Abstract
Knowing the normal cleanroom microbiota is the basis for ensuring microbiological quality; assess changes and the introduction of new sampling methods. During our study, we prepared a catalogue of cleanroom microorganisms located in four different cleanrooms in University Clinical Centre Ljubljana Pharmacy. Catalogue is prepared as a basis for assessing the suitability of the new rapid microbiological method and subsequent correlation of the results of both methods. The results of our study showed that 78% of isolated bacteria are Gram-positive. However, in more than 70% isolated bacteria were the part of the normal human microbiota, 10–15% of the microorganisms originated from the air, mainly spore-forming bacteria of the genus Bacillus and fungi, and 5–10% of the Gram-negative microorganisms that originated from the water and represent the potential endotoxins (pyrogens).
Keywords: Cleanroom, Environmental monitoring, Microbiota, Microorganisms, Colony forming unit, Bioluminescence, Adenosine triphosphate
1. Introduction
Pharmaceutical manufacturing, both nonsterile and in particular sterile processing, requires development and manufacturing in areas which minimize the potential for contamination through the control of environmental cleanliness and in minimizing the possibility of personnel introducing contamination into the process (Sandle, 2015a). In manufacturing facilities, i.e. cleanrooms (CR), the key aspect is that the level of cleanliness is controlled. CR are typically classified according to their use and are assured by the cleanliness of the air by the measurement of particles (Whyte, 2001).
The regulatory requirements for cleanrooms are detailed by EU GMP (European Commission, 2009) or cGMP in USA FDA and other guidelines. The way in which cleanrooms are qualified and assessed is assured by a series of ISO standards, where the ISO 14644 Part 1 sets the general standard for the classification of air cleanliness and Part 2 sets out the specifications for testing. In addition, ISO 14698 describes some of the standards and testing requirements for bio contamination control (Sandle, 2015a).
The routine environmental monitoring program is a critical aspect of documenting the state of control of the cleanroom facility (European Commission, 2009, Sutton, 2010b). It is a program, which evaluates the cleanliness of the manufacturing environment; the effectiveness of cleaning and disinfection programs and the operational performance of environmental controls (Cundell, 2004, Dixon, 2007, Sandle, 2011, Sandle, 2015a).
The monitoring program should be prepared on the base of qualification tests carried out in accordance with the relevant guidelines and standards, risk assessment and good knowledge of critical points of the controlled process. Critical points are the sites that represent the greatest microbiological risk for the aseptic process. Surface samples and air samples must therefore be taken at that phase and in the place where the product is mostly exposed and the risk is therefore the greatest. It is reasonable to prescribe a standard operating procedure (SOP) which defines frequency of sampling, sample quantities, equipment and sampling technique, warning and action limits and actions in case of deviations from the specified limits (European Commission, 2009, Sandle, 2015b). Such review should be carried out over an enough long period and so the complete picture can be revealed. This is important in order to understand if certain species which are recovered pose a product or environmental risk and to check if the cleaning and sanitization practices are effective (Sandle, 2011). The emphasis should always be upon environmental control rather than simply environmental monitoring. That is, where a risk is identified, the risk should be minimized as a part of the strategy of bringing the clean area into tighter control (Jimenez, 2007, Sandle, 2015a, Tršan and Pečar, 2010). In this way, we ensure that all the quality parameters are achieved and all necessary tests prescribed for the release of quality products are performed.
There are many sources of contamination and the types of microorganisms can indicate the origin of contamination. Microbiota in the environment and in the finished pharmaceutical product as well can originate from raw materials, including water, used equipment, facility air, personnel and production processes, and/ or from the primary packaging of the product (Resnik and Kerč, 2010, Sandle, 2011, Sandle, 2015a).
Microbiological monitoring program can be carried out in several ways. Visual assessment itself is not sufficient and classical microbiological methods require cultivation and isolation of microorganisms and as such are therefore not suitable for an immediate assessment. Nowadays, new alternative methods for measuring cellular components can be used as an alternative ones (D. Hussong and Mello, 2006).
There are different sampling methods, which are used for classical viable monitoring. These can be grouped into air and surface methods and into primary and secondary methods based on their theoretical efficiencies to recover microorganisms (Sandle, 2015a) (Table 1).
Table 1.
Microbiological viable monitoring methods.
| Air | Surface | |
|---|---|---|
| method 1 | Active air sampler (CFU/m3) |
Contact plate (CFU/25 cm2) |
| method 2 | Settle plate (CFU/90 mm over “×” time) |
Swab (CFU/surface) |
CFU - colony forming unit.
For air monitoring, this is undertaken using agar settle plates placed in the locations of greatest risk or alternative active (volumetric) air-samplers to provide a quantitative assessment of the number of microorganisms in the air per volume of air sampled (Dixon, 2007; David Hussong and Madsen, 2004, Sandle, 2014, Sandle, 2015a, Sutton, 2010a, Sutton, 2010b). A settle plate is an agar plate, placed in a defined location. For consistency of sampling, for aseptic filling, the EU GMP Guide recommends a 4 h exposure time. Active or volumetric air samplers are slightly different measurement of microorganisms in the air. Settle plates indicate the number of microorganisms that may deposit onto a surface; whereas, the active air-samplers indicate the number of microorganisms present in a given volume of air within the range of the air-sampler (Sandle, 2015a, Sandle, 2015b, Whyte, 1996).
