Abstract
Background:
Three main meniscal preservation methods have been used over the past decade: cryopreservation, freezing, and freezing with gamma irradiation.
Hypothesis:
All 3 preservation methods will result in similar biomechanical properties as defined by tensile and compression testing.
Study Design:
Controlled laboratory study.
Methods:
A total of 24 human lateral menisci were collected from patients who underwent total knee arthroplasty. Inclusion criteria were patients younger than 70 years with primary unilateral (medial) femorotibial knee osteoarthritis. Each meniscus was divided into 2 specimens cross-sectionally. One specimen was systematically cryopreserved and constituted the control (Cy; –140°C), and the other specimen was used for either the simple frozen group (Fr; –80°C) or the frozen+irradiated group (FrI; –80°C + 25-kGy irradiation). Compression and tensile tests were performed to analyze the elasticity modulus (Young modulus) in compression, the elasticity modulus in tension, the tensile force at failure, and the rupture profile of the tensile stress-strain curve.
Results:
A significant difference in the mean compression elasticity modulus was observed between the Cy and Fr groups (28.86 ± 0.77 vs 37.26 ± 1.08 MPa, respectively; P < .001) and between the Cy and FrI groups (28.86 ± 0.77 vs 45.92 ± 1.09 MPa, respectively; P < .001). A significant difference in the mean tensile elasticity modulus was also observed between the Cy and Fr groups (11.66 ± 0.97 vs 19.97 ± 1.37 MPa, respectively; P = .008) and between the Cy and FrI groups (11.66 ± 0.97 vs 45.25 ± 1.39 MPa, respectively; P < .001). There were no significant differences between the control and study groups in tensile force at failure. The analysis of the stress-strain curve revealed a slow-slope curve with a nonabrupt rupture (ductile material) for the Cy samples versus a clear rupture of the curve for the Fr and FrI samples (more fragile material).
Conclusion:
Cryopreservation allows for more elastic and less fragile tissue compared with simple freezing or freezing plus irradiation.
Clinical Relevance:
The study results exhibit the detrimental effect of simple freezing and freezing plus irradiation on human meniscal mechanical properties. If these effects occur in menisci prepared for allograft procedures, important differences could appear in the graft’s mechanical behavior and thus patient outcomes.
Keywords: meniscus, allograft, conservation, storage, irradiation, cryopreservation, freezing, mechanical properties
The long-term damaging effects of total meniscectomy include pain, potential instability, and osteoarthritis.12,13,16 Meniscal allografts have been advocated to treat these issues and potentially slow the onset of osteoarthritis. Midterm results of this procedure demonstrate significant improvement in patients’ pain scores26,27 as well as increasing the survivorship without failure (85%) of meniscal allografts.10,28 To play its biomechanical role, meniscal allograft tissue must resemble the qualities of native fibrocartilage.25 As such, graft preservation methods play a vital role in the biological, mechanical, and thus clinical success of meniscal allograft techniques.5 Three main meniscal preservation methods have been used over the past decade: freezing, freezing with gamma-irradiation, and cryopreservation.25
In a recent comparative study, Jacquet et al14 observed that cryopreservation does not cause significant histological alterations as compared with fresh tissue. On the other hand, significant differences were only found when comparing between freezing and freezing with irradiation processes with fresh tissue or cryopreserved samples.
These ex vivo microscopic findings need to be validated to estimate their clinical implication. This biomechanical study was designed to compare the mechanical properties of preserved meniscal allografts defined by the elasticity modulus during tensile and compression testing, as there is nothing in the literature to confirm that preserving the meniscal architecture preserves the biomechanical properties of the graft. We hypothesized that all preservation methods would result in similar biomechanical properties.
