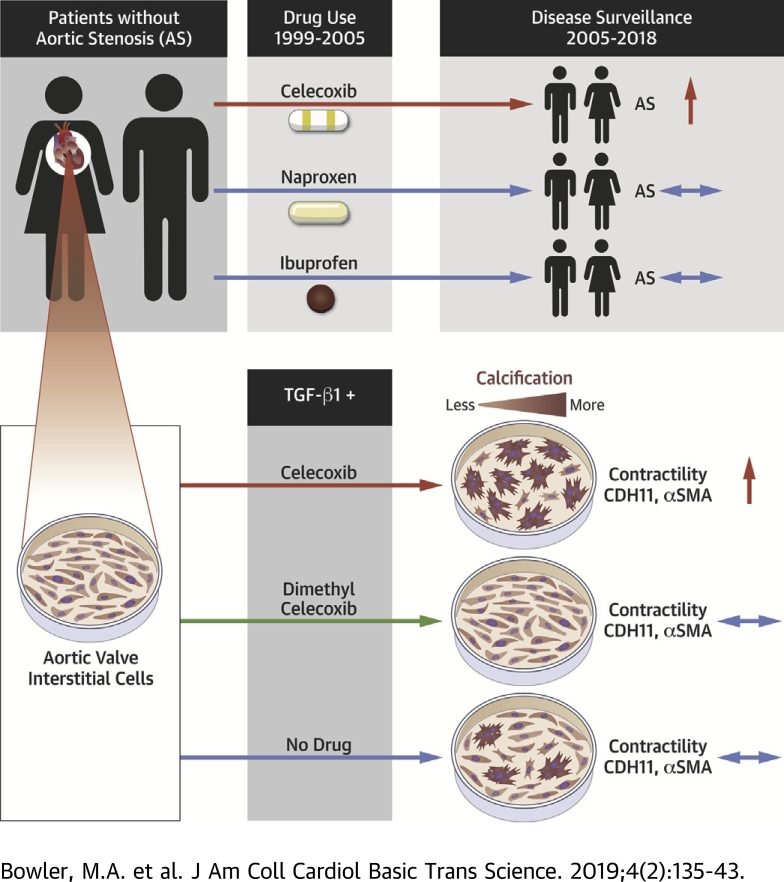
Visual Abstract
Key Words: aortic stenosis, aortic valve, calcification, celecoxib
Abbreviations and Acronyms: ANOVA, analysis of variance; AS, aortic stenosis; AVEC, aortic valve endothelial cell; AVIC, aortic valve interstitial cell; CAVD, calcific aortic valve disease; CDH11, cadherin-11; CN, calcific nodule; COX2, cyclooxygenase-2; EMR, electronic medical record; FDA, Food and Drug Administration; OR, odds ratio; SMA, smooth muscle actin; TGF, transforming growth factor; VUMC, Vanderbilt University Medical Center
Highlights
-
•
Celecoxib use is associated with diagnosis of aortic stenosis in analysis of electronic medical records.
-
•
Celecoxib treatment increases dystrophic calcification of aortic valve interstitial cells in vitro.
-
•
Dimethyl celecoxib, which binds CDH11, prevents TGF-β1–mediated calcification of aortic valve interstitial cells in vitro.
Summary
Calcific aortic valve disease is a progressive fibrocalcific process that can only be treated with valve replacement. Cadherin-11 has recently been identified as a potential therapeutic target for calcific aortic valve disease. The already approved drug celecoxib, a cyclooxygenase-2 inhibitor, binds cadherin-11, and was investigated as a therapeutic against calcific aortic valve disease. Unexpectedly, celecoxib treatment led to hallmarks of myofibroblast activation and calcific nodule formation in vitro. Retrospective electronic medical record analysis of celecoxib, ibuprofen, and naproxen revealed a unique association of celecoxib use and aortic stenosis.
