Abstract
Background
The treatment of acute hepatitis C (AHC) with direct-acting antiviral agents (DAAs) is considered a cornerstone of hepatitis C virus (HCV) elimination strategies, especially in human immunodeficiency virus (HIV)-infected individuals at high risk of onward transmission.
Objective
Optimal treatment regimens and duration for AHC in HIV-coinfected patients remain to be established. Thus, we aimed to evaluate the efficacy and safety of DAA treatment regimens in the setting of AHC.
Methods
All HIV-positive patients with a diagnosis of AHC according to the European AIDS Treatment Network (NEAT) consensus attending our clinic after 2014 were included. DAA treatment regimens and duration were based on current recommendations for chronic hepatitis C (CHC) at treatment initiation.
Results
Thirty-eight HIV/AHC patients (median age 42.0 years), mostly men who have sex with men (92%), were started on interferon-free regimens. HCV-genotype (GT) was predominately GT-1a (65%). The following DAA regimens were prescribed: ombitasvir/paritaprevir/ritonavir/dasabuvir (42%; 16/38), glecaprevir/pibrentasvir (29%; 11/38), sofosbuvir/ledipasvir (13%; 5/38), ombitasvir/paritaprevir/ritonavir (5%; 2/38), grazoprevir/elbasvir (5%; 2/38) and sofosbuvir/velpatasvir (5%; 2/38). All HIV/AHC patients achieved sustained virologic response 12 weeks after end of treatment (SVR12) (100%; 38/38). DAA-related adverse events were rare.
Conclusion
Interferon-free DAA regimens (including 34% pan-genotypic regimens) yielded 100% SVR12 in HIV/AHC individuals if treatment durations similar to CHC are applied.
Keywords: Hepatitis C virus, human immunodeficiency virus, coinfection, men who have sex with men
Key summary
Treatment with interferon (IFN)-free regimens resulted in 100% sustained virologic response rates 12 weeks after end of treatment (SVR12) in 38 human immunodeficiency virus (HIV)-positive individuals with acute hepatitis C virus (HCV) infection, if treatment durations similar to those recommended for chronic HCV are applied.
Introduction
Due to shared routes of transmission, people infected with HIV are also at increasing risk for coinfection with HCV.1 While acute hepatitis C (AHC) might spontaneously clear in men who have sex with men (MSM) with high-risk sexual practices and in persons who inject drugs (PWIDs) in 15–40% of cases, the majority of affected patients will develop chronic hepatitis C (CHC).2 Coinfection with HCV in HIV patients is typically associated with faster progression of liver fibrosis3 and a higher risk for cirrhosis, hepatocellular carcinoma and liver-related mortality.4,5 Thus, according to current European Association for the Study of the Liver (EASL) recommendations for the treatment of hepatitis C,6 IFN-free HCV treatment with direct-acting antiviral agents (DAAs) should be considered without delay in patients with liver fibrosis (≥F2) as well as in individuals at risk of transmitting HCV (i.e. MSM or PWIDs). Several studies reported high efficacy for IFN-free HCV regimens in HIV-infected patients, including sofosbuvir (SOF) plus ribavirin (RBV), SOF plus daclatasvir (DCV), SOF plus ledipasvir (LDV), ritonavir-boosted ombitasvir plus paritaprevir (2D) ± dasabuvir (3D), grazoprevir (GZV) plus elbasvir (EBV), SOF plus velpatasvir (VEL) or glecaprevir (G) plus pibrentasvir (P).7–10
However, existing data on IFN-free regimens for the treatment of AHC within the first six months of infection in the setting of HIV-coinfection is restricted to mostly SOF-based combinations, and studies included only a limited number of patients.11 Two studies investigating SOF/RBV for six (DARE-II) and 12 (SWIFT-C cohort I) weeks reported suboptimal SVR12 in 32% and 59% of HIV/AHC coinfected patients, respectively.12,13 Interestingly, in a small cohort of HIV-positive MSM with recent HCV infection (duration of infection <12 months), the combination of SOF/RBV achieved SVR12 in 92% (11/12).14 However, overall, only 56% (27/48) of HIV-infected individuals with acute or recent HCV infection were successfully treated using SOF/RBV. These results are in contrast to the high efficacy of SOF/LDV in AHC monoinfected patients who achieved 100% SVR12 after a short treatment duration of six weeks.15 A similar study conducted by Rockstroh et al. enrolled 26 HIV/AHC coinfected patients receiving SOF/LDV for six weeks and reported SVR12 rates of only 77% (20/26).16 A longer treatment duration of eight weeks with SOF/LDV was investigated in 27 HIV/AHC coinfected individuals by Naggie et al. (SWIFT-C cohort II) and resulted in an SVR12 rate of 100% (27/27).17 Another study reported comparable SVR12 results (97%; 29/30) following eight weeks of treatment with 3D ± RBV in HIV-positive patients with recent HCV infection.18 Nevertheless, the optimal duration and outcome of IFN-free therapy in HIV/AHC remain unclear, since all conducted studies had heterogeneous inclusion criteria and suffered from small sample size.19 Notably, previous data suggested that even a short duration of HCV infection might induce significant liver fibrosis and substantial liver damage in HIV-positive MSM,20 underlining the urge to initiate treatment early. Moreover, since HIV-positive MSM are at significant risk to transmit HCV,21 early initiation of highly efficient IFN-free DAA regimens might be particularly beneficial in this patient group (‘prevention of transmission’).11
The primary aim of this study was to evaluate the efficacy of different IFN- and RBV-free DAA regimens for AHC in HIV-positive subjects, given for similar durations as for CHC. Furthermore, we investigated the side effects of therapy and the course of liver stiffness before and after HCV treatment.
