Short abstract
In vitro follicular culture systems provide optimal culture models for research about the physiology of the ovary and support the clinical practices to achieve competent mature oocytes for in vitro fertilization. In vitro maturation of preantral follicles makes it possible to study the effects of therapeutic agents on various conditions or disorders of the ovary. Nowadays, preventive bioflavonoids against cancer, hypercholesterolemia, fatty liver, or a variety of toxic agents are in focus. The aim of this study was to design and investigate the impacts of different concentrations of hesperidin, a glycoside flavonoid, on the in vitro preantral follicle growth and maturation in the three-dimensional (3D) culture system which was made with sodium alginate. Preantral follicles (n = 1363) were mechanically isolated from immature mice ovaries, then, after capsulating, they were randomly divided into four groups: the control group received no concentration of hesperidin, and three experimental groups were supplemented with 10, 22.5, and 50 µmol/L of hesperidin. All groups were cultured for 12 days. At the end of the culture period, the percentage of survival rate, antrum formation, obtained metaphase II oocytes, and the secretion of 17β-estradiol and progesterone were significantly higher in the group Hesp 50 (50 µmol/L hesperidin). Moreover, the mean average of follicular diameter cultured in the group Hesp 50 was also increased and the mRNA expression levels of PCNA, FSH-R, and Bcl-2 genes were higher, while Bax mRNA expression was significantly reduced compared with the other groups. Follicles cultured in the presence of 50 µmol/L of hesperidin had a higher fertilization rate and embryo development. Adding hesperidin at the concentration of 50 µmol/L to the culture medium resulted in higher follicular growth and maturation and increased the rate of in vitro fertilization and embryo development.
Impact statement
It has been stated that hesperidin has many pharmacological effects, such as anti-inflammatory and antioxidant effects, antimicrobial activity, and anti-carcinogenic activity; but hesperidin and its derivatives have been under investigation as anti-fertility factors for a very long time. However, our results show that hesperidin can improve mice follicular growth and maturation during in vitro 3D culture. Hesperidin as an antioxidant factor could enhance the mRNA expression levels of two important genes involved in folliculogenesis, PCNA, and FSH-R. Our results prove for the first time that hesperidin not only has deleterious effects on follicular development but can also increase rates of in vitro fertilization and embryo development.
Keywords: Ovarian follicle culture, hesperidin, hormones, gene expression, in vitro maturation, in vitro fertilization
Introduction
Within the ovaries of rodents or humans, folliculogenesis, as a major issue in fertility, occurs as a complex and dynamic process, in which an interaction between the somatic cells of supporting cells (i.e. theca and granulosa cells (GCs)) and the oocyte leads to ovulation.1–4 Numerous factors affect ovulation and fertility, and it is a challenge to deal with infertility and infertility-causing diseases, such as polycystic ovary syndrome.5–7
In vitro maturation (IVM) of immature or preantral ovarian follicles can be used as an accepted model in order to perceive the mechanisms that may occur during folliculogenesis and also in order to study or find out the effects of multiple factors such as endocrines, antioxidants, nutrients, vitamins, and locally acting factors on follicular development.3 On the other hand, in vitro follicular culture systems, two-dimensional (2D) and three-dimensional (3D), not only provide optimal culture models for ovarian physiology research but also support the clinical practice to achieve competent mature oocytes for in vitro fertilization (IVF).3,8 Preantral follicle isolations from the ovary can be carried out by two common methods (mechanical and enzymatic) for culturing.3,4 In the 2D culture system, both forms of isolated follicles lose their integrity by the migration and attachments of GCs around the oocyte and to the culture dishes, respectively.3,4 Therefore, 2D systems cannot provide an excellent correlation with the in vivo conditions.3,8 However, isolated follicles can not only maintain their integrity in 3D cultures but also provide an encapsulated space around the follicles using hydrophobic membranes that prevent follicular attachment to the culture dishes. Hence, a good correlation with the in vivo conditions can be achieved in 3D systems.3,8
Numerous studies have been conducted to investigate fertility and infertility mechanisms and affecting factors, but these issues remain challenging.3,9 Additionally, the effects of diverse factors added to the culture media of the follicles can be investigated during IVM of preantral follicles.3
Nowadays, the search to assess the preventive effects of therapeutic agents, as potential health benefits, against various disorders is the target of excessive research; especially, preventive bioflavonoids against cancer, hypercholesterolemia, fatty liver, or a variety of toxic agents are in focus.10–20
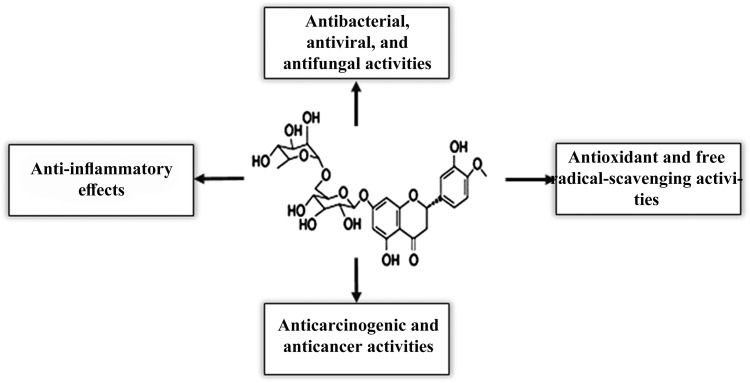
Hesperidin (Hesp) (Figure 1) (5, 7, 3′-trihydroxy-4′-methoxy-flavanone7-rhamnoglucoside) is a bioflavonoid, which is abundant in citrus fruits, such as orange and lemon, and plant-derived beverages, such as tea and olive oil commonly used in traditional medicines.21 It has been reported that Hesp has diverse pharmacological actions, such as antioxidant, anticarcinogenic, analgesic, antiviral, antibacterial, antifungal, antiulcer, anti-inflammatory, and anticancer activity (Figure 1).21 The anti-proliferative effect of Hesp against MCF-7 cells and its apoptotic effect on colon and pancreatic cancer cells have been reported.22–24 Interestingly, hesperidin was found to be safe during pregnancy; no side effects had been recorded even after the oral administration of the compounds in combination with diosmin to treat hemorrhoids.21 Recent epidemiological data reinforced the safety of hesperidin in pregnancy.25 Furthermore, one study reported the neuroprotective activity of Hesp in a murine model of aluminum-induced neurotoxicity.26 Toxicological studies have recently reported that Hesp can protect many tissues against toxic agents-induced oxidative injuries by its antioxidant and free radical-scavenging activity.21,27 It has been found that Hesp, by its strong cellular antioxidant protection, could decrease the damaging effects induced by cyclophosphamide (CP) treatment.28 Hesp can also facilitate the formation of vitamin C complex; hence, it can support the healthy immune system functions.29 Hesperidin, as a flavanone glycoside, comprises an aglycone, Hesperetin. It has been found that Hesperetin can inhibit the oxidative stress via estrogen receptor-mediated actions and, as an antioxidant, it can improve the quality of porcine oocytes during aging in vitro.30
Figure 1.
