Short abstract
Background
There is an increasing number of pediatric multiple sclerosis (MS) clinical trials occurring; however, data validating outcome metrics that accurately capture functional disability within pediatric cohorts are limited.
Objective
The aim of this study was to investigate the ability of the MS Functional Composite (MSFC) and Symbol Digit Modalities Test (SDMT) to distinguish functional disability in pediatric MS patients.
Methods
A total of 20 pediatric MS patients and 40 age and sex-matched controls completed the SDMT and MSFC components: a timed 25-foot walk (T25FW); 9-hole peg test (9HPT); and paced auditory serial addition test (PASAT). Z scores for MS patients were created for each test based on control means. MS patients underwent Expanded Disability Status Scale (EDSS) examination.
Results
Pediatric MS patients exhibited low levels of disability on EDSS, median [range]: 1.5 [1.0–3.0]. Compared with controls, MS patients performed significantly lower on SDMT (p = 0.0002) and all MSFC components: T25FW (p = 0.001), 9HPT (p = 0.01), and PASAT (p = 0.004). SDMT and MSFC performance were not correlated with EDSS.
Conclusions
Despite low levels of neurologic disability as measured by EDSS, pediatric patients with MS exhibit impaired performance in leg function, upper limb fine motor function, and auditory/visuospatial processing speeds, supporting the value of the MSFC and SDMT in this population. Longitudinal studies are needed to further validate their utility.
Keywords: Multiple sclerosis, pediatric, MSFC, SDMT, outcome measure
Introduction
Multiple sclerosis (MS) is an inflammatory-mediated demyelinating disease that affects the central nervous system and results in accumulating disability over time.1 Several approaches can be used to quantify and measure disease activity in a patient with MS. These include clinic-based metrics (e.g. annualized clinical relapse rate, timed walking and cognitive tests, and sustained increases in the Expanded Disability Status Scale (EDSS) score) and radiographic metrics (e.g. new/enlarging T2-weighted hyperintense or gadolinium-enhancing lesions on magnetic resonance imaging (MRI), brain volumetrics). These tests are important outcomes utilized within the realm of clinical trials in MS. In clinical practice, these measures can be beneficial in assessing treatment response to a given therapy and defining or prognosticating an individual’s disease course.
Pediatric MS patients often exhibit a more inflammatory disease course with more frequent clinical relapses, a greater burden of infratentorial lesions, and an overall higher brain lesion volume when compared with adult MS patients matched for disease duration.2–4 Despite this, pediatric MS patients often have good recovery with a distinct lack of marked disability progression in the first 10 years of disease,5 as measured by the EDSS. Despite the wide use of the EDSS in adult MS clinical trials, this metric appears less sensitive for measuring functional disability within a younger population as it is heavily weighted toward gait dysfunction with less emphasis on detecting neurocognitive impairments.6
With the recent commencement of clinical trials for pediatric MS therapies, validated outcome measures within this population have become an area of focus. Currently, pediatric MS clinical trials are employing outcome measures that are validated and established within the adult MS literature;7–11 however, these tests have a paucity of data supporting their meaningful use within a pediatric population. Standardized metrics that can reliably quantify physical and cognitive neurologic disability in pediatric MS patientsare needed. Perhaps the most studied outcome assessment in pediatric MS has been the Symbol Digit Modalities Test (SDMT), a test that measures visual processing speed.12–15 There is a paucity of data assessing the utility of the timed-25-foot walk (T25FW), paced auditory serial addition test (PASAT), and 9-hole peg test (9HPT) within a pediatric MS cohort.16 In this study, we aim to characterize the utility of current adult-validated MS assessments in differentiating pediatric MS patients from healthy, non-MS peers, which serves as a first step toward identifying outcome measures that may be more sensitive in detecting the impairments that are noted in pediatric MS.
Methods
Patients and recruitment
Pediatric MS patients were recruited from the Pediatric MS Clinic at the University of Virginia, USA from June 2016 through July 2017. All recruited patients met the 2010 McDonald diagnostic criteria for relapsing–remitting MS17 and the International Pediatric MS Study Group criteria for MS.18 Eligible patients had a current age of 18 years or younger and a disease duration of less than 10 years, with their first MS symptom(s) occurring prior to the age of 18 years. MS patients were excluded if they had experienced an MS relapse within the past 3 months of the study visit.
