Abstract
Nanotechnology has high potential in processing of industrial crops and by-products in order to extract valuable biological active compounds. The present study endeavored to take advantage of nanotech approach (i.e microemulsion, ME), as a novel green technique, for lutein extraction from marigold (Tagetes erecta) as an industrial crop. The pseudo-ternary phase diagrams confirmed the effect of surfactant type on the formation of mono-phasic lutein MEs. The combination of sucrose monopalmitate:1-poropanol (1:5) showed the highest efficiency in the presence of marigold petal powder (MPP, 18%) and water (42%). In addition, the efficiency of primitive MEs (without co-surfactants) was outstandingly increased as MPP was moistened by co-surfactants. Furthermore, different MEs resulted in various droplet size (14–250nm), PDI (0.05–0.32) and zeta potential (−1.96 to −38.50 mV). These findings revealed the outstanding importance of the surfactants and co-surfactants and their ratio on the extraction capability of MEs. These findings also proved the capability of microemulsion technique (MET) as a potential alternative to conventional solvent with possible applicability for extraction of lutein and other industrial plant based bio-compounds.
Keywords: Natural product chemistry, Physical chemistry, Food analysis, Food technology, Pharmaceutical science, Nanotechnology
1. Introduction
Carotenoids usually impart many biological activities that are already very well established in the literature. Lutein, as a xanthophyll, a subgroup of carotenoids, is one of these essential ingredients which is found in many plants but one of its major sources is marigold petals (Tagetes erecta). This industrial crop is cultivated all over the world especially in Mexico, Spain, China and India [1, 2]. Lutein is considered as coloring agent in food and feed [3] and widely known for its antioxidant activities, free radical scavenging, quenching of photo induced reactive oxygen species, inhibition of the auto-oxidation of cellular lipids [4], anti-inflammatory properties, neuroprotective effects, enhancing the immune function and prevention or improvement of cancers, atherosclerosis and cardiovascular diseases [5] alongside its beneficial health effect on visual acuity, reducing the risk of age-related macular degeneration (AMD) and cataract [6] and other health effects in central nervous system especially frontal and occipital cortex and hippocampus areas [7]. Owing to the potential therapeutic benefits of this xantophyllic pigment for sustenance of vital physiological functions and the inability of its synthesis in human body, its requirement needs to be fulfilled by daily diets [8]. Therefore, its extraction is greatly demanded for being utilized in formulation of foods, nutraceuticals, pharmaceuticals as well as supplements.
The chemical structure of lutein is constituted of eight isoprene, 40 carbon atoms and two hydroxyl groups. Due to the presence of conjugated double bonds, it is very susceptible to external stresses such as heat and light, which limit its industrial applications especially in food and nutraceutical formulations [9]. In addition, its poor solubility in aqueous solution leads to low absorption and inadequate bioavailability [10]. Therefore, especial measures must be taken during its extraction and application. With regard to its extraction, different methods mostly organic solvent extraction had been already examined [11]. Despite the high efficiency of solvent extraction, it offers some limitations and drawbacks such as solvent residues, health hazards for consumers and operators, lengthy processing, high energy consumption due to high temperature demand, thermal isomerization and degradation possibility as well as bioenvironmental issues [12, 13]. Furthermore, owning to its hydrophobicity, the solvent based extracted lutein cannot be easily re-dispersed in aqueous systems, the majority of food and drug systems, and its vulnerability to degradation and instability is another concern. Therefore, food, nutraceutical and pharmaceutical industries are looking for alternative techniques to extract and solubilize lutein with a high stability.
Among various alternative approaches, microemulsion technique (MET) as a novel approach, has high potential to extract different nutraceuticals and pharmaceuticals [12]. Microemulsions (ME) are defined as low viscose, isocratic and transparent systems which are thermodynamically stable. Their capability is highly depended to the surfactants and co-surfactants which are used in their formulations [14]. These surface active agents have high dissolution capacity indicating their potential capability to extract various ingredients in the form of MEs with advantages such as simplicity, low cost, ease of manufacturing, thermodynamic stability, stability against oxidation and protective effect on the extracted ingredients with high bioavailability [15]. The controlled release and feasibility at relatively low temperatures are also key scale-up criteria for commercialization [16] and could make this technique as a great candidate for extraction of sensitive phytochemicals such as lutein from natural (food) matrices. Its high potential on extraction and solubilization of different nutraceuticals and pharmaceuticals [12, 17, 18] has been proven. However, it has never been used for lutein extraction from marigold except handful reports on solubilization of lutein powder using microemulsion [19].
To meet the aforementioned drawbacks of carotenoid extraction, the present study thrived to design, develop and examine the potential capability of different microemulsion systems, as a green alternative method, on lutein extraction and solublization preferably on the basis of bio-natural surfactants and food grade co-surfactants from fresh and dried marigold petals.
2. Materials and methods
2.1. Materials
Span 20 (S20, HLB1: 8.6), Tween 20 (T20, HLB: 16.7), Tween 80 (T80, HLB: 15), sodium dodecyl sulfate (SDS, HLB: 40), ethanol (EtOH), 1-propanol (1-PrOH), glycerol (GlOH), propylene glycol (PGOH) and acetone (Merck Chemical Co., Darmstadt, Germany) were purchased and used without any further purification. Natural pharmaceutical grade Saponin (Sap, HLB: 13.5) and Rhamnolipid (Rhl, HLB: 9.5) were provided by Pioneer Biotech Co. (Shannxi, China), and sucrose monopalmitate (SMP, HLB: 15) was purchased from Compass Foods Company (Tuas, Singapore). Commercial soy lecithin was supplied by Behpak Company (Behshar, Iran) and further purification was done by removing the oil with acetone, preceded by fractionation with ethanol to produce lecithin (Lec, HLB: 7) with reasonably higher purity. Deionized water (18.2 MΩ cm) was used for preparation of solutions and extraction.
2.2. Methods
2.2.1. Preparation of marigold petals powder (MPP)
Fresh marigold flower (Tagetes erecta) was kindly supplied by municipality farm (Ardabil, Iran), and petals were manually separated and carefully washed. They were then air dried (domestic oven, UFE 500, Memmert, Germany, 8 h at 45 °C) away from light (final moisture content of 8%), ground (Hamilton domestic mill, FH-140, 230 W, China), sieved (#25, ENDECOTTS, London, England) and stored as powder at −18 °C.
2.2.2. Solvent extraction of lutein from MPP
Some 100 mg of MPP was added into 20 ml of acetone and mixed (3 h at 300 rpm) on a magnetic stirrer (MR3001, Heidolph, Germany) at room temperature (25 °C) under dim light. Then, the suspension was allowed to stand (5 min) and the supernatant was collected but the pellet was mixed (1h) again with acetone (10 ml) at the above mentioned conditions and former step was repeated two more times until the pellet turned colorless. Finally, the supernatants were pooled and stored at 4 °C for lutein content analysis. To prevent any photo-oxidation and isomerization, the samples were always covered with aluminum foil [20].
2.2.3. Optical measurement of lutein content
Lutein quantification was carried out based on its molar extinction coefficient [21]. The extract (sub-section 2.2.2) was centrifuged (3–30K, Sigma, Germany,15 min at 13000g and 25 °C), and its optical density (OD) was recorded against acetone as blank (UV-Vis Agilent Spectrophotometer, Cary60, path length = 1cm, US) at 446 nm, as the λmax of lutein had the least interference with other carotenoids. Lutein content of MPP was then calculated by:
| C = A446/(14.45 × 104) × (1/b) × 568.88 × V/M × 1L/103ml × 103 mg/g × Kg/103g |
Where C is the lutein content (mg/g), A446 is absorbance wavelength, b is path length (cm), 568.88 is molecular weight of lutein (gmol−1), V is the volume of MPP extract (ml), M is the weight of the consumed MPP (kg), and 14.45×104 is the molar extinction coefficient of lutein in acetone (L mol−1 cm−1).
2.2.4. Lutein extraction using microemulsion technique
2.2.4.1. Pseudoternary phase diagram
Alongside various dilution lines, different ratios of surfactants, co-surfactants, water and MPP were mixed and pseudoternary phase diagrams were plotted (Sigma Plot 14.0, Systat Software Inc., San Jose, USA). Then, the single-phase microemulsion region was determined based on macroscopic transparency or visual titration technique. In more detail, the deionized water was mixed with MPP, as lutein containing phase, at various ratios, followed by addition of the surfactant: co-surfactant (SCR) mixture (SCRmax of each surfactant). The mixtures were then agitated (2500 rpm, 25 °C, 20 min) and centrifuged (18000g, 25 °C, 15 min). Each point on pseudoternary phase diagram composed of various mass percent of three components (a, b, c) as:
| x%a + y%b + z%c = 100% |
Based on visual transparency, the single-phase ME region for various natural (Sap, Lec, Rhl, and SMP) and synthetic (S20, T20, T80 and SDS) surfactants were determined and phase diagrams were constructed.
