Abstract
Background: The present study aimed to evaluate serum 25-hydroxy vitamin D (25(OH) D) levels in Chinese patients with idiopathic benign paroxysmal positional vertigo (BPPV) and to investigate the possible relationship between the occurrence and recurrence of idiopathic BPPV and low 25(OH) D levels. Methods: Between 1 January 2017 and 31 May, 2018, consecutively older patients (age, older than 50 years) with idiopathic BPPV were recruited in the present study. For each patient, 2:1 sex and age matched healthy people were assigned as the control group. The influence of 25(OH) D levels on BPPV and recurrent BPPV were performed by binary logistic regression analysis. Results: In the present study, 174 patients with BPPV and 348 controls were included. The serum levels of 25(OH) D in those patients were lower than in those controls (P<0.001). One hundred eight patients were found to have vitamin D deficiency; thus, the prevalence was 62.1%, which was higher than that in the controls (42.8%). The data showed that patients with recurrent BPPV (N = 31) had lower serum levels of 25(OH) D compared with those who were not (11.2 ng/ml [interquartile range, 7.2–20.8 ng/ml] vs 18.7 ng/ml [14.2–24.8 ng/ml]). The regression analyses demonstrated that vitamin D deficiency was associated with BPPV and recurrent BPPV with an odds ratio of 2.15 (95% confidence interval [CI], 1.30–4.32; P=0.006) and 5.16 (95% CI, 1.00–34.12; P=0.05). Conclusion: Decreased serum levels of 25(OH)D were associated with the occurrence and recurrence of BPPV in a Chinese population, independent of other baseline markers.
Keywords: 25-hydroxy vitamin D, benign paroxysmal positional vertigo, recurrence, vitamin D deficiency
Introduction
Benign paroxysmal positional vertigo (BPPV) is the most commonly diagnosed type of vertigo and is characterized by short-duration vertigo, nausea, and/or positional nystagmus associated with changes in head position [1]. This condition presented as dizziness or vertigo of sudden onset that is provoked by certain changes in head position [2]. A previous epidemiology study found that the lifetime prevalence of BPPV was 2.4%, the 1-year prevalence was 1.6%, and the 1-year incidence was 0.6% [3]. However, only 8% of the affected participants had obtained effective treatment [3].
BPPV can cause severe impact, including reduced daily activities, falls, and depression on the quality of life, especially in elderly patients [4]. Patients with BPPV exhibited a 1.14-fold (95% confidence interval [CI], 1.04–1.25; P<0.01) risk of fracture than those without BPPV [5]. BPPV is thought to be caused by the presence of cupulolithiasis or canalithiasis in one or more semicircular canals. However, the exact etiology is unknown [1]. Calcium metabolism plays a primary role in the synthesis/absorption of otoconia made of calcium carbonate and thus might be an etiological factor in the onset of BPPV. Several studies indicated the association between BPPV with osteoporosis and vitamin D deficiency, implying that abnormal calcium metabolism may underlie BPPV [6].
A previous study found that the mean serum 25-hydroxy vitamin D (25(OH) D) levels were also significantly lower in female patients with BPPV than in healthy controls (P<0.001) [7]. Several studies found a correlation between vitamin D deficiency and the development, and the recurrence of BPPV [6, 8–9]. They suggested that vitamin D deficiency results in the production of abnormal otoconia, which results in otolith dysfunction [10]. Nevertheless, a few recent studies reported that a low vitamin D level was not associated with BPPV occurrence and/or recurrence [11–13]. Considering these inconsistencies, the relationship between BPPV and vitamin D deficiency is debatable.
The aim of the present study is to evaluate serum 25 (OH)D levels in Chinese patients with idiopathic BPPV and to investigate the possible relationship between the occurrence and recurrence of BPPV and low 25(OH) D levels.
Patients and Methods
Between 1 January 2017, and 31 May 2018, consecutively older patients (age, older than 50 years) with idiopathic BPPV at the Department of Neurology of Lanzhou General Hospital of PLA, China we recruited in the present study. The study was performed at geographic latitude 36.03°N. The BPPV diagnosis was based on a characteristic history and observation of typical nystagmus during the Dix-Hallpike maneuver, supine roll, and cephalic hyperextension tests [7]. A typical history of brief attacks of positional vertigo was obtained from all patients with BPPV in whom the apparent etiology was absent and described as idiopathic [14].
The exclusion criteria included (1) noncooperation; (2) having secondary factors for BPPV, such as a history of head trauma, vestibular neuritis, Meniere’s disease, migraines, ear surgery or sudden hearing loss, having a hip or lumbar spine fracture; (3) malignant, chronic renal, hepatic, stroke, cardiovascular or autoimmune diseases, gout, hypothyroidism or hyperthyroidism, the use of any drug, in particular allopurinol and/or diuretics, and a history of neurological diseases. The patients used vitamin and/or calcium supplements, and patients with systemic diseases influencing vitamin D levels during the study were excluded.
For each patient, 2:1 sex- and age-matched healthy people from the medical center at our hospital were assigned as the control group. The study protocol was approved by the Qilu Hospital of Shandong University Research Ethics Committee, and written informed consents were obtained from all included cases.
