Abstract
Background
Drug resistance remains as a challenge in the treatment of HER2-overexpressed breast cancer. Emerging evidence from clinical studies show relation of oxidized low density lipoprotein (LDL) and very low density lipoprotein (VLDL) level with drug resistance. However, the underlying molecular mechanisms for this effect remain unclear. Therefore, the aim of this study was to determine the effects of oxidized-LDL and VLDL in drug-resistant HER2-overexpressed breast cancer cells.
Methods
An in vitro cell model for tamoxifen-resistant HER2 overexpressed UACC732 cells was created using the pulse method. Cells were exposed to oxidized LDL (oxLDL) and very low density lipoprotein (VLDL) separately. Effects on cell morphology was studied using phase contrast microscopic changes. Percentage of cell viability was measured using proliferation assay kit. Development of tamoxifen resistance was determined based on P-gp expression with flow cytometry. Further analysis includedcell death measurement with flow cytometry method.
Results
UACC732 cells exposed to VLDL exhibited fibroblast-like morphology. This was further supported by proliferation assay, where the percentage of cell viability achieved more than 100% with 100 μg/ml of VLDL exposure, indicating cell proliferation. Findings also showed that VLDL caused reduction in expression of Pgp in resistant cells compared to resistant cells alone (p = 0.02).
Conclusion
Results of this study suggest that VLDL may play a role in growth of drug-resistant HER2-overexpressing cells. Lower expression of P-gp in presence of VLDL need to be investigated further.
Keywords: Biochemistry, Cancer research, Cell biology, Molecular biology
1. Introduction
Breast cancer is the second leading cause of cancer-related death among women worldwide [1]. Systemic agents to treat breast cancer are found to be effective in 90% of primary breast cancers and 50% of metastatic cases at the beginning of treatment. However, after a certain period of time, drug resistance develops in almost 60% of patients with breast cancer, which can lead to cancer recurrence. Drug resistance genes often are highly expressed in cancer patients. For example, multi-drug resistance gene 1 (MDR1) gene is linked to development of resistance in cancer. Currently, tamoxifen is the standard treatment in estrogen receptor (ER)-positive breast cancer mainly in premenopausal women. Although widely used, patients treated with tamoxifen still have high probability of cancer recurrence.
Emerging studies have reported a relationship between lipids and inflammation and cancer. For example, an increase in the serum level of oxidized low density lipoprotein (oxLDL) was noted in patients with breast or ovarian cancer, and thus it was reported that serum ox-LDL level predicts an increased risk of breast or ovarian cancer [2]. Other researchers found that fasting serum triglyceride and very low density lipoprotein (VLDL) cholesterol levels were significantly increased in Stage I and Stage IV breast cancer patients [2]. Additionally, patients with stage IV disease showed a significant increase in triglyceride and VLDL cholesterol levels and decreases in total, high density lipoprotein (HDL), and LDL cholesterol levels compared to patients with stage I breast cancer [3]. Due to their binding affinity to specific receptors, synthetic lipoproteins with simulated peptides are also being studied for their potential as drug delivery system and theranostic application [4, 5].
The ATP-binding cassette-B1 (ABCB1) transporter is also known as MDR1 or P-glycoprotein (Pgp). It promotes the efflux of drugs from cells. MDR is associated with multidrug resistance-associated protein 1 (ABCC1) and lung resistance-related protein (LRP), which is a human major vault protein. Most of the ABC family members are integral membrane proteins that could interact closely with membrane lipids. Development of cancer drug resistance is due to these transporter proteins [6]. Previous studies have linked ABCB1 functional activity with cholesterol [7, 8, 9]. These reports were based on studies that used CEM-resistant leukaemia cells and proteoliposomes. Breast cancer is a clinically and biologically heterogeneous disease, characterized by dysregulation of multiple cellular pathways and different sensitivities to treatment [10, 11] It is found to show more aggressive nature compared to estrogen receptor (ER) positive breast cancer cells [12]. Higher levels of LDL have been reported in HER2 positive breast cancer patients [13]. In human, lipid alterations are generally characterized by an increase in the level of very low-density lipoproteins (VLDL), small and dense low-density lipoproteins (LDL), as well as a high content of triglycerides in LDL and high-density lipoproteins (HDL), and an increased susceptibility of LDL to oxidation [14, 15]. Most of previous clinical studies highlight the role of VLDL in diseases [16, 17, 18]. Increased oxidative stress leads to LDL conversion into oxidized low density lipoprotein (oxLDL) [19]. It is important to emphasize the positive correlation between increase serum oxLDL and increased risk of cancer [2]. Cancer cells are usually exposed to higher reactive oxygen species levels which would further stimulate proliferation, death evasion, angiogenesis, invasiveness, and metastasis [20].
Because of the potential link between lipids and breast cancer, recent treatment approaches involve the use of statins, which target 3-hydroxy-3-methylglutaryl-CoA reductase. Statins currently are being evaluated in clinical trials for their anti-cancer efficacy [21]. However, this approach is limited to targeting a specific enzyme in the cholesterol synthesis pathway that is not related to drug resistance proteins such as P-gp and breast cancer resistant protein (BCRP). Additional work is needed to elucidate metabolic signalling responsible for the effects of oxLDL and VLDL. Thus, the roles of lipoproteins in drug-resistant HER2 overexpress breast cancer cells require further study.
2. Materials and methods
2.1. Chemicals
In this study, tamoxifen was purchased from Sigma-Aldrich (Missouri, US).
2.2. Cell culture medium
HER2-overexpressed (UACC732) cells were purchased from the American Type Culture Collection (Manassas, VA, USA). The cells were resistant to HER2 inhibitors such as trastuzumab and lapatinib. They were grown in L-glutamine RPMI-1640 media from Nacalai Tesque (Kyoto, Japan) with phenol red and supplemented with 10% heat-inactivated fetal bovine serum (FBS) purchased from Tico Europe (Amstelveen, Netherlands) and 1% antibiotics (10,000 U/ml of penicillin, 10,000 μg/ml of streptomycin) from Nacalai-Tesque (Kyoto, Japan).
2.3. oxLDL preparation
Human LDL was purchased from Merck Millipore (Temecula, CA, USA). The stock solution contained 150 mM NaCl, pH 7.4 with 0.01% EDTA, and LDL was isolated by ultracentrifugation. It was dissolved in phosphate buffered saline (PBS) (MP Biomedicals, IIIkirch Cedex, France) to 100 μg/ml. The LDL was then incubated with 5 μM of freshly prepared copper (II) sulfate solution for 18 h to allow oxidation to occur. This process was carried out in an incubator shaker (Certomat H Sartorius, Goettingen, Germany) that was maintained at 200 rotations per minute at 37 °C. After 18 h, the oxLDL was stored in a 4 °C refrigerator to terminate the oxidation [22, 23]. VLDL was purchased from Merck Millipore (Temecula, CA, USA). Experiments using VLDL was carried out as reported previously [24].