2.2.4.2. Effect of surfactants on lutein extraction yield
Considering the phase diagrams and single-phase ME regions for various surfactants, to evaluate their effects on lutein extraction yield, 40 g of double distilled water was mixed with different amounts of each surfactant separately in centrifuge tubes (50 ml). Then, MPP (20 mg) was added and the mixtures were mixed (30 min, at 25 °C, 175 rpm, under dim light). It is noteworthy that by using constant amounts of water and MPP but different concentrations of surfactants, various surfactant: lutein ratios (SOR) was achieved (100:1, 200:1, 500:1, 1000:1, 2000:1, 5000:1). The mixtures were centrifuged (13,000g, 15 min, room temperature) and supernatant (i.e., microemulsion) was separated. The remaining lutein content of pellet was also extracted (5 ml acetone, stirred at 90 rpm, 15 min at 25 °C) three times, the supernatants were pooled, filtered and analyzed for lutein content (sub-section 2.2.3). Microemulsion extraction yield was calculated through:
| Y (%) = (X3/X1) × 100 = [(X1−X2)/X1] × 100 |
Where Y is the microemulsion extraction yield (%), X1, X2 and X3 are the lutein content of MPP (mg/g), pellet phase of microemulsion extraction and supernatant phase, respectively.
2.2.4.3. Effect of co-surfactants on lutein extraction yield
The effect of four co-surfactants (EtOH, 1-PrOH, GlOH) and PGOH) at various surfactant: co-surfactant ratios or SCR (2:1, 1:1, 1:2, 1:5, 1:10, 1:20 and 1:50) was also evaluated and SORmax was the one which led to the highest lutein extraction efficiency (sub-section 2.2.4). To do so, two procedures were implemented as follows:
Method A: The optimum amount of each surfactant was separately mixed with 40 g of double distilled water then various amounts of each co-surfactant was added (water: surfactant: co-surfactant dispersion). Then, 20 mg of MPP was added and mixed thoroughly. The lutein extraction and quantification was conducted as previously described.
Method B: The optimum quantity of each surfactant was separately mixed with 40 g of double distilled water (surfactant solution). Then, 20 mg of MPP was soaked with various amounts of co-surfactants and mixed for thorough wetting (MPP: co-surfactant mixture). In the next step, the surfactant solution and MPP: co-surfactant mixture were mixed for a short while to complete the extraction process. The lutein extraction and quantification was opted as previously described.
2.2.5. Dynamic light scattering analysis
The mean droplet size (Z-Average), polydispersity index (PDI) and zeta potential (ZP) of MEs (method B) were measured (in an optical quality 1 ml borosilicate cell at 90° angle) by using a dynamic light scattering technique (Zetasizer Nano ZS90, Malvern Instruments, Worcestershire, UK). All measurements were opted at the set temperature (25 °C) without any diluting.
2.2.6. Statistical analysis
The mean lutein content of samples was determined and expressed as means with standard deviations (Mean ± SD) for at least three replicates per assay. Data were also checked for normality and the significance difference (p < 0.05) was tested by one-way ANOVA followed by Duncan's Multiple Range Test (DMRT) using SPSS software (IBM SPSS Statistics 21, Chicago, IL, USA). Curves were plotted using Excel 2013 (Microsoft Corporation, Redmond, WA, USA).
3. Results and discussion
3.1. Construction of phase diagrams
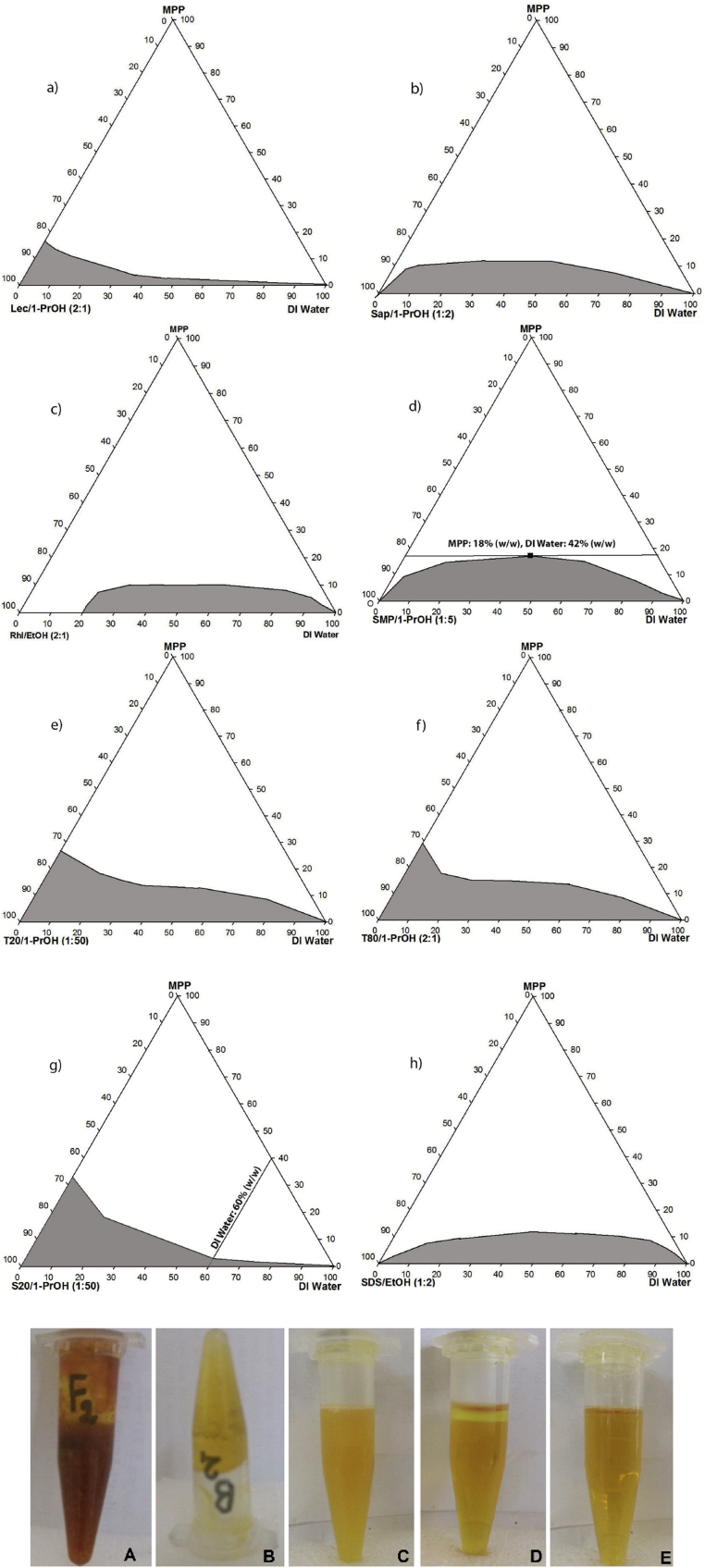
A pseudo-ternary phase diagram is an equilateral triangle including three vertices for three main components. It is the best way to predict the optimized compositions of surfactant/co-surfactant, oil and aqueous phases in the development of single-phase MEs. Therefore, in the present study, the pseudo-ternary phase diagrams of various MEs formula (using different surfactants and co-surfactants with various ratios of other components) were constructed. By plotting a borderline, the transparent monophasic ME regions were distinguished from multi-phase or turbid ones (Fig. 1. Upper panel). The uncolored regions indicate anisotropic turbid system, whereas the colored section represents isotropic, transparent, single-phase and thermodynamically stable MEs. It must be noted that, the observed differences in optical properties of transparent and turbid emulsions can be attributed to the variations in the light scattering behavior which is impressively influenced by particle size, PDI, ingredient fractional ratio and refractive index [22]. As MPP and some surfactants were used as powder (dried forms), therefore the available water was not sufficient for hydration. As a result, most of the mixtures in the uncolored regions were either incompletely hydrated or gelled (Fig. 1. Lower panel). Hence, it seems by using fresh marigold petals, one could expand the single-phase ME region but it was not tested in this study as MPP was more convenient to work with and the condition was easily controllable.
Fig.1.
Comparison of the pseudoternary phase diagrams and single-phase lutein microemulsion region (grey) using marigold petal powder (MPP), deionized water (DI water) and various surfactants in combination with the most efficient co-surfactant (SCRmax): a) Lec:1-PrOH (2:1), b) Sap: 1-PrOH (1:2), c) Rhl: EtOH (2:1), d) SMP: 1-PrOH (1:5), e) T20: 1-PrOH (1:50), f) T80: 1-PrOH (2:1), g) S20: 1-PrOH (1:50) and h) SDS: EtOH (1:2). Lower panel: An example of actual samples prepared with SMP: 1-PrOH (1:5): A) un-hydrated, B) gelled, C) turbid, D) multiphase, E) transparent and mono-phasic ME.