Patients and methods
Study design and population
All HIV-positive patients with a diagnosis of AHC in accordance with the European AIDS Treatment Network (NEAT) consensus conference22 attending our clinic at the Medical University of Vienna between January 2015 and January 2018 were included in this study. Patients with spontaneous clearance of AHC or chronification of HCV due to delayed treatment initiation or ongoing chemotherapy were not considered for this analysis. Regimens, dosage and duration of treatment for AHC were chosen in accordance with the current recommendations for CHC at the time of treatment initiation.23 However, local restrictions limited reimbursement of IFN-free therapy to patients with significant fibrosis or advanced liver disease until March 2017 for HCV genotype (GT) 1 and GT-4, and until October 2017 for GT-2 and GT-3. Patients eligible for reimbursement had to receive the most favorably priced regimens of those currently available.
Diagnosis of AHC: the NEAT consensus conference22
In accordance with the case definition criteria, AHC was defined as the first six months following HCV exposure. The diagnosis of AHC was established by one of the following criteria:
Preferred criteria (Grade A, Level II)
Positive anti-HCV Immunoglobulin G (IgG) in the presence or absence of a positive HCV Ribonucleic acid (RNA) and a documented negative anti-HCV IgG in the previous 12 months; or
Positive HCV RNA and a documented negative HCV RNA and negative anti-HCV IgG in the previous 12 months.
Alternative criteria (Grade B, Level III)
If historical data are lacking and relevant test results within the past year are unavailable, AHC may be diagnosed if the following criteria are met.
Positive HCV RNA regardless of anti-HCV IgG with:
(a) an acute rise in alanine transaminase (ALT) greater than 10 times the upper limits of normal (ULN); (b) an acute rise in ALT greater than five times the ULN, with documented normal ALT within 12 months. In individuals with a previously high ALT, an acute rise to 3.5 times their previous ALT is acceptable; and
anti-hepatitis A virus Immunoglobulin M (IgM) negative and anti-hepatitis B core IgM antibody negative, and exclusion of other causes of acute hepatitis.22
Assessed parameters
All parameters regarding patient characteristics were collected from the respective medical history. We used the VERSANT® HCV Genotype 2.0 Assay Line Probe Assay (LiPA) (Siemens Healthcare Diagnostics, Tarrytown, NY, USA) and the Abbott RealTime HCV assay (Abbott Molecular, Des Plaines, IL, USA) for HCV GT determination and HCV RNA quantification, respectively. The used assay is capable of quantifying HCV RNA to a lower limit of 12 IU/mL. The Fibrosis-4 (FIB-4)24 score was calculated as age (years) × aspartate transaminase (IU/l) × (platelet count (109/l) × alanine transaminase (IU/l)1/2)–1.
Liver stiffness
Transient elastography was used to measure liver stiffness with the Fibroscan® (Echosens, Paris, France), as described elsewhere.25 If suggested by the device, an XL-probe was used instead of M-probe. The following liver stiffness cut-offs were used for staging liver fibrosis (F): <7.1 kPa for F0/F1; ≥7.1 kPa and <9.5 kPa for ≥F2; ≥9.5 kPa and <12.5 kPa for ≥F3; and ≥12.5 kPa for ≥F4.25 Liver stiffness values ≥9.5 kPa denoted advanced liver fibrosis.
Study endpoints
Efficacy endpoints comprised SVR12. In addition, changes in liver stiffness and transaminases were assessed.
Adverse events (AEs) and serious adverse events (SAEs)
AEs were assessed and graded in accordance with the ‘Table for Grading the Severity of Adult and Pediatric Adverse Events’ provided by the US Department of Health and Human Services.26 The occurrence of one or more of the following events during treatment was considered as SAE: (1) if a patient died; (2) considerable risk of dying defining a life-threatening event; (3) any hospitalization; (4) persistent damage or a disability; (5) pregnancy during treatment with drug-associated congenital anomaly; or (6) other serious events requiring medical treatment but not leading to hospitalization.27
Alcohol intake
Patients were asked for alcohol consumption and advised to remain abstinent from alcohol during treatment. Based on interviews performed at the treatment visits, we are confident that the vast majority of patients remained abstinent from alcohol during the study period.
Statistical analysis
IBM SPSS Statistics 24 (SPSS Inc., USA) and GraphPad Prism 7.03 (GraphPad Software Inc., La Jolla, CA 92037, USA) were both used for statistical analyses. Parametrically and non-parametrically variables were analyzed by calculating the mean ± standard deviation and median (interquartile range), respectively. All nominal parameters were referred to as count of patients with/without (fraction of individuals with/without) the specific characteristic (including 95% confidence interval (CI)). Normality testing was carried out using the D’Agostino and Pearson normality test. Grouped parametric variables were compared using the independent sample t-test, while we used the Mann–Whitney U test for non-parametric variables. Differences in nominal parameters were calculated using the chi-squared test or Fisher’s exact test. For comparing paired measurements, the paired-samples t-test/Wilcoxon signed rank test or McNemar test were applied for continuous and categorical variables, respectively. For all analyses, a P-value of ≤0.05 determined statistical significance.