2.4. Morphological changes in cells due to lipoprotein exposure
Microscopic images of UACC732 cells were taken using a Zen 2 Lite Zeiss phase contrast microscope (Oberkochen, Germany). The objective was to identify the morphological changes that occurred in cells following exposure to oxLDL or VLDL. Images were captured from each well to show differences in growth of parent and resistant cells exposed to lipoproteins at 200x magnification. All experiments were carried out in triplicates.
2.5. Development of drug resistance in UACC 732 cells
To develop drug-resistant cells, UACC732 cells were grown in increasing concentrations of tamoxifen (from 3 to 14 μM) using the pulse method. The cells were exposed to tamoxifen for 3 days. This was followed by growth in drug-free complete culture medium until confluence was reached before the next cycle of treatment [25]. Development of resistance was confirmed by measuring P-gp expression using flow cytometry. All experiments were carried out in replicates.
2.6. Effects of lipoproteins on UACC732 cell viability determined using cell proliferation assay
After development of the tamoxifen-resistant UACC732 cell line, cells were exposed to ox-LDL and VLDL at 10 μg/ml concentration for 72 h [10]. The growth medium contained RPMI-1640, the antibiotics penicillin and streptomycin (1%), 3 mg/ml of lipoprotein deficient serum (LPDS), and 10 μg/ml of ox-LDL or VLDL [22, 26]. LPDS was used in the treatment instead of FBS to exclude the presence of other lipoproteins.
The experiment was done to determine effects of lipoproteins using the CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay (Promega). First, UACC732 parent and resistant cells were seeded separately in a 96-well flat-bottomed plate at a density of 4 x 103 cells per well in 100 μL of complete medium. They were left overnight to adhere to the plate before treatment with corresponding lipoproteins. Cytotoxicity of oxLDL and VLDL was tested on UACC732 cells at lipoprotein concentrations ranging from 0 to 100 μM for 3 days. The medium then was removed, and 100 μl of complete medium mixed with 20 μl of CellTiter 96® were pipetted into each well. The plate was incubated at 37 °C for 1.5 h in a humidified, 5% CO2 atmosphere, and absorbance was measured at 490 nm using the ELISA microplate reader. All experiments were performed in triplicates. Percentage of cell viability was calculated using the following formula:
The mean of cell viability was derived from triplicates for each concentration. A dose response curve was plotted. The IC50 inhibition of lipoproteins were determined as the concentration which reduced cell growth by 50% compared to untreated.
2.7. P-gp expression and cell death detection using flow cytometry analysis
P-gp expression was determined based the fluorescence intensity of anti-Pgp PE stain (BD Bioscience, California, US) by flow cytometry method. For measurement of cell viability and apoptotic cells, 7-Aminoactinomycin D (7-AAD) was purchased from BD Bioscience (California, US). It is a fluorescent DNA dye for apoptosis which can discriminate between viable cells, late and early apoptosis. Parent cells, resistant cells, resistant cells exposed to 10 μg/ml oxLDL and resistant cells exposed to 10 μg/ml VLDL were grown in 25-ml culture flasks in triplicates at 37 °C with 5% CO2. From each flask, a total of 1 x 106 cells was washed twice in 2 mL of PBS supplemented with 0.5% bovine serum albumin (BSA). Next, 0.1 mL of staining buffer (PBS, 0.5 % BSA, 0.1% sodium azide) and 0.02 mL of anti-Pgp phycoerythrin (PE) were added to the pelleted cells. The mixture was incubated at room temperature for 30 minutes, followed by centrifugation at 200 g for 10 minutes After centrifugation, the pellet was resuspended in 0.5 mL of staining buffer and 0.02 mL of 7AAD solution. It was gently mixed and incubated for 30 minutes at 4 °C in the dark. All the samples were analysed using the BD FACS Calibur flow cytometer (California, USA). Ten thousand events were collected, and debris and dead cells were gated out based on forward versus side scatter dot plots. More than three independent experiments were performed for parent, resistant, resistant cell exposed to oxLDL and resistant cells exposed to VLDL.
2.8. Statistical analysis
Graphpad Prism version 4 was used to perform one-way analysis of variance and Dunnet's Multiple Comparison for the cell proliferation assay. For analysis of P-gp expression, percentage of cell viability and percentage of cell death differences, parametric analysis (ANOVA test) was performed using SPSS version 24 to compare more than two groups. For two-group comparisons, the independent-sample T-test was used and p value less than 0.05 was considered as statistically significant.
3. Results
3.1. Morphological changes in cells due to lipoprotein exposure
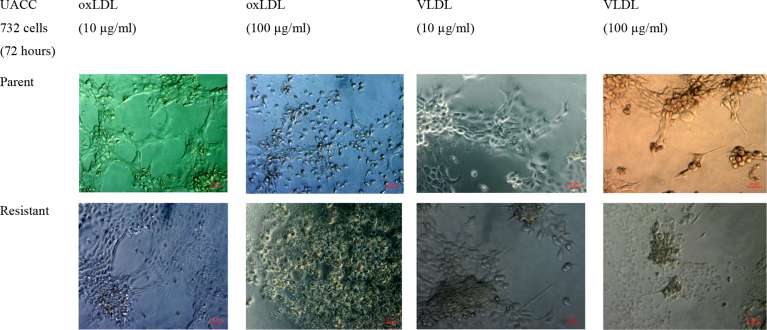
Changes in cell morphology were observed to determine effects of oxLDL and VLDL on cell size and growth. Cells were examined under a phase contrast microscope. Fig. 1 shows changes in cell morphology following exposure to lipoprotein. Cells exposed to oxLDL were spherical, whereas those exposed to VLDL had a fibroblast-like appearance. In addition, cell size was larger following exposure to high concentration of VLDL in both parent and resistant cells.
Fig. 1.
Morphological changes in parent and tamoxifen-resistant UACC732 cells after exposure to oxLDL and VLDL. Images were taken at 200x magnification with a Zeiss phase contrast microscope (n = 3).