Surface contact plates (RODAC - Replicate Organism Detection and Counting) are common sample type for surface contamination. Contact plate filled with microbiological agar is a quantifiable method, because after the contact between the plate and the manufacturing surface provides information relating to the number of microbial colonies and their relative position. The quantification is derived from the recording the number of colony forming units (CFU) per square centimetre (Sandle, 2015a).
Swabbing is performed by rubbing a surface while rotating the swab so that all parts of the tip are exposed through a number of strokes. Swabs are typically made up of sterile cotton tips, although swabs vary in the materials used for the applicator stick and the materials of the tip Some types of swabs require prewetting with diluent before use, other types of swabs are contained with a transport medium and there are either contained within a transport medium or require prewetting with a suitable recovery medium (Sandle, 2015a).
There are, however, some alternative rapid microbiological methods (Duguid et al., 2011, Easter, 2003) and as an example is the method for measuring the cellular components is the bioluminescent measurement of adenosine triphosphate (ATP). The essential benefit of this approach is that the results can be obtained within a few minutes (Sandle, 2015a). ATP can be in three forms; microbial inside living microorganisms, somatic or non-microbial within animals and plants and free ATP from cellular disorders or dead microorganisms. Its presence can be an indication of the overall contamination of both microbial and those derived from production raw materials, personnel and body fluids, which can also stimulate the growth of microorganisms. The method is based on the measurement of yellow-green visible light (550–570 nm) released in a specific enzyme reaction. The proportion of released light is proportional to the amount of intracellular ATP in the sample, and so we can roughly say, that the result, expressed in relative light units, is proportional to the number of microorganisms in the sample (Venkateswaran et al., 2003). The basic principle of the method is the oxidation of the organic compound of luciferin in the presence of the luciferase enzyme and ATP, which means that it is an enzyme-catalysed reaction of energy conversion to the light. High ATP values after cleaning and disinfection indicate inefficient cleaning and a high risk of contamination (Willis et al., 2007). The technique includes the sample collection, the implementation of the enzyme reaction and the detection of the light released during reaction by using a hand luminometer.
In addition to the rapid results, which are undoubtedly a significant advantage over the classical microbiological method, it is also advantageous that the implementation is not limited to trained laboratory personnel and can be carried out at the very place of sampling (Griffith et al., 2000). In the case of found defects in cleaning, quick accessibility of the results makes it possible to implement corrective measures immediately.
The purpose of this study was to prepare a catalogue of cleanroom microorganisms located in four different cleanrooms at the UKCL (University Clinical Centre Ljubljana) Pharmacy, a cleanroom microbiota, as a basis for assessing the suitability of the new rapid bioluminescent method and subsequent correlation its results with the results of classical microbiological (viable count) methods. It is the aim to investigate and establish whether this new method is aligned with the existing bioburden in raw materials, environment and finished products.
2. Experimental
For the present study samples were taken from four cleanrooms in UKCL Pharmacy in the period from 2011 to 2016. Three cleanrooms: a cleanroom 1 for the aseptic preparation, mostly for ophthalmic products (PA), cleanroom 2 for the preparation of a total parenteral nutrition (PPP) and cleanroom 3 for the preparations for cytostatic therapy (PCT) are used for aseptic preparation of individual therapy. The cleanroom 4 (PI) is used for the serial manufacturing of different parenterals and other sterile solutions with terminal sterilization.
All rooms are classified as ISO 14,644 class 7; EU GMP Grade C and laminar air flow chambers are classified as ISO 14,664 class 5; EU GMP Grade A).
Our cleanroom personal protective equipment was the same during the whole study. It consisted of disposable coat from synthetic particle-free materials, a sterile cap and a facemask covering hair and beard, sterilized footwear and double sterile gloves on hands without jewellery. During work, if necessary, but at least every hour, the upper pair of gloves is replaced. In the case of aseptic production, sterile coat is used and in the PI rooms the coverall.
2.1. Materials
Passive air sampling was performed by sedimentation method, by exposing settle plates with blood agar (90 mm diameter Petri dishes). Volumetric air sampling was conducted with RODAC plates with 3 different media: blood agar for bacteria, Sabouraud and DG18 (Dichloran glycerol agar) for fungi. For capture of 1 m3 of air for volumetric analysis, the air sampler has been used (SAS dual air sampler, VWR International S.r.l., Milan, Italy). Immediately prior to the swabbing surfaces and hands, swabs were put into the tubes with 5 ml of sterile physiological saline solution. When the surface to be controlled had previously been cleaned with a disinfectant, liquid transport medium contained the appropriate inactivator (Tween 80). All media used have been prepared and all the samples have been analysed at the Institute of Microbiology and Immunology, Faculty of Medicine in Ljubljana (IMI). For the disinfection of the used material, it was used a “spray and wipe” technique with a sterile 70% ethanol (Klerwipe 70/30®, Ecolab, Maribor, Slovenia).
2.2. Procedure
2.2.1. Swabbing
Swabbing was conducted with a sterile plastic template, which had a cut-out window with an area of 20 cm2. We carefully opened the test tube and with a slight pressure extracted excess liquid on the wall. The surface inside the template was wiped at least 3 times in a zigzag line vertically and at least 2 times horizontally. For hand sampling, we swabbed palms and tips of the fingers, especially around the nails, each hand separately. The swabs were returned back into the test tube and labelled it with the sample number and the date and time of collection (IMI, 2016). The samples were immediately submitted to the test laboratory and stored in an upright position, so that the liquid did not spill over the tube and the stopper. All swab samples were inoculated into Thioglycollate broth, Andrade Lactose Peptone water and on blood agar. Samples in Thioglycollate broth were incubated for 7 days at 35° C ± 1 °C, samples in the Lactose Medium were incubated for 3 days at 35° C ± 1 °C . All liquid media with observed growth were subcultured to solid media.