More than 25% of the U.S. population over 65 years of age is affected by calcific aortic valve disease (CAVD) (1). This degenerative disease is the most common cause of aortic stenosis (AS), which eventually requires surgical replacement of the aortic valve because there are no effective pharmaceutical treatments. This lack of medical therapy is a result of our inadequate understanding of the disease mechanism (2). CAVD is believed to be mediated by aortic valve interstitial cells (AVICs), which become activated by transforming growth factor (TGF)-β1 into myofibroblasts (3), characterized by increased contractility, collagen deposition, and expression of α-smooth muscle actin (SMA) and cadherin-11 (CDH11). When these myofibroblasts are subjected to strain, as is normal in the cardiac valve environment, this causes membrane tearing, leading to apoptosis-mediated cell death. This process has been termed the dystrophic pathway of calcification and was evident in 83% of excised human aortic valves (whereas only 13% of those showed osteogenic markers) (4), making dystrophic calcification the most prevalent mechanism of CAVD.
We recently identified and validated CDH11 as a possible therapeutic target for CAVD 5, 6, 7, 8. CDH11 is a mechanosensitive transmembrane cell adhesion protein known to have increased expression in calcified human aortic valves (7), to be increased in the AVICs of the Notch1+/− murine model of CAVD (5), and to be necessary for in vitro formation of the calcific nodules (CNs) characteristic of CAVD (7). Additionally, recent work has shown that blocking CDH11 with a monoclonal antibody in the Notch1+/− model prevents CAVD progression (6). These findings motivated us to evaluate current Food and Drug Administration (FDA)-approved drugs that may block CDH11 activity for CAVD, as the CDH11 antibody research program was recently halted by Roche after disappointing Phase II trials for rheumatoid arthritis. A review of the published reports revealed that celecoxib, brand name Celebrex (Pfizer, New York, New York), and its inactive analog, dimethyl celecoxib, bind CDH11 with high affinity (9). We therefore hypothesized that either of these drugs may prevent CAVD by blocking the homotypic CDH11 bonds between neighboring cells.
To evaluate this hypothesis, we treated porcine AVICs and aortic valve endothelial cells (AVECs) with celecoxib or dimethyl celecoxib. Cells were also treated with TGF-β1 to biochemically induce myofibroblast differentiation. Cells were then subjected to well-established functional assays of CAVD such as CN formation 5, 7, 10, 11, 12 and collagen gel contraction, as well as evaluated for expression of myofibroblast markers α-SMA and CDH11. To assess clinical relevance, we performed a retrospective analysis of celecoxib use and AS incidence in the electronic medical record (EMR) from Vanderbilt University Medical Center (VUMC).
Methods
In vitro experiments and statistical analysis
Porcine aortic valve cells were isolated as previously described 7, 13 and used between passages 3 and 11. Cells were evaluated with a combination of molecular and functional assays in order to understand the role of treatment with celecoxib, dimethyl celecoxib, and TGF-β1 in their propensity to calcify; details of the following in vitro assays are in the Supplemental Appendix. The nodule assay allows for rapid screening of potential drug strategies that may prevent dystrophic calcification in vitro 5, 7, 12. Briefly, cells were plated onto pronectin (AVICs) or collagen IV (AVECs) Flexcell plates (Flexcell International, Burlington, North Carolina), then treated with TGF-β1, and subsequently strained at 15% using the Flexcell Tension system, as previously described 10, 11. In a separate cohort, AVICs were treated with conditioned medium harvested from AVEC cultures after strain. AVICs were also evaluated for contractility using a free-floating collagen gel system in which cells were plated onto gels and imaged over time to quantify the gel area. Western blots and immunofluorescence were used to evaluate expression of myofibroblast markers CDH11 and α-SMA after various treatments. In all cases, cells were plated simultaneously with celecoxib (Tocris 3786, Tocris Bioscience, Bristol, United Kingdom), dimethyl celecoxib (Sigma-Aldrich D7196, Sigma-Aldrich, St. Louis, Missouri), or no drug to allow for interactions with CDH11 before homotypic bonds were formed. 10 μmol/l celecoxib and dimethyl celecoxib was chosen to match the plasma concentration found after typical doses of celecoxib in humans (9). For all experiments, n ≥ 3; more detailed methodology can be found in the Supplemental Appendix. All groups were compared with analysis of variance (ANOVA) in SigmaPlot software version 11.0 (Systat Software, San Jose, California), and a p value <0.05 was considered significant. Normality (Shapiro-Wilk) and equal variance were tested. Normal datasets with equal variance were analyzed via 1-way ANOVA with pairwise multiple comparisons made using the Holm-Sidak post hoc testing method. Non-normal datasets were analyzed via Kruskal-Wallis 1-way ANOVA on ranks with pairwise multiple comparisons made using Dunn’s post hoc testing method. In vitro data are presented as mean ± SEM.
