Abstract
Hepatitis C virus (HCV) infection remains a pressing public health issue. Identification of long term infection in primary care settings and community health centers can facilitate patients’ access to appropriate care. Given the increase in HCV prevalence in the United States, improving the HCV care continuum and expanding medication access to disproportionately affected populations can help reduce disease burden, health care system costs, and transmission. Innovative treatment programs developed in the primary care setting are needed to deliver quality care to meet the demand of those engaging in treatment. This article describes an HCV treatment program developed within a primary care federally qualified health center (FQHC) using physician assistants (PAs) and nurse practitioners (NPs) to address the high number of HCV positive patients identified at the clinic. An interdisciplinary care team was established to optimize patient experience around HCV care and treatment, using on-site primary care behavioral health consultants, an HCV treatment coordinator, and a 340B contracted specialty pharmacy. From January 2015 to April 2017, the Public Health Management Corporation (PHMC) Care Clinic medical providers referred 189 patients for HCV treatment. Of those referred, 102 patients successfully obtained a sustained virologic response (SVR), representing a 53.7% success rate from referral to cure. This treatment program successfully integrated HCV treatment in a patient population heavily affected by substance use and mental illness. Support and adoption of similar programs in primary care community health centers testing for HCV can help meet the clinical/behavioral needs of these marginalized populations.
Keywords: Hepatitis C, primary care, community health center, behavioral health, mental illness, substance use
Introduction
Hepatitis C virus (HCV) is the most common bloodborne infection in the United States, with an estimated 2.7 to 3.9 million Americans infected with long term HCV infection.1,2 Hepatitis C virus incidence has increased nearly 300% from 2010 to 2015, largely due to injection drug use among substance users.3,4 Identification and linkage to medical care is an urgent public health priority. Previous studies have found that implementation of routine HCV testing in a primary care community health center can increase the identification of patients infected with HCV and improve linkage to care rates.5 However, linkages to specialty care for HCV treatment among individuals accessing care at community health centers remain difficult due to limited resources of these clinics, patient transportation issues, and disengagement in care.5 With new direct-acting antiviral (DAA) medications, HCV treatment has become increasingly simplified, allowing primary care providers to provide treatment and disease management on-site instead of referral to a specialist. Primary care providers have been shown to be effective at prescribing HCV medications as well as successfully treating and curing HCV.6 However, primary care providers have yet to adopt on-site treatment due to multiple concerns, including time restraints and patient adherence. Patients with HCV may present with active substance use, mental health challenges, and/or homelessness, which are perceived by providers as risk factors for treatment nonadherence.7 Potential barriers to primary care treatment of HCV can be characterized by communication issues, providers demonstracted knowledge, and payment issues from government and other insurances.8 Treatment programs have been successful in urban communities with minority populations using primary care physicians (PCPs)9,10; however, there remains a need to develop treatment programs integrated in primary care community health centers using a mutlidiscplinary team approach to target HCV positive patients in a population with high rates of substance use and mental health comorbidities. The purpose of this article is to describe a novel approach to treating HCV in a federally qualified health center (FQHC) through integration of on-site specialty care.
Through a dual-routine, opt-out HIV/HCV testing initiative, high rates of HCV positive patients were identified within a network of five FQHCs.11 The HCV test used was an HCV antibody test via blood draw, which automatically reflexed to an HCV RNA polymerase chain reaction (PCR) to determine whether the patient was chronically infected. A comprehensive HCV treatment program was developed in one urban FQHC that serves a primarily low income, marginalized, Medicaid population. A third of the patient population seen at the clinic has been diagnosed with HIV, which is also managed at the clinic. We opted to analyze data from one of the five FQHC, the Public Health Management Corporation (PHMC) Care Clinic, due to the fact that the HCV treatment program was established at this clinic with referrals from other in-network clinics to the treatment program. Patients who were HCV positive were also referred to the clinic by community HCV testing sites (substance use treatment facilities and a needle exchange program) and affiliated halfway to substance use recovery programs. The population served at this health center reported 32% homelessness, 13% ever incarcerated, 67% intravenous drug use (IVDU), and 95% on either Medicaid or Medicare insurance programs.
