard deviation (SD). All Statistical level of significance except otherwise stated were determined using one-way analysis of variance (ANOVA) with Tukey’s post hoc test on a Graph pad Prism 5 (Graph Pad software). The mean difference was significant at 95% confidence interval (p < 0.05). The unbiased stereology was done using a two-way ANOVA and bonferorri posthoc test. The MWM behavioral data were expressed as the average number of seconds taken to find the platform per trial or per day (Carter et al., 2015). The data from MWM were subjected to a test for normality which was conducted using the D’Agostino & Pearson omnibus normality test and found to have a Gaussian distribution.
3. Results
3.1. Oxidative stress markers and tumor necrosis factor alpha
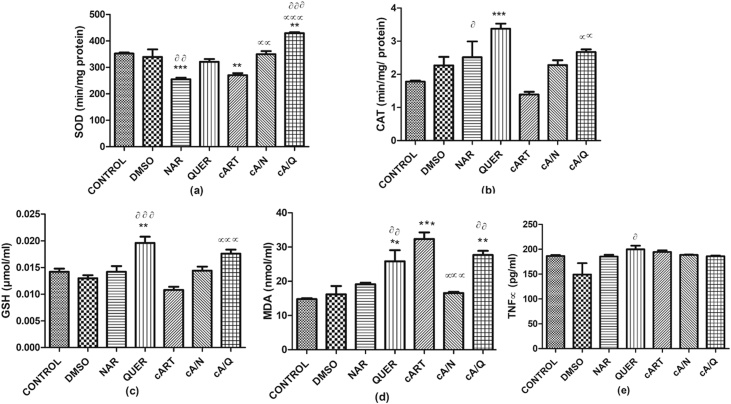
There was a significant decrease in the SOD of animals that received Naringenin compared to control (p < 0.0001) and compared to DMSO (p < 0.001). SOD also significantly decreased in animals that received cART compared to control (p < 0.001) but increased significantly in the group that received cART with quercetin compared to control (p < 0.001), DMSO (p < 0.0001), and cART (p < 0.0001). Animals that received a combination of cART and Naringenin also had a significant increase (p < 0.001) in SOD compared to cART only (Fig. 1a).
Fig. 1.
Showing Oxidative stress markers and Tumor necrosis factor alpha (TNFα). *p < 0.05 compared to control; αp < 0.05 compared to cART; ααp < 0.001 compared to cART, ᶞp < 0.05, ᶞᶞp < 0.001, ᶞᶞᶞp < 0.0001 compared to DMSO (n=5). Figures are represented as mean ± SD.
Fig. 1 There was a significant increase in catalase level in animals that received quercetin only compared to control (p < 0.0001) and those that received naringenin only compared to DMSO (p < 0.05). There was also a significant increase (p < 0.0001) of catalase in animals that received both cART and quercetin compared to those in cART only group (Fig. 1b).
There was a significant increase in GSH of animals that received quercetin compared to control (p < 0.001) and compared to DMSO (p < 0.0001). The animals that received a combination of cART and quercetin also had a significant increase (p < 0.0001) in GSH compared to those that had cART only (Fig. 1c).
There was a significant increase in MDA levels of animals that received cART (p < 0.0001), quercetin (p < 0.001), and a combination of cART and quercetin (p < 0.001) compared to control. There was also a significant increase (p < 0.001) in animals that received quercetin compared to DMSO. It was also observed that MDA decreased significantly (p < 0.0001) in animals that received both cART and naringenin compared with those that received cART only. (Fig. 1d).
There was a significant increase (p < 0.05) in TNF-α in quercetin compared to DMSO. The other groups had no significant difference in comparison with control or cART (Fig. 1e).