Clinical data and statistical analysis
AS patients 60 to 89 years of age on January 27, 2018, were identified using the Synthetic Derivative, a de-identified version of VUMC’s EMR containing >2.5 million unique records. Date gating and clinical covariates were identified a priori on the basis of celecoxib’s approval history and known risk factors for AS, respectively. Ibuprofen and naproxen were chosen for comparison due to their similar indications and pattern of use 14, 15, and their previous use as comparators for celecoxib in the PRECISION (Prospective Randomized Evaluation of Celecoxib Integrated Safety vs Ibuprofen or Naproxen) trial (16). Detailed cohort definition criteria can be found in the Supplemental Appendix. Mean available follow-up was 10.16 ± 3.14 years. Unadjusted odds ratios (ORs) and differences between cases and controls were calculated using the Fisher exact and Mann-Whitney U tests, respectively. Given the significant association of several clinical variables with incident AS in our preliminary models, a multivariable logistic regression based on age, sex, body mass index, hypertension, diabetes, and drug use was used to calculate adjusted ORs and p values (17). All analyses were performed using the statistical programming language R, version 3.4.4 (18). Clinical data are presented as mean ± SD. Use of the Synthetic Derivative is classified as nonhuman research by Vanderbilt University's institutional review board, and approval was given for this study.
Results
In vitro dystrophic calcification analysis
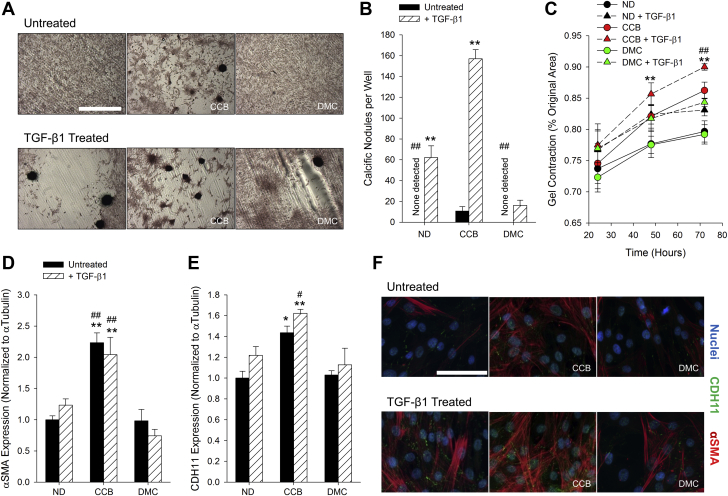
Alizarin Red staining of calcium shows the characteristic rounded morphology of CNs formed by AVICs (Figure 1A). As expected, treatment with TGF-β1 increases the number of CNs under all pre-treatment conditions (Figures 1A and 1B). Unexpectedly, celecoxib pre-treatment causes a greater increase in CN number, whereas dimethyl celecoxib pre-treatment, as hypothesized, prevents TGF-β1–induced CN formation (Figures 1A and 1B). A gel contraction assay reveals that celecoxib-treated AVICs appear more contractile than their untreated or dimethyl celecoxib–treated counterparts, though not significantly (Figure 1C). TGF-β1 treatment increases contractility as well and compounds with celecoxib treatment to cause significantly more contraction than the no drug pre-treated with TGF-β1 (Figure 1C). Expression of myofibroblast markers α-SMA and CDH11 were evaluated by Western blot (Figures 1D and 1E, Supplemental Figure S1) and immunofluorescence (Figure 1F, Supplemental Figure S2). Densitometry demonstrates a significant increase in both markers only in the celecoxib pre-treated AVICs (Figures 1D and 1F). We observe no calcification of AVECs alone, as was expected, and very little calcification in AVICs treated with AVEC conditioned medium (Supplemental Figure S3).
Figure 1.
