Short abstract
Synovial cells play a crucial part in gouty arthritis, with different features for the inflammation within the joint. However, there is no information about how the synoviocytes can mediate the activation of inflammation. We hypothesized that the process of monosodium urate (MSU) crystal uptake alters the inflammatory response of synoviocytes through regulation of unknown mechanisms. Synoviocytes were stimulated with MSU crystals, and the phagocytosis index (PhIx) was evaluated by counting of cells with MSU ingested using polarized light microscopy. Additionally, transmission electron microscopy and flow cytometry were performed. Secretion of cytokines was measured by a panel of immunoassays. Changes in gene expression of hypoxia-inducible factor-1 (HIF1A), von Hippel-Lindau (VHL), and vascular endothelial growth factor (VEGF) were evaluated by quantitative real-time PCR (qRT-PCR). Protein levels were detected by ELISA. MSU crystals induced a time-dependent increase in PhIx and the formation of numerous secretory vesicles and cavities located in the cytoplasm. Culture supernatants of MSU-treated cells had high levels of the cytokines IL-1β, IL-6, IL-8, TNF-α, and MCP-1, and the growth factors NGF and HGF. The decrease in HIF1A gene expression was 0.58-fold, and overexpression of VHL and VEGF genes was 1.98- and 4-fold, respectively, in MSU-treated synoviocytes compared to untreated cells. Additionally, VEGF levels were increased. The identification of phagocytosis of MSU crystals triggering an inflammatory cellular state in synoviocytes suggests a possible mechanism of synovial activation in the pathogenesis of crystal-induced arthritis.
Impact statement
Gout is distinguished by an inflammatory process that is mediated by phagocytosis of monosodium urate (MSU) crystals in synoviocytes by regulation of unknown mechanisms. Here we suggest that the synovial cells play a crucial role in gouty arthritis by activating inflammation by MSU uptake and increasing the secretion of pro-inflammatory cytokines IL-1β, IL-6, IL-8, TNF-α, MCP-1, and the growth factors NGF and HGF. We discuss some co-existing features in synoviocytes, including anomalous morphologies of the cells, and microvesicle formation, dysregulation in VEGF gene expression. We provide evidence that phagocytosis of MSU crystals triggers an inflammatory cellular state in synoviocytes in the pathogenesis of crystal-induced arthritis.
Keywords: Cytokines, HIF-1α signaling pathway, gout, synoviocytes, VEGF gene
Introduction
Although gout is the most common inflammatory arthropathy in young males, its prevalence is underestimated due to the long asymptomatic phases of the disease.1,2 Gout flares are characterized by acute burning arthritis with local hyperalgesia and pain caused by monosodium urate (MSU) crystals accumulated in the affected joint.3 In gout, resident macrophages phagocytose MSU crystals and induce the release of pro-inflammatory cytokines such as IL-8, IL-6, MCP-1, and INF-γ, as well as IL-1β via the assembly and activation of the NALP inflammasome.4,5 In turn, this secretion can promote the activation of synovial lining cells, which can participate in the inflammatory process of gout.6 Thus, synovial fibroblasts can phagocytose MSU crystals and respond by releasing arachidonic acid metabolites, such as PGE27 and MCP-1,8 besides contributing to neutrophil recruitment. These results suggest that synoviocytes also participate directly in the acute response to MSU crystals. However, there is no information on how the synoviocytes can mediate activation of inflammation within the joint.
A poorly studied pathway in gout is the hypoxia-inducible factor-1 (HIF-1α) pathway, which is a transcriptional factor that is expressed in many cells, including synoviocytes, and is activated in response to hypoxia.9,10 In normoxic conditions, HIF-1α is hydroxylated on proline residues and allows recognition by the von Hippel-Lindau protein (pVHL) that targets HIF-α for proteasomal degradation.11 However, during hypoxia, HIF-1α hydroxylation is inhibited and accumulates in the cytoplasm with subsequent translocation into nucleus and promote the gene expression hypoxia-sensitive like NOS2, VEGF, SOX9, COL2A1, among others, involved in various cellular and systemic adaptive responses to hypoxia, including angiogenesis, vasomotor regulation, cell proliferation and survival, cell death, and extracellular matrix metabolism.11,12
Additionally, HIF-1α is inducible by cytokines, including the hepatocyte growth factor (HGF), a cytokine produced from stromal cells that functions as a mitogen, morphogen.13,14 Also, HGF exhibits strong angiogenic properties through its ability to induce the expression of vascular endothelial growth factor (VEGF), which is another potent inflammatory and angiogenic factor and a classical mediator of sensitization of unmyelinated sensory nerves.15,16
Previous studies have demonstrated HIF-1α upregulation in the synovial membrane under pathological rheumatic conditions, including rheumatoid arthritis (RA) and osteoarthritis (OA).17,18 However, few studies about the role of HIF-1α in the inflammatory microenvironment promoted by the phagocytosis of MSU crystals and the relationship between inflammation and pain have been performed. Therefore, the present study aimed to evaluate the effect of the phagocytosis of MSU crystals on the gene expression levels of HIF1A, VHL, and VEGF in human fibroblast-like synoviocytes (FLS), with a view to evaluating the ability of the synoviocytes to mediate gout inflammation.
