Summary
Aims
Conventional dorsal root ganglion stimulation (DRGS) is known to achieve better pain‐paresthesia overlap of difficult‐to‐reach areas like the feet compared to dorsal column spinal cord stimulation (SCS). As in painful diabetic polyneuropathy (PDPN) pain is mostly present in the feet, we hypothesized that DRGS is more effective in relieving pain in PDPN when compared to SCS.
Methods
Diabetes was induced in female Sprague‐Dawley rats with an intraperitoneal injection of 65 mg/kg of streptozotocin (STZ; n = 48). Rats with a significant decrease in mechanical paw withdrawal response to von Frey filaments 4 weeks after injection were implanted with DRGS electrodes (n = 18). Rats were assigned to DRGS (n = 11) or sham‐DRGS (n = 7). Mechanical paw withdrawal thresholds (WT, measured in grams) in response to DRGS (50 Hz, 0.18 ± 0.05 mA) were assessed with von Frey testing. The results of the experiments on these animals were compared to the results of a previous study using exactly the same model on PDPN animals selected for SCS (n = 8) (40‐50 Hz, 0.19 ± 0.01 mA) and sham‐SCS (n = 3).
Results
In the SCS group, the log10 (10 000 × 50% WT) increased from 4910 to 5211 at t = 15 minutes (P < 0.05) and 5264 at t = 30 minutes (P = 0.11). In the DRGS group, the log10 (10,000 × 50% WT) increased from 4376 to 4809 at t = 15 minutes (P < 0.01) and 5042 at t = 30 minutes (P < 0.01). Both DRGS and SCS induced a similar and complete reversal of mechanical hypersensitivity. After cessation of stimulation (t = 60), the return of the log10 (10 000 × 50% WT) response was significantly faster with DRGS than that of SCS (P < 0.05).
Conclusions
We conclude that conventional DRGS is as effective as SCS in reduction of PDPN‐associated mechanical hypersensitivity in STZ‐induced diabetic rats. The wash‐in effect of DRGS and SCS was similar, but DRGS showed a faster washout course. Long‐term efficacy should be studied in future animal research.
Keywords: animal model, dorsal root ganglion stimulation, neuromodulation, painful diabetic polyneuropathy, spinal cord stimulation
1. INTRODUCTION
Diabetic polyneuropathy (DPN) is a chronic, symmetric, length‐dependent sensorimotor polyneuropathy and is present in up to 50% of patients with diabetes mellitus.1 One‐third of these DPN patients suffer from painful diabetic polyneuropathy (PDPN),2 which starts in the toes and spreads into the feet, legs, and hands.3 As PDPN can be debilitating and a severe handicap to the patient and since effectiveness of pharmacological drugs is limited, there is an urgent need for other treatment options. Conventional spinal cord stimulation of the dorsal columns (hereafter named SCS) has been shown to be such a treatment option, which as well can be supplementary to pharmacological therapy. SCS has shown to be effective on the short and long term in PDPN when pharmacological therapies have failed.4, 5, 6, 7, 8, 9, 10, 11, 12 However, conventional SCS often provides incomplete pain relief (50% pain reduction or even less),10, 11 which is restricted to 60% of PDPN patients and leaves 40% of the patients as nonresponders. In view of these limitations, a recently introduced and very promising option for treatment of PDPN might be conventional dorsal root ganglion stimulation. DRGS is known to achieve better pain‐paresthesia overlap of difficult‐to‐reach areas like the feet.13, 14 The results of a recently published retrospective case series suggest that DRGS improves painful symptoms in PDPN patients.15 As in PDPN pain is mostly present in the feet,16 we hypothesized that DRGS is more effective in pain relief in PDPN when compared to SCS.
In order to address this hypothesis, we implemented DRGS in an already operational and meticulously tested PDPN animal model and investigated the effectiveness of both DRGS and SCS. SCS and its resulting pain relief in streptozotocin (STZ)‐induced PDPN animals have recently been described and here it was shown that SCS normalizes STZ‐induced mechanical hypersensitivity.17 SCS resulted in “a clinically relevant reduction” of mechanical hypersensitivity in 70% of PDPN animals. DRGS in animals with peripheral nerve damage and chronic neuropathic pain has been described recently.18 DRGS did not cause any dorsal root ganglion (DRG) tissue damage as verified by histological examination and with the use of DRGS parameters closely replicating those in clinical use a significant reduction of mechanical hypersensitivity in chronic neuropathic animals was noted.18 In order to investigate the underlying pain‐relieving effect of DRGS in PDPN animals, we compared the pain‐relieving effect of DRGS vs SCS in PDPN‐associated mechanical hypersensitivity in STZ‐induced diabetic rats.