3.2. Development of tamoxifen resistance in UACC732 cells
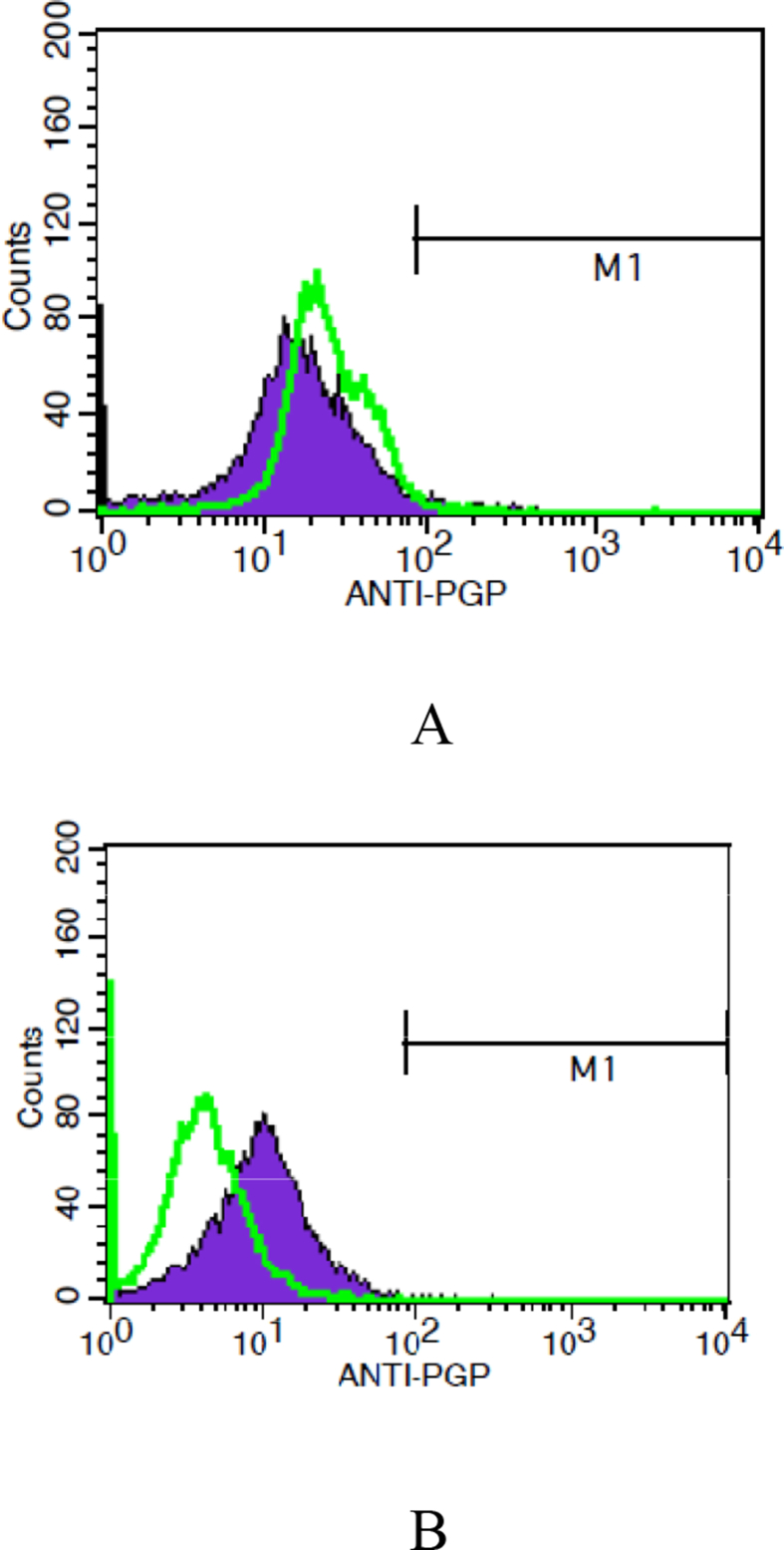
Parent UACC732 cells were exposed to a gradual increase of tamoxifen concentration (from 3 to 14 μM) using the pulse method. Flow cytometry analysis then was conducted to confirm the development of resistance upon exposure to tamoxifen. Fig. 2 shows the peak shifting towards the right along the x-axis, which indicates development of resistance to tamoxifen based on changes in P-gp expression over time (Fig. 2). The T-test test indicated no significant difference between parent and resistant cells (p = 0.394).
Fig. 2.
Expression of Pgp in UACC732 cells (A) before treatment and (B) after treatment with tamoxifen. Peaks in green represent parent cells. Each experiment was conducted in triplicates. Data analysis indicated no significant difference between parent and UACC 732 cells exposed to tamoxifen (p = 0.394).
3.3. Effects of lipoprotein on UACC732 cell viability determined using cell cell proliferation assay
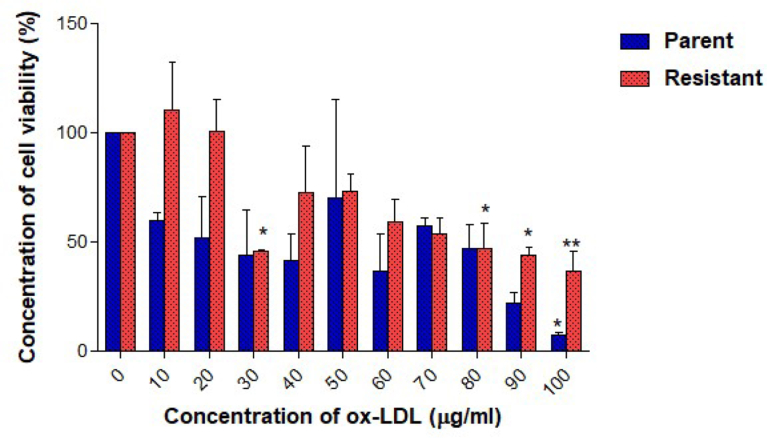
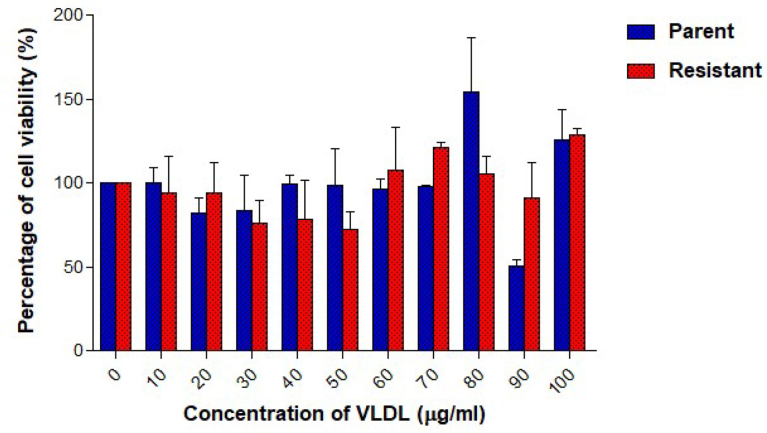
Cell viability was studied using the cell proliferation assay. Measurements were taken after treating UACC732 parent and resistant cells with lipoproteins. Tamoxifen-resistant UACC732 cells exposed to oxLDL had a higher IC50 value (73.8 μg/ml) than parent cells (30.9 μg/ml) (Fig. 3). Moreover, oxLDL inhibited cell growth at different concentrations in both types of cells. Resistant cells showed a significant reduction in percentage of viable cells when treated with oxLDL at 30 μg/ml (p < 0.05), 80 μg/ml (p < 0.05), 90 μg/ml (p < 0.05), and 100 μg/ml (p < 0.01) compared to untreated resistant cells. Parent cells showed significant (p < 0.05) reduction in percentage of viable cells only when treated with 100 μg/ml of oxLDL compared to untreated parent cells. Generally, cell viability was less than 100% with increasing dose of oxLDL. Parent cells were more sensitive to oxLDL than resistant cells, as the percentage of viability was lower at higher oxLDL concentrations. VLDL did not inhibit growth of parent or resistant UACC732 cells (Fig. 4). The IC50 was not achieved in these cells. Furthermore, no significant changes in cell viability were detected in either parent or resistant cells. Cell viability was greater than 100%, particularly with 100 μg/ml VLDL exposure.
