Abstract
lncRNA LOXL1 antisense RNA 1 (lncRNA LOXL1-AS1) was recently found to function as oncogenic lncRNA in glioblastoma, prostate cancer, and medulloblastoma. The role of LOXL1-AS1 in osteosarcoma was still unknown. In our study, we found LOXL1-AS1 expression levels were higher in osteosarcoma tissues and cell lines than normal bone tissues and normal osteoblast cell line, respectively. Moreover, high-expression of LOXL1-AS1 was correlated with Enneking stage, tumor size, distant metastasis, histological grade, and overall survival time in osteosarcoma patients. Furthermore, LOXL1-AS1 overexpression acted as an independent poor predictor for overall survival in osteosarcoma patients. The loss-of-function studies showed knockdown of LOXL1-AS1 dramatically inhibited osteosarcoma cell proliferation, migration, and invasion through suppressing PI3K-AKT pathway. In conclusion, LOXL1-AS1 predicts clinical progression and poor prognosis in osteosarcoma patients and functions as oncogenic lncRNA to regulate cell proliferation, cell cycle, migration, and invasion.
Keywords: biomarker, LncRNA, LOXL1-AS1, osteosarcoma
Introduction
Osteosarcoma is one of the most common malignant bone tumors in children and young adults and accounting for 20% of all primary bone sarcomas [1,2]. Osteosarcoma is derived from bone marrow mesenchymal cells and limited to the metaphysis of long bones [3,4]. Due to rapid growth and early metastasis, at least one of every four osteosarcoma patients had distant metastasis losing operation opportunity at the time of initial diagnosis [5]. Although a multidisciplinary approach of surgery combined with chemotherapy significantly improve the 5-year survival rate to 70% in osteosarcoma patients, the treatment and survival rates have shown very little improvement in the last decades [6,7]. Therefore, it is urgent to explore novel molecule target for developing new therapeutic drugs and improving clinical outcome in osteosarcoma patients.
LncRNAs (lncRNAs) are a member of non-coding RNAs family with longer than 200 nts and no protein-coding ability, and have been suggested to be involved in a wide range of physiological and pathological processes including carcinogenesis [8–10]. LncRNA LOXL1 antisense RNA 1 (LOXL1-AS1) is located on human chromosome 15q24.1 and consists of 10,781 nucleotides with five exons. Originally, oxidative stress and cyclic mechanical stress induced the dysregulation of LOXL1-AS1 expression in human lens epithelial cells and human Schlemm’s canal endothelial cells respectively [11]. Afterward, the role of LOXL1-AS1 has been investigated in several types of human cancer including lung cancer [12], hepatocellular carcinoma [13], glioma [14,15], breast cancer [16,17], prostate cancer [18], and medulloblastoma [19]. However, the expression pattern and function of LOXL1-AS1 in osteosarcoma is still unknown. Therefore, the purpose of the present study is to investigate the clinical significance and biological function of LOXL1-AS1 in osteosarcoma.
Materials and methods
Patient samples
Total 58 fresh osteosarcoma tissue specimens and 20 fresh adjacent normal tissue specimens were obtained from Beijing Friendship Hospital. In addition, 68 fresh osteosarcoma tissue specimens and 22 fresh adjacent normal tissue specimens were obtained from Tongliang Hospital of Traditional Chinese Medicine Affiliated to School of Chinese Medicine of Chongqing Medical University. There was no significant difference in sample information between the two hospitals. Fresh tissue specimens were frozen in liquid nitrogen immediately and stored at −80°C. Histological and pathological diagnosis of each sample was confirmed by at least two pathologists. All cases did not receive antitumor treatment before surgery or biopsy. This research was reviewed and approved by the Ethics Committee of Beijing Friendship Hospital (no.14009) and Tongliang Hospital of Traditional Chinese Medicine Affiliated to School of Chinese Medicine of Chongqing Medical University (no.C140032). Written consent of each osteosarcoma patient was obtained before participating in the present study.
Cell lines
Four human osteosarcoma cell lines (MG63, U-2 OS, Saos-2, and HOS) and a human normal osteoblast cell line (hFOB1.19) were purchased from American Type Culture Collection (ATCC, Manassas, VA, U.S.A.). All cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, NY, U.S.A.) containing 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, U.S.A.) at 37°C in a 5% CO2 humidified incubator.
