Abstract
Background
Ischemic heart disorders and accumulation of lipids in blood vessels could contribute to angina pectoris. Therefore, the aim of this study was to formulate sublingual tablets containing a novel combination of Atorvastatin calcium (ATOR) and Trimetazidine HCl (TMZ) for efficient treatment of coronary heart disorders.
Methods
The dissolution rate of water-insoluble ATOR was enhanced via complexation with sulfobutyl ether-β-cyclodextrin (SBE-β-CD) and addition of soluplus as a polymeric solubilizer excipient. The solubilized ATOR and TMZ were compressed into a sublingual tablets by direct compression technique and evaluated for their tableting characteristics. In addition, a new validated method based on High Performance Thin Layer Chromatography (HPTLC) was developed for simultaneous determination of both drugs in pure forms and sublingual tablets.
Results
The developed HPTLC method showed LODs of 0.056 and 0.013 μg/band and LOQs of 0.17, 0.040 μg/band for TMZ and ATOR, respectively and proved to be linear, accurate, precise and robust. The optimum formulation containing mixture of superdisintegrants; Ac-Di-Sol and crospovidone (4.8% w/w, each) showed the shortest disintegration time (65 s) and enhanced release profiles of both drugs.
Conclusions
The prepared sublingual tablets combining ATOR and TMZ will be a promising dosage form for coronary heart disease patients with an instant action and improved patient compliance.
Keywords: Atorvastatin, Trimetazidine, Sublingual tablets, HPTLC, Sulfobutylether-β-Cyclodextrin
1. Introduction
Coronary heart disease is the number-one killer of both men and women around the world. It causes dangerous thickening and narrowing of the coronary arteries that bring blood to the heart, which disrupts the flow of oxygen and nutrients to the heart, causing serious problems (Hao et al., 2015, Jiangyou et al., 2016). Nowadays, there are different strategies for the treatment of cardiovascular diseases. Statins family is one of the most recommended therapies for the primary and secondary prevention of cardiovascular disease. This could be attributed to their anti-hyperlipidemic effect through lowering the low-density lipoprotein (LDL) and to their pleiotropic effects including reduced vascular inflammation and platelet adhesion (Lim, 2013, Yildiz et al., 2007).
Atorvastatin calcium (ATOR) (Fig. 1.a) acts as a plaque stabilizer; therefore, it is used as a preventive treatment of strokes through its anti-inflammatory effect and possibly due to other mechanisms (Lim, 2013, Yildiz et al., 2007). Trimetazidine hydrochloride (TMZ) (Fig. 1b) is a cytoprotective drug acts mainly by inhibition of β-oxidation of free fatty acids (FFA), which in turn increases the metabolic rate of glucose (Piotr et al., 2014).
Fig. 1.
Chemical structures of Atorvastatin calcium (a) and Trimetazidine (b).
The combination therapy based on ATOR and TMZ could provide an effective improvement on cardiac functions; reduces the level of inflammatory factors and improves the endothelial function in patients with coronary heart disease which greatly reduce the risk of myocardial injury and enhance cure rates (Tian, 2016, Lin et al., 2013). Hence, combining both ATOR and TMZ into a single formulation will be beneficial for patients with coronary heart disease and can minimize the prevalence of postoperative myocardial injury as well as effectively improve their lipid profile (Song and Wang, 2018, Wang et al., 2018, Xu et al., 2017).
The combination of ATOR and TMZ needs pharmaceutical and analytical collaboration to get an optimized formulation. Hence, the lower solubility of ATOR and diminished dissolution could be an issue and how to quantitatively evaluate both drugs in the final formulation.
According to Biopharmaceutical Classification System (BCS), ATOR is classified under class II drugs, which are poorly water-soluble and highly permeable drug (Wadher et al., 2014). In addition, it has a very poor solubility in acidic pH (Ahjel and Lupuleasa, 2009). This leads to poor dissolution rate at gastric pH with consequent incomplete absorption and poor oral bioavailability of about 14% (Khan and Dehghan, 2011). Therefore, dissolution rate is the rate-limiting step for ATOR absorption. Whereas, TMZ HCL is a BCS Class-I drug which is highly soluble and highly permeable, so it doesn’t represent a challenge for formulation (Kumar et al., 2013). Several trials were reported to improve the aqueous solubility of ATOR including the formulation of solid dispersions (Wadher et al., 2014), the use of micro-environmental alkalinizing agents (Ahjel and Lupuleasa, 2009), self micro-emulsifying drug delivery systems (SMEDDS) and formulation of inclusion complexes (Hai and Ming, 2006, Palem et al., 2009).
Secondly, the different chemical nature and physico-chemical properties of both drugs as well as lack of analytical method to simultaneously determine the two drugs in the formulation or monitor their release profiles are a great challenge. Referring to the literature survey, there is no reported analytical method for simultaneous determination of ATOR and TMZ combination. ATOR has been determined using different analytical methods such as: HPLC (Goell et al., 2013, Hiral et al., 2014), capillary electrophoresis (Guihen et al., 2006, AlShehri, 2012), electrochemical (Rageh et al., 2018, Al-ghamdi, 2018), spectrofluorimetry (Ayad and Magdy, 2015, Sharaf El-Din et al., 2012), spectrophotometry (Llango and Shiji Kumar, 2012, Baghdady et al., 2013, Darwish et al., 2011). Densitometric methods have been also reported for determination of ATOR in combined dosage forms (Ramadan et al., 2014, Patole et al., 2011). Furthermore, there is a few number of reported methods is available for the quantification of TMZ hydrochloride. These include HPLC (Chowdhury et al., 2018), LC/ESI-MS (Xiong et al., 2011), spectrophotometric (Farzana et al., 2017, Aurelia Chiş et al., 2010), and electrochemical methods (Ghoneim et al., 2002). In addition, stability indicating HPTLC method of TMZ in the presence of its acid-induced degradation products has been reported (Bebawy et al., 2004) has been reported.
Comparing with other complicated separation techniques; HPLC and electrophoresis, densitometry is the most simple and cost-effective method with low volume consumption of solvents.
Hence, the aim of this study was to enhance the solubility of ATOR and hence its dissolution performance. Followed by combining both drugs in an oral sublingual formulation simultaneously with developing a simple, accurate and reproducible method for simultaneous quantification of both drugs the sublingual tablets. The solubility enhancement of ATOR was tried using different techniques such as grinding, physical mixing with a polymeric solubilizer; soluplus®, formulation of solid dispersion with a hydrophilic carrier; Pluronic F-68, formulation of loaded mixtures with SBE-β-CD and a combination of the most successful techniques was adopted. Then, sublingual tablets containing solubilized ATOR and TMZ were formulated and tested for their tableting performance. In addition, HPTLC analysis for simultaneous quantification of both drugs has been developed and validated according to International Conference on Harmonization (ICH) guidelines.
2. Materials and methods
2.1. Materials
Atorvastatin calcium and trimetazidine hydrochloride were kindly supplied by Eipico Company, Cairo, Egypt. Soluplus® and SBE-β-CD were obtained from Ligand Technology, USA. All pharmaceuticals were checked for purity by TLC. Acetonitrile, Ethyl acetate, hexane, chloroform and magnesium stearate were supplied by El-Nasr Pharmaceutical Chemicals Co. (Cairo, Egypt). HPTLC aluminum sheets pre-coated with silica gel G 60F254 plates were obtained from Fluka, 20 × 20 cm, 0.20 mm layer thickness. Pluronic F-68 was purchased from Sigma Aldrich Co., USA. All other chemicals and reagents used in this study were of analytical grade and used without further purification.
