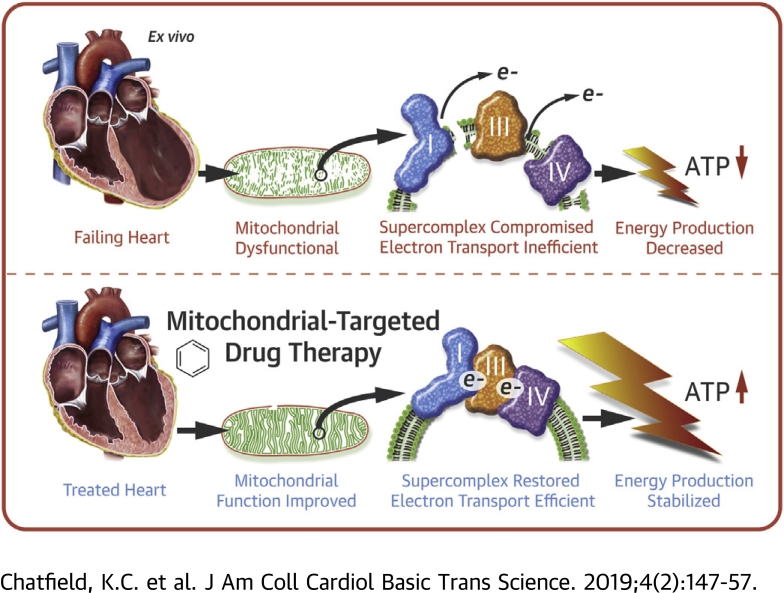
Visual Abstract
Key Words: heart failure, high-resolution respirometry, mitochondrial-targeted compounds, supercomplex
Abbreviations and Acronyms: ADP, adenosine diphosphate; BN-PAGE, blue native polyacrylamide gel electrophoresis; C, mitochondrial respiratory complex; FCCP, carbonyl cyanide p-trifluoromethoxyphenylhydrazone; HF, heart failure; RCR, respiratory control ratio; SC CCF, supercomplex coupling control factor
Highlights
-
•
Mitochondrial function is impaired in explanted failing pediatric and adult human hearts.
-
•
Elamipretide is a novel mitochondria-targeted drug that is targeted to cardiolipin on the inner mitochondrial membrane and improves coupling of the electron transport chain.
-
•
Treatment of explanted human hearts with elamipretide improves human cardiac mitochondrial function.
-
•
The study provides novel methods to evaluate the influence of compounds on mitochondria in the human heart and provides proof of principle for the use of elamipretide to improve mitochondrial energetics in failing myocardium due to multiple etiologies and irrespective of age.
Summary
Negative alterations of mitochondria are known to occur in heart failure (HF). This study investigated the novel mitochondrial-targeted therapeutic agent elamipretide on mitochondrial and supercomplex function in failing human hearts ex vivo. Freshly explanted failing and nonfailing ventricular tissue from children and adults was treated with elamipretide. Mitochondrial oxygen flux, complex (C) I and CIV activities, and in-gel activity of supercomplex assembly were measured. Mitochondrial function was impaired in the failing human heart, and mitochondrial oxygen flux, CI and CIV activities, and supercomplex-associated CIV activity significantly improved in response to elamipretide treatment. Elamipretide significantly improved failing human mitochondrial function.
Mitochondrial Dysfunction and Cardiolipin in Heart Failure
A substantial body of work has implicated abnormal mitochondrial function and consequent impaired energy production via oxidative phosphorylation as a factor in the development of heart failure (HF), leading to heightened interest in mitochondrial function as a therapeutic target 1, 2, 3. Efficient mitochondrial function is considered especially essential in the heart due to the sustained energy requirements of the myocardium. Cardiolipin, a unique phospholipid with 4 fatty acid side chains, is critical for maintaining normal adenosine triphosphate generation by anchoring the proteins of the electron transport chain onto the inner mitochondrial membrane architecture (4). In the heart, a mitochondrial inner membrane enriched with tetralinoleoyl cardiolipin (containing 4 linoleic acid side chains) facilitates the stability of the physical interaction between oxidative phosphorylation complex protein multimers in what has been termed the mitochondrial supercomplex, composed of complex (C) I, CIII, and CIV 5, 6. Abnormalities in cardiolipin composition lead to mitochondrial dysfunction in the failing heart through disruption of the supercomplex and can be improved with dietary interventions in rodent models 7, 8, 9. These abnormalities in mitochondrial function have led to heightened interest in mitochondria as a therapeutic target in HF (3).
Elamipretide, a Mitochondrial-targeted Peptide
Elamipretide (formerly referred to as Bendavia, MTP-131, and SS-31) is an aromatic-cationic, cell-permeable tetrapeptide in a new class of mitochondrial-targeted drugs and is the first in its class to enter clinical trials in patients with HF 10, 11, 12. The peptide is targeted to mitochondria via cardiolipin where it has been shown in animal models to improve energetics and decrease reactive oxygen species, possibly by stabilizing the mitochondrial membrane and cytochrome c 13, 14. Elamipretide rapidly enters tissue (within minutes of treatment) and has cardioprotective effects in ischemia/reperfusion injury in animal models 15, 16, 17, 18. To date, there are no data on the direct effects of elamipretide, or other members of this novel class, on mitochondrial function in the human heart.
Elamipretide in Human HF
The purpose of the present study was to investigate the effects of elamipretide on human cardiac mitochondrial function. Because this study was ex vivo and performed over the course of a few hours (too rapid to induce cardiolipin remodeling), any effects of the drug would likely be independent of cardiolipin molecular species alterations. Using freshly explanted human hearts, the current study found that: 1) elamipretide improves impaired mitochondrial function in HF, with no effect on normal mitochondrial function in nonfailing hearts; 2) elamipretide improves mitochondrial supercomplex function (CI, CIII, and CIV) but does not alter CII or CV activity; and 3) the short-term action of elamipretide is independent of any changes in cardiolipin side chain composition.