Results
Patient characteristics
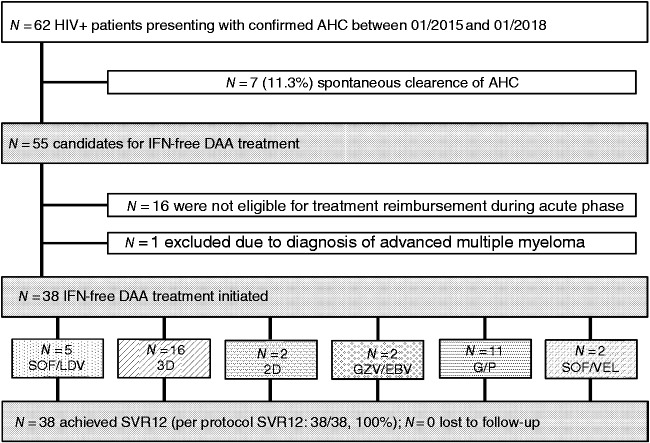
In total, 62 HIV-positive subjects had at least one visit within the first six months after recent exposure to HCV at our clinic between 2015 and 2018, and were classified as AHC infection applying the ‘NEAT criteria’ (A, B and C in 16, 31 and 13 individuals, respectively). Seven patients experienced spontaneous HCV clearance, leaving 55 candidates for IFN-free DAA-based therapy. DAA treatment was not reimbursed by patient insurance in 16 AHC cases due to restrictions set by healthcare providers. Additionally, one patient was excluded due to chemotherapy given for multiple myeloma. Finally, in 38 HIV/AHC coinfected patients, the following IFN- and RBV-free DAA treatments were initiated (Figure 1):
SOF/LDV in n = 5 (13%);
3D in n = 16 (42%);
2D in n = 2 (5%);
GZV/EBV in n = 2 (5%);
SOF/VEL in n = 2 (5%); and
G/P in n = 11 (29%).
Figure 1.
Patient flow chart.
The selection of different regimens was determined by HCV-GT, liver fibrosis stage, as assessed by transient elastography (TE) and availability of as well as reimbursement policies for DAA regimens during the study period.
Overall, the majority of patients were male (95%; 36/38) with a median age of 42 (8.8) years. In 92% (35/38) of AHC cases, MSM high-risk practices were considered as the main risk factor for HCV transmission, while the remaining three patients reported recent intravenous (IV) drug use. Some 21% (8/38) had a history of previous HCV therapy or spontaneous clearance (Table 1).
Table 1.
Patient characteristics at baseline.
| (n = 38) | |
|---|---|
| Epidemiological characteristics | |
| Sex | |
| Male (%, male/all) | 95% (36/38) |
| Age (years) | 42.0 (8.8) |
| Transmission (%, n/all) | |
| MSM | 92% (35/38) |
| PWID | 8% (3/38) |
| HCV reinfection (%, n/all) | 21% (8/38) |
| Weight (kg) | 73.9 ± 11.3 |
| BMI (kg m–2) | 23.4 ± 2.74 |
| Laboratory parameters | |
| Hemoglobin (g dL–1) | 15.1 (1.50) |
| Platelet count (G L–1) | 234 ± 66.3 |
| White blood cell count (G L–1) | 5.88 ± 1.97 |
| Prothrombin time (%) | 86.0 (23.5) |
| Albumin (g dL–1) | 44.9 (5.30) |
| Creatinine (mg dL–1) | 0.97 (0.26) |
| Bilirubin (mg dL–1) | 0.55 (0.35) |
| AST (U L–1) | 83.5 (131) |
| ALT (U L–1) | 169 (385) |
| GGT (U L–1) | 102 (271) |
| FIB-4 | 1.33 (0.75) |
| HIV infection parameters | |
| CD4+ T-lymphocyte count (cells µL–1) | 650 ± 271 |
| HIV-RNA <50 copies mL–1 | 84% (32/38) |
| HIV-RNA <400 copies mL–1 | 89% (34/38) |
| HCV infection parameters | |
| AHC infection to treatment initiation (days) | 118 (88.0) |
| HCV-RNA (log IU mL–1) | 5.41 (1.52) |
| HCV-GT (%, n/all) | |
| 1a | 66% (25/38) |
| 1b | 11% (4/38) |
| 2 | 3% (1/38) |
| 3 | 13% (5/38) |
| 4 | 8% (3/38) |
| Liver stiffness (%, n/all) | |
| F0/F1 (<7.1 kPa) | 47% (18/38) |
| F2 (≥7.1 kPa and <9.5 kPa) | 24% (9/38) |
| F3 (≥9.5 kPa and <12.5 kPa) | 18% (7/38) |
| F4 (≥12.5 kPa) | 11% (4/38) |
Continuous variables are reported as mean ± standard deviation or median (interquartile range).
AHC: acute hepatitis C; ALT: alanine transaminase; AST: aspartate transaminase; BL: baseline; BMI: body mass index; DAA: direct-acting antiviral agent; GGT: gamma-glutamyl transpeptidase; GT: genotype; HCV: hepatitis C virus; MSM: men who have sex with men; SVR12: sustained virologic response at week 12 after end of treatment; TE: transient elastography.
Prior to treatment initiation, median levels of ALT, aspartate transaminase and gamma-glutamyl transpeptidase (GGT) were elevated above the upper limit of normal in all patients, while median bilirubin levels were within the normal range.
