Abstract
Quxie capsule (QX), a herbal remedy used in traditional Chinese medicine, is routinely used in advanced colorectal cancer treatment in Xiyuan Hospital in Beijing, China. However, the mechanism(s) underlying the effect of QX in colorectal cancer remain unclear, which hampers the optimal use of QX for the treatment of the disease. The transcription factor forkhead box O1 (Foxo1) plays important roles in regulation of cell cycle, apoptosis, and immune response in various cancers. In this study, we examined the antitumor efficacy of QX in a mouse model of colorectal cancer and further investigated the mechanism by which QX regulated Foxo1 protein-mediated pathways. QX administered via gavage daily for 2 weeks in mice carrying CT26 mouse colon tumors resulted in significantly lower mean tumor weight (0.93 ± 0.32 g) compared with that in vehicle control-treated mice (1.57 ± 0.57 g, P <.05). Foxo1 protein expression in tumors was also higher in the QX group than that in the vehicle control group. Furthermore, QX treatment upregulated apoptotic proteins such as Fas, Bim, and cleaved caspase-3 in tumor tissue compared with those in the vehicle control group. Intriguingly, the ratios of Th1/Th2 and Th17/Treg cells and levels of T-bet protein (the key regulator of Th1 and Th2 cells) were higher while the level of Foxp3 (the key regulator of Treg cells) was lower in QX-treated mice compared to vehicle control mice, revealing that Foxo1 upregulated T-bet and downregulated Foxp3 and induced a shift in immune balance. This shift could be critical in the antitumor efficacy of QX. Furthermore, knocking down Foxo1 in human colon cancer HCT116 cells partially blocked the effect of QX-elicited antiproliferative activity. Together, these results suggest that QX exerts antitumor activity in CT26 mouse colon cancer model partially mediated by Foxo1-induced apoptosis and antitumor immune response.
Keywords: Quxie capsule, traditional Chinese medicine, colon cancer, Foxo1, T helper cells
Introduction
The incidence of colorectal cancer among cancers ranks the third in both women and men in China, whereas colorectal cancer is the third leading cause of cancer-related death in men and the fourth in women.1 Although the incidence of colorectal cancer has been declining in developed countries like the United States, it has been increasing in developing countries such as China because of increased exposure to risk factors such as increased consumption of red meat and smoking.2 Although the mortality rate and 5-year survival rate have improved during the past few decades due to emerging therapies such as molecular targeted therapy, the 5-year survival rate for patients with stage IV colorectal cancer is only about 12%.2 Developing novel and effective therapeutic strategies for advanced colorectal cancer are still urgently needed.
Traditional Chinese medicine has been applied to treat cancer or cancer-related symptoms for decades in China. Quxie capsule (QX) is a modified formula of Yinyanggongji pill, a herbal formula developed thousands of years ago. Compared with Yinyanggongji pill, QX has higher amounts of Coptis chinensis, Pinelliae rhizoma, Citri grandis exocarpium, Poria, Arecae semen, Magnoliae officnalis, Aurantii fructus immaturus, Acori tatarinowii, Corydalis rhizoma, Panax Ginseng, Lignum aquilariae resinatum, and Radix platycodonis. QX has been used for the treatment of advanced colorectal cancer in the traditional Chinese medicine oncology clinic in Xiyuan Hospital, Beijing, China. A randomized controlled trial conducted in this clinic has suggested that QX combined with conventional chemotherapy showed a significant survival benefit compared with chemotherapy alone in previously treated stage IV colorectal cancer patients at the age of 65 years or younger with left-sided colon disease.3 In addition, elevated level of apoptosis-related protein cleaved caspase-3 was found in QX-treated HCT116 cells,4 and elevated level of cytokine IFN-γ but reduced level of IL-4 were found in QX-treated mice.5 However, the molecular mechanisms by which QX induces colon cancer cell apoptosis and modulates host immune response still remain unclear.
Foxo1 is a member of the human mammalian class O of forkhead box transcription factors and plays important roles in regulation of cell cycle arrest, apoptosis, and immune response in various cancers.6-8 Mounting evidence suggests that Foxo1 functions as a tumor suppressor as it possess antiproliferative and proapoptotic activities in a variety of cancers.8-10 Foxo1 is known to be involved in mitochondria-dependent and -independent processes that stimulate the expression of death receptor ligands, including Fas ligand and Bcl-2 family members Bcl-XL, BNIP3, and Bim.11 Additionally, Foxo1 is important in regulating CD4+ T cell trafficking and homeostasis.12 Foxo1 was also reported to modulate Foxp3 expression and influence regulatory T (Treg) cell lineage commitment,13,14 as well as modulate T helper 1 (Th1) cell differentiation via T-bet.15,16 Significantly, in colon carcinoma-derived cells, inhibition of Foxo gene or protein via gene silencing or the pharmacological perturbation of signaling pathways such as EGFR, β-catenin, Wnt, or PI3K-AKT leads to CRC carcinogenesis.17
Understanding the effect of QX on Foxo1 could help optimize the use of QX in colorectal cancer treatment. In this study, we examined the antitumor efficacy of QX in a mouse model of colon cancer and human colon cancer cells and observed that QX can inhibit the growth of colorectal cancer potentially through regulating Foxo1 mediated pathways.
Materials and Methods
Preparation of QX
QX is composed of the following herbs: Evodiae fructus, Zingiberis rhizoma, Cortex cinnamomi, Radix aconiti, Coptis chinensis, Pinelliae rhizoma, Citri grandis exocarpium, Poria, Arecae semen, Magnoliae officnalis, Aurantii fructus immaturus, Acori tatarinowii rhizoma, Corydalis rhizoma, Panax Ginseng, Lignum aquilariae resinatum, Radix platycodonis, Succinum, Crotonis fructus, Galli Gigerii endothelium corneum, Hordei fructus germinatus, and Gleditsiae fructus abnormalis, at a ratio of 10:10:10:10:8:8:8:8:8:8:8:8:8:8:8:8:8:5:5:5:60. Gleditsiae fructus abnormalis was prepared by boiling this particular herb for 30 minutes twice followed with filtration and lyophilization. The rest of the herbs were powdered and mixed with the lyophilized Gleditsiae fructus abnormalis extraction thoroughly. QX was manufactured by the Pharmaceutical Center of Xiyuan Hospital (batch number 20170501, Beijing, China) and was dissolved in filtered (0.22 µm) water for the animal study.