Materials and methods
This study protocol was approved by the Research Committee of the Instituto Nacional de Rehabilitación “Luis Guillermo Ibarra Ibarra” of Mexico (Ref.02/13) and carried out according to The Code of Ethics of the World Medical Association (Declaration of Helsinki). A written informed consent was obtained from all patients.
MSU preparation
MSU crystals were synthesized and characterized according to a previous study19 and were sterilized at 180°C for 2 h. The absence of microbial contaminants was confirmed by negative cultures of microorganisms. The Limulus amebocyte cell-lysate assay (Sigma-Aldrich) demonstrated that they were bacterial endotoxin-free.19
Cell isolation and phenotyping
The FLS were obtained via mechanic-enzymatic breakdown of synovial membrane biopsies collected from five consecutive patients who underwent surgery for anterior cruciate ligament repair; none of them had history of gout, RA or clinically evidence of OA. FLS were isolated from tissue explants by digestion with 1 mg/mL collagenase type IA (Gibco, Life Technologies) for 2 h, with mixing at 37°C. The cells were seeded in T25 flasks at a density of 250,000/T25 flask until confluence. All cells were cultured in DMEM-F12 supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (P/S) (Gibco, Life Technologies). Subsequently, cells were incubated at 37°C and with 5% CO2, and the medium was changed every three days. The cells were harvested at confluence (TrypLE Express, Gibco, Life Technologies) and seeded into new flasks, one per patient; FLS were used at the fourth passage for the experiments. Phenotyping of synoviocytes was performed by surface markers with PE-conjugated monoclonal antibodies from BD PharMigenTM, California, USA, anti-CD166 was used for fibroblast (Figure 1(a)), anti-CD47 for mesenchymal cells (Figure 1(b)); and anti-CD14 associated to macrophage (Figure 1(c)). Data were collected through of FACSCalibur flow cytometer and analyzed with CellQuestTM PRO software (Becton–Dickinson). In addition, FLS analysis was evaluated by expression of prolyl-4-hydroxylase (P4H) by immunofluorescence assay (IFA) and Western blot (WB). For the IFA, cells were seeded into fixed and permeated chamber-slides. Typical morphology of FLS was observed (Figure 1(d)). Subsequently, cells were incubated with primary antibody P4H (ab108980, Abcam), followed by a secondary antibody (ab Alexa Fluor® 488, Abcam). Images were captured with an Ism 5 beta Carl Zeiss microscope (Figure 1(e)). Analysis of the protein content was performed by WB according to Zamudio-Cuevas et al.19 using secondary antibody ab97200 Goat pAb Rb, Abcam. Normalization was carried out with a β-actin antibody from Sigma (A3854). Blots were revealed using Immobilon Western Chemiluminescent HRP Substrate (Millipore Corporation, USA) and were scanned with an Amersham Imager 600 RGB (GE) (Figure 1(f)).
Figure 1.
Characterization of synoviocytes populations. (a) Positive cells anti-CD166 by flow cytometry. (b) Expression of CD47. (c) Low expression of CD14. Each image shows the average ± standard deviation of five independent experiments from different patients. (d) Typical morphology of FLS. (e) Expression of P4H+ in FLS by IFA using Alexa Fluor® 488 secondary anti-body. Original magnification ×20. (f) Protein expression of P4H+ by WB. Results are representative of one of five separate experiments. (A color version of this figure is available in the online journal.)
Experimental model
FLS were seeded in 24-wells treated cell culture plates at a density of 35,000/well, cultured in DMEM-F12, supplemented with 10% FBS and 1% P/S at 37° C, with 5% CO2 and an air humidity saturation of 95%. At 24 h, cells were treated with MSU crystals at 75 µg/mL in a DMEM-F12 supplemented medium with 2% FBS at different periods of incubation as follow: 5 min, 15 min, 20 min, 30 min, 60 min, 2 h, 4 h, 6 h, 12 h, 24 h, 48 h, and 72 h for phagocytosis assay. FLS incubated with medium alone served as controls. The 24 h time of exposure was one of the mainly used for subsequent analyzes.
Phagocytosis assay
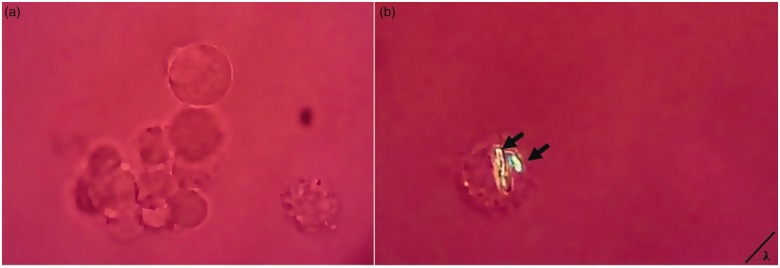
After incubation with MSU crystals, the adherent FLS monolayer was washed with PBS, and then it subjected to enzymatic disaggregation with TrypLE Express (Gibco, Life Technologies, USA). Intracellular crystal analysis was performed by placing a drop of cellular suspension between a cover slip and a slide using a polarized microscope with first-order red compensation (Axioskop 40, Carl Ziess). A measure of phagocytic activity of the FLS was determined by counting 100 successive cells in different sections and identified the number of cells that internalized at least one crystal through changes in the birefringence intensity of MSU (Figure 2). The percentage of FLS showing phagocytic activity (phagocytosis index) was calculated by number of FLS with MSU-crystals phagocytized per 100 FLS. Phagocytic activity was expressed as Phagocytic index (PhIx). Additionally, the phagocytosis of MSU crystals was examined by transmission electron microscopy, according to a previous study19 and also by flow cytometry analysis using side and forward scatter parameters.