The chemical structure and biological activities of hesperidin. Hesperidin possesses a wide range of bioactivities useful for clinical applications, such anti-inflammatory, antimicrobial, antioxidant, and anticancer activities.
However, based on our current knowledge, the efficiency of hesperidin on ovarian follicles in a long-term culture has not been described before. Therefore, the aim of this study was to investigate the effects of different concentrations of hesperidin on the follicular development of isolated preantral follicles in the 3D culture system made with sodium alginate hydrogel.
Material and methods
Animals, follicle isolation, and experimental design
Fifty NMRI (National Medical Research Institute) female mice (12–14 week old) were used with the permission of the Animal Research Ethical Committee of the Tabriz University of Medical Sciences (IR.TBZMED.REC.1396.555). Mice were terminated by cervical dislocation, and bilateral ovaries were dissected and placed immediately in α-minimal essential medium (α-MEM) (Gibco, UK), which was supplemented with 10% fetal bovine serum (FBS) (Gibco, UK). The preantral follicles were isolated mechanically under a laboratory stereomicroscope, then the intact follicles were chosen for 3D culture which had two or three layers of GCs with normal (i.e. round) and centrally located oocytes with the size of 125–135 µm.3 The preantral follicles (n = 1363) were divided into four groups. The control group (n = 286) was not treated with any additional supplementations, while groups Hesp 10 (n = 357), Hesp 22.5 (n = 369), and Hesp 50 (n = 351) were supplemented with 10, 22.5, and 50 µmol/L of hesperidin, respectively.31 The culture medium for all of the study groups was α-MEM medium, which was supplemented with 1% insulin-transferrin-selenium (ITS) (Gibco, UK), 100 mIU/mL of recombinant follicle-stimulating hormone (rFSH) (Serono, Switzerland), 100 IU/mL of penicillin, 50 μg/mL of streptomycin, and 5% FBS.3,4
3D in vitro culture of isolated follicles
For encapsulating isolated follicles, we used sodium alginate as has been previously described.3,4 In brief, 1 g of sodium alginate powder was dissolved in 10 mL of deionized water. Then, 0.5 g of charcoal was added to the solution to remove hydrogel impurities. The solution was centrifuged at 5000 r/min for 1 min, and then the removed transparent solution was stored at 4°C. Before each encapsulation, to prepare sodium alginate solution at a concentration of 0.5% (w/v), it was diluted with sterile phosphate buffer saline (PBS). Then, droplets of sodium alginate (8 mL) were put into the sterile culture dishes, and each isolated follicle was transferred inside each droplet of sodium alginate by using a micropipette. At the end of the process, they were immersed in the cross-linking solution containing CaCl2 (50 mM) and NaCl (140 mM) for 1 to 2 min. Then, after removing alginate beads from the cross-linking solution, they were washed with α-MEM media and transferred to a droplet (50 mL) of culture media in Petri dishes (SPL, Life Science, Korea), which was filled with 20 droplets of culture media. The encapsulated follicles were cultured under mineral oil (Sigma, Germany) for 12 days in an incubator (37°C, 5% CO2). Moreover, every other day, half of the media was changed.3,4
Assessment of follicular morphology
On days 0, 6, and 12 of the cell culture periods, the morphology of follicles was checked using an inverted microscope (Olympus, Japan), and the photo of the follicles was taken at 10× magnification. Then, follicle size was measured by ImageJ software (n = 40 for each group). On the last day (day 12) of the cell culture, 1.5 IU/mL of human chorionic gonadotropin (HCG) (Organon, The Netherlands) was added to the culture medium of all groups to induce oocyte maturation and ovulation. The released oocytes were considered as germinal vesicle (GV), germinal vesicle breakdown (GVBD), as well as metaphase II (MII), which had been described in our previous study.3,4
Hormonal assays
On the last day of the cell culture, half of the medium of each follicle culture medium from all subgroups (n = 5 for each subgroup, at least three times) was collected to investigate the levels of steroid hormones, 17-β estradiol (E2), and progesterone (P4). E2 and P4 were measured with a radioimmunoassay kit (Orion Diagnostica, Finland, sensitivity = 6.5 pg/mL) and an enzyme-linked immunosorbent assay kit (DiaPlus, USA, sensitivity = 0.1 ng/mL), respectively.3,4
RNA extraction, cDNA synthesis, and real-time RT-qPCR
The total cellular RNA from follicles (n = 12, last day of culture) was extracted using TRIzol reagent (Invitrogen, Karlsruhe, Germany) and was carried out according to the manufacturer’s protocol. The synthesis of cDNA was done according to the manufacturer’s instructions (Fermentas Inc., Hanover, MD, USA). The mRNA expression levels of Bax, Bcl-2, FSH-R, and PCNA genes were analyzed by GAPDH as a housekeeping gene. For PCR reactions, primers were adapted from other primers (as designed on the NCBI website or in our previous studies)3,32 and synthesized by Cinnagen (Table 1). PCRs were performed using Master Mix and SYBR Green I in an Applied Biosystems, StepOne™ thermal cycler (Applied Biosystems, USA). The program started with an initial melting cycle for 5 min at 95°C to activate the polymerase, followed by 40 cycles of melting (30 s at 95°C), annealing (30 s at 58°C) and expanse (30 s at 72°C). The quality of the PCR reactions was confirmed by melting curve analyses. For each sample, the reference gene (GAPDH) and target genes were amplified in the same run. Reference genes were almost equal. The target genes were normalized to a reference gene and calculated with the Pfaffl method (2−ΔΔCt, ΔΔCt = ΔCtSample–ΔCtControl).33
Table 1.