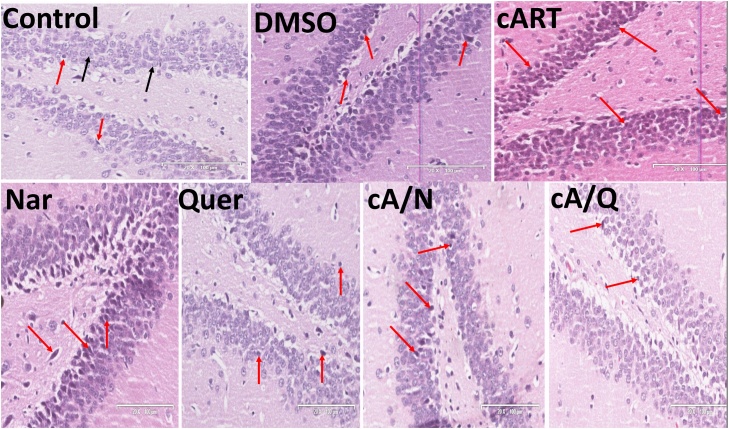
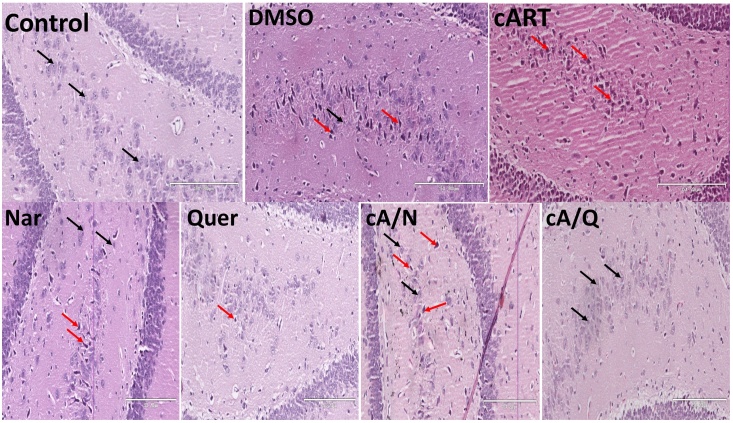
3.2. The cyto-architecture of the hippocampus
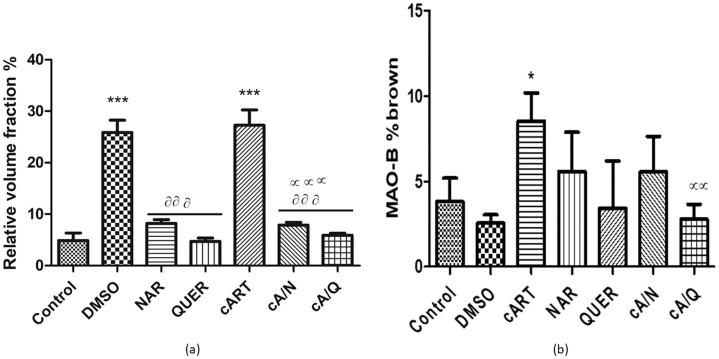
The normal neuronal pyramidal cells in the dentate gyrus, the Cornus Ammonis of the control group. The Histology of the dentate gyrus (Fig. 2) showed marked degenerative changes including shrinkage of the cytoplasm, extensively dark pyknotic nuclei (black arrows) and increased ferrugination of the neurons in the internal granule cells of animals administered cART only, and DMSO compared to other groups. Though there were slight degenerative changes observed in the group that received Naringenin only, and both Naringenin and cART simultaneously, there were still more normal neuronal cells in this group when compared to the DMSO, and cART only (Fig. 4a).
Fig. 2.
Dentate gyrus in the hippocampus of rats. Groups Control, Nar, Quer, cA/N, and cA/Q show normal histology with very few marked pyknotic neurons (red arrows). DMSO and cART show marked shrinkage of the cytoplasm, pyknotic nuclei and ferrugination of neurons (red arrows) (n = 5).
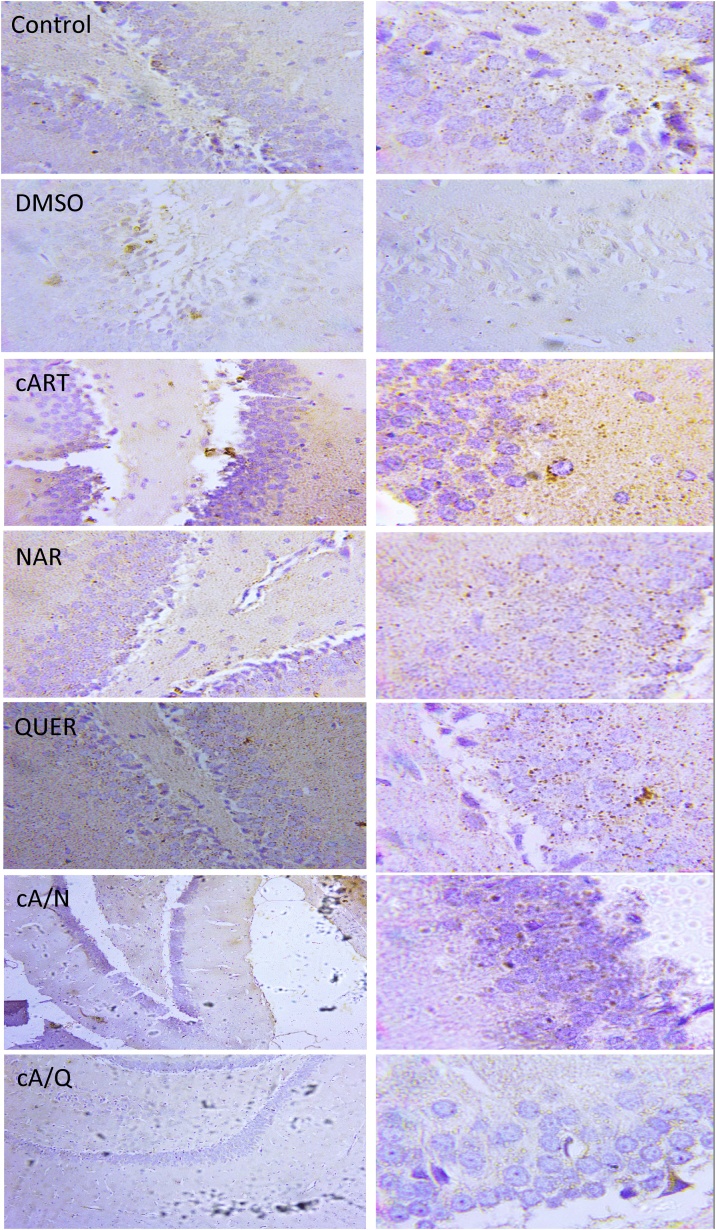
Fig. 4.