Patients starting DAA therapy during the first study period (when reimbursement of IFN-free therapy was still dependent on fibrosis stage) had more frequent fibrosis stages ≥F2 (68% vs. 26%, p = 0.014) and higher transaminase levels (ALT: 257 vs. 108 IU/mL, p = 0.022; GGT: 258 vs. 61, p = 0.008; Supplementary Table).
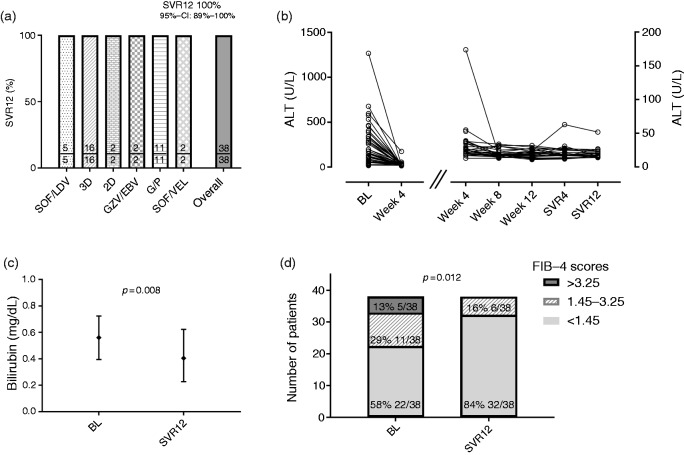
Treatment efficacy
All HIV positive patients receiving IFN-free treatment for AHC achieved SVR12 (100%; 38/38; 95% CI 89–100%; Figure 2(a)); thus, no patient was lost to follow-up (0%; 0/38). Median ALT levels decreased significantly from 169 IU/L (385) to 25 IU/L (15) within four weeks after treatment initiation (p < 0.001) and remained stable at normal levels (Figure 2(b)). Thirty-four patients had information on bilirubin levels 12 weeks after end of treatment. Although the median levels were within normal range before and after therapy, a significant decrease from 0.56 to 0.41 mg/dL (p = 0.008) was observed (Figure 2(c)). Furthermore, the proportions of patients with a FIB-4 index of <1.45, 1.45–3.25 and >3.25 significantly changed from 58% (22/38), 29% (11/38) and 13% (5/38) at baseline to 84% (32/38), 16% (6/38) and 0% (0/38) at SVR12 (p = 0.012), respectively (Figure 2(d)).
Figure 2.
SVR12 according to (a) ART regimen and (b) courses of ALT, as well as (c) bilirubin levels and (d) FIB-4 scores from baseline (BL) to SVR12 after interferon-free DAAs.
AEs
One third of all patients (34%; 13/38) experienced AEs of grade 1 or 2 during surveillance, while no grade 3/4 AEs were observed (Table 2). Fatigue was the most common AE affecting 13% (5/38) of all patients, and frequency distribution of AEs did not vary throughout different treatment regimens. However, a single SAE occurred, since a patient was hospitalized during HCV treatment due to the diagnosis of neurosyphilis to receive IV antibiotics. The SAE was considered to be treatment-unrelated. There were no treatment discontinuations due to AEs.
Table 2.
Adverse events (AEs).
| Events (%, n/all) | All patients (n = 38) |
|---|---|
| AE1 ≤ grade 2 | 34% (13/38) |
| Fatigue | 13% (5/38) |
| Fever | 5% (2/38) |
| Abdominal pain | 3% (1/38) |
| Arthralgia | 8% (3/38) |
| Rash | 8% (3/38) |
| Nausea | 3% (1/38) |
| Diarrhea | 3% (1/38) |
| Laboratory abnormalities | 3% (1/38) |
| AEa > grade 2 | 0% (0/38) |
| SAEb | 3% (1/38) |
| Treatment discontinuation due to AE | 0% (0/38) |
| Death | 0% (0/38) |
| AEs according to DAA regimen | |
| SOF/LDV (n = 5) | 20% (1/5) |
| 3D (n = 16) | 31% (5/16) |
| 2D (n = 2) | 50% (1/2) |
| GZV/EBV (n = 2) | 50% (1/2) |
| SOF/VEL (n = 2) | 50% (1/2) |
| G/P (n = 11) | 36% (4/11) |
AEs including fatigue, fever, abdominal pain, arthralgia, rash, nausea, diarrhea, ALT elevation.
Serious AEs including hospitalization for IV antibiotics (not related).
2D: ritonavir-boosted ombitasvir plus paritaprevir; 3D: ritonavir-boosted ombitasvir plus paritaprevir plus dasabuvir; AE: adverse event; ART: antiretroviral therapy; DAA: direct-acting antiviral agent; EBV: elbasvir; EI: entry inhibitors; G/P: glecaprevir plus pibrentasvir; GZV: grazoprevir; INSTI: integrase inhibitor; LDV: ledipasvir; NNRTI: non-nucleoside reverse-transcriptase inhibitors; N(t)RTIs: nucleos(t)idic reverse transcriptase inhibitors; PI: protease inhibitor; SAE: serious adverse event; SOF: sofosbuvir; VEL: velpatasvir.
Antiretroviral therapy
All patients (100%; 38/38) undergoing treatment for AHC were on antiretroviral therapy (ART) with HIV-1 RNA levels below 50 copies/mL in 84% (32/38) of subjects. However, in seven patients (18%), a switch of ART regimen prior to AHC treatment initiation was required to avoid relevant drug–drug interactions (DDIs). One patient (3%; 1/38) had to discontinue a HIV-protease inhibitor (PI), while the other six (16%; 6/38) had to discontinue non-nucleoside reverse-transcriptase inhibitors (Table 3). ART drug switches were necessary in one patient receiving G/P (9%; 1/11), in four patients receiving 3D (25%; 4/16), in one patient receiving 2D (50%; 1/2) and in one patient receiving SOF/VEL (50%; 1/2).