Moreover, the single-phase ME region is usually close to the base of diagram where deionized water (DI water) or surfactant: co-surfactant mixtures exhibited relatively high fluidity. Apart from water availability, the surfactant type was also very effective in the formation of mono-phasic transparent MEs. For instance, the Rhl (hygroscopic powder) in combination with EtOH (SCR, 2:1) formed ME with 10% of MPP at the presence of >20 wt% of water (Fig. 1c). In contrast, SMP: 1-PrOH (1:5) with 18 wt% of MPP and 42 wt% of water and both of T20: 1-PrOH (1:50) and T80: 1-PrOH (2:1) with 20 wt% of MPP and 16 and 10 wt% of water, formed single-phase MEs (Fig. 1d, e, f). These results demonstrated that for 18 wt% of MPP, only 40 wt% of SMP:1-PrOH (1:5) was required to form normal micelles to solubilize lutein and likely other carotenoids. It is clear that he water requirement was less than what was needed by T20 (64 wt%) and T80 (70 wt%). Apart from structural and functional differences, the high SCR (1:50) and the low viscosity of T20 and T80 could be another crucial factor on construction of MEs at the presence of high ratios of MPP (<20 wt%).
In addition, the hydrophobic surfactants (Lec, HLB = 7 and S20, HLB = 8.6) created smaller single-phase ME area at 50 and 60 wt% water ratios, respectively (Fig.1a and g). It seems in the case of S20 based ME, the high ratio of co-surfactant (SCR, 1:50) made it more appropriate to form ME with higher ratio of MPP (up to 33%). Moreover, the presence of large numbers of CH2 units in oxy-ethylene chains led to the higher affinity of the esterified lutein toward non-ionic surfactants (i.e., T20, T80 and S20). Therefore, in the presence of even lower water ratio, nonionic (i.e., T20, T80 and S20) and zwitterionic (i.e., Lec) surfactants formed isotropic domain (O/W) by interacting with MPP propitiously [23]. Finally the prepared pseudo-ternary phase diagrams enlightened the impact of MEs composition on the optical properties of designed systems. This property needs to be considered in producing functional delivery systems namely enriched beverages, where the optical transparency is the case. Moreover, adding lesser surfactants and achieving the highest extraction yield are very desired aspects from food application view point, therefore based on the knowledge achieved from pseudoternary phase diagrams, the influence of different ratios of various surfactants on the extraction yield of lutein (under constant ratios of MPP and water) were investigated in the following sub-sections.
3.2. Effect of surfactants and co-surfactants
Based on spectrophotometric analysis, the acetone extract of MPP contained 15.83 mg/g (dry weight) of lutein. This number is in a good agreement with 20.59 mg/g which was recently reported [24]. However, as is evident (Table.1), the oil/water MEs were only able to extract over the range 5.11% (Lec) to 13.22% (T80) using different surfactants when no co-surfactant was used. Oil in water (O/W) MEs are in fact the oil nano-droplets which are surrounded by a surfactant or surfactant–co-surfactant mono-layer which are dispersed in the aqueous phase. The mono-layer plays an important role in decreasing the interfacial tension and free energy as a result of which the hydrophobic interactions diminish and microemulsion formation facilitates. One might expect that the higher the SOR, the lower the interfacial tension. Moreover, by improving the mobility of the interface, the lutein extraction would become more efficient. Nevertheless, as can be seen (Table 1), the efficiency of MEs as a function of SOR was increased up to SORmax but with any further increase, the extraction efficiency did not improve. In other words, the yield–SOR dependency curve was very similar to an inverted U curve. This most likely can be attributed to the increased adsorption of surfactant molecules to the oil–water interface which eventually led to a decreased interfacial tension and the facilitated lutein extraction. Conversly, the ineffectiveness of the additional surfactant might be related to their higher viscosity at high SOR values which affects the interaction rate between lutein and surfactants.
Table 1.
Comparison of the extraction yiled (%) of different MEs (water 40 g, MPP 20 mg) under various SORs (surfactant: lutein ratios).
| Surfactant | SOR (%w/w) |
|||||
|---|---|---|---|---|---|---|
| 100:1 | 200:1 | 500:1 | 1000:1 | 2000:1 | 5000:1 | |
| Lec | 3.12 ± 0.14r | 5.11 ± 0.23pq | 5.06 ± 0.19pq | 4.99 ± 0.25q | 5.01 ± 0.06q | 4.73 ± 0.02q |
| Sap | 5.35 ± 0.16opq | 7.25 ± 0.34jkl | 6.94 ± 0.77klm | 6.56 ± 0.30lmn | 6.11 ± 0.54n | 5.78 ± 0.36nop |
| Rhl | 3.56 ± 0.09r | 4.74 ± 0.62q | 5.91 ± 0.24no | 6.20 ± 0.16n | 6.13 ± 0.40n | 5.89 ± 0.39no |
| SMP | 6.11 ± 0.37n | 8.11 ± 0.09i | 8.09 ± 0.24i | 8.00 ± 0.36i | 8.05 ± 0.19i | 7.91 ± 0.56ij |
| T20 | 10.76 ± 0.29de | 12.31 ± 0.74bc | 12.08 ± 0.18c | 12.00 ± 0.17c | 11.74 ± 0.05c | 11.87 ± 0.66c |
| T80 | 10.84 ± 0.13d | 12.19 ± 0.50c | 12.32 ± 0.04bc | 13.00 ± 0.23ab | 13.22 ± 0.60a | 13.04 ± 0.97a |
| S20 | 6.50 ± 0.91mn | 7.60 ± 0.34ijk | 9.06 ± 0.48h | 10.20 ± 0.43def | 10.04 ± 0.13ef | 9.31 ± 0.07gh |
| SDS | 8.36 ± 0.51i | 10.40 ± 0.52def | 10.21 ± 0.36def | 10.32 ± 0.15def | 9.94 ± 0.67fg | 9.69 ± 0.10fgh |
Different small letters represent significant difference (p < 0.05).
Efficiencies were reported in comparison to acetone extraction (lutein content of MPP = 15.83 mg/g).
Bold numbers represent the SORs at which maximum yield was achieved (i.e., SORmax).
Since lutein ME potentially needs to be utilized in the formulation of food or nutraceutical products. Therefore, its surfactant content should be kept at the minimum, just sufficient to reduce the interfacial tension to achieve thermodynamic stability with the highest extraction yield and the lowest adverse effect on the sensory properties. Consequently, the optimization is essential to develop a ME based extraction procedure. Though, the low efficiency of rudimentary formulas was a challenge that is why in the following steps, the role of various co-surfactants was examined.
Considering Table 2, it is obvious that all of co-surfactants led to enhanced extraction compared to those without co-surfactant (Table 1). These findings confirmed the crucial role of co-surfactants in formation of ME by lowering the interfacial tension between immiscible phases, providing superior flexibility of the interface via penetrating between the surfactants tails and consequently decreasing the continuous phase viscosity [25]. Furthermore, MEs prepared with different surfactants led to higher lutein extraction under different SCRs. In a more detailed manner, for each of ME, the extraction efficiency was significantly (p < 0.05) increased as SCR increased towards SCRmax. However, further increase of SCR, the efficiency either slightly increased (p > 0.05) or did not change and in some cases decreased (i.e., inverted U shape curve). This trend can be mainly attributed to the fact that at SCRmax, there was sufficient co-surfactant molecules available to facilitate surfactant–lutein interaction. Moreover, by thorough covering the O/W interface more efficient extraction could be performed. In contrast, an excess ratio of a co-surfactant (needed to self-assemble) was undesirable presumably due to its gradual leave of the interface and its partitioning (between the oil and water phases) and consequent solubilizing capacity decrease.
Table 2.
