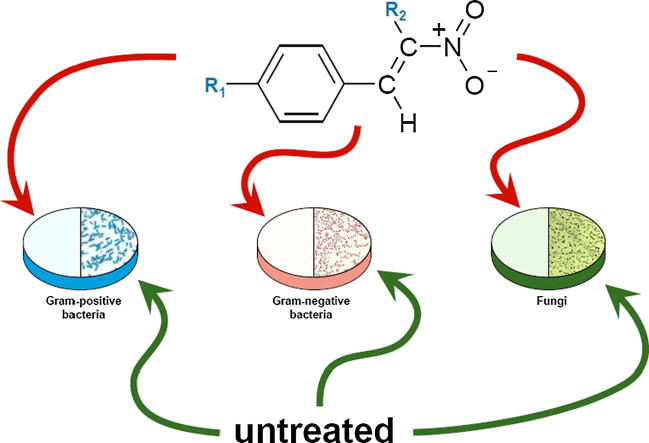
Graphical abstract
Keywords: Conjugated nitroalkenes, Antimicrobial activity, Fungicidal activity, Cytotoxicity, B. subtilis ATCC 6633, HepG2 cells, HaCaT cells
Abbreviations: CFU, colony forming unit; CNA, conjugated nitroalkenes; EWG, electron withdrawing group; HPLC, high-pressure liquid chromatography; MBC, minimal bactericidal concentration; MFC, minimal fungicidal concentration; MIC, minimal inhibitory concentration
Highlights
-
•
A homogenous series of β-EWG functionalyzed β-nitrostyrenes was synthesized and characterized by IR, UV, 1H NMR and 13C NMR spectra.
-
•
The obtained compounds were screened in vitro against a panel of the reference strains of bacteria and fungi - five of them exhibited significant antimicrobial activity against all tested reference bacteria and fungi belonging to yeasts.
-
•
Cytotoxicity of the compounds towards cultured human HepG2 and HaCaT cells was established – five of them displayed similar low pattern of activity in both cells lines.
Abstract
The process of searching for new antibacterial agents is more and more challenging due to the increasing drug resistance which has become a major concern in the field of infection management. Our study presents a synthesis and characterization by IR, UV, 1H NMR and 13C NMR spectra of a homogenous series of 1-EWG functionalized 2-aryl-1-nitroethenes which could prove good candidates for the replacement of traditional antibacterial drugs In vitro screening against a panel of the reference strains of bacteria and fungi and their cytotoxicity towards cultured human HepG2 and HaCaT cells was performed. Antimicrobial results indicated that four of the synthesized compounds exhibited a significant antimicrobial activity against all tested reference bacteria and fungi belonging to yeasts with a specific and strong activity towards B. subtilis ATCC 6633. Two of these compounds had no detectable cytotoxicity towards the cultured human cell lines, making them promising candidates for new antibacterial drugs.
1. Introduction
Heterocyclic nitrocompounds are becoming increasingly interesting for medicine, as they show extensive potency to fight bacteria, fungi and parasites (Raether et al., 2003). Unfortunately, some doubts have been raised as to their medical applications, as reports on their mutagenic and carcinogenic side-effects were published (González Borroto et al., 2005). However, it has been demonstrated that nitroalkene derivatives containing a nitro group separated from the aromatic ring by an alkene chain are deprived of genotoxic properties (Estrada, 1998).
Conjugated nitroalkenes (CNA) are valuable precursors in the organic synthesis. The presence of a strongly electron-withdrawing (EWG) nitro group at a vinyl moiety stimulates substantially the π-deficient character of their double bond. In consequence, CNA have a significant affinity to nucleophilic reagents as diazocompounds (Jasiński, 2015), nitrones (Jasiński and Mróz, 2015, Jasiński, 2015a), ylides (Jasiński, 2015b), 1,3-dienes (Łapczuk-Krygier et al., 2014, Jasiński, 2017), vinyl ethers (Jasiński et al., 2014) and many others. Additionally, the nitro group may be easily converted to many other functional groups (Ono, 2003). Subsequently, it was observed that the nitro group conjugated with the sp2 carbon atom stimulates many forms of biological activity (Gómez-Rivera et al., 2013, Lopes et al., 2011, Paraskevopoulos et al., 2015).
Taking these facts into consideration, we have decided to check a series of (E)-2-aryl-1-cyano-1-nitroethenes as potential antibacterial and antifungal agents, but without genotoxic properties. This work is a continuation of our comprehensive study on the synthesis (Jasiński, 2014, Boguszewska-Czubara et al., 2016, Jasiński and Kącka, 2015, Jasiński et al., 2016), physicochemical properties (Jasiński and Mróz, 2015, Jasiński, 2015a, Jasiński, 2015b, Jasiński, 2014, Jasiński and Kącka, 2015, Jasiński et al., 2016) and practical applications (Boguszewska-Czubara et al., 2016) of CNAs. In this paper we aim to extend our previous study (Boguszewska-Czubara et al., 2016) to a newly synthesized group of compounds with expected high antibacterial potential and low cytotoxicity. In particular, we present an improved protocol for the preparation of EWG-substituted CNA. The compounds obtained by this approach were spectrally characterized and screened for their antimicrobial activity against multiple bacterial and fungal strains. Their in vitro cytotoxicity was assessed in the HepG2 and HaCaT cells lines of human origin.
2. Results and discussion
2.1. Preparation of CNA
For this study we have selected six different conjugated nitroalkenes 1–6 (Table 1), whose properties were compared with the those, we had previously reported for compounds 7–12 (Boguszewska-Czubara et al., 2016). The first stage of the study involved the preparation of these compounds.
Table 1.
The exanimated nitroalkenes and their melting points.
| No | Compound | Yield [%] | Melting point [°C] |
|---|---|---|---|
| 1 | 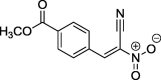 |
80 | 169–170 (ethanol) |
| 2 | 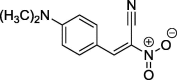 |
88 | 189–189.5 (benzene) |
| 3 | 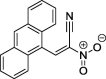 |
84 | 216–217 (ethanol) |
| 4 | 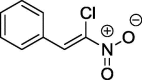 |
70 | 48–48.5 (ethanol) |
| 5 | 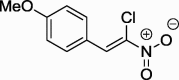 |
72 | 73–73.5 (ethanol) |
| 6 | 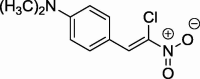 |
84 | 131–132 (ethanol) |
| 7 | 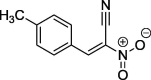 |
98a | 93–94 (benzene) |
| 8 | 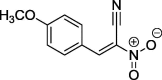 |
95a | 97–98 (benzene) |
| 9 | 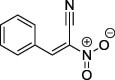 |
97a | 102–102 (benzene) |
| 10 | 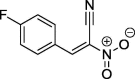 |
92a | 114–115 (benzene) |
| 11 | 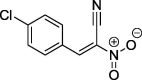 |
96a | 116–117 (benzene) |
| 12 | 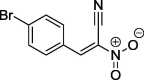 |
95a | 111–112 (benzene) |
The data was received from Boguszewska-Czubara et al. (2016).