Cell Culture
Both mouse colon carcinoma CT26 cells and human colon carcinoma HCT116 cells were purchased from National Infrastructure of Cell Line Resource of China (Beijing, China) or ATCC (Manassas, VA, USA). CT26 cells were cultured in RPMI-1640 medium, and HCT116 cells were cultured in McCoy’s 5A medium; both media were supplemented with 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin and incubated at 37°C in a humidified 5% CO2 atmosphere. For cell treatment, QX was boiled in hot water for 35 minutes, and then filtered and lyophilized. Lyophilized powder of QX was dissolved in cell culture medium and filtered by a 0.22 µM filter prior to the treatment.
Laboratory Animals
All animal studies were approved by The University of Texas MD Anderson Cancer Center Animal Care and Use Committee (IACUC protocol number: 00000669-RN02). Female Balb/c mice at 6 to 8 weeks old with body weight 25 ± 5 g were used. The animal facility was kept controlled at 23°C and 10% humidity, with a 12-hour light and 12-hour dark cycle. Mice were acclimated for 1 week in the animal facility prior to the experiment. Mice were injected with CT26 cells (1 × 105 cells/mouse) subcutaneously on the right flank and then randomly assigned to receive vehicle control or QX when tumor volume reached 50 mm3. Mice were treated with vehicle (ddH2O) or QX at 18.5 g/kg via gavage daily for 14 days. Tumor volume (mm3 = 1/2 × long diameter × short diameter2) was measured every other day. At the end of the 2-week treatment, the mice were euthanized, and the tumors were removed and either fixed in a 10% formalin-PBS (phosphate-buffered saline) solution or flash frozen in liquid nitrogen and stored at -80°C for further analysis. Spleens were collected and placed in ice-cold 1× HBSS (Hank’s balanced salt solution) for immune cell analysis.
TUNEL Assay Staining
To detect the in situ apoptosis in tumor tissue sections, we followed the TUNEL method as described by Resendes et al18 by using a TUNEL detection kit (Intergen Co., Oxford, UK).
Histopathology and Immunohistochemistry
Formalin-fixed tumor tissues were paraffin processed for biomarker identification by immunohistochemistry (IHC) staining. For IHC staining, slides were baked at 60°C for over 2 hours and then deparaffinized and rehydrated. Antigens were unmasked by heat-induced antigen retrieval. Slides were then immersed in 3% H2O2-methanol solution followed by blocking with 5% goat serum in 0.3% Triton X-100 PBS. Then slides were stained with Ki-67 antibody in a humidified chamber overnight at 4°C. Slides were washed thrice with PBS and then incubated with secondary antibody at room temperature for 45 minutes. Slides were incubated with ABC (Vector Laboratories, Burlingame, CA) followed by DAB (3,3′-diaminobenzidine) substrate for antibody visualization and counterstained with Mayer’s hematoxylin, dehydrated, and mounted with ClearMount Mounting Medium (American MasterTech, Lodi, CA).19
Western Blotting
Tumor and spleen tissues were placed in ice-cold lysis buffer (Thermo Fisher Scientific, Waltham, MA) and homogenized with tissue homogenizer (Precellys, Bertin Corp., Rockville, MD) followed by centrifugation at 10,000 g for 10 minutes at 4°C. Protein levels were quantified using the BCA protein assay. An equal amount of protein (20 µg) was applied to 10% to 15% SDS gel and then transferred onto polyvinyl membranes, according to standard procedure. Membranes were blocked with 5% nonfat dry milk blocking buffer prepared in Tris-buffered saline with 0.1% Tween 20 for 1 hour at room temperature. The membranes were then probed with primary antibodies of Foxo1, phosphorylated Foxo1 (p-Foxo1), caspase-3, cleaved caspase-3, Bim, FasL, T-bet, and GAPDH overnight at 4°C. The membranes were extensively washed and incubated with secondary antibodies (antirabbit IgG) prepared in 5% nonfat dry milk blocking buffer with 0.1% Tween 20 for 1 hour at room temperature. All antibodies were obtained from Affinity Biosciences (Cincinnati, OH). The membranes were washed again and then incubated with the ECL+ detection kit for 5 minutes. Membranes were scanned by Bio-Rad ChemiDoc Touch imaging system via chemiluminescence (Bio-Rad Laboratories, Carsland, CA). NIH ImageJ software was used for protein bands quantification.
Immune Cell Profiling
Spleen and tumor tissues were collected and placed in plain 1× HBSS. For isolation of lymphocytes from spleens and tumors, protocols described by Bartkowiak et al20 were used. In each sample, 1 × 106 cells were used for staining for immune cell surface markers. Cells were then incubated at 4°C for 1 hour with antibodies against mouse CD4 (BioLegend, San Diego, CA), CD3 (BD Biosciences, San Jose, CA), and CD8 (BioLegend). Subsequently, the cells were washed twice with PBS containing 2% FBS and then fixed and permeabilized with Foxp3 Fix/Perm Kit (ThermoFisher Scientific, Waltham, MA). Then cells were washed twice with wash buffer and incubated with intracellular markers: Foxp3 (eBioscience, San Diego, CA), IFN-γ (BioLegend), IL-17(BD Biosciences), and IL-4 (BioLegend) for 1 hour at 4°C. Antibodies were diluted according to the manufacturers’ recommendations. All the samples were collected on a BD Accuri C6 cytometer (BD Biosciences) and analyzed using FlowJo software (FlowJo v.10, Ashland, OR).
Detection of Apoptosis by Annexin V-FITC/PI Double Staining
Annexin V-FITC/PI staining (BD Biosciences) was used to quantify early and late apoptotic cells. Briefly, HCT116 cells (2.5 × 106) were treated with QX (0.3 and 0.6 mg/mL) for 48 hours. Cells were then harvested and washed with cold PBS twice and then stained with fluorescein isothiocyanate-conjugated annexin V and propidium iodide per the manufacturer’s instructions. Fluorescence was detected by a BD FACSCalibur flow cytometer and analyzed using CellQuest Pro software (BD Biosciences).