Control participants were recruited from the local community in addition to the University’s general pediatric and teen health clinics. Controls consisted of healthy, age and sex-matched participants without a medical condition (including neurologic, genetic, or psychiatric) that would preclude them from completing the testing being studied. The primary investigator (JNB) reviewed the medical history of all controls to confirm their eligibility for this study. Controls were screened prior to enrollment to ensure they had no symptoms currently or previously that were suggestive of inflammatory demyelinating disease.
Study procedures
The study was approved by the University of Virginia Institutional Review Board for Health Sciences Research, and all participants provided informed consent and assent, when applicable, prior to the conduct of any study-related procedures.
During a single visit, baseline demographics were recorded, including: age, sex, race/ethnicity. For patients with MS, disease duration (time from the first symptom consistent with MS) and past/current disease-modifying therapies were recorded. In addition, MS patients underwent a full neurologic assessment at the time of the single study visit, which included an EDSS, by the study’s primary investigator (JNB, a Neurostatus-certified pediatric neurologist).19–22 Following this assessment, all participants completed both the MSFC and SDMT in random order.
Multiple Sclerosis Functional Composite (MSFC)
The MS Functional Composite (MSFC) was administered according to manual protocol.23 The MSFC includes two trials of the T25FW, two timed trials of the 9HPT per hand (dominant and non-dominant), and a single trial of the PASAT. The T25FW is an ambulatory test with the score representing the time it took for the participant to walk 25 feet as quickly as possible. The 9HPT assesses upper extremity function and fine motor skills by having the participant pick up wooden pegs one at a time and place them into the nine holes as quickly as possible. Once all pegs are placed, the pegs must be quickly removed one at a time and placed back in the container. The test score reflects the time from start of the test to the time the last peg is placed back into the holding dish. Separate trials are held for the dominant and non-dominant hand. The PASAT assesses auditory processing speed and calculation. During this test, single digits are presented every 3 seconds and the patient must add each new digit to the one immediately prior to it. The score is equivalent to the number of correct answers out of 60 possible answers.
SDMT
The oral SDMT was completed according to manual protocol.24 The oral form (as opposed to the written form) was used to eliminate the impact of fine or gross motor impairments on SDMT performance. For the SDMT, participants use a key that correlates symbols with numbers in order to decode as many lines of symbols as possible within a timed 90-second interval. The participant’s score is equal to the number of symbols correctly decoded into the corresponding number within the time permitted.
Statistical analysis
All statistical analyses were conducted using SAS 9.4 software and R (version 1.1.456) Studio. A 1:2 ratio of pediatric patients with MS to non-MS peers was utilized to increase statistical power. For the T25FW and 9HPT, the scores were averaged between the two trials. All descriptive statistics were calculated and reported using standard appropriate statistics (e.g. means, frequencies, t statistic). Demographic factors were analyzed by cohort. Demographic traits were then compared between these two groups using a Student’s t test and Chi-square test as appropriate for continuous and ordinal variables, respectively. Each functional test was compared between MS patients and healthy, non-MS peers using univariate logistic regression to calculate odds ratio scores. Z scores were created for MS patients on each outcome measure and are representative of the standard deviation from the control group mean. To further analyze the correlation of the EDSS score with performance on the MSFC and SDMT, a Spearman’s correlation was utilized. A two-sided p value <0.05 was defined as statistically significant.
Results
A total of 60 participants completed the single study visit: 20 pediatric MS patients and 40 healthy, age- and sex-matched non-MS peers. The sample of 20 pediatric MS patients represents a convenience sample that could be recruited within the timeline of this particular study. Overall, two pediatric MS patients were excluded from this study as they had experienced a relapse within the 3 months prior to when study testing was to be performed. A single pediatric MS patient declined to participate in the study after being approached for recruitment. The majority of controls recruited came from the local community (95%, n = 38), while a minority were recruited from well-child visits (5%, n = 2). Baseline demographics were not significantly different between groups (Table 1). Pediatric MS patients were on a variety of disease-modifying therapies at the time of this study, though the most common therapies were the injectables (n = 9, 45%). Though MS patients had a median disease duration of 2 years, the EDSS scores for the MS cohort remained low, with a median score of 1.5 (range: 1.0–3.0), corresponding to a minimal level of neurologic disability.
Table 1.