2. METHODS
This study aimed to investigate the behavioral effect (mechanical hypersensitivity as measured by paw withdrawal to von Frey filaments) of one single stimulation paradigm in PDPN: a 30‐minute (min) conventional DRGS (hereafter named DRGS) being compared to a 30‐min SCS in rats with PDPN.16 This manuscript adheres to the applicable ARRIVE guidelines.
2.1. Animals
The experiments for this study were performed using 18 female Sprague‐Dawley rats, which were 8 weeks of age at the start of the experiment (170‐230 g). Eleven rats were selected for active DRGS, and seven rats were selected for sham‐DRGS. Animals were either housed in pairs before DRGS device implantation and individually after DRGS device implantation in transparent plastic cages with free access to food and water, in a 12‐hour light dark cycle. The experiments were approved by the Animal Research Committee of the Maastricht University Medical Centre (DEC‐protocol and approval (DEC 2013‐079)). The results of the experiments on these animals were compared to the results of a previous study investigating effect of conventional SCS but otherwise performed in an identical manner as this study (n = 8 for SCS and n = 3 for sham‐SCS).17
2.2. Induction of diabetes mellitus
DM was induced with a single intraperitoneal injection of 65 mg/kg streptozotocin (STZ, Sigma‐Aldrich, Schnelldorf, Germany) in 48 animals. Before STZ injection, rats were weighed and fasted overnight. STZ was freshly dissolved in sterile NaCl 0.9% to a solution of 65 mg/mL. Four days after STZ injection, blood glucose level was determined in blood derived from the saphenous vein of the leg using a standard blood glucose meter (Accu‐Chek Aviva®, Roche Diagnostics GmbH, Mannheim, Germany). Rats with a glucose level of ≥15 mmol/L were considered diabetic19 and were included in the study.
2.3. Behavioral testing
Pain behavior was assessed by testing mechanical hypersensitivity based on the hind limb paw withdrawal response to von Frey filaments. Before the start of behavioral testing, rats were placed in a transparent box on an elevated mesh floor and were given 15 minutes to acclimate to the surroundings. Mechanical hypersensitivity was assessed according to the “up‐down method,”20 as previously described.17, 21 A cutoff value of 28.84 g was defined. Thereafter, the registered 50% paw withdrawal thresholds (WT), measured in grams, were multiplied by 10 000 and then logarithmically transformed to conform with Weber's law22 and obtain a linear scale. The average of the mechanical hypersensitivity of both paws in the SCS animals of the previous study17 was compared to the average of the mechanical hypersensitivity of the ipsilateral (stimulated) paw in the DRGS animals, as we did not expect ipsilateral DRGS to have any effects on the contralateral hind paw.
2.4. Inclusion of animals
Animals were tested for mechanical hypersensitivity using von Frey hind limb withdrawal testing at baseline (before STZ injection), and once a week for 4 weeks following STZ injection, for the purpose of selecting animals that develop PDPN. Only animals showing mechanical hypersensitivity at 4 weeks postinjection were treated with either SCS or DRGS, whereas animals showing no mechanical hypersensitivity were excluded from the study. The presence of mechanical hypersensitivity was presumed if the log10 (10 000 × 50% WT) decreased by 0.2 units compared with pre‐STZ baseline.17, 21
2.5. Preparation electrode for DRG stimulation
The DRGS leads were manufactured from two platinum‐iridium wires of different gauges, where the larger diameter wire (0.010 in) contained the smaller center wire (0.005 in; PlasticsOne, Roanoke, VA, see Figure 1; see reference [18]) that were secured together at one end in a plastic connection hub. To prepare the electrode for DRGS implantation, the insulation from the terminal portion of the larger wire was removed, and the terminal portion of the wire was folded back upon itself to produce an atraumatic tip (Figure 1). The insulation was similarly removed from the tip of the smaller gauge wire, which was wrapped helically around the insulated portion of the central wire. This design produced an axially symmetric device that was insensitive to rotational movement. We added a few spots of dental cement to stabilize the structure of the electrode and to give a place for an encircling suture to grab when securing the inserted electrode. The lead was tested with an Ohmmeter to confirm that there was no contact between the two electrode poles and that there was suitable low resistance to the terminal contacts (ie, no break in the wires; Figure 1).