Dimethyl Celecoxib Prevents CN Formation in AVICs but Celecoxib Promotes CN Formation Through Myofibroblast Induction
(A) Cyclic biaxial strain and TGF-β1 induce CN formation, identified by Alizarin Red staining. (B) Treatment with celecoxib increases the number of CNs formed in the untreated and TGF-β1–treated cases. Dimethyl celecoxib treatment reduces the number of TGF-β1–induced CNs. (C) TGF-β1 treatment increases contractility. Celecoxib pre-treatment also increases contractility to the level of ND + TGF-β1. Treatment with celecoxib increases expression of α-SMA (D and F) and CDH11 (E and F). n ≥ 3. *p < 0.05 versus ND, #p < 0.05 versus ND + TGF-β1, **p < 0.001 versus same pre-treatment, ##p < 0.001 versus ND + TGF-β1. Scale bars indicate 1 mm (A) and 100 μm (F). AVIC = aortic valve interstitial cell; CCB = celecoxib; CN = calcific nodule; DMC = dimethyl celecoxib; ND = no drug; TGF = transforming growth factor.
Retrospective clinical analysis
The results obtained from these in vitro experiments led us to investigate possible clinical significance of celecoxib use. Approximately 8,300 deidentified patient records from VUMC met inclusion criteria and were queried for possible association of AS with celecoxib, naproxen, or ibuprofen use (Figure 2). In unadjusted analyses, celecoxib use is associated with an increased odds of developing AS (OR: 1.36; 95% confidence interval: 1.11 to 1.67; p = 0.003) (Table 1). After adjustment, this association persists (adjusted OR: 1.24; 95% confidence interval: 1.00 to 1.53; p = 0.046). Identical analyses were performed with ibuprofen and naproxen, and no associations were found. To assess the consistency of this cohort with those in other celecoxib studies, we cursorily examined unadjusted ORs of celecoxib with myocardial infarction and ischemic stroke, and found no association, as has been reported previously (Supplemental Table S1) (16).
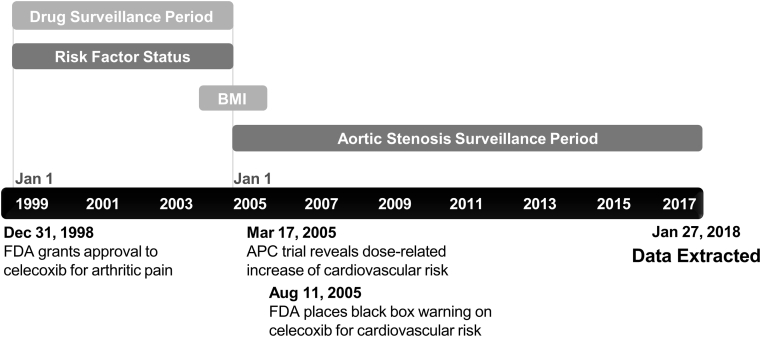
Figure 2.
Retrospective Cohort Study Design
The retrospective clinical analysis described here was designed based on the approval and clinical trial history of celecoxib. Celecoxib was approved on December 31, 1998, and clinical trial results revealed a potential cardiovascular risk in early 2005, defining our drug surveillance period. Body mass index (BMI) data were collected within 1 year of January 1, 2005. The aortic stenosis surveillance period extended from January 1, 2005, to January 27, 2018. APC = Adenoma Prevention With Celecoxib; FDA = Food and Drug Administration.
Table 1.