Immunohistochemistry of Monoamine oxidase B (MAO- B) expression seen in the brown stains x400 (left), x1000 (right) (n = 5).
The same findings were observed in cornus ammonis area 3 (CA3) (Fig. 3). Animals that received 1% v/v DMSO had cellular shrinkage, pyknotic nucleus and ferrugination of pyramidal cells in the fascia dentate and CA3 compared to control group. There were also few pyknotic nuclei found in Naringenin and quercetin groups.
Fig. 3.
Cornu ammonis area 3 (CA3) of hippocampus. Control: showing normal neurons (black arrows); DMSO: presence of pyknotic nuclei (red arrows). cART: multiple pyknotic nuclei and increased mineralization in soma bodies (red arrows); Nar, Quer, cA/N and cA/Q show normal cytoarchitecture with very few pyknotic nuclei (n = 5).
3.3. Immunohistochemistry of monoamine oxidase (MAO-B)
There was a significant (p < 0.05) increase in MAO- β expression in animals that received cART only compared to control. There was no significant difference in MAO- β expression between control and animals in the groups that received DMSO, Naringenin, and Quercetin. However, those animals that received cART and quercetin significantly (p < 0.001) decreased compared to cART group. (Fig. 4b).
3.4. Neurobehavioral test
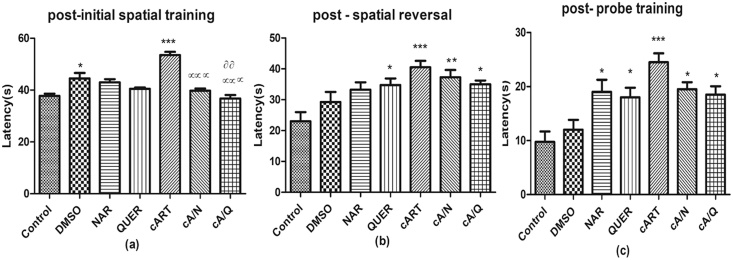
3.4.1. Initial spatial memory test
On comparison of the post-initial spatial training, there was a significant (p < 0.0001) increase in DMSO (44.50±4.20) and cART (53.50±2.52) compared to control (37.75±1.71). The animals that were co-administered cART with either Naringenin (39.75±1.71), or quercetin (36.75±2.75) decreased significantly (p < 0.0001) in spatial memory compared to animals that received cART only, and those that received cART+quercetin decreased significantly (p < 0.001) compared to DMSO. (Fig. 6a).
Fig. 6.
Neurobehavioral studies: Morris water maze test. *p < 0.05, **p < 0.001, ***p < 0.0001 compared to control; αp < 0.05, ααp < 0.001, αααp < 0.0001 compared to cART, ᶞp < 0.05, ᶞᶞp < 0.001, ᶞᶞᶞp < 0.0001 compared to DMSO (n=5). Figures are represented as mean ± SD.
3.4.2. Spatial reversal memory test
There was a significant decrease between control animals and animals treated with cART only, and quercetin (p < 0.0001, p < 0.05 respectively). However, when compared to the cART only group, there was a significant decrease in spatial reversal memory in animals that received both cART and Naringenin (p<0.001), and cART and quercetin (p<0.05) (Fig. 6b).
3.4.3. Probe test
There was a significant delay in the time to recognize the hidden platform in animals that received cART only, Naringenin, quercetin, (p < 0.0001, p < 0.05, p < 0.05 respectively) compared to control. It was also observed that animals that received cART alone took longer time to recognize the platform compared to cART with Naringenin, and cART with quercetin groups (Fig. 6c).
4. Discussion
In this study, the hippocampus-the cradle of cognition controlling several higher brain functions including, learning, memory, and spatial coding was studied to determine the therapeutic potential of Naringenin, and Quercetin following cART administration. Neurotoxic effects of cART was observed as evidenced in the increased MDA levels, representing a marked increase in lipid peroxidation as a result of excessive ROS production and an unopposed action by antioxidant enzymes within the hippocampus. Plausibly, cART inhibits the production of enzymatic antioxidants (GSH, SOD, and CAT) while increasing ROS leading to the induction of oxidative stress. Oxidative stress which is the biological basis for most diseases (Ichiishi et al., 2016) has been linked with neuro-inflammatory response and reported to inhibit neurotransmission and synaptic plasticity (Uttara et al., 2009). However, while the interplay between cART-induced oxidative stress and neuro-inflammation may elicit the release of cytokines, it certainly does not affect rat TNFα as observed from our results. A study by de Andrade et al. (2017) reports that increase in neuroinflammation as indicated by TNFα does not necessarily connote memory impairments. Therefore, the nonresponse to TNFα in this study does not translate to lack of memory loss.