Figure 1.
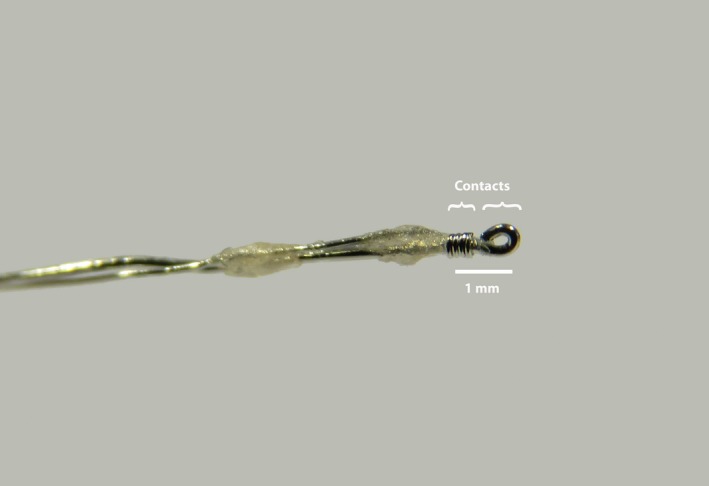
Bipolar electrode for dorsal root ganglion stimulation. Bipolar DRGS electrode. The insulation from the terminal portion of the larger wire was removed, and the terminal portion of the wire was folded back upon itself to produce an atraumatic tip. The insulation was similarly removed from the end of the smaller gauge wire, which was then wrapped helically around the insulated portion of the central wire (see reference [18]). Both the anode and the cathode were implanted at the L5 DRG
2.6. Implantation of DRGS device
A bipolar DRGS electrode was implanted unilaterally at the L5 DRG (adapted from reference [18]). In short: For implantation of the DRGS device, the lateral aspect of the intervertebral foramen was exposed via a paravertebral incision under general anesthesia. Subsequently, the foramen was gently opened by probing with a small, blunt nerve hook to provide a passage for the electrode to enter the foramen on the dorsolateral aspect of the DRG. Both the anode and the cathode were implanted at the L5 DRG. The electrode was secured into the transverse process caudal to the foramen using a stainless steel ligature and a small screw (diameter 0,86 mm, length 3,2 mm).
2.7. Implantation of dorsal column SCS device
The implantation of the dorsal column SCS device has been previously published, and the description is included here to document the difference between the techniques. For the implantation of the SCS device, performed under general anesthesia, a small laminectomy was made at level T13, and the cathode was inserted in the epidural space in caudal direction. Subsequently, the wire was secured to vertebra T12 with tissue adhesive (Histoacryl®, B Braun Medical BV, Oss, the Netherlands) to prevent migration of the electrode. The anode was placed subcutaneously in the left flank.16
2.8. SCS and DRGS
After implantation of a given device (DRGS or SCS) in the separate groups, the cables were tunneled subcutaneously through the neck of the animals, sutured to muscle and skin, and the connectors also attached to the skin. The wound was closed in layers. After implantation of the device, the rats were allowed to recover for 2 days before the start of SCS at day 3 following implantation.
The animals were stimulated in one session for 30 minutes at 3 days postimplantation. After the connector was attached to the wire of the pulse generator (for SCS: Grass S 88 stimulator fitted with a Grass SIU‐5 stimulus isolation unit and a Grass constant current unit [Astro Med, Grass, Warwick, RI, USA]; for DRGS: A‐M Systems MultiStim Model 3800, fitted with an A‐M Systems 3820 stimulus isolator [A‐M Systems, Sequim, WA, USA]), the motor threshold (MT) was determined at the following settings for both SCS and DRGS: frequency of 2 Hz and pulse width of 0.2 ms MT was defined as the current inducing contractions of the lower trunk or hind limb(s). Stimulation was applied for 30 minutes with an intensity of 66.7% of the MT, a pulse width of 0.2 ms, and with a frequency of 40‐50 Hz in the SCS animals (amplitude: 0.19 ± 0.01 mA) and 50 Hz in the DRGS animals (0.18 ± 0.05 mA). For the sham animals, the amplitude was set at zero.