Fig. 3.
UACC72 cell viability after exposure to oxLDL was determined using the cell proliferation assay. Data are presented as mean ± standard deviation (n = 3). One-way ANOVA was performed to compare data. *p < 0.05 and **p < 0.01. A significant reduction in percentage of cell viability was detected for 30, 80, 90, and 100 μg/ml in resistant cells compared to untreated resistant cells; only 100 μg/ml resulted in a significant reduction in percentage of cell viability of treated parent cells compared to control.
Fig. 4.
UACC72 cell viability after exposure to VLDL was determined using the cell proliferation assay. Data are presented as mean ± standard deviation (n = 3). One-way ANOVA revealed no significant difference between untreated and VLDL treated cells either in parent or resistant cells.
3.4. P-gp expression and cell death measurement using flow cytometry
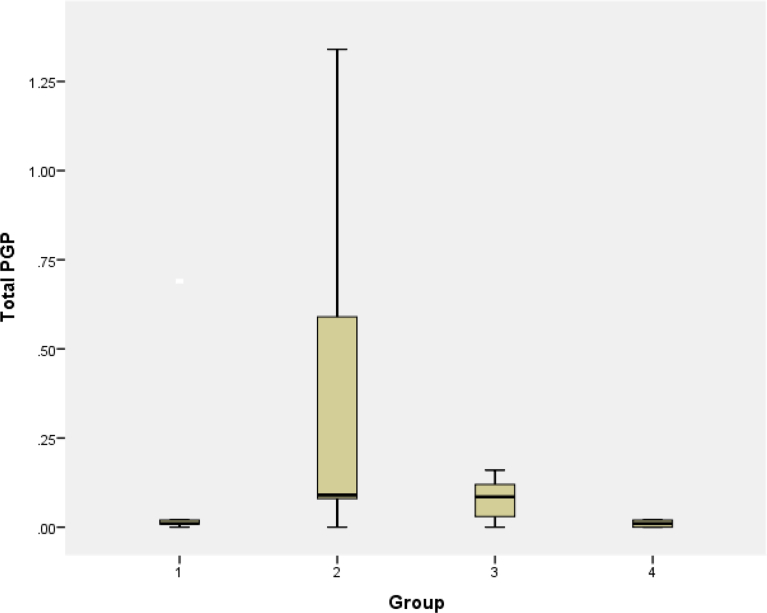
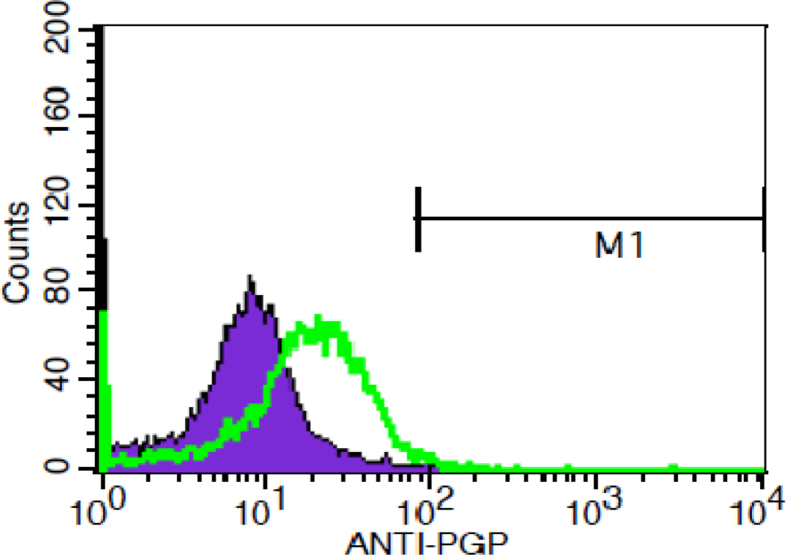
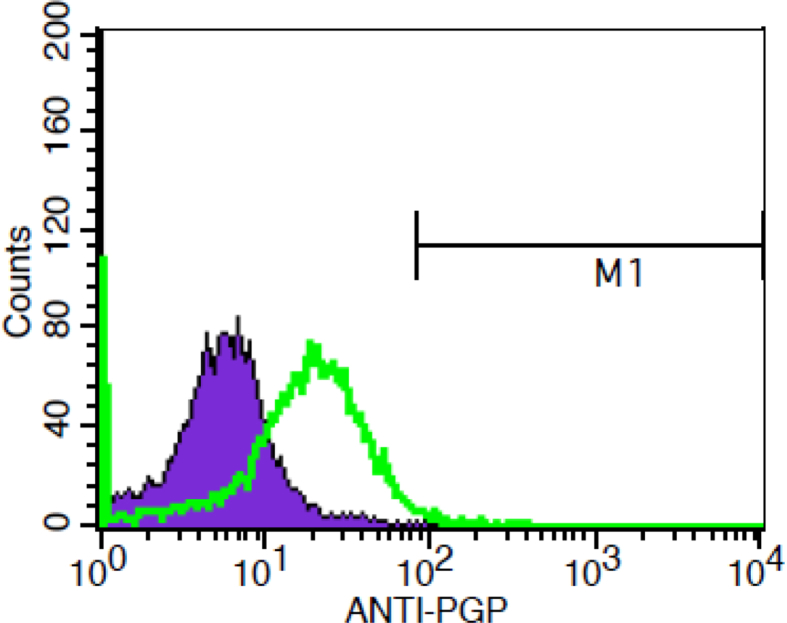
P-gp expression was significantly different between groups with ANOVA test (p = 0.088). Pgp expression was highest in resistant cells compared to parent and resistant cells that were exposed to lipoproteins (Table 1). Exposure to lipoproteins reduced expression of Pgp in resistant cells (Fig. 5). Pgp expression was reduced in resistant cells following exposure to oxLDL and VLDL compared to resistant cells, which illustrates a shift of the peak towards the left in treated cells. Further analysis was performed using independent T test. no significant difference in Pgp expression was detected between resistant UACC732 cells and resistant cells treated with oxLDL (p = 0.059) (Fig. 6). However, there was a significant difference in Pgp expression between resistant UACC732 cells and resistant cells exposed to VLDL (p = 0.02) (Fig. 7). Thus, VLDL reduced the expression of Pgp in resistant cells.