Methods
Chemicals and reagents
Elamipretide was provided by Stealth BioTherapeutics, Inc. (Newton, Massachusetts), resuspended in water at 10 mM, and stored at –80°C. The elamipretide ex vivo treatment concentration was determined by the plateau of a dose–response curve in a subset of samples (data not shown). Unless otherwise noted, all other chemicals were from MilliporeSigma (Burlington, Massachusetts).
Patient samples
Explanted cardiac ventricular tissue from children and adults of all races, sexes, and ethnic backgrounds with informed consent obtained from the subjects or their guardians were available for this study through the institutional review board–approved University of Colorado Pediatric and Adult Cardiac Tissue Banks. Fresh samples were harvested during sequential transplants at Children’s Hospital Colorado and University of Colorado Hospital. Additional experiments were performed in previously harvested frozen samples from the University of Colorado Heart Tissue Bank. Sample characteristics for each experiment are given in Table 1. More detailed information for each patient is provided in Supplemental Tables 1 and 2. Nonfailing tissue was procured from donors or individuals with normal systolic ventricular function. HF tissue was obtained from patients undergoing transplant for terminal HF with reduced systolic function. Heart tissue was dissected in the operating room immediately after explant and kept in BIOPS preservation solution at 4°C until processing (19). Mitochondria were freshly isolated within 6 h of explant for spectrophotometric assays. Time course experiments showed that after 3 days in BIOPS at 4°C, mitochondrial respiration was unchanged in nonfailing tissue, but increased in HF tissue (Supplemental Figure 1). High-resolution respirometry experiments were performed in fresh permeabilized cardiac fibers. Flash-frozen tissue at time of explant was used for in-gel activity staining using blue native polyacrylamide gel electrophoresis (BN-PAGE) separation.
Table 1.
Characteristics of Cardiac Samples According to Experiment
| High-Resolution Respirometry |
Enzyme Activity Assays |
BN-PAGE In-Gel Activity |
Cardiolipin Mass Spectrometry |
|||||
|---|---|---|---|---|---|---|---|---|
| Nonfailing | Failing | Nonfailing | Failing | Nonfailing | Failing | Nonfailing | Failing | |
| No. of heart samples | 13 | 23 | 11 | 12 | 19 | 21 | 5 | 10 |
| Male | 6 | 15 | 4 | 7 | 8 | 10 | 3 | 7 |
| Female | 7 | 8 | 7 | 5 | 11 | 11 | 2 | 3 |
| Age, yrs | 25 ± 7 | 31 ± 5 | 20 ± 8 | 29 ± 8 | 26 ± 5 | 25 ± 5 | 28 ± 13 | 29 ± 9 |
| Ejection fraction, % | 64 ± 5 | 24 ± 2 | 64 ± 4 | 27 ± 3 | 61 ± 3 | 22 ± 6 | 58 ± 2 | 20 ± 3 |
Values are n or mean ± SEM.
BN-PAGE = blue native polyacrylamide gel electrophoresis.
Mitochondrial isolation
Subpopulations of myocardial mitochondria, subsarcolemmal located beneath the plasma membrane and interfibrillar residing between the myofibrils, were isolated by using differential centrifugation and trypsin digestion as previously described with the following modifications for human tissue 20, 21. Briefly, freshly explanted cardiac tissue in pieces no larger than 5 mm was placed in ice-cold BIOPS solution. Tissue was trimmed of fat, placed into mitochondrial isolation buffer, and minced by using scissors. Low-speed spins were at 1,500g and high-speed spins at 4,200g. One milligram of trypsin was used per gram of tissue. Mitochondria were resuspended in potassium buffer and protein quantified (BCA, Thermo Fisher Scientific, Waltham, Massachusetts).
Enzymatic activities
Isolated mitochondria were diluted to a final protein concentration of 1 mg/ml and incubated with 100 μM elamipretide or an equal volume of vehicle (water) for 1 h on ice. Enzymatic activities of CI, CIV, and citrate synthase were performed at 30°C as previously described 22, 23.
Blue native polyacrylamide gel electrophoresis
Mitochondrial supercomplexes were separated by BN-PAGE using TGX 4-15% gels (Bio-Rad, Hercules, California). In-gel activity assays for CI, CII, CIV, and CV were performed with septal tissue processed according to published methods 22, 24. Samples were treated with 100 μM elamipretide for 1 h. Supercomplex and free protein bands were quantified by using ImageJ software (National Institutes of Health, Bethesda, Maryland). Total supercomplex activity was calculated as a sum of all supercomplex bands.
High-resolution respirometry
Respiration of permeabilized cardiac fibers was measured by high-resolution respirometry (Oxygraph, Oroboros Instruments, Innsbruck, Austria) using a stepwise protocol to evaluate various components of the electron transport system 19, 25. Approximately 30 mg of ventricular tissue was placed in BIOPS immediately after explant. One-half of the tissue was incubated in BIOPS containing 100 μM elamipretide at 4°C for 1 h. After incubation, tissue was cut into approximately 2-mg pieces and teased using forceps to separate fibers. The tissue was then placed in a solution of BIOPS containing 30 μg/ml saponin for 30 min to permeabilize the plasma membrane and to allow substrate delivery to the mitochondria. Fibers were washed for 10 min at 4°C in ice-cold mitochondrial respiration medium containing 25 μM blebbistatin ± 100 μM elamipretide (26). Samples were blotted on filter paper, weighed, and placed in the chambers of the Oroboros O2K apparatus at 37°C containing respiration medium and 25 μM blebbistatin ± 100 μM elamipretide. Standard protocols were followed for calibration of the chambers and followed by stepwise addition of 5 mM pyruvate, 1 mM malate, 4 mM adenosine diphosphate (ADP), 10 mM glutamate, 10 mM succinate, 10 μM cytochrome c, and either 2 μg/ml oligomycin or 0.5 μM steps of carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) (until the maximum rate was reached) followed by 2 μM rotenone. Oxygen flux rates were normalized per milligram of tissue wet weight.