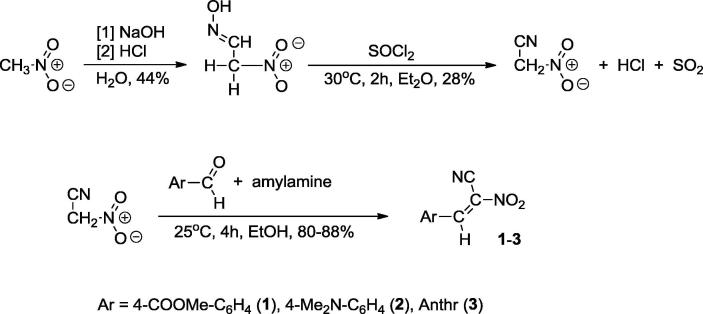
(E)-2-aryl-1-cyano-1-nitroethenes 1 and 2 were prepared using the procedure described earlier (Jasiński et al., 2016). For this purpose we used condensation between nitroacetonitrile and the appropriate aldehydes (Scheme 1). Analogously, we prepared one new compound: (E)-2-(9-anthryl)-1-cyano-1-nitroethene (3). Its constitution was confirmed by elemental analysis data as well as by the IR, UV, 1H NMR and 13C NMR spectra (see Experimental Section).
Scheme 1.
Preparation of conjugated nitroalkenes 1–3.
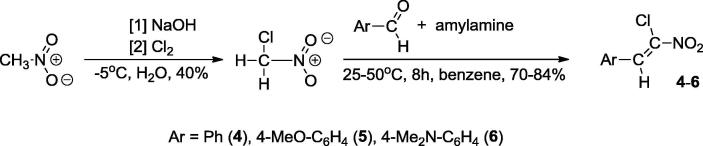
Then we decided to prepare a group of nitroalkenes 4–6. General methodologies for the preparation of (Z)-2-aryl-1-chloro-1-nitroethenes proceed via chlorination with dichloroiodobenzene and dehydrochlorination sequence (Liu et al., 2014). Alternatively, oxidative chlorination by hypochlorous acid may be used for this purpose (Kim et al., 1997). However, some years ago Aleksiev and Ivanova (Aлeкcиeв and Ивaнoвa, 1993) proposed another synthetic protocol for this group of compounds, via Henry condensation starting from chloronitromethane. Accordingly, we prepared (Z)-(4-dimethylamino)-1-chloro-1-nitroethene 6 using etylenediamine as the catalyst (Scheme 2).
Scheme 2.
Preparation of conjugated nitroalkenes 4–6.
We repeated these experiments, with the full optimization of the reaction parameters. As a result, we obtained the final product with the 84% yield using n-amylamine as the catalyst. In the same manner we also prepared two compounds with the phenyl and 4-methoxyphenyl substituents at position 2 of the nitrovinyl moiety (Scheme 2).
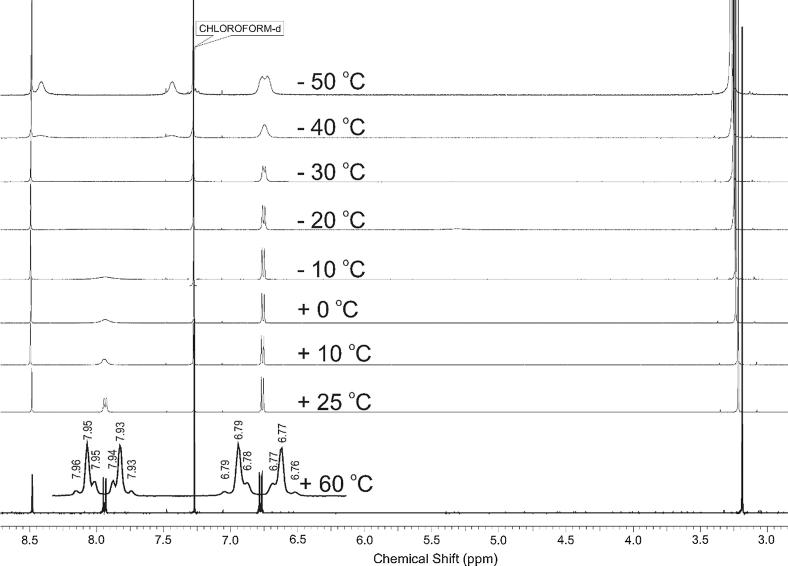
Unusual physical and chemical properties of the series of the obtained compounds were demonstrated on the example of compound 2 (Jasiński et al., 2016). It has been shown and rationalized, based on the results of quantumchemical calculations, NMR, UV/VIS spectroscopies and X-ray crystal structure analysis, that compounds of the studied structural motif display a significantly enhanced π electron delocalization along the molecule and a relatively lower level of aromaticity of the aryl substituent. In the most extreme case, in their solid state, such compounds exist in the zwitterionic form with the positive charge localised at the aryl side and the negative at the oxygen atom of the nitro group. The ionic nature of the compound may obviously influence its biological activity (Boguszewska-Czubara et al., 2016). Thus, from the biological point of view it was important to check whether the above mentioned zwitterionic properties are maintained at a temperature suitable for the living organisms. Variable-temperature NMR spectra were recorded and analysed. Then the 1H NMR spectra of the solution of 2 in CDCl3 were recorded at different temperatures, in the range from – 50 °C to +60 °C (Fig. 1).
Fig. 1.
The variable-temperature 1H NMR spectra of 2.
The results clearly show that at +60 °C the spectrum of compound 2 possess a typical AA’XX’ spin system, which may indicate its classical aromatic nature. At the same time in −50 °C temperature there are 2 different protons on the spectrum at position meta to the dimethylamine substituent with the NMR shift separation 491 Hz. This confirm the inherent zwitterionic nature of 2 in the solution at low temperature. The coalescence temperature for the interconversion of the canonical structures was measured as −25 °C (248 K) which corresponds to the interconversion rate constant (1090 s−1) and Gibbs activation energy at the coalescence temperature (11 kcal/mol) calculated with the assumption that the interconversion is a first-order process according to the dynamic NMR studies equations (Kemp, 1986).
For the observed first-order process, the value of the rate constant is inversely proportional to the interconversion time (t in seconds). At low temperature, when the interconversion is slow:
, were Δν is the NMR shift separation (in Hz) at low temperature, when the exchange does not occur.