The characteristic of primer sequences used in real-time quantitative reverse transcription polymerase chain reaction assays.
| Genes | Accession numbers | Primer sequence (5′–3′) | PCR product size (bp) |
|---|---|---|---|
| Bax | NM_017059.2 | F: 5′-TTTGCTACAGGGTTTCATCCAG-3′R: 5′-GTCCAGTTCATCGCCAATTC-3′ | 139 |
| Bcl-2 | NM_000633.2 | F: 5′-GAGAGCGTCAACAGGGAGAT-3′R: 5′-ACAGCCAGGAGAAATCAAACA-3′ | 169 |
| FSH-R | XM_011532735.2 | F: 5′-AGGTACAGCTCTGCCATGCT-3′R: 5′-GTACGAGGAGGGCCATAACA-3′ | 171 |
| PCNA | NM_011045.2 | F: 5′-GGAAGCTTAGAGTAGCTCTCATC-3′R: 5′-GGGAATTCGTGACAGAAAAGACCTC-3′ | 174 |
| GAPDH | NM-007393 | F: 5′-GGAAAAGAGCCTAGGGCAT-3′R: 5′-CTGCCTGACGGCCAGG-3′ | 64 |
IVF and embryo culture
For performing IVF, in the first stage, the cauda epididymis of male NMRI mice (eight-week old, n = 10) was dissected and placed immediately into human tubal fluid (HTF) medium (Sigma Aldrich, Germany), which had been supplemented with 5 mg/mL of bovine serum albumin (BSA) (Gibco, UK), for 1.5 h for sperm capacitation. In the second stage, after capacitation, the collected MII oocytes were transferred from all subgroups of the study to HTF medium, which contained capacitated spermatozoa and was supplemented with 15 mg/mL of BSA for a period of 6 h. In the third stage, after insemination, the oocytes were transferred to the global medium, which was supplemented with 5 mg/mL of BSA and were cultured for five days (120 h). During this period, the IVF and developmental rates for 2-cell embryos, morula, and hatching blastocyst embryos were assessed daily.34
Statistical analysis
Statistical analysis was carried out with SPSS software version 22 (SPSS, SPSS Inc., USA). Data were assessed for normality with the Kolmogorov–Smirnov test. All results are presented as mean ± SD and assessed with one-way ANOVA Tukey’s post hoc tests. p < 0.05 was considered as significant.
Results
The inverted microscope images and the mean diameter of cultured follicles
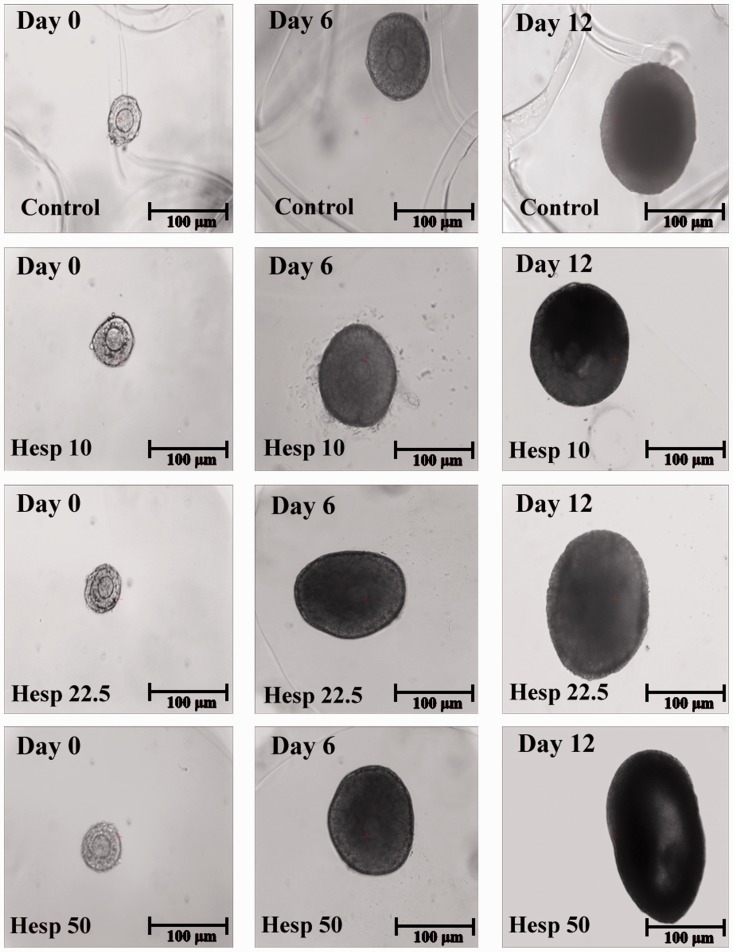
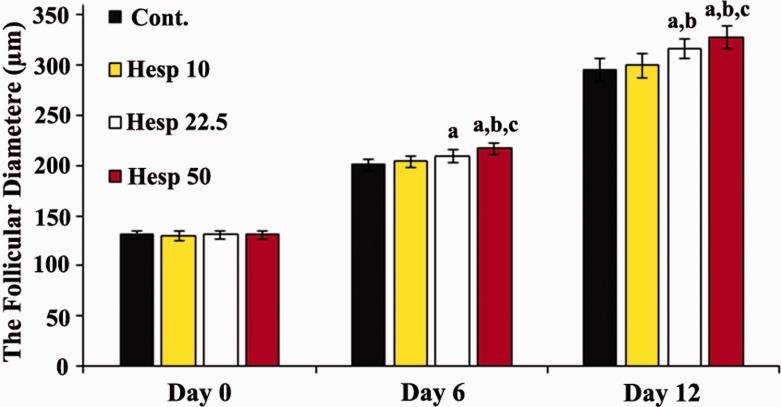
The inverted microscope images and the mean diameter of cultured isolated preantral follicles in all the groups are shown in Figures 2 and 3.
Figure 2.
Images of the inverted microscope during in vitro three-dimensional follicular development in the alginate droplet on day 0 (first column), day 6 (second column), and day 12 (third column) in different groups.
Figure 3.