Figure 2.
Assessment of MSU-crystal phagocytosis. Rounded typical morphology of the adherent cells when it detached and suspended. (a) Unstimulated FLS. (b) The arrows point to two engulfed MSU crystal in a FLS. Representative images of >20 fields from five independent experiments are shown. Original magnification ×40, polarized light microscopy. (A color version of this figure is available in the online journal).
Cytokine determination
Since IL-1β is one of the main inflammatory factors in the process of acute gout, we evaluated its production at different time points in supernatants from MSU-treated and MSU-untreated cultures. IL-1 was determined by the Human IL-1 Standard ABTS ELISA Development Kit (Peprotech, USA) according to the manufacturer’s instructions. Additional measurement of other cytokines levels (IL-6, TNF-α, IL-8, MCP-1, NGF, and HGF) were measured at 24 h time using the Milliplex Human Adipocyte Magnetic Panel (Merck-Millipore, Darmstadt, Germany). The quantification was performed by Magpix Merck-Millipore and results were expressed in pg/mL. VEGF was determined by the Human VEGF Standard ABTS ELISA Development Kit (Peprotech, USA).
Quantitative real-time PCR
Total RNA from the FLS with MSU crystals and controls was extracted by the Trizol method,20 and was treated with DNAse to remove contaminating genomic DNA, and the quantitative real-time (qRT-PCR) of the UGDH, HIF1A, VHL, and VEGF genes was performed in a RotorGene Q Thermocycler (Supplementary Table 1), using the RT2 First Strand Kit (Qiagen). The results were normalized to the housekeeping RPL27 gene, and the relative quantification was performed using the Relative Expression Tool software 2009 (REST-v09). At the end of the amplification, a melting assay was performed to confirm the specific size of each gene product.
Statistical analysis
Each experiment was performed in at least three independent experiments. Statistical analysis was performed using GraphPad Prism v 6.0 with analysis of variance (ANOVA), followed by a one-way post hoc Dunnett’s test. P values ≤0.05 were considered as statistically significant.
Results
FLS internalize MSU crystals
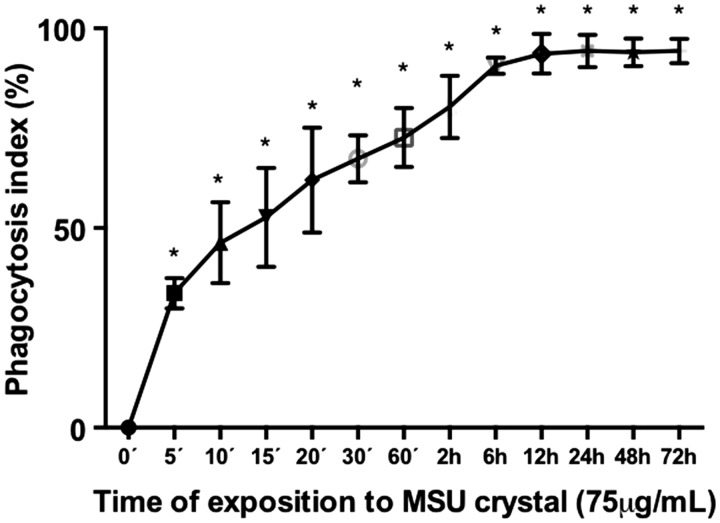
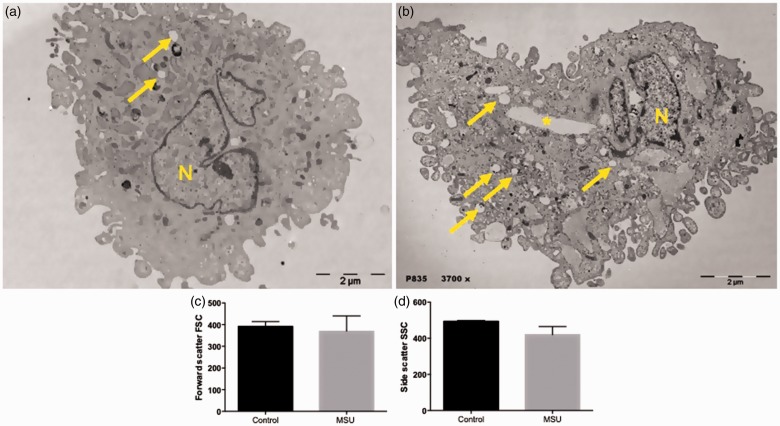
In response to MSU crystals, FLS showed a 33.6% increase in the PhIx for crystal internalization (P < 0.0001, in comparison to the control), starting at 5 min of stimulation. This internalization of the MSU crystals was time-dependent until reaching a maximum peak of 94% PhIx at 6 h (P < 0.0001, in comparison to the control), without showing changes at 12, 24, and 72 h when compared to 6 h (Figure 3). Electron micrographs showed structures representing cavities of MSU crystals deposited in the cytoplasm. They also evidenced numerous vacuoles of different shapes and sizes induced by MSU crystals, in comparison to non-stimulated FLS (Figure 4(a) and (b)). Flow cytometry analysis showed an increase in the forward scatter of the FLS, induced by MSU crystals, in contrast to a decrease in side scatter, when compared to untreated cells; however, these results were not significant (Figure 4(c) and (d)).