Small Interfering RNA Transfection
Small interfering RNA (siRNA) against the human FOXO1 gene or negative control siRNA (QIAGEN, Germantown, MD) were transiently transfected into HCT116 cells using Lipofectamine 3000 reagent (Invitrogen Life Technologies, Carlsbad, CA), according to the manufacturer’s instructions. The sequences of siRNA were as follows: 5′-CUG GAU CAC AGU UUU CCA AAUG-3′ (FOXO1) and 5′-GCA AGC UGA CCC UGA AGU UCAU-3′ (negative). After 48 hours, the cells were analyzed by Western blot assays or treated with QX (0.3 mg/mL) for 24 hours for cell count analysis.
Statistical Analysis
The Student’s t test was used to determine the statistical differences between control and treatment groups; a value of P ≤ .05 was considered significant. One-way ANOVA analysis of variance was used to determine statistical differences of the means in more than 2 groups. All analyses were performed using GraphPad Prism (version 7.0).
Results
QX Suppressed CT26 Tumor Growth in Syngeneic Mice
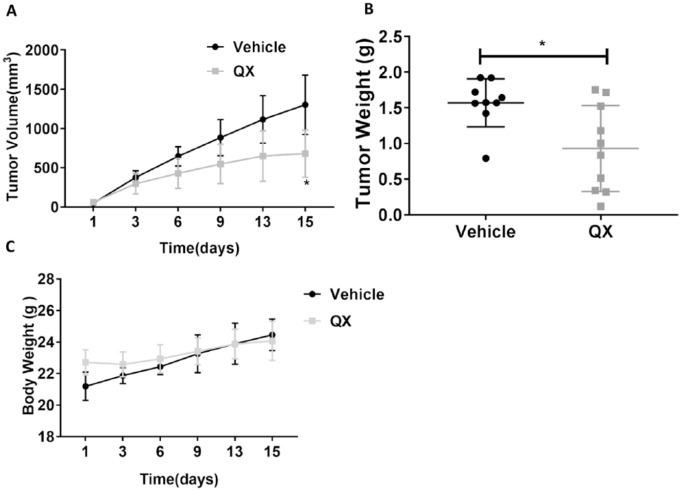
Tumor volume was measured every other day, and tumors were collected and weighed after the 2-week treatment. The tumor growth curve indicated that tumors grew more slowly in QX-treated mice compared with vehicle control-treated mice. At the end of the study, the mean tumor volume was 1302 ± 378 mm3 in the control group and 681 ± 300 mm3 in QX-treated mice (P < .05; Figure 1A). After 14 days of treatment, mean tumor weight in QX-treated mice (0.93 ± 0.32 g, n = 10) was significantly lower than in the vehicle control group (1.57 ± 0.57 g, n = 9, P < .05; Figure 1B). Limited body weight changes were observed between the QX-treated and vehicle control-treated mice (24.08 ± 1.00 g vs 24.46 ± 1.23 g, P > .05; Figure 1C). These results indicate that QX exerted antitumor activity in syngeneic mice without causing significant toxicity.
Figure 1.
Quxie capsule (QX) showed antitumor effects in a CT26 colon tumor syngeneic mouse model. (A) The growth curves of CT26 tumors in mice treated with QX or vehicle control for 14 days. (B) The mean terminal tumor weight in QX-treated mice was significantly lower than that in vehicle control-treated mice. (C) No difference was observed in the body weight of QX-treated and vehicle control-treated mice. Data are presented as mean ± SD. N = 9 to 10. *P < .05 for QX-treated mice compared with vehicle control-treated group.
QX Inhibited Cell Proliferation and Induced Apoptosis in Tumor Tissue
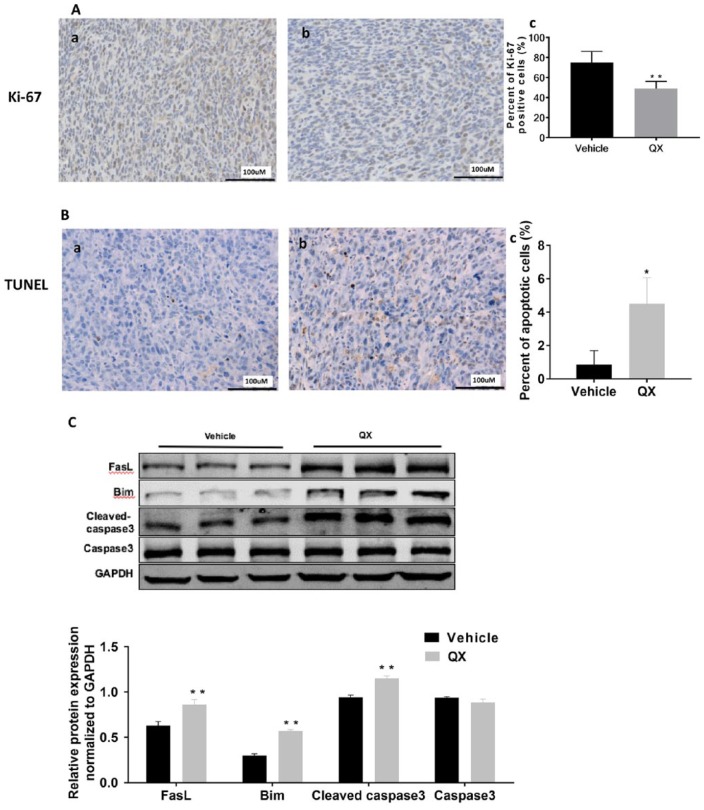
We used IHC staining for Ki-67 to determine the proliferation status of CT26 tumors. The Ki-67 expression was lower in CT26 tumor tissue derived from QX-treated mice than in tumor tissue from vehicle control-treated mice, and the reduction was statistically significant (P < .01; Figure 2A). To determine the underlying mechanism by which QX inhibits tumor growth, we measured apoptotic cell death with the TUNEL staining and the expression of apoptotic related protein with Western blotting. The apoptotic cell death was significantly higher in QX-treated mice than in vehicle control-treated mice (P < .05; Figure 2B). The levels of the proapoptotic proteins Bim, FasL, and cleaved caspase-3 were significantly higher in QX-treated CT26 tumors than in vehicle control-treated tumors (P < .01; Figure 2C), suggesting that QX inhibited the growth of CT26 tumor by reducing cell proliferation and inducing apoptosis in CT26 tumor cells.
Figure 2.