Program Description
Setting
The HCV treatment program was facilitated by four physician assistants (PAs) with supervision and as-needed-consultation by two part-time PCPs. There was no on-site specialist (ie hepatologist or gastroenterologist) present at the clinic. The care team included integrated primary care behavioral health consultants (BHCs), such as psychologists and licensed mental health counselors (LMHCs), an HCV treatment coordinator, and a contracted 340B specialty pharmacy to assist with prior authorization approvals, drug to drug interactions, and medication refills. This article will highlight the clinic’s interdisciplinary team approach to deliver comprehensive, wrap around services without referring patients to a specialist for their HCV treatment.
First provider visit
Once a patient was identified with long term HCV through the routine testing initiative, they were linked to care at the Care Clinic to receive medical evaluation. At the first provider visit after diagnosis, the medical provider (ie PA) conducted a thorough medical and social history. Pertinent HCV history includes the following factors: (1) first intravenous drug use (IVDU); (2) last IVDU; (3) alcohol intake history; (4) types and results of previous HCV tests; (5) sexual partners with HCV or IVDU history; (6) tattoo history; (7) HIV testing history; (8) history of hepatitis A virus (HAV) or hepatitis B virus (HBV) disease or immunizations; and (9) history of other HCV treatment or other evaluations of their liver health. A PA reviewed the current medications and completed a physical examination. Additional serum testing, including fibrosis scoring, HCV genotyping, and resistance testing, were ordered to further assess clinical evaluation of the patient’s long term HCV disease progression. A short list lab acquisition form was generated in the electronic medical record (EMR) based on Medicaid requirements for prior authorization approval. Previous medical records and labs were gathered, including HCV RNA confirmatory testing to confirm long term infection. The provider also provided initial education to the patient on lifestyle modifications to minimize progression of liver disease, reducing risk of transmission to others, as well as general knowledge on HCV disease and treatment education. The patient was then referred to the on-site primary care BHCs for further assessment and education.
Behavioral health consultation
The clinic was equipped with BHCs, a licensed clinical social worker and a psychologist, who provide on demand primary care behavioral health services at the request of the medical provider.12 When a patient is referred by their primary care providers for HCV treatment, information is shared to the BHCs about the patient’s intent to pursue HCV treatment, a brief biopsychosocial history, a review of the patient’s HCV treatment history, and lab results (eg viral load, genotype, and treatment regimen). The goal of the referral is to assess the patient’s well-being, readiness for HCV treatment, and appropriateness of HCV treatment. An assessment of current psychological distress and past/current substance use is conducted, often using a combination of clinical interviewing and briefly established screening tools. This was followed by an assessment of the patient’s knowledge about HCV disease and treatment. The BHCs provided further psychoeducation, reviewing the impact of HCV on the liver and the body, the importance of treatment, a brief description of how the medication effectively treats HCV using available visual aids and informational pamphlets, and referencing the patient’s available HCV laboratory values provided by the primary care providers.
The BHCs practiced the teach back method13 to have the patient share what they learned, such as (1) general knowledge about HCV; (2) HCV’s effect on the body; (3) the importance of daily medication adherence and attendance at follow-up medical visits, and (4) remaining substance free, especially alcohol free, during the course of the treatment. If the person does not exhibit clear risk factors (eg ongoing substance use, recent history of medication engagement difficulties, or substantial symptoms of psychological distress that may interfere with medical adherence), the BHCs will review the outcome of the assessment with the primary care providers and provide support for initiating treatment, sometimes with helpful suggestions to maximize the patient’s treatment engagement.