Table 3.
Management of antiretroviral therapy (ART) prior to and during interferon- and ribavirin-free direct-acting antiviral agent (DAA) therapy.
| Antiretroviral treatment (%, n/all) | All patients (n = 38) |
|---|---|
| PI | 3% (1/38) |
| N(t)RTI | 97% (37/38) |
| NNRTI | 21% (8/38) |
| INSTI/EI | 76% (29/38) |
| ART switch prior to DAA initiation | 18% (7/38) |
| discontinuation of (%, n/all) | |
| PI | 3% (1/38) |
| N(t)RTI | 0% (0/38) |
| NNRTI | 16% (6/38) |
| INSTI/EI | 0% (0/38) |
| Need for ART switch according to DAA regimen | |
| SOF/LDV (n = 5) | 0% (0/5) |
| 3D (n = 16) | 25% (4/16) |
| 2D (n = 2) | 50% (1/2) |
| GZV/EBV (n = 2) | 0% (0/2) |
| SOF/VEL (n = 2) | 50% (1/2) |
| G/P (n = 11) | 9% (1/11) |
2D: ritonavir-boosted ombitasvir plus paritaprevir; 3D: ritonavir-boosted ombitasvir plus paritaprevir plus dasabuvir; ART: antiretroviral therapy; DAA: direct-acting antiviral agent; EBV: elbasvir; EI: entry inhibitors; G/P: glecaprevir plus pibrentasvir; GZV: grazoprevir; INSTI: integrase inhibitor; LDV: ledipasvir; NNRTI: non-nucleoside reverse-transcriptase inhibitors; N(t)RTIs: nucleos(t)idic reverse transcriptase inhibitors; PI: protease inhibitor; SOF: sofosbuvir; VEL: velpatasvir.
Liver stiffness
According to initial liver stiffness assessments, 47% (18/38) had stage F0/1 fibrosis, whereas F2, F3 and F4 fibrosis were observed in 24% (9/38), 18% (7/38) and 11% (4/38), respectively. Additionally, all patients had paired liver stiffness measurements before and after the IFN-free DAA therapy available and a decrease in median levels from 7.3 kPa (4.25) to 5.9 kPa (2.30) (p = 0.003; Supplementary Figure). However, eight patients (21%; 8/38) showed liver stiffness values corresponding to liver fibrosis ≥F2 at SVR12. Interestingly, four of these patients even showed increases in liver stiffness from 1.3 kPa to 3.4 kPa, while four patients had decreases from 3.6 kPa to 11.1 kPa (Supplementary Figure).
Discussion
A total of 38 HIV-positive patients with AHC were treated with IFN- and RBV-free DAA regimens; all 38 patients (100%) achieved SVR12. Thus, 12 weeks of SOF/LDV, 2D, 3D, GZV/EBV, SOF/VEL and eight weeks of G/P treatment seems to be highly effective for the treatment of AHC/HIV coinfection. To our knowledge, this is the first study assessing non-SOF-based IFN- and RBV-free regimens in AHC also including pan-genotypic regimens.
While SOF/RBV is applicable among all HCV-GTs, DDIs with ART are rare13 and induction of resistance-associated variants of HCV is unlikely,28 insufficient efficacy was reported with shortened treatment durations of SOF/RBV in the DARE-II and SWIFT-C study (cohort I) in HIV-positive patients.12,13 Since the reimbursement of SOF/RBV was restricted to patients with advanced liver disease, SOF/RBV was not available for AHC treatment in Austria, despite HIV-positive MSM being at high risk for transmitting HCV.29
SOF/LDV, another highly effective SOF-based treatment regimen, was initially available in Austria in March 2015 for chronic HCV-GT1 monoinfection.30 Within the first year after approval, indications were extended to GT-4, GT-5, GT-6 and to patients with HIV/HCV coinfection. Thus, SOF/LDV was used for 13% (5/38) of our HIV/AHC coinfected cohort for 12 weeks, as recommended for HIV/CHC. Yet, more recent data suggest that HIV-positive patients with certain characteristics, such as treatment-naïve, non-cirrhotic HCV-GT1 and low baseline HCV-RNA level may be eligible for eight weeks of SOF/LDV.31 Accordingly, a shorter treatment duration of six weeks was investigated in AHC-monoinfected patients by Deterding et al.15 and in HIV/AHC by Rockstroh et al.,16 resulting in SVR12 rates of 100% (20/20), but only 79% (20/26) in HIV/AHC, respectively. Of the six patients in the HIV/AHC cohort not achieving SVR12, three patients had virologic relapse within the first four weeks post treatment, while one patient was reinfected and two patients were lost to follow-up.16 Compared with the AHC-monoinfected cohort, patients in the HIV/AHC cohort were all male (100% vs. 60%) and had lower ALT and bilirubin levels (95U/L vs. 225U/L and 0.7 mg/dL vs. 0.8 mg/dL).15,16 Since spontaneous virologic clearance during acute phase is more likely in women than in men, and considering the higher ALT and bilirubin levels suggestive of more recent AHC infection in the HIV-negative cohort, these covariates next to HIV status might explain the differences in SVR rates following abbreviated SOF/LDV treatment. However, patients in our HIV-coinfected cohort were also primarily male (95%) and had lower median ALT levels (169U/L) than patients in the HIV-negative study and still achieved SVR12 of 100%. A consecutive study performed by Naggie et al.17 (SWIFT-C cohort II) provided data on the efficacy of SOF/LDV administered for eight weeks in HIV/AHC and showed a SVR12 rate of 100%. The authors concluded that eight weeks of SOF/LDV for HIV/AHC coinfection is superior when compared with the 60% historical SVR rate achieved with IFN-based regimens, while having a better safety profile.17 In line with this finding, a series of previous studies, including HIV-positive patients with chronic HCV-GT1 and GT4 infection, reported excellent results for a treatment duration of eight weeks using SOF/LDV in patients fulfilling certain baseline criteria.31–33