The animals were tested for mechanical hypersensitivity immediately before stimulation (= 4 weeks after STZ injection), 15 (t = 15 minutes) and 30 minutes (t = 30 minutes) during stimulation, and 30 minutes after stimulation (t = 60 minutes). The measurements at 15 and 30 minutes during stimulation were performed with the pulse generator switched on. The effect of stimulation was assessed as follows: Firstly, the mean log10 (10 000 × 50% WT) after the start of stimulation (at 15 and 30 minutes) was compared with the mean log10 (10 000 × 50% WT) before stimulation onset. Secondly, the mean log10 (10 000 × 50% WT) after the start of stimulation (at 15 and 30 minutes) was compared with the mean pre‐STZ log10 (10 000 × 50% WT). Thirdly, the effect size was assessed by calculation of the difference between the pre‐STZ mean log10 (10 000 × 50% WT) and the mean log10 (10 000 × 50% WT) at 15 and 30, and 60 minutes after the start of stimulation. Lastly, the percentage of responders to stimulation treatment was also calculated. A responder to stimulation was defined as an animal with an increase of the log10 (10 000 × 50%WT) ≥ 0,2 during stimulation at 15 and 30 minutes after the start of stimulation treatment.17
2.9. Statistical analysis
Data are presented as means and standard error of mean (SEM). Within‐group analysis of changes of mechanical WT over time was analyzed using the Wilcoxon signed‐rank test. Between‐group comparisons of mechanical WT and group characteristics were analyzed using the Mann‐Whitney U test and Kruskal‐Wallis test. Statistical significance was defined as P < 0.05.
3. RESULTS
3.1. Description of cohorts of animals
Starting from 48 animals, which were injected with STZ, 43 developed DM (blood glucose ≥15 mmol/L; 90%). PDPN developed in 22 animals (log10 (10 000 × 50% WT) decreased ≥0.2); 51%), and those animals were implanted with a DRGS device. Four of these 22 animals were excluded from the study, due to connector breakage (n = 2) and due to having a too high MT (>1 mA) (n = 2). The remaining 18 animals were included for this study and divided into two groups: DRGS (n = 11) and sham‐DRGS (n = 7). For SCS, 11 PDPN animals were used as a historical cohort and as described by Pluijms et al17 From these 11 rats, 8 were stimulated with SCS (mid‐frequency SCS group; 50 Hz) and 3 control rats underwent sham‐SCS. No significant differences were found between groups with respect to glucose levels [SCS 25.4 ± 1.5 mmol/L, sham‐SCS 23.4 ± 4.0 mmol/L, DRGS 27.8 ± 1.2 mmol/L, and sham‐DRGS 29.2 ± 0.9 mmol/L (P = 0.12)].
3.2. Development of mechanical hypersensitivity
In the SCS group, the log10 (10 000 × 50% WT) decreased from 5412 before STZ injection to 4910 before the start of stimulation (P < 0.01). In the DRGS group, the log10 (10 000 × 50% WT) decreased from 5059 before STZ injection to 4376 before the start of stimulation (P < 0.01). In the sham‐SCS group, the log10 (10 000 × 50% WT) showed a trend toward a decrease from 5404 at pre‐STZ baseline to 4918 before the start of the sham‐SCS therapy (P = 0.25). The log10 (10 000 × 50% WT) in the sham‐DRGS group significantly decreased from 5041 before STZ injection to 4416 before the start of sham‐DRGS therapy (P < 0.05).
3.3. Effect of SCS/DRGS on mechanical hypersensitivity
In the SCS group, stimulation resulted in an increase of the log10 (10 000 × 50% WT) from 4910 before SCS to 5211 at t = 15 minutes (P < 0.05) and a nonsignificant trend toward an increase to 5264 at t = 30 minutes (relative to the pre‐stimulation baseline P = 0.11). No differences were observed between the 15‐minute and 30‐minute time point with SCS (P = 0.69). After SCS was stopped, the log10 (10 000 × 50% WT) returned to pre‐SCS values at t = 60 minutes (5093 g; P = 0.20). In the DRGS group, the log10 (10 000 × 50% WT) increased from 4376 before the start of DRGS treatment to 4809 at t = 15 minutes (P < 0.01) and increased to 5042 at t = 30 minutes relative to the pre‐stimulation baseline (P < 0.01). A significant increase was observed between the 15‐min and 30‐min time point with DRGS (P < 0.05). After cessation of DRGS therapy, the log10 (10 000 × 50% WT) returned to pre‐DRGS values at t = 60 minutes (4451; P = 0.90). After cessation of the stimulation (t = 60 minutes) the washout effect, the log10 (10 000 × 50% WT) with DRGS was significantly lower than that of SCS (P < 0.05) (data presented as % of pre‐STZ; Figure 2).