Comparison of the extraction yiled (%) of MEs (water 40 g, MPP 20 mg) prepared with various SCRs (surfactant: co-surfactant ratios) of different co-surfactants under SORmax (Lec; 200:1, Sap; 200:1, Rhl; 1000:1, SMP; 200:1, T20; 200:1, T80; 2000:1, S20; 1000:1 and SDS; 200:1).
| S∗/CS∗∗ | SCR (%w/w) |
||||||
|---|---|---|---|---|---|---|---|
| 2:1 | 1:1 | 1:2 | 1:5 | 1:10 | 1:20 | 1:50 | |
| Lec: GlOH | 7.67 ± 0.20gh | 5.55 ± 0.64i | 7.72 ± 0.50gh | 7.93 ± 0.35gh | 7.26 ± 0.31h | 8.15 ± 0.42gh | 9.79 ± 0.11cde |
| Lec: PGOH | 10.65 ± 0.21abc | 10.23 ± 0.33bcde | 10.40 ± 0.52bcde | 11.03 ± 0.63ab | 10.81 ± 0.39abc | 10.32 ± 0.15bcde | 10.00 ± 0.52bcde |
| Lec: 1-PrOH | 11.71 ± 0.49a | 8.50 ± 0.92fg | 9.26 ± 0.72ef | 9.59 ± 0.38de | 9.77 ± 0.17cde | 9.93 ± 0.72bcde | 10.37 ± 0.89bcde |
| Lec: EtOH | 10.27 ± 0.92bcde | 10.04 ± 1.07bcde | 10.35 ± 0.53bcde | 10.59 ± 0.76abc | 10.68 ± 0.46abc | 10.79 ± 0.89abc | 11.10 ± 0.73ab |
| Sap: GlOH | 10.84 ± 0.66gh | 9.43 ± 0.83i | 11.02 ± 0.37fgh | 13.30 ± 0.51ab | 12.95 ± 0.73abcd | 12.16 ± 0.44bcdef | 11.71 ± 0.62defg |
| Sap: PGOH | 10.81 ± 0.48gh | 10.20 ± 0.22hi | 11.63 ± 0.64efg | 13.24 ± 0.90abc | 13.33 ± 0.71ab | 12.82 ± 0.58abcde | 12.01 ± 0.49cdefg |
| Sap: 1-PrOH | 10.99 ± 0.55fgh | 10.25 ± 0.46hi | 13.71 ± 0.83a | 13.69 ± 0.29a | 13.74 ± 0.83a | 13.67 ± 0.69a | 13.70 ± 0.25a |
| Sap: EtOH | 10.27 ± 0.04hi | 10.14 ± 0.72hi | 12.35 ± 0.17bcde | 12.49 ± 0.14abcde | 12.68 ± 1.06abcde | 12.78 ± 0.62abcde | 12.80 ± 1.42abcde |
| Rhl: GlOH | 7.69 ± 0.34lm | 6.64 ± 0.84n | 7.03 ± 0.72mn | 7.92 ± 0.41lm | 8.48 ± 0.83kl | 9.51 ± 0.77ij | 10.97 ± 0.20def |
| Rhl: PGOH | 10.63 ± 0.81efgh | 9.18 ± 0.20jk | 9.58 ± 0.63hij | 10.79 ± 0.62efg | 10.83 ± 0.51efg | 11.70 ± 0.91abcde | 12.00 ± 0.37abcd |
| Rhl: 1-PrOH | 10.64 ± 0.73efgh | 9.82 ± 0.67ghij | 10.96 ± 0.16def | 11.00 ± 0.26def | 11.09 ± 0.29cdef | 10.45 ± 0.31fghi | 11.29 ± 0.44bcdef |
| Rhl: EtOH | 12.57 ± 0.95a | 11.24 ± 0.39cdef | 11.42 ± 0.10bcdef | 11.55 ± 0.82abcdef | 12.17 ± 59abc | 12.38 ± 30ab | 12.61 ± 0.63a |
| SMP: GlOH | 11.38 ± 0.21ijk | 10.63 ± 0.86jkl | 12.07 ± 0.41ghi | 12.78 ± 0.66defgh | 12.73 ± 0.42efgh | 12.23 ± 0.23ghi | 11.34 ± 1.00ijkl |
| SMP: PGOH | 10.89 ± 0.51jkl | 8.93 ± 0.43m | 10.29 ± 0.29l | 12.43 ± 0.31ghi | 12.11 ± 0.96ghi | 11.68 ± 0.41hij | 10.84 ± 0.40jkl |
| SMP: 1-PrOH | 11.67 ± 0.77hij | 10.42 ± 0.32kl | 12.55 ± 0.60fgh | 15.19 ± 0.72a | 14.89 ± 0.91ab | 14.51 ± 0.82abc | 14.57 ± 0.37abc |
| SMP: EtOH | 12.92 ± 0.22defg | 12.09 ± 0.63ghi | 13.82 ± 0.13bcd | 14.09 ± 0.49bc | 14.12 ± 0.27bc | 13.57 ± 0.65cdef | 13.70 ± 0.71cde |
| T20: GlOH | 15.67 ± 1.72klm | 15.06 ± 1.03m | 15.62 ± 0.82klm | 16.28 ± 0.79jklm | 16.49 ± 0.89ijklm | 16.86 ± 1.05hijkl | 16.64 ± 0.65hijklm |
| T20: PGOH | 16.73 ± 0.14hijkl | 15.49 ± 0.28lm | 15.75 ± 1.37klm | 16.75 ± 0.96hijkl | 16.96 ± 0.54hijkl | 17.23 ± 1.21ghijk | 17.73 ± 0.73fghij |
| T20: 1-PrOH | 18.91 ± 0.56def | 18.25 ± 0.87efgh | 18.75 ± 1.43defg | 19.89 ± 0.54bcd | 20.08 ± 0.65bcd | 21.24 ± 0.54b | 22.94 ± 1.06a |
| T20: EtOH | 18.26 ± 0.42efgh | 17.84 ± 0.64fghij | 18.02 ± 0.72fghi | 19.03 ± 0.77cdef | 19.65 ± 1.02bcde | 20.52 ± 0.17bc | 21.00 ± 0.32b |
| T80: GlOH | 19.65 ± 0.17ghij | 18.19 ± 0.85k | 19.12 ± 0.55hijk | 19.08 ± 0.10hijk | 18.63 ± 0.28jk | 19.01 ± 0.63ijk | 19.86 ± 0.13ghi |
| T80: PGOH | 19.87 ± 0.09ghi | 19.01 ± 0.62ijk | 19.65 ± 1.16ghij | 19.87 ± 1.04ghi | 20.08 ± 0.72ghi | 20.19 ± 0.37gh | 20.41 ± 0.28g |
| T80: 1-PrOH | 27.76 ± 1.17a | 24.92 ± 0.97ef | 25.20 ± 0.38de | 25.86 ± 0.08bcde | 26.09 ± 0.53bcd | 26.48 ± 0.18bc | 26.97 ± 0.12ab |
| T80: EtOH | 26.19 ± 0.47bcd | 23.92 ± 0.85f | 24.15 ± 0.75f | 25.59 ± 0.46cde | 26.16 ± 0.18bcd | 26.37 ± 0.22bc | 26.89 ± 0.26ab |
| S20: GlOH | 15.32 ± 0.29n | 15.20 ± 0.76n | 15.63 ± 0.56mn | 17.26 ± 0.53hijkl | 17.65 ± 0.45ghij | 17.83 ± 1.02fghij | 18.04 ± 1.57efgh |
| S20: PGOH | 15.82 ± 0.67klmn | 15.67 ± 0.82lmn | 15.75 ± 1.12lmn | 16.23 ± 1.43jklmn | 16.36 ± 0.21ijklmn | 17.01 ± 0.62hijklm | 17.67 ± 1.18fghij |
| S20: 1-PrOH | 18.56 ± 1.24efgh | 18.02 ± 0.78efgh | 19.09 ± 1.10efg | 19.56 ± 0.80cde | 20.86 ± 0.67bc | 21.58 ± 0.53b | 23.46 ± 0.99a |
| S20: EtOH | 17.54 ± 0.97ghij | 17.36 ± 0.75hijk | 17.98 ± 0.86efghi | 18.27 ± 0.87efgh | 19.29 ± 0.24def | 20.65 ± 0.48bcd | 21.77 ± 0.22b |
| SDS: GlOH | 14.34 ± 0.45jk | 14.27 ± 0.67k | 14.68 ± 0.37ijk | 15.08 ± 1.08ghijk | 15.37 ± 0.21fghij | 15.85 ± 0.30efgh | 16.26 ± 0.42def |
| SDS: PGOH | 15.12 ± 0.52ghijk | 14.87 ± 0.63hijk | 15.13 ± 0.86ghijk | 15.47 ± 0.46fghi | 15.70 ± 0.17efghi | 16.07 ± 0.61efg | 16.36 ± 0.45def |
| SDS: 1-PrOH | 16.43 ± 1.06def | 16.02 ± 0.21efg | 16.65 ± 0.62de | 17.18 ± 0.86d | 18.56 ± 0.18c | 19.26 ± 0.88bc | 20.59 ± 0.06a |
| SDS: EtOH | 19.12 ± 0.23bc | 18.86 ± 0.39bc | 20.85 ± 0.92a | 20.65 ± 0.18a | 20.74 ± 0.26a | 20.79 ± 0.42a | 19.89 ± 0.72ab |
Different small letters represent significant difference (p < 0.05) among MEs based on the same surfactant.
Efficiencies were reported in comparison to acetone extraction (lutein content of MPP = 15.83 mg/g).
Bold numbers represent the SCR at which maximum yield was achieved (i.e., SCRmax).
Surfactant.
Co-surfactant.
Comparing certain ratios of different co-surfactants for a specific surfactant, 1-PrOH was recognized as the most effective co-surfactant for most MEs except Rhl and SDS where ethanol was the choice due to the higher efficiency. It is reported that effective co-surfactants (i.e., EtOH and 1-PrOH), due to their smaller volume, more surface tension reduction and ease of redistribution in the interface, could enhance the interface consequently lutein extraction [26]. Matching the efficiency of various ratios of certain co-surfactant for specific surfactant showed that an optimum content (SCRmax) of co-surfactant is necessary to achieve the high possible extraction yield. This probably occurred due to the high polarity of co-surfactants with the tendency to incorporate into water. At the optimum SCR, the co-surfactant molecules are readily allocated between the surfactant molecules on the interface to improve its flexibility as a result of which the solubilizing capacity could be improved [27].