Thus, at the coalescence temperature:
On the other hand, this constant could be defined according to the Eyring equation:
, were KB is the Boltzmann constant (3.299 · 10-24); R – the universal gas constant (1.9872); a – transmission coefficient equal to 1; K – temperature in Kelvin, h – the Planck constant (1.584 · 10-34).
Consequently,
or
Therefore it seems that in the solution at the temperature above 0 °C thermal vibrations interrupt the overlapping of the π-orbitals of aromatic, olefin, nitro and nitrile systems, which makes the conjugations inefficient and, as a result, at the temperature of biological studies, compound 2 oscillates between its 2 canonic forms freely.
(E)-2-(4-methylphenyl)-1-cyano-1-nitroethene (7) was likewise examined (Boguszewska-Czubara et al., 2016), nevertheless, in this case no differences in the NMR spectra recorded at the temperatures from −60 and +60 °C were found because of a purely classical character of the substance.
Since compound 2 represents an extreme case with the maximal electron donating effect of the substituent on the aromatic ring (Jasiński et al., 2016), we could assume classical structures for all the tested compounds at the temperatures of biological studies. Nevertheless, the tested compounds displaying a low isomerization barrier may possess a highly polar flat zwitterionic configuration when it is required for a better binding to the target enzyme. This observation allows to compare the biological properties of all presented compounds 1–6 directly, without any need to consider the electronic structures of individual compounds and resulting specific biochemical interactions.
2.2. In vitro antimicrobial assays
In the course of our continuous studies on new candidates for antibiotics efficient against antibiotics resistant microorganisms, we have already found that some (E)-2-aryl-1-cyano-1-nitroethenes 7–12 exhibit antimicrobial and fungicidal activities (Boguszewska-Czubara et al., 2016). As the conclusions of our previous work were promising, the evaluation of other substituted nitroethylenes, including the rare class of such compounds: 1-chloro-1-nitroethenes, was necessary. Our results indicated that the newly synthesized compounds 1–6 had an inhibitory effect on the growth of both reference strains of Gram-positive and Gram-negative bacteria and the reference strains of yeasts belonging to Candida spp. (Table 2).
Table 2.
The activity data expressed as MIC (MBC or MFC) [µg/mL] against the reference strains of microorganisms for the tested compounds.
| Species | MIC (MBC or MFC) [µg/mL] of the tested compounds |
|||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | CIP/FLU** | ||
|
Staphylococcus aureus ATCC 6538 |
1000 –* |
125 (500) |
31.25 (125) |
500 (500) |
62.5 (500) |
250 (250) |
0.244 | |
| Gram-positive bacteria |
Staphylococcus aureus ATCC 25923 |
1000 – |
31.25 (250) |
7.81 (15.62) |
125 (250) |
31.25 (250) |
62.5 (250) |
0.488 |
|
Staphylococcus aureus ATCC 43300 |
1000 – |
62.5 (500) |
31.25 (125) |
250 (250) |
31.25 (125) |
62.5 (125) |
0.244 | |
| Staphylococcus epidermidis ATCC 12228 | 250 (1000) |
62.5 (500) |
31.25 (125) |
500 (500) |
62.5 (125) |
125 (250) |
0.122 | |
|
Bacillus subtilis ATCC 6633 |
250 – |
62.5 (500) |
3.91 (7.81) |
125 (125) |
31.25 (62.5) |
7.81 (15.62) |
0.031 | |
|
Bacillus cereus ATCC 10876 |
1000 – |
125 (250) |
7.81 (31.25) |
250 - |
62.5 (250) |
62.5 - |
0.061 | |
|
Micrococcus luteus ATCC 10240 |
500 – |
31.25 (125) |
62.5 (250) |
500 (1000) |
62.5 (250) |
500 (1000) |
0.976 | |
| Gram-negative bacteria | Bordetella bronchiseptica ATCC 4617 | – | – | 500 – |
125 (500) |
62.5 (1000) |
125 (1000) |
0.976 |
|
Klebsiella pneumoniae ATCC 13883 |
– | – | – | 62.5 (125) |
31.25 (250) |
31.25 (62.5) |
0.122 | |
|
Escherichia coli ATCC 25922 |
– | – | 1000 – |
125 (1000) |
250 (500) |
125 (250) |
0.004 | |
|
Proteus mirabilis ATCC 12453 |
– | – | – | 125 (250) |
125 (500) |
125 (250) |
0.031 | |
|
Salmonella typhimurium ATCC 14028 |
– | – | – | 125 (500) |
250 (500) |
125 (250) |
0.061 | |
|
Pseudomonas aeruginosa ATCC 9027 |
– | – | – | 31.25 - |
31.25 - |
31.25 (1000) |
0.488 | |
| Fungi |
Candida albicans ATCC 2091 |
500 (1000) |
31.25 (125) |
7.81 (31.25) |
250 (250) |
125 (500) |
31.25 (31.25) |
0.245* |
|
Candida albicans ATCC 10231 |
500 (1000) |
31.25 (31.25) |
7.81 (15.62) |
125 (250) |
125 (500) |
31.25 (62.5) |
0.976* | |
|
Candida parapsilosis ATCC 22019 |
250 (500) |
31.25 (125) |
31.25 (125) |
125 (500) |
31.25 (500) |
31.25 (125) |
1.953* | |
‘–’ concentration above 1000 µg/mL.
Standard antibiotics used as positive controls: ciprofloxacin (CIP) for bacteria and fluconazole (FLU) for fungi.
Minimum concentrations of these compounds, obtained by the broth microdilution method, which inhibited the growth of Gram-positive bacteria belonging to staphylococci (Staphylococcus aureus ATTC strains and Staphylococcus epidermidis ATCC 12228), micrococci and bacteria from Bacillus spp. ATTC, ranged from 3.91 to 500 µg/mL, while the MBC values were 7.81–>1000 µg/mL. Five compounds 2–6 indicated a good or very good activity with a bacteriostatic or bactericidal effect against these bacteria. Compound 3 showed the most potent bactericidal activity against Gram-positive bacteria (MBC/MIC = 2–4). This substance had a very strong effect against S. aureus ATCC 25,923 and both strains of Bacillus spp. ATCC with MIC = 3.91–7.81 µg/mL (MBC = 7.81–31.25 µg/mL). The bioactivity of 3 towards the remaining staphylococci and micrococci was good (31.25–62.5 µg/mL and MBC = 125–250 µg/mL). In addition to this, compound 6 also showed a very intense bactericidal activity towards B. subtilis ATCC 6633 (MIC = 7.81 µg/mL).