Cardiolipin quantitation
Cardiolipin was quantified in a subset of the total cohort by using previously published methods with liquid chromatography coupled to electrospray ionization mass spectrometry in an API 4000 Mass Spectrometer (SCIEX, Framingham, Massachusetts) (20). After BIOPS or BIOPS Plus 100 μM elamipretide treatment for 4 h, heart tissue was frozen without buffer at –80°C. Tissue pieces were homogenized by using a glass-on-glass homogenizer in phosphate-buffered saline and lipids extracted according to previously published methods with 1 mmol tetramyristoyl-cardiolipin as an internal standard (Avanti Polar Lipids, Alabaster, Alabama) 20, 27. Cardiolipin species were quantified per milligram of protein.
Statistical analysis
Statistical analyses were performed by using Prism software version 6.0 (GraphPad Software, La Jolla, California). Treatment effects were analyzed by using a ratio-paired Student’s t-test, with p < 0.05 being significant and p < 0.1 reported as trending toward significance (28). HF or time-based effects were analyzed by using an unpaired Student’s t-test. Datasets were tested for Gaussian distribution with the D’Agostino and Pearson omnibus test or the Shapiro-Wilk normality test. Data that did not conform to a Gaussian distribution (glutamate and supercomplex coupling control factor data) were log-transformed before analysis.
Graphs with bars show SEM for unpaired Student’s t-test data, and graphs with paired data show means plus SDs (chosen for clarity) flanked on either side of the paired data. Linear regression analysis was performed to assess for an association between age and significant outcomes (no associations were demonstrated).
Results
Elamipretide improves respiration of intact mitochondria from the failing human heart
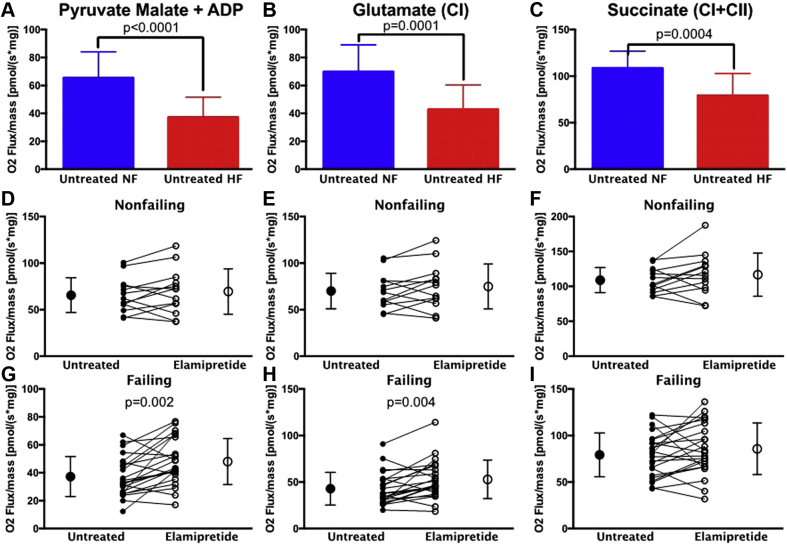
Quantitation of the results of high-resolution respirometry in all samples is shown in Figure 1 with sample traces shown in Supplemental Figure 2. There was significantly lower mitochondrial normalized oxygen flux in HF vs nonfailing samples using pyruvate malate + ADP, glutamate (CI), and succinate (CI + CII) as substrates (Figures 1A to 1C). There were also significantly lower oxygen fluxes in HF with inhibition of flux through CI + CII using oligomycin or the uncoupler FCCP (p < 0.01) but not combined with the CI inhibitor rotenone (data not shown).
Figure 1.
High-resolution Respirometry of Treated and Untreated Heart Fibers
Oxygen (O2) flux normalized to mass in permeabilized heart fibers from untreated heart failure (HF) versus nonfailing (NF) human hearts with additions of: (A) pyruvate malate plus adenosine diphosphate (ADP); (B) subsequent addition of glutamate acting through complex (C) I; and (C) subsequent addition of succinate additionally activating CII. (D to F) Elamipretide treatment had no effect on oxygen flux of NF samples (G to I) but significantly improved oxygen flux in HF samples after additions of the aforementioned substrates. Untreated hearts in (D to I) are shown with closed circles and treated hearts with open circles. NF, n = 13; HF, n = 23.
Elamipretide treatment of nonfailing permeabilized fibers did not alter mitochondrial oxygen flux (Figures 1D to 1F). However, in HF, there was a significant increase in oxygen flux with elamipretide treatment using the substrates pyruvate malate + ADP (Figure 1G) and glutamate (CI) (Figure 1H). Notably, there was no significant difference with elamipretide treatment with the addition of succinate (CI + CII) in HF (Figure 1I). Addition of pyruvate/malate, FCCP, rotenone, or oligomycin did not significantly affect oxygen flux through CI + II (data not shown).
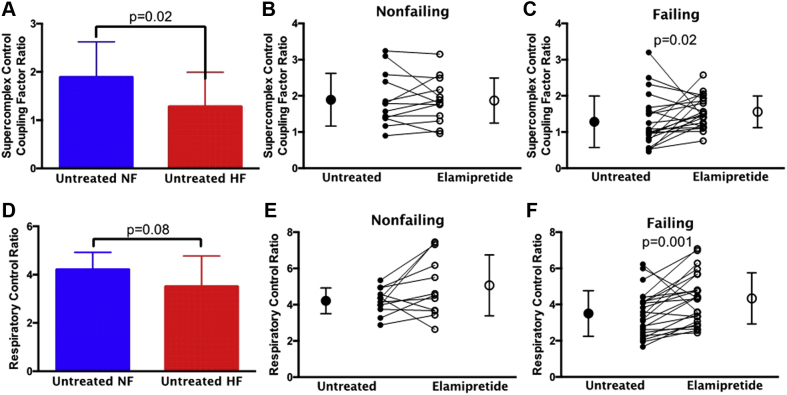
Elamipretide increases the supercomplex coupling control factor and respiratory control ratio in failing mitochondria
The supercomplex coupling control factor ratio (SC CCF) is a mathematical construct to calculate the influence of the supercomplex on a given respirometry measurement and is directly proportional to supercomplex integrity (derivation is given in the Supplemental Methods). The SC CCF was significantly lower with HF (Figure 2A). Elamipretide treatment in nonfailing hearts did not alter SC CCF (Figure 2B). Conversely, in HF, elamipretide treatment significantly increased SC CCF (Figure 2C). The respiratory control ratio (RCR) indicates a trend toward decreasing in HF (Figure 2D). There was no change in RCR with elamipretide treatment in nonfailing hearts (Figure 2E), whereas in HF, RCR was significantly increased with elamipretide treatment (Figure 2F).