Quxie capsule (QX) inhibited cell proliferation and induced apoptosis in tumor tissues. (A) Ki-67 staining of tumor sections obtained from mice treated with (a) vehicle control or (b) QX. Quantification of Ki-67-positive cells in the tumor sections (c). (B) TUNEL staining of tumor sections obtained from mice treated with (a) vehicle control or (b) QX. Quantification of TUNEL-positive cells in the tumor sections (c). (C) Western blotting of proapoptotic proteins Bim, FasL, and cleaved caspase-3 expression in tumor tissues of QX-treated mice or vehicle control-treated mice. Data are presented as mean ± SD. *P < .05, **P < .01 versus vehicle control.
QX Regulated Foxo1 and p-Foxo1 Expression in Tumor Tissues
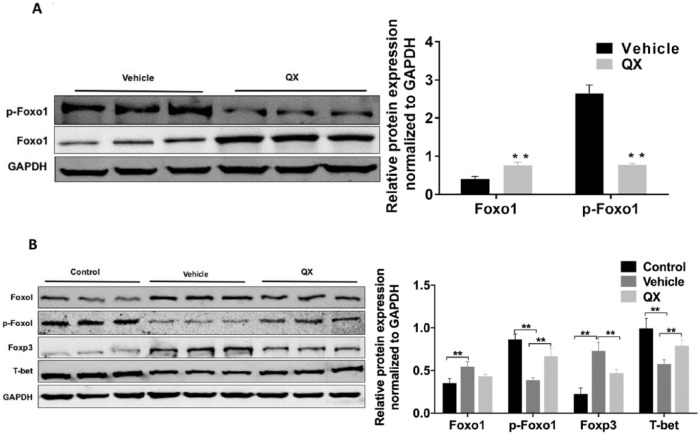
To determine whether QX-induced apoptosis could be mediated through Foxo1 alteration, we measured the protein levels of Foxo1 and p-Foxo1 in both tumor and spleen tissues with Western blotting. As shown in Figure 3A, Foxo1 expression in tumor tissues from QX-treated mice was 1.89-fold of that treated with vehicle control (P < .01), while p-Foxo1, the inactive form of Foxo1, was 71% lower in tumor tissues from QX-treated mice compared with tumor tissues from control-treated mice (P < .01). Given Foxo1 acts as a tumor suppressor and can regulate proapoptosis-related pathways, these data suggest that QX upregulates the active form of Foxo1 protein, which may contribute to its antitumor effects.
Figure 3.
Quxie capsule (QX) regulates the expression of Foxo1 and its regulatory proteins in mouse tumor and spleen tissues. (A) Western blots of Foxo1 and p-Foxo1 protein expression in mouse CT26 colon tumor tissues. (B) Foxo1, p-Foxo1, Foxp3, and T-bet protein expression in spleen tissues of mice bearing CT26 tumor. Mice in the control group were ordinary Balb/c mice without tumor and treatment. Data are presented as mean ± SD. **P < .01 versus vehicle control.
QX Regulated Foxo1 and p-Foxo1 Expression in Spleen Tissue
We measured the expression of Foxo1, p-Foxo1, T-bet (the key regulator of Th1/Th2 cell differentiation), and Foxp3 (the key regulator of Treg cell differentiation) protein by Western blotting in spleen tissues from QX-treated and vehicle control-treated mice. Foxo1 was lower in spleen tissues from QX-treated mice than in tissues from vehicle control-treated mice (0.43 ± 0.01 vs 0.54 ± 0.03, P > .05), while p-Foxo1 expression was significantly higher in the spleen tissues from QX-treated mice than in tissues from vehicle control-treated mice (0.67 ± 0.05 vs 0.39 ± 0.02, P < .01; Figure 3B). T-bet expression was significantly higher (0.79 ± 0.04 vs 0.57 ± 0.04, P < .01), while Foxp3 was significantly lower (0.47 ± 0.03 vs 0.74 ± 0.07, P < .01), in spleen tissues from QX-treated mice than in tissues from vehicle control-treated mice (Figure 3B). Collectively, these data suggested that QX downregulates the expression of Foxo1 in spleen tissues of the CT26 tumor-bearing mice, which in turn, modulates key regulators of T helper cell differentiation.
QX Modulated Immune Cell Populations in Tumor and Spleen Tissues
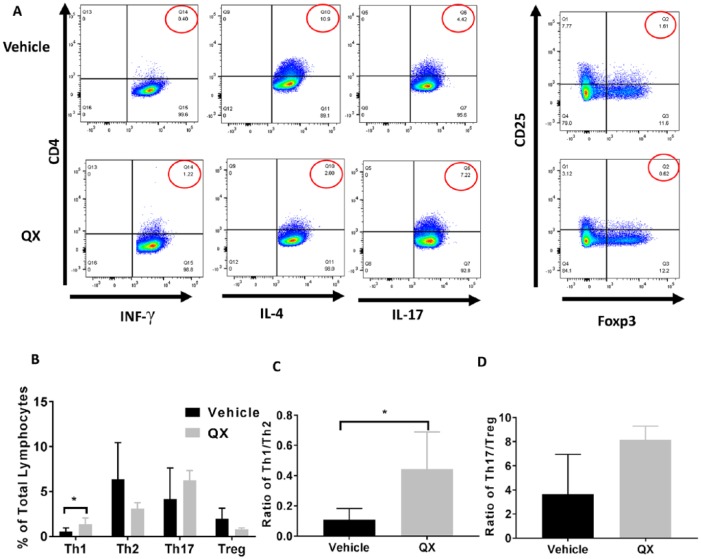
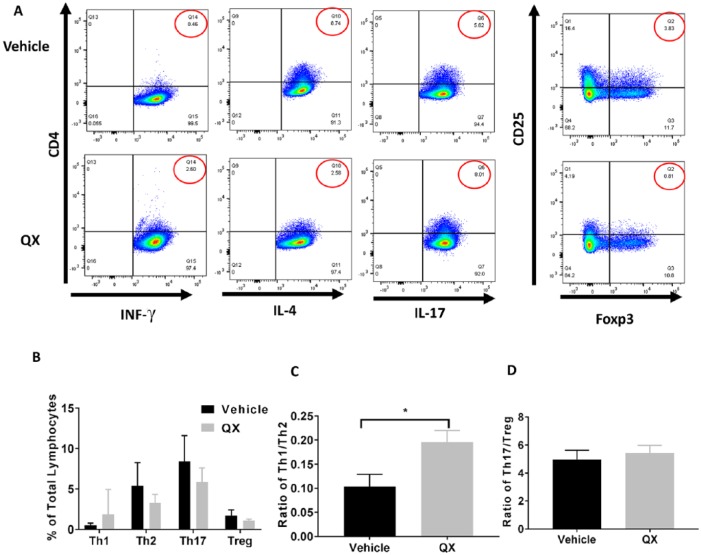
We measured immune cell populations in both tumor and spleen tissues from QX- and vehicle control-treated mice by flow cytometry, respectively (Figure 4A&5A). The percentage of Th1 cells in tumor tissue from QX-treated mice was 2.1 times higher than that in vehicle control-treated mice (P < .05), and the proportion of Th2 cells was 48.9% lower in tumor tissues from QX-treated mice than in tissues from vehicle control-treated mice (P > .05; Figure 4B). There was no difference in the proportion of Th17 cells in tumor tissues from QX-treated mice compared with that in tissues from vehicle control-treated mice (P > 0.05; Figure 4B). Additionally, the proportion of Treg cells was 61.4% lower in tumor tissues from QX-treated mice than in tumor tissues from vehicle control-treated mice (P > .05; Figure 4B). We also calculated the ratios of Th1/Th2 and T17/Treg. The Th1/Th2 ratio was 3.1 times higher in tumor tissues from QX-treated mice than in tissues from vehicle control-treated mice (P < .05), whereas less modulation of the Th17/Treg ratio by QX was observed (P > 0.05; Figure 4C and D)
Figure 4.