Figure 3.
Dose response curve of PhIx of MSU crystal by FLS. Quantification of cells with internalized MSU crystals was determined by polarized light microscopy. Data are presented as mean value ± standard deviation of at least three independent experiments. *P value ≤0.05 in comparison to zero time. PhIx: phagocytic index.
Figure 4.
Phagocytosis of MSU crystals by FLS. (a) Scanning electron micrograph of untreated FLS exhibiting cell nuclear (N), and vesicular structures (yellow arrows). (b) FLS treated with MSU crystals exhibiting swollen vesicular structures (yellow arrows), MSU crystal cavity (yellow asterisk) and cell nuclear (N) structures. Images are representative of one of five separate experiments with FLS from different patients. Phagocytosis analysis by flow cytometry. (c) Forward scatter, (d) Side scatter. Each bar represents the mean value ± standard deviation of at least three independent experiments. (A color version of this figure is available in the online journal).
FLS activation following MSU crystal uptake
MSU-treated FLS increased the production of IL-1β at 12 and 24 h (Figure 5), as well as other inflammatory cytokines such as IL-6, TNF-α, and HGF and chemokines such as IL-8, MCP-1, and the nerve growth factor (NGF) respect to absence of stimulus. FLS treated with MSU crystals released increased levels of IL-6, 18,024.6 ± 9093.2 pg/mL vs. 3070.8 ± 4766 (P = 0.0171); TNF-α, 1.77 ± 0.47 pg/mL vs. 0.88 ± 0.33 pg/mL (P < 0.0613); HGF, 48.1 ± 24.1 pg/mL vs. 11.3 ± 7.83 pg/mL (P = 0.0238); IL-8, 4608 ± 819.3 pg/mL vs. 1803.7 ± 2250.1 pg/mL (P = 0.0124); MCP-1, 4264.8 ± 3317.1 pg/mL vs. 224.9 ± 128.6 pg/mL (P = 0.0648); and NGF to 3.13 ± 0.9 pg/mL vs. 0.43 ± 0.13 pg/mL (P = 0.0057) compared to non-stimulated FLS (Figure 6).
Figure 5.
IL-1β production in FLS exposed to MSU-crystals. Columns show quantification of IL-1β secretion on FLS treated with MSU at different time. Quantification of IL-1β production was by microplate reader. Values are expressed as the mean ± standard deviation of at least three independent experiments. *P value ≤ 0.05 vs. control.
Figure 6.
Production of cytokines on FLS treated with MSU crystals. Cytokines were evaluated by the Milliplex Human Adipocyte Magnetic Panel. (a) Levels of IL-6, (b) TNF-α, (c) HGF, (d) IL-8, (e) MCP-1, (f) NGF. Each point represents the mean value ± standard deviation of at least three independent experiments. *P value ≤0.05 in comparison to control.
MSU crystals induce inflammation through the VEGF gene and protein expression in FLS
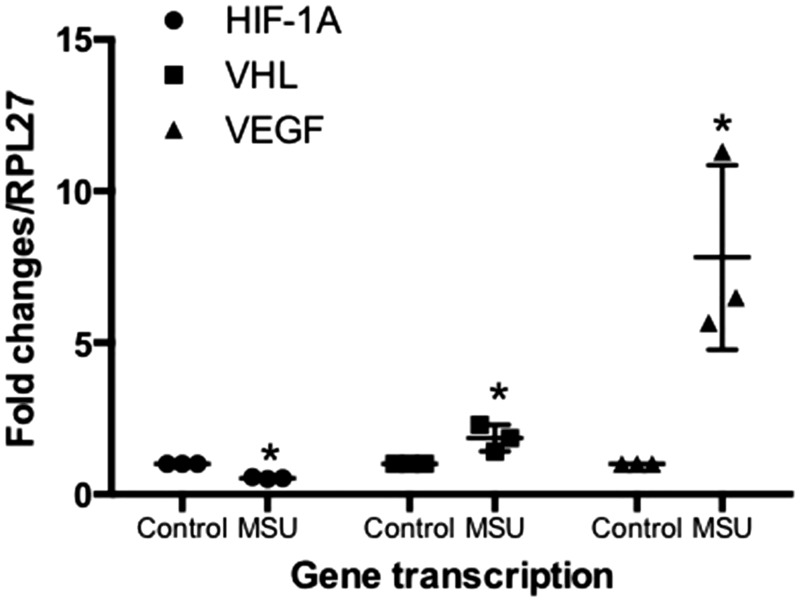
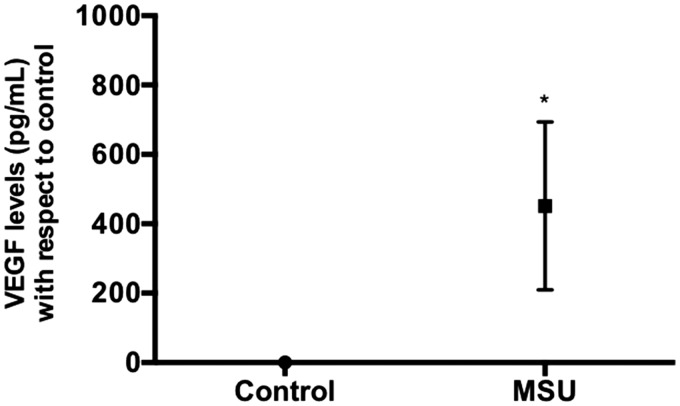
qRT-PCR analysis indicated that the expression of the VEGF gene in the presence of MSU crystals was increased 7.82-fold, when compared with non-treated cells. Similarly, the VHL expression (the negative regulator of HIF1A) showed a 1.85-fold augmentation, compared with non-stimulated FLS. Finally, the FLS exposed to MSU crystals decreased the mRNA expression of the HIF1A gene by 0.53-fold compared with non-treated FLS (Figure 7). Concomitantly, VEGF secretion was increased at 24 h after MSU crystal stimulation (Figure 8).