RNA extraction and quantitative real-time PCR
Total RNAs from tissues and cells were extracted with TRIzol (Invitrogen, Carlsbad, CA, U.S.A.) and reversely transcribed into complementary DNA by using Revert Aid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, U.S.A.) with random primers. Then, the PCR process was performed with Power SYBR Green PCR Master Mix (Life Technologies, Carlsbad, CA, U.S.A.) on an ABI 7500 (Applied Biosystems, Foster City, CA, U.S.A.). The specific primer sequences of LOXL1-AS1 and GAPDH were as follow: LOXL1-AS1 forward primer, 5′-TTCCCATTTACCTGCCCGAAG-3′; LOXL1-AS1 reverse primer, 5′-GTCAGCAAACACATGGCAAC-3′; GAPDH forward primer, 5′-GGAAGGACTCATGACCACAGTCC-3′; GAPDH reverse primer, 5′-TCGCTGTTGAAGTCAGAGGAGACC -3′. GAPDH was used as an internal control.
Cell transfection
siRNA targetted to LOXL1-AS1 (si-LOXL1-AS1) and corresponding negative control (si-NC) purchased from Genechem (Shanghai, China) for knocking down LOXL1-AS1 expression and transferred into osteosarcoma cells using Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA, U.S.A.) according to the manufacturer’s instructions. After successful transfection for 48 h, the osteosarcoma cells were collected for the following in vitro experiments.
MTT assay
MTT assay was used to detect the cell proliferation. After culture for 48, 48, 72, or 96 h, transfected osteosarcoma cells were incubated with 20 μl (10 mg/ml) MTT solution for 4 h. Afterward, 150 μl DMSO was added to dissolve the crystals. The optical density of each well was measured at the wavelength of 490 nm. All experiments were performed in triplicate.
Cell cycle analysis
Cell cycle analysis was determined by flow cytometry using Cell Cycle Staining Ki (MultiSciences, Hangzhou, China). Transfected osteosarcoma cells were fixed with ethanol, and RNase was added for RNA degradation. Samples were stained with propidium iodide and analyzed by flow cytometry according to the manufacturer’s guidelines. All experiments were performed in triplicate.
Cell migration and invasion assays
24-well transwell chambers (Corning, Kennebunk, ME, U.S.A.) were used to conduct cell migration assay. For cell invsion assay, the transwell chambers were coated with Matrigel (BD Biosciences, U.S.A.). Briefly, transfected osteosarcoma cells were suspended in FBS-free DMEM and seeded into the upper well. Then, the lower well was added with DMEM containing 20% FBS. Following 24 h incubation, cells on the lower surface of filter were fixed with methanol and stained with crystal violet, while cells on the upper surface of filter were removed with a cotton swab. The cell numbers on the lower surface of filter were counted in five random microscopic fields under a microscope. The experiments were repeated in triplicate.
Protein isolation and western blot
The cells were lysed in RIPA lysis buffer (Beyotime, Shanghai, China) with protease inhibitor. Then, equal proteins were separated on 10% SDS-PAGE and transferred to polyvinylidene fluoride membrane. PI3K, phosphorylated PI3K (p-PI3K), AKT, phosphorylated AKT (p-AKT) proteins were detected by monoclonal antibodies for PI3K, p-PI3K, AKT, p-AKT (1:1,000; Cell Signaling Technology, Beverly, MA, U.S.A.) and visualized by the enhanced chemiluminescence system (Amersham, Arlington Heights, IL, U.S.A.). The density of the bands was quantitated using the imaging system (Bio-Rad, Hercules, CA, U.S.A.). All experiments were performed in triplicate.
Statistical analysis
SPSS 18.0 software (IBM, Armonk, NY, U.S.A.) and GraphPad Prism 5.0 software (La Jolla, CA, U.S.A.) were utilized to analyze all the statistical data. Student’s ttest was used to analyze the difference between two groups. Correlations between LOXL1-AS1 expression and clinicpathological features of osteosarcoma patients were estimated by chi-square test. Survival curves were made by Kaplan–Meier method, and log-rank test was used for comparing the survival distributions. Univariate and multivariate Cox regression analyses were used for identifying independent prognostic factors in osteosarcoma patients. A P value <0.05 was considered statistically significant.