For each included case, age, sex, body mass index (BMI), blood pressure, the seasons into study, regular physical activity habits (walking at a brisk pace for 30 min or more, three times a week) and medication status (diabetes mellitus, hypertension, hyperlipidemia, smoking, drinking) were recorded. BMI was calculated as weight in kilograms divided by the square of height in meters. The intensity of BPPV was assessed by the patients and was expressed as visual analogue scale (VAS) score (0–10), where 0 indicated no vertigo and 10 indicated severe attacks of vertigo [15]. The posterior semicircular canal (PSC) type of BPPV and the horizontal semicircular canal (HSC) were diagnosed [4]. The patients were divided into two groups according to the recurrence or nonrecurrence of BPPV. Recurrent BPPV was defined when the patients reported two or more previous episodes of positional vertigo similar to those experienced at the time of diagnosis, with at least 1-month interval [16].
The serum samples were prospectively drawn from the antecubital vein at the first morning after admission. After centrifugation, the samples were immediately stored at −80°C before assay. Serum level of 25(OH) D was measured with competitive chemiluminescent immunoassay in a calibrated Elecsys 2010 (Roche diagnostics GmbH, Germany).
Statistical analysis
Results were expressed as percentages for categorical variables and as medians (interquartile ranges, [IQRs]). Univariate data on demographic and clinical features were compared by Mann–Whitney U-test or χ2 test as appropriate. Correlations among continuous variables were assessed by the Spearman rank-correlation coefficient.
The influence of 25(OH) D levels on BPPV and recurrent BPPV were performed by binary logistic regression analysis, which allows adjustment for possible confounding factors (age, sex, BMI, season, diabetes mellitus, hypertension, hyperlipidemia, smoking, drinking, VAS score, regular exercise habit, and different semicircular canals). Results were expressed as adjusted odds ratios (OR) with the corresponding 95% CI. In addition, multivariate analysis models were applied to further explore the relation of BPPV and vitamin D state. The 25(OH) D levels are therefore used to classify the vitamin D status into vitamin D deficiency (<20 ng/ml), vitamin D insufficiency (20–29 ng/ml) and vitamin D sufficiency (≥30 ng/ml) [17]. We also conducted subgroup analyses separately among men and women cases.
Receiver operating characteristic (ROC) curves were utilized to evaluate the accuracy of serum 25(OH) D to predict BPPV. Area under the curve (AUC) was calculated as measurements of the accuracy of the test. All statistical analysis was performed with SPSS for Windows, version 20.0 (SPSS Inc., Chicago, IL, U.S.A.). Statistical significance was defined as P<0.05.
Results
Baseline clinical characteristics
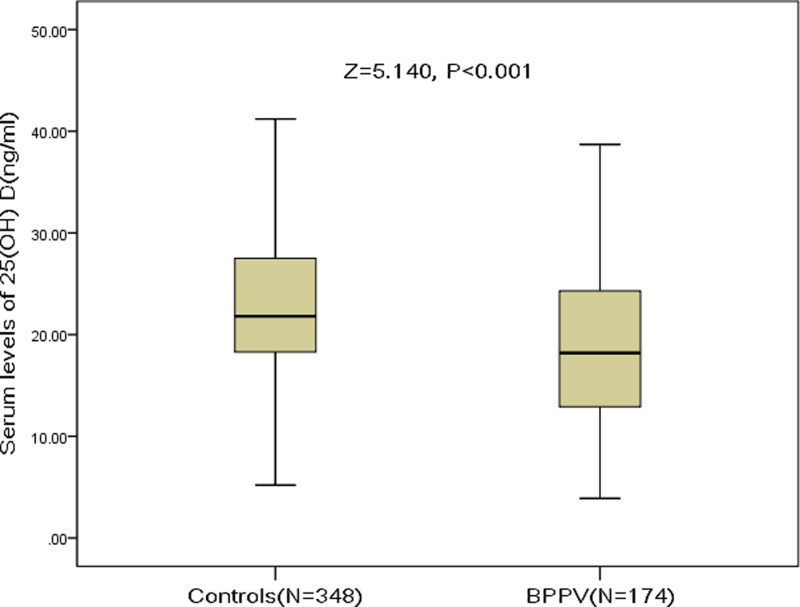
In the present study, 174 patients with BPPV and 348 controls were included. The serum levels of 25(OH) D in those patients were lower than those controls (18.2 ng/ml [IQR, 12.7–24.3 ng/ml] vs 21.8 ng/ml [IQR, 18.3–27.6 ng/ml]; P<0.001; Figure 1). One hundred eight patients were diagnosed to have vitamin D deficiency; thus, the prevalence was 62.1%, which was higher than that in the controls (42.8%) (Table 1). Most (83.3%) of the patients were evaluated within 2 weeks from symptom onset. Baseline clinical characteristics of patients and controls are listed in Table 1.
Figure 1. Comparisons of serum 25(OH) D levels between patients with BPPV and controls.
Mann–Whitney U-test. All data are medians and IQR.