2.2. Methods
2.2.1. Preparation of standard solutions of synthetic mixture
Standard stock solutions containing 0.25 and 1.00 mg/mL of ATOR and TMZ respectively was prepared in 10 ml volumetric flask by dissolving 2.50 mg ATOR and 10.00 mg TMZ in methanol. The stock solutions were stored at 4 °C in amber glass vessels and can be used within 2 days. The working solutions were prepared by further dilutions of the standard solution with methanol.
2.2.2. Chromatographic conditions
2.2.2.1. General procedure
A Camag-HPTLC system comprising of Linomat-5 automatic sample applicator and Camag TLC scanner III with winCAT'S version 4.0 software (Muttenz, Switzerland) was used. A Camag 100 µL Sample syringe (Hamilton, Bonaduz, Switzerland) was used for sample application as bands (band size: 4 mm) under a stream of nitrogen on aluminum plates coated with silica gel G 60F254 (20 × 5 cm). UV lamp (short wavelength 254 nm, Vilber Louranate 220 V 50 Hz, Marne-la-Vallee Cedex, France) and TLC tank (standard type, 27.0 cm width × 26.5 cm height × 7.0 cm diameter, Sigma-Aldrich Co., USA) were used.
TLC was performed on 20 × 20 cm aluminum foil-backed plates pre-coated with 0.20 mm layers of silica gel G 60F254. Before use, the plates were cut into 20 × 5 cm pieces. Chloroform–methanol–glacial acetic acid (68: 11.2: 0.8 v/v/v) was used as a mobile phase. The HPTLC plates were developed in a 27.0 cm W × 26.5 cm H × 7.0 cm D conventional TLC tank lined with thick filter paper for chamber saturation. Before use, the tank covered with its lid was pre-saturated with mobile phase (80 ml) vapor for at least 20 min at room temperature (25 ± 5 °C). Four microliters of the working standard solutions and/or sample solutions were applied to the plates, 1 cm from the lower edge, with a Linomat-5 automatic sample applicator. Plates were allowed to dry in air for 5 min. then transferred to the TLC tank and developed with the optimized mobile phase till the ascended solvent front moved about three-fourths the length of the plate from the origin (approximately 5 min). After development, plates were removed, dried in air for 5 min. and scanned by a Camag Dual-wavelength TLC scanner III with winCAT’s version 4.0 software in the absorbance mode at 246 nm and 230 nm for ATOR and TMZ, respectively.
2.2.2.2. Construction of calibration curves
Four microliters from the working standard solutions containing different concentrations of the studied drugs in the range of (0.05–1.00 and 0.2–4.00) µg/band of ATOR and TMZ; respectively were applied to HPTLC plates. The working synthetic mixture solutions of the binary mixture were spotted in triplicate on HPTLC plates. The plate was then developed and densitometric analysis was achieved as described under general procedure. The data of peak area versus drug concentration was treated by linear least square regression analysis.
2.2.2.3. Validation of the analytical method
Validation studies were conducted using the optimized assay conditions based on the principles of validation described in the ICH guidelines. Key analytical parameters including linearity, lower limit of detection (LLOD), lower limit of quantification (LLOQ), precision, accuracy and robustness were evaluated. Results were expressed as percentages, with n; representing the number of samples.
2.2.2.3.1. Linearity
Linearity of the peak area response was verified at six concentration levels with triplicate measurements. The obtained data were then treated by linear least square regression analysis.
2.2.2.3.2. Lower limits of detection (LLOD) and quantification (LLOQ)
LLOD and LLOQ were calculated as 3.3σ/S and 10σ/S, respectively, where σ is the standard deviation of the intercept of the regression equation and S is the slope of the calibration curve.
2.2.2.3.3. Precision and accuracy
Intra-day precision of the proposed method was tested by replicate analysis of the working standard mixture of ATOR-TMZ at three different concentration levels; low, medium and higher ranges of the calibration curve. This study was repeated for three days to determine the inter-day precision (n = 9). Precision was expressed in %RSD. Accuracy was expressed as percentage recovery:
| (1) |
2.2.2.3.4. Robustness
The robustness of the proposed method was determined by studying the effect of minor changes on the method performance such as the composition of mobile phase, saturation time and analytical wavelength.
2.2.3. Preformulation studies
2.2.3.1. Compatibility studies
Compatibility studies for ATOR, TMZ and the used excipients were carried out using Differential Scanning Calorimetry (DSC). DSC thermograms were obtained using a Shimadzu DSC-50 (Japan) equipped with a software computer program TA50. Approximately 5 mg of each sample of both drugs and their physical mixtures with the investigated excipients were placed in an aluminum pan of 50 µL capacity and 0.1 mm thickness, press-sealed with aluminum cover of 0.1 mm thickness. An empty pan sealed in the same way was used as a reference. Samples were heated from 30° to 300 °C at a rate of 10 °C min−1 and nitrogen flow of 40 ml/min. Indium was used as a standard for calibrating temperature.
2.2.3.2. In vitro dissolution performance of pure ATOR
USP dissolution apparatus II (Erweka, Germany) was used at 50 r. p. m. rotation speed. Ten milligrams of pure ATOR samples were sprinkled to the dissolution medium of 250 ml phosphate buffer solution with pH 6.8, equilibrated at 37 ± 0.5 °C (European Pharmacopoeia, 2014). At time intervals of 5, 15, 30, 45 and 60 min, samples (5 ml) of the solution were withdrawn by a volumetric pipette with 0.45 μm membrane filter (Agilent Technologies, Santa Clara, CA) and replaced with an equal volume of fresh dissolution medium equilibrated at 37 ± 0.5 °C. The samples were analyzed for the released amount of ATOR spectrophotometrically (JENWAY spectrophotometer-model 6305, UK) at λmax of 238 nm. The experiment was performed in triplicate and the average recordings were used for calculations.
2.2.3.3. Enhancement of ATOR dissolution rate
2.2.3.3.1. Effect of grinding
Pure ATOR was ground in a vibrating uniball mill (VEB Leuchten bau-KM1, Germany) for 15 min. Then, 10 mg samples were tested for the in vitro dissolution as described above.
2.2.3.3.2. Effect of polymeric solubilizer
ATOR was physically-mixed with soluplus® in ratios 1:1 and 1:2 w/w and the in vitro dissolution of ATOR from samples equivalent to 10 mg drug dose was determined as described above.
2.2.3.3.3. Formulation of ATOR solid dispersion
Solid dispersion of ATOR with Pluronic F-68 was prepared in weight ratio of 1:7 w/w by fusion method. Pluronic F-68 ratio was selected on basis of previous study (Aboutaleb et al., 2016a, Aboutaleb et al., 2016b). Briefly, the calculated amount of Pluronic F-68 was placed in a porcelain dish and heated till melting over steam bath. ATOR amount was dispersed gradually into molten carrier using a glass rod. After complete dispersion, the dish was removed from steam bath and left aside to cool at room temperature till solidification of its contents. Then, the solid dispersion formed was pulverized and samples equivalent to 10 mg ATOR were tested for in vitro dissolution rate as described above.
2.2.3.3.4. Formulation of ATOR loaded mixtures with SBE-β-CD
Loaded mixtures of ATOR with SBE-β-CD in molar ratios 1:1 and 1:2 were prepared by solvent evaporation method. The calculated amount of ATOR was dissolved in minimum amount of methanol while the required amount of SBE- β-CD was dissolved in a co-solvent system of methanol and dichloromethane (1:1 v/v). Both were kept with continuous stirring over a magnetic stirrer (Gallenkamp, UK). The drug solution was added gradually to SBE-β-CD solution with continuous stirring. The solvents were removed under reduced pressure at 40 °C in a thermostatically controlled Vacuum oven drier (Horyzont SPT-200, Poland) till constant weight was obtained. The prepared mixtures were pulverized and samples equivalent to 10 mg ATOR tested for the in vitro dissolution rate as described.