Figure 2.
SC CCF and RCR With Elamipretide Treatment
(A) Supercomplex coupling control factor (SC CCF) ratio is lower in untreated HF than NF controls. Elamipretide treatment does not alter SC CCF in (B) NF hearts but improves SC CCF in (C) HF. (D) The respiratory control ratio (RCR) trends lower in untreated NF hearts versus HF hearts. Elamipretide treatment has no effect on RCR in (E) NF hearts but increases RCR in (F) HF hearts. Untreated hearts in B, C, E and F are shown with closed circles and treated hearts with open circles. NF, n = 13; HF, n = 23. Abbreviations as in Figure 1.
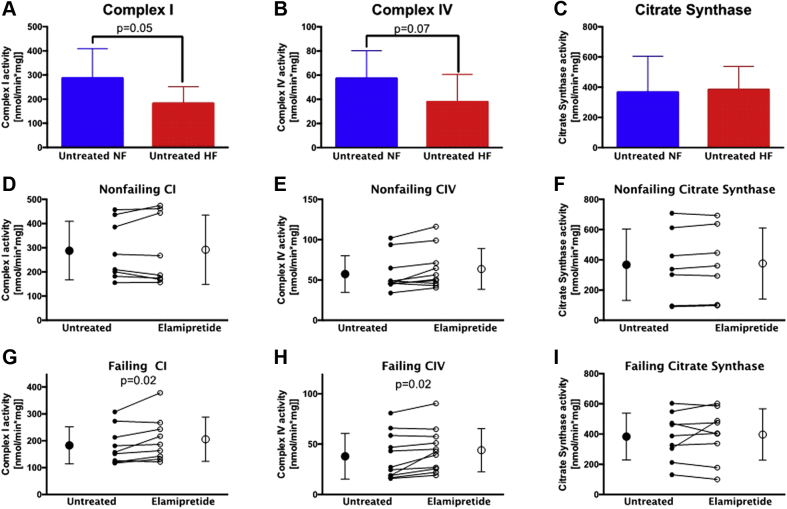
Activities of mitochondrial CI and CIV in subsarcolemmal mitochondria are increased with elamipretide
Freshly isolated subsarcolemmal and interfibrillar mitochondria treated with elamipretide were used to measure total activity of CI and CIV as well as citrate synthase, a soluble enzyme in the mitochondrial matrix (29). Total CI activity was significantly lower in HF compared with nonfailing samples (Figure 3A), whereas CIV activity in subsarcolemmal mitochondria showed a trend toward a decrease in HF (Figure 3B). No difference in citrate synthase activity was observed between HF and the nonfailing group (Figure 3C). In nonfailing subsarcolemmal mitochondria, activities of these 3 enzyme complexes were unaffected by elamipretide treatment (Figures 3D to 3F). However, in failing subsarcolemmal mitochondria, both CI and CIV activities exhibited a trend for increase by elamipretide treatment (Figures 3G and 3H), while citrate synthase activity was unchanged (Figure 3I). None of the enzyme activities was altered in the elamipretide-treated interfibrillar mitochondria (data not shown).
Figure 3.
Individual Total Activity Measurements of CI, CIV, and Citrate Synthase in Subsarcolemmal Mitochondria
(A to C) Enzyme activities of CI, CIV, and citrate synthase are lower, trending lower, and unchanged, respectively, in untreated HF versus NF controls. (D to F) Activities of CI, CIV, and citrate synthase are unaltered with elamipretide treatment in NF hearts. (G to I) Individual activities of CI and CIV increase with elamipretide treatment in HF, with citrate synthase unchanged. Untreated hearts in (C to G) are shown with closed circles and treated hearts with open circles. NF, n = 11; HF, n = 12. Abbreviations as in Figure 1.
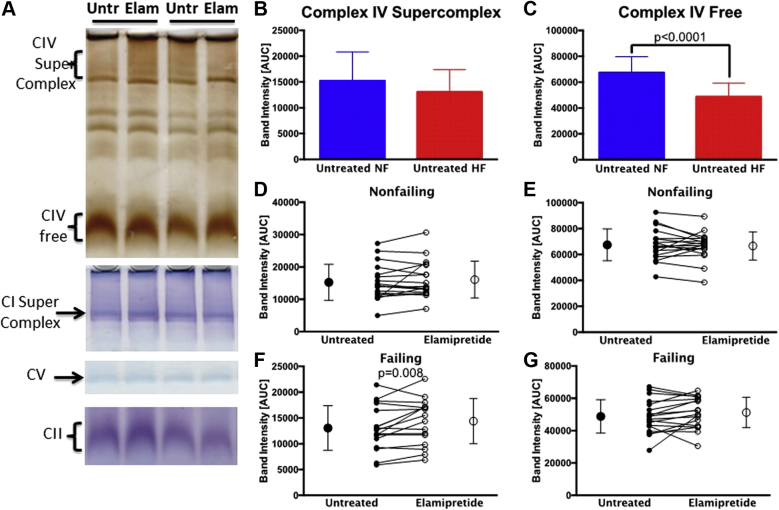
Supercomplex-associated CIV activity is altered with elamipretide
BN PAGE in-gel activity was used to separate supercomplex from free CIV. Figure 4A shows representative gels for CI, CII, CIV, and CV activities. CIV activity is present in a free state, within the supercomplex, and in intermediate molecular weight forms not analyzed in this study. CI is only present in its supercomplex form. When these bands were quantified, the quantity of CIV in the supercomplex was not altered in HF (Figure 4B), whereas the free CIV activity was significantly lower (Figure 4C). In nonfailing samples, elamipretide treatment had no effect on CIV regardless of whether it is bound or free (Figures 4D and 4E). By contrast, in HF, the activity of CIV in the supercomplex was significantly higher after elamipretide treatment, whereas activity of free CIV was unchanged (Figures 4F and 4G). There were no significant changes detected in the activities of CI, CII, or CV in HF or with elamipretide treatment according to this assay (data not shown).