Clinical characteristics of study participants.
| MS | Controls | p value | |
|---|---|---|---|
| Number | 20 | 40 | |
| Sex, n (%) female | 16 (80) | 32 (80) | --- |
| Age current, median (range) | 16 (12–18) | 16 (12–18) | --- |
| Race, ethnicity, n (%) | 0.35 | ||
| White, non-Hispanic | 14 (70) | 29 (72.5) | |
| White, Hispanic | 2 (10) | 2 (5) | |
| White + African | 1 (5) | 3 (7.5) | |
| African | 3 (15) | 3 (7.5) | |
| Asian | 0 (0) | 3 (7.5) | |
| Disease onset (years), median (IQR) | 13 (13–14.5) | ||
| Disease duration (years), median (IQR) | 2 (1–3.5) | ||
| Current DMT, n (%) | |||
| No DMT | 1 (5) | ||
| Interferon beta 1-a | 5 (25) | ||
| Glatiramer acetate | 4 (20) | ||
| Teriflunomide | 1 (5) | ||
| Dimethyl fumarate | 2 (10) | ||
| Fingolimod | 2 (10) | ||
| Natalizumab | 2 (10) | ||
| Rituximab | 3 (15) | ||
| Number of prior DMT attempts, median (range) | 0 (0–4) | ||
| EDSS score, median (IQR) | 1.5 (1.5–2.0) |
DMT: disease-modifying therapy; EDSS: Expanded Disability Status Scale; IQR: interquartile range; MS: multiple sclerosis.
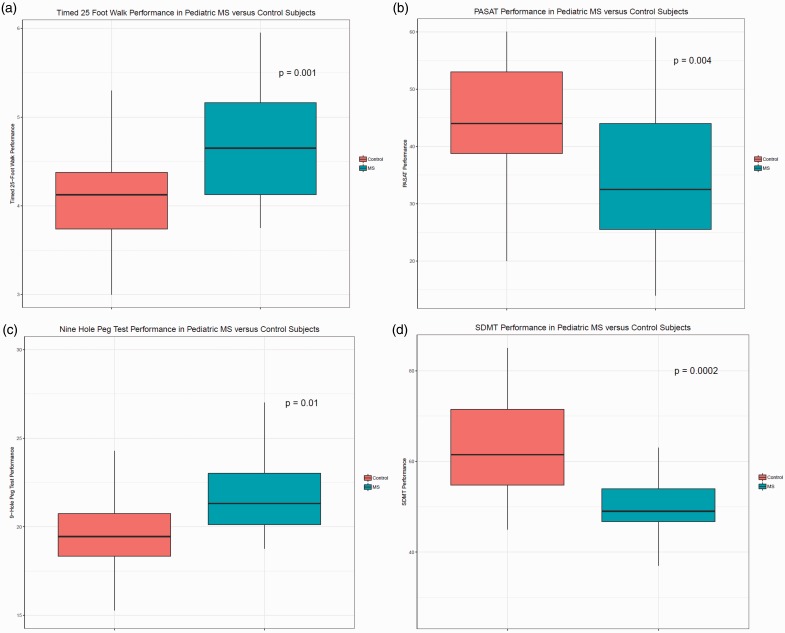
For outcome measure results, a comparison of the raw scores for each individual test is presented in Table 2. The average scores for all metrics assessed were significantly different between the two cohorts (Figure 1). MS patients took longer on the T25FW (p = 0.001) and the 9HPT (p = 0.01). The T25FW analyses, when adjusted for height, were unchanged. MS patients had significantly lower scores on both the timed PASAT (p = 0.004) and the SDMT (p = 0.0002). Of note, the variability among MS patients on the SDMT was quite low compared with controls and MS patient performance on other metrics. Overall, pediatric MS patients (compared with healthy peers) were more likely to perform lower on the T25FW (odds ratio (OR) 4.28; p = 0.004), 9HPT (OR 1.29; p = 0.01), PASAT (OR: 0.93; p = 0.008), and SDMT (OR 0.90; p = 0.002), as reported in Table 3.
Table 2.
Performance (mean ± standard deviation) on MSFC and SDMT in patients with MS versus controls.
| MS | Controls | p-value | |
|---|---|---|---|
| Timed 25-Foot Walk | 4.67 ± 0.69 | 4.11 ± 0.57 | 0.001 |
| Nine-Hole Peg Test | 23.4 ± 5.4 | 19.9 ± 2.9 | 0.01 |
| Nine-Hole Peg Test (dominant hand) | 22.7 ± 4.3 | 19.7 ± 3.2 | 0.01 |
| Nine-Hole Peg Test (non-dominant hand) | 24.5 ± 6.9 | 20.2 ± 2.8 | 0.01 |
| PASAT | 35.6 ± 12.9 | 44.7 ± 10.1 | 0.004 |
| SDMT | 51.4 ± 11.9 | 63.9 ± 11.0 | 0.0002 |
MS: multiple sclerosis; MSFC: Multiple Sclerosis Functional Composite; PASAT: Paced Auditory Serial Addition Test; SDMT: Symbol Digit Modalities Test.