Celecoxib Use Is Associated With AS
| Cases | Controls | Unadjusted OR (95% CI) | Unadjusted p Value | Adjusted OR (95% CI) | Adjusted p Value | |
|---|---|---|---|---|---|---|
| Celecoxib | (n = 574) | (n = 6,397) | ||||
| Male | 57.49 (330) | 43.71 (2,796) | 1.73 (1.46–2.06) | <0.001 | 1.70 (1.43–2.03) | <0.001 |
| Age, yrs | 76.70 ± 4.80 | 72.89 ± 7.91 | 1.06 (1.05–1.07) | <0.001 | 1.06 (1.05–1.07) | <0.001 |
| BMI, kg/m2 | 30.67 ± 6.94 | 28.04 ± 6.05 | 1.02 (1.01–1.03) | 0.002 | 1.02 (1.01–1.03) | 0.003 |
| Hypertension | 54.88 (315) | 40.24 (2,574) | 1.81 (1.52–2.14) | <0.001 | 1.42 (1.19–1.70) | <0.001 |
| Type 2 diabetes | 24.74 (142) | 15.85 (1,014) | 1.75 (1.43–2.13) | <0.001 | 1.35 (1.09–1.67) | 0.006 |
| Celecoxib use | 23.34 (134) | 18.21 (1,165) | 1.36 (1.11–1.67) | 0.003 | 1.24 (1.00–1.53) | 0.046 |
| Ibuprofen | (n = 427) | (n = 4,724) | ||||
| Male | 57.14 (244) | 44.86 (2,119) | 1.64 (1.34–2.00) | <0.001 | 1.59 (1.30–1.95) | <0.001 |
| Age, yrs | 76.86 ± 7.82 | 73.15 ± 7.87 | 1.06 (1.05–1.07) | <0.001 | 1.06 (1.05–1.07) | <0.001 |
| BMI, kg/m2 | 30.83 ± 6.89 | 29.93 ± 6.94 | 1.02 (1.00–1.03) | 0.011 | 1.02 (1.01–1.04) | 0.006 |
| Hypertension | 54.33 (232) | 40.60 (1,918) | 1.74 (1.43–2.12) | <0.001 | 1.40 (1.13–1.72) | 0.002 |
| Type 2 diabetes | 25.76 (110) | 16.49 (779) | 1.76 (1.40–2.21) | <0.001 | 1.38 (1.08–1.77) | 0.010 |
| Ibuprofen use | 26.46 (113) | 30.25 (1,429) | 0.83 (0.66–1.04) | 0.102 | 0.98 (0.78–1.23) | 0.852 |
| Naproxen | (n = 509) | (n = 5,342) | ||||
| Male | 57.37 (292) | 45.17 (2,413) | 1.63 (1.36–1.96) | <0.001 | 1.55 (1.29–1.87) | <0.001 |
| Age, yrs | 76.50 ± 7.86 | 73.07 ± 7.88 | 1.06 (1.04–1.07) | <0.001 | 1.05 (1.04–1.07) | <0.001 |
| BMI, kg/m2 | 30.58 ± 6.81 | 29.83 ± 6.96 | 1.01 (1.00–1.03) | 0.021 | 1.02 (1.00–1.03) | 0.025 |
| Hypertension | 55.80 (284) | 40.21 (2,148) | 1.88 (1.56–2.25) | <0.001 | 1.55 (1.28–1.88) | <0.001 |
| Type 2 diabetes | 24.75 (126) | 16.17 (864) | 1.71 (1.38–2.11) | <0.001 | 1.35 (1.07–1.88) | 0.010 |
| Naproxen use | 16.50 (84) | 18.12 (968) | 0.89 (0.70–1.14) | 0.364 | 0.92 (0.71–1.18) | 0.498 |
Values are % (n) or mean ± SD. Odds ratios (ORs) for age and BMI are reported per unit increase.
AS = aortic stenosis; BMI = body mass index; CI = confidence interval.
Discussion
Our investigation was motivated by the need for pharmaceutical alternatives to aortic valve replacement and the unique ability of celecoxib and dimethyl celecoxib to bind CDH11, a recently identified target for CAVD and AS. We have previously demonstrated that targeting CDH11 in vivo prevents the pathological increase in aortic jet maximum velocity (6), a clinical metric used to define the severity of AS. Others have found that celecoxib and its inactive analog, dimethyl celecoxib, were able to bind CDH11 (9), presenting an opportunity to exploit the off-target effects of celecoxib to treat CAVD with an already FDA-approved drug. The main finding of this work was unexpected. Primarily, celecoxib, the FDA-approved drug we anticipated being a potential therapeutic for CAVD, causes calcification in vitro and is associated with AS in patients. Conversely, the inactive analog dimethyl celecoxib showed the expected benefit of CDH11 blockade. Although further studies of dimethyl celecoxib are warranted, the new risk of celecoxib, a commonly prescribed drug, is the focus of our studies and discussion.