The mean average of follicular diameter (μm) on days 0, 6, and 12 of the experiment. a, b, and c show significant differences compared with the control group, Hesp 10, and Hesp 22.5, respectively. On day 6, the administration of hesperidin at the dose of 22.5 µmol/L caused greater increase in follicular diameter in comparison with the control group (ap < 0.05); however, in the Hesp 50 group, the mean diameter of follicles was significantly increased compared with the control, Hesp 10, and Hesp 22.5 (ap < 0.05, bp < 0.05, and cp < 0.001, respectively). On day 12, the administration of hesperidin at the dose of 22.5 µmol/L could increase the follicular diameter in comparison with the control and Hesp 10 groups (ap < 0.05 and bp < 0.05, respectively), while treatment with 50 µmol/L hesperidin (Hesp 50) caused greater increase in follicular diameter in comparison with the control, Hesp 10, and Hesp 22.5 (ap < 0.05, bp < 0.05, and cp < 0.001, respectively). (A color version of this figure is available in the online journal.)
At the initiation of the cell culture (day 0), the mean diameter of encapsulated follicles was around 131 μm. On day 6, the diameters of follicles increased in all cultured groups (200 ± 5.9, 204 ± 5.69, 209 ± 6.11, and 2.16 ± 5.19 µm), and a significant increase was found in groups Hesp 22.5 and Hesp 50 as compared with the control group (p < 0.05). In this part, the largest diameter was observed in group Hesp 50 (p < 0.05). Furthermore, at the end of the culture period—day 12—the mean diameter of follicles in groups Hesp 22.5 and Hesp 50 was significantly increased (316 ± 9.27 and 327 ± 11.03 µm, respectively) as compared with the control and Hesp 10 groups (295 ± 11.2 and 299 ± 11.9 µm, respectively) (p < 0.05). However, the largest diameter was observed in the follicles of group Hesp 50 (p < 0.05).
There was no significant difference between the control group and experimental 1 group on days 6 and 12 of culture.
Follicular developmental rate
Developmental rates of encapsulated cultured follicles in all cultured groups are summarized in Table 2. The rates of surviving follicles, antrum formation, and MII oocytes were significantly increased in groups Hesp 22.5 and Hesp 50 in comparison with two other groups of the culture (p < 0.05). Among all groups, the highest rate was observed in group Hesp 50 (p < 0.05). There was no significant difference between the control and Hesp 10 (10 µmol/L) groups in these parameters.
Table 2.
Developmental rates of cultured isolated preantral follicles.
| Groups | Number of follicles(1363) | Number of survived(% ± SD) | Number of antrum formation (% ± SD) | Number of MII oocytes (% ± SD) |
|---|---|---|---|---|
| Cont. | 286 | 192 (67.13 ± 2.31) | 83 (43.22 ± 3.09) | 19 (22.89 ± 1.19) |
| Hesp 10 | 357 | 249 (69.74 ± 2.88) | 114 (45.78 ± 2.43) | 29 (25.43 ± 1.08) |
| Hesp 22.5 | 369 | 271 (73.44 ± 2.71)a,b | 133 (49.07 ± 2.56)a,b | 41 (30.82 ± 1.11)a,b |
| Hesp 50 | 351 | 275 (78.34 ± 2.01)a,b,c | 149 (54.18 ± 2.92)a,b,c | 56 (37.58 ± 1.15)a,b,c |
Note: The control (Cont.) group contained 5% fetal bovine serum (FBS) without hesperidin, Hesp 10 was treated with 10 µmol/L hesperidin, Hesp 22.5 was treated with 22.5 µmol/L hesperidin, and Hesp 50 was treated with 50 µmol/L hesperidin. Values are given as mean ± SD.
ap < 0.05 as compared with the control group.
bp < 0.05 as compared with Hesp10 group.
cp < 0.002 as compared with Hesp 22.5 group.
Hormonal assays
Table 3 shows the concentrations of E2 as well as P4. As shown in Table 3, in groups Hesp 22.5 and Hesp 50, there was a significant increase in hormone levels compared with the control group and group Hesp 10 (p < 0.05). Moreover, a significant difference was observed in the level of E2 when group Hesp 10 was compared with the control group (p < 0.05). The highest hormonal productions were observed in group Hesp 50 as compared with other groups of the study.
Table 3.
The levels of steroid hormones, 17-β estradiol (E2), and progesterone (P4), in collected media on day 12 of the culture.
| Groups | 17-β estradiol (Mean ± SD) (pg/mL) | Progesterone (Mean ± SD) (ng/mL) |
|---|---|---|
| Cont. | 1872.81 ± 62.35 | 16.93 ± 2.21 |
| Hesp 10 | 1987.74 ± 58.12a | 19.34 ± 1.11 |
| Hesp 22.5 | 2189.93 ± 81.38a,b | 27.69 ± 2.02a,b |
| Hesp 50 | 2785.94 ± 45.98a,b,c | 35.67 ± 1.08a,b,c |
Note: The control (Cont.) group contained 5% fetal bovine serum (FBS) without hesperidin, Hesp 10 was treated with 10 µmol/L hesperidin, Hesp 22.5 was treated with 22.5 µmol/L hesperidin, and Hesp 50 was treated with 50 µmol/L hesperidin. Values are given as mean ± SD.
ap < 0.05 as compared with the control group.
bp < 0.05 as compared with Hesp10 group.
cp < 0.001 as compared with Hesp 22.5 group.
Gene expression in follicles
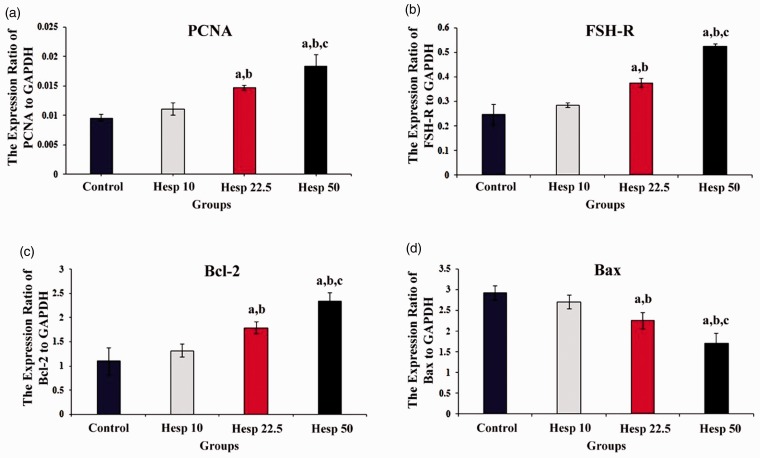
The impacts of hesperidin on the expression levels of Bax, Bcl-2, PCNA, as well as FSH-R mRNA were investigated by RT-qPCR. Figure 4 shows the relative expression levels, which were normalized against the level of endogenous GAPDH as an internal control. As shown in Figure 4, although the expression of FSH-R as well as PCNA genes was significantly higher in experimental groups 2 and 3 than in other groups (p < 0.05), the highest mRNA expression of FSH-R and PCNA genes was observed in experimental group 3 supplemented with 50 µmol/L hesperidin. On the other hand, the mRNA expression of the pro-apoptotic gene, Bax, was significantly decreased in groups Hesp 22.5 and Hesp 50 when compared with the control and Hesp 10 groups (p < 0.05), while the lowest Bax mRNA expression was observed in group Hesp 50 (p < 0.05). The relative expression of the Bcl-2 gene in experimental groups 2 and 3 was significantly different from that of the control and Hesp 10 groups (p < 0.05). However, the highest mRNA expression of Bcl-2, as one of the anti-apoptotic genes, was observed in group Hesp 50 (p < 0.05).