The brain is particularly susceptible to oxidative damage due to its high amounts of unsaturated fatty acids whose double bonds are radico-philic in addition to its low antioxidant defense system (Floyd and Carney, 1992). Thus, triggering a cascade of deleterious reactions on neighbouring fatty acids culminating to neurodegeneration. It has been reported that the byproducts of lipid peroxidation have high affinity for amino acids, hampering glutamate and sugar uptake blocking neurotransmitters and activating c-Jun and MAP kinase pathways to invoke cellular apoptosis (Keller et al., 1997).
On the contrary, animals that received co-administration of cART with either Naringenin or Quercetin had improved memory compared to those with cART alone.
Furthermore, in this study, histology showed marked neurodegeneration in the hippocampal neurons as evidenced in the cytoplasm shrinkage and pyknotic nuclei in the granule cells of the dentate gyrus and CA3 regions in cART treated animals. Dentate gyrus consist of a complex neuronal circuit which acts as a gate to the propagation of limbic activities (Dyhrfjeld-Johnsen et al., 2009). Granule cells in dentate gyrus receive excitatory neuronal inputs from entorhinal cortex and sends it to CA3 (Witter, 1993). Therefore, it plays a critical role in both cognition and in regulation of induction and propagation of pathological activities (Dengler and Coulter, 2016). Increased lipid peroxidation which reduces membrane fluidity and compartmentation (Gurel et al., 2005) most likely resulted in impaired cellular function and senescence of the granule cells (Fig. 1). Though these effects were mitigated by Quercetin and Naringenin, it is not quite clear why naringenin alone had some deleterious effects on the histology of the hippocampus (Fig. 5 a) However, this finding could be related to the toxicity of 1% DMSO which has been reported to impair cell viability, mitochondrial integrity and glutamate transporter expression of astrocytes (Yuan et al., 2014). This is also in concert with the report of Ranawat and Bakshi (2017). It appears most of the toxicity Monoamine oxidase- β (MAO- β), a mitochondrial enzyme which metabolizes/inhibits dopamine neurotransmitters has been reported to enhance the production of ROS culminating in cell senescence (Maggiorani et al., 2017). Though there exist some opposing views (Damier et al., 1996; Sanz et al., 2009), our study tends to conform with the former. In this study, it was observed that MAO-B was expressed majorly in the synaptic cleft. Whether or not MAO-B is involved in the pathophysiology of ROS generation is not clear, however, the upregulation of MAO-B expression and the marked increase in MDA levels in addition to the decrease in antioxidant enzymes suggest that MAO-B is directly or indirectly involved with oxidative stress and subsequent neurodegeneration.
Fig. 5.
(a) quantification of pyknotic neurons (b) Quantification of Immunohistochemistry of Monoamine oxidase B (MAO- B) expression. Values are represented as mean ± SD. *p < 0.05, **p < 0.001, ***p < 0.0001 compared to control; αp < 0.05, ααp < 0.001, αααp < 0.0001 compared to cART, ᶞp < 0.05, ᶞᶞp < 0.001, ᶞᶞᶞp < 0.0001 compared to DMSO.
The consequence of these neurodegenerative changes in cART-treated animals was evident in the post neurobehavioral studies. The resultant delay in initial spatial reversal and probe tests in cART-treated animals concurs with numerous studies supporting the hypothesis that oxidative stress could culminate in dementia and other memory loss diseases (Halliwell, 2006; García-Mesa et al., 2015). Furthermore, the inhibition of dopamine in the hippocampus would inhibit synapses and transfer of excitatory information from the entorhinal cortex to the limbic system thereby resulting in memory impairments (Rajagopalan et al., 2017; Dickerson, 2017). Moreover, the numerous necrotic cells seen in the dentate gyrus and the Cornus ammonis region of the hippocampus of animals treated with cART is in sync with the outcomes of the neurobehavioural studies. Whereas, loss of spatial memory and impaired learning may be due to a plethora of factors, nevertheless, the role of mitochondria-induced oxidative stress is pivotal in such neurodegenerative changes.
Thus, in pursuance of therapeutic measures, a balance in the redox reaction may be the lee way. The use of Naringenin and Quercetin – bioflavonoids rich in antioxidants have been explored in this study to mitigate the neurodegenerative effects of cART and restore/enhance memory. Our results also clearly show a potentiation of endogenous antioxidant enzymes in the hippocampus by Quercetin and Naringenin, mitigating lipid peroxidation by neutralizing the cART-induced free radicals. This is consistent with previous reports on the rich antioxidant and free radical scavenging properties of Quercetin (Weng et al., 2011) and Naringenin (Al-Rejaie et al., 2015).
Furthermore, the consequence of generating endogenous antioxidant enzymes and the ROS-neutralizing potential of these bioflavonoids (especially quercetin) is observed in the seamless neurogenesis ongoing in the dentate gyrus, as well as prevalence of pyramidal neurons in the CA3 of co-treated groups. The effect therefore, is the unattenuated transmission of excitatory impulse from the hippocampus to other higher centers (Witter, 1993). Perhaps, this culminated to the improvement of spatial memory as observed in this study.