T helper cell profiling in Quxie capsule (QX)-treated tumor tissues. (A) Representative flow cytometry of T cells in tumor tissues. Numbers circled in red indicate percentage of Th1 (CD4+ IFN-γ+) cells, Th2 (CD4+ IL-4+) cells, Th17 (CD4+ IL-17+) cells (left panel), and Treg (CD25+ Foxp3+) cells (right panel). (B) Percentages of Th1, Th2, Th17, and Treg cells among tumor-infiltrated lymphocytes. (C) The Th1/Th2 ratio in vehicle control- and QX-treated tumor tissues. (D) The Th17/Treg ratio in vehicle control- and QX-treated tumor tissues. Data are presented as mean ± SD. *P < .05 versus vehicle control.
Figure 5.
T helper cell profiling in Quxie capsule (QX)-treated spleen tissues. (A) Representative flow cytometry of T cells in spleen tissue. Numbers circled in red indicate percentage of Th1 (CD4+ IFN-γ+) cells, Th2 (CD4+ IL-4+) cells, Th17 (CD4+ IL-17+) cells (left panel), and Treg (CD25 + Foxp3+) cells (right panel). (B) Percentages of Th1, Th2, Th17, and Treg cells in spleen tissues. (C) The Th1/Th2 ratio in vehicle control- and QX-treated spleen tissues. (D) The Th17/Treg ratio in vehicle control- and QX-treated spleen tissues. Data are presented as mean ± SD. *P < .05 versus vehicle control.
There was no difference in the population of IFN-γ-positive Th1 cells in spleen tissues from QX-treated mice compared with the spleen tissues from vehicle control-treated mice. The population of Th2 cells in QX-treated mouse spleen was 39.1% lower than that in vehicle control-treated mouse spleen, but the difference was not significant (P > .05; Figure 5B). The proportion of Th17 cells was also lower in spleen tissues from QX-treated mice than in spleen tissues from vehicle control-treated mice (5.88% and 8.39%, respectively; P > .05). Furthermore, the proportion of Treg cells was lower in spleen tissues from QX-treated mice than in spleen tissues from vehicle control-treated mice (1.06% and 1.77%, respectively; P > .05). The ratio of Th1/Th2 was significantly higher in spleen tissues of QX-treated mice than that of veihicel-control group whereas only limited differences in Th17/Treg ratios were observed in spleen tissues from QX-treated and vehicle control-treated mice (Figure 5C and D). These data suggested that QX is capable of modulating the immune suppressive tumor microenvironment by increasing the population of Th1 cells and reducing the Treg immune cells.
QX-Induced Cell Death Was Mediated by Foxo1
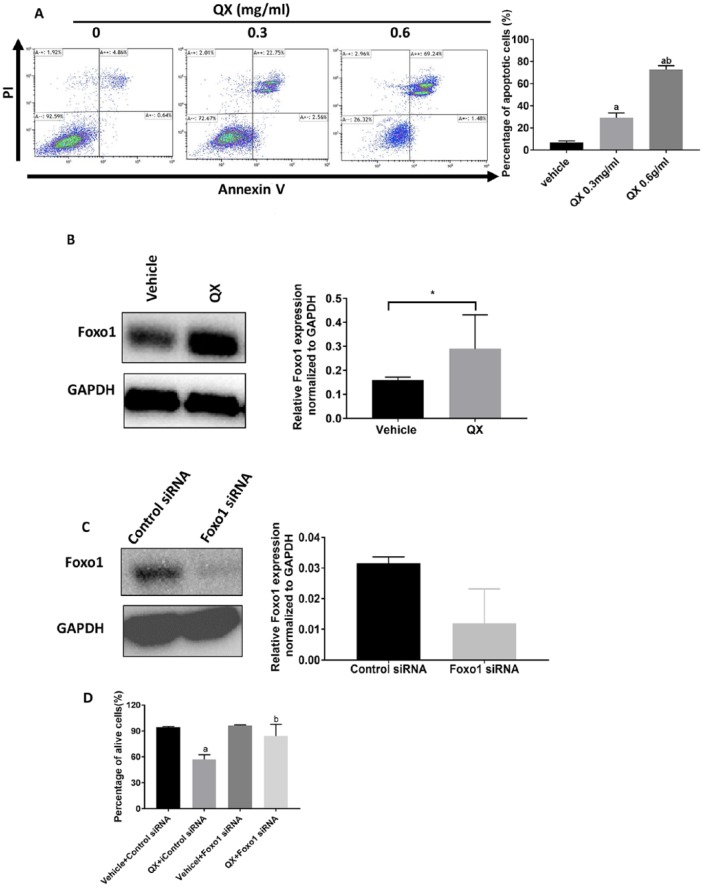
Because we found that apoptosis-related proteins such as cleaved caspase-3, FasL, and Bim were elevated in QX-treated tumor tissue, we sought to confirm the induction of apoptosis by Annexin V-FITC/PI staining in human colon cancer HCT116 cells in vitro. The results showed that cells treated with QX (0.3 mg/mL and 0.6 mg/mL, respectively) for 48 hours underwent apoptotic cell death in a dose-dependent manner compared with vehicle control-treated cells (29.15%, 72.69%, and 7.00%, respectively; P < .01; Figure 6A). Also, Foxo1 protein expression was significantly higher in QX-treated cells than in vehicle control-treated cells (Figure 6B).