Figure 4.
In-gel Activities of Supercomplex-associated and Free Electron Transport Complexes in Subsarcolemmal Mitochondria
(A) Representative lanes from blue native polyacrylamide gel electrophoresis gels from failing hearts with in-gel activity stains for electron transport complexes CIV, CI, CV, and CII either free or in the supercomplex with arrows/brackets showing quantifiable bands on each gel. The 4 lanes represent 2 samples with untreated or elamipretide-treated mitochondria. (B and C) Supercomplex CIV activity is unchanged in untreated failing samples, but free CIV is decreased. (D to G) Elamipretide treatment of nonfailing samples had no effect on CIV activity (D, E) but in failing hearts improves (F) supercomplex-associated CIV but (G) not free CIV. Untreated hearts in (D to G) are shown with closed circles and treated hearts with open circles. NF, n = 19; HF, n = 21. AUC = area under the curve; Elam = elamipretide treated; Untr = untreated; other abbreviations as in Figure 1.
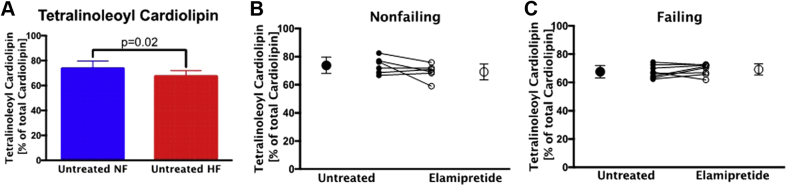
Cardiolipin composition is not altered with elamipretide treatment
Tetralinoleoyl cardiolipin, the predominant cardiac cardiolipin species, with 4 linoleic acids and a mass-to-charge ratio of 1448, is shown as a percentage of the total (11 major species were used for total) cardiolipin content. The percentage of tetralinoleoyl cardiolipin was significantly lower in HF, consistent with our previously reported findings (Figure 5A), but elamipretide treatment had no effect on tetralinoleoyl cardiolipin in nonfailing or HF samples (Figures 5B and 5C) 7, 30. Cardiolipin absolute amounts, percent totals of 8 major cardiolipin species, monolysocardiolipin, or the sum of all cardiolipin species were unaltered after elamipretide treatment (data not shown).
Figure 5.
Tetralinoleoyl Cardiolipin With Elamipretide Treatment
(A) The effect of HF on tetralinoleoyl cardiolipin (mass-to-charge ratio, 1,448) in untreated samples is expressed as a percentage of total cardiolipin. Elamipretide-treated (B) NF hearts or (C) HF hearts showed no changes in tetralinoleoyl cardiolipin. Untreated hearts in B and C are shown with closed circles and treated hearts with open circles. NF, n = 5; HF, n = 10. Abbreviations as in Figure 1.
Discussion
Overview of novel findings
To the best of our knowledge, this study is the first to investigate the effect of elamipretide on mitochondrial respiration in explanted human heart tissue. Elamipretide treatment improves left ventricular volumes in adults with HF (12), suggesting a potentially new avenue for therapeutic agents in HF (31). We extended these clinical trial data by showing that impaired mitochondrial function in the failing pediatric and adult human heart can be improved with elamipretide treatment. Elamipretide treatment improved the function of components of the electron transport chain when they were associated with the supercomplex in human cardiac mitochondria from the failing ventricle. Finally, acute elamipretide treatment of human cardiac tissue improved mitochondrial and supercomplex function even though the treatment was too rapid to allow remodeling of cardiolipin. This comprehensive analysis in human heart tissue provides additional insight into the mechanism by which elamipretide treatment improves the function of the respiratory chain in human cardiac mitochondria. These findings support the use of supercomplex stabilizing compounds for the treatment of human disease 16, 32.
Elamipretide in human disease
Clinical trials have used elamipretide to address renal, cardiac, and skeletal muscle disease, but this study is the first to show reversal of mitochondrial dysfunction in HF. Elamipretide improved 6-min walk test distances in patients with mitochondrial myopathy (33) and improved left ventricular volumes in patients with HF and left ventricular dysfunction (12). Similarly, elamipretide was associated with improved kidney function after renal angioplasty (34). To our knowledge, there is only 1 other study exploring the influence of elamipretide directly on human mitochondria. Wijermars et al. (35) examined the influence of SS-31 on mitochondrial function in normal renal biopsy samples subjected to ischemic stress. An important difference between their protocol and ours was that the biopsy specimens were pretreated with the compound during the ischemic stress followed by a demonstration of attenuation of mitochondrial dysfunction in the ex vivo study. Similar to our findings in the heart, there was no effect of elamipretide on mitochondrial function in the control renal biopsy specimen. Importantly, our study is the first to report the ability to improve mitochondrial dysfunction in human disease with acute treatment with elamipretide.
Elamipretide’s mechanism of action works through the enzymes of the mitochondrial supercomplex
Results of high-resolution respirometry showed that elamipretide improves human cardiac mitochondrial function through improved coupling of the supercomplex-associated enzyme complexes CI, CIII, and CIV. Oxygen flux after the addition of supercomplex-specific substrates, pyruvate malate, ADP, and glutamate was impaired in the failing heart. Elamipretide improved oxygen flux in failing samples under these conditions, indicating direct activity on the supercomplex. Succinate, the next substrate added in our experimental protocol, forces the majority of electron flow through CII, a component of the respiratory chain not associated with the supercomplex 36, 37. CII-mediated oxygen flux was not influenced by HF, nor was it altered by elamipretide treatment. Furthermore, addition of rotenone, which inhibits CI and blocks all supercomplex-mediated respiration, permitted CII-mediated oxygen flux that cannot be augmented by elamipretide treatment of tissues (Supplemental Figure 3). These data show that the effect of elamipretide treatment occurs via its effect on CI/CIII/CIV supercomplex activity.