However, if a patient exhibited possible barriers to successful treatment completion with HCV, a comprehensive treatment plan was designed through collaboration among the patient, their primary care providers, and the BHCs to increase readiness for treatment. A brief motivational interviewing intervention was enacted by the BHCs to explore patient readiness to consider sobriety and explore what supports might be needed to make HCV treatment an attainable goal.14 In other cases, a brief problem solving intervention may be needed to address obstacles to medication adherence and modifications of their daily routine, such as matching up medication times with a meal time or using an automated cell phone reminder. Further assistance was provided throughout the patient’s course of treatment to promote adherence and engagement, as requested by the primary care providers. Patients were linked to mental health and substance use treatment services, as needed, regardless of potential barrier to HCV treatment.
Second provider visit
Serum tests from the first provider visit were reviewed and informed the provider regarding the severity of disease as well as best case treatment choice. The patient was also referred for an abdominal ultrasound and transient elasography (a FibroScan), as needed. Hepatitis C virus education was reiterated and expanded by the medical provider. The medical provider determined the correct medication to prescribe while the BHC drafted a letter to the insurance company reviewing their behavioral assessment and advocating for medication approval. Materials, including updated lab work, patient medical history, and current medication list, were gathered by the HCV treatment coordinator and submitted to the 340B contracted pharmacy to start the insurance prior authorization process. Once approved by the insurance company, the medication was delivered by the specialty pharmacy directly to the clinic, along with all subsequent medication refills.15 For patients without insurance, on-site social service personnel investigated possible insurance options for the person and worked to initiate health coverage. Medicaid is often an option for this patient population, especially with expanded Medicaid coverage in Pennsylvania, but other options at times needed to be explored.
Follow-up provider visits
Once approved by the payer and delivered by the co-located 340b pharmacy, a start date was initiated, and adherence education was given to the patient. The medication was shipped to the clinic from the pharmacy and then dispensed to the patient on-site at the clinic by the provider. Patients were then seen every 2 to 4 weeks to receive a primary care visit, medication refills, and laboratory tests to monitor treatment adherence and response. Each primary care visit involved reviewing the patient’s comprehensive health and well-being, including mental health status and substance use. Conversations about risk reduction and maintaining proper liver care through lifestyle modifications were continued throughout the entire course of treatment. Patients received reminder phone calls for their upcoming appointments. If a patient missed their appointment, a staff member could visit their home address and providing transportation assistance to re-engage the patient. Medication refills, clinic appointments, and laboratory results were closely tracked by the treatment coordinator to ensure proper engagement and that patients did not miss any medication doses during the treatment regimen. Referral to BHC is repeated as needed.
The treatment coordinator also maintained an active registry of HCV patients across the different phases of the identified HCV pretreatment and treatment continuums, follow patients from referral to diagnosis to cure, insurance approval status, starting treatment, finishing treatment, and obtaining sustained virologic response (SVR) blood work. In addition, they reviewed population management with the clinic’s HCV treatment team during brief weekly interdisciplinary meetings. These weekly care team meetings also allowed the treatment coordinator to discuss quality improvement (QI) needs and initiatives, review patients in the pipeline for prior authorization submission, and discuss current patients on treatment that needed additional resources and attention.