Celecoxib promotes myofibroblast differentiation and calcification in vitro
Although AVICs are widely believed to be the cells driving CAVD, we evaluated the effects of celecoxib and dimethyl celecoxib on both AVICs and AVECs, as well as effects on AVICs from drug-treated AVEC conditioned medium. Because AVECs showed no response to celecoxib or dimethyl celecoxib (Supplemental Figure S3), we focused on direct effects of the drugs on AVICs. We show here that celecoxib causes an increase in both α-SMA and CDH11 expression, pointing to an induction of the myofibroblast phenotype, which then leads to CN formation. Conversely, although dimethyl celecoxib treatment does not appear to change the AVIC phenotype—contractility, α-SMA expression, and CDH11 expression remain unchanged—it does significantly reduce CN formation. This supports our hypothesis that this beneficial effect is likely the result of dimethyl celecoxib preventing homotypic interactions of CDH11 between neighboring cells. With CDH11’s cell–cell adhesions blocked, the tension between AVICs is reduced, which prevents the membrane tearing and subsequent apoptosis-mediated cell death that leads to CN formation. Given that activity in the cyclooxygenase-2 (COX2) axis is the key difference between celecoxib and dimethyl celecoxib, we attribute celecoxib’s promyofibroblast effect to COX2 inhibition, which supports the notion of a protective role for COX2 in dystrophic CN formation.
Celecoxib is associated with AS
This is not the first investigation into the impact of COX2 inhibitors on heart disease. Most COX2 inhibitors were pulled from the market because of adverse cardiovascular effects by 2005 19, 20. Celecoxib had not displayed the same adverse effects and has retained FDA approval; however, the FDA mandated a cardiovascular safety trial. This study showed no increased risk of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke with celecoxib use when compared with ibuprofen or naproxen (16). However, these outcomes focus on acute, relatively short-term, and thrombotic events, and do not include valvular pathologies. On the basis of our in vitro data, rather than an acute thrombotic event, we suspected an increase in long-term risk of AS in these patients. Therefore, we tested our hypothesis using longitudinal clinical data. In retrospective analysis, we observed a unique association of celecoxib use with the presence of AS. The association of celecoxib and AS remained significant when adjusted for age, sex, body mass index, and known AS risk factors 17, 21. The same is not observed in patients taking ibuprofen or naproxen, which have comparable major indications and clinical uses to celecoxib (acute pain, inflammatory or rheumatoid disorders, osteoarthritis, and primary dysmenorrhea), but inhibit COX1 in addition to COX2. This suggests there is something unique about celecoxib or selective COX2 inhibition that is associated with AS.
The unknown role of COX2 in AS
COX2 expression is increased in calcified human aortic valves (22), yet there are conflicting data as to whether it is a disease initiator or part of a protective response. COX2 inhibition has been shown in the Klotho-deficient mouse to lead to decreased aortic valve calcification via an osteogenic mechanism assessed by cell- and tissue-level pathology (22). Although calcification is the most common pathology finding in AS, clinical decision making is driven by functional measures (such as aortic jet maximum velocity). However, clinical studies of celecoxib have not yet focused on valvular function or pathology, or long-term effects (>4 years) of the drug 16, 19, 23. We have shown that COX2 inhibition can promote CN formation in porcine AVICs through the more prevalent dystrophic pathway of calcification, and a significant association between celecoxib use and AS in humans.
Collectively, these findings support further investigation of celecoxib or COX2’s role in other models of CAVD and AS, such as Notch1+/− or Apoe−/− mice, to clarify whether COX2 is protective or disease-driving. COX2 also plays a key role in modulating various immune processes, and investigating the impact of celecoxib in immunocompetent CAVD models may provide new insights into the in vivo mechanisms implicated. Clinically, a multifaceted retrospective study of functional and imaging-defined AS progression with celecoxib use may further clarify this risk. It is still unclear whether celecoxib introduces novel risk or is a modifying risk factor in those already at risk. Additionally, although the efficacy of targeting CDH11 in vivo has been shown (6), further studies of dimethyl celecoxib in relevant murine models and eventually humans could reveal a novel therapeutic for CAVD.
Study limitations
Our in vitro experiments rely on porcine cells, which are a standard model for CAVD research and are potentially better examples of healthy valve cells than samples from humans, because most heart valve donors are not free of other cardiovascular pathologies. Future work in human AVICs or a variety of in vivo mouse models could confirm our proposed mechanism.