In the complementary spectrophotometry studies, total mitochondrial enzymatic activities of complexes CI and CIV were increased with elamipretide in the failing heart, with no effect on citrate synthase, a soluble enzyme not in the membrane that serves as a marker of mitochondrial content and nonmembrane-associated mitochondrial activity. Enzyme activities of CI and CIV assayed by using BN-PAGE analysis revealed increased supercomplex-associated CIV activity but not CI activity with elamipretide treatment. This result may indicate a stronger effect of elamipretide on recruitment of CIV to the supercomplex. Alternatively, BN-PAGE may not have the sensitivity to detect improvement in CI activity recorded with the enzymatic assay. Taken together, these data suggest that elamipretide improves mitochondrial function by directly affecting respiratory complexes specifically associated with the mitochondrial supercomplex, perhaps by recruiting free CIV into the supercomplex.
Improvement of mitochondrial function by elamipretide is independent of cardiolipin remodeling
Elamipretide reportedly rapidly targets mitochondria through the attraction between the positively charged residues of the peptide with the negatively charged cardiolipin head group, which is believed to improve phospholipid-dependent bioenergetics (13). Indeed, long-term treatment with elamipretide in animal models has been associated with increased mitochondrial cardiolipin remodeling to its tetralinoleoyl form 16, 38. To date, however, it is unclear if cardiolipin remodeling after chronic treatment is a cause or a consequence of elamipretide’s mechanism of action. We would not anticipate that elamipretide could acutely alter cardiolipin isoforms in an ex vivo system, and in fact we report here that drug treatment can improve mitochondrial function in the absence of any change in cardiolipin species.
We instead hypothesize that elamipretide may elicit its acute effects by improving the stabilization of cardiolipin–protein interactions, allowing the maintenance of the mitochondrial supercomplex.
Improvement of mitochondrial function is independent of age and HF etiology
There is a growing body of literature characterizing the unique adaptations that exist between the failing pediatric heart and the adult heart 39, 40, 41, 42, 43, 44, 45. These studies, combined with the differential response of children to adult-based HF therapies and limited improvement in pediatric HF outcomes over the past decade, suggest that investigations of pediatric-specific HF treatments are needed 46, 47, 48. Importantly, the present study included both pediatric and adult failing hearts and showed that elamipretide improves mitochondrial function across a number of different pathologic phenotypes on current evidence-based therapy. This finding is unique and provides encouraging evidence that the mitochondria may represent one final common pathway in HF and that mitochondrial-targeted therapies may have more generalizable efficacy complementary to existing HF therapies.
Study limitations
First, this study was unable to elucidate the mechanisms underlying chronic exposure to elamipretide. Although this study concluded that elamipretide does have a positive effect on mitochondrial function via the enzymes of the supercomplex, and this mechanism does not involve changes in quantity or quality of cardiolipin, the exposure time to elamipretide was limited to a few hours. Future studies are needed to determine the influence of chronic treatment on human mitochondrial function. Second, this study was performed with all hearts that were sequentially collected by our tissue bank over the course of several years. Additional research in larger populations will be necessary to determine if specific disease phenotypes derive greater benefit than others. Third, systemic administration of elamipretide could affect all other mitochondrial-containing tissues and could cause off-target effects not measured by these assays. Fourth, because the experiments were performed in heart tissue in isolation, concentrations of elamipretide in the ex vivo experiments cannot be extrapolated to in vivo treatment. Lastly, because elamipretide binds to the mitochondrial membrane, other membrane-dependent processes such as cross-talk with the endoplasmic reticulum and calcium signaling could be involved; however, this study was limited in scope to the effect of elamipretide on the supercomplex and electron transport system. Any additional effects of elamipretide on human mitochondrial calcium transport and endoplasmic reticulum–mitochondria interactions would need to be analyzed in future studies.
Conclusions
A new class of mitochondrial-targeted drugs, which includes elamipretide, represents a promising new strategy for the treatment of HF (49). A central problem in HF is the inability of the heart to adequately meet its own metabolic demands due to altered mitochondrial function and metabolism. These drugs offer targeted benefits through improvement of mitochondrial function and energy production. The present study is the first to assess the impact of a member of this new class of compounds directly on human heart tissue. The findings of the current study are the first to report rapid improvement in mitochondrial function in the human heart, likely through improved coupling of the mitochondrial supercomplex. These data support the use of mitochondrial-targeted pharmaceutical agents to improve energetics and mitochondrial function in the failing heart.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Elamipretide, a premier member of a new class of mitochondrial-targeted compounds, improves mitochondrial function in failing myocardium via increased activity of respiratory CI and IV, and efficiency of these complexes in a respiratory supercomplex, improving overall mitochondrial oxygen flux. These data support the use of mitochondrial-targeted therapies to augment oxidative phosphorylation and provide a novel approach to the treatment of human HF.
TRANSLATIONAL OUTLOOK: Mitochondrial-targeted drugs such as elamipretide show promise as novel therapies that improve efficiency of mitochondrial energy production in HF caused by structural or myocardial disease.