Table 1.
Pgp expression in (1) parent cells; (2) resistant cells; (3) resistant cells exposed to oxLDL; (4) resistant cells exposed to VLDL.
| Total P-gp |
|||||
|---|---|---|---|---|---|
| 1 (n = 4) | 2 (n = 13) | 3 (n = 12) | 4 (n = 6) | p | |
| Mean | 0.1460 | 0.3346 | 0.0800 | 0.0100 | 0.088 |
| Std deviation | 0.3042 | 0.4385 | 0.05705 | 0.0089 | |
No significant differences between any of the cell types.
Fig. 5.
Effects of treatment with lipoproteins on Pgp expression: (1) parent cells; (2) resistant cells; (3) resistant cells exposed to oxLDL; (4) resistant cells exposed to VLDL. Data are presented as mean ± standard deviation. The T test revealed no significant differences between parent cells and resistant cells (p = 0.394), resistant cells alone and resistant cells treated with oxLDL (p = 0.059). Significant difference was observed only resistant cells alone and resistant cells treated with VLDL (p = 0.02). All experiments were performed in replicates (parent n = 4, resistant n = 13, resistant oxLDL n = 12, resistant VLDL n = 6).
Fig. 6.
Difference in P-gp expression between resistant UACC732 cells and resistant cells exposed to oxLDL. T test results indicated no significant difference between the cell types (p = 0.059). Green line represents parent cells and purple represent resistant cells. All experiments were performed in replicates resistant (n = 13) and resistant exposed to oxLDL (n = 12).
Fig. 7.
Difference in Pgp expression between resistant UACC732 cells and resistant cells exposed to VLDL. T test results indicated a significant difference between cell types (p = 0.02). Green line represents parent cells and purple represent resistant cells. All experiments were performed in replicates, resistant (n = 13) and resistant exposed to VLDL (n = 6).
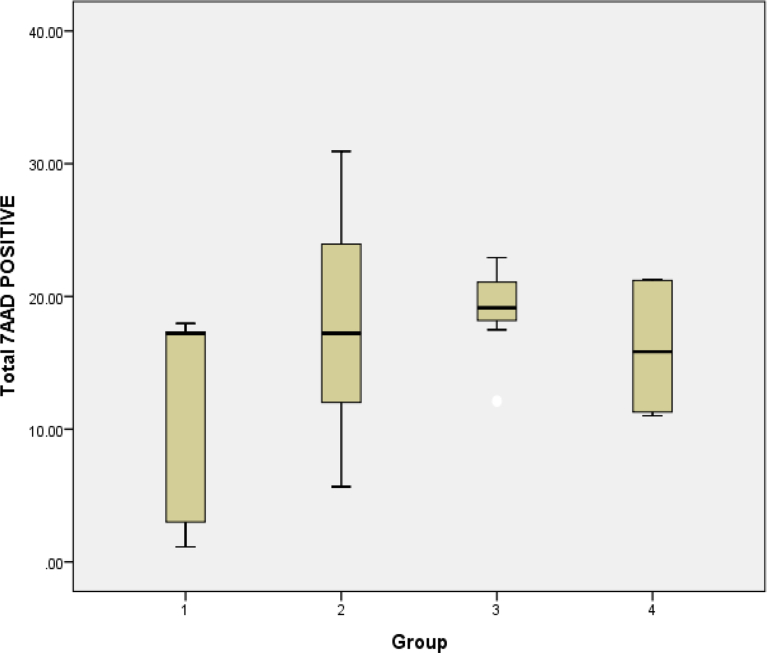
For cell death, the percentage of 7ADD expression was highest in resistant cells exposed to oxLDL compared to the other cell types studied (Table 2). However, no significant difference was noted between all cells with ANOVA analysis (p = 0.19). Exposure to the lipoproteins increased mean expression of 7AAD in resistant cells compared to parent cells (Fig. 8). However, the no significant difference was noted (p = 0.152). Effect of lipoproteins was studied. Percentage was higher in resistant cells exposed to oxLDL (18.79%) compared to cells exposed to VLDL (16.08%). However, the further analysis with T test showed no significant difference in expression of 7AAD in resistant cells after exposure to oxLDL (p = 0.789) and after exposure to VLDL (0.607).
Table 2.
Percentage cell death of different cell types: (1) parent cells; (2) resistant cells; (3) resistant cells exposed to oxLDL; (4) resistant cells exposed to VLDL.
| Total 7AAD Positive |
|||||
|---|---|---|---|---|---|
| 1 (n = 5) | 2 (n = 12) | 3 (n = 12) | 4 (n = 6) | p | |
| Mean | 11.33 | 18.05 | 18.79 | 16.08 | 0.19 |
| Std deviation | 8.485 | 8.335 | 3.544 | 5.298 | |
No significant differences between any of the cell types.
Fig. 8.