Figure 6.
Quxie capsule (QX) had an anticancer effect in human colorectal cancer HCT116 cells. (A) Flow cytometry showed apoptotic cells in QX- or vehicle control-treated HCT116 cells with Annexin V-FITC/PI double staining. The lower right quadrant showed annexin-positive cells (early apoptosis), and the upper right quadrant shows cells positive for both annexin and PI (late apoptosis). aP < .01 for QX-treated compared with vehicle-treated HCT116 cells. bP < .01 for QX (0.6 mg/ml)-treated compared with QX (0.3 mg/mL)-treated HCT116 cells. (B) Foxo1 protein expression in QX-treated HCT116 cells. *P < .05 versus vehicle control. (C) Foxo1 protein expression in FOXO1 siRNA-silenced HCT116 cells. (D) Cell viability in QX-treated Foxo1 siRNA and control siRNA-transfected HCT116 cells. aP < .05 for QX-treated control siRNA-transfected cells compared with vehicle-treated controls. bP < .05 for QX-treated FOXO1 siRNA transfected cells compared with QX-treated control siRNA-transfected cells. Data are presented as mean ± SD.
To validate whether the antiproliferative effect of QX was mediated by Foxo1, we evaluated cell viability by counting the viable cells in QX-treated Foxo1 siRNA and control siRNA-silenced HCT116 cells. As shown in Figure 6C and D, the antiproliferative effect of QX was less pronounced in FOXO1-knockdown HCT116 cells (<10% inhibition) than in control siRNA-transfected cells (29% inhibition). These results suggested that upregulating Foxo1 might be responsible for QX-elicited inhibition of cell proliferation and induction of apoptosis in colon cancer cells.
Discussion
In this study, we demonstrated that QX inhibited colon tumor growth through induction of apoptosis and that this inhibition might be mediated through the Foxo1 pathway. Foxo1 acts as a tumor suppressor, and it has been shown to inhibit cell migration and invasion in prostate cancer in vitro.21 Foxo1 protein stability and transcriptional activity are affected by phosphorylation modification.7,22 Foxo1 localizes in the nucleus and transcriptionally regulates cellular functions and activities, and phosphorylation of Foxo1 leads to its nuclear export, degradation, and loss of transcriptional activity.23 Because Foxo1 inactivation is common in many human cancer types, restoring Foxo1 activity is a potential approach for cancer treatment.24 In our study, increased Foxo1 protein and decreased inactive form of p-Foxo1 expression were observed in QX-treated tumor tissue, suggesting the apoptotic cell death induced by QX in colon tumors might be mediated by Foxo1-related signaling pathway(s).
In addition to tumor suppression, Foxo1 is also involved in regulation of T cell differentiation,16,25-27 particularly, CD4+ T helper cell differentiation.12,28 CD4+ T cell differentiation toward Th1 or Th2 lineage is driven by T-bet and GATA3, respectively.27 Foxo1 has been found to repress T-bet in T cells, which inhibits the expression of T-bet without direct DNA binding, indicating that inactivation of Foxo1 is essential for enhanced expression of T-bet.16 Treg cells typically express Foxp3, and Foxo1 was demonstrated to be involved in the induction of Foxp3 expression in Treg cells.13 Thus, the inactivated Foxo1 in the immune cells plays a pivotal role in induction of Th1 cells and repression of Treg cells. Taken together, these findings show that Foxo1 is an upstream regulator of T helper cell differentiation. Tumor-infiltrating lymphocytes are critical in antitumor immune response.29 The abundance of tumor-infiltrating T cells has been linked to prognosis in colorectal cancer patients.30 For example, it has been reported that a higher Th1 cell presence was associated with prolonged disease-free survival,31 excessive Treg cells suppress antitumor immune responses in colon cancer, and a higher level of Treg cells in tumors has been associated with poor prognosis in colorectal cancer patients.32 An imbalance of Th1 and Th2 or gradual loss of Th1 populations and increase in Th2 cytokine profile occur during progressive tumor growth in mouse models of renal cell carcinoma and colon adenocarcinoma.33-36 It was reported that transcription factor Foxo1 plays a critical role in controlling the development and function of Foxp3+ Treg cells as well as the T-bet-mediated differentiation from Th0 to Th1 and Th2 cells.14,27,34,37 In our study, we found that Foxp3 was downregulated and T-bet was upregulated in the spleen tissue of mice treated with QX, thus leading to increased ratios of Th1 to Th2 and Th17 to Treg cells.
In addition to Foxo1-mediated pathways, other anticancer mechanisms of the components of QX have been reported. For example, Gleditsia saponin C (GSC), an extract of Gleditsiae fructus abnormalis, is believed to induce cell death by increasing the ratio of Bax to Bcl-2 and inhibiting the ERK and Akt signaling pathways.38 Additionally, GSC was shown to suppress TNF-α-induced NF-κB activation, which in turn raised the susceptibility of lung cancer cells to TNF-α-induced apoptosis.38 GSC can also lead to cell cycle arrest at the G2/M phase and inhibit the growth of human colon cancer (HCT116) both in vitro and in vivo through increased p53 levels, downregulation of cyclins and cyclin-dependent kinases, and phosphorylation of ERK, p38 MAP kinase, and JNK.39-41 Crotonis fructus, another important component of QX, was reported to inhibit 12-O-tetradecanoylphorbol-13-acetate-induced cell invasion and upregulate matrix metalloproteinase-9 expression in MCF-7 cells via protein kinase C/p38/c-Jun N-terminal kinase/AP-1 pathway.42 Another herb in QX, Coptis chinensis, was shown to exert an anticancer effect by inhibiting the proliferation of vascular smooth muscle cells.43 In addition to its direct antitumor effect, Coptis chinensis also has shown anti-inflammatory effects in mouse models by downregulating nitric oxide and inducible nitric oxide synthase via its suppression of NF-κB and MAPK activation.43,44
The Chinese herbal formula QX has been routinely used in colorectal cancer treatment in Xiyuan Hospital in Beijing, China. In general, a limited number of patients reported abdominal pain or diarrhea that might be caused by QX treatment because some of the herbs, such as Crotonis fructus or its component, has a relatively strong purgative effect45,46 and has been reported to cause abdominal pain in animal study in a dose-dependent manner.47 However, these side effects are usually manageable and allow patients to continue to use this particular formula for the treatment of their colorectal cancer. A randomized controlled clinical trial conducted in Xiyuan hospital showed that no severe hematological toxicity (grade III or higher), liver toxicity, or kidney toxicity was observed in all 30 patients treated with QX.3 One patient in QX treatment group reported abdominal pain as an adverse event.3 Here, we further confirmed that QX at clinical relevant dose did not reduce the body weight of the mice bearing CT26 tumor or cause any gastrointestinal tract-associated symptoms. Given that the duration of QX treatment in the current study is less than 1 month, studies of various doses of QX and long-term administration to further explore its efficacy, safety, and toxicity are needed.