In order to be able to calculate the number of bacteria on the sampled surface, the volume of the liquid should remain constant 5 ml. Before transport samples were stored in the refrigerator, and not longer than 2 h (IMI, 2016).
2.2.2. Settle plates
Petri dishes filled with blood agar were exposed for a certain period of time (1–3 h) in a given area. The exposure time depended on whether the space was at that time operational or not in use. We recorded the exact time of exposure and the data was used for a 4-hour exposure calculation. After the exposure, agars were transported to the laboratory and were kept at room temperature. Plates were incubated for 18–24 h at 35° C ± 1° C and then for additional 18–24 h at room temperature.
2.2.3. Volumetric sampling
1 m3 of air in the room was actively collected with the sampler in three different media; blood agar for bacteria, Sabouraud and DG18 for fungi. Bacteriological air control samples on blood agar were incubated 18–24 h at 35 °C ± 1 °C and for a further 18–24 h at room temperature. The selective media for yeasts and moulds; DG18 were incubated 5–7 days at 30 °C and Sabouraud for 5–7 days at 37 °C. In parallel, a negative control from the machine and quality control personnel has been taken as well.
2.3. Sites and sampling frequency
Selection of surface sampling sites was conducted for each facility, considering the specificity of processes taking place in them. Based on a risk assessment approach we defined the sites, at which the microbial contamination would most likely have a negative effect on the product quality: places where the highest contamination is expected, or where the drug product was exposed to the potential contamination for the longest time. In other words, we wanted to establish the worst conditions. In sampling plane, the obligatory sampling sites were determined, where the sampling was performed always and additional places that were intended for more general review of the state of cleanliness of the cleanroom (Table 2).
Table 2.
Sampling sites and frequency of sampling.
| Samples | Frequency |
|---|---|
| Swabs from working surfaces and hands | |
|
Obligatory sampling sites: Sampling device, working surface in LAF chamber, operators hands, assistents hands |
monthly |
|
Additional sampling sites: Other working surfaces (outside LAF), trays, syringe, bottles and vials, floors, walls, material airlock system, devices, filling needles, filters… |
monthly |
| Air samples | |
| Settle plates: inside the LAF chamber | daily |
| Active – RODAC plates: in the middle of cleanroom | monthly |
All collected and accordingly labelled samples were sent for analysis to IMI. Samples collected with swabbing were split into appropriate media and incubated for up to seven days. In the lab the presence of growth on the media was read daily, counted the growing colonies and identified them up to the species. The result for each swab had information on the total number of CFU/dm2 and the type and number of bacteria or fungi individual species (Švent-Kučina et al., 2013). The results obtained from air control were presented as a total number of CFU/m3 or CFU/l of air. In addition, the species and number of bacteria and fungi were identified (Švent-Kučina et al., 2013).
3. Results and discussion
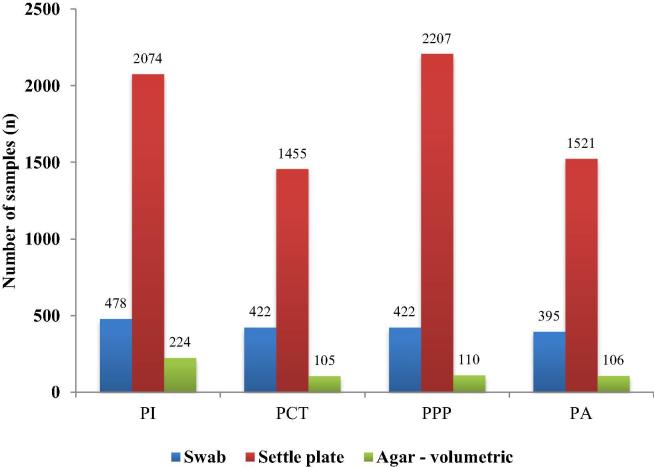
From 2011 to 2016, a total of 9.519 different samples were collected according to the environmental monitoring program in four cleanrooms. Number of samples and samples types taken from individual cleanroom are shown in Fig. 1. Of these, there were 1.717 swabs of working surfaces and hands, 7.257 settle plates and 545 RODAC plates, which were used for active air sampling. Surface control with swabs and air control by volumetric method was carried out once a month in all cleanrooms, while sedimentation plates were exposed daily from 2012 in all operating rooms including laminar air flow chambers. On the average, there were between six to eight samples per day.
Fig. 1.
All types and numbers of microbiological samples in the years between 2011 and 2016 and from different cleanrooms (CR). PI: for parenteral preparations, PA: for ophthalmics, PCT: for cytostatic therapy, PPP: for parenteral nutrition.
During the first year, 10.2% samples were positive. As soon we started with daily exposures of settle plates, the number of positive samples decreased and remained stable at 4.0%. The number of positive samples, where microbial growth was detected, were somewhat different depending on used method and cleanroom where the samples were taken. It is shown in Table 3.
Table 3.