Figure 2.
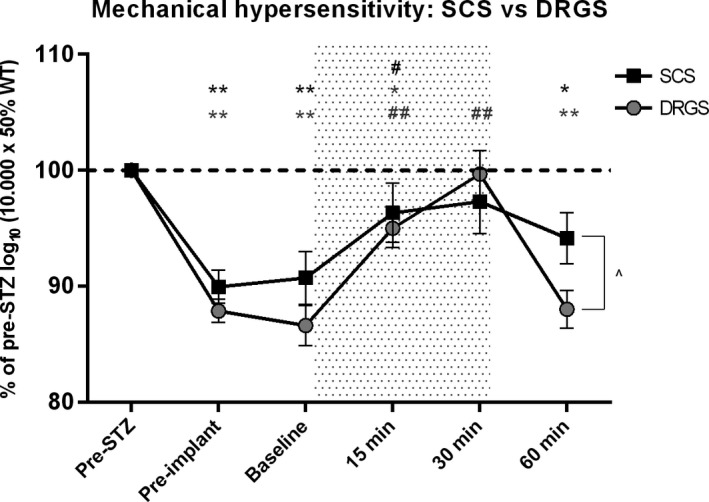
Effect of SCS (n = 8) and DRGS (n = 11) on mechanical hypersensitivity. Data are presented as mean % of pre‐STZ log10 (10 000 × 50% WT) ± SEM. Data are compared to pre‐STZ values and pre‐stimulation baseline values. The stippled area denotes the stimulation period. (also in Figure 3). *P < 0.05, **P < 0.01 compared to pre‐STZ values; # P < 0.05, ## P < 0.01 compared to pre‐SCS baseline; P < 0.05. SCS, spinal cord stimulation; DRGS, dorsal root ganglion stimulation; STZ, streptozotocin; SEM, standard error of mean; min, minutes
3.4. Effect of sham‐SCS/sham‐DRGS on mechanical hypersensitivity
Sham therapy—as expected—did not result in a significant increase of the log10 (10 000 × 50% WT) in the sham‐SCS group at t = 15 minutes (4778, P = 0.75) and at t = 30 minutes (4796, P = 0.99), and neither in the sham‐DRGS group at t = 15 minutes (4354, P = 0.56) and at t = 30 minutes (4382, P = 0.69). No significant differences in terms of log10 (10 000 × 50% WT) were found between the DRGS and SCS groups (data presented as % of pre‐STZ; Figure 3).
Figure 3.
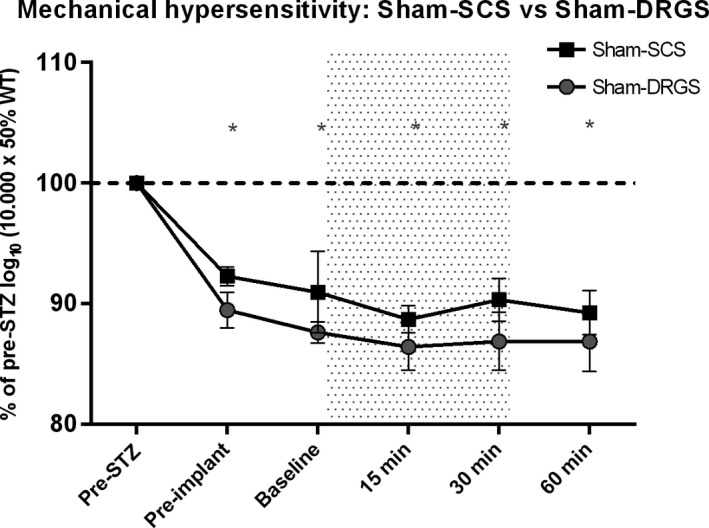
Effect of sham‐SCS (n = 3) and sham‐DRGS (n = 7) on mechanical hypersensitivity. Data are presented as mean % of pre‐STZ log10 (10 000 × 50% WT) ± SEM. Data are compared to pre‐STZ values and pre‐stimulation baseline values. *P < 0.05 compared to pre‐STZ values. SCS, spinal cord stimulation; DRGS, dorsal root ganglion stimulation; STZ, streptozotocin; SEM, standard error of mean; min, minutes
3.5. Percentage responders
In the SCS cohort, the percentage of responders to stimulation was 75% (six out of eight) at t = 15 and 30 minutes, whereas in the DRGS cohort, the percentage of responders was 73% (eight out of eleven) at t = 15 minutes and 91% (ten out of eleven) at t = 30 minutes. Sham‐SCS did not result in a response on mechanical hypersensitivity both at t = 15 minutes (0/3) and at t = 30 minutes (0/3), whereas sham‐DRGS resulted in 1 responder only at t = 30 minutes (1/7, 14%) (Table 1).