Figure 7.
Gene expression of HIF1A, VHL, and VEGF in FLS. The cells were treated with MSU crystals (75 µg/mL) at 24 h, and gene expression was evaluated by qRT-PCR. Each point represents the mean value ± standard deviation of at least three independent experiments. *P value ≤0.05 compared to untreated FLS.
Figure 8.
VEGF protein expression in FLS. The synoviocytes were treated with MSU crystals (75 µg/mL) at 24 h, and the protein expression was evaluated by ELISA. Each point represents the mean value ± standard deviation of at least three independent experiments. *P value ≤0.05 compared to untreated FLS (control).
Discussion
Gout is a paradigm for acute inflammation triggered by interaction between MSU crystals and the local tissue environment. The cells that are most commonly studied in vitro in gouty arthritis are neutrophils and macrophages.21 However, our current results suggest that FLS may also participate by uptake of crystals through phagocytosis.
The current study provides further insight into the activation of the MSU crystals in the phagocytic capacity of human FLS, as well as their regulating role in the development of the inflammatory response mediated by MSU crystals.
Firstly, our findings showed a marked increase in PhIx in FLS stimulated with MSU crystals in a time-dependent manner. Visualization with polarized light microscopy showed that 95% of the FLS can phagocytize MSU crystals after 24 h. Moreover, the morphostructural scanning electron microscopy analysis showed traces left behind by the phagocytized crystals, as well as the presence of several secretory vesicles in the cytoplasm, which suggest the production of pro-inflammatory cytokines in response to MSU crystals. Results from experiments in which leukocytes phagocytized MSU crystals show that the crystals, once phagocytized, may be released by cell disruption, whereas the cytokines leak out into the cytoplasm.22–24 Even though MSU crystal phagocytosis induces morphostructural changes in FLS, these could not be detected with the side and forward dispersion indices of the cells by flow cytometry, even though this method has previously revealed significant differences between side-scatter and forward-scatter profiles in monocyte/macrophage cell lines.25 In addition, previous studies shown that phagocytosis of MSU crystals increase of side scatter in neutrophils, eosinophils, and basophils due to induction of extracellular DNA traps26 or by a phenotype change in CD14+ monocytes.27
Since IL-1β is one of the main inflammatory factors in the process of phagocytosis of MSU crystals in acute gout,21 detection of IL-1β level was verify at different time points. Our results showed that MSU crystals increased the production of IL-1β at 6, 12, and 24 h, suggesting an association with times in which greater phagocytosis of MSU crystals is detected. Upon discovering the formation of secretory vesicles, we decided to evaluate their secretion into the supernatant of the MSU crystal-activated FLS using a low-density array for pro-inflammatory cytokine detection. We identified cytokines (IL-6, TNF-α), chemokines (IL-8, MCP-1), and growth factors (NGF, HGF) with an inflammatory activity that have already been associated with the acute inflammatory phase of gout.28
The greater than two-fold increased secretion of IL-6, TNF-α, IL-8, and MCP-1 found here is consistent with other in vitro studies, where the same phenomenon was observed.29,30 These cytokines have been reported to be increased, in vivo, in the synovial fluids and the synovial membranes of patients with joint disease.31 Our study demonstrates an increase of NGF, a nociceptive pain modulator that, in addition to its neuronal effects, is known to act on inflammatory cells as a critical factor in inflammation-associated hyperalgesia.32 NGF is a growth factor associated with inflammation in chondrocytes and synovial fluid from OA patients,33 and is upregulated in the FLS of inflammatory arthritis, such as RA and psoriatic arthritis. This suggests that dysregulated production of NGF has the potential to induce inflammation and joint damage.34
The close relationship between inflammation and angiogenesis has been established in the pathogenesis of joint diseases, including crystal-induced arthritis.21 We observed that MSU crystal stimulated FLS secrete higher levels of HGF, a multifunctional factor that has been identified in osteoarticular tissues as a key molecule for bone and cartilage replacement after these have suffered damage.35 Increased production of HGF in the synovial fluid has been reported in damage mechanisms like RA, which has been associated with the vasculoproliferative phase of inflammation.36–38
The capacity of HGF to trigger vascularity is based on its ability to activate HIF1A transcription and reestablish normal oxygen conditions under hypoxic conditions.13 In rheumatic diseases, particularly in OA, HIF-1α has a role in the transcriptional response regulating angiogenesis.39 Therefore, we decided to identify whether HIF1A is overexpressed in inflammation induced by MSU crystals in FLS. We identified a decrease in HIF1A levels in the FLS treated with MSU crystals, compared with the controls, in contrast to the increase in VHL expression.