Nowadays, the vast majority of HIV/CHC can also be treated with pan-genotypic DAA regimens, such as G/P or SOF/VEL, yielding excellent SVR rates.8,9 Thus, G/P and SOF/VEL may also be used for HIV/AHC coinfection, and, indeed, the results of our study with SVR12 in 100% (11/11 and 2/2) of patients, respectively, suggest optimal efficacy in this setting. Large, prospective phase-3 studies on pan-genotypic DAA regimens for AHC are now recruiting and the results are expected in the upcoming years.34
Interestingly, according to baseline TE measurements, only 47% of our patients were classified as F0/F1 fibrosis and 11% presented with values corresponding to F4 fibrosis. However, during the first study period, we had a ‘biased’ selection of patients with higher liver stiffness (≥F2 fibrosis) since reimbursement of DAA therapy was limited to advanced fibrosis with TE values ≥7.1 kPa being accepted. Nonetheless, these unexpectedly high ‘fibrosis stages’ result from the limited capability of TE in the setting of acute hepatitis as hepatic necro-inflammation profoundly impacts on liver stiffness measurement, even if ‘true’ fibrosis is not present. Still, while overall liver stiffness decreased following HCV eradication, eight patients showed liver stiffness values corresponding to liver fibrosis ≥F2 at SVR12. This is in line with the findings of studies using IFN-based regimens35; however, it is unclear whether this observation indicates high rates of liver fibrosis progression in HIV/AHC20, the presence of concomitant liver disease35 or whether these patients just need a longer period of time to resolve hepatic inflammation/fibrosis.36
We favor a strategy of early treatment initiation of IFN- and RBV-free DAA regimens in HIV/AHC coinfected patients, since the observed SVR12 rates of 100% suggest optimal HCV eradication results. Especially in MSM with ongoing high-risk sexual practices and PWIDs, the high efficacy of these regimens clearly supports a ‘treatment as prevention’ strategy to further reduce HCV prevalence in high-risk populations. While shortened SOF/RBV and SOF/LDV durations of six weeks yielded suboptimal results in the setting of HIV-positive individuals with acute HCV coinfection, it remains to be investigated if novel, potentially more effective pan-genotypic regimens such as G/P or SOF/VEL may be used for shorter durations in the context of AHC. However, there is a body of evidence derived from studies in CHC monoinfected patients that treatment durations shorter than eight weeks compromise treatment efficacy.19
Early treatment initiation has been shown to be crucial for reducing the prevalence of hepatitis C among HIV-positive patients with high-risk behavior for spreading HCV.37
In conclusion, given SVR12 rates of 100%, our results strongly support the early use of IFN- and RBV-free DAA regimens for AHC in HIV-coinfected patients. Future studies should evaluate shorter treatment durations and determine the impact of this early ‘treatment as prevention’ strategy in high-risk populations.
Declaration of conflicting interests
David Chromy received travel support from AbbVie and consulted for Gilead and MSD; Mattias Mandorfer received honoraria for consulting and payments for lectures from AbbVie, Bristol-Myers Squibb, Gilead, and Janssen, as well as travel support from AbbVie and Gilead; Theresa Bucsics received travel support from AbbVie and Gilead; Philipp Schwabl received payments for lectures from Roche, Böhringer Ingelheim and Bristol-Myers Squibb and travel support from AbbVie, Gilead, Janssen, and Roche; Bernhard Scheiner received travel support from Gilead; Caroline Schmidbauer has nothing to disclose; Maximilian Christopher Aichelburg received honoraria for consulting and payments for lectures from Gilead and MSD and travel support from AbbVie, Gilead, Janssen and MSD; Peter Ferenci received unrestricted research grants from Gilead, as well as honoraria for board membership and consulting from AbbVie and MSD; Michael Trauner received grants from MSD, honoraria for consulting from AbbVie, Gilead, Janssen and MSD, payments for lectures from Gilead, MSD and Roche, as well as travel support from Gilead; Markus Peck-Radosavljevic received grants from Albireo, Cymabay, Falk, Gilead, Intercept, MSD, and Takeda; advisory board fees from Albireo, Gilead, Falk, Novartis, Intercept, MSD, Phenex and Regulus; and speaker and/or travel fees from Intercept, Gilead, Falk, and MSD. He is also listed as coinventor on patents on medical use of nor-UDCA (filed by the medical University of Graz, Austria); Thomas Reiberger TR received grant support from Abbvie, Boehringer-Ingelheim, Gilead, MSD, Philips Healthcare, Gore; speaking honoraria from Abbvie, Gilead, Gore, Intercept, Roche, MSD; consulting/advisory board fee from Abbvie, Bayer, Boehringer-Ingelheim, Gilead, MSD, Siemens; and travel support from Boehringer-Ingelheim, Gilead and Roche.