Methods
After local institutional review board approval, 24 human lateral menisci were collected from patients who underwent total knee arthroplasty from September to October 2017. All patients gave written consent before their inclusion in the study. Inclusion criteria were patients aged <70 years undergoing total knee arthroplasty with isolated medial femorotibial arthritis or femoropatellar and medial femorotibial joint degeneration (but with a lateral femorotibial compartment with Kellgren-Lawrence grade <215) and no prior surgery, trauma, or developmental disease of the operated knee. Magnetic resonance imaging was systematically performed 1 month preoperatively to verify the absence of radiological meniscal lesions. If a grade <1 lesion was detected, the patient was not included in the study. Patient characteristics are summarized in Table 1.
TABLE 1.
Patient Characteristics
| Patient | Age, y | Sex | Weight, kg | Height, cm | Body Mass Index, kg/m2 |
|---|---|---|---|---|---|
| 1 | 63 | Male | 77 | 182 | 23.2 |
| 2 | 65 | Male | 82 | 186 | 23.7 |
| 3 | 61 | Female | 68 | 175 | 22.2 |
| 4 | 64 | Female | 56 | 158 | 22.4 |
| 5 | 66 | Male | 84 | 186 | 24.3 |
| 6 | 67 | Male | 79 | 181 | 24.1 |
| 7 | 60 | Male | 77 | 184 | 22.7 |
| 8 | 59 | Female | 63 | 161 | 24.3 |
| 9 | 64 | Male | 79 | 178 | 24.9 |
| 10 | 62 | Female | 57 | 159 | 22.5 |
| 11 | 63 | Female | 61 | 164 | 24.3 |
| 12 | 61 | Female | 63 | 165 | 22.7 |
| 13 | 67 | Female | 62 | 164 | 23.1 |
| 14 | 63 | Female | 56 | 159 | 22.2 |
| 15 | 62 | Male | 74 | 182 | 22.3 |
| 16 | 69 | Male | 77 | 179 | 24.0 |
| 17 | 68 | Male | 79 | 180 | 24.4 |
| 18 | 62 | Male | 77 | 177 | 24.6 |
| 19 | 62 | Female | 63 | 162 | 24.0 |
| 20 | 64 | Female | 59 | 167 | 21.2 |
| 21 | 67 | Male | 73 | 177 | 23.3 |
| 22 | 68 | Female | 64 | 164 | 23.8 |
| 23 | 67 | Male | 80 | 180 | 24.7 |
| 24 | 61 | Female | 68 | 169 | 23.8 |
Preparation of Samples
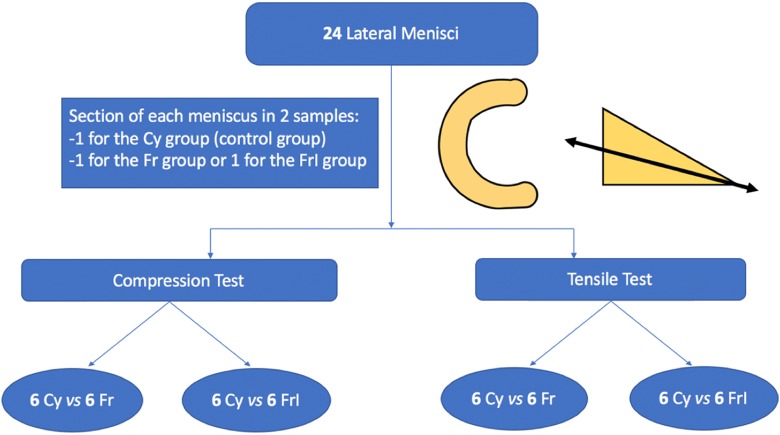
Each meniscus was divided cross-sectionally, extending from the end of the central edge of the peripheral/capsular attachment to obtain 2 similar segments: 1 superior and 1 inferior (Figure 1). One segment was systematically cryopreserved and constituted the control group (Cy), and the other segment was used for either the simple frozen group (Fr) or the frozen+irradiated group (FrI) (Figure 1). The choice of segment between the superior and inferior fragments was made randomly for each group.
Figure 1.