Table 1. Characteristics of patients and controls.
| BPPV | Controls | P‡ | |
|---|---|---|---|
| N | 174 | 348 | |
| Age, median (IQR), years | 61 (54–69) | 61 (54–69) | NS |
| Sex-female, n (%) | 102 (58.6) | 204 (58.6) | NS |
| BMI, median (IQR), kg/m2 | 25.8 (24.3–27.4) | 26.0 (24.4–27.6) | NS |
| Systolic blood pressure, median (IQR), mmHg | 125 (115–135) | 123 (114–130) | NS |
| Diastolic blood pressure, median (IQR), mmHg | 80 (75–86) | 78 (72–85) | NS |
| Including season-winter, n (%) | 51 (29.3) | 105 (30.2) | NS |
| Diabetes mellitus, n (%) | 25 (14.4) | 55 (15.8) | NS |
| Hypertension, n (%) | 31 (17.8) | 66 (19.0) | NS |
| Hyperlipidemia, n (%) | 44 (25.3) | 94 (27.0) | NS |
| Smoking, n (%) | 28 (16.1) | 53 (15.2) | NS |
| Drinking, n (%) | 19 (10.9) | 35 (10.1) | NS |
| Regular exercise habit, n (%) | 21 (12.1) | 40 (11.5) | NS |
| VAS score median (IQR) | 4 (1–6) | – | |
| 25(OH) D, ng/ml | 18.2 (12.7–24.3) | 21.8 (18.3–27.6) | <0.001 |
| Vitamin D deficiency, n (%)† | 108 (62.1) | 149 (42.8) | <0.001 |
| Vitamin D insufficiency, n (%)† | 42 (24.1) | 132 (37.9) | 0.002 |
| Vitamin D sufficiency, n (%)† | 24 (13.8) | 67 (19.3) | 0.12 |
| Different semicircular canals, n (%) | – | ||
| Posterior | 122 (70.1) | ||
| Horizontal | 45 (25.9) | ||
| Anterior | 7 (4.0) |
the P-value was tested by Mann–Whitney U-test or χ2 test.
The 25(OH) D levels are therefore used to classify the vitamin D status into vitamin D deficiency (<20 ng/ml), vitamin D insufficiency (20–29 ng/ml) and vitamin D sufficiency (≥ 30 ng/ml).
Abbreviation: DM: diabetes mellitus.
Characteristics of BPPV
BPPV most commonly involved the posterior canal (n=122, 70.1%), followed by the horizontal (n=45, 25.9%) and anterior canal (n=7, 4.0%). The 25(OH) D levels did not differ statistically in patients with different semicircular canals (P>0.05). With respect to the most frequent involvement (PSC), the right side was affected in 72 (59.0%) patients and the left side in 50 (41.0%) patients. The 25(OH) D levels did not differ statistically in patients with PSC BPPV for either the right or left side (P>0.05). The median score of VAS was 4 (IQR, 1–6). As a continuous variable, it was found that there was a correlation between VAS score and serum levels of 25(OH) D (r = −0.348; P<0.001). Recurrent attacks of BPPV were reported in 31 patients.
Serum 25(OH) D levels and risk of BPPV
As a continuous variable, 25(OH) D was associated with the decreased risk of BPPV (OR, 0.96; 95% CI, 0.94–0.98; P=0.006) in the univariate model. Multiple-logistic regression analyses adjusted for age, sex, BMI, season, diabetes mellitus, hypertension, hyperlipidemia, smoking, drinking, and regular exercise habit suggested that 25(OH) D was still associated with the decreased risk of BPPV (OR, 0.98; 95% CI, 0.96–0.99; P=0.009). In addition, as a categorical variable, the results demonstrated that vitamin D deficiency was associated with BPPV (OR, 2.15; 95% CI, 1.30–4.32, P=0.006; Table 2).
Table 2. Univariate and multivariate analyses for BPPV according to vitamin D state.
| Vitamin D state† | BPPV/All†† | Crude OR (95% CI), P# | Multivariable-adjusted OR‡, P# |
|---|---|---|---|
| Vitamin D deficiency | 108/232 | 2.43 (1.43–4.14), 0.001 | 2.15 (1.30–4.32), 0.006 |
| Vitamin D insufficiency | 42/199 | 0.75 (0.42–1.33), 0.32 | 0.70 (0.37–1.55), 0.48 |
| Vitamin D sufficiency | 24/91 | Reference | Reference |
The 25(OH) D levels are therefore used to classify the vitamin D status into vitamin D deficiency (<20 ng/ml), vitamin D insufficiency (20–29 ng/ml) and vitamin D sufficiency (≥30 ng/ml).
All included BPPV and controls.
Adjusted for factors including age, sex, BMI, season, diabetes mellitus, hypertension, hyperlipidemia, smoking, drinking, and regular exercise habit.
P-value for the trend <0.001.