Other obtained substances 4–6 exhibited a similar effect towards Gram-negative rods (MIC = 31.25–250 µg/mL and MBC = 62.5–>1000 µg/mL), with a particularly high bacteriostatic activity at the concentration of 31.25 µg/mL against P. aeruginosa ATCC 9027. Among them, compounds 4 and 6 indicated a good effect against these bacteria, whereas in the case of substance 5, the activity was moderate or good (Table 2).
According to our study, the growth of the reference yeast strains belonging to Candida spp. was also inhibited by all compounds 1–6. Compounds 2 and 3 showed the strongest fungicidal effect (MFC/MIC = 1–4). On the basis of minimal inhibitory concentration values it was shown that 3 displayed a very strong activity against C. albicans ATCC with MIC = 7.81 µg/mL and MFC = 15.62–31.25 µg/mL and a good activity against C. parapsilosis ATCC 22,019 (MIC = 31.25 µg/mL and MFC = 125 µg/mL). Substance 2 indicated a good activity against all tested Candida spp. ATCC (MIC = 31.25 µg/mL and MFC = 31.25–125 µg/mL) (Table 2).
On the basis of minimal inhibitory concentration values it was also shown that substance 6 displayed the most potent fungicidal activity against these microorganisms with MIC = 31.25 µg/mL. Substances 4 and 5 showed a little less potent effect towards Candida spp. ATTC (MIC = 31.25–250 µg/mL, MFC = 250–500 µg/mL, MFC/MIC = 1–16) (Table 2). Compound 1 showed the weakest activity of all the synthesized compounds against Gram-positive bacteria (MIC = 200–1000 µg/mL), fungi belonging to Candida spp. ATCC (MIC = 250–500 µg/mL) and no activity at all against Gram-negative bacteria.
In comparison to other (E)-2-aryl-1-cyano-1-nitroethenes 7–12 possessing EWG and EDG, which demonstrated a wide spectrum of antimicrobial activity in a line with a low cytotoxicity towards cultured human cells (Boguszewska-Czubara et al., 2016), compound 3 exhibited very strong fungicidal activity (MIC = 7.81 µg/mL MFC = 15.62–31.25 µg/mL). Moreover, compound 3 showed very strong bacteriostatic and bactericidal activity against Gram-positive bacteria (MIC = 3.91–7.81 µg/mL, MBC = 7.81–31.25 µg/mL) while compounds 4–6 were found to be sensitive to Gram-negative bacteria (MIC = 31.25 µg/mL).
2.3. Cytotoxicity
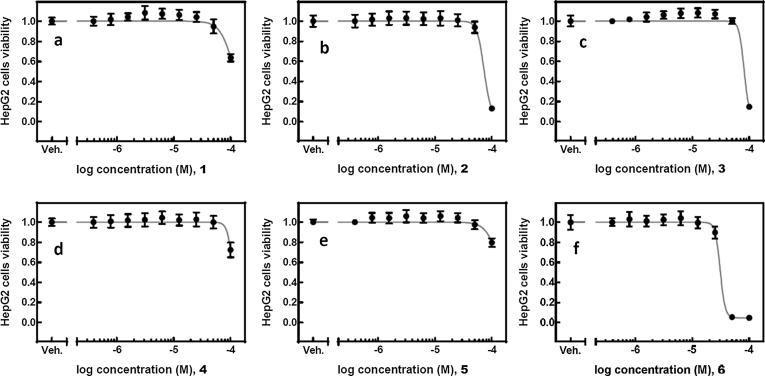
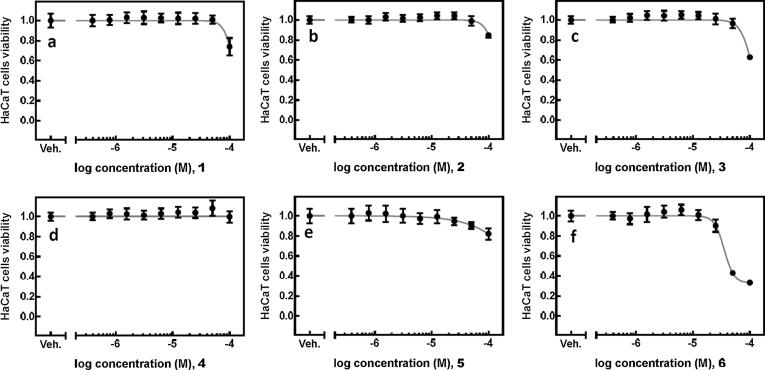
In order to evaluate the cytotoxicity of compounds 1–6, we utilized the plate-based tetrazolium reduction assay as well as human HepG2 and HaCaT cells of liver and skin origin, respectively. Compounds 1–5 displayed a similar pattern of activity in both cells lines (Figs. 2 and 3).
Fig. 2.
Cytotoxicity of compounds 1–6 (a–f) in HepG2 cells.
Fig. 3.
Cytotoxicity of compounds 1–6 (a–f) in HaCaT cells.
HepG2 cells (Fig. 2) were treated with a range of concentrations (0.39–100.00 µM) of the respective compounds (1–6, a–f) or vehicle (DMSO, 0.1%) for 24 h. Cell viability was measured using the tetrazolium-based assay. The mean absorbance value for the control (DMSO-treated) cells was assigned as 1. The data are expressed as mean ± SD from three independent experiments conducted in sextuplicates.
Compounds 1 (Fig. 2a), 4 (Fig. 2d), and 5 (Fig. 2e) were cytotoxic to HepG2cells only when used at the highest dose of 100 µM, and no effect on cellular viability was observed at lower concentrations. Compounds 2 (Fig. 2b) and 3 (Fig. 2c) were slightly more toxic towards HepG2cells and inhibited the cell growth with logIC50 of –4.093 ± 0.026 M (80.7 µM) and –4.140 ± 0.009 M (72.4 µM), respectively.
In order to make a direct comparison of the MICs and cytotoxicity, the µM concentrations of compounds 1–6 used to define cytotoxicity were transformed into the µg/mL, common for IC50 measurements (Table 3).
Table 3.
The cytotoxic activities of compounds 1–6 in HepG2 and HaCaT cell lines.
| HepG2 |
HaCaT |
|||||
|---|---|---|---|---|---|---|
| ID | logIC50 ± SD [M] | IC50 [µM] | IC50 [µg/mL] | logIC50 ± SD [M] | IC50 [µM] | IC50 [µg/mL] |
| 1 | >−4 | >100 | >23.2 | >−4 | >100 | >23.2 |
| 2 | −4.093 ± 0.026 | 80.7 | 17.51 | >−4 | >100 | >21.7 |
| 3 | −4.140 ± 0.009 | 72.4 | 19.84 | >−4 | >100 | >27.4 |
| 4 | >−4 | >100 | >18.35 | – | – | – |
| 5 | >−4 | >100 | >21.45 | >−4 | >100 | >21.45 |
| 6 | −4.510 ± 0.023 | 30.9 | 7.03 | −5.089 ± 0.04 | 8.1 | 1.84 |
IC50 values were obtained by curve fitting of the sigmoidal equation to experimental data.