2.2.3.3.5. Effect of combination of loaded mixture and polymeric solubilizer
The optimum ratio of loaded mixtures prepared in the previous step was physically mixed with soluplus® in 1:2 w/w, loaded mixture to solubilizer; respectively and samples equivalent to 10 mg ATOR were tested for in vitro dissolution.
2.2.4. Formulation and evaluation of ATOR-TMZ sublingual tablets
2.2.4.1. Formulation of sublingual tablets
It was found that combination between ATOR-SBE-β-CD loaded mixture (1:2 M ratio) and soluplus® (1:2 w/w loaded mixture to soluplus®, respectively) showed the highest dissolution rate. Therefore, it was selected for sublingual tablets formulation. A fixed amount (50.8 mg) of this optimum formulation equivalent to 10 mg ATOR together with 20 mg of pure TMZ HCl were used in the formulation of sublingual tablets. Different diluents were used including soluplus®, anhydrous lactose and Avicel pH101. Ac-Di-Sol (Croscarmellose sodium), Crospovidone and Explotab (Sodium starch glycolate) were used as superdisintegrants to promote fast disintegration of tablets and they were incorporated in different concentrations ranged from 4.0 to 9.6 %w/w of the final tablet weight of 250 mg (Sheshala et al., 2011, Tawfeek et al., 2015, Tawfeek et al., 2017, Tawfeek et al., 2018). Magnesium stearate was used as a lubricant in a concentration of 1% (w/w). Cherry flavor was used as flavoring agent in concentration of 3.2% (w/w). Saccharin sodium was used as a sweetener agent in a concentration of 9.6% (w/w). All powders were mixed by trituration in a glass mortar with a pestle to obtain a uniform mixture. The blended powders were compressed into tablets weighing 250 mg using a single punch tablet machine (Erweka, Germany) having a die set of 8 mm diameter. Table 1. summarizes the prepared formulations.
Table 1.
Composition of the prepared formulations of sublingual tablets containing ATOR-TMZ combination.
| Ingredients | Formula composition (mg)a |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | F11 | F12 | |
| Solubilized ATORb | 50.8 | 50.8 | 50.8 | 50.8 | 50.8 | 50.8 | 50.8 | 50.8 | 50.8 | 50.8 | 50.8 | 10 |
| TMZ | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| Mg stearate | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Cherry flavor | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| Saccharin sodium | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 |
| Ac-Di-Sol | – | 10 | 20 | – | – | 10 | 12 | 12 | 12 | 24 | – | – |
| Explotab | – | – | – | 10 | 20 | 10 | 12 | 12 | – | – | 24 | – |
| Crospovidone | – | – | – | – | – | – | – | – | 12 | – | – | 24 |
| Soluplus | 145.2 | 135.2 | 125.2 | 135.2 | 125.2 | – | – | – | – | – | – | – |
| Anhydrous lactose | – | – | – | – | – | 125.2 | 121.2 | – | – | – | – | |
| Avicel pH 101 | – | – | – | – | – | – | – | 121.2 | 121.2 | 121.2 | 121.2 | 121.2 |
Total tablet weight was 250 mg.
Solubilized ATOR is ATOR-SBE- β-CD loaded mixture (1:2 M ratio).
2.2.4.2. Physico-chemical evaluation of the formulated sublingual tablets
2.2.4.2.1. Uniformity of tablets weight
Uniformity of tablets weight was determined according to (European Pharmacopoeia, 2014). Twenty tablets from each formulation were randomly selected and weighed individually. As stated by pharmacopoeia, for tablets weighing 80–250 mg, actual tablet weight should not be deviated from claimed value by more than 7.5%.
2.2.4.2.2. Uniformity of drug content
The drug content of the prepared sublingual tablets was determined using the (European Pharmacopoeia, 2014). Ten randomly selected tablets from each formulation were assayed individually. Each tablet was powdered and the active ingredients were extracted by continuous stirring with methanol in a 100 ml volumetric flask. The contents of flask were filtered and after suitable dilution, the solution was assayed by HPTLC according to the developed method described above. Drug content was expressed as a percentage of claimed labels and should be 100 ± 15% as stated in European Pharmacopeia.
2.2.4.2.3. Tablet friability
Twenty tablets were randomly selected from each formulation and revolved in a friabilator (Erweka, Germany) at 25 r.p.m. for 4 min. The tablets were brushed gently and the percentage of weight loss was calculated and should not exceed 1% according to (European Pharmacopoeia, 2014).
2.2.4.2.4. Tablet hardness
Erweka hardness tester (Erweka, Germany) was used for determination of tablet hardness. Ten randomly-selected tablets were tested individually and the average was considered (Aboutaleb et al., 2016a, Aboutaleb et al., 2016b).
2.2.4.2.5. Thickness and diameter of the prepared tablets
Twenty tablets from each formulation were investigated using a micrometer (Mitutoyo Co., Japan) and the average thickness and diameter were determined.
2.2.4.2.6. In vitro disintegration time of the prepared tablets
In vitro disintegration time study was carried out on randomly selected 6 tablets from each batch using the apparatus specified in the European Pharmacopoeia, 2014 (Erweka, Germany). A volume of 250 ml phosphate buffer solution (pH 6.8) equilibrated at 37 ± 0.5 °C was used as a disintegration medium and the time taken for complete disintegration of the tablet with no solid mass remaining in the apparatus was measured. According to Pharmacopoeial specifications for sublingual tablets, disintegration time should be less than 3 min (European Pharmacopoeia, 2014).
2.2.4.2.7. In vitro dissolution performance
Three randomly selected tablets from each formulation were studied using the same procedure and criteria described above in Section 2.2.3.2. The only difference was that ATOR and TMZ in samples were simultaneously quantified using the developed HPTLC method described above.
3. Results and discussion
In this study the combination of TMZ and ATOR into a sublingual tablet which would provide a synergistic improvement on the cardiac function as well as giving a faster action for a large population of patients has been developed. The interested drugs in their combination were determined using a novel dual wavelength TLC – densitometry which facilitates their quantification simultaneously in the formulated tablets.
3.1. Optimization of chromatographic conditions
3.1.1. Selection of the optimum detection wavelengths
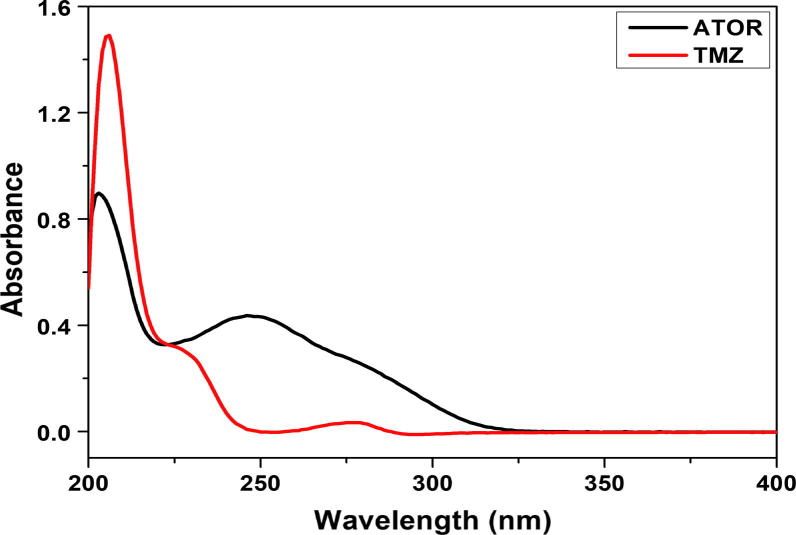
According to the UV absorption spectra of the methanolic solutions of TMZ and ATOR (Fig. 2) a dual wavelength detection mode at λmax of 230 and 246 nm respectively, was used. This mode of detection improves the method sensitivity as well as specificity with minimized noise level.