Although we have imposed strict time gates and cohort selection criteria, retrospective EMR study does not allow for controlled assessment or follow-up of study participants. The retrospective nature of the clinical analysis does not allow us to quantify dosage of patients included, but contemporary published reports conclude that >80% of users at the time had standard 200 mg prescriptions 14, 15. It is difficult to confidently rule in or rule out AS for patients in this large deidentified cohort of clinical records, but we tried to use definitions that would increase the accuracy of these designations. We cannot rule out that CAVD may have been present in some individuals during the drug exposure period. In addition, the retrospective nature of the study precludes conclusions about causality of CAVD. The various differences between the celecoxib and control cohorts are adjusted for when possible, but may imply additional underlying differences that are better controlled in a randomized controlled trial. For example, in our preliminary models, we assessed the impact of hyperlipidemia on AS incidence, but it was not significantly associated with AS and had no effect on the model. This may be due to incomplete retrospective data or the lack in the era queried of consistent laboratory values such as lipoprotein(a), which has since proven a reliable biomarker for the association of dyslipidemia with AS (21). Additionally, valve morphology is a highly prevalent risk factor for CAVD, but it could not be accurately assessed in this retrospective study without consistent imaging for all subjects. An echo-driven study may provide more clarity on the impact of celecoxib in patients with a bicuspid aortic valve. Reanalysis of the PRECISION trial data may be an effective option for assessing the potential risk outlined in this analysis.
Conclusions
Overall, these data suggest that celecoxib use is associated with the development of CAVD. Although further studies are necessary, it is likely that dimethyl celecoxib or a monoclonal antibody against CDH11 would be safer therapeutic options than celecoxib to pursue for patients with CAVD or other CDH11-mediated diseases. Considering the indications for celecoxib, these results suggest that physicians must carefully balance the risks of COX1 inhibition in the gut with those of COX2-specific inhibition in the aortic valve when choosing a pain control regimen, and use celecoxib with caution in elderly patients with risk factors for AS.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: A recent cardiovascular safety trial showed that celecoxib is noninferior to ibuprofen and naproxen with regard to cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke, and is associated with a lower risk of gastrointestinal bleeding than those medications. Those findings may encourage more widespread use of celecoxib to treat inflammatory pain. Our findings, including both in vitro studies and a retrospective review of the electronic medical record, indicate that celecoxib is associated with calcific nodule formation and the development of aortic stenosis.
TRANSLATIONAL OUTLOOK: Additional clinical studies are needed to confirm the link between celecoxib use and the development or progression of aortic stenosis, as this could influence prescribing patterns for medications that relieve inflammatory pain. Our studies show that dimethyl celecoxib impedes calcific nodule formation in vitro. Thorough in vivo animal studies should be performed to assess dimethyl celecoxib’s translational potential for the prevention or treatment of calcific aortic valve disease.
Footnotes
This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH), the National Science Foundation (NSF), or Vanderbilt University. This work was funded by NIH grants HL135790, HL115103, and GM007347, American Heart Association grant 18PRE34070125, and NSF grants 1055384 and 2013170175. The datasets used for the analyses described were obtained from Vanderbilt University Medical Center’s BioVU, which is supported by numerous sources: institutional funding, private agencies, and federal grants. These include the NIH-funded Shared Instrumentation grant S10RR025141, and CTSA grants UL1TR002243, UL1TR000445, and UL1RR024975. The clinical datasets used for the analyses described were obtained from Vanderbilt University Medical Center’s Synthetic Derivative. Dr. Lindman has received research grants from Edwards Lifesciences and Roche Diagnostics; served on scientific advisory boards for Roche Diagnostics; and has been a consultant to Medtronic and Roche Diagnostics. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
References