Percentage of positive samples with different sampling method; PI: for parenteral preparations, PA: for ophthalmics, PCT: for cytostatic therapy, PPP: for parenteral nutrition.
| Swabs |
Settle plates |
Active air samples |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PI | PCT | PPP | PA | PI | PCT | PPP | PA | PI | PCT | PPP | PA | |
| 2011 | 7.27% | 3.08% | 0.00% | 5.26% | 7.69% | 0.00% | 0.00% | 9.09% | 48.15% | 41.67% | 33.33% | 38.46% |
| 2012 | 13.75% | 14.89% | 8.64% | 0.00% | 0.29% | 0.00% | 0.00% | 1.45% | 60.42% | 56.52% | 50.00% | 33.33% |
| 2013 | 8.22% | 7.79% | 6.94% | 10.00% | 0.00% | 0.00% | 0.00% | 0.83% | 50.00% | 26.32% | 30.00% | 35.00% |
| 2014 | 17.02% | 6.38% | 5.36% | 7.41% | 0.00% | 0.00% | 0.00% | 0.71% | 44.44% | 81.25% | 33.33% | 20.00% |
| 2015 | 11.40% | 0.00% | 1.52% | 10.96% | 0.00% | 0.00% | 0.00% | 0.98% | 78.05% | 57.89% | 35.00% | 22.22% |
| 2016 | 11.93% | 4.35% | 9.09% | 15.94% | 0.00% | 0.00% | 0.00% | 0.87% | 75.00% | 62.50% | 37.50% | 50.00% |
| (2011–2016) | 11.51% | 6.64% | 4.50% | 8.35% | 0.14% | 0.00% | 0.00% | 1.05% | 59.82% | 54.29% | 37.27% | 33.02% |
| n (2011–2016) | 55 | 28 | 19 | 33 | 3 | 0 | 0 | 16 | 134 | 57 | 41 | 35 |
Most positive samples were established with the volumetric air sampling as shown in Table 3. This is explainable as this method is actively collecting larger (air) samples and thus provides results that are more representative. This method enables determination of the number of microorganisms in a cubic meter of air (free and that bound to particles in the air). With sedimentation method, on the other hand, we can identify any microorganism that falls on the medium due to the free fall only. Although most of the “whole” particles (microbe with a carrier) are usually greater than 12 μm, the time during which the particles remain suspended in the air is highly dependent on the air turbulence in a room. This may affect the reliability of these results, because the greater the turbulence of air in the room and the smaller the particles are, the longer is time for the particles to fall on the test medium (Sandle, 2015b). This claim can be confirmed by particle size data in the annual validation report.
It is shown on the Table 3 that the highest and almost doubled number samples with microbial growth were determined in PI. The possible reason lies in the room volume, which is much bigger than in others and that there is a need for more samples for the overall assessment. The results are, however, not as critical as many samples were taken at sites and at times when the product was no longer in direct contact with the air. In the PI, unlike in other cleanrooms, all the products are manufactured serially and terminally sterilized and analysed for bacterial endotoxines.
The very low number of positive samples in the sedimentation method over all six years monitoring time, as shown in Table 3, is due to the fact that the sedimentation plates were exposed in working places with highest grade of cleanliness – Class A (Laminar Air Flow Chambers).
The advantage of the passive method is the possibility of continuous exposure of the medium to up to four hours (European Commission, 2009), however, the time of the maximum allowed exposure of the medium must be validated with appropriate tests (Sutton, 2010b), otherwise the problem with drying of the medium and hence, its “cultivation” properties may become questionable. Since the microorganisms respond differently to the stress they are experienced during sampling, their numbers may be inaccurately assessed due to the reduced cultivation capacity of the medium (Fakruddin et al., 2013, Stewart et al., 1995). The type and amount of grown microbes are also affected by the type of growing culture media and the conditions of cultivation. We can expose only one, for bacteria and fungi universal growth medium, which is then, incubated under two different conditions or we can expose two selective media (Sandle, 2014). Because passive sampling does not intervene in the immediate environment, the media can be exposed in close proximity to critical control points, which is useful primarily for chambers with laminar air flow (Sandle, 2015b, Whyte, 1996). The results obtained are expressed in the number of colonies after 4 h exposure. However, when interpreting the results obtained from sedimentation plates, one colony may mean one microorganism, one pair of microorganisms, a chain, or a whole cluster of microorganisms transmitted on a sedimentary particle (Sandle, 2015b).
The classical methods have certain disadvantages; especially with regard to the ability of the organisms to grow on the microbiological medium and be identified. Most of the techniques prescribe the use of a sterile brush consisting of a more or less flexible holder with fibrous tip, wetted with a suitable wetting solution. Sample is collected by rubbing the sterile brush over the sampling point (Moore and Griffith, 2002a, Moore and Griffith, 2007). The bacteria are thus removed and transferred to the solid medium directly or via liquid medium. Only these can support the survival or recovery of microorganisms with their properties, or neutralize any used cleansers and disinfectants (Moore and Griffith, 2007). It is important, however, that the solution used during the sampling and counting period neither qualitatively nor quantitatively changes the population of microorganisms (Moore and Griffith, 2007).
Efficacy of sample collection technique and the proportion of microorganisms captured can be reduced in all three main stages of testing, namely, sampling the surface, release of microorganisms from swabs and subsequent cultivation (Moore and Griffith, 2007). Since the technique itself and also the force used with sampling is very difficult to standardize, the sampling phase is critical, that is, the successful capture of the microorganism from the working surface. It has been found, that the fraction of the bacteria captured by the swab is strongly dependent on the type of material of the swab, wetness and the type of liquid medium, wetness/dryness of the sampled area, previous use of disinfectants etc. The proportion of microorganisms that is later released into the microbiological medium from the swab depends on the same factors. The speed of the transport, the transport itself and sample storage conditions before adjusting the growing conditions are also important. It was previously proven, that despite the fact that all the optimal parameters were provided, the effectiveness of the sample collecting can be achieved from a minimum of 0.5 to up to a maximum of 25%, where the main limiting factor was defined as the release phase of the bacteria from the swabs to the medium (Davidson et al., 1999, Moore and Griffith, 2002b).