Fig. 2.
UV absorption spectra of the methanolic solutions of TMZ and ATOR.
3.1.2. Mobile phase composition
A laboratory prepared mixture of TMZ and ATOR was used to investigate the optimum separation conditions. Developing systems of different composition and ratios were tried (Table 2). Various band dimensions were tested in order to obtain sharp and symmetrical peaks. The separation of the two drugs was a challenging task as ATOR and TMZ possess a wide discrepancy in their physicochemical properties. ATOR is a weak acid with a pKa of 4.33 (carboxylic group) and log P (n-octanol-water partition coefficient) of 5.7. On the other side, TMZ is a weak base with a pKa1 of 4.45 and pKa2 of 9.14 (two nitrogen atoms of the piperidine ring) and log P of 1.04 (Wishart et al., 2006).
Table 2.
Different trials for selection of the optimum mobile phase for separation of TMZ - ATOR mixture.
| No. | Different trials | TMZ (Rf) | ATOR (Rf) |
|---|---|---|---|
| 1 | Ethyl acetate: hexane5: 5 (v/v) |
0.00 | With SFa |
| 2 | Ethyl acetate: hexane: ammonia5: 5: 0.1 (v/v/v) |
0.00 | With SF |
| 3 | Ethyl acetate: hexane: glacial acetic acid5: 5: 0.1 (v/v/v) |
0.00 | With SF |
| 4 | Ethyl acetate: hexane: glacial acetic acid8: 2: 0.1 (v/v/v) |
0.00 | 0.91 |
| 5 | Ethyl acetate: hexane: glacial acetic acid9: 1: 0.1 (v/v/v) |
0.00 | With SF |
| 6 | Ethyl acetate: hexane: water: glacial acetic acid8: 1: 1: 0.1 (v/v/v) |
0.05 | 0.90 |
| 7 | Ethyl acetate: methanol: glacial acetic acid8: 2: 0.1 (v/v/v) |
0.1 | 0.82 |
| 8 | Ethyl acetate: methanol: water: glacial acetic acid4: 4: 2: 0.2 (v/v/v) |
0.55 | 0.95 |
| 9 | Ethyl acetate: methanol: water: glacial acetic acid5: 4: 1: 0.1 (v/v/v) |
0.28 | 0.85 |
| 10 | Ethyl acetate: methanol: water: glacial acetic acid5.5: 3: 1.5: 0.1 (v/v/v/v) |
0.15 | 0.82 |
| 11 | Ethyl acetate: methanol: water: glacial acetic acid4: 4: 2: 0.1 (v/v/v/v) |
0.32 | With SF |
| 12 | Ethyl acetate: methanol: water: glacial acetic acid 5: 4: 1: 0.2 (v/v/v/v) |
0.23 | With SF |
| 13 | Ethyl acetate: methanol: water: glacial acetic acid6: 3.5: 0.5: 0.2 (v/v/v/v) |
0.14 | 0.98 |
| 14 | Ethyl acetate: methanol: glacial acetic acid6.5: 3.5: 0.5 (v/v/v) |
0.09 | 0.91 |
| 15 | Ethyl acetate: methanol: water: glacial acetic acid6: 3.5: 0.5: 0.1 (v/v/v/v) |
0.07 | 0.91 |
| 16 | Ethyl acetate: methanol: water: Ammonia6: 3.5: 0.5: 0.1 (v/v/v/v) |
0.20 | 0.84 |
| 17 | Chloroform: methanol: glacial acetic acid8.5: 1: 0.5 (v/v/v) |
0.16 | 0.95 |
| 18 | Chloroform: methanol: water: glacial acetic acid6: 3.5: 0.5: 0.2 (v/v/v/v) |
0.75 | With SF |
| 19 | Phosphate buffer (pH 6.9): ethyl acetate: methanol: water0.5: 1.3: 3.3: 4.8 (v/v/v/v) |
0.70 | 0.82 |
| 20 | Chloroform: methanol: glacial acetic acid7.5: 1.5: 1 (v/v/v) |
0.38 | With SF |
| 21 | Chloroform: methanol: glacial acetic acid8.5: 1: 0.1 (v/v/v) |
0.15 | 0.63 |
| 22 |
Chloroform: methanol: glacial acetic acid 8.5: 1.4: 0.1 (v/v/v) |
0.3 | 0.72 |
| 23 | Chloroform: methanol: glacial acetic acid8.5: 2.8: 0.2 (v/v/v) |
0.23 | 0.77 |
SF: solvent front.
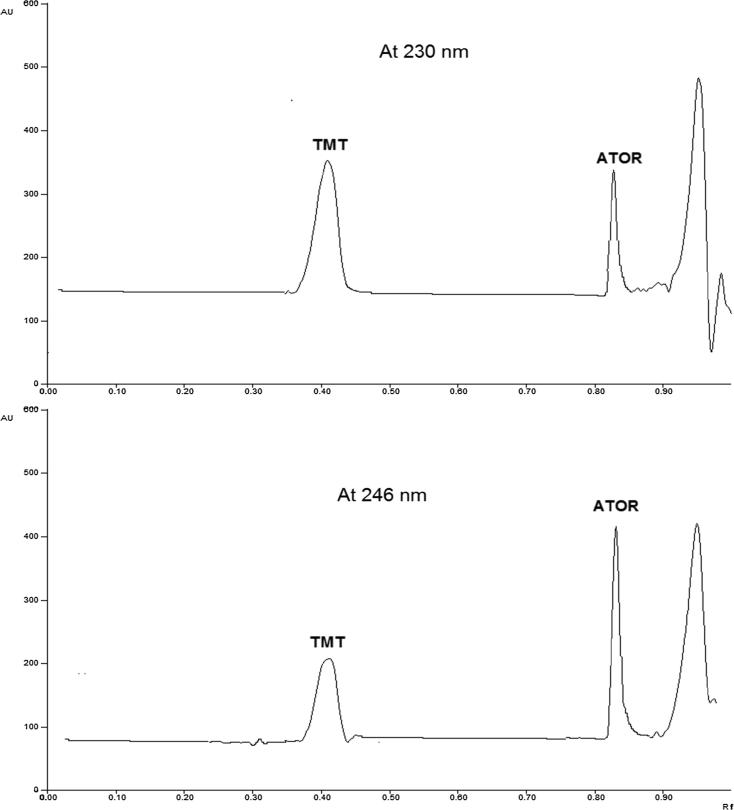
The typical well-known conditions for normal phase TLC with (1:1) ethylacetate: hexane as a mobile phase was studied first to investigate the separation of the two drugs. Being non-polar, ATOR migrates with the solvent front, leaving the positively charged TMZ, which is strongly attached to the negatively charged silica, at the start line. The situation was not improved upon trying to use organic modifiers such as ammonia or glacial acetic acid (Table 2). Upon trying to replace hexane with methanol or methanol/water mixture in order to render the mobile phase system more polar and to improve Rf of TMZ, still the Rf of ATOR is significantly high, which renders the accurate quantitation of ATOR impossible. This can be attributed to the high solubility of ATOR in methanol. The same holds true when using phosphate buffer, pH 6.9 (instead of glacial acetic acid), which was tried to minimize the degree of dissociation of TMZ, making it more non-polar. Although, this approach successfully increases Rf of TMZ and slightly reduces that of ATOR (due to dissociation of carboxylic acid moiety), however, there is no base line separation of the two compounds, even when trying different concentrations and ratios of phosphate buffer. Then, trying to replace ethylacetate with chloroform improves greatly the situation and reduces Rf of ATOR and after fine tuning the percentage of chloroform: methanol: glacial acetic acid (8.5: 1.4: 0.1 (v/v/v)) was selected as the optimum mobile phase system giving compact bands for each drug, with suitable Rf values, and used for subsequent spectro-densitometric analysis. The typical chromatograms for the tested drugs in the mixture were presented in (Fig. 3) at λmax of 230 and 246 nm, respectively.