Our study demonstrated that QX elicited antitumor efficacy in a CT26 syngeneic mouse model partially via Foxo1-mediated apoptosis and immune cell regulation. Given Foxo1 can be modulated by a number of regulating factors, such as reactive oxygen species,48,49 AKT, and SIRT1,50 further study is needed to explain how QX regulates Foxo1 by examining the aforementioned regulating factors in colorectal tumor. Furthermore, because QX is composed of multiple herbs, there might be other signaling pathways involved in QX-elicited antitumor efficacy. Thus, screening methods such as RPPA or RNAseq need to be performed to identify other possible pathways that might be involved in QX-elicited antitumor activity in further study. In addition, determining how each individual herb contributes to antitumor effect of QX in colorectal cancer might further optimize the therapeutic potential of QX in this particular cancer. Collectively, in light of its observed antitumor efficacy and relatively good safety profile, further investigation of the antitumor mechanism of QX in colorectal cancer is warranted.
Acknowledgments
We thank Bin Yang for helping with immunohistochemistry staining in tumor tissue.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported in part by Capital Developing Foundation (2016_1_4171) (YFY) and discretionary funds (PY).
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177-193. [DOI] [PubMed] [Google Scholar]
- 3. Zhang T, Yang Y-f, He B, Yi D-h, Hao J, Zhang D. Efficacy and safety of quxie capsule (祛邪胶囊) in metastatic colorectal cancer: a double-blind randomized placebo controlled trial. Chin J Integr Med. 2018;24:171-177. [DOI] [PubMed] [Google Scholar]
- 4. Ning D. A Mechanism Study of Quxie Capsule in Treating Colon Cancer [dissertation]. Bejing, China: Xiyuan Hospital of China Academy of Chinese Medical Sciences; 2014. [Google Scholar]
- 5. Ning D, Yufei Y, Yinan L, Yun X, Bin H. The mechanism study of Qingxue granule and Quxie capsule in inhibiting HCT-116 proliferation. Chin J Basic Med Tradit Chin Med. 2013;19:38-40. [Google Scholar]
- 6. Kim SY, Ko YS, Park J, et al. Forkhead transcription factor FOXO1 inhibits angiogenesis in gastric cancer in relation to SIRT1. Cancer Res Treat. 2016;48:345-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Y, Zhou Y, Graves DT. FOXO transcription factors: their clinical significance and regulation. Biomed Res Int. 2014;2014:925350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782-787. [DOI] [PubMed] [Google Scholar]
- 9. Schmidt M, de Mattos SF, van der Horst A, et al. Cell cycle inhibition by FoxO forkhead transcription factors involves downregulation of cyclin D. Mol Cell Biol. 2002;22:7842-7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Strasser A, Cory S, Adams JM. Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases. EMBO J. 2011;30:3667-3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Farhan M, Wang H, Gaur U, Little PJ, Xu J, Zheng W. Foxo signaling pathways as therapeutic targets in cancer. Int J Biol Sci. 2017;13:815-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Newton RH, Shrestha S, Sullivan JM, et al. Maintenance of CD4 T cell fitness through regulation of Foxo1. Nat Immunol. 2018;19:838-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harada Y, Harada Y, Elly C, et al. Transcription factors Foxo3a and Foxo1 couple the E3 ligase Cbl-b to the induction of Foxp3 expression in induced regulatory T cells. J Exp Med. 2010;207:1381-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kerdiles YM, Stone EL, Beisner DL, et al. Foxo transcription factors control regulatory T cell development and function. Immunity. 2010;33:890-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hedrick SM, Michelini RH, Doedens AL, Goldrath AW, Stone EL. FOXO transcription factors throughout T cell biology. Nat Rev Immunol. 2012;12:649-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rao RR, Li Q, Bupp MRG, Shrikant PA. Transcription factor Foxo1 represses T-bet-mediated effector functions and promotes memory CD8+ T cell differentiation. Immunity. 2012;36:374-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Laissue P. The forkhead-box family of transcription factors: key molecular players in colorectal cancer pathogenesis. Mol Cancer. 2019;18:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Resendes AR, Majó N, Segalés J, et al. Apoptosis in normal lymphoid organs from healthy normal, conventional pigs at different ages detected by TUNEL and cleaved caspase-3 immunohistochemistry in paraffin-embedded tissues. Vet Immunol Immunopathol. 2004;99:203-213. [DOI] [PubMed] [Google Scholar]
- 19. Pan Y, Rhea P, Tan L, et al. PBI-05204, a supercritical CO2 extract of Nerium oleander, inhibits growth of human pancreatic cancer via targeting the PI3K/mTOR pathway. Invest New Drugs. 2015;33:271-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bartkowiak T, Singh S, Yang G, et al. Unique potential of 4-1BB agonist antibody to promote durable regression of HPV+ tumors when combined with an E6/E7 peptide vaccine. Proc Nat Acad Sci U S A. 2015;112:E5290-E5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang H, Pan Y, Zheng L, et al. Foxo1 inhibits Runx2 transcriptional activity and prostate cancer cell migration and invasion. Cancer Res. 2011;71:3257-3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xie Q, Chen J, Yuan Z. Post-translational regulation of FOXO. Acta Biochim Biophys Sin (Shanghai). 2012;44:897-901. [DOI] [PubMed] [Google Scholar]