Results
The LOXL1-AS1 expression in human osteosarcoma tissues and cell lines
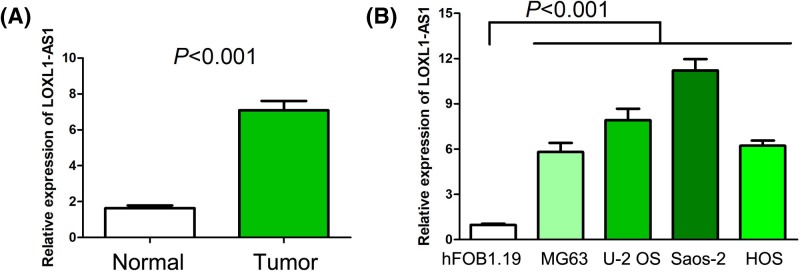
To initially measure the LOXL1-AS1 expression in osteosarcoma, we collected 96 osteosarcoma tissues specimens and 24 normal bone tissue specimens. Compared with normal bone tissue specimens, LOXL1-AS1 expression was remarkably increased in osteosarcoma tissues specimens (P<0.001, Figure 1A). Moreover, we detected LOXL1-AS1 expression in osteosarcoma cell lines and a human normal osteoblast cell line and observed that levels of LOXL1-AS1 expression in osteosarcoma cell lines (MG63, U2OS, Saos-2, and HOS) was profoundly higher than human normal osteoblast cell line (hFOB1.19) (P<0.001, Figure 1B).
Figure 1. The LOXL1-AS1 expression in human osteosarcoma tissues and cell lines.
(A) LOXL1-AS1 expression was remarkably increased in osteosarcoma tissues specimens compared with normal bone tissue specimens. (B) Levels of LOXL1-AS1 expression in osteosarcoma cell lines (MG63, U2OS, Saos-2, and HOS) was profoundly higher than human normal osteoblast cell line (hFOB1.19).
The correlation between LOXL1-AS1 expression and clinicopathological characteristics in osteosarcoma
For estimating the clinical significance of LOXL1-AS1 expression in osteosarcoma, all cases in this research were classified into high LOXL1-AS1 expression group (n=63) and low LOXL1-AS1 expression group (n=63) in accordance to the median value of LOXL1-AS1 expression. Then, we performed chi-square test to assess correlations between LOXL1-AS1 expression and clinicopathological characteristics of osteosarcoma, and observed high-expression of LOXL1-AS1 was correlated with Enneking stage (P<0.001, Table 1), tumor size (P=0.004, Table 1), distant metastasis (P=0.001, Table 1) and histological grade (P=0.001, Table 1). However, there was no obvious correlation between LOXL1-AS1 expression and other of clinicopathological characteristics including gender (P=0.353, Table 1), age (P=0.139, Table 1), and tumor site (P=0.348, Table 1).
Table 1. Associations between LOXL1-AS1 expression and clinicopathological characteristics in osteosarcoma cases.
| Characteristics | n | High expression | Low expression | P |
|---|---|---|---|---|
| Age (y) | ||||
| ≤18 | 46 | 27 | 19 | 0.139 |
| >18 | 80 | 36 | 44 | |
| Gender | ||||
| Female | 45 | 20 | 25 | 0.353 |
| Male | 81 | 43 | 38 | |
| Enneking stage | ||||
| I-IIA | 46 | 13 | 33 | <0.001 |
| IIB-III | 80 | 50 | 30 | |
| Tumor size | ||||
| ≤8 cm | 76 | 30 | 46 | 0.004 |
| >8 cm or discontinuous tumors | 50 | 33 | 17 | |
| Distant metastasis | ||||
| Absence | 104 | 45 | 59 | 0.001 |