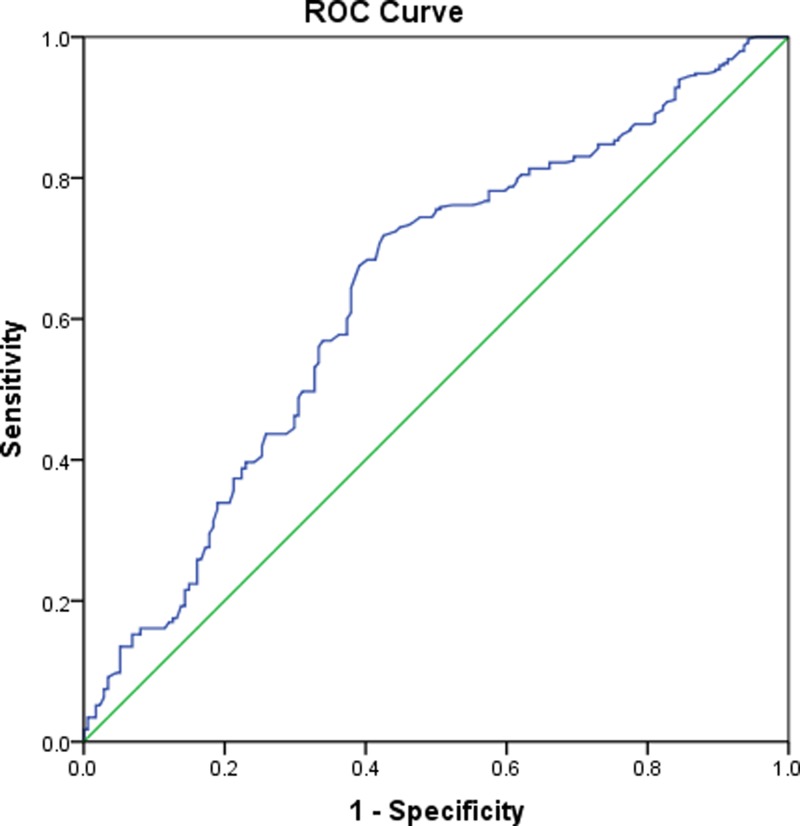
Based on the ROC curve, the projected optimal cut-off value of serum 25(OH) D levels as an indicator for the diagnosis of BPPV was 16.8 ng/ml, which yielded a sensitivity of 71.5% and a specificity of 64.5%, with an AUC of 0.64 (95% CI, 0.59–0.69). See Figure 2.
Figure 2. Receiver operator characteristic curve demonstrating sensitivity as a function of 1-specificity for predicting the BPPV based on serum level of 25(OH) D.
Serum 25(OH) D levels and risk of recurrent BPPV
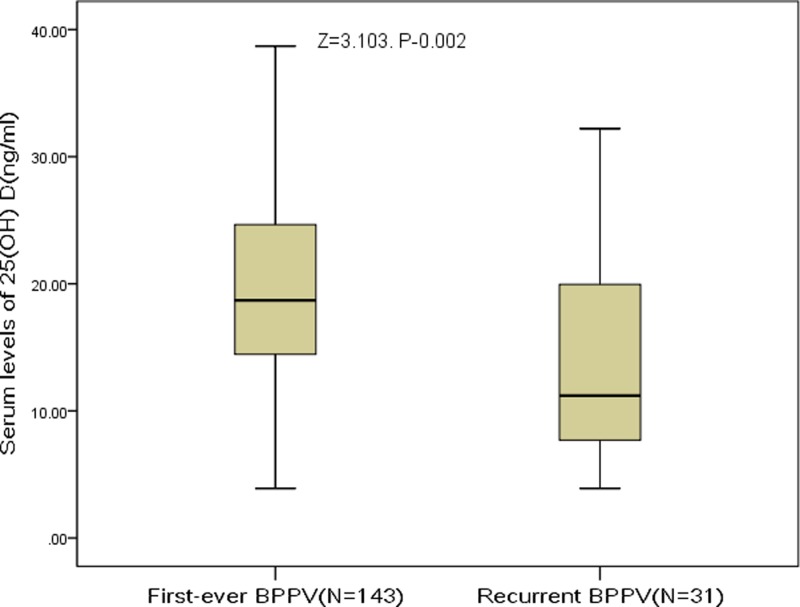
The patients were divided into two groups according to the recurrence or nonrecurrence of BPPV. Figure 3 showed that patients with recurrent BPPV had lower serum levels of 25(OH) D compared with nonrecurrent BPPV (11.2 ng/ml; IQR, 7.2–20.8 ng/ml vs 18.7 ng/ml; 14.2–24.8 ng/ml). As a continuous variable, 25(OH) D was associated with decreased risk of recurrent BPPV (OR, 0.92; 95% CI, 0.87–0.97; P=0.002) in the univariate model. Multiple-logistic regression analyses adjusted for age, sex, BMI, season, diabetes mellitus, hypertension, hyperlipidemia, smoking, drinking, VAS score, regular exercise habit, and different semicircular canals showed that 25(OH) D was still associated with decreased risk of recurrent BPPV (OR, 0.94; 95% CI, 0.90–0.98; P=0.006). In addition, as a categorical variable, the results suggested that vitamin D deficiency was associated with recurrent BPPV with the ORs of 5.16 (95% CI, 1.00–34.12; P=0.05) (Table 3).
Figure 3. Comparisons of serum 25(OH) D levels between patients with recurrent BPPV and de novo BPPV.
Mann–Whitney U-test. All data are medians and IQR.