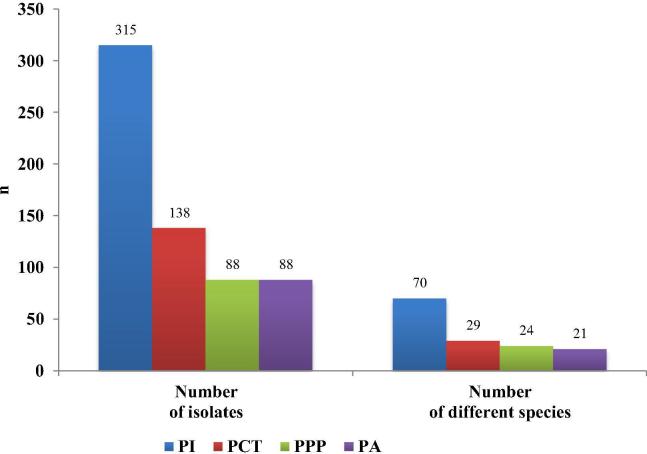
The highest diversity was observed in PI. Production process as it is in PI cleanroom and the equipment itself can have a strong impact on microbiota and microbiological quality of the products. The data are shown in Figs. 2 and 3. Generally speaking, more phases the production process has, the greater the number of equipment and personnel, the greater is the number of microorganism identified. Because of this reason, the equipment must be designed from stainless materials with as much flat surfaces as possible, with as few dead spots as possible and with the possibility of continuous cleaning and disinfection. Due to difficult cleaning, microbial contamination is the most problematic with scrubbers, pumps, valves and various connecting pipes. A great possibility for contamination is also the state of wet equipment (Tršan and Pečar, 2010).
Fig. 2.
Number of isolates in individual CRs and the number of different isolated species. PI: for parenteral preparations, PA: for ophthalmics, PCT: for cytostatic therapy, PPP: for parenteral nutrition.
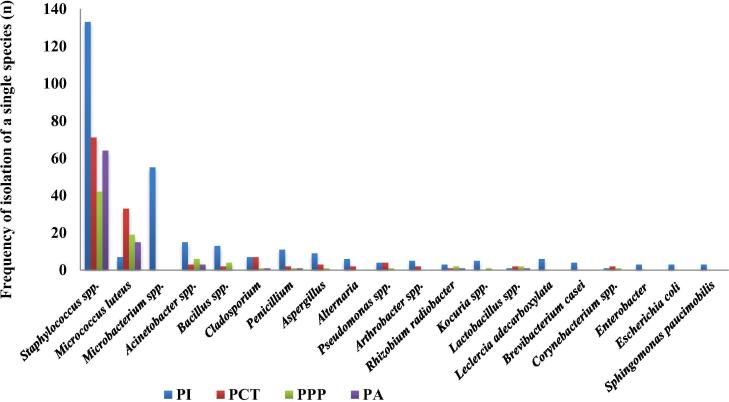
Fig. 3.
The frequency of isolation of individual species (more than 0.5%) in the various CRs. PI: for parenteral preparations, PA: for ophthalmics, PCT: for cytostatic therapy, PPP: for parenteral nutrition.
In PA Staphylococcus spp were the most frequent – 72.7% among 88 isolates. 17% were Micrococcus bacteria, which was also expected because they are an integral part of normal human flora, especially skin and the upper respiratory tract, and are the main source of contamination of cleanrooms (Tršan and Pečar, 2010). They together represent 89.7% of the isolated population, while the remaining 10.3% consist of bacteria Acinetobacter spp, moulds from the air and some other individual species.
The distribution of bacterial species/genus was similar in all cleanrooms. The most commonly isolated microorganisms in PI were from genus Staphylococcus, Micrococcus and Acinetobacter. Some microbes, including Gram-negative bacteria and fungi, appear only once. This is undoubtedly a consequence of the presence of a larger number of personnel in the preparation process and the increased number of cleaning personnel, while the staffs in PA are relatively small and constant.
The situation is similar in the PCT room, although the number of staff and fluctuation is even greater. Due to the specifics of the prepared products in both rooms, the quantity of the medical equipment and consumables used in short period of time is also inappropriately higher. Unfortunately, a lot of this material is not designed and properly packaged in a double dust-free package that would be suitable for cleanrooms. The presence of other microbes is much smaller, in less than 0.5% of cases and because of that they are not shown in the figure. Due to space limitations, both cleanrooms are located nearby transport corridor, which additionally contribute to the number and diversity of detected microorganisms.