| Presence | 22 | 18 | 4 | |
| Histological grade | ||||
| G1–G2 | 57 | 19 | 38 | 0.001 |
| G3–G4 | 69 | 44 | 25 | |
| Tumor site | ||||
| Femur/tibia | 104 | 50 | 54 | 0.348 |
| Other | 22 | 13 | 9 |
The correlation between LOXL1-AS1 expression and overall survival in osteosarcoma
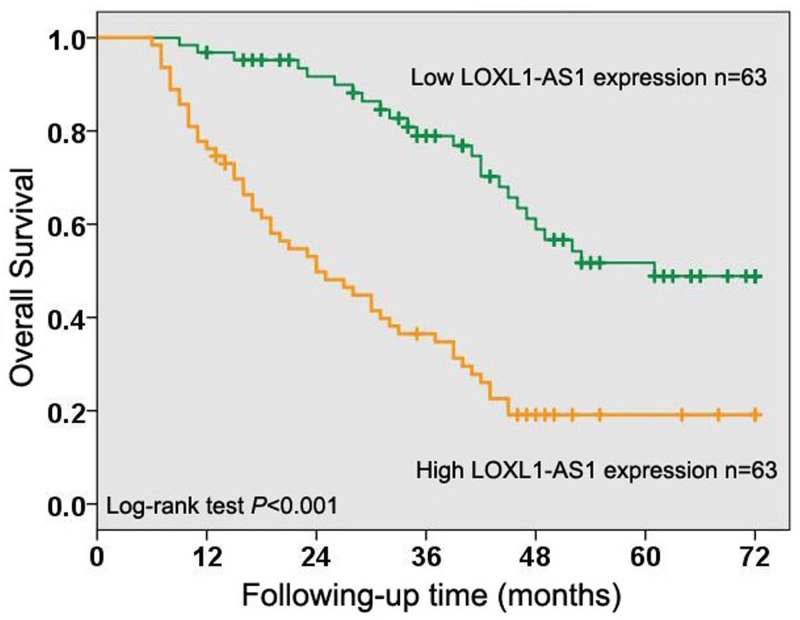
In order to evaluate the importance of LOXL1-AS1 expression for the clinical outcome in osteosarcoma patients, Kaplan–Meier method and log-rank test were used to analyze the correlation between LOXL1-AS1 expression and overall survival in osteosarcoma. The results showed osteosarcoma cases with high levels of LOXL1-AS1 expression had poorer overall survival than those with low levels of LOXL1-AS1 expression (P<0.001, Figure 2). Moreover, we performed univariate Cox regression analysis and identified Enneking stage (P<0.001, Table 2), tumor size (P=0.012, Table 2), distant metastasis (P<0.001, Table 2), histological grade (P<0.001, Table 2), and LOXL1-AS1 expression (P<0.001, Table 2) as prognostic factors for overall survival in osteosarcoma cases. Furthermore, the multivariate Cox regression analysis suggested high LOXL1-AS1 expression was an independent poor predictor in osteosarcoma patients (P=0.024, Table 2).
Figure 2. The correlation between LOXL1-AS1 expression and overall survival in osteosarcoma.
Kaplan–Meier method and log-rank test were used to analyze the correlation between LOXL1-AS1 expression and overall survival in osteosarcoma and showed that osteosarcoma cases with high levels of LOXL1-AS1 expression had poorer overall survival than those with low levels of LOXL1-AS1 expression.
Table 2. Univariate and multivariate Cox regression of prognostic factors in osteosarcoma patients.
| Parameter | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age (y) | ||||||
| (≤18 vs >18) | 0.698 | 0.441–1.104 | 0.124 | |||
| Gender | ||||||
| (Female vs ≥male) | 1.351 | 0.832–2.195 | 0.224 | |||
| Enneking stage | ||||||
| (I–II A vs II B–III) | 2.949 | 1.723–5.045 | <0.001 | 1.134 | 0.486–2.646 | 0.770 |
| Tumor size | ||||||
| (≤8 vs >8 cm or discontinuous tumors) | 1.808 | 1.140–2.868 | 0.012 | 1.227 | 0.744–2.023 | 0.423 |
| Distant metastasis | ||||||
| (Absence vs presence) | 6.007 | 3.334–10.823 | <0.001 | 3.592 | 1.906–6.767 | <0.001 |
| Histological grade | ||||||
| (G1–G2 vs G3–G4) | 2.578 | 1.572–4.227 | <0.001 | 1.589 | 0.772–3.272 | 0.209 |
| Tumor site | ||||||
| (Femur/tibia vs other) | 1.586 | 0.909–2.766 | 0.104 | |||
| LOXL1-AS1 expression | ||||||
| (Low vs high) | 3.374 | 2.071–5.496 | <0.001 | 1.985 | 1.096–3.597 | 0.024 |
Abbreviation: HR, hazard ratio.