Fig. 3.
Typical chromatograms for the tested drugs in their synthetic mixture at λmax of 230 and 246 nm, respectively using the optimized chromatographic conditions.
3.2. Method validation
The last step of the present study was to check method’s validation for linearity intra/inter-day precision, accuracy, and robustness according to ICH guidelines. For the statistical analysis, Microsoft Office Excel 2016 (Microsoft Corporation) was used. An excellent linearity was established at six levels in the range of 0.2–4.0, 0.05–1.0 μg/band for TMZ & ATOR respectively with R2 of more than 0.999 for the two analytes. The slope and intercept of the calibration curve were 2527.7 and 959.55 for TMZ, 4947.20 and 936.13 for ATOR, respectively. The LLODs were 0.056 and 0.013 μg/band and the LLOQs were 0.17, 0.040 μg/band for TMZ and ATOR respectively (Table 3). The intra and inter-day precision was confirmed since, the %RSD was found to be ≤1.938 and ≤1.995 respectively (Table 4). In addition, the accuracy was presented as % recovery within the range of 94.00–98.80 % indicating the good accuracy of the developed method. Robustness study reveals that small changes did not alter the retardation factor or the peaks resolution and therefore it can be concluded that the method conditions are robust (Table 5).
Table 3.
Optimum conditions and quantitative parameters of the proposed spectrodensitometric method.
| Studied mixture | TMZ – ATOR | |
|---|---|---|
| Component drugs | TMZ | ATOR |
| Analytical wavelength (nm) | 230 | 246 |
| Rf | 0.39 | 0.77 |
| Linearity range (µg/band) | 0.2–4.0 | 0.05–1.0 |
| Correlation coefficient (R) | 0.9997 | 0.9996 |
| R2 | 0.9995 | 0.9994 |
| Intercept (a) ± SD* | 959.55 ± 42.91 | 936.13 ± 20.18 |
| Slope (b) ± SD* | 2527.7 ± 38.26 | 4947.20 ± 45.14 |
| LOD** | 0.056 | 0.013 |
| LOQ*** | 0.17 | 0.040 |
Average of six replicates.
Limit of detection (µg/band).
Limit of quantitation (µg/band)
Table 4.
Intra-day and Inter-day precision of the proposed spectrodensitometric method for TMZ – ATOR synthetic mixture.
| Drug | Added Conc. (µg/band) | Intra-day precision (n = 3) |
Inter-day precision (n = 9) |
||||
|---|---|---|---|---|---|---|---|
| Found Conc. (µg/band) | RSD% | Accuracy% | Found Conc. (µg/band) | RSD% | Accuracy% | ||
| TMZ | 0.4 | 0.391 | 1.938 | 97.833 | 0.387 | 0.779 | 94.5 |
| 1.6 | 1.530 | 1.414 | 95.626 | 1.573 | 1.304 | 98.337 | |
| 3.2 | 3.151 | 0.446 | 98.453 | 3.162 | 0.437 | 98.803 | |
| ATOR | 0.2 | 0.188 | 1.737 | 94.00 | 0.187 | 1.821 | 93.333 |
| 0.4 | 0.384 | 1.688 | 96.00 | 1.573 | 1.995 | 95.083 | |
| 0.8 | 0.784 | 0.522 | 98.417 | 3.162 | 0.691 | 98.043 | |
Table 5.
Robustness of the proposed spectrodensitometric method for determination of TMZ - ATOR mixture.
| Component drugs | TMZ | ATOR |
|---|---|---|
| No variation Chloroform/methanol/glacial acetic acid (68: 11.2: 0.8 v/v/v) |
99.23 ± 0.45 | 98.21 ± 1.09 |
| Chloroform; +5% | 97.11 ± 0.72 | 96.34 ± 0.54 |
| −5% | 99.11 ± 0.65 | 100.21 ± 1.23 |
| Methanol; +5% | 97.13 ± 1.11 | 99.08 ± 0.50 |
| −5% | 99.12 ± 0.88 | 99.34 ± 0.34 |
| Glacial acetic acid +5% | 97.09 ± 1.22 | 100.32 ± 1.02 |
| −5% | 100.88 ± 1.24 | 99.24 ± 1.23 |
| Saturation time; +5 (min) | 99.11 ± 0.78 | 100.07 ± 0.76 |
| − 5 | 99.09 ± 1.13 | 100.22 ± 1.87 |
| Analytical wavelength; +5(nm) | 96.28 ± 0.99 | 99.72 ± 1.32 |
| −5 | 99.15 ± 1.13 | 97.23 ± 0.39 |
*Average of three replicates, Results are expressed as % Recovery ± standard deviation.
3.3. Preformulation studies
3.3.1. Compatibility studies
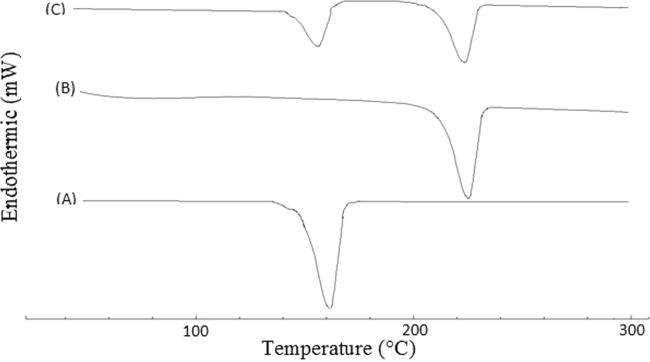
Fig. 4 shows DSC thermograms for pure ATOR, TMZ and their physical mixture at a weight ratio of 1:2, which was selected to simulate the same ratio of the two drugs in the prepared tablets. Both drugs showed sharp melting endothermic peaks at 161.19 and 228.0 °C for ATOR and TMZ (Fig. 4, Traces, A and B), respectively. Physical mixture of the two drugs showed the same peaks without significant shift indicating absence of incompatibilities between them. In addition, it was also found that DSC thermograms for the two drugs combination with all investigated excipients didn’t show significant shift in these peaks confirming absence of incompatibilities between them and their suitability to be used in this study. Detailed DSC thermograms and their interpretation are shown in the supplementary data file (Figs. S1-S10 and Table S1).
Fig. 4.
DSC thermograms of pure ATOR (A), pure TMZ (B) and their 1:2 w/w physical mixture (C).