Series flowchart. Cy, cryopreservation; Fr, frozen; FrI, frozen+irradiated.
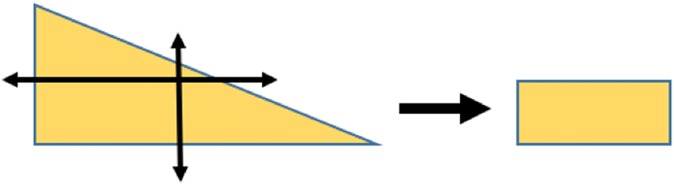
For compression testing, a parallelepiped specimen was harvested from each sample to obtain parallel flat surfaces at the central region of the meniscus (Figure 2). Tensile testing did not require further preparation. Each sample was measured with a digital caliper (Absolute Solar Digimatic Caliper; Mitutoyo) (resolution U = 0.01 mm) and underwent either tensile or compression testing.
Figure 2.
Preparation of samples for compression testing.
Meniscal samples were plunged into a physiological saline solution and then placed in a cryo-kit (8°C) (Macopharma) for transportation to a local tissue bank (<6 hours). Specimens were prepared with the following steps: (1) graft reception in a clean room (controlled atmosphere zone), (2) decontamination of the graft with an antibiotic solution (rifampicin + thiophenicol), (3) rinsing with 0.1 M cacodylate buffer for 5 minutes, and (4) bacteriological sampling. After preparation, different preservation methods were applied:
Cy group: a cryoprotective solution (10% of DMSO + SCOT 30) (Macopharma) was added, and the bag was vacuumed to extract the residual air while progressively decreasing the temperature (starting at –4°C and decreasing at –2°C per minute to –40°C and then –5°C per minute to –140°C). Samples were stored in a nitrogen tank in a vapor phase at –145°C.
Fr group: a simple freezing process was used while progressively decreasing the temperature (starting at –4°C and decreasing at –2°C per minute to –40°C and then –5°C per minute to –80°C).
FrI group: simple congelation with a progressive decrease in temperature (starting at –4°C and decreasing at –2°C per minute to –40°C and then –5°C per minute to –80°C) was performed.
The grafts were then transported in a dry ice–controlled container (stored at –80°C) to be irradiated by gamma rays at a separate facility. The doses received ranged between 22.7 and 27.8 kGy (2.2-2.7 Mrad). After this treatment, the samples were again stored at –80°C until an analysis was undertaken. All samples were stored for at least 1 month before biomechanical testing.
Biomechanical Testing
The compression and tensile tests were performed on a universal testing machine (5566; Instron) with a measurement error in displacement of 0.05%, and the force transducer had a measurement error of 0.2% in tension and compression.
Compression Testing
Each sample was subjected to 5 relaxation compression cycles with a maximum load of 50 N (Figure 3). The speed of progression was 3 mm/min. The stress-strain curve was then obtained using the pretesting relaxed measurements of section and thickness. The elasticity modulus (Young modulus) was calculated in the relaxation elastic phase of the fifth cycle.23
Figure 3.

Compression testing.
Tensile Testing
Each sample was attached to the ends of the tensile testing machine by jaws dedicated to handle soft tissue to prevent inadvertent movement (2716-015; Instron) (maximum force of 30 kN with a jaw face that was 25 mm wide × 57 mm high)21 (Figure 4). The positioning required one-third of the specimen’s length in each jaw, with the central third defining the initial length before tension. An increasing load (10 mm/min) was applied until the specimens failed. A stress-strain curve was obtained for each specimen using the dimensions of the samples. Then, we calculated the Young modulus in the elastic phase of the testing curve. Moreover, tensile force at failure was noted.
Figure 4.

Tensile testing.