HaCaT cells (Fig. 3) were treated with a range of concentrations (0.39–100.00 µM) of the respective compounds (1–6, a–f) or vehicle (DMSO, 0.1%) for 24 h. Cell viability was measured using the tetrazolium-based assay. The mean absorbance value for the control (DMSO-treated) cells was assigned as 1. The data are expressed as mean ± SD from three independent experiments conducted in sextuplicates.
Compound 6 (Figs. 2f and 3f) displayed the most cytotoxic character and inhibited the viability of HepG2 cells with logIC50 of –4.510 ± 0.023 M (30.9 µM) and HaCaT cells with logIC50 of –5.089 ± 0.021 M (8.1 µM). Compound 4 (Fig. 3d) displayed no cytotoxic properties up to the concentration of 100 mM in HaCaT cells. Compounds 1–3 (Fig. 3a–c) and 5 (Fig. 3e) showed some level of cytotoxicity, however, only at the highest dose used in this experiment.
Again in comparison with compounds 7–12 we have found compounds 4 and 5 to possess no or very low cytotoxicity against HaKaT and HepG2 cells (>100 µM), what makes them promising compounds for further clinical studies.
3. Conclusions
Our results indicate that newly synthesized compounds 2 and 3 exhibited a potent antimicrobial activity against reference Gram-positive bacteria and fungi belonging to yeasts. However, these compounds markedly inhibited the viability of HepG2 cells, whereas compounds 4 and 5 exhibited a good or very good antimicrobial activity against all tested reference bacteria and fungi belonging to yeasts with no or minimal cytotoxicity towards cultured human HepG2 or HaCaT cells. Compound 6 displayed a specific and strong activity towards B. subtilis ATCC 6633, however, it was also the most cytotoxic compound tested in this study. The determination of the mechanism of action and molecular target of the presented compounds are currently in process.
Taking into account strong antimicrobial and antifungal activities, as well as antibacterial properties expressed by the compounds together with their low cytotoxic effect against healthy human cells, (HaKaT) compounds 4 and 5 seem to be promising candidates for new antibacterial drugs, which might replace nitrofurane-containing antibiotics.
4. Author contribution statement
Initiated and supervised the work: ABC, RJ.
Participated in research design: RJ, ABC.
Conducted the experiments: KK, DM, AB, ŁP, AW, OMD.
Wrote or contributed to the writing of the manuscript: ABC, AW, RJ, OMD.
5. Experimental Section
5.1. General Section
Melting points were determined on a Boetius apparatus and are uncorrected. Elemental analyses were performed on a Perkin-Elmer PE-2400 CHN apparatus. IR spectra were recorded on a Bio-Rad spectrophotometer in the CCl4 solution (Łojewska et al., 2013). 1H NMR (500 MHz) and 13C NMR (125 MHz) spectra were recorded on a Bruker AMX 500 spectrometer. High-pressure liquid chromatography (HPLC) was done using a Knauer apparatus equipped with a UV–VIS detector. To monitor the reaction progress, a LiChrospher 18-RP 5 μm column (4 × 240 mm) and 75% methanol as the eluent at the flow rate of 1.0 cm3/min were used.
5.2. Materials
Commercially available (Sigma-Aldrich) reagents and solvents were used. All solvents had been tested by means of gas chromatography immediately before use. Conjugated nitroalkenes were prepared according to the procedures listed below.
5.2.1. Nitroacetonitrile
Methazonic acid (60 g) (Jasiński, 2013) was dissolved in diethyl ether (300 mL) in a four-neck glass flask (750 mL), equipped with a mechanical stirrer, dropping funnel, thermometer and reflux condenser, Next, the reaction mixture was heated to boiling, and thionyl chloride (43 mL) was dropped in slowly. While introducing thionyl chloride to the reaction system, the temperature should be higher than 30 °C. The solution was stirred for 120 min, filtered and evaporated in the argon atmosphere. Oil residue was treated with diethyl ether (240 mL) and water (90 mL). The organic layer was separated, dried over anhydrous calcium chloride and evaporated in the argon atmosphere. The oil residue was purified by column chromatography. Silica gel was used as the stationary phase, and benzene as the eluent. Pure nitro acetonitrile was obtained as a dark yellow liquid with the 28% yield.
5.2.2. (E)-2-aryl-1-cyano-1-nitroethenes – General procedure
Firstly, an appropriate aldehyde (0.100 mol) was dissolved in ethanol (2 mL). Next, molecular sieves (0.1 g of 4 Å), nitroacetonitrile (0.11 mol) and a catalytic amount of n-amylamine (55 µL) were added. The reaction mixture was stirred for 4 h at room temperature. The product was separated by filtration and recrystallized from ethanol or benzene. Spectral characteristics of the products are listed below:
Methyl 4-[(E)-2-cyano-2-nitroethenyl]benzoate (1): m.p.: 169–170; 1H NMR (500 MHz, CDCl3) δ: 4.01 (s, 3H, OCH3), 8.10 (d, 2H, Ar), 8.24 (d, 2H, Ar), 8.70 (s, 1H, C—H); 13C NMR (126 MHz, CDCl3) δ: 52,82, 110.42, 130.75, 130.94, 131.83, 135.59, 146.96, 165.41; IR (cm−1) vmax = 1332, 1542, 1624, 2233; UV/VIS (MeOH, nm) λmax = 229, 322. The analysis of the product spectra confirmed its identity with literature data (Jasiński et al., 2016: ESI).
(2E)-3-[4-(Dimethylamino)phenyl]-2-nitroprop-2-enenitrile (2): m.p.: 189–189.5; 1H NMR (500 MHz, CDCl3) δ: 3.20 (s, 6H, NCH3), 6.76 (d, 2H, Ar), 7.93 (d, 2H, Ar), 8.49 (s, 1H, C—H); 13C NMR (126 MHz, CDCl3) δ: 40.25, 112.25, 113.38, 113.44, 114.97, 135.86, 147.79, 155.20; IR (CCl4, cm−1) vmax = 1373, 1575, 1737; UV/VIS (MeOH, nm) λmax = 275, 481. The analysis of the product spectra confirmed its identity with literature data (Jasiński et al., 2016).