End of treatment
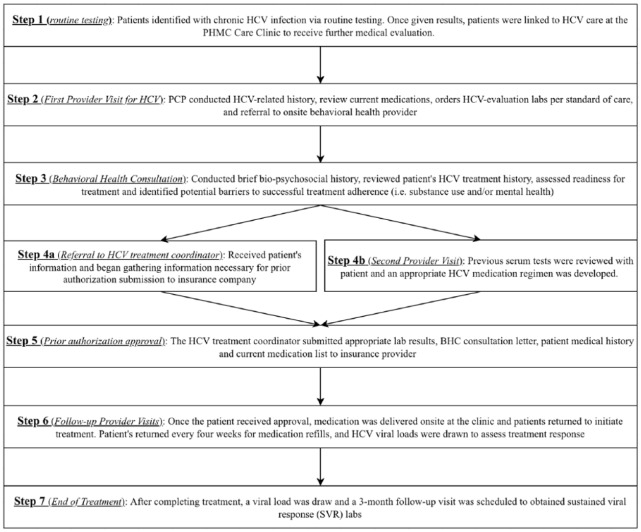
Once the patient completed the treatment regimen, viral load was measured to assure successful treatment completion. The patient was counseled to continue liver health modifications as well as risk reduction. The patient was then asked to return for their 12-week post-treatment SVR lab work. If the patient was cirrhotic, they are counseled about hepatocellular carcinoma screening, which will continue for life, regardless of HCV status. Patients were also informed that re-infection is possible if they engage in high risk behavior. A flowchart of the HCV treatment model can be seen in Figure 1.
Figure 1.
Flowchart of integrated HCV treatment model.
Program Outcomes
Between January 2015 and April 2017, 190 patients were referred by their medical providers to start HCV treatment through the on-site treatment program after testing positive for long term HCV (Table 1). Most of the patients were male (81.6%) and African American (66.8%). More than four-fifths of the patients had a current behavioral health diagnosis and/or a history of substance use, with half of the patient population having documented IVDU (Table 1). In addition, 35.8% enrolled in the program had a history of homelessness and 34.2% were co-infected with HIV. Nearly half (45.79%) of patients had a fibrosis score of F2 or lower.
Table 1.
Patient demographics characteristics of whom were referred to the on-site treatment program.
| Patient demographics | Number of patients referred to HCV treatment program, n (%) |
|---|---|
| Gender | |
| Female | 35 (18.4) |
| Male | 155 (81.6) |
| Race | |
| African American | 127 (66.8) |
| White/Caucasian | 37 (19.5) |
| Asian | 2 (1.1) |
| Declined to respond | 24 (12.6) |
| Ethnicity | |
| Hispanic or Latino | 25 (13.2) |
| Non-Hispanic or Latino | 158 (83.2) |
| Undefined | 7 (3.6) |
| Age | |
| 18-39 | 12 (6.3) |
| 40-59 | 112 (58.9) |
| >60 | 66 (34.7) |
| Insurance | |
| Medicaid | 151 (79.5) |
| Medicare | 28 (14.7) |
| Private | 11 (5.8) |
| Mental health diagnosis | |
| Bipolar | 26 (13.7) |
| Schizophrenia | 14 (7.4) |
| Depression/mood disorder | 110 (57.9) |
| Anxiety | 70 (36.8) |
| Adjustment disorder | 12 (6.3) |
| PTSD | 14 (7.4) |
| Other (insomnia and panic disorder) | 34 (17.9) |
| HCV risk factor | |
| Baby boomer (1945-1965) | 146 (76.8) |
| History of IVDU | 105 (55.3) |
| Unknown history of IVDU | 40 (21) |
| Homelessness | 68 (35.8) |
| Unlicensed tattoo | 3 (1.6) |
| Incarceration | 31 (16.3) |
| HCV positive partner | 2 (1.1) |
| Co-infection | |
| HIV | 65 (34.2) |
| HBV | 3 (1.6) |
| HCV mono-infected | 122 (64.2) |
| Drug use history | |
| Heroin | 69 (36.3) |
| Cocaine | 72 (37.9) |
| Crack cocaine | 35 (18.4) |
| Marijuana | 28 (14.7) |
| Other (methadone, amphetamines, and so on) | 30 (15.8) |
| Genotype | |
| 1a | 130 (68.4) |
| 1b | 30 (15.8) |
| 2 | 14 (7.4) |
| 3 | 15 (7.9) |
| 4 | 1 (0.5) |
| Fibrosis Score | |
| F0 | 17 (8.9) |
| F0-F1 | 14 (7.4) |
| F1 | 5 (2.6) |
| F1-F2 | 35 (18.4) |
| F2 | 16 (8.4) |
| F2-F4 | 13 (6.8) |
| F3 | 32 (16.8) |
| F3-F4 | 7 (3.7) |
| F4 | 51 (26.8) |
| Treatment length | |
| 8 weeks | 14 (9.9) |
| 12 weeks | 116 (81.7) |
| 24 weeks | 12 (8.4) |
| Treatment experienceda | 36 (18.9) |
| Treatment naiveb | 154 (81.1) |
Abbreviations: HCV, hepatitis C virus; HBV, hepatitis B virus; PTSD, post-traumatic stress disorder; IVDU, intravenous drug use.