Statistical Analysis
Before the initiation of the study, a sample analysis estimated that 6 samples for each group would be necessary to be powered (80%) to distinguish a difference of 5 ± 3–nm Young modulus values. Patient characteristics were expressed using the appropriate descriptive statistics for the type of variables. Descriptive statistics included the mean ± SD for continuous variables. The Student t test was used to compare the distribution of continuous parameters between groups (or the Mann-Whitney test when data were not normally distributed or when the homoscedasticity assumption was rejected). All reported P values are 2-sided, with a significance threshold of 0.05. Statistical analyses were performed using SPSS/JMP software (v 13; IBM/SAS Institute).
Results
Compression Testing
A significant difference in the compression elasticity modulus was observed between the Cy and Fr groups (28.86 ± 0.77 vs 37.26 ± 1.08 MPa, respectively; mean difference, 8.40 ± 1.33 MPa; P < .001). A significant difference in the compression elasticity modulus was also found between the Cy and FrI groups (28.86 ± 0.77 vs 45.92 ± 1.09 MPa, respectively; mean difference, 17.06 ± 1.33 MPa; P < .001) (Table 2).
TABLE 2.
Compression Elasticity Modulus (Young Modulus)
| Mean Difference (95% CI), MPa | P | |
|---|---|---|
| Cryopreserved vs frozen | 8.40 (5.40-11.41) | <.001 |
| Cryopreserved vs frozen+irradiated | 17.06 (14.05-20.07) | <.001 |
Tensile Testing
A significant difference in the tensile elasticity modulus was observed between the Cy and Fr groups (11.66 ± 0.97 vs 19.97 ± 1.37 MPa, respectively; mean difference, 8.31 ± 1.68 MPa; P = .008). A significant difference in the tensile elasticity modulus was also noticed between the Cy and FrI groups (11.66 ± 0.97 vs 45.25 ± 1.39 MPa, respectively; mean difference, 33.59 ± 1.59 MPa; P < .001) (Table 3).
TABLE 3.
Tensile Elasticity Modulus
| Mean Difference (95% CI), MPa | P | |
|---|---|---|
| Cryopreserved vs frozen | 8.31 (4.50-12.12) | .008 |
| Cryopreserved vs frozen+irradiated | 33.59 (29.78-37.39) | <.001 |
With the numbers available, we did not find any significant difference regarding force at failure between the different groups, with the mean difference being 78.33 N between the Cy and Fr groups (P = .186) and 40.50 N between the Cy and FrI groups (P = .199) (Table 4).
TABLE 4.
Force at Failure
| Mean Difference (95% CI), N | P | |
|---|---|---|
| Cryopreserved vs frozen | 78.33 (16.02-131.33) | .186 |
| Cryopreserved vs frozen+irradiated | 40.50 (28.95-107.25) | .199 |
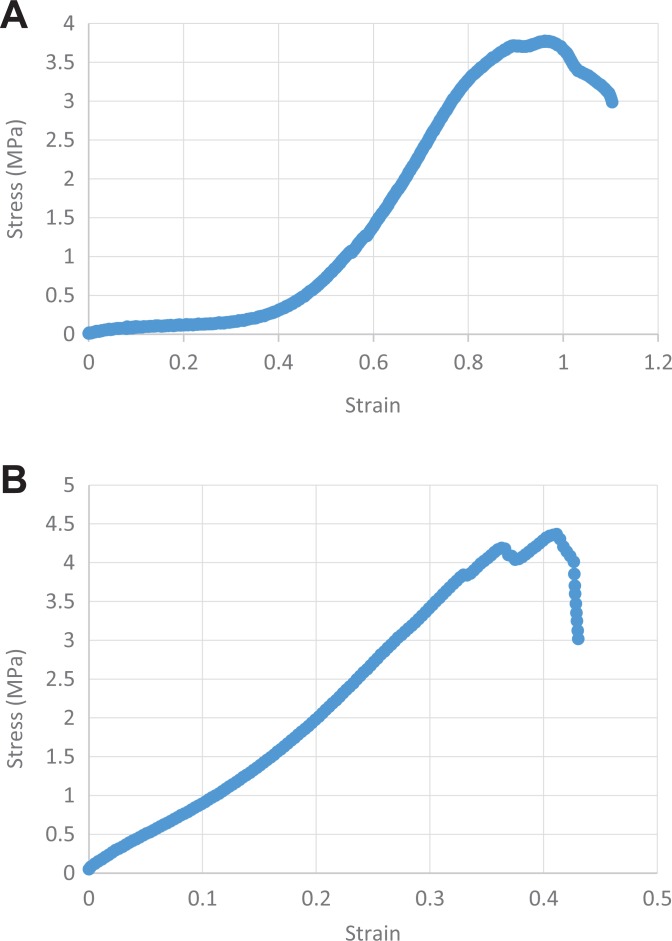