The biological function of LOXL1-AS1 in osteosarcoma
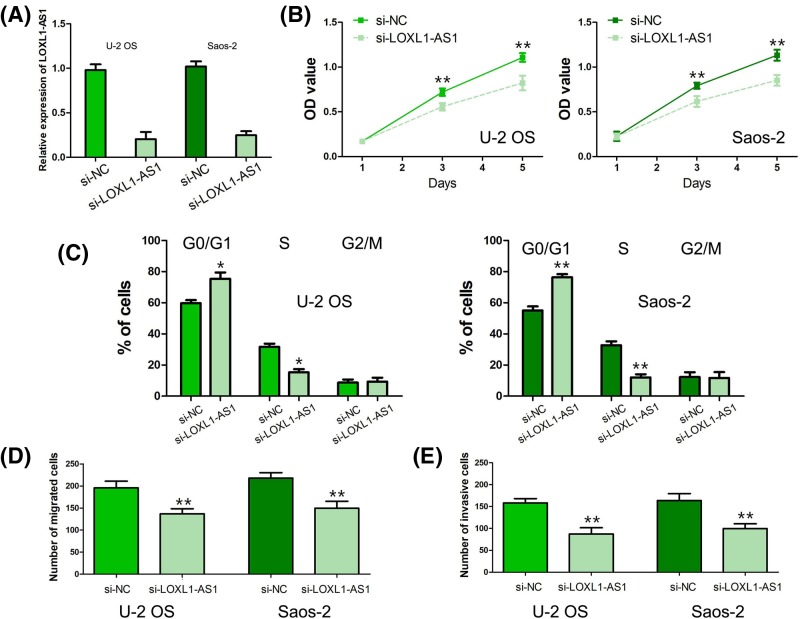
To explore the biological function of LOXL1-AS1 in osteosarcoma, U-2 OS and Saos-2 cells were transfected with si-LOXL1-AS1 and the efficiency of si-LOXL1-AS1 in osteosarcoma cells was verified by qRT-PCR (Figure 3A). The results of MTT assay showed that osteosarcoma cell proliferation was obviously depressed after U-2 OS and Saos-2 cells were transfected with si-LOXL1-AS1 (P<0.001, Figure 3B). The cell cycle analysis indicated that the cell proportion was significantly increased in the G1 phase and markedly decreased in the S phase after U-2 OS and Saos-2 cells were transfected with si-LOXL1-AS1, which suggested that knockdown of LOXL1-AS1 led to cell cycle arrest at G0/G1 phase (P<0.01, Figure 3C). Moreover, the results of transwell migration and invasion assays revealed that knockdown of LOXL1-AS1 dramatically inhibited osteosarcoma cell migration and invasion abilities in U-2 OS and Saos-2 cells (P<0.001, Figure 3D,E).
Figure 3. The biological function of LOXL1-AS1 in osteosarcoma.
(A) The efficiency of si-LOXL1-AS1 in osteosarcoma cells was verified by qRT-PCR. (B) Osteosarcoma cell proliferation was obviously depressed after U-2 OS and Saos-2 cells were transfected with si-LOXL1-AS1. (C) Knockdown of LOXL1-AS1 led to cell cycle arrest at G0/G1 phase in osteosarcoma cells. (D,E) Knockdown of LOXL1-AS1 dramatically inhibited osteosarcoma cell migration and invasion abilities. (*: P<0.01, **: P<0.001).
The molecular mechanism of LOXL1-AS1 in osteosarcoma
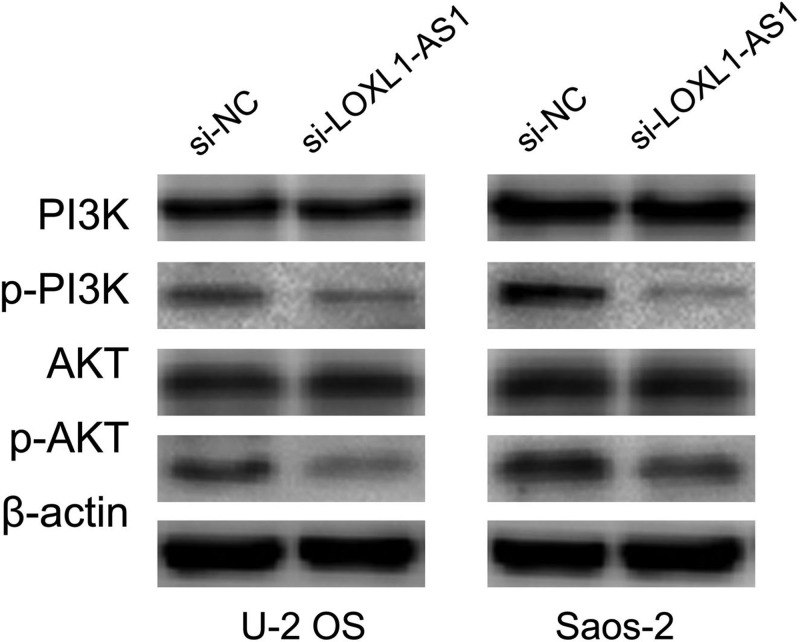
The published reported showed that LOXL1-AS1 enhanced medulloblastoma cell proliferation and motility via activating PI3K-AKT pathway [19]. Meanwhile, PI3K-AKT pathway has been suggested to be involved in osteosarcoma cell proliferation, cell cycle, migration, and invasion [20–22]. Therefore, we measure the effect of LOXL1-AS1 on PI3K and AKT expression through western blot. The results suggested knockdown of LOXL1-AS1 significantly inhibited p-PI3K and p-AKT expression but had no effect on PI3K and AKT expression in U-2 OS and Saos-2 cells (Figure 4).