Moreover, the downregulation of MAO-B as observed in the quercetin and cART co-treated groups portrays quercetin as a MAO-B inhibitor. MAO-B inhibitors has been explored as a therapeutic intervention in chronic neurodegenerative diseases including Parkinson`s disease (Riederer and Müller, 2017). Though dopamine levels were not assessed in this study, it is plausible that quercetin prevented its metabolism allowing for good neuronal synapses.
Firm conclusions have not been reached on the bioavailability of flavonoids in the human brain, however, its ability to penetrate the BBB seem to be premised on lipophilicity of individual flavonoids/metabolites, less polar flavonoids/metabolites are capable of greater brain uptake than the more polar ones (i.e., sulfated and glucuronidated derivatives) (Youdim et al., 2004). In addition, their brain entry is dependent on their interactions with specific efflux transporters (such as P-glycoproteins) located in the BBB (Faria et al., 2010). Thus, flavonoids on penetrating the brain induce a cascade of reactions involving the impairment of excess ROS production. ROS has been reported to regulate the PI3 kinase signaling pathway by inhibiting the phosphatase and tensin homolog (PTEN), which in turn antagonizes the PI3k product phosphoinositide‐3‐phosphate (Kim et al., 2009; Oswald et al., 2018). Flavonoids have been reported to induce phosphorylation of hippocampal PI3k/Akt, the activation downstream of mTOR, and the increased expression of activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) (Williams et al., 2008). Such changes most likely underpin the enhancements in spatial memory, plausibly, through the downregulation of MAO-B and the upregulation of dopamine neurotransmitters facilitating changes in synaptic strength and the induction of morphological changes of the neurons (Oswald et al., 2018).
In congruence with this allusion, the effective neuronal preservation and synaptic plasticity enhanced by Naringenin and Quercetin is seen in the improved post initial spatial tests but for the post probe test and the post reversal test, the mechanism underpinning the delayed latency remains unclear.and may require further studies.
5. Conclusion
In conclusion, cART increases ROS production, MAO-B expression, and impairments on spatial memory and learning. This study for the first time shows that a combination of either Naringenin or Quercetin with cART could preserve hippocampal neurons from the diverse toxicities of cART via the modulation of oxidant-antioxidant pathway.
Ethics approval and consent to participate
All experiments with Wistar rats were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals with the approval (protocol number: CMUL/HREC/03/17/113) of the Health Research Ethics Committee of the College of Medicine of the University of Lagos, Lagos, Nigeria.
Consent for publication
Not applicable.
Availability of data and materials
All data generated or analyzed during this study are available from the corresponding author on reasonable request.
Conflict of interests
The authors declare that they have no competing interests.
Funding
Research reported in this publication was supported by the Fogarty International Center of the National Institutes of Health under Award Number D43TW010134. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This study was also supported by the following co-funding partners: Fogarty International Center (FIC), NIH Common Fund, Office of Strategic Coordination, Office of the Director (OD/OSC/CF/NIH), Office of AIDS Research, Office of the Director (OAR/NIH), Office of Research on Women's Health, Office of the Director (ORWH/NIH), National Institute on Minority Health and Health Disparities (NIMHD/NIH), National Institute of Neurological Disorders and Stroke (NINDS/NIH).
Authors’ contributions
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: ENA, OOD, OOA, AAO. Acquisition of data: AMF, ITU. Analysis and interpretation of data: ENA, AMF, ITU, DO, OOA, Statistical Analysis: AMF, ENA. Drafting of manuscript: AMF, ENA, DO, OOD. Critical revision of manuscript for important intellectual content: ENA, OOD, AAO, ASA. Obtained funding: ENA. Administrative, technical and material support: DO, AAO, OOD, OOA, ASA. Project supervisors: AAO, and ASA.
Acknowledgements
The authors appreciate the AIDS Prevention Initiative in Nigeria (APIN) center of Lagos University Teaching Hospital (LUTH) for the provision of combinatioin antiretroviral therapy.