The analysis of the stress-strain curve between groups revealed a slow-slope curve with a nonabrupt rupture (ductile material) for Cy samples (Figure 5A). A clear rupture of the stress-strain curve was observed for Fr and FrI samples (more fragile material) (Figure 5B). In addition, failure seemed to happen more quickly for the Fr and FrI samples than for Cy samples, in which failure was more gradual. The mode of failure in the Fr and FrI samples was most probably caused by delamination of the fibers.
Figure 5.
Stress-strain curve of a (A) cryopreserved sample and (B) frozen sample.
Discussion
The key finding of this study is that cryopreservation allows for more elastic and less fragile tissue than simple freezing or freezing plus irradiation. We rejected our hypothesis that all preservation methods would result in similar biomechanical properties. We observed a significant change in the Young modulus in both compression and tensile testing when comparing specimens from the Cy and Fr groups. These findings were more obvious when comparing differences between the Cy and FrI specimens. All of our findings might be explained by an increased rigidity of meniscal tissue related to the freezing and/or irradiation procedures.
The relatively large variability in tensile and compression stiffness among different preservation processes is multifactorial. In general, the tensile mechanical properties of biological materials depend on the relative contents of major extracellular matrix constituents, the organization of the matrix constituents, and the interactions of these constituents. Prior studies have reported that different preservation methods can alter the meniscal ultrastructure,8,9 which corroborates the differences that we saw between cryopreservation, freezing, and freezing plus irradiation.
While conducting this study, we were also able to examine the meniscal tensile force at failure and the rupture profile of the tensile stress-strain curve. This is also defined as the ability of collagen tissue to absorb energy until it fractures. The tensile force at failure of the Fr and FrI samples was lower than for Cy samples, even if this difference was not statistically significant. This decrease in tensile force at failure could lead to more frequent lesions of Fr and FrI grafts during transverse stresses occurring during flexion-extension movements.17
Our analysis of the stress-strain curves demonstrated that the Cy samples had a very gradual rupture profile, reflecting ductile material, whereas the Fr and FrI samples presented an acute rupture profile, often found in fragile material. This means that Cy samples have the ability to deform without breaking at higher absorbed energy levels than Fr samples and FrI samples during extreme tension.20
No data were found in the literature with regard to estimating the elasticity modulus of fresh menisci (in compression or tension) or the force at failure. Regarding the tensile elasticity modulus, the available data (from frozen specimens) are summarized in Table 5.
TABLE 5.