It is noteworthy that EtOH and 1-PrOH are already recognized as GRAS and permitted as direct food additives (Ref. 21 CFR 172.515 and 184–1293, 1990) though their concentrations would never exceed the recommended levels in final products. Overall, the lutein extraction yield was improved with suitable co-surfactants over the range 5.55 (Lec: GlOH, 1:1) to 27.67% (T80: 1-PrOH, 2:1). Despite these significant improvements, the highest extraction efficiency (∼28%) was still much lessr than conventional organic solvent. For any further improvements, in the next step the SCRmax were selected and the influence of preparation methods (see sub section of 2.2.6) was evaluated.
The comparison of preparation methods proved the outstanding role of co-surfactant, as the extraction efficiency was significantly (p < 0.05) increased when MPP was moistened in co-surfactant prior to be mixed by surfactant: water dispersion. It is interesting that these changes were more pronounced for natural surfactants than synthetic ones (Table 3). It can be partly attributed to the moistening effect, which likely facilitated the lutein extraction and mobility as well as the flexibility of the amphiphilic monolayer. Owing to effective incorporation of co-surfactant into surfactant monolayer, they are required to achieve better solubilizing and extraction yield [15, 28]. These findings showed that the extraction yield was increased from ∼27.7 to 39.4%, the highest compared to the others, with T80:1-PrOH at SCRmax (2:1). This could be attributed to the well-balanced molecular structure of T80 (a poly-oxy-ethylene with intermediate hydrocarbon chain). From physiological point of view, oleic acid is the major intestinal digestion metabolite of T80 which could enhance chylomicron secretion, consequently the lymphatic transport and oral bioavailability of the loaded nutraceuticals (e.g., lutein). Hence, T80 as a permitted food additive (FDA, 21CFR172.840) could be likely recommended as a choice for food grade MEs formulation. In addition, the formation of stable MEs using minimum amount of natural surfactants and co-surfactants is the major challenge for food applications. Therefore, alongside the non-toxic and non-irritant T80 based MEs [29], Sap:1-PrOH (at its SORmax and SCRmax) showed reasonably high extraction yield (33%) which is an original valuable finding that may promise the application of MET in extraction and solubilizing of lipophilic compound into food industry. It must be noted that the amphiphilic characteristic of Sap is derived from the simultaneous presence of hydrophilic (e.g., sugars) and hydrophobic (e.g., phenolics) groups within a single molecule. Therefore, beside its remarkable capability it may synergistically amplify the antioxidant activity of lutein due to the presence of hydrophobic phenolic moiety too.
Table 3.
Effect of preparation methods on lutein extraction efficiency (%) of different microemulsions (water 40 g, MPP 20 mg) under SORmax and SCRmax.
| Surfactant | SORmax (%w/w) | Co-surfactant | SCRmax (%w/w) | Extraction yield (%)∗ |
|
|---|---|---|---|---|---|
| Method A | Method B | ||||
| Lec | 200:1 | 1-PrOH | 2:1 | 11.71 ± 0.49k | 25.36 ± 0.76f |
| Sap | 200:1 | 1-PrOH | 1:2 | 13.71 ± 0.83j | 33.00 ± 0.43c |
| Rhl | 1000:1 | EtOH | 2:1 | 12.57 ± 0.95jk | 26.50 ± 0.82ef |
| SMP | 200:1 | 1-PrOH | 1:5 | 15.19 ± 0.72i | 25.52 ± 0.24f |
| T20 | 200:1 | 1-PrOH | 1:50 | 22.94 ± 1.06g | 32.91 ± 0.11c |
| T80 | 2000:1 | 1-PrOH | 2:1 | 27.76 ± 1.17e | 39.41 ± 0.40a |
| S20 | 1000:1 | 1-PrOH | 1:50 | 23.46 ± 0.99g | 35.67 ± 0.53b |
| SDS | 200:1 | EtOH | 1:2 | 20.85 ± 0.92h | 29.54 ± 0.79d |
Different small letters represent significant difference (p< 0.05).
Efficiencies were reported in comparison to acetone extraction (lutein content of MPP = 15.83 mg/g).
Moreover, the extraction efficiency of surfactants is very depended to their distinct molecular characteristics and therefore their critical micellization concentrations (CMCs). CMC is the limit of surfactant concentration above which micelles form. Therefore, different SORmax might be partially attributed to different CMC values of various surfactants (Table 4). In addition, the high lutein extraction efficiency of synthetic surfactants compared to their natural counterparts at SORmax (Tables 1 and 2) may also be connected with their CMCs. For instance, a relationship can be seen between the high efficiency of T80 (39.41%) and S20 (35.67%) with their low CMCs (0.017 and 0.024 mM L−1) consequently high Cmax2/CMC ratios (710.82 and 953.16), respectively [30, 31]. It is reported that at high concentrations, slightly higher than CMC, the micelles usually aggregate or even coalesce [32]. On the other hand, below CMC, the bilayer might be formed instead of micelles. Considering the data presented in Table 4, it could be concluded that, except Lec and SDS, for other MEs, reverse micelles were the most probable nano-structures for lutein entrapment when concentrations of Lec and SDS were lower than CMC (20 and 7.4 mM L−1, respectively), and appreciable fraction of surfactant was not adsorbed to the interface. Therefore, the bi-layer lamellar distribution was the most expected one [31, 33]. Unilamellar vesicles of phospholipids (100 nm) was already reported [34]. The smaller molecular weight (288.3 g/M−1) of SDS could lead to even lower surface load therefore its partial micellization [35].
Table 4.
Comparison of molecular weight (MW), and critical micelle concentration (CMC) of various surfactants used in the MEs (40 ml water, 20 mg MPP) with their corresponding SORmax, Cmax and Cmax/CMC ratio.
| Characteristic | Surfactant |
|||||||
|---|---|---|---|---|---|---|---|---|
| Lec | Sap | Rhl | SMP | T20 | T80 | S20 | SDS | |
| HLB | 7.0 | 13.5 | 9.5 | 15 | 16.7 | 15 | 8.6 | 40 |
| Viscositya (mPa.s) | 10000 | - | - | - | 250–450 | 375–480 | 4250 | - |
| MW (g M−1) | 644 | 635 | 651 | 580.8 | 1228 | 1310 | 346 | 288.3 |
| CMCb (mM L−1) | 20 | 0.603 | 0.230 | 0.055 | 0.060 | 0.012 | 0.024 | 7.4 |
| SORmax | 200:1 | 200:1 | 1000:1 | 200:1 | 200:1 | 2000:1 | 1000:1 | 200:1 |
| Cmaxc (mM L−1) | 2.000 | 2.493 | 12.158 | 2.725 | 1.290 | 12.084 | 22.876 | 5.497 |
| Cmax/CMC | 0.10 | 4.13 | 52.86 | 6.81 | 26.33 | 710.82 | 953.16 | 0.74 |
Viscosity reported at 25°C for commercial pure surfactants.
CMC values were adopted from various refrenses as cited in the text.
Cmax represents the surfactant concentration at SORmax.
Another important factor in surfactant selection for ME formulation is the HLB value as a rough guide. In other words, the solubility of surfactants either in water or oil phase is dependent on their HLB values. However, comparison of Lec (HLB = 7) based ME with S20 (HLB = 8.6) indicates other than HLB, factors like the surfactant viscosity, CMC, molecular weight (Table 4) and the joining capability of target molecules (e.g., lutein) with surfactant are also very determining parameters which are needed to be carefully considered. As is shown (Table 4), the extraction efficiency of Lec (Table 3) likely due to its high viscosity (10000 mPa.s), high (20 mM L−1) CMC [33] and molar weight (644) was comparably lower than S20 which had lower (0.024 mM L−1) CMC [30], smaller molecular size (346), lower viscosity (4250 mPa.s), higher SOR (1000:1) and SCR (1:50). In contrast, despite the CMC and low molecular weight similarities [36, 37, 38] and also the high Cmax/CMC of Rhl (52.86) in comparson to Sap (4.13), it was more likely due to the intermediate HLB (13.5) value of the latter that its lutein extraction efficiency was much better than Rhl (Table 3). Furthermore, despite the similarity (0.055 and 0.060 mM L−1) of CMC [39, 40], HLB (15 and 16.7) and SOR (200:1), the higher SCR (1:50 versus 1:5) as well as Cmax/CMC (26.33 versus 6.81) of T20 brought about better extraction capability for T20 (32.91%) in comparison to SMP (25.52%).