(2E)-3-(Anthracen-9-yl)-2-nitroprop-2-enenitrile (3): m.p.: 216–217; 1H NMR (500 MHz, CDCl3) δ: 7.63 (dd, 2H, Ar), 7.72 (dd, 2H, Ar), 7.99 (d, 2H, Ar), 8.13 (d, 2H, Ar), 8.72 (s, 1H, C—H), 9.77 (s, 1H, Ar); 13C NMR (126 MHz, CDCl3) δ: 109.73, 120.1, 124.13, 126.25, 128.75, 129.15, 129.79, 129.86, 131.02, 133.72, 147.6; IR (KBr, cm−1) vmax = 1364, 1560; UV/VIS (MeOH, nm) λmax = 251, 470. Elemental analysis calculated for C17H10N2O2: 74.44%C, 3.67%H, 10.21%N, Found: 74.28%C, 3.65%H, 10.15%N.
5.2.3. Chloronitromethane
Nitromethane (54 mL) was placed in a three-necked flask equipped with a mechanical stirrer, dropping funnel and thermometer, and then, after cooling the contents of the flask to below −5 °C, a solution of sodium hydroxide (20 g) in water (160 mL) was added. The precipitated sodium salt of nitromethane was filtered and washed with cold diethyl ether. Then the prepared suspension of this salt in diethyl ether (200 mL) was placed in a scrubber in an ice bath and chlorine gas was bubbled through the suspension for approximately 10 min, cooling the contents of the scrubber down to below −5 °C. The resulting mixture was filtered and then diethyl ether was distilled from the filtrate on a rotary evaporator. The residue was distilled under the reduced pressure. Pure chloronitromethane was obtained as a pale yellow liquid with the 40% yield.
5.2.4. (Z)-2-aryl-1-chloro-1-nitroethenes – General procedure
Firstly, an appropriate aldehyde (0.1 mol) was dissolved in benzene (2 mL). Next, chloronitromethane (0.2 mol) and n-amylamine (55 µL) were added. The reaction mixture was stirred for 1 h at 50 °C and then for 7 h at room temperature. The product was separated by filtration and recrystallized from ethanol. Spectral characteristics of the products are listed below:
[(Z)-2-Chloro-2-nitroethenyl]benzene (4): m.p.: 48–48.5; 1H NMR (500 MHz, CDCl3) δ: 7.45–7.40 (m, 3H, Ar), 7.79–7.77 (m, 2H, Ar), 8.30 (s, 1H, C—H); 13C NMR (126 MHz, CDCl3) δ: 129.11, 129. 68, 131.22, 131.67, 131.96, 137.60; IR (KBr, cm−1) vmax = 764, 1307, 1532, 1610; UV/VIS (MeOH, nm) λmax = 226, 325. The analysis of the product spectra confirmed its identity with literature data (Liu et al., 2014).
1-[(Z)-2-Chloro-2-nitroethenyl]-4-methoxybenzene (5): m.p.: 73–73.5; 1H NMR (500 MHz, CDCl3) δ: 3.82 (s, 3H, OCH3), 6.93 (d, 2H, Ar), 7.79 (d, 2H, Ar), 8.29 (s, 1H, C—H); 13C NMR (126 MHz, CDCl3) δ: 55.56, 114.71, 122.10, 131.69, 133.57, 135.32, 162.68; IR (KBr, cm−1) vmax = 829, 1344. 1260, 1525; UV/VIS (MeOH, nm) λmax = 240, 357. The analysis of the product spectra confirmed its identity with literature data (Dauzonne and Demerseman, 1990).
4-[(Z)-2-Chloro-2-nitroethenyl]-N,N-dimethylaniline (6): m.p.: 131–132; 1H NMR (500 MHz, CDCl3) δ: 3.03 (s, 6H, NCH3), 6.65 (d, 2H, Ar), 7.75 (d, 2H, Ar), 8.29 (s, 1H, C—H); 13C NMR (126 MHz, CDCl3) δ: 40.02, 111.71, 116.85, 132.17, 133.05, 134.05, 152.72; IR (KBr, cm−1) vmax = 1529, 1308, 1260, 812; UV/VIS (MeOH, nm) λmax = 269, 346, 447. The analysis of the product spectra confirmed its identity with literature data (Łojewska et al., 2013).
5.3. In vitro antimicrobial assays
The examined compounds 1–6 were screened in vitro for their antibacterial and antifungal activities using the broth microdilution method (according to the guidelines of the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (EUCAST, 2003) and Clinical and Laboratory Standards Institute (CLSI, 2012)) against a panel of the reference and clinical or saprophytic strains of microorganisms, including Gram-positive bacteria (Staphylococcus aureus ATCC 6538, Staphylococcus aureus ATCC 25923, Staphylococcus aureus ATCC 43300, Staphylococcus epidermidis ATCC 12228, Bacillus subtilis ATCC 6633, Bacillus cereus ATCC 10876, Micrococcus luteus ATCC 10240), Gram-negative bacteria (Bordetella bronchiseptica ATCC 4617, Klebsiella pneumoniae ATCC 13883, Escherichia coli ATCC 25922, Proteus mirabilis ATCC 12453, Salmonella typhimurium ATCC 14028, Pseudomonas aeruginosa ATCC 9027) and fungi belonging to yeasts (Candida albicans ATCC 2091, Candida albicans ATCC 10231, Candida parapsilosis ATCC 22019). The microorganisms belonging to ATCC came from the American Type Culture Collection, routinely used for the evaluation of antimicrobials. All the used microbial cultures were first subcultured on nutrient agar or Sabouraud agar at 35 °C for 18–24 h or 30 °C for 24–48 h for bacteria and fungi, respectively.
The surfaces of Mueller-Hinton agar (for bacteria) and RPMI 1640 with MOPS (for fungi) were inoculated with the suspensions of bacterial or fungal species. Microbial suspensions were prepared in sterile saline (0.85% NaCl) with an optical density of 0.5 McFarland standard scale–, approximately 1.5 × 108 CFU (colony forming units)/mL for the bacteria and 0.5 McFarland standard scale, approximately 5 × 105 CFU/mL) for the fungi.
Samples containing the examined compounds were dissolved in 1 mL of dimethyl sulfoxide (DMSO). Furthermore, bacterial and fungal suspensions were put onto Petri dishes with solid media containing the tested compounds 1–6 (2 mg/mL) and followed incubation at 37 °C for 24 h and 30 °C for 48 h for bacteria and fungi, respectively. The inhibition of the microbial growth was evaluated by comparison with the control culture prepared without any sample tested. Ciprofloxacin or fluconazole (Sigma) were used as the reference antibacterial or antifungal compounds, respectively.
Subsequently, the MIC (minimal inhibitory concentration) of the compounds was examined by the microdilution broth method (Wiegand et al., 2008), using their two-fold dilutions in Mueller-Hinton broth (for bacteria) and the RPMI 1640 medium with MOPS (for fungi) prepared in 96-well polystyrene plates. Final concentrations of the compounds ranged from 1000 to 0.488 µg/mL. Microbial suspensions were prepared in sterile saline (0.85% NaCl) with an optical density of 0.5 McFarland standard. Next, each bacterial or fungal suspension (2 µL) was added to each well containing broth (200 µL) and various concentrations of the examined compounds. After incubation (37 °C for 24 h for bacteria and 30 °C for 24 h for yeasts), the MIC was assessed spectrophotometrically as the lowest concentration of the samples showing complete bacterial or fungal growth inhibition. Appropriate DMSO, growth and sterile controls were carried out. The medium with no tested substances was used as control.