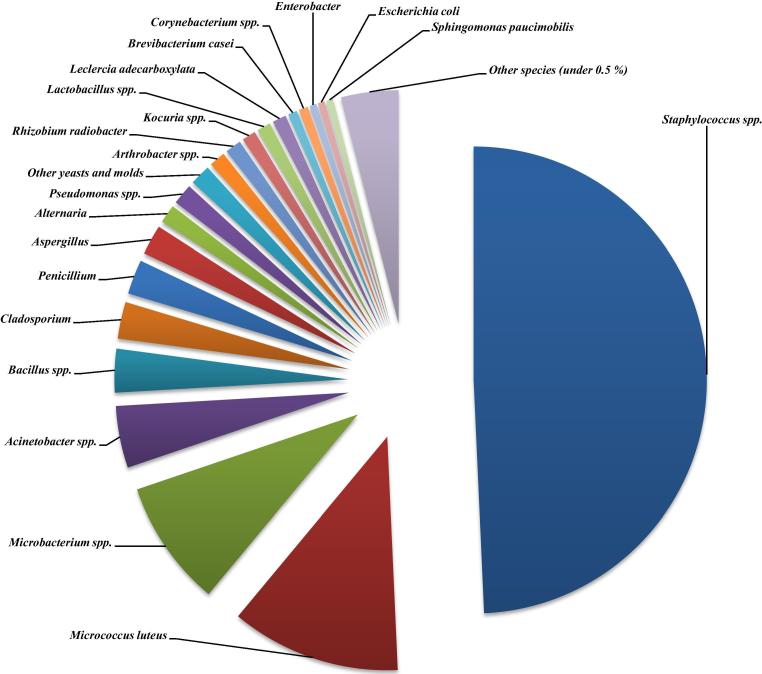
The total number of samples taken was 9519. There were 421 positive samples. A total of 629 isolates were identified from the positives representing 45 different microbial species. It is evident from Fig. 4 that working staff is the source for more than 70% isolates. There were 78.8% of Gram-positive bacteria, 11.2% Gram-negative and the rest 10.0% were fungi.
Fig. 4.
Catalogue of cleanrooms' microbiota – most commonly occurring species from all samples.
The presence of Gram-positive cocci is an indication of personnel cleanroom contamination. The personnel are by far the biggest threat for the contamination, since many microbes are part of a human microbiota, and many of them are facultative pathogens. On 1 cm2 of the skin on the human hand, there is about 32 000 bacteria; therefore, when sitting, a person releases 10,000 particles into the environment and even 1,000,000 when moving. The source of contamination can be skin, which constantly regenerates and loses dead epidermal cells, as about 10% of these cells carry with them microbes. These skin particles represent a source of food and moisture for these microorganisms, so they can be further reproduced. Due to all these reasons it is very important that the skin surface is protected by protective clothing, caps, footwear, mask and gloves (Tršan and Pečar, 2010).
The most commonly isolated species of the genus Staphylococcus were coagulase negative Staphylococci which are usually non-pathogenic, however, in immune compromised subjects they can cause serious, especially catheter-related infections (Becker et al., 2014). In 50% of cases, S. hominis, S. epidermidis and S. haemolyticus were identified (Fig. 4).
The presence of gram negative bacteria may be connected with water or moisture. The vast majority of bacteria found in the pharmaceutical water systems, where they enter with drinking water, are Gram-negative no fermentative rods (Resnik and Kerč, 2010, Tršan and Pečar, 2010). Their multilayer cell wall, which is also a source of endotoxines, protects them from an extremely hypotonic environment that is characteristic for pharmaceutical waters (Tršan and Srčič, 2016). Another surviving mechanism in the case of unsuitable living conditions for the bacteria is their ability to be attached to the surface, where they form a biofilm, a very resistant form of microbial growth, which is hardly destroyed even with disinfection (Resnik and Kerč, 2010). Gram-positive microorganisms are extremely rarely identified in pharmaceutical waters, because, in contrast to Gram-negative, they require larger concentration of organic matter.
We are usually mainly concerned with the presence of Enterobacteriacae, because they are the main indicator of faecal contamination (Tršan and Pečar, 2010). In our cases there were detected Enterobacter, Erwinia spp., Escherichia coli and Klebsiella oxytoca. Among Gram-negative bacteria Pseudomonas and pathogenic species of the genus Acinetobacter have been detected too.
Air is not a normal living environment for the microorganisms and is primarily used only as a transfer medium. Most microorganisms are associated with physical particles in air and represent mainly endospore-forming microorganisms: Bacillus and Clostridium. Others are Staphylococcus spp., Streptococcus spp. Corynebacterium and fungi Aspergillus and Penicillium (Resnik and Kerč, 2010). The recovery of endospore-forming bacteria and fungi, for example, could indicate a problem with HEPA filters or insufficient pressure difference between a clean and less clean area.
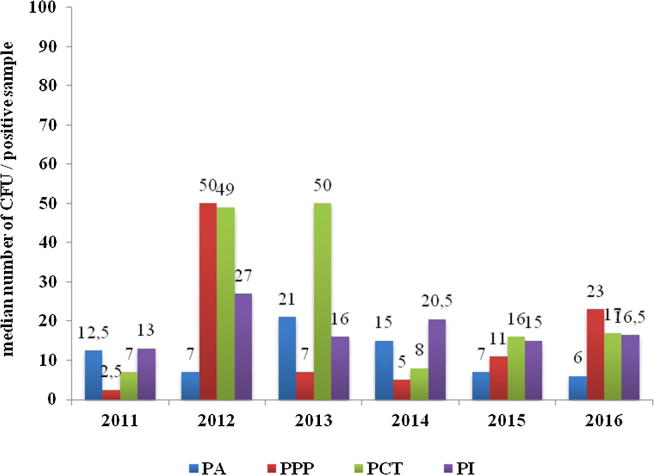
After examining all the positive results, it was established that in the most cases and in all cleanrooms the results were on average between 23 and 109 colonies per unit (CFU/dm2) (Fig. 5). The lowest values obtained were measured in the aseptic product preparation area (PA). Two values, namely 2012 in PCT and 2016 in PI, stand out from the average. In both cases, the samples from the additional sampling point were collected and non-pathogenic Staphylococcus spp. and Microbacterium spp. have been isolated Additional sampling points are usually not critical sites for the product, however, they are very important for cleaning efficacy assessment.