Figure 4. The molecular mechanism of LOXL1-AS1 in osteosarcoma.
Knockdown of LOXL1-AS1 significantly inhibited p-PI3K and p-AKT expression but had no effect on PI3K and AKT expression in osteosarcoma cells.
Discussion
LOXL1-AS1 is a lncRNA encoded on the opposite strand of lysyl oxidase-like 1 (LOXL1), which was found to strongly associated with exfoliation glaucoma and exfoliation syndrome [23,24]. Subsequently, the dysregulation of LOXL1-AS1 was gradually discovered in human cancers. Wang et al. analyzed a non-small cell lung cancer microarray (GSE18842) and observed that LOXL1-AS1 expression was elevated in tumor tissues compared with normal tissues [12]. Moreover, Wang et al. analyzed 160 glioblastoma RNA-seq data at the Chinese Glioma Genome Atlas and found that the mesenchymal subtype of glioblastoma had high level of LOXL1-AS1 expression [15]. In medulloblastoma, Gao et al. found levels of LOXL1-AS1 expression were notably increased in tumor samples compared with adjacent noncancerous samples [19]. However, Xu et al. also analyzed the genome-wide lncRNA expression profiles of breast cancer at The Cancer Genome Atlas (TCGA) database and found that there was no statistical difference of LOXL1-AS1 expression between breast cancer tissues and normal mammary tissues [16]. Up to now, there was no report about the expression pattern of LOXL1-AS1 in osteosarcoma. In our study, we first found LOXL1-AS1 expression levels were higher in osteosarcoma tissues and cell lines than normal bone tissues and normal osteoblast cell line, respectively. Meanwhile, we further assessed the clinical significance of LOXL1-AS1 expression in osteosarcoma patients via analyzing correlations between LOXL1-AS1 expression and clinicopathological characteristics, and observed that high-expression of LOXL1-AS1 was correlated with Enneking stage distant metastasis and histological grade. In medulloblastoma patients, high-expression of LOXL1-AS1 was associated with advanced clinical stage [19]. In addition, Zhang et al. classified hepatocellular carcinoma samples into epithelial and mesenchymal subtypes and screened differential lncRNAs expression, and found that LOXL1-AS1 was overexpressed in the mesenchymal subtype comparing with the epithelial subtype [13]. Besides, Mathias et al. indentified LOXL1-AS1 as a specific lncRNA in basal-like breast cancer [17].
The prognostic value of LOXL1-AS1 was seldom reported in human cancers. Wang et al. reported high LOXL1-AS1expression acted as unfavorable prognostic factor for overall survival in glioblastoma patients [15]. In our study, we found osteosarcoma patients with high levels of LOXL1-AS1 expression had poorer overall survival than those with low levels of LOXL1-AS1 expression, and LOXL1-AS1 overexpression as an independent poor predictor in osteosarcoma patients. Moreover, we tried to the prognostic significance of LOXL1-AS1 expression in thirty types of human cancer at TCGA database and found that LOXL1-AS1 expression was associated with clinical outcome in nine kinds of tumor. LOXL1-AS1 overexpression was found to be favorable prognostic biomarker in adrenocortical carcinoma, uterine corpus endometrial carcinoma, and uveal melanoma, and to be unfavorable prognostic biomarker in cholangio carcinoma, glioblastoma multiforme, kidney renal clear cell carcinoma, brain lower grade glioma, mesothelioma, and pancreatic adenocarcinoma. Generally, more studies would be needed to verify the prognostic value of LOXL1-AS1 expression in human cancers.
LOXL1-AS1 has been suggested to act as oncogenic lncRNA in glioblastoma [15], prostate cancer [18], and medulloblastom [19]. Wang et al. showed knockdown of LOXL1-AS1 inhibited glioblastoma cell proliferation and weakened mesenchymal characteristics through decreasing NF-κB pathway [15]. In prostate cancer, silencing of LOXL1-AS1 suppressed cell proliferation and arrested cell cycle progression via regulating miR-541-3p and CCND1 [18]. Moreover, Gao et al. reported inhibition of LOXL1-AS1 depressed cell proliferation, migration, tumor growth, and induced cell cycle arrest and cell apoptosis through modulating PI3K-AKT pathway [19]. In our study, we found knockdown of LOXL1-AS1 dramatically inhibited osteosarcoma cell proliferation, migration, and invasion through suppressing PI3K-AKT pathway. Besides, PI3K/AKT activation has been suggested to involve in the stemness maintenance in human cancers [25–27]. Therefore, we will try to explore the effect of LOXL1-AS1 on the stemness maintenance in osteosarcoma for overcoming the drug resistance and relapse [28].