Table 3. Univariate and multivariate analyses for recurrent BPPV according to vitamin D state.
| Vitamin D state † | RBPPV/BPPV | Crude OR (95% CI), P# | Multivariable-adjusted OR‡, P# |
|---|---|---|---|
| Vitamin D deficiency | 25/108 | 6.93 (1.03–33.12), 0.03 | 5.16 (1.00–34.12), 0.05 |
| Vitamin D insufficiency | 5/42 | 3.11 (0.34–28.31), 0.29 | 2.85 (0.36–28.55), 0.42 |
| Vitamin D sufficiency | 1/24 | Reference | Reference |
The 25(OH) D levels are therefore used to classify the vitamin D status into vitamin D deficiency (<20 ng/ml), vitamin D insufficiency (20–29 ng/ml) and vitamin D sufficiency (≥30 ng/ml).
Adjusted for factors including Age, sex, BMI, season, diabetes mellitus, hypertension, hyperlipidemia, smoking, drinking, VAS score, regular exercise habit, and different semicircular canals.
P-value for the trend <0.001.
Abbreviations: RBPPV, recurrent BPPV.
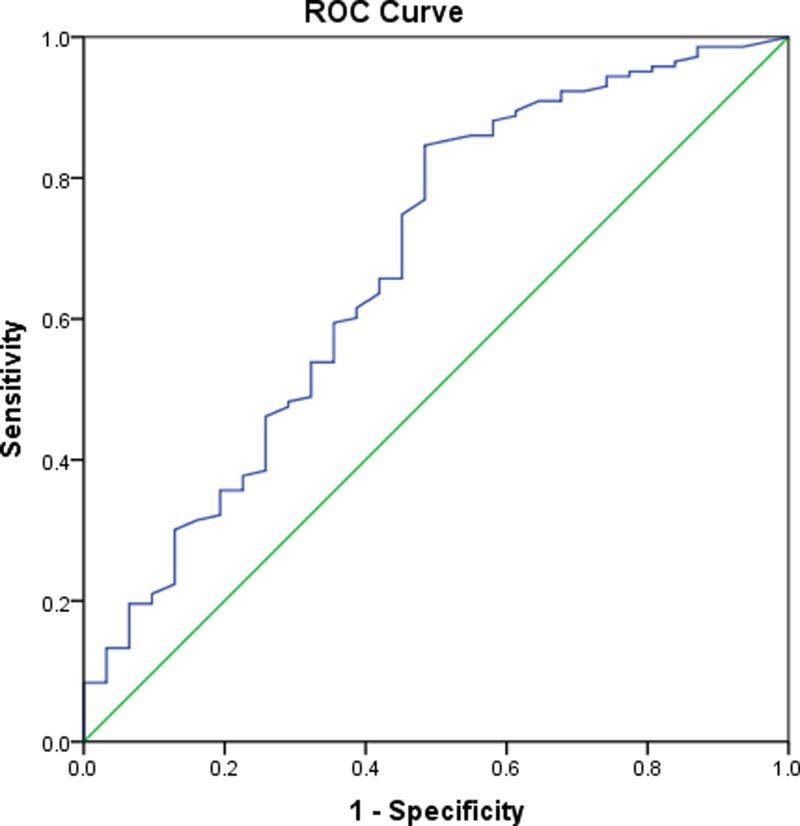
Based on the ROC curve, the optimal cut-off value of serum 25(OH) D levels as an indicator for diagnosis of BPPV was projected to be 14.2 ng/ml, which yielded a sensitivity of 84.5% and a specificity of 57.4%, with an AUC of 0.68 (95% CI, 0.58–0.79). See Figure 4.
Figure 4. Receiver operator characteristic curve demonstrating sensitivity as a function of 1-specificity for predicting the recurrent BPPV based on serum level of 25(OH) D.
Sub-group analysis
Furthermore, we also conducted analyses separately among male and female cases. In the multivariate regression analysis, the date demonstrated that for each 1 ng/ml increase of serum concentration of 25(OH) D, the association with risk of BPPV was stronger among female cases (OR, 0.95; 95% CI, 0.93–0.97; P=0.001) versus male cases (OR, 0.98; 95% CI, 0.96–0.99; P=0.012). Interestingly, the predictive value of 25(OH) D to predict recurrent BPPV (OR, 0.92; 95% CI, 0.89–0.97; P=0.002) was also stronger in female cases than in male cases (0.95 [0.92–0.99]; P=0.015).
Discussion
Research on the relationship between vitamin D deficiency and vertigo is scarce, and this context has not been adequately investigated. In the present study, we evaluated serum 25 (OH)D levels in Chinese patients with idiopathic BPPV and to investigate the possible relationship between the occurrence and recurrence of BPPV and low 25(OH) D levels. The present study clarified that (1) serum levels of 25(OH) D were lower in BPPV than that in controls; (2) serum levels of 25(OH) D were lower in the recurrence of BPPV than in the de novo BPPV; (3) the regression analyses demonstrated that vitamin D deficiency was associated with BPPV and recurrent BPPV with an OR of 2.15 (95% CI, 1.30–4.32; P=0.006) and 5.16 (95% CI, 1.00–34.12; P=0.05), suggesting that low 25(OH) D may be a risk factor for BPPV and recurrent BPPV; (4) this correlation between vitamin D deficiency wand BPPV was stronger in women than in men; (5) serum levels of 25(OH) D were negative associated with intensity of BPPV (assessed by VAS score).