We found that VEGF was overexpressed in synoviocytes stimulated with MSU crystals. Therefore, MSU crystals did not activate VEGF by the canonical HIF1A pathway, at least for a time after 24 h. Additionally, it is possible that other transcription factors such as nuclear factor-kappa B (NF-kB) and activator protein-1 (AP-1) may be involved in the activation of VEGF.40,41 Our data showed that MSU crystals could decrease the gene expression of HIF1A in FLS, contrasting with the overexpression of HIF1A in the inflamed synovial membrane, due to the increased oxygen demand caused by the inflammatory process and tissue damage.42 All studies published so far have pointed to the synovial lining layer as the main site of HIF1A expression. It is not clear whether this expression is due to other lineages, as HIF1A is upregulated in RA fibroblasts, CD3+ T cells, and CD68+ synovial macrophages.18 However, the precise role of HIF1A remains to be defined. Little was known about the contributions of the different HIF-1 subunits, but recent studies have shown that HIF-2α is mainly overexpressed in fibroblasts, where it enhances their osteoclastogenic potential and regulates cell proliferation, expression of receptor activator of NF-kB ligand, and induction of several catabolic factors12; additionally, HIF-2α subunit is also involved in cartilage destruction.43 Therefore, other subunits not evaluated in this study such as HIF-3α,44 that may be playing a role as regulators of the synovial inflammation in gout.
In conclusion, we confirmed that MSU crystals phagocytosis activates FLS as mediators of inflammation, producing cytokines and chemokines that mediate the recruitment and activation of leukocytes. Further studies are needed to achieve a better understanding of the phagocytosis mechanism triggered by MSU crystals, as well as to elucidate the pathway of the inflammation process that takes place on the synovial compartment, and which could contribute to the angiogenesis and pain experienced during an acute gout attack. This model of inflammation caused by MSU crystals phagocytosis in FLS is crucial for determining the role of anti-inflammatory molecules during the development of therapeutic targets to block synovial inflammation.
Supplemental Material
Supplemental Material for Phagocytosis of monosodium urate crystals by human synoviocytes induces inflammation by Yessica Zamudio-Cuevas, Javier Fernández-Torres, Gabriela Angélica Martínez-Nava, Karina Martínez-Flores, Adriana Ramírez Olvera, Daniel Medina-Luna, Alma Delia Hernández Pérez, Carlos Landa-Solís and Alberto López-Reyes: on behalf of the EUROASPIRE Investigators* in Experimental Biology and Medicine
ACKNOWLEDGMENTS
The authors thank Mónica Guadalupe Santamaría-Olmedo for performed the quantitative real-time PCR, as well as Roberto Sánchez-Sánchez, PhD, of Biotechnology Laboratory at the Instituto Nacional de Rehabilitación “Luis Guillermo Ibarra Ibarra” for the Human IL-1 and VEGF Standard ABTS ELISA Development Kits (Peprotech, USA) obtained for this study.
Authors’ contributions
YZC and JFT contributed equally to this work. They undertook experiments and managed data, wrote the manuscript, and conceived and designed experiments. ALR conceived the study and experimental designed, contributed with reagents and materials. GAMN conducted statistical analysis and data processing. KMF, ARO, DML, CLS, ADHP conducted experimental guidance, data verification and contributed analysis tools. All authors read and critically revised the manuscript and approved the final version.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval and consent to participate
Ethical approval was obtained from the institutional review board (INR-LGII, Ref: 02/13). All participants gave a written informed consent prior to inclusion in the study. The study protocol was approved by the local ethical committee CONBIOETICA-09-CEI-031–20171207.
FUNDING
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M. Rising burden of gout in the UK but continuing suboptimal management: a nationwide population study. Ann Rheum Dis 2015; 74:661–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, Giannini EH, Heyse SP, Hirsch R, Hochberg MC, Hunder GG, Liang MH, Pillemer SR, Steen VD, Wolfe F. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum 1998; 41:778–99 [DOI] [PubMed] [Google Scholar]
- 3.Moilanen LJ, Hämäläinen M, Lehtimäki L, Nieminen RM, Moilanen E. Urate crystal induced inflammation and joint pain are reduced in transient receptor potential Ankyrin1 deficient mice potential role for transient receptor potential Ankyrin 1 in gout. PLoS One 2015; 10:e0117770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cronstein BN, Terkeltaub R. The inflammatory process of gout and its treatment. Arthritis Res Ther 2006; 8:S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006; 440:237–41 [DOI] [PubMed] [Google Scholar]