Conclusion
LOXL1-AS1 is a novel biomarker for predicting clinical progression and poor prognosis, and functions as tumor promoter to regulate osteosarcoma cell proliferation, migration, and invasion through PI3K-AKT pathway.
Abbreviations
- AS1
antisense RNA 1
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
fetal bovine serum
- LOXL1
lysyl oxidase-like 1
- qRT-PCR
quantitative real-time PCR
- TCGA
The Cancer Genome Atlas
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
The authors declare that there are no sources of funding to be acknowledged.
Author contribution
A.G. conceived and designed the experiments. S.C. and W.L. conducted the experiments and analyzed the data.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A. and Jemal A. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Morrow J.J. and Khanna C. (2015) Osteosarcoma genetics and epigenetics: emerging biology and candidate therapies. Crit. Rev. Oncog. 20, 173–197 10.1615/CritRevOncog.2015013713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saraf A.J., Fenger J.M. and Roberts R.D. (2018) Osteosarcoma: accelerating progress makes for a hopeful future. Front. Oncol. 8, 4 10.3389/fonc.2018.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taran S.J., Taran R. and Malipatil N.B. (2017) Pediatric osteosarcoma: an updated review. Indian J. Med. Paediatr. Oncol. 38, 33–43 10.4103/0971-5851.203513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y., Yang J., Zhao N., Wang C., Kamar S., Zhou Y.. et al. (2018) Progress in the chemotherapeutic treatment of osteosarcoma. Oncol. Lett. 16, 6228–6237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isakoff M.S., Bielack S.S., Meltzer P. and Gorlick R. (2015) Osteosarcoma: current treatment and a collaborative pathway to success. J. Clin. Oncol. 33, 3029–3035 10.1200/JCO.2014.59.4895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrison D.J., Geller D.S., Gill J.D., Lewis V.O. and Gorlick R. (2018) Current and future therapeutic approaches for osteosarcoma. Expert Rev. Anticancer Ther. 18, 39–50 10.1080/14737140.2018.1413939 [DOI] [PubMed] [Google Scholar]
- 8.Smolle M.A. and Pichler M. (2018) The role of long non-coding RNAs in osteosarcoma. Noncoding RNA 4, pii:E7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang N., Meng X., Liu Y., Chen Y. and Liang Q. (2019) LPS promote osteosarcoma invasion and migration through TLR4/HOTAIR. Gene 680, 1–8 10.1016/j.gene.2018.09.031 [DOI] [PubMed] [Google Scholar]
- 10.Gu W., Zhang E., Song L., Tu L., Wang Z., Tian F.. et al. (2018) Long noncoding RNA HOXD-AS1 aggravates osteosarcoma carcinogenesis through epigenetically inhibiting p57 via EZH2. Biomed. Pharmacother. 106, 890–895 10.1016/j.biopha.2018.06.173 [DOI] [PubMed] [Google Scholar]
- 11.Hauser M.A., Aboobakar I.F., Liu Y., Miura S., Whigham B.T., Challa P.. et al. (2015) Genetic variants and cellular stressors associated with exfoliation syndrome modulate promoter activity of a lncRNA within the LOXL1 locus. Hum. Mol. Genet. 24, 6552–6563 10.1093/hmg/ddv347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D. and Hu Y. (2018) Silenced long non-coding RNA PVT1 increased the radiosensitivity of non-small cell lung cancer through the microRNA-424-5p/lncRNA PVT1/CARM1 signaling pathway. Mol. Ther. Nucleic Acids 16, 130–140 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Zhang Z., Wang S. and Liu W. (2018) EMT-related long non-coding RNA in hepatocellular carcinoma: a study with TCGA database. Biochem. Biophys. Res. Commun. 503, 1530–1536 10.1016/j.bbrc.2018.07.075 [DOI] [PubMed] [Google Scholar]