3.3. Droplet size and zeta potential
The droplet size is a critical characteristic of MEs due to its drastic influence on physicochemical properties (absorption and releasing rate) of target (e.g., lutein in the present study) agent [41]. In addition, it is a crucial factor to be considered in determining the stability mechanism [13]. Moreover, zeta potential (ZP) is a determinative parameter in stabilization of emulsified systems such as MEs. The droplet size, polydispersity index (PDI) and ZP of the optimized lutein MEs were therefore determined using dynamic light scattering. It must be noted that, the dynamic light scattering technique measures the intensity of scattered light under particular scattering angle and converts it either through mathematical calculations into droplet size distribution or via cumulative analysis into mean hydrodynamic diameter reported as Z-average. The type of surfactant usually has an appreciable effect on the mean droplet diameter, PDI and ZP. As it can be seen (Table 5), the mean droplet size of all MEs were <200 nm except one with Lec. The T80 and Lec based MEs also had the lowest (14.37 nm) and highest (250.80 nm) mean droplet sizes, respectively. In addition, there was not a direct correlation between HLB numbers and the droplet sizes, but it was obvious that hydrophobic surfactants including; Lec (HLB = 7) and S20 (HLB = 8.6) led to a coarse MEs (250.80 and 183 nm mean droplet sizes) likely due to their poor solubility. Furthermore, T80 and SMP with intermediate HLB (HLB = 15) created smaller droplets (14.37 and 40.04 nm). This likely implies the possible importance of HLB on droplet size. The small droplet size of T80 based ME can be partially attributed to its relatively large (2000:1) SOR [42]. Alongside this, the bioavailability of lutein may be increased as the droplet size of ME decreased. Apart from the emulsifying capability of surfactants and co-surfactants, these differences imply that there are a number of other physicochemical properties which contribute on droplet size too. It can be concluded that in some cases the smaller molecular size, lower CMC, higher surface activity, lower surface load and high purity led to much smaller droplets with likely higher zeta potentials. Considering the CMC and the actual concentration (Cmax) of various surfactants (Table 4), it could be speculated that, the higher droplet size of Lec based ME could be attributed to its lower Cmax/CMC value (0.1). It is already pointed out that for designing a nano-size stable emulsion it is necessary to work above the CMC value of surfactants [43] which means this should be > 1. However, in spite of its droplet size (250.80 nm) and impurity, it sufficiently prevented droplet coalescence or phase separation via its high (−38.50 mV) electrostatic repulsion [44]. In addition, fractionation of commercial soy lecithin led to high purity (high phosphatidylcholine) so that its emulsifying capabilities improved [45]. On the contrary, the relatively small (−1.61 mV) zeta potential and large droplet size (183 nm) of S20 based ME indicated that such system could not be considered as thermodynamically stable but kinetically stable one and formation of a layer of free lutein may occur due to coalescence after long term storage.
Table 5.
Comparison of mean droplet size, PDI and zeta potential of different microemulsions (water 40 g, MPP 20 mg) under SORmax and SCRmax (method B).
| Surfactant | SORmax (%w/w) | Co-surfactant | SCRmax (%w/w) | Z-Average (nm) | PDI | ZP (mV) |
|---|---|---|---|---|---|---|
| Lec | 200:1 | 1-PrOH | 2:1 | 250.8 | 0.32 | −38.5 |
| Sap | 200:1 | 1-PrOH | 1:2 | 104.9 | 0.24 | −29.3 |
| Rhl | 1000:1 | EtOH | 2:1 | 110.8 | 0.23 | −26.2 |
| SMP | 200:1 | 1-PrOH | 1:5 | 40.0 | 0.28 | −11.4 |
| T20 | 200:1 | 1-PrOH | 1:50 | 119.1 | 0.24 | −12.2 |
| T80 | 2000:1 | 1-PrOH | 2:1 | 14.4 | 0.21 | −10.9 |
| S20 | 1000:1 | 1-PrOH | 1:50 | 183.0 | 0.05 | −2.0 |
| SDS | 200:1 | EtOH | 1:2 | 113.4 | 0.29 | −36.4 |
As mentioned, the differences in mean droplet size of lutein MEs could also partly attributed to the molecular size diversity of various surfactants (Table 4). In addition, as can be seen (Tables 1 and 2), the highest extraction efficiency was occurred at distinct SORs and SCRs (i.e., SORmax and SCRmax) under which the MEs had different droplet size. To have a clear understanding of MEs droplet size, all aforementioned influential parameters must be considered as a bundle. As such, the steadiness or unsteadiness of each parameter could lead to a certain behavior either in favor or against nano-emulsification and eventually thermodynamic stability. For example, with regard to MEs which fabricated by T80, the high solubility (HLB = 15), high SOR (2000:1), low CMC, high Cmax/CMC ratio (∼710) and thorough covering of the surface area were the promising parameters to form stable lutein MEs (14.37 nm). It is reported that the high surfactant adsorption can decrease the interfacial tension and this could promote formation of small droplets [46]. The mobility of surfactant is another important characteristic and it is clear that T80, due to the presence of a monooleate tail (C18 = 1), could bring about high mobility and consequently spontaneous formation of ME [47]. Similarly, the high water solubility of SMP (HLB = 15) beside its moderate zeta potential (−11.40 mV) and relative mobility, due to the presence of a monopalmitate tail (C16 = 1), led to lutein ME with relatively small droplets (40.04 nm). Regarding SDS, it should be highlighted that despite its small molar mass (288 g/mol), superior water solubility (HLB = 40) and completely charged sulfated head groups (−36.40 mV), the mean droplet size (113 nm) was larger than SMP and T80. This was likely occurred as a consequence of its lower Cmax/CMC (0.74) compared to that of SMP (6.81) and especially T80 (710.82) which is under micellization level (≥1). Moreover, by considering T20's molecular size (1227 g/mol) and its droplet size similarity (119.10 nm) to SDS, its behavior could be likely attributed to higher solubility in continuous phase (HLB = 16.7), high ratio of 1-PrOH (SCR, 1:50) and its relatively low negative charge (−12.20 mV). The other important factor on the formation of thermodynamically stable MEs is critical packing parameter (CPP) at the oil–water interface, which can be characterized by their molecular geometry [48]. CPP can usually control ME construction by determining the optimum curvature of the interface monolayer that a specific surfactant tends to form. Consequently, it can influence interfacial characteristics such as surface energy, thermodynamic balance and rheology which may impact the formation of MEs by spontaneous emulsification methods. From CPP point of view, T20 with saturated linear chains (C12 = 0) should afford lower CPP than T80 with unsaturated bended chains (C18 = 1), therefore higher droplet size. This difference will affect the packing of the surfactant molecules at the oil–water interface, which may influence the tendency for spontaneous formation of ultrafine droplets. Due to the lack of information regarding the molecular structure and size, the relatively fair water solubility of Sap (HLB = 13.5) and Rhl (HLB = 9.5) alongside their high negative electrostatic charges (−29.30 and −26.20 mV, respectively) one may speculate these as possible reasons for their droplet size (104.90 and 110.80 nm, respectively). In contrast, the partial water solubility of S20 (HLB = 8.6), weak electrostatic charge (−1.96 mV) and high surfactant concentration (SOR, 1000:1) were probably the major driving forces to lead to large droplets (183 nm) via depletion-flocculation mechanism of adsorbed surfactant.
The PDI as the width of droplet size distribution uniformity index is variable between 0.05 and 1, the smaller the value the more uniform the droplet size distribution [49]. The PDI value of most MEs was over the range 0.21–0.32 except S20 (0.05) with the highest uniformity. Moreover, our results confirmed that there was not a direct relationship between the MEs droplet size and their electrical characteristics. The other noticeable point (Table 3) is the influence of surfactant molecules nature on surface charge density. As it can be seen, some of MEs had higher negative charge (Lec, Sap, Rhl and SDS) than others (SMP, T20 and T80). The low surface charge (−1.96 mV) of S20 would be expected because of its non-ionic nature. The droplets coated with T20 and T80 also had relatively low surface potential (−12.20 and −10.86 mV respectively) despite their non-ionic nature. Such behavior could be explained either by the preferential adsorption of hydroxyl ions from lutein and water or anionic impurities in the surfactants such as free fatty acids [50, 51]. The high surface charge of Sap, Rhl and SDS could be credited to their glucuronic acid, carboxylic acid and sulfated head groups, respectively [28, 42, 52, 53]. The negative charge of Lec could be also attributed to the minor presence of anionic phospholipids namely phosphatidylinositol and phosphatidic acid [18]. Another possible explanation to Lec based ME surface charge could be the potential hydrogen bond of lutein hydroxyls with cholines (phosphatidylcholine) which draw positive choline into micelle and negative phosphatidyls toward interface consequently high surface charge [54]. Regarding SMP, despite its non-ionic nature, the superiority of the pH (∼7) of prepared ME to the pKa value of palmitic acid (4.9) resulted in conversion of carboxylic acid to its deprotonated form (R-COO‒) and consequently negative surface charge [28]. A strongly negative electrical surface charge usually leads to a strong electrostatic repulsion between MEs droplets, consequently good stability against aggregation, coalescence and phase separation [44, 52, 55]. This is why in Lec based lutein ME, despite its relatively coarse droplets, the electrostatic repulsion (−38.50 mV) was sufficient to prevent any coalescence brought about therefore its ME was quite stable.