Figure 4.
The mRNA expression levels of PCNA, FSH-R, Bcl-2, and Bax genes in all groups of the study–control, Hesp 10, Hesp 22.5, and Hesp 50—on the last day of the culture period, day 12. Values are mean ± standard deviation (SD). Hesp: Hesperidin. (a) The mRNA expression of PCNA; a, b, and c show significant differences compared with the control group (p < 0.05), Hesp 10 group (p < 0.04), and Hesp 50 group (p < 0.003), respectively. (b) The mRNA expression of FSH-R; a, b, and c show significant differences compared with the control group (p < 0.05), Hesp 10 group (p < 0.05), and Hesp 50 group (p < 0.001), respectively. (c) The mRNA expression of Bcl-2; a, b, and c show significant differences compared with the control group (p < 0.05), Hesp 10 group (p < 0.05), and Hesp 50 group (p < 0.02), respectively. (d) The mRNA expression of Bax; a, b, and c show significant differences compared with the control group (p < 0.05), Hesp 10 group (p < 0.003), and Hesp 50 group (p < 0.001), respectively. (A color version of this figure is available in the online journal.)
Fertilization rate and embryo development
The fertilization rates of MII oocytes and embryo development are summarized in Table 4. In addition, the development of embryos is shown in Figure 5. As shown in Table 4, the percentage of fertilization rate increased in all experimental groups, while the highest percentage of fertilization rate was found in group Hesp 50 (p < 0.05). Moreover, embryo development—2-cell, morula, and hatched—was higher in all groups receiving hesperidin compared with the control group, but the highest percentage of embryo development was observed in group Hesp 50 (p < 0.05).
Table 4.
Fertilization rate and embryo development in all study groups.
| Groups | Number of MII | Number of fertilized (% ± SD) | Number of 2-cell (%± SD) | Number of morula (% ± SD) | Number of hatched (% ± SD) |
|---|---|---|---|---|---|
| Cont. | 19 | 13 (68.42 ± 0.3) | 8 (61.53 ± 0.6) | 4 (30.76 ± 0.4) | 3 (23.07 ± 0.8) |
| Hesp 10 | 27 | 19 (70.37 ± 0.5) | 12 (63.15 ± 0.6) | 6 (31.57 ± 0.7) | 5 (26.31 ± 1.1) |
| Hesp 22.5 | 38 | 27 (71.05 ± 0.5)a | 18 (66.66 ± 0.9)a | 9 (33.33 ± 1.2)a | 8 (29.62 ± 1.1)a |
| Hesp 50 | 51 | 39 (76.47 ± 0.9)a,b,c | 29 (74.35 ± 1.4)a,b,c | 16 (41.02 ± 0.9)a,b,c | 13 (33.33 ± 1.2)a,b,c |
Note: The control (Cont.) group contained 5% fetal bovine serum (FBS) without hesperidin, Hesp 10 was treated with 10 µmol/L hesperidin, Hesp 22.5 was treated with 22.5 µmol/L hesperidin, and Hesp 50 was treated with 50 µmol/L hesperidin. Values are given as mean ± SD.
ap < 0.05 as compared with the control group.
bp < 0.05 as compared with Hesp10 group.
cp < 0.001 as compared with Hesp 22.5 group.
Figure 5.
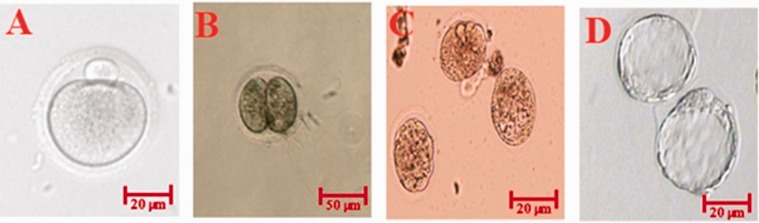
Effect of hesperidin on embryo development. (a) a healthy MII oocyte. (b, c, and d) Embryo development in the stages of 2-cell, morula, and hatched, respectively. (A color version of this figure is available in the online journal.)