Consistent with our finding, one study demonstrated that vitamin D deficiency was associated with BPPV with an OR of 2.1 (95% CI, 1.1–3.1; P=0.031) in postmenopausal women [7], another study showed that vitamin D deficiency was associated with BPPV with an odds ratio of 2.054 (95% CI, 1.088–3.877; P=0.026) in female patients [18]. Similarly, Talaat et al. [6] found that low levels of vitamin D were related to development of BPPV, whereas very low levels were associated with recurrence of BPPV. Furthermore, Jeong et al. [8] reported that decreased vitamin D only showed a significant relationship with BPPV occurrence, but the vitamin D level did not differ between the de novo and recurrent groups. Yang et al. [19] showed that serum 25(OH) D levels in men were associated with the occurrence of BPPV. Furthermore, a recent meta-analysis study showed that low vitamin D level was significantly evident among patients with recurrent episodes of BPPV; however, there was a failure in establishing a relationship between the occurrence of BPPV and low vitamin D level [20]. These differences might be caused by different clinical settings. In addition, serum 25(OH) D levels were also affected by various factors, such as age, sex, seasonal factors, hormonal factors, location, nutrition and lifestyle habits, and preexisting metabolic disorders [21].
The relationship between decreasing levels of 25(OH) D and BPPV was reported in the present study. A more meaningful study was whether vitamin supplementation can improve the BPPV, especially for BPPV patients with vitamin D deficiency. However, our cross-sectional design could not obtain any causal relation. One study showed that the treatment of BPPV with 1α-hydroxyvitamin D3 could effectively improve the symptoms of the patients, and the level of vitamin D3 and the occurrence of osteopenia/osteoporosis were the clinical indexes of whether the BPPV treatment was effective [22]. Another study indicated that the normalization of serum vitamin D significantly reduces BPPV recurrences [23]. Similarly, the recurrences of BPPV were improved after correction of vitamin D deficiency [24]. The beneficial effect of vitamin D therapy on severity of BPPV may be attributed to direct the effect of vitamin D on vestibular system or indirect effect of vitamin D, on muscle strength, fall, balance, and musculoskeletal system [25–27]. The establishment of the causal link between BPPV and decreased vitamin would require animal experiments and eventually a clinical trial that looks for preventive effects of vitamin D supplementation on recurrence of BPPV in patients with BPPV and low serum vitamin D.
The relationship between decreasing levels of 25(OH) D and BPPV reported in the present study was no proof of a causal relation due to the cross-sectional design; however, there are mechanisms through which low levels of 25(OH) D could have harmful effects on the BPPV. First, the epithelial Ca2+ channel transport system, Na+/Ca2+ exchangers, and plasma membrane Ca2+ pumps expressed in the inner ear contribute to this critical balance of calcium levels by transepithelial absorption of Ca2+ from the endolymph of the inner ear [28]. Vitamin D regulates the expression of some Ca2+ binding proteins via vitamin D receptors (VDR) in the epithelial cells of the inner ear [28]. Therefore, it has been speculated that vitamin D deficiency also contributes to the development of BPPV by abnormal calcium metabolism in the inner ear [19]. Second, VDR-deficient mice showed a balance dysfunction [29]. Previous studies found VDR in epithelial cell of crista ampullaris, membranous semicircular canals, and surrounding bone cells in mice [22]. In addition, the accelerated rotation, inclined platform, rotation, and swimming tests show that the equilibrium function of mice with mutant VDR is decreased, suggesting that vitamin D insufficiency may cause the vestibular dysfunction, such as BPPV [29–30]. Subjects with vitamin D deficiency have abnormal ocular and cervical vestibular evoked myogenic potentials, indicating that vitamin D deficiency results in otolith dysfunction [10].
One limitation of the present study was the relatively small sample size (N = 174), which made it impossible to draw firm conclusions in the sub-group analysis. Especially, the recurrent case was so small (N = 31), and the multivariate analysis using these data may have some problem. For the generalization of the results, the sample size should be larger. Second, we only tested the serum levels of 25(OH) D once time and did not perform follow-up to detect the values of 25(OH) D at different stages of the disease (e.g., acute stage or remission stage). Third, because our study was cross-sectional, we were unable to establish a causal relationship between 25(OH) D and the development of BPPV. In addition, we evaluated only idiopathic BPPV. We were unable to identify the association between comorbidities and BPPV, both of which are affected by aging. Last, because of some technical limitations, we did not test the biomarkers of osteoporosis and VDR, which should be considered in future studies.
Conclusion
In summary, decreased serum levels of 25(OH)D were associated with the occurrence and recurrence of BPPV in a China population, independent of other baseline markers. The measurement of serum levels of vitamin D might be helpful in diagnosing and managing BPPV patients. Because it plays an important role at multiple stages of BPPV progression, vitamin D will be worthy of further research as a possible therapeutic target.