Previously Published Data for Tensile Elasticity Modulus
| Mean ± SD (95% CI), MPa | |
|---|---|
| Bursac et al2 (2009) Frozen specimens from deceased donors Storage time: not disclosed |
80.9 ± 24.6 (20.3-129.1) |
| Tissakht and Ahmed24 (1995) Frozen specimens from deceased donors Storage time: not disclosed |
72.85 ± 22.91 (3.59-151.80) |
| Ahmad et al1 (2017) Frozen specimens from living donors Storage time: 6 weeks |
54.17 ± 19.54 |
Our values were slightly lower than elastic moduli presented in similar published literature. Those differences can be explained by the fact that most of the studies2,24 utilized samples harvested from deceased donors without any information on sampling sequences and storage time. In our study, all samples were from living donors. To limit the deleterious effects of prolonged exposure to ambient temperature, the samples were immediately placed in a cryo-kit at 8°C, and the preservation process was carried out in less than 6 hours.7 Using tissue from living donors instead of cadaveric tissue avoids bias related to death-induced hypoxia, which could adversely affect the biomechanical properties of tissue.19
In the Ahmad et al1 study, meniscal samples came from a patient with a tumor near the knee, which required a prosthetic replacement. No information was disclosed regarding the possible radiotherapy treatment received, which would likely modify the biomechanical properties of the meniscus. In 3 studies,1,2,24 no information was provided on the freezing process utilized, in particular the rate of descent of temperature, which has been described as a factor that may cause tissue damage.22
For compression testing, the only data identified from the literature come from the Chia and Hull3 study, which described a highly variable Young modulus (0.135-1.130 MPa) according to the preconditioning strain level (3%, 6%, 9%, or 12% strain). In that study, only 10 cadaveric medial menisci were studied (in our study, we only considered lateral menisci). The authors did not indicate the time between death and freezing, the existence of degenerative or traumatic abnormalities, or the freezing process used. These differences may contribute to and explain the greater variability of these published results in comparison with the current study.
One of the limitations of our study is the lack of a fresh tissue group. However, it was impossible to obtain 3 different samples from the same meniscus because the amount of material was insufficient to perform the mechanical tests. Moreover, it is in our mind impossible to harvest, create and attach specimens onto the loading device before ischemia. We did not find solutions in the actual literature to avoid this limitation. Most of the authors froze their specimens before testing and did not estimate fresh tissue properties.
We recognize another limitation of our study: The mean age of the patients from whom specimens were harvested was in comparison older than donors in other studies (63.8 years in our study vs 53.5 years in the literature4). Because of this, menisci evaluated during our analyses might have been altered by aging and degenerative processes. We tried to avoid any limitation related to this methodological bias by excluding menisci with significant degenerative lesions and studying only nonarthritic joints (lateral compartment) from patients suffering from only medial femorotibial degeneration. It has also been described by Bursac et al2 that there are no significant correlations between either the biochemical composition or tensile mechanical properties and the donor age of lateral or medial menisci. Another difficulty encountered in this study was the formation of 2 samples from the same meniscus. Although there are no data in the literature that assert that the superior and inferior parts of a meniscus have different biomechanical properties, we assigned each fragment (superior or inferior) to each group randomly to limit this potential bias.
Finally, our study only approximates the physiological biomechanical environment of the meniscus. The compression tests simulated loading of the meniscus during walking and thus its ability to absorb axial shocks during several loading cycles.6,11 Yet, the compression forces are not distributed uniformly over the entire surface of the meniscus and essentially only concern the middle segment.18 Our tensile tests simulated the transverse stresses applied to the horn-root junction of the meniscus during flexion-extension movements.29 Yet, in vivo tensile strains are predominantly located at the root-horn junction, where the meniscus adheres to the tibial plate.29 We tried to reproduce this anatomic representation by placing the fixed point of the jaws at the ends of the menisci, near the insertion of the roots. During weightbearing and movement, the menisci are normally subjected to a combination of tension, compression, and shear forces. Shear forces could not be evaluated in this study because no device allowed us to reproduce in vitro the impact of these forces. Thus, the ability of a meniscal allograft to withstand these forces after transplantation would appear to be a key element in the successful outcome of such a procedure.
Conclusion
Cryopreserved meniscal sections demonstrated superior stress-strain, tension, and compression biomechanics compared with frozen and frozen+irradiated specimens. Cryopreservation allows the preservation of an elastic and less fragile meniscal allograft compared with the freezing and freezing+irradiation processes.