Table 1.
Percentage responders to stimulation
| Group | T = 15 min | T = 30 min |
|---|---|---|
| SCS | 6/8 (75%) | 6/8 (75%) |
| DRGS | 8/11 (73%) | 10/11 (91%) |
| Sham‐SCS | 0/3 (0%) | 0/3 (0%) |
| Sham‐DRGS | 0/7 (0%) | 1/7 (14%) |
DRGS, dorsal root ganglion stimulation; SCS, spinal cord stimulation; WT, withdrawal threshold; min, minutes.
A responder to stimulation is defined as an animal with an increase of the log10 (50%WT) ≥ 0.2 during stimulation.
3.6. Effect size of SCS and DRGS: comparison with pre‐STZ values
In the SCS group, the log10 (10 000 × 50% WT) returned to pre‐STZ values after t = 15 minutes (P = 0.31) and t = 30 minutes (P = 0.69). In the DRGS group, the log10 (10 000 × 50% WT) returned to pre‐STZ values after t = 30 minutes (P > 0.99) (data presented as % of pre‐STZ; Figure 2).
3.7. Intergroup comparison of effect size of SCS and DRGS on mechanical hypersensitivity
Intergroup comparison of the effect size showed no differences between the SCS cohort and the DRGS cohort at 15 minutes (P = 0.30) and at 30 minutes (P = 0.13), and 60 minutes (P = 0.59) (Figures 2, 3, 4). (data presented as % of pre‐STZ (Figures 2, 3, 4).
Figure 4.
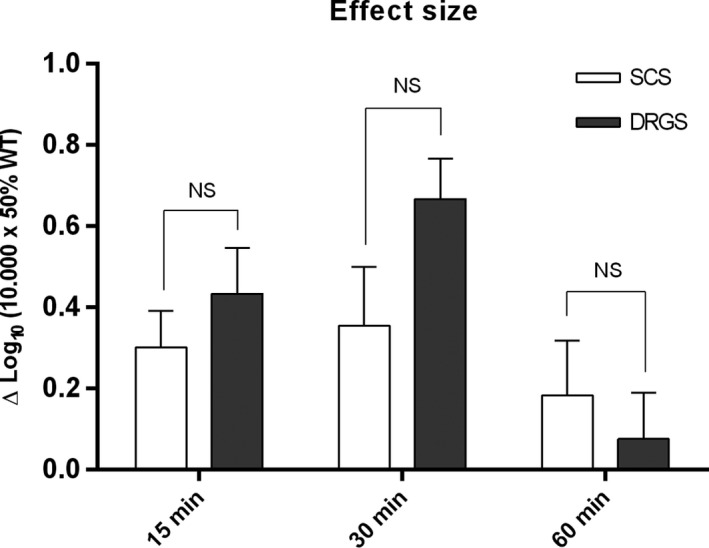
Reversal of mechanical hypersensitivity after 15, 30, and 60 minutes of SCS or DRGS. Data of SCS and DRGS at 15, 30, and 60 minutes minus their respective pre‐stimulation baseline at each time point. Data are presented as mean Δlog10 (10 000 × 50% WT) ± SEM. N = 8 for SCS and n = 11 for DRGS. SCS, spinal cord stimulation; DRGS, dorsal root ganglion stimulation; SEM, standard error of mean, NS, not significant; min, minutes
4. DISCUSSION
This is the first study that analyzes the behavioral pain‐relieving effect of DRGS in PDPN animals. From our results, we conclude that DRGS is as effective as SCS for PDPN‐associated mechanical hypersensitivity in STZ‐induced diabetic rats. Both DRGS and SCS induced a complete reversal of mechanical hypersensitivity and a return to pre‐STZ values. The complete reversal was obtained already at 15 minutes in the SCS group vs 30 minutes in the DRGS group. The percentage of responders was similar in both treatment groups. The wash‐in effect of DRGS and SCS was also similar, with the exception that the therapeutic effect of SCS remained stable between 15 minutes and 30 minutes, while in the DRGS group, the therapeutic effect increased between these time points. DRGS showed a faster washout course 30 minutes after cessation of stimulation in comparison with SCS.