- 6.Zheng SC, Zhu XX, Xue Y, Zhang LH, Zou HJ, Qiu JH, Liu Q. Role of the NLRP3 inflammasome in the transient release of IL-1β induced by monosodium urate crystals in human fibroblast-like synoviocytes. J Inflamm (Lond) 2015; 12:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalbeth N, Haskard DO. Mechanisms of inflammation in gout. Rheumatology (Oxford) 2005; 44:1090–6 [DOI] [PubMed] [Google Scholar]
- 8.Scanu A, Oliviero F, Gruaz L, Sfriso P, Pozzuoli A, Frezzato F, Agostini C, Burger D, Punzi L. High-density lipoproteins downregulate MCP-1 production in human fibroblast-like synoviocytes stimulated by urate crystals. Arthritis Res Ther 2010; 12:R23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brouwer E, Gouw AS, Posthumus MD, van Leeuwen MA, Boerboom AL, Bijzet J, Bos R, Limburg PC, Kallenberg CG, Westra J. Hypoxia inducible factor-1-alpha (HIF-1alpha) is related to both angiogenesis and inflammation in rheumatoid arthritis. Clin Exp Rheumatol 2009; 27:945–51 [PubMed] [Google Scholar]
- 10.Gaber T, Dziurla R, Tripmacher R, Burmester GR, Buttgereit F. Hypoxia inducible factor (HIF) in rheumatology: low O2! See what HIF can do! Ann Rheum Dis 2005; 64:971–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernández-Torres J, Hernández-Díaz C, Espinosa-Morales R, Camacho-Galindo J, Galindo-Sevilla NC, López-Macay A, Zamudio-Cuevas Y, Martínez-Flores K, Santamaría-Olmedo MG, Pineda C, Granados J, Martínez-Nava GA, Gutiérrez M, López-Reyes AG. Polymorphic variation of hypoxia inducible factor-1 A (HIF1A) gene might contribute to the development of knee osteoarthritis: a pilot study. BMC Musculoskelet Disord 2015; 16:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernández-Torres J, Zamudio-Cuevas Y, Martínez-Nava GA, López-Reyes AG. Hypoxia-inducible factors (HIFs) in the articular cartilage: a systematic review. Eur Rev Med Pharmacol Sci 2017; 21:2800–10 [PubMed] [Google Scholar]
- 13.Tacchini L, Dansi P, Matteucci E, Desiderio MAH. Hepatocyte growth factor signalling stimulates hypoxia inducible factor-1 (HIF-1) activity in HepG2 hepatoma cells. Carcinogenesis 2001; 22:1363–71 [DOI] [PubMed] [Google Scholar]
- 14.Kitajima Y, Ide T, Ohtsuka T, Miyazaki K. Induction of hepatocyte growth factor activator gene expression under hypoxia activates the hepatocyte growth factor/c-Met system via hypoxia inducible factor-1 in pancreatic cancer. Cancer Sci 2008; 99:1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin YM, Huang YL, Fong YC, Tsai CH, Chou MC, Tang CH. Hepatocyte growth factor increases vascular endothelial growth factor-A production in human synovial fibroblasts through c-Met receptor pathway. PLoS One 2012; 7:e50924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamilton JL, Nagao M, Levine BR, Chen D, Olsen BR, Im HJ. Targeting VEGF and its receptors for the treatment of osteoarthritis and associated pain. J Bone Miner Res 2016; 31:911–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pichiule P, Chavez JC, Schmidt AM, Vannucci SJ. Hypoxia-inducible factor-1 mediates neuronal expression of the receptor for advanced glycation end products following hypoxia/ischemia. J Biol Chem 2007; 282:36330–40 [DOI] [PubMed] [Google Scholar]
- 18.Quiñonez-Flores CM, González-Chávez SA, Pacheco-Tena C. Hypoxia and its implications in rheumatoid arthritis. J Biomed Sci 2016; 23:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zamudio-Cuevas YE, Martínez-Flores K, Fernández-Torres J, Loissell-Baltazar YA, Medina-Luna D, López-Macay A, Camacho-Galindo J, Hernández-Díaz C, Santamaría-Olmedo MG, López-Villegas EO, Oliviero F, Scanu A, Cerna-Cortés JF, Gutiérrez M, Pineda C, López-Reyes A. Monosodium urate crystals induce oxidative stress in human synoviocytes. Arthritis Res Ther 2016; 18:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987; 162:156–9 [DOI] [PubMed] [Google Scholar]
- 21.Busso N, So A. Gout. Mechanisms of inflammation in gout. Arthritis Res Ther 2010; 12:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riddle JM, Bluhm GB, Barnhart MI. Ultrastructural study of leucocytes and urates in gouty arthritis. Ann Rheum Dis 1967; 26:389–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shirahama T, Cohen AS. Ultrastructural evidence for leakage of lysosomal contents after phagocytosis of monosodium urate crystals: a mechanism of gouty inflammation. Am J Pathol 1974; 76:501–20 [PMC free article] [PubMed] [Google Scholar]
- 24.Landis RC, Yagnik DR, Florey O, Philippidis P, Emons V, Mason JC, Haskard DO. Safe disposal of inflammatory monosodium urate monohydrate crystals by differentiated macrophages. Arthritis Rheum 2002; 46:3026–33 [DOI] [PubMed] [Google Scholar]
- 25.Yagnik DR, Hillyer P, Marshall D, Smythe CD, Krausz T, Haskard DO, Landis RC. Noninflammatory phagocytosis of monosodium urate monohydrate crystals by mouse macrophages. Implications for the control of joint inflammation in gout. Arthritis Rheum 2000; 43:1779–89 [DOI] [PubMed] [Google Scholar]