3.3.2. Enhancement of ATOR in vitro dissolution by different techniques
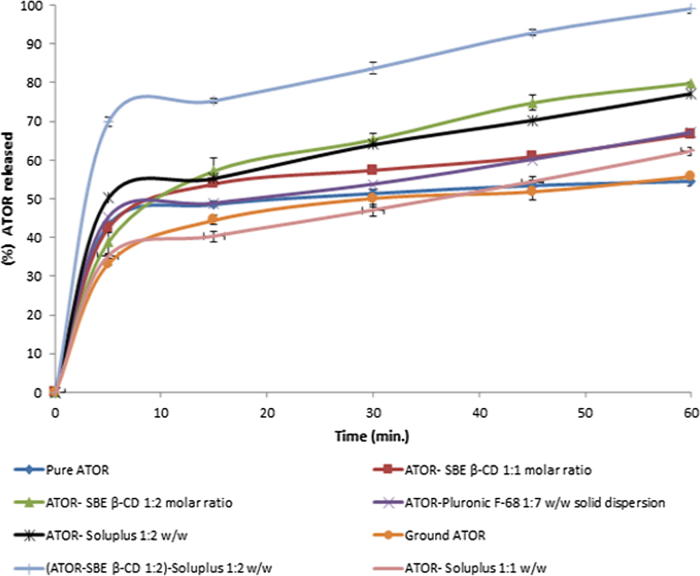
Fig. 5 shows release profiles of ATOR from different preparations attempted to improve its dissolution performance. Pure ATOR showed poor dissolution rate (54% after 60 min) due to its limited aqueous solubility (Ahjel and Lupuleasa, 2009). Grinding of pure ATOR didn’t show significant effect on its dissolution rate. Formulation of solid dispersion with Pluronic F-68 showed some improvement in the dissolution rate (67% after 60 min), but it was not considered enough to build on. Using soluplus® as a solubilizer or formulation of inclusion complex with SBE-β-CD at 1:1 w/w and 1:1 M ratio, respectively resulted in some improvement in ATOR release profile (62.23% and 66.57% after 60 min, respectively) which was markedly enhanced upon doubling drug to carrier ratio to 1:2 w/w and 1:2 M ratio for soluplus® and SBE-β-CD, respectively (77.23 and 80% released after 60 min, respectively). Soluplus® is known to have a powerful solubilizing effect and has shown excellent improvement in the solubility of BCS class II drugs (Shamma and Basha, 2013). In addition, SBE-β-CD is a novel derivative of cyclodextrins family that also showed promising successes with several poorly-soluble drugs through inclusion of the drug within its hydrophobic core that forms excellent environment for water-insoluble drugs, while its hydrophilic surface imparts good aqueous solubility (McIntosh et al., 2004, Cushing et al., 2009, Kim et al., 1998). Furthermore, combining both Soluplus® and SBE-β-CD in the formulation with ATOR to exploit their advantages by solubilizing the drug using different mechanisms was also investigated. Surprisingly, we found that combination of ATOR-SBE-β-CD loaded mixture (1:2 M ratio) together with soluplus® (1:2 w/w loaded mixture to Soluplus®, respectively) achieved the highest dissolution rate (approximately 100% after 60 min); therefore it was selected to be incorporated into the formulated sublingual tablets.
Fig. 5.
In vitro release profiles of ATOR from different formulations adopted to enhance its dissolution rate.
3.4. Physico-chemical evaluation of the formulated sublingual tablets
3.4.1. Physical properties
Results revealed that all the prepared tablets had uniform weight ranging from (245–255.5 mg), thickness (2.56–2.76 mm) and diameter (7.92–8.11 mm). Results of drug content (90–95%) and friability (weight loss 0.233–0.656%) studies were acceptable according to the Pharmacopoeial specifications mentioned above. Tablet hardness values ranging from 2.4 to 5.5 kg/cm2 which were suitable values for sublingual tablets (Aboutaleb et al., 2016a, Aboutaleb et al., 2016b). So, the tablets were accepted to be used for further studies.
Formulation F1 containing no superdisintegrants showed higher disintegration time more than 3 min. The addition of 4.0% (w/w) superdisintegrant (Ac-Di-Sol, crospovidone or Explotab) reduced disintegration time owing to wicking and capillary effects exerted by superdisintegrant leading to faster disintegration of tablet upon contact with water (Aboutaleb et al., 2016a, Aboutaleb et al., 2016b). The disintegration time was inversely proportional to superdisintegrant concentration in tablets in the investigated concentration range (4.0–9.6% w/w) as published before (Tawfeek et al., 2015). Tablets containing Ac-Di-Sol showed a shorter disintegration time than those containing Explotab or crospovidone which could be attributed to slower water uptake and the gelling tendency of both Explotab and crospovidone which delaying their effect (Aboutaleb et al., 2016a, Aboutaleb et al., 2016b, Shazly et al., 2013). Whereas, using a mixture of Ac-Di-Sol (4.8% w/w) and crospovidone (4.8% w/w) in formulation (F9), a faster disintegration time has been obtained (65 sec). Probably, this can be explained on the basis of different mechanisms of actions of the used superdisintegrants. Ac-Di-Sol works by both wicking and swelling mechanisms due to its rapid wetting upon contact with water resulting from its high hydrophilicity, while crospovidone works mainly by wicking mechanism due to its capillarity nature with little wetting properties. Thus; their combination may act synergistically better than using one of them separately. Furthermore, it is reported that incorporation of excessive amount of a given superdisintegrant may form a gel that inhibits water penetration into the tablet core and thus; inhibiting tablet disintegration (Pabari and Ramtoola, 2012, Tanuwijaya and Karsono, 2013). Upon investigation of the effect of diluent type on the tablet properties, it was found that Avicel pH 101 showed shorter disintegration time than soluplus® or anhydrous lactose and was more efficient in tablet formulation for adjusting both tablet hardness and disintegration time. Table S2 in the supplementary data file shows disintegration time for all the prepared formulations.
3.4.2. In vitro dissolution performance
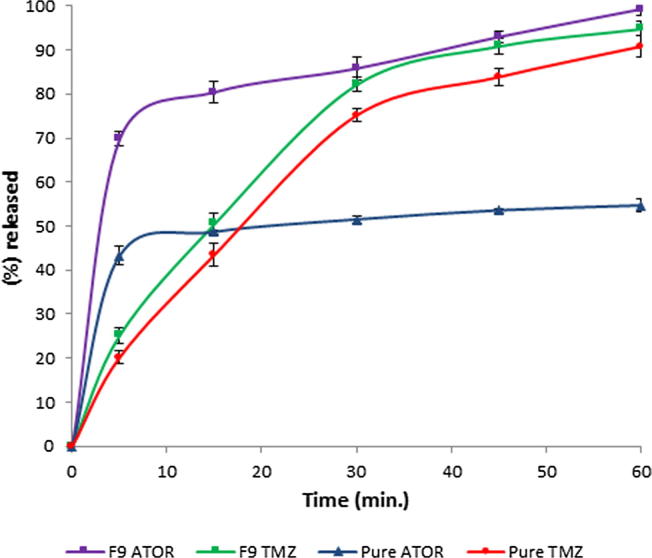
Fig. 6 shows the in vitro release profiles of both ATOR and TMZ from formulation (F9) which was selected as the optimum formulation regarding its superior physical properties. Both drugs in the optimized formulation showed enhanced release profiles compared to their pure forms. ATOR and TMZ showed 85.77 ± 1.6 and 82.16 ± 2.5 percentage released after 30 min and achieved almost complete release at the end of the investigated time span (60 min.). This improvement was obvious in case of water-insoluble drug; ATOR which showed about 2-fold enhancement in its dissolution rate relative to its pure status. This could be attributed to the solubilizing effect of SBE-β-CD and soluplus® as well as the fast tablet disintegration imparted by superdisintegrants which cooperated collectively to give enhanced release profile.