Ethics approval
This study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee of the Medical University of Vienna (EK number 1527/2017) on 4 July 2017.
Funding
This work was supported by grants from AbbVie and Gilead to TR.
Informed consent
The need for written informed consent was waived by the local ethics committee due to the retrospective nature of the study and the anonymized reporting of the results.
Supplemental Material
Supplemental Material for High efficacy of interferon-free therapy for acute hepatitis C in HIV-positive patients by David Chromy, Mattias Mandorfer, Theresa Bucsics, Philipp Schwabl, Bernhard Scheiner, Caroline Schmidbauer, Maximilian Christopher Aichelburg, Peter Ferenci, Michael Trauner, Markus Peck-Radosavljevic and Thomas Reiberger in United European Gastroenterology Journal
References
- 1.Boesecke C, Grint D, Soriano V, et al. Hepatitis C seroconversions in HIV infection across Europe: which regions and patient groups are affected? Liver Int 2015; 35: 2384–2391. [DOI] [PubMed] [Google Scholar]
- 2.Seaberg EC, Witt MD, Jacobson LP, et al. Spontaneous clearance of the hepatitis C virus among men who have sex with men. Clin Infect Dis 2015; 61: 1381–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reiberger T, Ferlitsch A, Sieghart W, et al. HIV-HCV co-infected patients with low CD4+ cell nadirs are at risk for faster fibrosis progression and portal hypertension. J Viral Hepat 2010; 17: 400–409. [DOI] [PubMed] [Google Scholar]
- 4.Degos F, Christidis C, Ganne-Carrie N, et al. Hepatitis C virus related cirrhosis: time to occurrence of hepatocellular carcinoma and death. Gut 2000; 47: 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith C, Sabin CA, Lundgren JD, et al. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study. AIDS 2010; 24: 1537–1548. [DOI] [PubMed] [Google Scholar]
- 6.European Association for the Study of the Liver. Electronic address EEE and European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2018. J Hepatol 2018. 69: 461–511. [DOI] [PubMed] [Google Scholar]
- 7.Mandorfer M, Schwabl P, Steiner S, et al. Interferon-free treatment with sofosbuvir/daclatasvir achieves sustained virologic response in 100% of HIV/hepatitis C virus-coinfected patients with advanced liver disease. AIDS 2016; 30: 1039–1047. [DOI] [PubMed] [Google Scholar]
- 8.Kwo PY, Poordad F, Asatryan A, et al. Glecaprevir and pibrentasvir yield high response rates in patients with HCV genotype 1-6 without cirrhosis. J Hepatol 2017; 67: 263–271. [DOI] [PubMed] [Google Scholar]
- 9.Wyles D, Brau N, Kottilil S, et al. Sofosbuvir and velpatasvir for the treatment of hepatitis C virus in patients coinfected with human immunodeficiency virus type 1: an open-label, phase 3 study. Clin Infect Dis 2017; 65: 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rockstroh JK, Lacombe K, Viani RM, et al. Efficacy and safety of glecaprevir/pibrentasvir in patients co-infected with hepatitis C virus and human immunodeficiency virus-1: the EXPEDITION-2 study. Clin Infect Dis 2018. 2: 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinello M, Hajarizadeh B, Grebely J, et al. Management of acute HCV infection in the era of direct-acting antiviral therapy. Nat Rev Gastroenterol Hepatol 2018. 15: 412–424. [DOI] [PubMed] [Google Scholar]
- 12.Martinello M, Gane E, Hellard M, et al. Sofosbuvir and ribavirin for 6 weeks is not effective among people with recent hepatitis C virus infection: the DARE-C II study. Hepatology 2016; 64: 1911–1921. [DOI] [PubMed] [Google Scholar]
- 13.Naggie S, Marks KM, Hughes M, et al. Sofosbuvir plus ribavirin without interferon for treatment of acute hepatitis C virus infection in HIV-1-infected individuals: SWIFT-C. Clin Infect Dis 2017; 64: 1035–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Sayed A, Barbati ZR, Turner SS, et al. Sofosbuvir in the treatment of early HCV infection in HIV-infected men. HIV Clin Trials 2017; 18: 60–66. [DOI] [PubMed] [Google Scholar]
- 15.Deterding K, Spinner CD, Schott E, et al. Ledipasvir plus sofosbuvir fixed-dose combination for 6 weeks in patients with acute hepatitis C virus genotype 1 monoinfection (HepNet Acute HCV IV): an open-label, single-arm, phase 2 study. Lancet Infect Dis 2017; 17: 215–222. [DOI] [PubMed] [Google Scholar]
- 16.Rockstroh JK, Bhagani S, Hyland RH, et al. Ledipasvir-sofosbuvir for 6 weeks to treat acute hepatitis C virus genotype 1 or 4 infection in patients with HIV coinfection: an open-label, single-arm trial. Lancet Gastroenterol Hepatol 2017; 2: 347–353. [DOI] [PubMed] [Google Scholar]
- 17.Naggie S, Fierer DS, Hughes M, et al. 100% SVR with 8 weeks of ledipasvir/sofosbuvir in HIV-infected men with acute HCV infection: results from the SWIFT-C trial (sofosbuvir-containing regimens without interferon for treatment of acute HCV in HIV-1 infected individuals). Hepatology 2017; 66: 113A–113A. [Google Scholar]