Effects of lipoproteins on cell death determined based 7AAD staining using flow cytometry analysis: (1) parent cells; (2) resistant cells; (3) resistant cells exposed to oxLDL; (4) resistant cells exposed to VLDL. Data are presented as mean ± standard deviation. There was no significant difference between parent and resistant (p-0.152), resistant alone and resistant exposed to oxLDL (p = 0.783) and resistant alone with resistant exposed to VLDL (p = 0.607). All experiments were performed in replicates (parent n = 5, resistant n = 12, resistant oxLDL n = 12, resistant VLDL n = 6).
4. Discussion
Currently many anti-cancer treatments are effective at the beginning but over time lose efficacy as cancer cells begin to develop resistance to drugs. MDR is one of the major and most common obstacles to the effective treatment of cancer. One of the important mechanisms underlying MDR is cellular overproduction of P-gp, which is encoded by the MDR1 gene and acts as an efflux pump for various hydrophobic anticancer drugs. P-gp belongs to the superfamily of ATP-binding cassette (ABC) transporters which also comprises other proteins, such as the MDR-associated proteins (MRPs) or the half transporter ABCG2 (other definitions MXR, mitoxantrone-resistance protein, and BCRP, breast cancer resistance protein), also associated with MDR. P-gp overexpression in cancer cells has become a therapeutic target for circumventing MDR [27]. Therefore, it is important to study the mechanisms that underlie development of drug resistance. Emerging studies suggest that lipoproteins might be involved in cancer and in development of resistance to anti-cancer drugs. The transport of cholesterol in blood circulation occurs through lipoproteins, mainly in LDL. It was reported to increase cell proliferation in ER-positive and ER-negative cells. In contrast, HDL only increased the proliferation of ER-negative cells [28, 29]. Apart from that, serum oxidized low density lipoprotein increased the risk of colon cancer [30]. Similarly, increased level of oxidized low density lipoprotein was also noted in breast cancer patients [31, 32]. Studies suggest that the intracellular lipid accumulation is linked with aggressive nature of ER-breast cancer and prostate cancer [32,33]. Besides cholesterol transport, lipoproteins are also involved in transport of triglycerides. VLDL is the major transporter of triglycerides [34]. A population-based study suggests that pre-diagnostic triglycerides and high-density lipoprotein-cholesterol/total cholesterol ratio may independently provide unique information in regard to prognostic outcome among patients with triple negative breast cancer [35]. Membrane lipid research show the varied roles of lipids in regulating membrane Pgp function, membrane trafficking, apoptotic pathways, drug transport, and endocytic functions, particularly endocytosis, the primary mechanism of cellular uptake of nanoparticle-based drug delivery systems [36]. In this study, we focused the role of lipids in HER2 overexpressed subtype as it is one of the most commonly studied proto-oncogene in human cancer studies. It plays a pivotal role in oncogenic transformation, tumorigenesis, and metastasis. HER2 gene is amplified and/or overexpressed in ∼20%–30% of invasive breast carcinomas and is associated with unfavorable prognosis, shorter relapse time, and decreased overall survival [37,38]. A better knowledge of mechanisms responsible for primary and acquired resistance may improve treatment sensitivity [39]. In vitro study in HER2 overexpressed cells indicated that elevated StAR-related lipid transfer protein 3 (StARD3), may contribute to breast cancer aggressiveness by increasing membrane cholesterol and enhancing oncogenic signalling [40]. However, there is still limited knowledge on effects of lipids and lipoproteins in HER2 cell proliferation and development of resistance. This study was conducted to compare the effects of oxLDL and VLDL on HER2 overexpressed cell proliferation and resistance.
Morphology changes of resistant and parent UACC732 cells were compared following exposure to oxLDL and VLDL. After 24 h of exposure to 10 μg/ml of oxLDL or VLDL, cells remained spherical in shape. However, exposure to 100 μg/ml of VLDL resulted in the presence of fibroblast-like cells in both parent and resistant cells. This finding suggests that exposure to VLDL promoted epithelial to mesenchymal transition (EMT) characteristics in the cells. Previous studies have reported evidence of EMT involvement in drug resistance and metastasis of cancers [41]. For example, continuous exposure of A549 lung adenocarcinoma cells to the drug gefitinib resulted in induction of the EMT [23]. Phenotypic changes indicative of the EMT, including spindle cell shape and increased pseudopodia formation, were detected in these A549 cells, and these changes were accompanied by a decrease of E-cadherin and an increase of vimentin, a mesenchymal marker.
Effects of lipoproteins on cell proliferation also were investigated in both parent and resistant UACC732 cells. The IC50 value of oxLDL in resistant cells (73.8 μg/ml) was higher than that in parent cells (30.9 μg/ml), which showed that the resistant cells required more oxLDL compared to parent cells to achieve 50% cell death. The dose-response curve indicated that exposure to 100 μg/ml of oxLDL reduced cell viability to less than 10% in parent cells and to approximately 40% in resistant cells based on the proliferation assay. Similar finding was reported previously, where oxLDL can initiate cell death in cancer cells [42]. In contrast, a study of MDA MB 231 breast cancer cells showed that exposure to 100 μg/ml of LDL had a growth stimulatory effect; however, HDL did not promote cell growth [43]. In this study, VLDL caused increase in cell proliferation in parent and resistant UACC732 cells especially with 100 μg/ml of VLDL. Previous study with 10–25 μg/ml of VLDL in MCF-7 and MDA-MB-231 cells also increased cell viability [34].
P-gp, also known as ABCB1 and MDR1 in human cells, mediates the efflux of drugs and toxic agents from cells and plays a key role in the MDR of cancer cells [44]. It functions as a transporter via excretion of drugs, toxic agents, and xenobiotics from cells. Several natural compounds, dietary phytochemicals, and herbal medicines have the ability to modulate P-gp function and cause effects such as drug-drug, herb-drug, and herb-toxicant interactions [45]. In this study, P-gp expression in UACC732 cells treated with lipoproteins was assessed using flow cytometry. P-gp expression was high in resistant cells compared to parent cells, but exposure of resistant cells to lipoproteins reduced P-gp expression. A significant reduction in P-gp expression was detected among VLDL-treated resistant cells (p = 0.02). These findings suggest VLDL may regulate P-gp expression in HER2-overexpressed cells. Additionally, possible role of triglycerides, the major component of VLDL in downregulating expression of P-gp functions needs further investigation.