Fig. 6.
In vitro release profiles of ATOR and TMZ from the optimized formulation (F9) in comparison with the pure drugs.
4. Conclusions
A novel HPTLC-based analytical method was successfully developed for simultaneous determination of ATOR and TMZ in both pure state and sublingual tablet formulation. The developed method was sensitive, accurate, precise and robust in terms of validation. ATOR-sulfobutyl ether-β-CD/soluplus® showed the highest dissolution rate compared to the other investigated methods. ATOR/TMZ sublingual tablets showed acceptable physical properties and the optimized formulation showed excellent release profiles of both drugs and in vitro disintegration time of 65 s. The novel ATOR/TMZ sublingual tablets would be promising for coronary heart disease patients merging instant action and improved patient compliance.
Conflict of interest
The authors report no conflicts of interest. If the research data used in preparation of the manuscript is available, and if so, where to access this data.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jsps.2019.02.001.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Aboutaleb A.E., Abdel-Rahman S.I., Ahmed M.O., Younis M.A. Design and evaluation of domperidone sublingual tablets. Int. J. Pharm. Pharm. Sci. 2016;8(6):195–201. [Google Scholar]
- Aboutaleb A.E., Abdel-Rahman S.I., Ahmed M.O., Younis M.A. Improvement of domperidone solubility and dissolution rate by dispersion in various hydrophilic carriers. J. App. Pharm. Sci. 2016;6(7):133–139. [Google Scholar]
- Ahjel S.W., Lupuleasa D. Enhancement of solubility and dissolution rate of different forms of atorvastatin calcium in direct compression tablet formulas. Farmacia. 2009;57(3):290–300. [Google Scholar]
- Al-ghamdi A.F. High sensitivity determination of atorvastatin calcium in pharmaceuticals and biological fluids using adsorptive anodic stripping voltammetry onto surface of ultra-trace graphite electrode. Curr. Anal. Chem. 2018;14(2):92–100. [Google Scholar]
- AlShehri M.M. Validated capillary electrophoresis method for simultaneous determination of ezetimibe and atorvastatin in pharmaceutical formulations. Saudi Pharm. J. 2012;20(2):143–148. doi: 10.1016/j.jsps.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurelia Chiş A., Gabriela Gligor F., Cormoş G., Curea E., Bojiţă M. Spectrophotometric method for the determination of trimetazidine dihydrochloride from pharmaceutical forms. Farmacia. 2010;58(5):629–636. [Google Scholar]
- Ayad M., Magdy N. Application of new spectrofluorometric techniques for determination of atorvastatin and ezetimibe in combined tablet dosage form. Chem. Pharm. Bull. 2015;63(6):443–449. doi: 10.1248/cpb.c15-00023. [DOI] [PubMed] [Google Scholar]
- Baghdady Y., Al-Ghobashy M., Abdel-Aleem A., Weshahy S. Spectrophotometric and TLC-densitometric methods for the simultaneous determination of ezetimibe and atorvastatin calcium. J. Adv. Res. 2013;4(1):51–59. doi: 10.1016/j.jare.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebawy L.I., El Tarras M.F., El Sabour S.A. Determination of trimetazidine dihydrochloride in the presence of its acid-induced degradation products. J. AOAC Int. 2004;87(4):827–833. [PubMed] [Google Scholar]
- Chowdhury M.I., Ullah A., Al Maruf A., Islam M.S., Ahmed M.U., Shohag H., Azad M.K., Hasnat A. Validation and optimization of a simple RP-HPLC method for determination of trimetazidine in human serum and its application in a pharmacokinetic study with healthy bangladeshi male volunteers. Dhaka University J. Pharm. Sci. 2018;10(2):71–78. [Google Scholar]
- Council of Europe, editor. European Pharmacopoeia. 8th ed. Strasbourg: European Directorate for the Quality of Medicines and Health Care; 2014.
- Cushing D.J., Kowey P.R., Cooper W.D., Massey B.W., Gralinski M.R., Lipicky R.J. PM101: A cyclodextrin-based intravenous formulation of amiodarone devoid of adverse hemodynamic effects. Eur. J. Pharmacol. 2009;607(1–3):167–172. doi: 10.1016/j.ejphar.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Darwish H.W., Hassan S.A., Salem M.Y., El-Zeiny B.A. Three different spectrophotometric methods manipulating ratio spectra for determination of binary mixture of amlodipine and atorvastatin. Spectrochim. Acta Part A. 2011;83(1):140–148. doi: 10.1016/j.saa.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Farzana H., Mukhlasy H., Hussain I., Hossain M., Hasan M. Validation of spectrophotometric dissolution method for modified release trimetazidine pharmaceutical dosage form. J. Innov. Pharm. Biolog. Sci. 2017;4(2):68–73. [Google Scholar]
- Ghoneim M.M., Khashaba P.Y., Beltagi A.M. Determination of trimetazidine HCl by adsorptive stripping square-wave voltammetry at a glassy carbon electrode. J. Pharm. Biomed. Anal. 2002;27(1–2):235–241. doi: 10.1016/s0731-7085(01)00525-8. [DOI] [PubMed] [Google Scholar]
- Goell A., Baboota S., Sahni J., Srinivas K., Gupta R., Gupta A., Semwal V., Ali J. Development and validation of stability-indicating assay method by UPLC for a fixed dose combination of atorvastatin and ezetimibe. J. Chromatogr. Sci. 2013;51(3):222–228. doi: 10.1093/chromsci/bms131. [DOI] [PubMed] [Google Scholar]
- Guihen E., Sisk G., Scully N., Glennon J. Rapid analysis of atorvastatin calcium using capillary electrophoresis and microchip electrophoresis. Electrophoresis. 2006;27(12):2338–2347. doi: 10.1002/elps.200500899. [DOI] [PubMed] [Google Scholar]
- Hai R.S., Ming K.Z. Preparation and evaluation of self-micro emulsifying drug delivery systems (SMEDDS) containing atorvastatin. J. Pharm. Pharmacol. 2006;58(9):1183–1191. doi: 10.1211/jpp.58.9.0004. [DOI] [PubMed] [Google Scholar]
- Hao J., Du H., Li W., Zhao Z., Liu F., Lu J., Yang C., Cui W. Effects of atorvastatin combined with trimetazidine on myocardial injury and inflammatory mediator in unstable angina patients during perioperative of percutaneous coronary intervention. Eur. Rev. Med. Pharmacol Sci. 2015;19(23):4642–4646. [PubMed] [Google Scholar]
- Hiral S., Charmy K., Deepak K., Priti M. Simultaneous determination of atorvastatin calcium and olmesartan medoxomil in a pharmaceutical formulation by reversed phase high-performance liquid chromatography, high-performance thin-layer chromatography and UV spectrophotometric methods. J. AOAC Int. 2014;97(3):791–797. doi: 10.5740/jaoacint.11-204. [DOI] [PubMed] [Google Scholar]
- International Conference on Harmonization, Topic Q2 (R1) Validation of Analytical Procedures: Text and Methodology, n.d. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf.