Figure 1.
Performance on MSFC (A–C) and SDMT (D) for pediatric patients with multiple sclerosis versus controls. Boxplots demonstrate median with interquartile range. Whiskers represent the range.
MS: multiple sclerosis; MSFC: Multiple Sclerosis Functional Composite; PASAT: Paced Auditory Serial Addition Test; SDMT: Symbol Digit Modalities Test.
Table 3.
Odds ratios and 95% confidence intervals for tests distinguishing pediatric MS subjects from healthy peers.
| Odds ratio | 95% confidence interval | p value | |
|---|---|---|---|
| Timed 25-Foot Walk | 4.28 | 1.59–11.53 | 0.004 |
| Nine-Hole Peg Test | 1.29 | 1.06–1.58 | 0.01 |
| PASAT | 0.93 | 0.88–0.98 | 0.008 |
| SDMT | 0.90 | 0.84 – 0.96 | 0.002 |
PASAT: Paced Auditory Serial Addition Test; SDMT: Symbol Digit Modalities Test
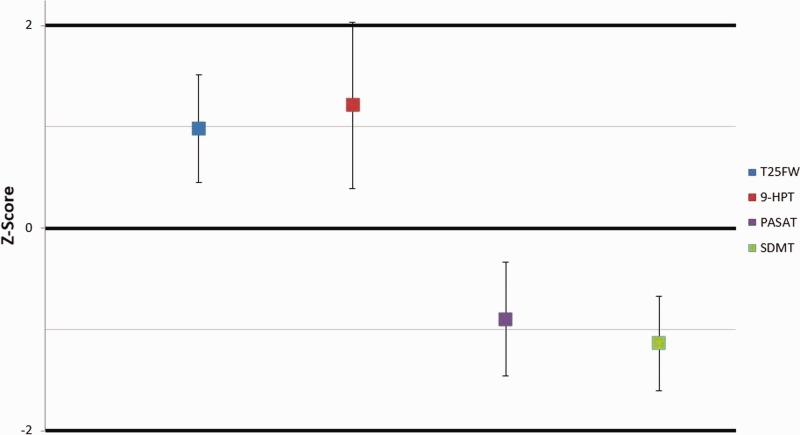
Z scores for each outcome measure are presented in Figure 2. Patients with MS had significantly elevated scores on the T25FW and 9HPT, both tests where a higher score represents a slowed performance. Likewise, the patients with MS received consistently lower scores on the PASAT and SDMT, tests where a lower-numbered score represents a smaller percentage of correct answers.
Figure 2.
Z scores for pediatric patients with multiple sclerosis with 95th percentile confidence intervals. Presented z scores for patients with multiple sclerosis are representative of the standard deviation from the control group mean per outcome measure.
9HPT: 9-hole peg test; PASAT: Paced Auditory Serial Addition Test; SDMT: Symbol Digit Modalities Test; T25FW: timed 25-foot walk.
To further analyze the relationship between the EDSS score and the MSFC and SDMT metrics, correlation testing was performed between MSFC z scores and EDSS in addition to SDMT z scores and EDSS. No significant correlation was found between MSFC or SDMT and EDSS testing. Performance on SDMT and MSFC were strongly correlated (r = 0.61, p = 0.004).
Discussion
Validated metrics of pediatric MS-related functional disability are lacking.6 Clinically-based adult measures (e.g. EDSS) are often used in pediatric patients but are not reliably or routinely employed. Our study is consistent with the current literature, suggesting that EDSS scores in pediatric MS patients typically remain quite low within the first 10 years of disease onset. All patients in this study had a disease duration of 10 years or less, and EDSS scores did not surpass 3.0 in any patient. We hypothesized that pediatric MS patients may have a functionally meaningful disability that is poorly captured by the standardized EDSS assessment, a tool that is heavily weighted in physical function, but less sensitive in measuring cognitive impairment and fine motor skills.