Previously had been treated for hepatitis C.
Never received treatment for hepatitis C.
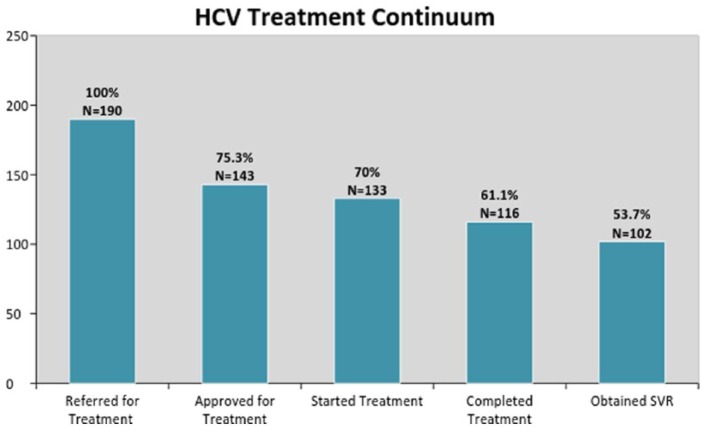
The number and percentage of referred patients who received a long term HCV diagnosis and referred for treatment (n = 190), approved for treatment (n =143 ), started treatment (n = 133), completed treatment (n = 116), and attained SVR (n = 102) as explained in the care continuum in Figure 2.
Figure 2.
Continuum of care for long term hepatitis C patients referred for treatment: January 2015 to April 2017.
In this care continuum, 75% of patients who were referred for treatment obtained prior authorization approval from their insurance providers to receive HCV medication. Of those approved for treatment, the care team obtained a 93% success rate at initiating patients on treatment regimens. Of the patients who started treatment, 116 (87.2%) patients successfully completed their course of treatment. At the time of data collection completion, 12 patients were still receiving active treatment, and 5 patients started treatment but failed to finish the prescribed regimen due to loss of contact. Of those who have finished treatment and received SVR lab work, 102 (98.1%) patients have been cured. A total of 12 patients were due for SVR labs but have yet to return to the clinic for lab work. For those that failed to return for SVR but had end of treatment labs, we estimated that 96% of them achieved SVR.
One patient failed after a full course of treatment, and one patient cleared the virus but was re-infected due to active IVDU. In addition, most of the patients (79%) enrolled in the treatment program kept the clinic as their primary health care home after SVR is achieved.