Footnotes
This work was supported by the Addison Scott Memorial Fund, the Boedecker Foundation Award, the Jack Cooper Millisor Chair in Pediatric Heart Disease, a gift from the Nair Family, research support from Stealth BioTherapeutics, Inc., and the following National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute grants: K08 HL127294, R01 HL107715 (to Dr. Stauffer) and R01 HL126928 (to Dr. Miyamoto). This study was supported by NIH/National Center for Advancing Translational Sciences Colorado CTSA Grant Number UL1 TR001082. The contents are the authors’ sole responsibility and do not necessarily represent official NIH views. Drs. Sucharov, Miyamoto, and Stauffer are founders and scientific advisors for CoramiR Biomedical, LLC. Dr. Stauffer received research support from Stealth BioTherapeutics, Inc. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
References
- 1.Ingwall J.S., Weiss R.G. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ Res. 2004;95:135–145. doi: 10.1161/01.RES.0000137170.41939.d9. [DOI] [PubMed] [Google Scholar]
- 2.Bayeva M., Gheorghiade M., Ardehali H. Mitochondria as a therapeutic target in heart failure. J Am Coll Cardiol. 2013;61:599–610. doi: 10.1016/j.jacc.2012.08.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown D.A., Perry J.B., Allen M.E. Expert consensus document: mitochondrial function as a therapeutic target in heart failure. Nat Rev Cardiol. 2017;14:238–250. doi: 10.1038/nrcardio.2016.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu Y., Phoon C.K., Berno B. Loss of protein association causes cardiolipin degradation in Barth syndrome. Nature Chem Biol. 2016;12:641–647. doi: 10.1038/nchembio.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mileykovskaya E., Dowhan W. Cardiolipin-dependent formation of mitochondrial respiratory supercomplexes. Chem Phys Lipids. 2014;179:42–48. doi: 10.1016/j.chemphyslip.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang M., Mileykovskaya E., Dowhan W. Gluing the respiratory chain together. Cardiolipin is required for supercomplex formation in the inner mitochondrial membrane. J Biological Chemistry. 2002;277:43553–43556. doi: 10.1074/jbc.C200551200. [DOI] [PubMed] [Google Scholar]
- 7.Chicco A.J., Sparagna G.C. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol. 2007;292:C33–C44. doi: 10.1152/ajpcell.00243.2006. [DOI] [PubMed] [Google Scholar]
- 8.Paradies G., Paradies V., Ruggiero F.M., Petrosillo G. Cardiolipin and mitochondrial function in health and disease. Antioxidants Redox Signaling. 2014;20:1925–1953. doi: 10.1089/ars.2013.5280. [DOI] [PubMed] [Google Scholar]
- 9.Chicco A., Sparagna G., McCune S. Linoleate-rich high-fat diet decreases mortality in hypertensive heart failure rats compared to lard and low-fat diets. Hypertension. 2008;52:549–555. doi: 10.1161/HYPERTENSIONAHA.108.114264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ajith T.A., Jayakumar T.G. Mitochondria-targeted agents: future perspectives of mitochondrial pharmaceutics in cardiovascular diseases. World J Cardiol. 2014;6:1091–1099. doi: 10.4330/wjc.v6.i10.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szeto H.H. First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br J Pharmacol. 2014;171:2029–2050. doi: 10.1111/bph.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daubert M.A., Yow E., Dunn G. Novel mitochondria-targeting peptide in heart failure treatment: a randomized, placebo-controlled trial of elamipretide. Circ Heart Fail. 2017;10 doi: 10.1161/CIRCHEARTFAILURE.117.004389. [DOI] [PubMed] [Google Scholar]
- 13.Zhao K., Zhao G.M., Wu D. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem. 2004;279:34682–34690. doi: 10.1074/jbc.M402999200. [DOI] [PubMed] [Google Scholar]
- 14.Birk A.V., Chao W.M., Bracken C., Warren J.D., Szeto H.H. Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis. Br J Pharmacol. 2014;171:2017–2028. doi: 10.1111/bph.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai W., Shi J., Gupta R.C., Sabbah H.N., Hale S.L., Kloner R.A. Bendavia, a mitochondria-targeting peptide, improves postinfarction cardiac function, prevents adverse left ventricular remodeling, and restores mitochondria-related gene expression in rats. J Cardiovasc Pharmacol. 2014;64:543–553. doi: 10.1097/FJC.0000000000000155. [DOI] [PubMed] [Google Scholar]
- 16.Sabbah H.N., Gupta R.C., Kohli S., Wang M., Hachem S., Zhang K. Chronic therapy with elamipretide (MTP-131), a novel mitochondria-targeting peptide, improves left ventricular and mitochondrial function in dogs with advanced heart failure. Circ Heart Fail. 2016;9:e002206. doi: 10.1161/CIRCHEARTFAILURE.115.002206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi J., Dai W., Hale S.L. Bendavia restores mitochondrial energy metabolism gene expression and suppresses cardiac fibrosis in the border zone of the infarcted heart. Life Sci. 2015;141:170–178. doi: 10.1016/j.lfs.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho J., Won K., Wu D. Potent mitochondria-targeted peptides reduce myocardial infarction in rats. Coron Artery Dis. 2007;18:215–220. doi: 10.1097/01.mca.0000236285.71683.b6. [DOI] [PubMed] [Google Scholar]
- 19.Veksler V.I., Kuznetsov A.V., Sharov V.G., Kapelko V.I., Saks V.A. Mitochondrial respiratory parameters in cardiac tissue: a novel method of assessment by using saponin-skinned fibers. Biochim Biophys Acta. 1987;892:191–196. doi: 10.1016/0005-2728(87)90174-5. [DOI] [PubMed] [Google Scholar]
- 20.Sparagna G.C., Johnson C.A., McCune S.A., Moore R.L., Murphy R.C. Quantitation of cardiolipin molecular species in spontaneously hypertensive heart failure rats using electrospray ionization mass spectrometry. J Lipid Res. 2005;46:1196–1204. doi: 10.1194/jlr.M500031-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Palmer J.W., Tandler B., Hoppel C.L. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem. 1977;252:8731–8739. [PubMed] [Google Scholar]
- 22.Chatfield K.C., Coughlin C.R., 2nd, Friederich M.W. Mitochondrial energy failure in HSD10 disease is due to defective mtDNA transcript processing. Mitochondrion. 2015;21:1–10. doi: 10.1016/j.mito.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friederich M.W., Erdogan A.J., Coughlin C.R., 2nd Mutations in the accessory subunit NDUFB10 result in isolated complex I deficiency and illustrate the critical role of intermembrane space import for complex I holoenzyme assembly. Hum Mol Genet. 2017;26:702–716. doi: 10.1093/hmg/ddw431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Coster R., Smet J., George E. Blue native polyacrylamide gel electrophoresis: a powerful tool in diagnosis of oxidative phosphorylation defects. Pediatr Res. 2001;50:658–665. doi: 10.1203/00006450-200111000-00020. [DOI] [PubMed] [Google Scholar]