Cognitive impairment occurs in approximately 30–40% pediatric MS patients,25 typically noted at a time when peak performance for academic achievements should be occurring. Thus, it is not surprising that neurocognitive ability plays a highly relevant role (arguably, to a greater extent than physical performance) in the daily function of these patients.26 Perhaps the most frequently observed deficit of cognition in pediatric MS is diminished informational processing speed. SDMT has been considered a valid tool to assess this particular impairment in patients with MS and has been an outcome measure of interest, particularly for pediatric MS clinical trials.12–14,16 Like these previous publications, our data support the use of the SDMT in discriminating between healthy, non-MS peers and pediatric MS patients. Interestingly, performance variability among the MS cohort was relatively low, despite variable disease durations. This likely reflects the impact of pediatric-onset MS on several cognitive functions important for SDMT performance (processing speed, memory, and visual scanning) and the subsequent failure of age-expected maturational growth following MS onset.
There is less literature evaluating the use of other metrics in the pediatric population, including those metrics contained as part of the MSFC. The T25FW and PASAT, though extensively studied within the adult MS population, have not been well studied within a pediatric population. However, Waldman and colleagues did assess its value in 20 pediatric MS patients versus 13 healthy controls and found no discriminatory value between their cohorts.16 Our data supports the individual abilities of the T25FW and PASAT to significantly discriminate pediatric MS patients from age- and sex-matched, non-MS peers. These contrasting findings may be secondary to our study population, which includes a larger age- and sex-matched control population. Consistent with a prior report,16 our data support the utility of the 9HPT to reliably discriminate pediatric MS patients from healthy controls. With a median disease duration of 2 years in this cohort, our collective data would suggest that early impairments in several neurocognitive and physical domains are present and quantifiable via the metrics studied. Our data did not demonstrate a significant correlation in SDMT or MSFC performance and EDSS scores. This would support the idea that the SDMT and MSFC are sensitive at capturing functional impairments that are not captured by EDSS testing.
Our study has some limitations. Firstly, the median patient age at the time of testing was 16 years, and the youngest patient within this cohort was aged 11 years, thus the data may not be reflective of the youngest pediatric MS population (e.g. those that are prepubertal). Additionally, our cohort was one of relatively low physical disability (as measured by the EDSS), though we consider this to be largely consistent with the literature of pediatric MS. Still, given the low and narrow range of our MS patients' EDSS scores, we could not evaluate the utility of the MSFC and SDMT as it relates to pediatric MS patients with higher EDSS scores. Finally, it is important to consider the duration of MS disease when interpreting the results of studies like ours. With a median disease duration of 2 years, our study results may not be readily generalizable to pediatric patients with MS at the time of diagnosis or to those several years post-diagnosis.
In conclusion, validation of and consensus agreement on important outcome measures for future pediatric MS trials remains in question. Both SDMT and MSFC are sensitive in capturing functional disability that is present, yet difficult to characterize, within the pediatric MS population. Our results support the potential utility of these specific metrics for pediatric MS outcome assessment. These findings also suggest that pediatric MS patients experience deficits in processing speed and lower limb/upper limb function that would not be typically captured on the EDSS or a cursory neurologic examination. Our data are supportive of next step longitudinal assessments of these metrics to specifically determine their utility in quantifying accumulating functional disability that is not currently being captured by traditional adult-based metrics. These next step longitudinal studies may help to identify the utility of these measures as reliable, clinic-based metrics with improved sensitivity for quantifying MS-related disability in youth with MS.
Acknowledgments
The authors would like to thank Emily Lethyam, S. Grace Herod, and Casey Engel for their contribution to acquiring data on the study participants.
Conflict of Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This study was funded by the ZiMS Foundation, a private volunteer-based foundation that raises money for research in MS. Student participation (HK) in this study was funded via a grant from the American Academy of Neurology.