Fig. 5.
Median number of total colony count. PI: for parenteral preparations, PA: for ophthalmics, PCT: for cytostatic therapy, PPP: for parenteral nutrition.
Numerical limits for microbiological tests are questionable because levels have to be reasonable in terms of the capability of the method (Sutton, 2012). Questionable are linear range of plate counts and a lower limit of quantification, which are approximately 25 colonies per plate. This is opposed to the limit of detection of one colony per plate. Alert and action levels in the 1–10 CFU range are therefore also of questionable accuracy.
For that reason a new CRR (Contamination Recovery Rate) approach is more convenient. In a given data set of samples a certain percentage of those samples can be expected to exhibit non-zero recoveries of contamination. The percentage of samples presenting contamination is then defined as the CRR. United States Pharmacopoeia (USP) considers that CFU counts in excess of 15 from a single ISO 5 environmental sample generally indicate that a significant change has occurred in that environment, as the occurrence of counts of that magnitude should generally be infrequent. USP stresses that the number of colonies, is not so important, but rather environmental changes (Sutton, 2012).
However, in our case, the mean total colony count data might be of importance, as the amount of ATP that can be expected in a given environment or a given cleanroom. Median total colony count per square dm calculated from all recoveries from all 9519 collected samples during last 6 years of environmental monitoring program is shown in Fig. 5.
The average value of ATP in Gram-negative bacteria, Gram-positive bacteria and fungi (Candida albicans) is 1.43, 12.28 and 212.81 femtogram (fg)/cell (Kodaka et al., 1996). This means that the level of ATP in the fungi is approximately 100 times greater than in the Gram-negative bacteria and the 10 times greater than amount of ATP in the Gram-positive bacteria, both the bacillus and the coccus. The amount of ATP in spores is very low due to lower metabolic activity.
However, taking into account the new CRR approach and as a certain degree of microbiota stability for a particular working environment has been confirmed, than the knowledge of the actual amount of microorganisms or present number of colonies would be not so important to assess the suitability of working environment anymore. In many cases, the magnitude of an individual deviation over the limit is less informative than the frequency with which contamination occurs.
4. Conclusion
Knowing the cleanrooms' microbiota is of the greatest importance for the quality control, for detecting changes and for analysing the trends. The results we have obtained provide information about facility performance, personnel cleanliness, gowning practices, the equipment and cleaning procedures. The main purpose of our study was to establish the microbiota catalogue that will be the foundations for introducing an alternative and fast method based on the measurement of the ATP presence. Although we used various techniques, sampling points, disinfection neutralizers, various types of media that were incubated in different conditions for a different periods of time and however, also various identification methods, we have actually came up with results that support and confirm the previous literature results. The microbiota in all cleanrooms was more or less constant and the results confirmed that it is closely related to the human, whose skin is the main source of the contaminants, i.e. Gram-positive bacteria of the genus Staphylococcus and Micrococcus. As the third major contaminant, a Gram-negative bacteria Acinetobacter was determined, and is originating from the air. Just in a few percent Gram-negative bacteria from water, fungi and others were confirmed. The isolated number of microorganisms varied in the individual samples, and is due to the known limitations and weaknesses of the methods used in the individual cleanrooms. However, on the average it was stable. Taking into account that with classical microbiological methods only about 10% of the microorganisms is detected and that Gram-positive bacteria are relatively large, with a high content of ATP in their cell; the average value of ATP 10–15 fg/cell (Kodaka et al., 1996), it is expected that a new bioluminescence method might be successfully implemented and would thus contribute to the fast and effective quality assurance system. Although the method will not meet the expectations of recognizing the trends of microorganisms by identity or characteristics, it will, however, be able to identify changes in trends and levels exceeding alert and action levels as part of the environmental monitoring program.
Footnotes
Peer review under responsibility of King Saud University.