- 25.Letellier T., Malgat M., Coquet M., Moretto B., Parrot-Roulaud F., Mazat J.P. Mitochondrial myopathy studies on permeabilized muscle fibers. Pediatr Res. 1992;32:17–22. doi: 10.1203/00006450-199207000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Gnaiger E.K.A., Schneeberger S., Seiler R., Brandacher G., Steurer W., Margreiter R. Mitochondria in the cold. In: Heldmaier G.K.M., editor. Life in the Cold. Springer; Berlin, Germany: Heidelberg/New York, NY: 2000. pp. 431–442. [Google Scholar]
- 27.Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 28.Curran-Everett D., Benos D.J. Guidelines for reporting statistics in journals published by the American Physiological Society. Am J Physiol Regulatory Integrative Comparative Physiol. 2004;287:R247–R249. doi: 10.1152/ajpregu.00346.2004. [DOI] [PubMed] [Google Scholar]
- 29.Lanza I.R., Nair K.S. Functional assessment of isolated mitochondria in vitro. Methods Enzymol. 2009;457:349–372. doi: 10.1016/S0076-6879(09)05020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chatfield K.C., Sparagna G.C., Sucharov C.C. Dysregulation of cardiolipin biosynthesis in pediatric heart failure. J Mol Cell Cardiol. 2014;74:251–259. doi: 10.1016/j.yjmcc.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mann D.L. Targeting myocardial energetics in the failing heart: are we there yet? Circ Heart Fail. 2017;10 doi: 10.1161/CIRCHEARTFAILURE.117.004658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai D.F., Chen T., Szeto H. Mitochondrial targeted antioxidant peptide ameliorates hypertensive cardiomyopathy. J Am Coll Cardiol. 2011;58:73–82. doi: 10.1016/j.jacc.2010.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karaa A., Haas R., Goldstein A., Vockley J., Weaver W.D., Cohen B.H. Randomized dose-escalation trial of elamipretide in adults with primary mitochondrial myopathy. Neurology. 2018;90:e1212–e1221. doi: 10.1212/WNL.0000000000005255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saad A., Herrmann S.M.S., Eirin A. Phase 2a clinical trial of mitochondrial protection (elamipretide) during stent revascularization in patients with atherosclerotic renal artery stenosis. Circ Cardiovasc Interv. 2017;10 doi: 10.1161/CIRCINTERVENTIONS.117.005487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wijermars L.G., Schaapherder A.F., de Vries D.K. Defective postreperfusion metabolic recovery directly associates with incident delayed graft function. Kidney Int. 2016;90:181–191. doi: 10.1016/j.kint.2016.02.034. [DOI] [PubMed] [Google Scholar]
- 36.Lapuente-Brun E., Moreno-Loshuertos R., Acin-Perez R. Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science (New York, NY) 2013;340:1567–1570. doi: 10.1126/science.1230381. [DOI] [PubMed] [Google Scholar]
- 37.Lancaster C.R., Kroger A. Succinate: quinone oxidoreductases: new insights from X-ray crystal structures. Biochimica et biophysica acta. 2000;1459:422–431. doi: 10.1016/s0005-2728(00)00180-8. [DOI] [PubMed] [Google Scholar]
- 38.Eirin A., Ebrahimi B., Zhang X. Mitochondrial protection restores renal function in swine atherosclerotic renovascular disease. Cardiovasc Res. 2014;103:461–472. doi: 10.1093/cvr/cvu157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyamoto S.D., Stauffer B.L., Polk J. Gene expression and beta-adrenergic signaling are altered in hypoplastic left heart syndrome. J Heart Lung Transplant. 2014;33:785–793. doi: 10.1016/j.healun.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakano S.J., Miyamoto S.D., Movsesian M., Nelson P., Stauffer B.L., Sucharov C.C. Age-related differences in phosphodiesterase activity and effects of chronic phosphodiesterase inhibition in idiopathic dilated cardiomyopathy. Circ Heart Fail. 2015;8:57–63. doi: 10.1161/CIRCHEARTFAILURE.114.001218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakano S.J., Sucharov J., van Dusen R. Cardiac adenylyl cyclase and phosphodiesterase expression profiles vary by age, disease, and chronic phosphodiesterase inhibitor treatment. J Card Fail. 2017;23:72–80. doi: 10.1016/j.cardfail.2016.07.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stauffer B.L., Russell G., Nunley K., Miyamoto S.D., Sucharov C.C. miRNA expression in pediatric failing human heart. J Mol Cell Cardiol. 2013;57:43–46. doi: 10.1016/j.yjmcc.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sucharov C.C., Sucharov J., Karimpour-Fard A., Nunley K., Stauffer B.L., Miyamoto S.D. Micro-RNA expression in hypoplastic left heart syndrome. J Card Fail. 2015;21:83–88. doi: 10.1016/j.cardfail.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tatman P.D., Woulfe K.C., Karimpour-Fard A. Pediatric dilated cardiomyopathy hearts display a unique gene expression profile. JCI Insight. 2017;2 doi: 10.1172/jci.insight.94249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel M.D., Mohan J., Schneider C. Pediatric and adult dilated cardiomyopathy represent distinct pathological entities. JCI Insight. 2017;2 doi: 10.1172/jci.insight.94382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rossano J.W., Shaddy R.E. Update on pharmacological heart failure therapies in children: do adult medications work in children and if not, why not? Circulation. 2014;129:607–612. doi: 10.1161/CIRCULATIONAHA.113.003615. [DOI] [PubMed] [Google Scholar]
- 47.Shaddy R.E., Boucek M.M., Hsu D.T. Carvedilol for children and adolescents with heart failure: a randomized controlled trial. JAMA. 2007;298:1171–1179. doi: 10.1001/jama.298.10.1171. [DOI] [PubMed] [Google Scholar]
- 48.Kantor P.F., Abraham J.R., Dipchand A.I., Benson L.N., Redington A.N. The impact of changing medical therapy on transplantation-free survival in pediatric dilated cardiomyopathy. J Am Coll Cardiol. 2010;55:1377–1384. doi: 10.1016/j.jacc.2009.11.059. [DOI] [PubMed] [Google Scholar]
- 49.Steggall A., Mordi I.R., Lang C.C. Targeting metabolic modulation and mitochondrial dysfunction in the treatment of heart failure. Diseases (Basel, Switzerland) 2017;5 doi: 10.3390/diseases5020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.