References
- 1.Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Ann Rev Neurosci 2008; 31: 247. [DOI] [PubMed] [Google Scholar]
- 2.Ghassemi R, Narayanan S, Banwell B, et al. Quantitative determination of regional lesion volume and distribution in children and adults with relapsing-remitting multiple sclerosis. PLoS One 2014; 9: e85741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verhey LH, Signori A, Arnold DL, et al. Clinical and MRI activity as determinants of sample size for pediatric multiple sclerosis trials. Neurology 2013. ; 81: 1215–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorman MP, Healy BC, Polgar-Turcsanyi M, et al. Increased relapse rate in pediatric-onset compared with adult-onset multiple sclerosis. Arch Neurol 2009; 66: 54–59. [DOI] [PubMed] [Google Scholar]
- 5.Renoux C, Vukusic S, Mikaeloff Y, et al. Natural history of multiple sclerosis with childhood onset. N Engl J Med 2007; 356: 2603–2613. [DOI] [PubMed] [Google Scholar]
- 6.Waldman A, Ness J, Pohl D, et al. Pediatric multiple sclerosis: Clinical features and outcome. Neurology 2016; 87: S74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kornek B, Aboul-Enein F, Rostasy K, et al. Natalizumab therapy for highly active pediatric multiple sclerosis. JAMA Neurol 2013; 70: 469–475. [DOI] [PubMed] [Google Scholar]
- 8.Ghezzi A, Immunomodulatory Treatment of Early Onset MS (ITEMS) Group. Immunomodulatory treatment of early onset multiple sclerosis: results of an Italian co-operative study. Neurol Sci 2005; 26: S183–186. [DOI] [PubMed] [Google Scholar]
- 9.Kornek B, Bernert G, Balassy C, et al. Glatiramer acetate treatment in patients with childhood and juvenile onset multiple sclerosis. Neuropediatrics 2003; 34: 120–126. [DOI] [PubMed] [Google Scholar]
- 10.Mikaeloff Y, Moreau T, Debouverie M, et al. Interferon-beta treatment in patients with childhood-onset multiple sclerosis. J Pediatr 2001; 139: 443–446. [DOI] [PubMed] [Google Scholar]
- 11.Tenembaum SN, Segura MJ. Interferon beta-1a treatment in childhood and juvenile-onset multiple sclerosis. Neurology 2006; 67: 511–513. [DOI] [PubMed] [Google Scholar]
- 12.Akbar N, Signori A, Amato MP, et al. Maturational Trajectory of Processing Speed Performance in Pediatric Multiple Sclerosis. Dev Neuropsychol 2017; 42: 299–308. [DOI] [PubMed] [Google Scholar]
- 13.Bigi S, Marrie RA, Till C, et al. The computer-based Symbol Digit Modalities Test: establishing age-expected performance in healthy controls and evaluation of pediatric patients with MS. Neurol Sci 2017; 38: 635–642. [DOI] [PubMed] [Google Scholar]
- 14.Charvet LE, Beekman R, Amadiume N, et al. The Symbol Digit Modalities Test is an effective cognitive screen in pediatric onset multiple sclerosis (MS). J Neurol Sci 2014; 341: 79–84. [DOI] [PubMed] [Google Scholar]
- 15.Amato MP, Goretti B, Ghezzi A, et al. Neuropsychological features in childhood and juvenile multiple sclerosis: five-year follow-up. Neurology 2014; 83: 1432–1438. [DOI] [PubMed] [Google Scholar]
- 16.Waldman AT, Chahin S, Lavery AM, et al. Binocular low-contrast letter acuity and the symbol digit modalities test improve the ability of the Multiple Sclerosis Functional Composite to predict disease in pediatric multiple sclerosis. Mult Scler Relat Disord 2016; 10: 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krupp LB, Tardieu M, Amato MP, et al. International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler 2013; 19: 1261–1267. [DOI] [PubMed] [Google Scholar]
- 19.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 20.Hirst C, Ingram G, Pearson O, et al. Contribution of relapses to disability in multiple sclerosis. J Neurol 2008; 255: 280–287. [DOI] [PubMed] [Google Scholar]
- 21.Lublin FD, Baier M, Cutter G. Effect of relapses on development of residual deficit in multiple sclerosis. Neurology 2003; 61: 1528–1532. [DOI] [PubMed] [Google Scholar]
- 22.Mowry EM, Pesic M, Grimes B, et al. Demyelinating events in early multiple sclerosis have inherent severity and recovery. Neurology 2009; 72: 602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer JS. Multiple Sclerosis Functional Composite (MSFC): administration and scoring manual. New York: National Multiple Sclerosis Society; 2001. [Google Scholar]
- 24.Smith A. Symbol Digit Modalities Test: Manual. Los Angeles, CA, USA: Western Psychological Services; 1982. [Google Scholar]
- 25.Amato MP, Goretti B, Ghezzi A, et al. Cognitive and psychosocial features in childhood and juvenile MS: two-year follow-up. Neurology 2010; 75: 1134–1140. [DOI] [PubMed] [Google Scholar]
- 26.Banwell BL, Anderson PE. The cognitive burden of multiple sclerosis in children. Neurology 2005; 64: 891–894. [DOI] [PubMed] [Google Scholar]