4. Conclusions
Apart from toxicity, health and environmental hazards of solvent extraction, from application point of view, the solvent extracted compounds (e.g., lutein) cannot be easily dissolved in food or pharmaceutical systems that are mostly hydrophilic. Therefore, MET, as green and environmentally friendly technique, due to its potential capability in simultaneous extraction and solubilizing of carotenoids is of great interests. Hence, in the present study, based on pseudo-ternary studies the capability of various synthetic and natural surfactants in combination with different co-surfactants on formulation of mono-phasic isotropic MEs was investigated. In addition, MEs were successfully developed for extraction and solubilizing of lutein from plant source (MPP). The specifications of surfactant, surfactant: lutein (SORs) and surfactant: co-surfactant ratios (SCRs) were pointed out as key parameters to achieve high extraction efficiency with nano-sized droplets characterized using zetasizer. Moreover, the zeta potential confirmed their stability against aggregation and coalescences due to the strong electrostatic repulsion. These findings provided a platform for possibility of designing MEs with high lutein extraction capacity which could be beneficial in production of lutein enriched commercial functional foods, supplements, personal care and pharmaceutical products. However, for this purpose its efficiency needs to be further improved in order to be comparable and commercially feasible in comparison to the existing technique. For that, we believe the application of pretreatments such as enzymatic hydrolysis and sonication as well as incorporating another component (e.g., sunflower oil) in ME formulation could somehow improve its efficiency. These aspects have been accomplished in our food colloids and rheology laboratory and will be reported in the near future.
Declarations
Author contribution statement
Mehdi Jalali-Jivan: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Soliman Abbasi: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Footnotes
Hydrophilic–Lipophilic Balance.
Cmax represents the surfactant concentration at SORmax.
References
- 1.Lin J.H., Lee D.J., Chang J.S. Lutein production from biomass: marigold flowers versus microalgae. Bioresour. Technol. 2015;184:421–428. doi: 10.1016/j.biortech.2014.09.099. [DOI] [PubMed] [Google Scholar]
- 2.Piccaglia R., Marotti M., Grandi S. Lutein and lutein ester content in different types of Tagetes patula and T. erecta. Ind. Crops Prod. 1998;8:45–51. [Google Scholar]
- 3.Yang C., Fischer M., Kirby C., Liu R., Zhu H., Zhang H., Chen Y., Sun Y., Zhang L., Tsao R. Bioaccessibility, cellular uptake and transport of luteins and assessment of their antioxidant activities. Food Chem. 2018;249:66–76. doi: 10.1016/j.foodchem.2017.12.055. [DOI] [PubMed] [Google Scholar]
- 4.Jung H.Y., Ok H.M., Park M.Y., Kim J.Y., Kwon O. Bioavailability of carotenoids from chlorella powder in healthy subjects: a comparison with marigold petal extract. J. Funct. Foods. 2016;21:27–35. [Google Scholar]
- 5.Madaan T., Choudhary A.N., Gyenwalee S., Thomas S., Mishra H., Tariq M., Vohora D., Talegaonkar S. Lutein, a versatile phyto-nutraceutical: an insight on pharmacology, therapeutic indications, challenges and recent advances in drug delivery. PharmaNutr. 2017;5:64–75. [Google Scholar]
- 6.Peng M.-L., Chiu H.-F., Chou H., Liao H.-J., Chen S.-T., Wong Y.-C., Shen Y.-C., Venkatakrishnan K., Wang C.-K. Influence/impact of lutein complex (marigold flower and wolfberry) on visual function with early age-related macular degeneration subjects: a randomized clinical trial. J. Funct.Foods. 2016;24:122–130. [Google Scholar]
- 7.Picone S., Ritieni A., Fabiano A., Graziani G., Paolillo P., Livolti G., Galvano F., Gazzolo D. Lutein levels in arterial cord blood correlate with neuroprotein activin A in healthy preterm and term newborns: a trophic role for lutein? Clin. Biochem. 2018;52:80–84. doi: 10.1016/j.clinbiochem.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 8.Grudzinski W., Piet M., Luchowski R., Reszczynska E., Welc R., Paduch R., Gruszecki W.I. Different molecular organization of two carotenoids, lutein and zeaxanthin, in human colon epithelial cells and colon adenocarcinoma cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018;188:57–63. doi: 10.1016/j.saa.2017.06.041. [DOI] [PubMed] [Google Scholar]
- 9.Ranganathan A., Hindupur R., Vallikannan B. Biocompatible lutein-polymer-lipid nanocapsules: acute and subacute toxicity and bioavailability in mice. Mater. Sci. Eng. C. 2016;69:1318–1327. doi: 10.1016/j.msec.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 10.Donsì F., Sessa M., Mediouni H., Mgaidi A., Ferrari G. Encapsulation of bioactive compounds in nanoemulsion- based delivery systems. Procedia Food Sci. 2011;1:1666–1671. [Google Scholar]
- 11.Wang T., Han J., Tian Y., Zhang D., Wang Y., Wu Y., Ni L. Combined process of reaction, extraction, and purification of lutein in marigold flower by isopropanol–KOH aqueous two-phase system. Separ. Sci. Technol. 2016;51:1490–1498. [Google Scholar]
- 12.Radi M., Abbasi S. Optimization of novel oil extraction technique from canola seeds: lecithin-based microemulsion. Eur. J. Lipid Sci. Technol. 2018;0:1700267. [Google Scholar]
- 13.Amiri-Rigi A., Abbasi S. Stability assessment of lycopene microemulsion prepared using tomato industrial waste against various processing conditions. J. Sci. Food Agric. 2017;97:4922–4928. doi: 10.1002/jsfa.8368. [DOI] [PubMed] [Google Scholar]
- 14.Mondal M.H., Malik S., Roy A., Saha R., Saha B. Modernization of surfactant chemistry in the age of gemini and bio-surfactants: a review. RSC Adv. 2015;5:92707–92718. [Google Scholar]
- 15.Prévost S., Gradzielski M., Zemb T. Self-assembly, phase behaviour and structural behaviour as observed by scattering for classical and non-classical microemulsions. Adv. Colloid Interface Sci. 2017;247:374–396. doi: 10.1016/j.cis.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 16.Xu Z., Jin J., Zheng M., Zheng Y., Xu X., Liu Y., Wang X. Co-surfactant free microemulsions: preparation, characterization and stability evaluation for food application. Food Chem. 2016;204:194–200. doi: 10.1016/j.foodchem.2016.01.073. [DOI] [PubMed] [Google Scholar]
- 17.Amiri-Rigi A., Abbasi S., Scanlon M.G. Enhanced lycopene extraction from tomato industrial waste using microemulsion technique: optimization of enzymatic and ultrasound pre-treatments. Innov. Food Sci. Emerg. Technol. 2016;35:160–167. [Google Scholar]
- 18.Abbasi S., Radi M. Food grade microemulsion systems: canola oil/lecithin:n-propanol/water. Food Chem. 2016;194:972–979. doi: 10.1016/j.foodchem.2015.08.078. [DOI] [PubMed] [Google Scholar]
- 19.Amar I., Aserin A., Garti N. Solubilization Patterns of lutein and lutein esters in food grade nonionic microemulsions. J. Agric. Food Chem. 2003;51:4775–4781. doi: 10.1021/jf026222t. [DOI] [PubMed] [Google Scholar]
- 20.Jalali Jivan M., Abbasi S. An attempt to cast light into lutein extraction and its alkali optimization. J.Food Meas.Charact. 2018;13:1–8. [Google Scholar]
- 21.Fish W.W., Perkins-Veazie P., Collins J.K. A quantitative assay for lycopene that utilizes reduced volumes of organic solvents. J. Food Compos. Anal. 2002;15:309–317. [Google Scholar]
- 22.Komaiko J., Sastrosubroto A., McClements D.J. Encapsulation of ω-3 fatty acids in nanoemulsion-based delivery systems fabricated from natural emulsifiers: sunflower phospholipids. Food Chem. 2016;203:331–339. doi: 10.1016/j.foodchem.2016.02.080. [DOI] [PubMed] [Google Scholar]
- 23.Panigrahi S., Padhi R., Sahoo L., Misra P.K. Organization of amphiphiles, Part VII: effect of variation of composition and salt on the phase behavior of some ethoxylated surfactants. J. Dispersion Sci. Technol. 2007;26:275–283. [Google Scholar]