Discussion
In a primary care setting without a specialist on staff, PAs and nurse practitioners (NPs) as primary care providers were successful at delivery HCV treatment and managing comorbidities through behavioral health providers. This program had a 93% success rate at initiating patients on treatment regimens after insurance approval, showing a level of success comparable to those with traditional medical doctors (MDs) with the presence of a specialist.16 This program is unique in at least two ways. First, the treatment program was developed by mid-level providers to treat HCV-infected patients, without the active involvement of a hepatologist. Second, the program was developed in an community health center that treats a medically underserved minority population disproportionately affected by homelessness, mental illness, and substance use. The program’s success can be attributed to three practices. First, offering opt-out laboratory-based HCV reflex testing reduced the time that patients waited to receive their test results, enabling patients to be engaged faster into the treatment process and may have helped to maximize initial patient motivation to engage in treatment. The opt-out testing in the high risk population captured positive tests that may have been missed with standard screening (ie baby boomers and people who inject drugs). Second, establishing immediate patient-provider relationships within the primary care setting helped HCV treatment uptake. Third, integrating behavioral health services into standard, routine care for HCV positive patients showed promise as an effective intervention in HCV treatment adherence and health outcomes, especially in a vulnerable population. This program achieved a 98.1% sustained virologic rate (SVR) in a high risk population, with most patients having a history of substance use and/or mental health problems (85.3% and 82.6%, respectively). The BHCs play a crucial role in assessing and connecting high risk patients to care resources for substance abuse and mental health disorders, likely increasing patient engagement in HCV treatment.17,18
Integrating this type of program into additional community health centers is plausible. Screening for HCV in the primary care setting is becoming more common, and on-site primary care behavioral health services continue to be supported in community health centers.19 For community health centers with limited funding and resources for their patients, creating an HCV “test and treat” model within their existing clinic infrastructure is not only beneficial for the patient population that they serve but also fiscally sustainable.20,21 Federally qualified health centers have the ability to generate revenue by using 340b drug pricing on HCV medications that can be used to cover testing on uninsured/underinsured patients, hiring of treatment support staff (such as a treatment coordinator, outreach staff, and behavioral health providers), and QI.15
Limitations of the program include restrictive policies regarding patients on Medicaid receiving HCV treatment. Most of this population (76%) reported receiving Medicaid benefits. Restrictions regarding certain needed Fibrosis Score levels22 (eg greater than F2 Fibrosis Score for treatment approval) and barriers toward treatment approval for those with recent alcohol and drug use were two factors that reduced the total number of patients who were effectively able to be approved for treatment by their insurance. For example, 18.9% of identified HCV positive patients (n = 36) did not meet the existing fibrosis score cut-off for treatment approval. However, newly enacted statewide policy changes eliminating the fibrosis restrictions on publicly insured plans will allow for more patients to access DAA medications in the near future. It is likely that psychosocial evaluation will become even more important for insurance approval: individuals with recent substance use history may be considered “too high risk” (55% of our population) and denied for cost containment purposes. However, further research is needed to assess to impact of behavioral health integration into HCV treatment protocols.
Conclusions
With the proper support and integrated BHCs, treating HCV in a primary care setting with high rates of substance abuse and mental health illness is possible and effective. As access to HCV medications improves, community health centers with the staff of PAs and NPs are well positioned to screen and treat HCV positive patients in communities with high prevalence rates. Further dissemination of this HCV treatment method that uses PAs as the primary care provider is needed to assess the benefit of integrating primary care behavioral health services into routine HCV treatment strategies to treat long term infection in active substance users and patients diagnosed with co-occurrring mental health illnesses.
Acknowledgments
The authors would like to thank the dedicated providers and care team at the PHMC Care Clinic.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The primary author of this publication was partially funded as a consultant on a Gilead FOCUS program. This funding did not support the research activities presented in this publication.
Author Contributions: TSB developed the concept, design, data collection and analysis, including the writing of the article. KG contributed to writing and reviewing of the article. KH contributed to the concept development and writing of the article. TC developed the concept, desgin, and writing of the article.
Ethical Approval: This study expedited approval by Public Health Management Corporation (PHMC) Institutional Review Board (IRB), IRB #0002451. Data were collected via a retrospective chart review of electronic health records.
References
- 1. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, national health and nutrition examination survey 2003 to 2010. Ann Intern Med. 2014;160:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mitchell AE, Colvin HM. Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C. Washington, DC: National Academies Press; 2010. [PubMed] [Google Scholar]
- 3. Control CFD and Prevention. Viral Hepatitis Surveillance, United States, 2014. Published June, 2017. https://www.cdc.gov/hepatitis/statistics/2014surveillance/pdfs/2014hepsurveillancerpt.pdf.