- Jiangyou W., Chen H., Song D., Peng J., Su X. The clinical short-term outcome of atorvastatin and trimetazidine combination treatment in patients with non-ST segment elevation acute coronary syndromes undergoing percutaneous coronary intervention. Int. Cardiovasc. Forum J. 2016;8:68–72. [Google Scholar]
- Khan F.N., Dehghan M.H.G. Enhanced bioavailability of atorvastatin calcium from stabilized gastric resident formulation. AAPS Pharm. Sci. Tech. 2011;12(4):1077–1086. doi: 10.1208/s12249-011-9673-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Oksanen D.A., Massefski J., Blake J.F., Duffy E.M., Chrunyk B. Inclusion complexation of ziprasidone mesylate with beta-cyclodextrin sulfobutyl ether. J. Pharm. Sci. 1998;87(12):1560–1567. doi: 10.1021/js980109t. [DOI] [PubMed] [Google Scholar]
- Kumar V., Khurana L., Ahmed S., Singh R.B. Application of assumed IVIVC in product life cycle management: a case study of trimetazidine dihydrochloride extended release tablet. J. Bioequiv. Biovailab. 2013;5(1):6–15. [Google Scholar]
- Lim S.Y. Role of statins in coronary artery disease. Chonnam. Med. J. 2013;49(1):1–6. doi: 10.4068/cmj.2013.49.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Ma A., Zhang W., Bai X. Cardioprotective effects of atorvastatin plus trimetazidine in percutaneous coronary intervention. Pak. J. Med. Sci. 2013;29(2):545–548. doi: 10.12669/pjms.292.2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llango K., Shiji Kumar P. Validated spectrophotometric methods for the simultaneous determination of telmisartan and atorvastatin in bulk and tablets. Pharm. Methods. 2012;3(2):112–116. doi: 10.4103/2229-4708.103892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh M.P., Schwarting N., Rajewski R.A. In vitro and in vivo evaluation of a sulfobutyl ether β-cyclodextrin enabled etomidate formulation. J. Pharm Sci. 2004;93(10):2585–2594. doi: 10.1002/jps.20160. [DOI] [PubMed] [Google Scholar]
- Pabari R., Ramtoola Z. Effect of a disintegration mechanism on wetting, water absorption, and disintegration time of orodispersible tablets. J Young Pharm. 2012;4(3):157–163. doi: 10.4103/0975-1483.100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palem C.R., Patel S., Pokharkar B.V. Solubility and stability enhancement of atorvastatin by cyclodextrin complexation. PDA. J. Pharm. Sci. Technol. 2009;63(3):217–225. [PubMed] [Google Scholar]
- Patole S., Khodke A., Potale L., Damle M. A validated densitometric method for analysis of atorvastatin calcium and metoprolol tartarate as bulk drugs and in combined capsule dosage forms. J. Young Pharm. 2011;3(1):55–59. doi: 10.4103/0975-1483.76420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotr C., Rysz J., Banach M. Defining the role of trimetazidine in the treatment of cardiovascular disorders: some insights on its role in heart failure and peripheral artery disease. Drugs. 2014;74(9):971–980. doi: 10.1007/s40265-014-0233-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rageh A.H., El-Zahry M.R., Atia N.N. Utility of ionic liquid-based surfactant in enhancement of oxidation peak signal of atorvastatin at pencil graphite electrode. Curr. Anal. Chem. 2018;14(2):101–110. [Google Scholar]
- Ramadan A., AL-Akraa H., Maktabi M. TLC simultaneous determination of amlodipine, atorvastatin, rosuvastatin and valsartan in pure form and in tablets using phenyl-modified aleppo bentonite. Int. J. Pharm. Pharm. Sci. 2014;6(3):180–188. [Google Scholar]
- Shamma R.N., Basha M. Soluplus®: A novel polymeric solubilizer for optimization of Carvedilol solid dispersions: Formulation design and effect of method of preparation. Powder Technol. 2013;237(1):406–414. [Google Scholar]
- Sharaf El-Din M., Salama F., Nassar M., Attia K., Kaddah M. Validated spectrofluorimetric method for the determination of atorvastatin in pharmaceutical preparations. J. Pharm. Anal. 2012;2(3):200–205. doi: 10.1016/j.jpha.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shazly G.A., Tawfeek H.M., Ibrahium M.A., Auda S.A., El-Mahdy M. Formulation and evaluation of fast dissolving tablets containing taste masked microspheres of diclofenac sodium for sustained release. Digest J. Nano. Bio. 2013;8(3):1281–1293. [Google Scholar]
- Sheshala R., Khan N., Chitneni M., Darwis Y. Formulation and in vivo evaluation of ondansetron orally disintegrating tablets using different superdisintegrants. Arch. Pharmacal. Res. 2011;34(11):1945–1956. doi: 10.1007/s12272-011-1115-y. [DOI] [PubMed] [Google Scholar]
- Song J.D., Wang L.L. Clinical effect of atorvastatin-trimetazidine combined treatment of coronary heart disease. Biomed. Res. 2018;29(5):962–965. [Google Scholar]
- Tanuwijaya J., Karsono The effects of crospovidone and croscarmellose sodium as superdisintegrants on the characteristics of piroxicam nanoparticles ODT (orally disintegrating tablet) Int. J. Pharm. Tech. Res. 2013;5(4):1590–1597. [Google Scholar]
- Tawfeek H.M., Abdel-Aleem J.A., Ahmed M.M. Development and optimization of itopride hydrochloride fast disintegrating tablets using factorial design and response surface methodology. Int. J. Pharm. Sci. Res. 2015;6(4):1661–1672. [Google Scholar]
- Tawfeek H.M., Abdellatif A.A.H., Mahmmoud A., Saleem I.Y. Colonic delivery of indometacin loaded PGA-co-PDL microparticles coated Eudragit L100 from fast disintegrating tablets. Int. J. Pharm. 2017;531(1):80–89. doi: 10.1016/j.ijpharm.2017.08.069. [DOI] [PubMed] [Google Scholar]
- Tawfeek H.M., Faisal W., Soliman G.M. Enalapril maleate orally disintegrating tablets: tableting and in vivo evaluation in hypertensive rats. Pharm. Dev. Tech. 2018;23(5):496–503. doi: 10.1080/10837450.2017.1329318. [DOI] [PubMed] [Google Scholar]
- Tian L. Effects of atorvastatin combined with trimetazidine on cardiac function, inflammatory factors and endothelial function in patients with coronary artery disease. J. Hainan Med. University. 2016;22(14):19–22. [Google Scholar]
- Wadher S.J., Martande S.S., Kalyankar T.M. Solubility enhancement of poorly water soluble drug atorvastatin calcium by solid dispersion technique using natural carrier. Pharmacophore. 2014;5(4):563–576. [Google Scholar]
- Wang J.Y., Chen H., Yan H., Su X. Effect of atorvastatin and trimetazidine combination treatment in patients with NSTE-ACS undergoing PCI. Cardiology Plus. 2018;3(2):41–46. [Google Scholar]
- Wishart D.S., Knox C., Guo A.C., Shrivastava S., Hassanali M., Stothard P., Chang Z., Woolsey J. Drug Bank: A comprehensive resource for in silico drug discovery and exploration. Nucl. Acids Res. 2006;34(1):D 668–672. doi: 10.1093/nar/gkj067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X., Yang L., Zhai S. Validation of a rapid and simple LC-MS/MS method to determine trimetazidine in rat plasma. J. Liq. Chrom. Rel. Technol. 2011;34(16):1645–1653. [Google Scholar]
- Xu H., Chen L.P., Li H.J., Li J.L., Sun W.D., Xin L., Wang B.S. Atorvastatin/trimetazidine combination therapy in patients with chronic cardiac failure. Trop. J. Pharm. Res. 2017;16(8):2013–2018. [Google Scholar]
- Yildiz A., Cakar M.A., Baskurt M., Okcun B., Guzelsoy D., Coskun U. The effects of atorvastatin therapy on endothelial function in patients with coronary artery disease. Cardiovas. Ultrasound. 2007;5:51–56. doi: 10.1186/1476-7120-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.