Discussion
It has been reported that preantral follicles can be successfully cultured and matured in 2D or 3D culture systems.3 IVC and IVM of immature ovarian follicles have been considered as a model to study the mechanisms of folliculogenesis at the genetic level.3 These methods are an alternative for breast cancer patients who are unable to undergo ovarian stimulation and for the investigation of the actions of hormones, toxicants, drugs, antioxidants on oocyte, or somatic cells.3
For a very long time, hesperidin has been under investigation as an antifertility factor.21 In 1948, it was reported that phosphorylated hesperidin (PH) acts as an antifertility agent by inhibiting the sperm enzyme hyaluronidase.35 Another study reported that the intraperitoneal administration of PH (20 mg/kg) to both male and female mice for eight days reduced the percentage of pregnancies on mating.36
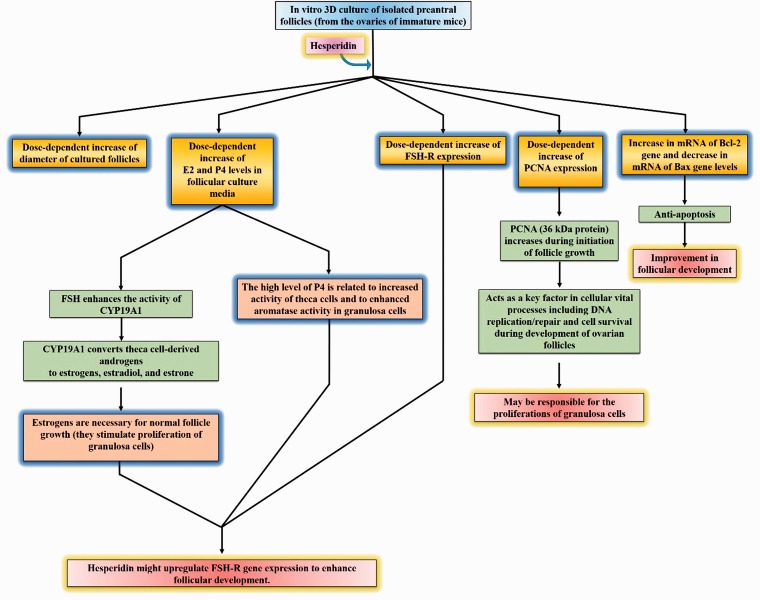
However, the present study was the first to demonstrate that the addition of hesperidin positively affected the in vitro 3D culture of preantral follicles isolated from the ovaries of immature mice (Figure 6). Our results showed that the diameter of cultured follicles increased in all study groups during 12 days of culture, and this effect of the compound was dose dependent. On the other hand, adding hesperidin to the culture media could enhance follicular development rate, fertilization rate, and embryo development in a dose-dependent manner.
Figure 6.
In vitro 3D culture of isolated preantral follicles (from the ovaries of immature mice) by adding hesperidin. (A color version of this figure is available in the online journal.)
During in vitro culture and maturation of preantral follicles, they are maintained under higher concentrations of O2; therefore, continuously in aerobic cells, free radicals are produced and result in decreasing follicular development.3 However, ROS may affect follicular growth and maturation.3 On the other hand, it may be a major cause of poor oocyte quality and may thereby decrease the developmental competence of oocytes in vivo and in vitro.37 The balance between ROS and antioxidants influences follicular growth and maturation because antioxidant supplements, by reducing reactive oxygen species and inhibiting lipid peroxidation, can lead to preventing cell damage, enhance follicular development, and increase the fertilization rate and embryo development.30 One study reported that hesperidin, through its antioxidant action, provides the protection of fertility against oxidative stress induced by CP in ovaries of rats.38 Another study showed that treatment with Hesperetin could enhance the in vitro development of aging porcine oocytes and the mRNA expression of some cytoplasmic maturation marker genes, such as bone morphogenetic protein 15 (BMP15) and growth differentiation factor-9 (GDF9).30
Our results also showed that the gene expression of PCNA, which can be found in fetal and adult ovaries of several mammals, was significantly increased by hesperidin. It has been reported that PCNA is a 36 kDa protein, which increases during the initiation of follicle growth and acts as one of the key factors in cellular vital processes including DNA replication and repair as well as cell survival during the development of ovarian follicles. Hence, the hesperidin-induced increase in PCNA gene expression can be partially responsible for the proliferations of GCs.
On the other hand, our results showed that treatment with hesperidin increases the expression of FSH-R mRNA and the levels of E2 and P4 in the media. FSH-R is exclusively located on GCs, and is essential for the ovarian function and fertility. FSH-R is a glycoprotein that belongs to the family of G protein-coupled receptors and is expressed in growing follicles.31 On the other hand, FSH plays an important role in ovarian follicular maturation, oogenesis, and the production of steroid hormones; hence, it is crucial for fertility39; in other words, when the FSH binds its cognate receptor, it induces the follicular transition from preantral to antral follicles and promotes cytochrome P450.3 FSH can also increase the transcription of CYP19A1 in GCs involved in converting theca cell-derived androgens, namely dehydroepiandrosterone, androstenedione, androstenediol, and testosterone into estrogens, estradiol, and estrone.40,41 It has been indicated that estrogens stimulate the proliferation of GCs and are absolutely necessary for the normal follicle growth.40 We have also found that hesperidin increased E2 and P4 levels in follicular culture media dose dependently. The high level of P4 is related to increased activity of theca cells and to enhanced aromatase activity in GCs.3,40 Therefore, it seems that the hesperidin-induced increase in FSH-R gene expression can be partially responsible for the elevated estrogen hormones. Interestingly, one study found a potential impact of hesperidin against formaldehyde (FA, 2 mg/kg) toxicity in pregnant rats; the results also showed that the level of E2 (17β-estradiol) was significantly higher in mice that orally received 50 mg/kg hesperidin and in Hesp + FA groups than in the control and FA groups, while the level of progesterone (P4) did not show a significant difference.42 Hence, we hypothesize that hesperidin might upregulate FSH-R gene expression to enhance follicular development.
It has been interestingly proved that the accumulation of ROS influences apoptosis.16,43 Flavonoids as antioxidants can neutralize reactive oxygen metabolites, and therefore protect apoptosis-induced cell death.44 We have found that hesperidin treatment led to an increase and a decrease in the mRNA levels of Bcl-2 gene (anti-apoptotic) and Bax gene (pro-apoptotic), respectively. Hesperidin was also found to inhibit the generation of ROS.45 One study reported that treatment with Hesperetin decreased the level of ROS in aging porcine oocytes in vitro and significantly increased the expression of antioxidant genes, such as peroxiredoxin 5, nuclear factor erythroid 2-like 2, and superoxide dismutase 1 and 2.30 Thus, the improvement in follicular development by hesperidin can partially be explained by its anti-apoptotic activity.
Conclusion
According to these results, our study provides a mechanistic link between the enhancement of follicular development and the functions of E2, P4, PCNA, FSH-R, Bcl-2, and Bax. The dose-dependent increase in the diameter of cultured follicles, the increase of E2 and P4 levels in follicular culture media, the increase of PCNA and FSH-R expression, the increase of mRNA of Bcl-2 gene and the decrease in mRNA of Bax gene levels might help follicular development, offering a novel approach to developing a new class of follicle developing and follicle enhancing agents.
Authors’ contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript; HS, MB, MMK, MS, and MSGF have collected the data; MB has written the paper; MA, MMHT, RG and HP-T have contributed data or analysis tools; ME and OA have performed the analysis; RG and HP-T have conceived and designed the analysis.
DECLARATION OF CONFLICT OF INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
Ministry of Human Capacities, Hungary grant 20391–3/2018/FEKUSTRAT is acknowledged.