In this study, oxLDL-exposed resistant cells also exhibited lower P-gp expression compared to resistant cells alone. In contrast, a previous study of doxorubicin-resistant uterine sarcoma cells showed that LDL, depending on its concentration, caused an increase in ABCB1, ABCC1, and LRP expression, whereas ABCB1 expression increased at low HDL and decreased at high HDL concentrations [27]. Nevertheless, another study reported that 100 μg of LDL significantly decreased ABCB1-associated ATPase activity in a vinblastine-resistant human lymphoblastic leukemia cell line [46]. This shows the expression of P-gp may be influenced by level of LDL oxidation and cancer types. It was reported that the reduction in P-gp expression could relate to cholesterol homeostasis. When a cell contains sufficient cholesterol, LDL receptor synthesis is reduced so that additional LDL molecules cannot be taken up [47]. In another study focused on the relationship between LDL and P-gp expression, the drug atorvastatin was more effective at reducing LDL cholesterol in ABCB1 high expressors than in low expressors [31]. This suggests a possible interaction between P-gp and lipoprotein metabolism.
Staining for dead cells with 7AAD showed that the 10 μg/ml oxLDL-exposed resistant cells had the highest percentage of dead cells compared to resistant cells exposed to 10 μg/ml VLDL. However, the difference was not significant compared to resistant cells alone. Lu et al. (2016) compared the effects of LDL subfractions 1 and 5 (L1 and L5) and VLDL on MCF7 and MDA-MD-231 cells [13]. In MCF7 cells, only VLDL (1–10 μg/ml) increased the number of viable cells, whereas HDL (10 and 25 μg/ml) decreased the number of viable cells. In MDA-MD-231 cells, L1 (5–25 μg/ml), L5 (5–25 μg/ml), and VLDL (25 μg/ml) all caused a significant increase of viable cells. However, the viability of MDA-MD-231 cells was not affected by HDL. They reported that only VLDL provided a survival advantage and that it promoted lung metastasis in MDA-MD-231-injected mice [34]. Therefore, this shows that VLDL was found to promote cell viability in different subtypes of breast cancer.
5. Conclusion
The study was done to compare the effects of different lipoproteins in HER2 overexpressed breast cancer cells on cell growth dan development of resistance. Findings suggest that VLDL particularly caused morphological changes and increased cell viability in HER2 overexpressed resistant and parent cells. Interestingly, P-gp expression was found to be reduced in these cells following exposure to lipoprotein which renders further investigation.
Declarations
Author contribution statement
Siti Zainab Salim: Performed the experiments; Analyzed and interpreted the data.
Seoparjoo Azmel Md Isa: Analyzed and interpreted the data.
Shahrul Hamid: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported Fundamental Research Grant Scheme from the Ministry of Education (Higher Education), Malaysia (203/CIPPT/6711540).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Sun Y.-S. Risk factors and preventions of breast cancer. Int. J. Biol. Sci. 2017;13(11):1387–1397. doi: 10.7150/ijbs.21635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delimaris I. Oxidized LDL, serum oxidizability and serum lipid levels in patients with breast or ovarian cancer. Clin. Biochem. 2007;40(15):1129–1134. doi: 10.1016/j.clinbiochem.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Kokoglu E. Alterations of serum lipids and lipoproteins in breast cancer. Cancer Lett. 1994;82(2):175–178. doi: 10.1016/0304-3835(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X., Huang G. Synthetic lipoprotein as nano-material vehicle in the targeted drug delivery. Drug Deliv. 2017;24(2):16–21. doi: 10.1080/10717544.2017.1384518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee J.Y. Low-density lipoprotein-mimicking nanoparticles for tumor-targeted theranostic applications. Small. 2015;11(2):222–231. doi: 10.1002/smll.201303277. [DOI] [PubMed] [Google Scholar]
- 6.Jiao X. MiR-181a enhances drug sensitivity in mitoxantone-resistant breast cancer cells by targeting breast cancer resistance protein (BCRP/ABCG2) Breast Canc. Res. Treat. 2013;139(3):717–730. doi: 10.1007/s10549-013-2607-x. [DOI] [PubMed] [Google Scholar]
- 7.Gayet L. Control of P-glycoprotein activity by membrane cholesterol amounts and their relation to multidrug resistance in human CEM leukemia cells. Biochemistry. 2005;44(11):4499–4509. doi: 10.1021/bi048669w. [DOI] [PubMed] [Google Scholar]
- 8.Eckford P.D., Sharom F.J. Interaction of the P-glycoprotein multidrug efflux pump with cholesterol: effects on ATPase activity, drug binding and transport. Biochemistry. 2008;47(51):13686–13698. doi: 10.1021/bi801409r. [DOI] [PubMed] [Google Scholar]
- 9.Garrigues A., Escargueil A.E., Orlowski S. The multidrug transporter, P-glycoprotein, actively mediates cholesterol redistribution in the cell membrane. Proc. Natl. Acad. Sci. U. S. A. 2002;99(16):10347–10352. doi: 10.1073/pnas.162366399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esserman L.J. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL—CALGB 150007/150012, ACRIN 6657. J. Clin. Oncol. 2012;30(26):3242–3249. doi: 10.1200/JCO.2011.39.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arpino G., Milano M., De Placido S. Features of aggressive breast cancer. Breast. 2015;24(5):594–600. doi: 10.1016/j.breast.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Rodrigues dos Santos C. Plasma level of LDL-cholesterol at diagnosis is a predictor factor of breast tumor progression. BMC Canc. 2014;14(1):132. doi: 10.1186/1471-2407-14-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vergès B. Pathophysiology of diabetic dyslipidaemia: where are we? Diabetologia. 2015;58(5):886–899. doi: 10.1007/s00125-015-3525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugden M., Holness M. Pathophysiology of diabetic dyslipidemia: implications for atherogenesis and treatment. Clin. Lipidol. 2011;6(4):401–411. [Google Scholar]
- 16.Choi S.H., Ginsberg H.N. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends Endocrinol. Metabol. 2011;22(9):353–363. doi: 10.1016/j.tem.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen S., Karpe F. Determinants of VLDL-triglycerides production. Curr. Opin. Lipidol. 2012;23(4):321–326. doi: 10.1097/MOL.0b013e3283544956. [DOI] [PubMed] [Google Scholar]
- 18.Schwab U. Effect of the amount and type of dietary fat on cardiometabolic risk factors and risk of developing type 2 diabetes, cardiovascular diseases, and cancer: a systematic review. Food Nutr. Res. 2014;58(1):25145. doi: 10.3402/fnr.v58.25145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Couillard C. Circulating levels of oxidative stress markers and endothelial adhesion molecules in men with abdominal obesity. J. Clin. Endocrinol. Metab. 2005;90(12):6454–6459. doi: 10.1210/jc.2004-2438. [DOI] [PubMed] [Google Scholar]