The pathophysiology of diabetic neuropathy includes metabolic changes, which affect nerve fibers and conduction velocity as well as blood microvessel alterations.1 Painful stimuli are transmitted by peripheral nerves along small nonmyelinated (C‐type) and thinly myelinated (A δ) fibers. These fibers are involved in diabetic polyneuropathy, as along with large myelinated fibers (A α and A β).23 It is conceived that SCS activates fast‐conducting thick A β fibers24 and that electrical stimulation in the dorsal column results in antidromic activation of the descending collateral branches. According to the gate control theory, this stimulation of Aβ fibers results in modulation of the incoming C‐ or A δ‐mediated nociceptive signal.25 Animal studies have shown that SCS provides a suppressive action on dorsal horn neuronal hyperexcitability.26
The DRG mediates nociception from the peripheral nerves to the central nervous system27 and is an appealing site for neurostimulation as it represents the sensory gateway to the spinal cord, containing sensory neuron somata for all sensory modalities and fiber types. At the DRG, it is theoretically possible to modulate not only the non‐nociceptive A β fibers, but also the nociceptive A δ‐ and C‐type fibers.25 Furthermore, the DRG is of great importance in the development and maintenance of chronic pain, as it exhibits pathophysiologic changes during chronic pain states, like altered electrophysiological membrane properties, changes in the expression of integral membrane proteins, and altered gene expression.28, 29, 30 Elevated excitability of sensory neurons in the DRG contributes to the pathogenesis of chronic pain that follows peripheral nerve injury,31 and treatment at this site may interact with the pathogenic processes. It is known that DRGS reduces the generation of action potentials by the sensory neurons during membrane depolarization, which suggest that DRGS provides its analgesic effect via reducing sensory neuron excitability, both on spontaneous activation within the DRG and exerting an inhibitory influence on small diameter fiber activity passage at the T‐junction.25
Conventional SCS (pulse width 0.2‐0.5 ms; amplitude of 3.6‐8.5 mA; frequency of 40‐80 Hz)32, 33 has been shown to be effective in patients having a variety of neuropathic pain conditions, including PDPN.10, 11, 12 DRGS is, with proper lead placement, effective for pain localized to the back, groin, legs, and feet.13, 34, 35, 36 While SCS is often unable to cover difficult‐to‐reach areas like the feet and the groin without generating extensive paresthesias or motor side effects,13 conventional DRGS (settings: pulse width 0.2‐0.4 ms; amplitude of 800‐900 µA; frequency of 20‐70 Hz)13, 34 has been shown to be able to cover these difficult‐to‐reach areas, without generating large unwanted areas of paresthesia. DRGS offers several other potential benefits over SCS systems like lack of positional and movement effects on stimulation and reduced migration rate, because of better lead stability.1, 13, 27 Additionally, as the anatomical location of the DRG offers a closer proximity to the electrodes compared to the spinal cord and its dorsal columns, reduced power is required.1, 13
As PDPN pain is mostly located in the feet, we expected DRGS to be more effective for PDPN pain relief then SCS. Nevertheless, our results showed that effectiveness for pain relief with DRGS and SCS is similar in PDPN animals, with use of conventional stimulation settings. It needs to be stressed that these experiments were based on short‐term stimulation paradigms. Therefore, long‐term efficacy should be studied in future animal research to analyze and detect possible differences between the effect of DRGS and SCS in PDPN. In this study, the therapeutic pain‐relieving effect of SCS remained stable between 15 minutes and 30 minutes, while in the DRGS group, the therapeutic effect increased between these time points. This suggests that a longer stimulation time would probably benefit mostly for DRGS, as treatment with SCS reached maximal pain relief effects after 30 minutes (or possibly even earlier). A possible advantage of DRGS as compared to SCS in PDPN could well appear only in a long‐term stimulation study.15