References
- Becker K., Heilmann C., Peters G. Coagulase-negative Staphylococci. Clin. Microbiol. Rev. 2014;27(4):870–926. doi: 10.1128/CMR.00109-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundell A.M. Microbial testing in support of aseptic processing. Pharm. Technol. 2004;28:56–67. [Google Scholar]
- Davidson C.A., Griffith C.J., Peters A.C., Fielding L.M. Evaluation of two methods for monitoring surface cleanliness-ATP bioluminescence and traditional hygiene swabbing. Luminescence. 1999;14(1):33–38. doi: 10.1002/(SICI)1522-7243(199901/02)14:1<33::AID-BIO514>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Dixon A.M. CRC Press; 2007. Environmental monitoring for cleanrooms and controlled environments. [Google Scholar]
- Duguid J., Balkovic E., du Moulin G.C. RMM-rapid microbiological methods: where are they now? Am. Pharm. Rev. 2011;14(7):18. [Google Scholar]
- Easter M.C. CRC Press; 2003. Rapid microbiological methods in the pharmaceutical industry. [Google Scholar]
- European Commission . Volume 4. European commission; Brussels: 2009. Guidelines to good manufacturing practice medicinal products for human and veterinary use, Annex 1: manufacture of sterile medicinal products. (Eudralex). [Google Scholar]
- Fakruddin M., Mannan K.S.B., Andrews S. Viable but nonculturable bacteria: food safety and public health perspective. ISRN Microbiol. 2013 doi: 10.1155/2013/703813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith C.J., Cooper R.A., Gilmore J., Davies C., Lewis M. An evaluation of hospital cleaning regimes and standards. J. Hosp. Inf. 2000;45(1):19–28. doi: 10.1053/jhin.1999.0717. [DOI] [PubMed] [Google Scholar]
- Hussong D., Madsen R.E. Analysis of environmental microbiology data from cleanroom samples. Pharm. Technol. 2004;28(5 SUPP):10–15. [Google Scholar]
- Hussong D., Mello R. Alternative microbiology methods and pharmaceutical quality control. Am. Pharm. Rev. 2006;9(1):62–68. [Google Scholar]
- IMI . Ljubljana: Medicinska fakulteta. Inštitut za mikrobiologijo in imunologijo; 2016. Splošna navodila za odvzem in transport vzorcev za mikrobiološke preiskave. [Google Scholar]
- Jimenez L. Microbial diversity in pharmaceutical product recalls and environments. PDA J. Pharm. Sci. Technol. 2007;61(5):383–399. [PubMed] [Google Scholar]
- Kodaka H., Fukuda K., Mizuochi S., Horigome K. Adenosine triphosphate content of microorganisms related with food spoilage. Japan. J. Food Microbiol. 1996;13(1):29–34. [Google Scholar]
- Moore G., Griffith C. A comparison of traditional and recently developed methods for monitoring surface hygiene within the food industry: an industry trial. Int. J. Environ. Health Res. 2002;12(4):317–329. doi: 10.1080/0960312021000056429. [DOI] [PubMed] [Google Scholar]
- Moore G., Griffith C. Factors influencing recovery of microorganisms from surfaces by use of traditional hygiene swabbing. Dairy Food Environ. Sanitation. 2002;22(6):410–421. [Google Scholar]
- Moore G., Griffith C. Problems associated with traditional hygiene swabbing: the need for in-house standardization. J. Appl. Microbiol. 2007;103(4):1090–1103. doi: 10.1111/j.1365-2672.2007.03330.x. [DOI] [PubMed] [Google Scholar]
- Resnik M., Kerč J. Mikrobioloska kakovost farmacevtskih izdelkov = microbiological quality of pharmaceutical products. Farmacevtski vestnik. 2010;61:23–29. [Google Scholar]
- Sandle T. A review of cleanroom microflora: types, trends, and patterns. PDA J. Pharm. Sci. Technol. 2011;65(4):392–403. doi: 10.5731/pdajpst.2011.00765. [DOI] [PubMed] [Google Scholar]
- Sandle T. Examination of the order of incubation for the recovery of bacteria and fungi from pharmaceutical-grade cleanrooms. Int. J. Pharm. Compd. 2014;18(3):242–247. [PubMed] [Google Scholar]
- Sandle T. Woodhead Publishing; 2015. Pharmaceutical microbiology: essentials for quality assurance and quality control. [Google Scholar]
- Sandle T. Settle plate exposure under unidirectional airflow and the effect of weight loss upon microbial growth. Eur. J. Parenteral Pharm. Sci. 2015;20(2):45–50. [Google Scholar]
- Stewart S.L., Grinshpun S.A., Willeke K., Terzieva S., Ulevicius V., Donnelly J. Effect of impact stress on microbial recovery on an agar surface. Appl. Environ. Microbiol. 1995;61(4):1232–1239. doi: 10.1128/aem.61.4.1232-1239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton S. Qualification of an environmental monitoring program. J. Valid. Technol. 2010;16(2):78. [Google Scholar]
- Sutton S. The environmental monitoring program in a GMP environment. J. GXP Compl. 2010;14(3):22. [Google Scholar]
- Sutton S. USP< 1116> and contamination recovery rates. J. Valid. Technol. 2012;18(4):79. [Google Scholar]
- Tršan M., Pečar S. Dobra proizvodna praksa sterilnih izdelkov v bolnišnični lekarni s poudarkom na testiranju bakterijskih endotoksinov. specialistična naloga: M. Tršan. 2010 [Google Scholar]
- Tršan M., Srčič S. Implementacija avtomatiziranega testnega sistema testiranja pirogenosti v bolnisnicni proizvodnji parenteralnih raztopin = the implementation of the automatic test system for pyrogenicity testing in hospital parenteral production. Farm. Vestn. 2016;2016(67):25–37. [Google Scholar]
- Venkateswaran K., Hattori N., La Duc M.T., Kern R. ATP as a biomarker of viable microorganisms in clean-room facilities. J. Microbiol. Methods. 2003;Vol. 52:67–377. doi: 10.1016/s0167-7012(02)00192-6. Netherlands. [DOI] [PubMed] [Google Scholar]
- Whyte W. In support of settle plates. PDA J. Pharm. Sci. Technol. 1996;50(4):201–204. [PubMed] [Google Scholar]
- Whyte W. Testing, Operation. Johnson Wiley & Sons; England: 2001. Cleanroom technology-fundamentals of design; pp. 243–246. [Google Scholar]
- Willis C., Morley R., Westbury J., Greenwood M., Pallett A. Evaluation of ATP bioluminescence swabbing as a monitoring and training tool for effective hospital cleaning. British J. Infect. Control. 2007;8(5):17–21. [Google Scholar]
- Švent-Kučina N., Kofol R., Pirš M., Mrvič T., Germ J., Triglav T.K., Matos T. Vzorčenje bolnišničnega okolja in rok zdravstvenih delavcev = assessment of nosocomial surface and air contamination and contamination of hands of healtcare workers. Okuzbe, povezane z zdravstvom. 2013;2013(52):167–178. [Google Scholar]