References
- Adana M., Akang E., Peter A., Jegede A., Naidu E., Tiloke C., Chuturgoon A., Azu O. Naringenin attenuates highly active antiretroviral therapy-induced sperm DNA fragmentations and testicular toxicity in Sprague-Dawley rats. Andrology. 2018;6:166–175. doi: 10.1111/andr.12439. [DOI] [PubMed] [Google Scholar]
- Akang E., Oremosu A., Osinubi A., Dosumu O., Kusemiju T., Adelakun S., Umaru M. Histomorphometric studies of the effects of Telfairia occidentalis on alcohol-induced gonado-toxicity in male rats. Toxicol. Rep. 2015;2:968–975. doi: 10.1016/j.toxrep.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akay C., Cooper M., Odeleye A., Jensen B.K., White M.G., Vassoler F., Gannon P.J., Mankowski J., Dorsey J.L., Buch A.M. Antiretroviral drugs induce oxidative stress and neuronal damage in the central nervous system. J. Neurovirol. 2014;20:39–53. doi: 10.1007/s13365-013-0227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Rejaie S.S., Aleisa A.M., Abuohashish H.M., Parmar M.Y., Ola M.S., Al-Hosaini A.A., Ahmed M.M. Naringenin neutralises oxidative stress and nerve growth factor discrepancy in experimental diabetic neuropathy. Neurol. Res. 2015;37:924–933. doi: 10.1179/1743132815Y.0000000079. [DOI] [PubMed] [Google Scholar]
- Anthony I.C., Bell J.E. Springer; 2009. Neuropathological Findings Associated With Long-term HAART. HIV and the Brain. [Google Scholar]
- Anthony I., Ramage S., Carnie F., Simmonds P., Bell J. Influence of HAART on HIV-related CNS disease and neuroinflammation. J. Neuropathol. Exp. Neurol. 2005;64:529–536. doi: 10.1093/jnen/64.6.529. [DOI] [PubMed] [Google Scholar]
- Carter S.D., Mifsud K.R., Reul J.M. Distinct epigenetic and gene expression changes in rat hippocampal neurons after Morris water maze training. Front. Behav. Neurosci. 2015;9:156. doi: 10.3389/fnbeh.2015.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho F.B., Gutierres J.M., Beckmann D., Santos R.P., Thomé G.R., Baldissarelli J., Stefanello N., Andrades A., Aiello G., Ripplinger A. Quercetin treatment regulates the Na+, K+-ATPase activity, peripheral cholinergic enzymes, and oxidative stress in a rat model of demyelination. Nutr. Res. 2018;55:45–56. doi: 10.1016/j.nutres.2018.04.004. [DOI] [PubMed] [Google Scholar]
- Damier P., Kastner A., Agid Y., Hirsch E.C. Does monoamine oxidase type B play a role in dopaminergic nerve cell death in Parkinson’s disease? Neurology. 1996;46 doi: 10.1212/wnl.46.5.1262. 1262-1262. [DOI] [PubMed] [Google Scholar]
- De Andrade A.M., Da Cruz Fernandes M., De Fraga L.S., Porawski M., Giovenardi M., Guedes R.P. Omega-3 fatty acids revert high-fat diet-induced neuroinflammation but not recognition memory impairment in rats. Metab. Brain Dis. 2017;32:1871–1881. doi: 10.1007/s11011-017-0080-7. [DOI] [PubMed] [Google Scholar]
- Dengler C.G., Coulter D. Progress in Brain Research. Elsevier; 2016. Normal and epilepsy-associated pathologic function of the dentate gyrus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson B.C. 2017. Memory: Systems, Abilities, Deficits, Disorders. [Google Scholar]
- Djidja M.-C., Claude E., Scriven P., Allen D.W., Carolan V.A., Clench M.R. Antigen retrieval prior to on-tissue digestion of formalin-fixed paraffin-embedded tumour tissue sections yields oxidation of proline residues. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics. 2017;1865:901–906. doi: 10.1016/j.bbapap.2016.11.019. [DOI] [PubMed] [Google Scholar]
- Dong Z., Bai Y., Wu X., Li H., Gong B., Howland J.G., Huang Y., He W., Li T., Wang Y.T. Hippocampal long-term depression mediates spatial reversal learning in the Morris water maze. Neuropharmacology. 2013;64:65–73. doi: 10.1016/j.neuropharm.2012.06.027. [DOI] [PubMed] [Google Scholar]
- Dyhrfjeld-Johnsen J., Morgan R.J., Soltesz I. Double trouble? Potential for hyperexcitability following both channelopathic up-and downregulation of Ih in epilepsy. Front. Neurosci. 2009;3:5. doi: 10.3389/neuro.01.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Faria A., Pestana D., Teixeira D., Azevedo J., Freitas V., Mateus N., Calhau C. Flavonoid transport across RBE4 cells: a blood-brain barrier model. Cell. Mol. Biol. Lett. 2010;15:234. doi: 10.2478/s11658-010-0006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd R.A., Carney J.M. Free radical damage to protein and DNA: mechanisms involved and relevant observations on brain undergoing oxidative stress. Ann. Neurol. 1992;32 doi: 10.1002/ana.410320706. [DOI] [PubMed] [Google Scholar]
- García-Mesa Y., Colie S., Corpas R., Cristòfol R., Comellas F., Nebreda A.R., Giménez-Llort L., Sanfeliu C. Oxidative stress is a central target for physical exercise neuroprotection against pathological brain aging. J. Gerontol. Ser. A: Biomed. Sci. Med. Sci. 2015;71:40–49. doi: 10.1093/gerona/glv005. [DOI] [PubMed] [Google Scholar]