- 4. Zibbell JE, Iqbal K, Patel RC, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged ⩽30 years—Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. MMWR Morb Mortal Wkly Rep. 2015;64:453–458. [PMC free article] [PubMed] [Google Scholar]
- 5. Coyle C, Viner K, Hughes E, et al. Identification and linkage to care of HCV-infected persons in five health centers—Philadelphia, Pennsylvania, 2012–2014. MMWR Morb Mortal Wkly Rep. 2015;64:459–463. [PMC free article] [PubMed] [Google Scholar]
- 6. Kattakuzhy S, Gross C, Emmanuel B, et al. Expansion of treatment for hepatitis C virus infection by task shifting to community-based nonspecialist providers: a nonrandomized clinical trial. Ann Intern Med. 2017;167:311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rogal SS, McCarthy R, Reid A, et al. Primary care and hepatology provider—perceived barriers to and facilitators of hepatitis C treatment candidacy and adherence. Dig Dis Sci. 2017;62:1933–1943. [DOI] [PubMed] [Google Scholar]
- 8. McGowan CE, Fried MW. Barriers to hepatitis C treatment. Liver Int. 2012;32::151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lasser KE, Heinz A, Battisti L, et al. A hepatitis C treatment program based in a safety-net hospital patient-centered medical home. Ann Fam Med. 2017;15:258–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trooskin SB, Navarro VJ, Winn RJ, et al. Hepatitis C risk assessment, testing and referral for treatment in urban primary care: role of race and ethnicity. World J Gastroenterol. 2007;13:1074–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coyle C, Moorman AC, Bartholomew T, et al. The HCV care continuum: linkage to HCV care and treatment among patients at an urban health network, Philadelphia, PA: [published online ahead of print January 11, 2019]. Hepatology. doi: 10.1002/hep.30501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Robinson P, Reiter J. Behavioral Consultation and Primary Care: A Guide to Integrating Services. Cham, Switzerland: Springer International Publishing; 2016. [Google Scholar]
- 13. Tamura-Lis W. Teach-back for quality education and patient safety. Urol Nurs. 2013;33:267–271. [PubMed] [Google Scholar]
- 14. Chambers JE, Brooks AC, Medvin R, et al. Examining multi-session brief intervention for substance use in primary care: research methods of a randomized controlled trial. Addict Sci Clin Pract. 2016;11:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Veterans Health Care Act of 1992. 2nd ed.; 1992:4943. [Google Scholar]
- 16. Edlin BR. Perspective: test and treat this silent killer. Nature. 2011;474:S18–S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364:2199–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sylvestre DL, Zweben JE. Integrating HCV services for drug users: a model to improve engagement and outcomes. Int J Drug Policy. 2007;18:406–410. [DOI] [PubMed] [Google Scholar]
- 19. Fireman M, Indest DW, Blackwell A, Whitehead AJ, Hauser P. Addressing tri-morbidity (hepatitis C, psychiatric disorders, and substance use): the importance of routine mental health screening as a component of a comanagement model of care. Clin Infect Dis. 2005;40:S286–S291. [DOI] [PubMed] [Google Scholar]
- 20. Miller BF, Petterson S, Burke BT, Phillips RL, Jr, Green LA. Proximity of providers: colocating behavioral health and primary care and the prospects for an integrated workforce. Am Psychol. 2014;69:443–451. [DOI] [PubMed] [Google Scholar]
- 21. Valdiserri R, Khalsa J, Dan C, et al. Confronting the emerging epidemic of HCV infection among young injection drug users. Am J Public Health. 2014;104:816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bota S, Sirli R, Sporea I, et al. A new scoring system for prediction of fibrosis in chronic hepatitis C. Hepat Mon. 2011;11:548–555. [PMC free article] [PubMed] [Google Scholar]