- 26.Schorn C, Janko C, Latzko M, Chaurio R, Schett G, Herrmann M. Monosodium urate crystals induce extracellular DNA traps in neutrophils, eosinophils, and basophils but not in mononuclear cells. Front Immunol 2012; 3:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeong JH, Hong S, Kwon OC, Ghang B, Hwang I, Kim YG, Lee CK, Yoo B. CD14+ cells with the phenotype of infiltrated monocytes consist of distinct populations characterized by anti-inflammatory as well as pro-inflammatory activity in gouty arthritis. Front Immunol 2017; 8:1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin WJ, Harper JL. Innate inflammation and resolution in acute gout. Immunol Cell Biol 2010; 88:15–9 [DOI] [PubMed] [Google Scholar]
- 29.Chen DP, Wong CK, Tam LS, Li EK, Lam CW. Activation of human fibroblast-like synoviocytes by uric acid crystals in rheumatoid arthritis. Cell Mol Immunol 2011; 8:469–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guerne PA, Terkeltaub R, Zuraw B, Lotz M. Inflammatory microcrystals stimulate interleukin-6 production and secretion by human monocytes and synoviocytes. Arthritis Care Res 1987; 32:1443–52 [DOI] [PubMed] [Google Scholar]
- 31.Lioté F, Champy R, Moenner M, Boval-Boizard B, Badet J. Elevated angiogenin levels in synovial fluid from patients with inflammatory arthritis and secretion of angiogenin by cultured synovial fibroblasts. Clin Exp Immunol 2003; 132:163–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuligoi R. Effect of colchicine on nerve growth factor-induced leukocyte accumulation and thermal hyperalgesia in the rat. Naunyn-Schmiedeberg's Arch Pharmacol 1998; 358:264–9 [DOI] [PubMed] [Google Scholar]
- 33.Pecchi E, Priam S, Gosset M, Pigenet A, Sudre L, Laiguillon MC, Berenbaum F, Houard X. Induction of nerve growth factor expression and release by mechanical and inflammatory stimuli in chondrocytes: possible involvement in osteoarthritis pain. Arthritis Res Ther 2014; 16:R16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raychaudhuri SK, Raychaudhuri SP. Functional significance of nerve growth factor and its receptor (TrkA) in inflammatory arthritis. Arthritis Res Ther 2010; 12:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonnet CS, Walsh DA. Osteoarthritis, angiogenesis and inflammation. Rheumatology (Oxford) 2005; 44:7–16 [DOI] [PubMed] [Google Scholar]
- 36.Adamopoulos IE, Athanasou NA. Hepatocyte growth factor in normal and diseased bone and joint tissues. Curr Rheumatol Rev 2006; 2:1–7 [DOI] [PubMed] [Google Scholar]
- 37.Yukioka K, Inaba M, Furumitsu Y, Yukioka M, Nishino T, Goto H, Nishizawa Y, Morii H. Levels of hepatocyte growth factor in synovial fluid and serum of patients with rheumatoid arthritis and release of hepatocyte growth factor by rheumatoid synovial fluid cells. J Rheumatol 1994; 21:2184–9 [PubMed] [Google Scholar]
- 38.Koch AE, Halloran MM, Hosaka S, Shah MR, Haskell CJ, Baker SK, Panos RJ, Haines GK, Bennett GL, Pope RM, Ferrara N. Hepatocyte growth factor. A cytokine mediating endothelial migration in inflammatory arthritis. Arthritis Rheum 1996; 39:1566–75 [DOI] [PubMed] [Google Scholar]
- 39.Chu H, Xu ZM, Yu H, Zhu KJ, Huang H. Association between hypoxia-inducible factor-1a levels in serum and synovial fluid with the radiographic severity of knee osteoarthritis. Genet Mol Res 2014; 13:10529–36 [DOI] [PubMed] [Google Scholar]
- 40.Bancroft CC, Chen Z, Yeh J, Sunwoo JB, Yeh NT, Jackson S, Jackson C, Van Waes C. Effects of pharmacologic antagonists of epidermal growth factor receptor, PI3K and MEK signal kinases on NF-kappa B and AP-1 activation and IL-8 and VEGF expression in human head and neck squamous cell carcinoma lines. Int J Cancer 2002; 99:538–48 [DOI] [PubMed] [Google Scholar]
- 41.Fujioka S, Niu J, Schmidt C, Sclabas GM, Peng B, Uwagawa T, Li Z, Evans DB, Abbruzzese JL, Chiao PJ. NF-κB and AP-1 connection: mechanism of NF-κB-dependent regulation of AP-1 activity. Mol Cell Biol 2004; 24:7806–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henroit Y, Pesesse L, Lambert C. Targetin the synovial angiogenesis as a novel treatment approach to osteoarthritis. Ther Adv Musculoskelet Dis 2014; 6:20–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ryu JH, Chae CS, Kwak JS, Oh H, Shin Y, Huh YH, Lee CG, Park YW, Chun CH, Kim YM, Im SH, Chun JS. Hypoxia-inducible factor-2α is an essential catabolic regulator of inflammatory rheumatoid arthritis. PLoS Biol 2014; 12:e1001881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka T, Wiesener M, Bernhardt W, Eckardt KU, Warnecke C. The human HIF (hypoxia-inducible factor)-3alpha gene is a HIF-1 target gene and may modulate hypoxic gene induction. Biochem J 2009; 424:143–51 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Phagocytosis of monosodium urate crystals by human synoviocytes induces inflammation by Yessica Zamudio-Cuevas, Javier Fernández-Torres, Gabriela Angélica Martínez-Nava, Karina Martínez-Flores, Adriana Ramírez Olvera, Daniel Medina-Luna, Alma Delia Hernández Pérez, Carlos Landa-Solís and Alberto López-Reyes: on behalf of the EUROASPIRE Investigators* in Experimental Biology and Medicine