- 23. de Brachène AC, Demoulin JB. FOXO transcription factors in cancer development and therapy. Cell Mol Life Sci. 2016;73:1159-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lu H, Liu P, Pan Y, Huang H. Inhibition of cyclin-dependent kinase phosphorylation of FOXO1 and prostate cancer cell growth by a peptide derived from FOXO1. Neoplasia. 2011;13:854-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luo CT, Liao W, Dadi S, Toure A, Li MO. Graded Foxo1 activity in regulatory Treg cells differentiates tumor immunity from spontaneous autoimmunity. Nature. 2016;529:532-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ouyang W, Liao W, Luo CT, et al. Novel Foxo1-dependent transcriptional programs control T(reg) cell function. Nature. 2012;491:554-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Evans CM, Jenner RG. Transcription factor interplay in T helper cell differentiation. Brief Funct Genomics. 2013;12:499-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ouyang W, Beckett O, Flavell RA, Li MO. An essential role of the Forkhead-box transcription factor Foxo1 in control of T cell homeostasis and tolerance. Immunity. 2009;30:358-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hodi FS, Dranoff G. The biologic importance of tumor-infiltrating lymphocytes. J Cutan Pathol. 2010;37(suppl 1):48-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nosho K, Baba Y, Tanaka N, et al. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol. 2010;222:350-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tosolini M, Kirilovsky A, Mlecnik B, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, Th2, Treg, Th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263-1271. [DOI] [PubMed] [Google Scholar]
- 32. Zhang X, Kelaria S, Kerstetter J, Wang J. The functional and prognostic implications of regulatory T cells in colorectal carcinoma. J Gastrointest Oncol. 2015;6:307-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ghosh P, Komschlies KL, Cippitelli M, et al. Gradual loss of T-helper 1 populations in spleen of mice during progressive tumor growth. J Natl Cancer Inst. 1995;87:1478-1483. [DOI] [PubMed] [Google Scholar]
- 34. Sakaguchi S, Miyara M, Costantino CM, Hafler DA. Foxp3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490-500. [DOI] [PubMed] [Google Scholar]
- 35. Wing JB, Sakaguchi S. Multiple treg suppressive modules and their adaptability. Front Immunol. 2012;3:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shurin M, Lu L, Kalinski P, Stewart-Akers AM, Lotze MT. Th1/Th2 balance in cancer, transplantation and pregnancy. Springer Semin Immunopathol. 1999;21:339-359. [DOI] [PubMed] [Google Scholar]
- 37. Jenner RG, Townsend MJ, Jackson I, et al. The transcription factors T-bet and GATA-3 control alternative pathways of T-cell differentiation through a shared set of target genes. Proc Natl Acad Sci U S A. 2009;106:17876-17881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cheng Y, He W, He Y. Gleditsia saponin C induces A549 cell apoptosis via caspase-dependent cascade and suppresses tumor growth on xenografts tumor animal model. Front. Pharmacol. 2018;8:988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee SJ, Park K, Ha SD, Kim WJ, Moon SK. Gleditsia sinensis thorn extract inhibits human colon cancer cells: the role of ERK1/2, G2/M-phase cell cycle arrest and p53 expression. Phytother Res. 2010;24:1870-1876. [DOI] [PubMed] [Google Scholar]
- 40. Yu J, Li G, Mu Y, Zhou H, Wang X, Yang P. Anti-breast cancer triterpenoid saponins from the thorns of Gleditsia sinensis [published online February 23, 2018]. Nat Prod Res. doi: 10.1080/14786419.2018.1443092 [DOI] [PubMed] [Google Scholar]
- 41. Lee SJ, Ryu DH, Jang LC, Cho SC, Kim WJ, Moon SK. Suppressive effects of an ethanol extract of Gleditsia sinensis thorns on human SNU-5 gastric cancer cells. Oncol Rep. 2013;29:1609-1616. [DOI] [PubMed] [Google Scholar]
- 42. Song HK, Lee GS, Park SH, et al. Crotonis fructus extract inhibits 12-O-tetradecanoylphorbol-13-acetate-induced expression of matrix metalloproteinase-9 via the activator protein-1 pathway in MCF-7 cells. J Breast Cancer. 2017;20:234-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tang F, Mei W, Tian D, Huang D. An evidence-based perspective of Coptis chinensis (Chinese goldthread) for cancer patients. In: Cho WCS, ed. Evidence-based Anticancer Materia Medica. Dordrecht, Netherlands: Springer; 2011:111-130. [Google Scholar]
- 44. Choi YY, Kim MH, Han JM, et al. The anti-inflammatory potential of cortex Phellodendron in vivo and in vitro: down-regulation of NO and iNOS through suppression of NF-κB and MAPK activation. Int Immunopharmacol. 2014;19:214-220. [DOI] [PubMed] [Google Scholar]
- 45. Asuzu IU, Gray AI, Waterman PG. The extraction, isolation and identification of the purgative component of Croton penduliflorus seed oil. J Ethnopharmacol. 1988;23:267-271. [DOI] [PubMed] [Google Scholar]
- 46. Hu J, Gao WY, Gao Y, Ling NS, Huang LQ, Liu CX. M3 muscarinic receptor- and Ca2+ influx-mediated muscle contractions induced by croton oil in isolated rabbit jejunum. J Ethnopharmacol. 2010;129:377-380. [DOI] [PubMed] [Google Scholar]
- 47. Xin W, Hong W, Dan L, Fang T. Study on the pharmacological actions of gradated dose of defatted croton seed powder. Tianjin J Tradit Chin Med. 2009;26:72-74. [Google Scholar]
- 48. Klotz LO, Steinbrenner H. Cellular adaptation to xenobiotics: interplay between xenosensors, reactive oxygen species and FOXO transcription factors. Redox Biol. 2017;13:646-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Klotz LO, Sánchez-Ramos C, Prieto-Arroyo I, Urbánek P, Steinbrenner H, Monsalve M. Redox regulation of FoxO transcription factors. Redox Biol. 2015;6:51-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shi F, Li T, Liu Z, et al. FOXO1: another avenue for treating digestive malignancy? Semin Cancer Biol. 2018;50:124-131. [DOI] [PMC free article] [PubMed] [Google Scholar]