References
- 1.Epifano O, Dean J. Genetic control of early folliculogenesis in mice. Trends Endocrinol Metab 2002; 13:169–73 [DOI] [PubMed] [Google Scholar]
- 2.Alikani M, Schimmel T, Willadsen SM. Cytoplasmic fragmentation in activated eggs occurs in the cytokinetic phase of the cell cycle, in lieu of normal cytokinesis, and in response to cytoskeletal disorder. Mol Hum Reprod 2005; 11:335–44 [DOI] [PubMed] [Google Scholar]
- 3.Shoorei H, Khaki A, Ainehchi N, Hassanzadeh Taheri MM, Tahmasebi M, Seyedghiasi G, Ghoreishi Z, Shokoohi M, Khaki AA, Abbas Raza SH. Effects of Matricaria chamomilla extract on growth and maturation of isolated mouse ovarian follicles in a three-dimensional culture system. Chin Med J 2017; 131:218–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shoorei H, Shokoohi M, Ghorbani S, Eyni H, Hassanzadeh Taheri MM, Kalarestaghi H, Khaki A, Khaki A, Roshangar L, Majdi Seghinsara A, Riahi Rad K, Tahmasebi M. Panax ginseng extract improves follicular development after in vitro 3D mouse preantral follicle culture. Cell J 2019; 21:210–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohammad MB, Seghinsara AM. Polycystic ovary syndrome (PCOS), diagnostic criteria, and AMH. Asian Pacific J Cancer Prevent 2017; 18:17–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majdi Seghinsara A, Banimohammad M. New facts about ovarian stem cells: the origin and the fate. Int J Women’s Health Reprod Sci 2018; 6:127–33 [Google Scholar]
- 7.Abtahi-Eivari SH, Moghimian M, Soltani M, Shoorei H, Asghari R, Hajizadeh H, Shokoohi M, Alami S, Ghaderi FK. The effect of Galega officinalis on hormonal and metabolic profile in a rat model of polycystic ovary syndrome (PCOS). Int J Women's Health Reprod Sci 2018; 6:276–82 [Google Scholar]
- 8.Brito IR, Lima IMT, Xu M, Shea LD, Woodruff TK, Figueiredo JR. Three-dimensional systems for in vitro follicular culture: overview of alginate-based matrices. Reprod Fertil Dev 2014; 26:915–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munne S, Alikani M, Ribustello L, Colls P, Martinez-Ortiz PA, McCulloh DH. Euploidy rates in donor egg cycles significantly differ between fertility centers. Human Reprod 2017; 32:743–9 [DOI] [PubMed] [Google Scholar]
- 10.Pazoki-Toroudi HR, Hesami A, Vahidi S, Sahebjam F, Seifi B, Djahanguiri B. The preventive effect of captopril or enalapril on reperfusion injury of the kidney of rats is independent of angiotensin II AT1 receptors. Fundam Clin Pharmacol 2003; 17:595–8 [DOI] [PubMed] [Google Scholar]
- 11.Pazoki-Toroudi HR, Ajami M, Habibey R. Pre-medication and renal pre-conditioning: a role for alprazolam, atropine, morphine and promethazine. Fundam Clin Pharmacol 2010; 24:189–98 [DOI] [PubMed] [Google Scholar]
- 12.Ajami M, Davoodi SH, Habibey R, Namazi N, Soleimani M, Pazoki-Toroudi H. Effect of DHA+EPA on oxidative stress and apoptosis induced by ischemia-reperfusion in rat kidneys. Fundam Clin Pharmacol 2013; 27:593–602 [DOI] [PubMed] [Google Scholar]
- 13.Ghadernezhad N, Khalaj L, Pazoki-Toroudi H, Mirmasoumi M, Ashabi G. Metformin pretreatment enhanced learning and memory in cerebral forebrain ischaemia: the role of the AMPK/BDNF/P70SK signalling pathway. Pharm Biol 2016; 54:2211–9 [DOI] [PubMed] [Google Scholar]
- 14.Amani H, Habibey R, Hajmiresmail SJ, Latifi S, Pazoki-Toroudi H, Akhavan O. Antioxidant nanomaterials in advanced diagnoses and treatments of ischemia reperfusion injuries. J Mater Chem B 2017; 5:9452–76 [DOI] [PubMed] [Google Scholar]
- 15.Javdan N, Ayatollahi SA, Iqbal Choudhary M, Al-Hasani S, Pazoki-Toroudi H. FOXO1 targeting by capsaicin reduces tissue damage after testicular torsion. Andrologia 2018;e12987. [DOI] [PubMed] [Google Scholar]
- 16.Shoorei H, Khaki A, Khaki AA, Hemmati AA, Moghimian M, Shokoohi M. The ameliorative effect of carvacrol on oxidative stress and germ cell apoptosis in testicular tissue of adult diabetic rats. Biomed Pharmacother 2018; 111:568–78 [DOI] [PubMed] [Google Scholar]
- 17.Shokoohi M, Olad Saheb Madarek E, Khaki A, Shoorei H, Khaki AA, Soltani M, Ainehchi N. Investigating the effects of onion juice on male fertility factors and pregnancy rate after testicular torsion/detorsion by intrauterine insemination method. Int J Women’s Health Reprod Sci 2018; 6:499–505 [Google Scholar]
- 18.Soltani M, Moghimian M, Abtahi-Eivari SH, Shoorei H, Khaki A, Shokoohi M. Protective effects of Matricaria chamomilla extract on torsion/detorsion-induced tissue damage and oxidative stress in adult rat testis. Int J Fertil Steril 2018; 12:242–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tejada S, Manayi A, Daglia M, Nabavi SF, Sureda A, Hajheydari Z, Gortzi O, Pazoki-Toroudi H, Nabavi SM. Wound healing effect of curcumin: a review. Curr Pharm Biotechnol 2016; 17:1002–7 [PubMed] [Google Scholar]
- 20.Mehrjerdi FZ, Aboutaleb N, Pazoki-Toroudi H, Soleimani M, Ajami M, Khaksari M, Safari F, Habibey R. The protective effect of remote renal preconditioning against hippocampal ischemia reperfusion injury: role of KATP channels. J Mol Neurosci 2015; 57:554–60 [DOI] [PubMed] [Google Scholar]
- 21.Garg A, Garg S, Zaneveld LJD, Singla AK. Chemistry and pharmacology of the citrus bioflavonoid hesperidin. Phytother Res 2001; 15:655–69 [DOI] [PubMed] [Google Scholar]