- 1.Rajamannan N.M., Evans F.J., Aikawa E. Calcific aortic valve disease: not simply a degenerative process: a review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: calcific aortic valve disease-2011 update. Circulation. 2011;124:1783–1791. doi: 10.1161/CIRCULATIONAHA.110.006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hutcheson J.D., Aikawa E., Merryman W.D. Potential drug targets for calcific aortic valve disease. Nat Rev Cardiol. 2014;11:218–231. doi: 10.1038/nrcardio.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker G.A., Masters K.S., Shah D.N., Anseth K.S., Leinwand L.A. Valvular myofibroblast activation by transforming growth factor-beta: implications for pathological extracellular matrix remodeling in heart valve disease. Circ Res. 2004;95:253–260. doi: 10.1161/01.RES.0000136520.07995.aa. [DOI] [PubMed] [Google Scholar]
- 4.Mohler E.R., 3rd, Gannon F., Reynolds C., Zimmerman R., Keane M.G., Kaplan F.S. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–1528. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 5.Chen J., Ryzhova L.M., Sewell-Loftin M.K. Notch1 mutation leads to valvular calcification through enhanced myofibroblast mechanotransduction. Arterioscler Thromb Vasc Biol. 2015;35:1597–1605. doi: 10.1161/ATVBAHA.114.305095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark C.R., Bowler M.A., Snider J.C., Merryman W.D. Targeting cadherin-11 prevents notch1-mediated calcific aortic valve disease. Circulation. 2017;135:2448–2450. doi: 10.1161/CIRCULATIONAHA.117.027771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hutcheson J.D., Chen J., Sewell-Loftin M.K. Cadherin-11 regulates cell-cell tension necessary for calcific nodule formation by valvular myofibroblasts. Arterioscler Thromb Vasc Biol. 2013;33:114–120. doi: 10.1161/ATVBAHA.112.300278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowler M.A., Bersi M.R., Ryzhova L.M., Jerrell R.J., Parekh A., Merryman W.D. Cadherin-11 as a regulator of valve myofibroblast mechanobiology. Am J Physiol Heart Circ Physiol. 2018;315:H1614–H1626. doi: 10.1152/ajpheart.00277.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dakshanamurthy S., Issa N.T., Assefnia S. Predicting new indications for approved drugs using a proteochemometric method. J Med Chem. 2012;55:6832–6848. doi: 10.1021/jm300576q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher C.I., Chen J., Merryman W.D. Calcific nodule morphogenesis by heart valve interstitial cells is strain dependent. Biomech Model Mechanobiol. 2013;12:5–17. doi: 10.1007/s10237-012-0377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J., Peacock J.R., Branch J., David Merryman W. Biophysical analysis of dystrophic and osteogenic models of valvular calcification. J Biomech Eng. 2015;137:020903. doi: 10.1115/1.4029115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutcheson J.D., Ryzhova L.M., Setola V., Merryman W.D. 5-HT(2B) antagonism arrests non-canonical TGF-beta1-induced valvular myofibroblast differentiation. J Mol Cell Cardiol. 2012;53:707–714. doi: 10.1016/j.yjmcc.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gould R.A., Butcher J.T. Isolation of valvular endothelial cells. J Vis Exp. 2010;46:2158. doi: 10.3791/2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blondell R.D., Azadfard M., Wisniewski A.M. Pharmacologic therapy for acute pain. Am Fam Physician. 2013;87:766–772. [PubMed] [Google Scholar]
- 15.Fosbøl E.L., Gislason G.H., Jacobsen S. The pattern of use of non-steroidal anti-inflammatory drugs (NSAIDs) from 1997 to 2005: a nationwide study on 4.6 million people. Pharmacoepidemiol Drug Saf. 2008;17:822–833. doi: 10.1002/pds.1592. [DOI] [PubMed] [Google Scholar]
- 16.Nissen S.E., Yeomans N.D., Solomon D.H. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med. 2016;375:2519–2529. doi: 10.1056/NEJMoa1611593. [DOI] [PubMed] [Google Scholar]
- 17.Yan A.T., Koh M., Chan K.K. Association between cardiovascular risk factors and aortic stenosis: the CANHEART aortic stenosis study. J Am Coll Cardiol. 2017;69:1523–1532. doi: 10.1016/j.jacc.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 18.R Development Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2018. R: a language and environment for statistical computing. [Google Scholar]
- 19.Bresalier R.S., Sandler R.S., Quan H. Adenomatous Polyp Prevention on Vioxx (APPROVe) Trial Investigators. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092–1102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 20.U.S. Food and Drug Administration. COX-2 Selective (Includes Bextra, Celebrex, and Vioxx) and Non-Selective Non-Steroidal Anti-Inflammatory Drugs (NSAIDs). February 6, 2018. Available at: https://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm429364.htm. Accessed April 10, 2018.
- 21.Lindman B.R., Clavel M.A., Mathieu P. Calcific aortic stenosis. Nat Rev Dis Primers. 2016;2:16006. doi: 10.1038/nrdp.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wirrig E.E., Gomez M.V., Hinton R.B., Yutzey K.E. COX2 inhibition reduces aortic valve calcification in vivo. Arterioscler Thromb Vasc Biol. 2015;35:938–947. doi: 10.1161/ATVBAHA.114.305159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solomon S.D., McMurray J.J., Pfeffer M.A. Adenoma Prevention with Celecoxib (APC) Study Investigators. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;52:1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.