- Gurel A., Coskun O., Armutcu F., Kanter M., Ozen O.A. Vitamin E against oxidative damage caused by formaldehyde in frontal cortex and hippocampus: biochemical and histological studies. J. Chem. Neuroanat. 2005;29:173–178. doi: 10.1016/j.jchemneu.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress and neurodegeneration: where are we now? J. Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- Harricharan R., Thaver V., Russell V.A., Daniels W.M. Tat-induced histopathological alterations mediate hippocampus-associated behavioural impairments in rats. Behav. Brain Funct. 2015;11:3. doi: 10.1186/s12993-014-0047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton R.K., Franklin D.R., Ellis R.J., Mccutchan J.A., Letendre S.L., Leblanc S., Corkran S.H., Duarte N.A., Clifford D.B., Woods S.P. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J. Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L., Liu K., Li Y., Ma S., Ji X., Liu L. Necrotic pyknosis is a morphologically and biochemically distinct event from apoptotic pyknosis. J. Cell. Sci. 2016;129:3084–3090. doi: 10.1242/jcs.184374. [DOI] [PubMed] [Google Scholar]
- Howard V., Reed M. Unbiased stereology: three-dimensional measurement in microscopy. Garland Sci. 2004 [Google Scholar]
- Ichiishi E., Li X.-K., Iorio E.L. Oxidative stress and diseases: clinical trials and approaches. Oxid. Med. Cell. Longev. 2016;2016 doi: 10.1155/2016/3458276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J.N., Pang Z., Geddes J.W., Begley J.G., Germeyer A., Waeg G., Mattson M.P. Impairment of glucose and glutamate transport and induction of mitochondrial oxidative stress and dysfunction in synaptosomes by amyloid β‐peptide: Role of the lipid peroxidation product 4‐hydroxynonenal. J. Neurochem. 1997;69:273–284. doi: 10.1046/j.1471-4159.1997.69010273.x. [DOI] [PubMed] [Google Scholar]
- Kim Y.-C., Kitaura H., Taira T., Iguchi-Ariga S.M., Ariga H. Oxidation of DJ-1-dependent cell transformation through direct binding of DJ-1 to PTEN. Int. J. Oncol. 2009;35:1331–1341. [PubMed] [Google Scholar]
- Li P., Bai J., Dong B., Lu Y., Zhang S., Guo S., Tan N., Zhao M., Du S., Cao P. In vivo pharmacokinetics of puerarin via different drug administration routes based on middle cerebral artery occlusion model. Eur. J. Drug Metab. Pharmacokinet. 2017;42:719–727. doi: 10.1007/s13318-016-0388-4. [DOI] [PubMed] [Google Scholar]
- Lian G.-Y., Wang Q.-M., Tang P.M.-K., Zhou S., Huang X.-R., Lan H.-Y. Combination of asiatic acid and naringenin modulates NK cell anti-cancer immunity by rebalancing Smad3/Smad7 signaling. Mol. Ther. 2018;26:2255–2266. doi: 10.1016/j.ymthe.2018.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke W.M., Proudfoot J.M., Stewart S., Mckinley A.J., Needs P.W., Kroon P.A., Hodgson J.M., Croft K.D. Metabolic transformation has a profound effect on anti-inflammatory activity of flavonoids such as quercetin: lack of association between antioxidant and lipoxygenase inhibitory activity. Biochem. Pharmacol. 2008;75:1045–1053. doi: 10.1016/j.bcp.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Maggiorani D., Manzella N., Edmondson D.E., Mattevi A., Parini A., Binda C., Mialet-Perez J. Monoamine oxidases, oxidative stress, and altered mitochondrial dynamics in cardiac ageing. Oxid. Med. Cell. Longev. 2017;2017 doi: 10.1155/2017/3017947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mane D.R., Kale A.D., Belaldavar C. Validation of immunoexpression of tenascin-C in oral precancerous and cancerous tissues using ImageJ analysis with novel immunohistochemistry profiler plugin: An immunohistochemical quantitative analysis. J. Oral Maxillofacial Pathol. JOMFP. 2017;21:211. doi: 10.4103/jomfp.JOMFP_234_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Oswald M.C., Garnham N., Sweeney S.T., Landgraf M. Regulation of neuronal development and function by ROS. FEBS Lett. 2018;592:679–691. doi: 10.1002/1873-3468.12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan M.-H., Lai C.-S., Ho C.-T. Anti-inflammatory activity of natural dietary flavonoids. Food Funct. 2010;1:15–31. doi: 10.1039/c0fo00103a. [DOI] [PubMed] [Google Scholar]
- Rajagopalan A., Jinu K., Sailesh K.S., Mishra S., Reddy U.K., Mukkadan J.K. Understanding the links between vestibular and limbic systems regulating emotions. J. Nat. Sci. Biol. Med. 2017;8:11. doi: 10.4103/0976-9668.198350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranawat P., Bakshi N. Naringenin; a bioflavonoid, impairs the reproductive potential of male mice. Toxicol. Mech. Methods. 2017;27:417–427. doi: 10.1080/15376516.2017.1296048. [DOI] [PubMed] [Google Scholar]
- Rendeiro C., Guerreiro J.D., Williams C.M., Spencer J.P. Flavonoids as modulators of memory and learning: molecular interactions resulting in behavioural effects. Proc. Nutr. Soc. 2012;71:246–262. doi: 10.1017/S0029665112000146. [DOI] [PubMed] [Google Scholar]
- Riederer P., Müller T. Use of monoamine oxidase inhibitors in chronic neurodegeneration. Expert Opin. Drug Metab. Toxicol. 2017;13:233–240. doi: 10.1080/17425255.2017.1273901. [DOI] [PubMed] [Google Scholar]