- 20.Hecht F. The role of oxidative stress on breast cancer development and therapy. Tumor Biol. 2016;37(4):4281–4291. doi: 10.1007/s13277-016-4873-9. [DOI] [PubMed] [Google Scholar]
- 21.Pandyra A.A. Genome-wide RNAi analysis reveals that simultaneous inhibition of specific mevalonate pathway genes potentiates tumor cell death. Oncotarget. 2015;6(29):26909–26921. doi: 10.18632/oncotarget.4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuhrman B. Cell-induced copper ion-mediated low density lipoprotein oxidation increases during in vivo monocyte-to-macrophage differentiation. Free Radic. Biol. Med. 2004;37(2):259–271. doi: 10.1016/j.freeradbiomed.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 23.Lian T.-W. Fisetin, morin and myricetin attenuate CD36 expression and oxLDL uptake in U937-derived macrophages. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2008;1781(10):601–609. doi: 10.1016/j.bbalip.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Tsai Y.-Y. VLDL-activated cell signaling pathways that stimulate adrenal cell aldosterone production. Mol. Cell. Endocrinol. 2016;433:138–146. doi: 10.1016/j.mce.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coley H.M. Cancer Cell Culture. Springer; 2004. Development of drug-resistant models; pp. 267–273. [Google Scholar]
- 26.Khaidakov M., Mehta J.L. Oxidized LDL triggers pro-oncogenic signaling in human breast mammary epithelial cells partly via stimulation of MiR-21. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0046973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nobili S. Overcoming tumor multidrug resistance using drugs able to evade P-glycoprotein or to exploit its expression. Med. Res. Rev. 2012;32(6):1220–1262. doi: 10.1002/med.20239. [DOI] [PubMed] [Google Scholar]
- 28.Antalis C.J. High ACAT1 expression in estrogen receptor negative basal-like breast cancer cells is associated with LDL-induced proliferation. Breast Canc. Res. Treat. 2010;122(3):661–670. doi: 10.1007/s10549-009-0594-8. [DOI] [PubMed] [Google Scholar]
- 29.dos Santos C.R. LDL-cholesterol signaling induces breast cancer proliferation and invasion. Lipids Health Dis. 2014;13(1):16. doi: 10.1186/1476-511X-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki K. Serum oxidized low-density lipoprotein levels and risk of colorectal cancer: a case-control study nested in the Japan Collaborative Cohort Study. Cancer Epidemiol. Biomark. Prev. 2004;13(11 Pt 1):1781–1787. [PubMed] [Google Scholar]
- 31.Badid N. Oxidant/antioxidant status, lipids and hormonal profile in overweight women with breast cancer. Pathol. Oncol. Res. 2010;16(2):159–167. doi: 10.1007/s12253-009-9199-0. [DOI] [PubMed] [Google Scholar]
- 32.Yan X.L. Mesenchymal stem cells from primary breast cancer tissue promote cancer proliferation and enhance mammosphere formation partially via EGF/EGFR/Akt pathway. Breast Cancer Res. Treat. 2011 doi: 10.1007/s10549-011-1577-0. [DOI] [PubMed] [Google Scholar]
- 33.Yue S. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metabol. 2014;19(3):393–406. doi: 10.1016/j.cmet.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu C.-W. VLDL and LDL, but not HDL, promote breast cancer cell proliferation, metastasis and angiogenesis. Cancer Lett. 2017;388:130–138. doi: 10.1016/j.canlet.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 35.Lofterød T. Impact of pre-diagnostic triglycerides and HDL-cholesterol on breast cancer recurrence and survival by breast cancer subtypes. BMC Canc. 2018;18(1):654. doi: 10.1186/s12885-018-4568-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peetla C., Vijayaraghavalu S., Labhasetwar V. Biophysics of cell membrane lipids in cancer drug resistance: implications for drug transport and drug delivery with nanoparticles. Adv. Drug Deliv. Rev. 2013;65(13):1686–1698. doi: 10.1016/j.addr.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hudis C.A. Trastuzumab — mechanism of action and use in clinical practice. N. Engl. J. Med. 2007;357(1):39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 38.Gabos Z. Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. J. Clin. Oncol. 2006;24(36):5658–5663. doi: 10.1200/JCO.2006.07.0250. [DOI] [PubMed] [Google Scholar]
- 39.Minuti G. Increased MET and HGF gene copy numbers are associated with trastuzumab failure in HER2-positive metastatic breast cancer. Br. J. Canc. 2012;107:793. doi: 10.1038/bjc.2012.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vassilev B. Elevated levels of star-related lipid transfer protein 3 alter cholesterol balance and adhesiveness of breast cancer cells: potential mechanisms contributing to progression of HER2-positive breast cancers. Am. J. Pathol. 2015;185(4):987–1000. doi: 10.1016/j.ajpath.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 41.Nurwidya F. Epithelial mesenchymal transition in drug resistance and metastasis of lung cancer. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2012;44(3):151–156. doi: 10.4143/crt.2012.44.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zabirnyk O. Oxidized low-density lipoproteins upregulate proline oxidase to initiate ROS-dependent autophagy. Carcinogenesis. 2009;31(3):446–454. doi: 10.1093/carcin/bgp299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodrigues dos Santos C. LDL-cholesterol signaling induces breast cancer proliferation and invasion. Lipids Health Dis. 2014;13(1):16. doi: 10.1186/1476-511X-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamidovic A., Hahn K., Kolesar J. Clinical significance of ABCB1 genotyping in oncology. J. Oncol. Pharm. Pract. 2010;16(1):39–44. doi: 10.1177/1078155209104380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rocejanasaroj A., Tencomnao T., Sangkitikomol W. Thunbergia laurifolia extract minimizes the adverse effects of toxicants by regulating P-glycoprotein activity, CYP450, and lipid metabolism gene expression in HepG2 cells. Genet. Mol. Res. 2014;13(1):205–219. doi: 10.4238/2014.January.10.12. [DOI] [PubMed] [Google Scholar]
- 46.Kamau S.W. Effect of the modulation of the membrane lipid composition on the localization and function of P-glycoprotein in MDR1-MDCK cells. In Vitro Cell. Dev. Biol. Anim. 2005;41(7):207–216. doi: 10.1290/0502016.1. [DOI] [PubMed] [Google Scholar]
- 47.Brown M.S., Goldstein J.L. Regulation of the activity of the low density lipoprotein receptor in human fibroblasts. Cell. 1975;6(3):307–316. doi: 10.1016/0092-8674(75)90182-8. [DOI] [PubMed] [Google Scholar]