Acknowledgment
The authors acknowledge Mr Sebastien Bodrero for taking care of all storage/conservation procedures at Etablissement Français du Sang.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: S.P. and M.O. are educational consultants for Newclip. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the Comité de Protection des Personnes Sud-Méditerranée II.
References
- 1. Ahmad S, Singh VA, Hussein SI. Cryopreservation versus fresh frozen meniscal allograft: a biomechanical comparative analysis. J Orthop Surg (Hong Kong). 2017;25(3):2309499017727946. [DOI] [PubMed] [Google Scholar]
- 2. Bursac P, York A, Kuznia P, Brown LM, Arnoczky SP. Influence of donor age on the biomechanical and biochemical properties of human meniscal allografts. Am J Sports Med. 2009;37(5):884–889. [DOI] [PubMed] [Google Scholar]
- 3. Chia HN, Hull ML. Compressive moduli of the human medial meniscus in the axial and radial directions at equilibrium and at a physiological strain rate. J Orthop Res. 2008;26(7):951–956. [DOI] [PubMed] [Google Scholar]
- 4. Cohen J, Bistritz Y, Ashkenazi T. Deceased organ donor characteristics and organ utilization in Israel, 2004-2013. Isr Med Assoc J. 2015;17(6):365–369. [PubMed] [Google Scholar]
- 5. Fabbriciani C, Lucania L, Milano G, Schiavone Panni A, Evangelisti M. Meniscal allografts: cryopreservation vs deep-frozen technique. An experimental study in goats. Knee Surg Sports Traumatol Arthrosc. 1997;5(2):124–134. [DOI] [PubMed] [Google Scholar]
- 6. Fithian DC, Kelly MA, Mow VC. Material properties and structure-function relationships in the menisci. Clin Orthop Relat Res. 1990;252:19–31. [PubMed] [Google Scholar]
- 7. Fölsch C, Mittelmeier W, Bilderbeek U, Timmesfeld N, von Garrel T, Peter Matter H. Effect of storage temperature on allograft bone. Transfus Med Hemother. 2012;39(1):36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gelber PE, Gonzalez G, Lloreta JL, Reina F, Caceres E, Monllau JC. Freezing causes changes in the meniscus collagen net: a new ultrastructural meniscus disarray scale. Knee Surg Sports Traumatol Arthrosc. 2008;16(4):353–359. [DOI] [PubMed] [Google Scholar]
- 9. Gelber PE, Gonzalez G, Torres R, Garcia Giralt N, Caceres E, Monllau JC. Cryopreservation does not alter the ultrastructure of the meniscus. Knee Surg Sports Traumatol Arthrosc. 2009;17(6):639–644. [DOI] [PubMed] [Google Scholar]
- 10. Hannon MG, Ryan MK, Strauss EJ. Meniscal allograft transplantation: a comprehensive historical and current review. Bull Hosp Jt Dis (2013). 2015;73(2):100–108. [PubMed] [Google Scholar]
- 11. Haut Donahue TL, Hull ML, Rashid MM, Jacobs CR. The sensitivity of tibiofemoral contact pressure to the size and shape of the lateral and medial menisci. J Orthop Res. 2004;22(4):807–814. [DOI] [PubMed] [Google Scholar]
- 12. Higuchi H, Kimura M, Shirakura K, Terauchi M, Takagishi K. Factors affecting long-term results after arthroscopic partial meniscectomy. Clin Orthop Relat Res. 2000;377:161–168. [DOI] [PubMed] [Google Scholar]
- 13. Horský I, Huraj E, Huraj E, Sklovský A. [Degenerative changes in the knee joint after meniscectomy]. Acta Chir Orthop Traumatol Cech. 1987;54(6):517–521. [PubMed] [Google Scholar]
- 14. Jacquet C, Erivan R, Argenson J-N, Parratte S, Ollivier M. Effect of 3 preservation methods (freezing, cryopreservation, and freezing + irradiation) on human menisci ultrastructure: an ex vivo comparative study with fresh tissue as a gold standard. Am J Sports Med. 2018;46(12):2899–2904. [DOI] [PubMed] [Google Scholar]