Ethics, consent, and permissions
Written informed consents were obtained from all patients; and, the present study conformed to the principles of the Declaration of Helsinki was approved by the investigational review board of the Qilu Hospital of Shandong University.
Data Availability
Please contact corresponding author for data requests.
Acknowledgments
We are grateful to the staff in the Department of Cardiology the Linyi People’s Hospital for their support with patient recruitment. We also grateful to the patients who were included in the present study.
Abbreviations
- 25(OH) D
25-hydroxy vitamin D
- AUC
area under the curve
- BMI
body mass index
- CI
confidence interval
- BPVV
benign paroxysmal positional vertigo
- OR
odd ratio
- PSC
posterior semicircular canal
- ROC
receiver operating characteristic
- VAS
visual analogue scale
- VDR
vitamin D receptor
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
The authors declare that there are no sources of funding to be acknowledged.
Author Contribution
L.X. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: D.J., L.L., K.W.K., C. X.B., L.X.; Acquisition of data: D.J., L.L.; Analysis and interpretation of data: D.J., L.L., K.W.K., C.X.B., L.X.; Drafting of the manuscript: D.J., L.L., K.W.K.; Critical revision of the manuscript for important intellectual content: C.X.B., L.X.; Administrative, technical, or material support: L.L., K.W.K., C.X.B.; and Study supervision: L.X.
References
- 1.Yu S., Liu F., Cheng Z.. et al. (2014) Association between osteoporosis and benign paroxysmal positional vertigo: a systematic review. BMC Neurol. 14, 110 10.1186/1471-2377-14-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furman J.M. and Cass S.P. (1999) Benign paroxysmal positional vertigo. N. Engl. J. Med. 341, 1590–1596 10.1056/NEJM199911183412107 [DOI] [PubMed] [Google Scholar]
- 3.Von Brevern M., Radtke A., Lezius F.. et al. (2007) Epidemiology of benign paroxysmal positional vertigo: a population-based study. J. Neurol. Neurosur. Psychiatry 78, 710–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celikbilek A., Gencer Z K., Saydam L.. et al. (2014) Serum uric acid levels correlate with benign paroxysmal positional vertigo. Eur. J. Neurol. 21, 79–85 10.1111/ene.12248 [DOI] [PubMed] [Google Scholar]
- 5.Liao W.L., Chang T.P., Chen H.J.. et al. (2015) Benign paroxysmal positional vertigo is associated with an increased risk of fracture: a population-based cohort study. J. Orthop. Sports Phys. Ther. 45, 406–412 [DOI] [PubMed] [Google Scholar]
- 6.Talaat H.S., Abuhadied G., Talaat A.S.. et al. (2015) Low bone mineral density and vitamin D deficiency in patients with benign positional paroxysmal vertigo. Eur. Arch. Otorhinolaryngol. 272, 2249–2253 10.1007/s00405-014-3175-3 [DOI] [PubMed] [Google Scholar]
- 7.Han W., Fan Z., Zhou M.. et al. (2018) Low 25-hydroxyvitamin D levels in postmenopausal female patients with benign paroxysmal positional vertigo. Acta Otolaryngol. 138, 443–446 10.1080/00016489.2017.1416168 [DOI] [PubMed] [Google Scholar]
- 8.Jeong S.H., Kim J.S., Shin J.W.. et al. (2013) Decreased serum vitamin D in idiopathic benign paroxysmal positional vertigo. J. Neurol. 260, 832–838 10.1007/s00415-012-6712-2 [DOI] [PubMed] [Google Scholar]
- 9.Talaat H.S., Kabel A.M.H., Khaliel L.H.. et al. (2016) Reduction of recurrence rate of benign paroxysmal positional vertigo by treatment of severe vitamin D deficiency. Auris Nasus Larynx 43, 237–241 10.1016/j.anl.2015.08.009 [DOI] [PubMed] [Google Scholar]
- 10.Sanyelbhaa H. and Sanyelbhaa A. (2015) Vestibular-evoked myogenic potentials and subjective visual vertical testing in patients with vitamin D deficiency/insufficiency. Eur. Arch. Otorhinolaryngol. 272, 3233–3239 10.1007/s00405-014-3395-6 [DOI] [PubMed] [Google Scholar]
- 11.Parham K., Leonard G., Feinn R.S.. et al. (2013) Prospective clinical investigation of the relationship between idiopathic benign paroxysmal positional vertigo and bone turnover: a pilot study. Laryngoscope 123, 2834–2839 10.1002/lary.24162 [DOI] [PubMed] [Google Scholar]
- 12.Maslovara S., Butkovic Soldo S., Sestak A.. et al. (2018) 25 (OH) D3 levels, incidence and recurrence of different clinical forms of BPPV. Braz. J. Otorhinolaryngol. 84, 453–459 10.1016/j.bjorl.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karatas A., Yuceant G.A., Yuce T.. et al. (2017) Association of benign paroxysmal positional vertigo with osteoporosis and vitamin D deficiency: a case-controlled study. J. Int. Adv. Otol. 13, 259–266 10.5152/iao.2016.2640 [DOI] [PubMed] [Google Scholar]