The MBC (minimal bactericidal concentration) or MFC (minimal fungicidal concentration) are defined as the lowest concentration of the compounds required to kill a particular bacterial or fungal species. The MBC or MFC were determined as described above (Popiołek et al., 2016). In brief, the cultures (20 μL) used for the MIC determination were removed from each well and spotted onto an appropriate agar medium. The plates were incubated at 37 °C for 24 h and at 30 °C for 48 h for bacteria and fungi, respectively. The lowest compound concentrations with no visible growth observed were established as bactericidal or fungicidal concentrations. All the experiments were repeated three times and representative data are presented.
In this study, no bioactivity was defined as the MIC > 1000 µg/mL, mild bioactivity as the MIC in the range of 501–1000 µg/mL, moderate bioactivity as the MIC ranging from 126 to 500 µg/mL, good bioactivity as the MIC in the range 26–125 µg/mL, strong bioactivity with the MIC between 10 and 25 µg/mL and very strong bioactivity as the MIC < 10 µg/mL. The MBC/MIC or MFC/MIC ratios were calculated in order to determine the bactericidal/fungicidal (MBC/MIC ≤ 4, MFC/MIC ≤ 4) or bacteriostatic/fungistatic (MBC/MIC > 4, MFC/MIC > 4) effects of the tested compounds (O’Donnell et al., 2010).
5.4. Cell cultures
HepG2 and HaCaT cells were purchased from ATCC (#HB-8065, Rockville, MD) and CLS (#300493, Eppelheim, Germany), respectively. HepG2 cells were cultured in the Eagle’s minimal essential medium modified with the fetal bovine serum (FBS) (10%), l-glutamine (2 mM), sodium pyruvate (1 mM), penicillin (100 U/mL) and streptomycin (0.1 mg/mL). HaCaT cells were maintained in high-glucose (4.5 g/L) Dulbecco’s Modified Eagle’s Medium supplemented with FBS (10%) and l-glutamine (2 mM), penicillin (100 U/mL) and streptomycin (0.1 mg/mL). The cell culture media and the supplements were obtained from Life Technologies (Carlsbad, CA). The cells were cultured at 37 °C in the humidified atmosphere of 5% CO2 in air.
5.5. MTS assay
Cytotoxic effects of compounds 1–6 were assessed using the CellTiter MTS reagent (Promega, Mannheim, Germany) as described previously (Boguszewska-Czubara et al., 2016). As vehicle (Veh) only cell culture medium with DMSO content equal to prepared solutions was used.
5.6. Statistical analysis
Data on the cellular viability were curve-fitted to the four-parameter sigmoidal equation and plotted as the mean ± standard deviation (SD) using the GraphPad Prism v6 (GraphPad Software, Inc., San Diego, CA). Concomitantly, half-maximal inhibitory concentrations (IC50) were calculated where possible.
6. Ethical issue
Not applicable.
Declaration of interest
The authors declare no conflict of interest. This work was partially supported by the Polpharma Scientific Foundation scholarship (to AW). In part the research was carried out with the equipment purchased thanks to the financial support of the European Regional Development Fund under the framework of the Operational Program Development of Eastern Poland 2007–2013 (Contract No. POPW.01.03.00-06-009/11-00, equipping the laboratories of the Faculties of Biology and Biotechnology, Mathematics, Physics and Informatics, and Chemistry for studies of biologically active substances and environmental samples) as well as Polish National Science Centre research grant (2012/05/B/ST5/00362).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Anna Boguszewska-Czubara, Email: anna.boguszewska-czubara@umlub.pl.
Oleg M. Demchuk, Email: O.Demchuk@IFarm.eu.
Radomir Jasiński, Email: radomir@chemia.pk.edu.pl.
References
- A Aлeкcиeв, Д. И., Ивaнoвa, C. M. Cинтeз 2-гaлoгeн-2-нитpoэтeнилapeнoв. 1993. ЖOpX 29(11), pp. 2226–2229.
- Boguszewska-Czubara A., Lapczuk-Krygier A., Rykala K., Biernasiuk A., Wnorowski A., Popiolek L., Maziarka A., Hordyjewska A., Jasiński R. Novel synthesis scheme and in vitro antimicrobial evaluation of a panel of (E)-2-aryl-1-cyano-1-nitroethenes. J. Enzyme Inhib. Med. Chem. 2016;31(6):900–907. doi: 10.3109/14756366.2015.1070264. [DOI] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI), 2012. Reference method for broth dilution antifungal susceptibility testing of yeasts. In:”4th Informational Supplement M27–S4. Clinical and Laboratory Standards Institute, Wayne, PA. ASIN: 1562388649, ISBN: 1-56238-826-6.
- Dauzonne D., Demerseman P. A convenient synthesis of 3-chloro-3,4-dihydro-4-hydroxy-3-nitro-2-phenyl-2H-1-benzopyrans. Synthesis. 1990;01:66–69. doi: 10.1055/s-1990-26791. [DOI] [Google Scholar]
- Estrada E. Structure-mutagenicity relationships in 2-furylethylene derivatives. A molecular orbital study of the role of nitro groups. Mutat. Res. - Genet. Toxicol. Environ. Mutagen. 1998;420(1–3):67–75. doi: 10.1016/S1383-5718(98)00141-7. [DOI] [PubMed] [Google Scholar]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infection. 2003;9(8):9–15. doi: 10.1046/j.1469-0691.2003.00790.x. [DOI] [PubMed] [Google Scholar]
- Gómez-Rivera A., Aguilar-Mariscal H., Romero-Ceronio N., Roa-De La Fuente L.F., Lobato-García C.E. Synthesis and anti-inflammatory activity of three nitro chalcones. Bioorg. Med. Chem. Lett. 2013;23(20):5519–5522. doi: 10.1016/j.bmcl.2013.08.061. [DOI] [PubMed] [Google Scholar]
- González Borroto J.I., Machado G.P., Creus A., Marcos R. Comparative genotoxic evaluation of 2-furylethylenes and 5-nitrofurans by using the comet assay in TK6 cells. Mutagenesis. 2005;20(3):193–197. doi: 10.1093/mutage/gei026. [DOI] [PubMed] [Google Scholar]
- Jasiński, R., 2013. Preparatyka alifatycznych nitrozwiązków. Radomskie Towarzystwo Naukowe. Radom. ISBN: 978-83-88100-67-3.