- 14.Xiong Z., Wang L., Wang Q. and Yuan Y. (2018) LncRNA MALAT1/miR-129 axis promotes glioma tumorigenesis by targeting SOX2. J. Cell. Mol. Med. 10.1111/jcmm.13667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H., Li L. and Yin L. (2018) Silencing LncRNA LOXL1-AS1 attenuates mesenchymal characteristics of glioblastoma via NF-kappaB pathway. Biochem. Biophys. Res. Commun. 500, 518–524 10.1016/j.bbrc.2018.04.133 [DOI] [PubMed] [Google Scholar]
- 16.Xu S., Kong D., Chen Q., Ping Y. and Pang D. (2017) Oncogenic long noncoding RNA landscape in breast cancer. Mol. Cancer 16, 129 10.1186/s12943-017-0696-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathias C., Zambalde E.P., Rask P., Gradia D.F. and de Oliveira J.C. (2019) Long non-coding RNAs differential expression in breast cancer subtypes: what do we know? Clin. Genet. 10.1111/cge.13502 [DOI] [PubMed] [Google Scholar]
- 18.Long B., Li N., Xu X.X., Li X.X., Xu X.J., Liu J.Y.. et al. (2018) Long noncoding RNA LOXL1-AS1 regulates prostate cancer cell proliferation and cell cycle progression through miR-541-3p and CCND1. Biochem. Biophys. Res. Commun. 505, 561–568 10.1016/j.bbrc.2018.09.160 [DOI] [PubMed] [Google Scholar]
- 19.Gao R., Zhang R., Zhang C., Liang Y. and Tang W. (2018) LncRNA LOXL1-AS1 promotes the proliferation and metastasis of medulloblastoma by activating the PI3K/AKT pathway. Anal. Cell. Pathol. (Amst.) 2018, 9275685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song L., Zhou Z., Gan Y., Li P., Xu Y., Zhang Z.. et al. (2018) Long noncoding RNA OIP5-AS1 causes cisplatin resistance in osteosarcoma through inducing the LPAATbeta/PI3K/AKT/mTOR signaling pathway by sponging the miR-340-5p. J. Cell. Biochem. 120, 9656–9666 [DOI] [PubMed] [Google Scholar]
- 21.Zhang J., Yu X.H., Yan Y.G., Wang C. and Wang W.J. (2015) PI3K/Akt signaling in osteosarcoma. Clin. Chim. Acta 444, 182–192 10.1016/j.cca.2014.12.041 [DOI] [PubMed] [Google Scholar]
- 22.Liu B., Xu L., Dai E.N., Tian J.X. and Li J.M. (2018) Anti-tumoral potential of MDA19 in human osteosarcoma via suppressing PI3K/Akt/mTOR signaling pathway. Biosci. Rep. 38, 10.1042/BSR20181501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aboobakar I.F., Johnson W.M., Stamer W.D., Hauser M.A. and Allingham R.R. (2017) Major review: exfoliation syndrome; advances in disease genetics, molecular biology, and epidemiology. Exp. Eye Res. 154, 88–103 10.1016/j.exer.2016.11.011 [DOI] [PubMed] [Google Scholar]
- 24.Wan P., Su W. and Zhuo Y. (2017) The role of long noncoding RNAs in neurodegenerative diseases. Mol. Neurobiol. 54, 2012–2021 10.1007/s12035-016-9793-6 [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y., Cheng H., Li W., Wu H. and Yang Y. (2019) Highly-expressed P2X7 receptor promotes growth and metastasis of human HOS/MNNG osteosarcoma cells via PI3K/Akt/GSK3beta/beta-catenin and mTOR/HIF1alpha/VEGF signaling. Int. J. Cancer 10.1002/ijc.32207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong P., Konno Y., Watari H., Hosaka M., Noguchi M. and Sakuragi N. (2014) The impact of microRNA-mediated PI3K/AKT signaling on epithelial-mesenchymal transition and cancer stemness in endometrial cancer. J. Transl. Med. 12, 231 10.1186/s12967-014-0231-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He D., Wang R.X., Mao J.P., Xiao B., Chen D.F. and Tian W. (2017) Three-dimensional spheroid culture promotes the stemness maintenance of cranial stem cells by activating PI3K/AKT and suppressing NF-kappaB pathways. Biochem. Biophys. Res. Commun. 488, 528–533 10.1016/j.bbrc.2017.05.080 [DOI] [PubMed] [Google Scholar]
- 28.Su J., Zhang L., Zhang W., Choi D.S., Wen J., Jiang B.. et al. (2014) Targeting the biophysical properties of the myeloma initiating cell niches: a pharmaceutical synergism analysis using multi-scale agent-based modeling. PLoS ONE 9, e85059 10.1371/journal.pone.0085059 [DOI] [PMC free article] [PubMed] [Google Scholar]