- 14.Sheikhzadeh M., Lotfi Y., Mousavi A.. et al. (2016) Influence of supplemental vitamin D on intensity of benign paroxysmal positional vertigo: a longitudinal clinical study. Caspian J. Internal Med. 7, 93–98 [PMC free article] [PubMed] [Google Scholar]
- 15.Kansu L., Avci S., Yilmaz I. and Ozluoglu L.N. (2010) Long-term follow-up of patients with posterior canal benign paroxysmal positional vertigo. Acta Otolaryngol. 130, 1009–1012 10.3109/00016481003629333 [DOI] [PubMed] [Google Scholar]
- 16.Jeong S.H., Choi S.H., Kim J.Y., Koo J.W., Kim H.J. and Kim J.S. (2009) Osteopenia and osteoporosis in idiopathic benign positional vertigo. Neurology 72, 1069–1076 10.1212/01.wnl.0000345016.33983.e0 [DOI] [PubMed] [Google Scholar]
- 17.Tu W.J., Zhao S.J., Xu D.J.. et al. (2014) Serum 25-hydroxyvitamin D predicts the short-term outcomes of Chinese patients with acute ischaemic stroke. Clin. Sci. 126, 339–346 10.1042/CS20130284 [DOI] [PubMed] [Google Scholar]
- 18.Wu Y.Q., Lu X.X., Fan Z.Y.. et al. (2018) Relationship between 25-hydroxyvitamin D levels and idiopathic benign paroxysmal positional vertigo in female patients. Zhonghua Yi Xue Za Zhi 98, 1223–1226 [DOI] [PubMed] [Google Scholar]
- 19.Yang C.J., Kim Y., Lee H.S.. et al. (2017) Bone mineral density and serum 25-hydroxyvitamin D in patients with idiopathic benign paroxysmal positional vertigo. J. Vestib. Res. 27, 287–294 10.3233/VES-170625 [DOI] [PubMed] [Google Scholar]
- 20.AlGarni M.A., Mirza A.A., Althobaiti A.A.. et al. (2018) Association of benign paroxysmal positional vertigo with vitamin D deficiency: a systematic review and meta-analysis. Eur. Arch. Otorhinolaryngol. 275, 2705–2711 10.1007/s00405-018-5146-6 [DOI] [PubMed] [Google Scholar]
- 21.Yu S., Fang H., Han J.. et al. (2015) The high prevalence of hypovitaminosis D in China: a multicenter vitamin D status survey. Medicine (Baltimore) 94, e585 10.1097/MD.0000000000000585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu X., Dong F. and Gu J. (2018) Analysis of effect of 1α-hydroxyvitamin D3 on benign paroxysmal positional vertigo and risk factors. Exp. Ther. Med. 15, 2321–2326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheikhzadeh M., Lotfi Y., Mousavi A.. et al. (2016) The effect of serum vitamin D normalization in preventing recurrences of benign paroxysmal positional vertigo: a case-control study. Caspian J. Intern.l Med. 7, 173–177 [PMC free article] [PubMed] [Google Scholar]
- 24.Büki B., Ecker M., Jünger H.. et al. (2013) Vitamin D deficiency and benign paroxysmal positioning vertigo. Med. Hypotheses 80, 201–204 10.1016/j.mehy.2012.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silva C., Amorim A.M. and Paiva A. (2015) Benign paroxysmal positional vertigo-a review of 101 cases. Acta Otorrinolaringol. Esp. 66, 205–209 10.1016/j.otorri.2014.09.003 [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Escamez J.A., Gamiz M.J., Fernandez-Perez A. and Gomez-Fiñana M. (2005) Long-term outcome and health-related quality of life in benign paroxysmal positional vertigo. Eur. Arch. Otorhinolaryngol. 262, 507–511 10.1007/s00405-004-0841-x [DOI] [PubMed] [Google Scholar]
- 27.Mastaglia S.R., Seijo M., Muzio D.. et al. (2011) Effect of vitamin D nutritional status on muscle function and strength in healthy women aged over sixty-five years. J. Nutr. Health Aging 15, 349–354 10.1007/s12603-010-0287-3 [DOI] [PubMed] [Google Scholar]
- 28.Yamauchi D., Raveendran N.N., Pondugula S.R.. et al. (2005) Vitamin D upregulates expression of ECaC1 mRNA in semicircular canal. Biochem. Biophys. Res. Commun. 331, 1353–1357 10.1016/j.bbrc.2005.04.053 [DOI] [PubMed] [Google Scholar]
- 29.Minasyan A., Keisala T., Zou J., Zhang Y., Toppila E., Syvälä H.. et al. (2009) Vestibular dysfunction in vitamin D receptor mutant mice. J. Steroid Biochem. Mol. Biol. 114, 161–166 10.1016/j.jsbmb.2009.01.020 [DOI] [PubMed] [Google Scholar]
- 30.Lips P., Binkley N., Pfeifer M., Recker R., Samanta S., Cohn D.A.. et al. (2010) Once-weekly dose of 8400 IU vitamin D(3) compared with placebo: effects on neuromuscular function and tolerability in older adults with vitamin D insufficiency. Am. J. Clin. Nutr. 91, 985–991 10.3945/ajcn.2009.28113 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please contact corresponding author for data requests.