- 15. Kohn MD, Sassoon AA, Fernando ND. Classifications in brief: Kellgren-Lawrence classification of osteoarthritis. Clin Orthop Relat Res. 2016;474(8):1886–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krüger-Franke M, Siebert CH, Kugler A, Trouillier HH, Rosemeyer B. Late results after arthroscopic partial medial meniscectomy. Knee Surg Sports Traumatol Arthrosc. 1999;7(2):81–84. [DOI] [PubMed] [Google Scholar]
- 17. Lento PH, Akuthota V. Meniscal injuries: a critical review. J Back Musculoskelet Rehabil. 2000;15(2):55–62. [DOI] [PubMed] [Google Scholar]
- 18. Makinejad MD, Abu Osman NA, Wan Abas WAB, Bayat M. Preliminary analysis of knee stress in full extension landing. Clinics. 2013;68(9):1180–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Makris EA, Responte DJ, Paschos NK, Hu JC, Athanasiou KA. Developing functional musculoskeletal tissues through hypoxia and lysyl oxidase-induced collagen cross-linking. Proc Natl Acad Sci U S A. 2014;111(45):e4832–e4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nawathe S, Yang H, Fields AJ, Bouxsein ML, Keaveny TM. Theoretical effects of fully ductile versus fully brittle behaviors of bone tissue on the strength of the human proximal femur and vertebral body. J Biomech. 2015;48(7):1264–1269. [DOI] [PubMed] [Google Scholar]
- 21. Ollivier M, Sbihi J, Sbihi A, Pithioux M, Parratte S, Argenson J-N. Ropivacaine alters the mechanical properties of hamstring tendons: in vitro controlled mechanical testing of tendons from living donors. Orthop Traumatol Surg Res. 2017;103(7):1027–1030. [DOI] [PubMed] [Google Scholar]
- 22. Pegg DE. Mechanisms of freezing damage. Symp Soc Exp Biol. 1987;41:363–378. [PubMed] [Google Scholar]
- 23. Sweigart MA, Athanasiou KA. Tensile and compressive properties of the medial rabbit meniscus. Proc Inst Mech Eng H. 2005;219(5):337–347. [DOI] [PubMed] [Google Scholar]
- 24. Tissakht M, Ahmed AM. Tensile stress-strain characteristics of the human meniscal material. J Biomech. 1995;28(4):411–422. [DOI] [PubMed] [Google Scholar]
- 25. Vangsness CT, Garcia IA, Mills CR, Kainer MA, Roberts MR, Moore TM. Allograft transplantation in the knee: tissue regulation, procurement, processing, and sterilization. Am J Sports Med. 2003;31(3):474–481. [DOI] [PubMed] [Google Scholar]
- 26. Verdonk PCM, Demurie A, Almqvist KF, Veys EM, Verbruggen G, Verdonk R. Transplantation of viable meniscal allograft: survivorship analysis and clinical outcome of one hundred cases. J Bone Joint Surg Am. 2005;87(4):715–724. [DOI] [PubMed] [Google Scholar]
- 27. Verdonk PCM, Verstraete KL, Almqvist KF, et al. Meniscal allograft transplantation: long-term clinical results with radiological and magnetic resonance imaging correlations. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):694–706. [DOI] [PubMed] [Google Scholar]
- 28. Wirth CJ, Peters G, Milachowski KA, Weismeier KG, Kohn D. Long-term results of meniscal allograft transplantation. Am J Sports Med. 2002;30(2):174–181. [DOI] [PubMed] [Google Scholar]
- 29. Yao J, Lancianese SL, Hovinga KR, Lee J, Lerner AL. Magnetic resonance image analysis of meniscal translation and tibio-menisco-femoral contact in deep knee flexion. J Orthop Res. 2008;26(5):673–684. [DOI] [PubMed] [Google Scholar]