- 24.Ingkasupart P., Manochai B., Song W.T., Hong J.H. Antioxidant activities and lutein content of 11 marigold cultivars (Tagetes spp.) grown in Thailand. Food Sci. Technol. 2015;35:380–385. [Google Scholar]
- 25.Amiri-Rigi A., Abbasi S. Microemulsion-based lycopene extraction: Effect of surfactants, co-surfactants and pretreatments. Food Chem. 2016;197:1002–1007. doi: 10.1016/j.foodchem.2015.11.077. [DOI] [PubMed] [Google Scholar]
- 26.Campo L., Yaghmur A., Garti N., Leser M.E., Folmer B., Glatter O. Five-component food-grade microemulsions: structural characterization by SANS. J. Colloid Interface Sci. 2004;274:251–267. doi: 10.1016/j.jcis.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 27.Kawakami K., Yoshikawa T., Moroto Y., Kanaoka E., Takahashi K., Nishihara Y., Masuda K. Microemulsion formulation for enhanced absorption of poorly soluble drugs: I. Prescription design. J. Control. Release. 2002;81:65–74. doi: 10.1016/s0168-3659(02)00049-4. [DOI] [PubMed] [Google Scholar]
- 28.Rao J., McClements D.J. Food-grade microemulsions, nanoemulsions and emulsions: fabrication from sucrose monopalmitate & lemon oil. Food Hydrocolloids. 2011;25:1413–1423. [Google Scholar]
- 29.Kaur G., Mehta S.K. Developments of Polysorbate (Tween) based microemulsions: Preclinical drug delivery, toxicity and antimicrobial applications. Int. J. Pharm. 2017;529:134–160. doi: 10.1016/j.ijpharm.2017.06.059. [DOI] [PubMed] [Google Scholar]
- 30.Wu Y., Iglauer S., Shuler P., Tang Y., Goddard W.A. Alkyl Polyglycoside-sorbitan ester formulations for improved oil recovery. Tenside Surfactants Deterg. 2010;47:280–287. [Google Scholar]
- 31.Mandal A.B., Nair B.U., Ramaswamy D. Determination of the critical micelle concentration of surfactants and the partition coefficient of an electrochemical probe by using cyclic voltammetry. Langmuir. 1988;4:736–739. [Google Scholar]
- 32.Lee, Vishnyakov A., Neimark A.V. Calculations of critical micelle concentration by dissipative particle dynamics simulations: the role of chain rigidity. J. Phys. Chem. B. 2013;117:10304–10310. doi: 10.1021/jp4042028. [DOI] [PubMed] [Google Scholar]
- 33.Wu Y., Wang T. Soybean lecithin fractionation and functionality. J. Am. Oil Chem. Soc. 2003;80:319–326. [Google Scholar]
- 34.Lu, Nielsen N.S., Baron C.P., Jensen L.H.S., Jacobsen C. Physico-chemical properties of marine phospholipid emulsions. J. Am. Oil Chem. Soc. 2012;89:2011–2024. [Google Scholar]
- 35.McClements D.J. Critical review of techniques and methodologies for characterization of emulsion stability. Crit. Rev. Food Sci. Nutr. 2007;47:611–649. doi: 10.1080/10408390701289292. [DOI] [PubMed] [Google Scholar]
- 36.Chrzanowski L., Wick L.Y., Meulenkamp R., Kaestner M., Heipieper H.J. Rhamnolipid biosurfactants decrease the toxicity of chlorinated phenols to Pseudomonas putida DOT-T1E. Lett. Appl. Microbiol. 2009;48:756–762. doi: 10.1111/j.1472-765X.2009.02611.x. [DOI] [PubMed] [Google Scholar]
- 37.Mitra S., Dungan S.R. Micellar properties of quillaja Saponin. 1. Effects of temperature, salt, and pH on solution properties. J. Agric. Food Chem. 1997;45:1587–1595. [Google Scholar]
- 38.Mondal M.H., Sarkar A., Maiti T.K., Saha B. Microbial assisted (pseudomonas sp.) production of novel bio-surfactant rhamnolipids and its characterisation by different spectral studies. J.Molec. Liq. 2017;242:873–878. [Google Scholar]
- 39.Xiao D., Ye R., Davidson P.M., Hayes D.G., Golden D.A., Zhong Q. Sucrose monolaurate improves the efficacy of sodium hypochlorite against Escherichia coli O157:H7 on spinach. Int. J. Food Microbiol. 2011;145:64–68. doi: 10.1016/j.ijfoodmicro.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 40.Patist A., Bhagwat S.S., Penfield K.W., Aikens P., Shah D.O. On the measurement of critical micelle concentrations of pure and technical-grade nonionic surfactants. J. Surfactants Deterg. 2000;3:53–58. [Google Scholar]
- 41.Cho H.J., Lee D.W., Marasini N., Poudel B.K., Kim J.H., Ramasamy T., Yoo B.K., Choi H.G., Yong C.S., Kim J.O. Optimization of self-microemulsifying drug delivery system for telmisartan using Box–Behnken design and desirability function. J. Pharm. Pharmacol. 2013;65:1440–1450. doi: 10.1111/jphp.12115. [DOI] [PubMed] [Google Scholar]
- 42.Uluata S., McClements D.J., Decker E.A. Physical stability, autoxidation, and Photosensitized oxidation of ω-3 oils in nanoemulsions prepared with natural and synthetic surfactants. J. Agric. Food Chem. 2015;63:9333–9340. doi: 10.1021/acs.jafc.5b03572. [DOI] [PubMed] [Google Scholar]
- 43.Mattei M., Kontogeorgis G.M., Gani R. Modeling of the critical micelle concentration (CMC) of nonionic surfactants with an extended group-contribution method. Ind. Eng. Chem. Res. 2013;52:12236–12246. [Google Scholar]
- 44.Azarikia F., Abbasi S. Efficacy of whey protein–tragacanth on stabilization of oil-in-water emulsions: comparison of mixed and layer by layer methods. Food Hydrocolloids. 2016;59:26–34. [Google Scholar]
- 45.Guiotto E.N., Cabezas D.M., Diehl B.W.K., Tomás M.C. Characterization and emulsifying properties of different sunflower phosphatidylcholine enriched fractions. Eur. J. Lipid Sci. Technol. 2013;115:865–873. [Google Scholar]
- 46.Saberi A.H., Fang Y., McClements D.J. Fabrication of vitamin E-enriched nanoemulsions: factors affecting particle size using spontaneous emulsification. J. Colloid Interface Sci. 2013;391:95–102. doi: 10.1016/j.jcis.2012.08.069. [DOI] [PubMed] [Google Scholar]
- 47.Ostertag F., Weiss J., McClements D.J. Low-energy formation of edible nanoemulsions: factors influencing droplet size produced by emulsion phase inversion. J. Colloid Interface Sci. 2012;388:95–102. doi: 10.1016/j.jcis.2012.07.089. [DOI] [PubMed] [Google Scholar]
- 48.Guttoff M., Saberi A.H., McClements D.J. Formation of vitamin D nanoemulsion-based delivery systems by spontaneous emulsification: factors affecting particle size and stability. Food Chem. 2015;171:117–122. doi: 10.1016/j.foodchem.2014.08.087. [DOI] [PubMed] [Google Scholar]
- 49.Jivan M.J., Madadlou A., Yarmand M. An attempt to cast light into starch nanocrystals preparation and cross-linking. Food Chem. 2013;141:1661–1666. doi: 10.1016/j.foodchem.2013.04.071. [DOI] [PubMed] [Google Scholar]
- 50.Hur S.J., Decker E.A., McClements D.J. Influence of initial emulsifier type on microstructural changes occurring in emulsified lipids during in vitro digestion. Food Chem. 2009;114:253–262. [Google Scholar]
- 51.Cui L., Cho H.T., McClements D.J., Decker E.A., Park Y. Effects of salts on oxidative stability of lipids in Tween-20 stabilized oil-in-water emulsions. Food Chem. 2016;197:1130–1135. doi: 10.1016/j.foodchem.2015.11.099. [DOI] [PubMed] [Google Scholar]
- 52.Liang L., Chen F., Wang X., Jin Q., Decker E.A., McClements D.J. Physical and oxidative stability of flaxseed oil-in-water emulsions fabricated from sunflower lecithins: impact of blending lecithins with different phospholipid Profiles. J. Agric. Food Chem. 2017;65:4755–4765. doi: 10.1021/acs.jafc.7b01469. [DOI] [PubMed] [Google Scholar]
- 53.Nguyen T.T., Sabatini D.A. Characterization and emulsification properties of Rhamnolipid and sophorolipid biosurfactants and their applications. Int. J. Mol. Sci. 2011;12:1232–1244. doi: 10.3390/ijms12021232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller W.L. Steroidogenic acute regulatory protein (StAR), a novel mitochondrial cholesterol transporter. BBA-Mol. Cell Biol. L. 2007;1771:663–676. doi: 10.1016/j.bbalip.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 55.Zou L., Zhang Z., Zhang R., Liu W., Liu C., Xiao H., McClements D.J. Encapsulation of protein nanoparticles within alginate microparticles: impact of pH and ionic strength on functional performance. J.Food Engin. 2016;178:81–89. [Google Scholar]