- 22.Natarajan N, Thamaraiselvan R, Lingaiah H, Srinivasan P, Maruthaiveeran Periyasamy B. Effect of flavonone hesperidin on the apoptosis of human mammary carcinoma cell line MCF-7. Biomed Prevent Nutr 2011; 1:207–15 [Google Scholar]
- 23.Johnson JL, de Mejia EG. Flavonoid apigenin modified gene expression associated with inflammation and cancer and induced apoptosis in human pancreatic cancer cells through inhibition of GSK-3beta/NF-kappaB signaling cascade. Mol Nutr Food Res 2013; 57:2112–27 [DOI] [PubMed] [Google Scholar]
- 24.Park HJ, Kim M-J, Ha E, Chung J-H. Apoptotic effect of hesperidin through caspase3 activation in human colon cancer cells, SNU-C4. Phytomedicine 2008; 15:147–51 [DOI] [PubMed] [Google Scholar]
- 25.Lacroix I, Beau AB, Hurault-Delarue C, Bouilhac C, Petiot D, Vayssière C, Vidal S, Montastruc JL, Damase-Michel C. First epidemiological data for venotonics in pregnancy from the EFEMERIS database. Phlebology 2016; 31:344–8 [DOI] [PubMed] [Google Scholar]
- 26.Jangra A, Kasbe P, Pandey SN, Dwivedi S, Gurjar SS, Kwatra M, Mishra M, Venu AK, Sulakhiya K, Gogoi R. Hesperidin and silibinin ameliorate aluminum-induced neurotoxicity: modulation of antioxidants and inflammatory cytokines level in mice hippocampus. Biol Trace Elem Res 2015; 168:462–71 [DOI] [PubMed] [Google Scholar]
- 27.Sahu BD, Kuncha M, Sindhura GJ, Sistla R. Hesperidin attenuates cisplatin-induced acute renal injury by decreasing oxidative stress, inflammation and DNA damage. Phytomedicine 2013; 20:453–60 [DOI] [PubMed] [Google Scholar]
- 28.Wilmsen PK, Spada DS, Salvador M. Antioxidant activity of the flavonoid hesperidin in chemical and biological systems. J Agric Food Chem 2005; 53:4757–61 [DOI] [PubMed] [Google Scholar]
- 29.Peterson JJ, Beecher GR, Bhagwat SA, Dwyer JT, Gebhardt SE, Haytowitz DB, Holden JM. Flavanones in grapefruit, lemons, and limes: a compilation and review of the data from the analytical literature. J Food Compost Anal 2006; 19:S74–80 [Google Scholar]
- 30.Kim W-J, Lee S-E, Park Y-G, Jeong S-G, Kim E-Y, Park S-P. Antioxidant hesperetin improves the quality of porcine oocytes during aging in vitro. Mol Reprod Dev 2019; 86:32–41 [DOI] [PubMed] [Google Scholar]
- 31.Liu WY, Liou S-S, Hong T-Y, Liu I-M. Protective effects of hesperidin (citrus flavonone) on high glucose induced oxidative stress and apoptosis in a cellular model for diabetic retinopathy. Nutrients 2017; 9:1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazoochi T, Salehnia M, Pour Beiranvand S, Forouzandeh M, Mowla SJ, Hajizadeh E. Analysis of apoptosis and expression of genes related to apoptosis in cultures of follicles derived from vitrified and non-vitrified ovaries. Mol Hum Reprod 2009; 15:155. [DOI] [PubMed] [Google Scholar]
- 33.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001; 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Behbahanian A, Eimani H, Zeinali B, Rezazadeh Valojerdi M, Eftekhari YP, Shahverdi A, Gourabi H, Golkar-Narenji A. In vitro maturation, fertilization and embryo culture of oocytes obtained from vitrified auto-transplanted mouse ovary. Int J Fertil Steril 2013; 6:278–85 [PMC free article] [PubMed] [Google Scholar]
- 35.Beiler JM, Martin GJ. Inhibition of hyaluronidase action by derivatives of hesperidin. J Biol Chem 1948; 174:31–5 [PubMed] [Google Scholar]
- 36.Martin GJ, Beiler JM. Effect of phosphorylated hesperidin, a hyaluronidase inhibitor, on fertility in the rat. Science 1952; 115:402. [DOI] [PubMed] [Google Scholar]
- 37.Goto Y, Noda Y, Mori T, Nakano M. Increased generation of reactive oxygen species in embryos cultured in vitro. Free Radic Biol Med 1993; 15:69–75 [DOI] [PubMed] [Google Scholar]
- 38.Khedr NF. Protective effect of mirtazapine and hesperidin on cyclophosphamide-induced oxidative damage and infertility in rat ovaries. Exp Biol Med (Maywood) 2015; 240:1682–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fortune JE. Ovarian follicular growth and development in mammals. Biol Reprod 1994; 50:225–32 [DOI] [PubMed] [Google Scholar]
- 40.Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev 2004; 25:947–70 [DOI] [PubMed] [Google Scholar]
- 41.Conley AJ, Bird I. The role of cytochrome P450 17 alpha-hydroxylase and 3 beta-hydroxysteroid dehydrogenase in the integration of gonadal and adrenal steroidogenesis via the delta 5 and delta 4 pathways of steroidogenesis in mammals. Biol Reprod 1997; 56:789. [DOI] [PubMed] [Google Scholar]
- 42.Merzoug S, Toumi ML. Effects of hesperidin on formaldehyde-induced toxicity in pregnant rats. Excli J 2017; 16:400–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shokoohi M, Shoorei H, Soltani M, Abtahi-Eivari S-H, Salimnejad R, Moghimian M. Protective effects of the hydroalcoholic extract of Fumaria parviflora on testicular injury induced by torsion/detorsion in adult rats. Andrologia 2018;e13047. [DOI] [PubMed] [Google Scholar]
- 44.Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem 2002; 13:572–84 [DOI] [PubMed] [Google Scholar]
- 45.Hirata A, Murakami Y, Shoji M, Kadoma Y, Fujisawa S. Kinetics of radical-scavenging activity of hesperetin and hesperidin and their inhibitory activity on COX-2 expression. Anticancer Res 2005; 25:3367–74 [PubMed] [Google Scholar]