- Robak J., Gryglewski R.J. Flavonoids are scavengers of superoxide anions. Biochem. Pharmacol. 1988;37:837–841. doi: 10.1016/0006-2952(88)90169-4. [DOI] [PubMed] [Google Scholar]
- Robertson K., Su Z., Margolis D., Krambrink A., Havlir D., Evans S., Skiest D., Team A.S. Neurocognitive effects of treatment interruption in stable HIV-positive patients in an observational cohort. Neurology. 2010;74:1260–1266. doi: 10.1212/WNL.0b013e3181d9ed09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Gonzalez P.D., Lopez-Hernandez F.J., Perez-Barriocanal F., Morales A.I., Lopez-Novoa J.M. Quercetin reduces cisplatin nephrotoxicity in rats without compromising its anti-tumour activity. Nephrol. Dial. Transplant. 2011;26:3484–3495. doi: 10.1093/ndt/gfr195. [DOI] [PubMed] [Google Scholar]
- Sanz E., Quintana A., Valente T., Manso Y., Hidalgo J., Unzeta M. Monoamine oxidase‐B activity is not involved in the neuroinflammatory response elicited by a focal freeze brain injury. J. Neurosci. Res. 2009;87:784–794. doi: 10.1002/jnr.21892. [DOI] [PubMed] [Google Scholar]
- Satyanarayana P., Singh D., Chopra K. Quercetin, a bioflavonoid, protects against oxidative stress-related renal dysfunction by cyclosporine in rats. Methods Find. Exp. Clin. Pharmacol. 2001;23:175–182. doi: 10.1358/mf.2001.23.4.634641. [DOI] [PubMed] [Google Scholar]
- Sinha A.K. Colorimetric assay of catalase. Anal. Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- Solomon I.H., De Girolami U., Chettimada S., Misra V., Singer E.J., Gabuzda D. Brain and liver pathology, amyloid deposition, and interferon responses among older HIV-positive patients in the late HAART era. BMC Infect. Dis. 2017;17:151. doi: 10.1186/s12879-017-2246-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern A.L., Lee R.N., Panvelker N., Li J., Harowitz J., Jordan-Sciutto K.L., Akay-Espinoza C. Differential effects of antiretroviral drugs on neurons in vitro: roles for oxidative stress and integrated stress response. J. Neuroimmune Pharmacol. 2017:1–13. doi: 10.1007/s11481-017-9761-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal. Biochem. 2017;524:13–30. doi: 10.1016/j.ab.2016.10.021. [DOI] [PubMed] [Google Scholar]
- UNAIDS . Joint United Nations Programme on HIV/AIDS (UNAIDS); Geneva, Switzerland: 2017. Fact Sheet—Latest Statistics on the Status of the AIDS Epidemic. [Google Scholar]
- UNAIDS . UNAIDS; Geneva, Switzerland: 2017. Global AIDS Monitoring 2018 Indicators for Monitoring the 2016 United Nations Political Declaration on Ending AIDS. Guidance. [Google Scholar]
- Uttara B., Singh A.V., Zamboni P., Mahajan R. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vauzour D., Vafeiadou K., Rodriguez-Mateos A., Rendeiro C., Spencer J.P. The neuroprotective potential of flavonoids: a multiplicity of effects. Genes Nutr. 2008;3:115. doi: 10.1007/s12263-008-0091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees C.V., Williams M.T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006;1:848. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng C.-J., Chen M.-J., Yeh C.-T., Yen G.-C. Hepatoprotection of quercetin against oxidative stress by induction of metallothionein expression through activating MAPK and PI3K pathways and enhancing Nrf2 DNA-binding activity. N. Biotechnol. 2011;28:767–777. doi: 10.1016/j.nbt.2011.05.003. [DOI] [PubMed] [Google Scholar]
- WHO . World Health Organization; 2016. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. [PubMed] [Google Scholar]
- Williams R.J., Spencer J.P. Flavonoids, cognition, and dementia: actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radic. Biol. Med. 2012;52:35–45. doi: 10.1016/j.freeradbiomed.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Williams C.M., El Mohsen M.A., Vauzour D., Rendeiro C., Butler L.T., Ellis J.A., Whiteman M., Spencer J.P. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radic. Biol. Med. 2008;45:295–305. doi: 10.1016/j.freeradbiomed.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Witter M.P. Organization of the entorhinal—hippocampal system: A review of current anatomical data. Hippocampus. 1993;3:33–44. [PubMed] [Google Scholar]
- Yew W.-W., Chan D.P., Singhal A., Zhang Y., Lee S.-S. Does oxidative stress contribute to adverse outcomes in HIV-associated TB? J. Antimicrob. Chemother. 2018 doi: 10.1093/jac/dkx509. [DOI] [PubMed] [Google Scholar]
- Youdim K.A., Qaiser M.Z., Begley D.J., Rice-Evans C.A., Abbott N.J. Flavonoid permeability across an in situ model of the blood–brain barrier. Free Radic. Biol. Med. 2004;36:592–604. doi: 10.1016/j.freeradbiomed.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Yuan C., Gao J., Guo J., Bai L., Marshall C., Cai Z., Wang L., Xiao M. Dimethyl sulfoxide damages mitochondrial integrity and membrane potential in cultured astrocytes. PLoS One. 2014;9 doi: 10.1371/journal.pone.0107447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are available from the corresponding author on reasonable request.