- 18.Martinello M, Bhagani S, Gane E, et al. Shortened therapy of eight weeks with paritaprevir/ritonavir/ombitasvir and dasabuvir is highly effective in people with recent HCV genotype 1 infection. J Viral Hepat 2018. 25: 1180–1188. [DOI] [PubMed] [Google Scholar]
- 19.Gaeta GB, Puoti M, Coppola N, et al. Treatment of acute hepatitis C: recommendations from an expert panel of the Italian Society of Infectious and Tropical Diseases. Infection 2017. 46: 183–188. [DOI] [PubMed] [Google Scholar]
- 20.Steininger K, Boyd A, Dupke S, et al. HIV-positive men who have sex with men are at high risk of development of significant liver fibrosis after an episode of acute hepatitis C. J Viral Hepat 2017; 24: 832–839. [DOI] [PubMed] [Google Scholar]
- 21.Mandorfer M, Schwabl P, Steiner S, et al. Advances in the management of HIV/HCV coinfection. Hepatol Int 2016. 10: 424–435. [DOI] [PubMed] [Google Scholar]
- 22.European ATNAHCICP. Acute hepatitis C in HIV-infected individuals: recommendations from the European AIDS Treatment Network (NEAT) consensus conference. AIDS 2011; 25: 399–409. [DOI] [PubMed] [Google Scholar]
- 23.European Association for the Study of the Liver. Electronic address EEE. EASL recommendations on treatment of hepatitis C 2016. J Hepatol 2017; 66: 153–194. [DOI] [PubMed] [Google Scholar]
- 24.Chromy D, Schwabl P, Bucsics T, et al. Non-invasive liver fibrosis assessment and HCV treatment initiation within a systematic screening program in HIV/HCV coinfected patients. Wien Klin Wochenschr 2018; 130: 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castera L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 2005; 128: 343–350. [DOI] [PubMed] [Google Scholar]
- 26.US Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases. Division of AIDS (DAIDS) table for grading the severity of adult and pediatric adverse events. Corrected version 2.1 ed. 2017. Available at: https://rsc.niaid.nih.gov/clinical-research-sites/daids-adverse-event-grading-tables (accessed 27 February 2019).
- 27.Administration USFaD. MedWatch the FDA Safety Information and Adverse Event Reporting Program, https://www.fda.gov/safety/medwatch/howtoreport/ucm053087.htm (2016, accessed 31 January 2019).
- 28.Svarovskaia ES, Gane E, Dvory-Sobol H, et al. L159F and V321A sofosbuvir-associated hepatitis C virus NS5B substitutions. J Infect Dis 2016; 213: 1240–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinello M, Grebely J, Petoumenos K, et al. HCV reinfection incidence among individuals treated for recent infection. J Viral Hepat 2017; 24: 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014; 370: 1889–1898. [DOI] [PubMed] [Google Scholar]
- 31.Ingiliz P, Christensen S, Kimhofer T, et al. Sofosbuvir and ledipasvir for 8 weeks for the treatment of chronic hepatitis C virus (HCV) infection in HCV-monoinfected and HIV-HCV-coinfected individuals: results from the German hepatitis C cohort (GECCO-01). Clin Infect Dis 2016; 63: 1320–1324. [DOI] [PubMed] [Google Scholar]
- 32.Isakov V, Gankina N, Morozov V, et al. Ledipasvir-sofosbuvir for 8 weeks in non-cirrhotic patients with previously untreated genotype 1 HCV infection +/− HIV-1 co-infection. Clin Drug Investig 2018; 38: 239–247. [DOI] [PubMed] [Google Scholar]
- 33.Boerekamps A, Vanwolleghem T, Van der Valk M, et al. Treatment of non-cirrhotic HCV genotype 4 infected patients with 8 weeks of ledipasvir/sofosbuvir: an open-label, multicenter clinical trial. J Hepatol 2018; 68: S105–S364. [Google Scholar]
- 34.Lampejo T, Agarwal K, Carey I. Interferon-free direct-acting antiviral therapy for acute hepatitis C virus infection in HIV-infected individuals: a literature review. Dig Liver Dis 2018; 50: 113–123. [DOI] [PubMed] [Google Scholar]
- 35.Mandorfer M, Steiner S, Schwabl P, et al. Treatment intensification with boceprevir in HIV-positive patients with acute HCV-genotype 1 infection at high risk for treatment failure. Wien Klin Wochenschr 2016; 128: 414–420. [DOI] [PubMed] [Google Scholar]
- 36.Mandorfer M, Kozbial K, Freissmuth C, et al. Interferon-free regimens for chronic hepatitis C overcome the effects of portal hypertension on virological responses. Aliment Pharmacol Ther 2015; 42: 707–718. [DOI] [PubMed] [Google Scholar]
- 37.Martin NK, Jansen K, Boesecke C, et al. Can HCV be eliminated among HIV-infected MSM in Berlin? Modeling a setting with increasing and high treatment rates. J Hepatol 2018; 68: S105–S364. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for High efficacy of interferon-free therapy for acute hepatitis C in HIV-positive patients by David Chromy, Mattias Mandorfer, Theresa Bucsics, Philipp Schwabl, Bernhard Scheiner, Caroline Schmidbauer, Maximilian Christopher Aichelburg, Peter Ferenci, Michael Trauner, Markus Peck-Radosavljevic and Thomas Reiberger in United European Gastroenterology Journal