- Jasiński R. Molecular mechanism of thermal decomposition of fluoronitroazoxy compounds: DFT computational study. J. Fluor. Chem. 2014;160:29–33. doi: 10.1016/j.jfluchem.2014.01.007. [DOI] [Google Scholar]
- Jasiński R. Nitroacetylene as dipolarophile in [2 + 3] cycloaddition reactions with allenyl-type three-atom components: DFT computational study. Monatsh. Chem. 2015;146(4):591–599. doi: 10.1007/s00706-014-1389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasiński R. A stepwise, zwitterionic mechanism for the 1,3-dipolar cycloaddition between (Z)-C-4-methoxyphenyl-N-phenylnitrone and gem-chloronitroethene catalysed by 1-butyl-3-methylimidazolium ionic liquid cations. Tetrahedron Lett. 2015;56(3):532–535. doi: 10.1016/j.tetlet.2014.12.007. [DOI] [Google Scholar]
- Jasiński R. In the searching for zwitterionic intermediates on reaction paths of [3 + 2] cycloaddition reactions between 2,2,4,4-tetramethyl-3-thiocyclobutanone S-methylide and polymerizable olefins. RSC Adv. 2015;5(122):101045–101048. doi: 10.1039/c5ra20747a. [DOI] [Google Scholar]
- Jasiński R. One-step versus two-step mechanism of Diels-Alder reaction of 1-chloro-1-nitroethene with cyclopentadiene and furan. J. Mol. Graph. Model. 2017;75:55–61. doi: 10.1016/j.jmgm.2017.04.008. [DOI] [PubMed] [Google Scholar]
- Jasiński R., Kącka A. A polar nature of benzoic acids extrusion from nitroalkyl benzoates: DFT mechanistic study. J. Mol. Model. 2015;21(3):59. doi: 10.1007/s00894-015-2592-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasiński R., Kubik M., Łapczuk-Krygier A., Kącka A., Dresler E., Boguszewska-Czubara A. An experimental and theoretical study of the hetero Diels-Alder reactions between (E)-2-aryl-1-cyano-1-nitroethenes and ethyl vinyl ether: one-step or zwitterionic, two-step mechanism? Reaction Kinet., Mech. Catal. 2014;113(2):333–345. doi: 10.1007/s11144-014-0753-8. [DOI] [Google Scholar]
- Jasiński R., Mirosław B., Demchuk O.M., Babyuk D., Łapczuk-Krygier A. In the search for experimental and quantumchemical evidence for zwitterionic nature of (2E)-3-[4-(dimethylamino)phenyl]-2-nitroprop-2-enenitrile - An extreme example of donor-π-acceptor push-pull molecule. J. Mol. Struct. 2016;1108:689–697. doi: 10.1016/j.molstruc.2015.12.056. [DOI] [Google Scholar]
- Jasiński R., Mróz K. Kinetic aspects of [3+2] cycloaddition reactions between (E)-3,3,3-trichloro-1-nitroprop-1-ene and ketonitrones. React. Kinet. Mech. Catal. 2015;116(1):35–41. doi: 10.1007/s11144-015-0882-8. [DOI] [Google Scholar]
- Kemp W. Published by Macmillan International Higher Education LTD; London: 1986. Nuclear Magnetic Resonance in Chemistry: A Multinuclear Introduction. ISBN 978-0-333-37292-0. [Google Scholar]
- Kim J.N., Son J.S., Lee H.J., Jung K.S. An expedient synthesis of β-chloro-β-nitroolefin derivatives. Synth. Commun. 1997;27(11):1885–1891. doi: 10.1080/00397919708006789. [DOI] [Google Scholar]
- Łapczuk-Krygier A., Ponikiewski Ł., Jasiński R. The crystal structure of (1RS,4RS,5RS,6SR)-5-cyano-5-nitro-6-phenyl-bicyclo[2.2.1]hept-2-ene. Crystallogr. Rep. 2014;59(7):961–963. doi: 10.1134/S1063774514070128. [DOI] [Google Scholar]
- Liu L., Zhang-Negrerie D., Du Y., Zhao K. PhICl2 and wet DMF: An efficient system for regioselective chloroformyloxylation/a-chlorination of alkenes/a, β-unsaturated compounds. Org. Lett. 2014;16(2):436–439. doi: 10.1021/ol403321n. [DOI] [PubMed] [Google Scholar]
- Łojewska J., Knapik A., Jodłowski P., Łojewski T., Kołodziej A. Topography and morphology of multicomponent catalytic materials based on Co, Ce and Pd oxides deposited on metallic structured carriers studied by AFM/Raman interlaced microscopes. Catal. Today. 2013;216:11–17. doi: 10.1016/j.cattod.2013.05.008. [DOI] [Google Scholar]
- Lopes M.S., De Souza Pietra R.C.C., Borgati T.F., Romeiro C.F.D., Júnior P.A.S., Romanha A.J., Alves R.J., Souza-Fagundes E.M., Fernandes A.P.S.M., De Oliveira R.B. Synthesis and evaluation of the anti parasitic activity of aromatic nitro compounds. Eur. J. Med. Chem. 2011;46(11):5443–5447. doi: 10.1016/j.ejmech.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Ono, N., 2003. The Nitro Group in Organic Synthesis. Wiley-VCH. ISBN: 9780471458463.
- O’Donnell F., Smyth T.J.P., Ramachandran V.N., Smyth W.F. A study of the antimicrobial activity of selected synthetic and naturally occurring quinolines. Int. J. Antimicrob. Agents. 2010;35(1):30–38. doi: 10.1016/j.ijantimicag.2009.06.031. [DOI] [PubMed] [Google Scholar]
- Paraskevopoulos G., Krátký M., Mandíková J., Trejtnar F., Stolaříková J., Pávek P., Besra G., Vinšová J. Novel derivatives of nitro-substituted salicylic acids: Synthesis, antimicrobial activity and cytotoxicity. Bioorganic Med. Chem. 2015;23(22):7292–7301. doi: 10.1016/j.bmc.2015.10.029. [DOI] [PubMed] [Google Scholar]
- Popiołek Ł., Biernasiuk A., Malm A. Synthesis and in vitro antimicrobial activity of nalidixic acid hydrazones. J. Heterocy. Chem. 2016;53(5):1589–1594. doi: 10.1002/jhet.2468. [DOI] [Google Scholar]
- Raether W., Hänel H., Ha H. Nitroheterocyclic drugs with broad spectrum activity. Parasitol. Res. 2003;90(Supp 1):S19–S39. doi: 10.1007/s00436-002-0754-9. [DOI] [PubMed] [Google Scholar]
- Wiegand I., Hilpert K., Hancock R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008;3(2):163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]