Furthermore, as the concept underlying DRGS differs from the SCS (DRGS likely to modulate also nociceptive C and Aδ fibers, whereas SCS only stimulates Aβ fibers), it is of the utmost importance to test various stimulation settings in an experimental model to optimize the pain‐relieving effect. Novel advances in neurostimulation frequencies have emerged, like high‐frequency SCS (HF SCS, with frequencies up to 10 kHz) and Burst‐SCS (frequency 40 Hz provided in bursts of five pulses, with an internal frequency of 500 Hz.25 Van Beek et al21 evaluated the effect of SCS frequency (5‐500 Hz) on mechanical hypersensitivity in the chronic phase of experimental PDPN. A higher frequency (500 Hz) SCS resulted in a delayed effect on the pain‐related behavioral outcome in chronic PDPN. The effect of HF DRGS and Burst DRGS and/or other DRGS settings on pain relief in PDPN animals need to be tested in an experimental model, where also operational measures (eg, preference of location in cage due to active or placebo stimulation) are included in the final outcomes for the treatments. At present, it is not known which amplitude (:or percentage of MT in experimental models) is most adequate and effective with SCS and DRGS. A first attempt to study the effect of intensity of SCS and pain relief has been made by Meuwissen et al,37 who compared the effect of the intensity of Burst‐SCS vs conventional SCS in a model of peripheral neuropathy. From this study, it was concluded that Burst‐SCS requires significantly more mean charge per second in order to achieve similar pain relief, as compared with conventional SCS. In humans, the amplitude in conventional DRGS is usually 4‐10 times less (0.8‐0.9 mA) than the amplitude used for conventional SCS (3.6‐8.5 mA). On the contrary, in the current animal study, amplitudes for both SCS and DRGS were very close (67% MT, 0.19 ± 0.01 mA and 0.18 ± 0.05 mA, respectively). As our study was the first study to compare SCS and DRGS in an experimental animal model for PDPN, we preferred using similar settings for DRGS and SCS. However, we cannot completely exclude that with an amplitude of 67% MT, as in our protocol, also the dorsal columns in the spinal cord were stimulated during DRGS, thereby potentially causing a similar pain relief in the animals. The use of lower DRGS stimulation amplitudes (more like the amplitudes used clinically in humans—ie, about 15% of the conventional output of an SCS system)34 and effect on pain relief unquestionably need to be tested in the near future.
4.1. Limitations
For translation of our experimental data to the clinic, where long‐term SCS protocols are used for treatment of pain in PDPN, we underline that our experiments and results deal with use of short‐term stimulation paradigms and that thus long‐term efficacy was not studied.
We conclude that DRGS is as effective as SCS for PDPN‐associated mechanical hypersensitivity in STZ‐induced diabetic rats. At the same time, a faster washout course after cessation of the stimulation is noted with DRGS as compared to SCS. The development of the present model for DRGS in PDPN animals allows future research on mechanism and effectiveness of other clinically relevant stimulation paradigms, especially DRGS with different frequencies, lower amplitudes, and longer stimulation time.
CONFLICTS OF INTEREST
This work was financially supported by a research grant from St. Jude Medical (to P. Maino and EAJ Joosten). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
B. Linderoth serves as a consultant to Medtronic; St. Jude Medical; Boston Scientific, and Elektra AB. EAJ Joosten serves as a consultant for Boston Scientific and Salvia BioElectronics.
Koetsier E, Franken G, Debets J, et al. Effectiveness of dorsal root ganglion stimulation and dorsal column spinal cord stimulation in a model of experimental painful diabetic polyneuropathy. CNS Neurosci Ther. 2019;25:367–374. 10.1111/cns.13065
REFERENCES
- 1. Tesfaye S, Selvarajah D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes Metab Res Rev. 2012;28(Suppl. 1):8‐14. [DOI] [PubMed] [Google Scholar]
- 2. Abbott CA, Malik RA, Van Ross E, Kulkarni J, Boulton AJM. Prevalence and characteristics of painful diabetic neuropathy in a large community‐based diabetic population in the U.K. Diabetes Care. 2011;34:2220‐2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peltier A, Goutman SA, Callaghan BC. Painful diabetic neuropathy. BMJ. 2014;348(1):g1799–g1799. [DOI] [PubMed] [Google Scholar]
- 4. Kumar K, Toth C, Nath RK. Spinal cord stimulation for chronic pain in peripheral neuropathy. Surg Neurol. 1996;46:363‐369. [DOI] [PubMed] [Google Scholar]
- 5. Tesfaye S, Watt J, Benbow SJ, Pang KA, Miles J, MacFarlane IA. Electrical spinal‐cord stimulation for painful diabetic peripheral neuropathy. Lancet. 1996;348:1698‐1701. [DOI] [PubMed] [Google Scholar]
- 6. Daousi C, Benbow SJ, MacFarlane IA. Electrical spinal cord stimulation in the long‐term treatment of chronic painful diabetic neuropathy. Diabet Med. 2005;22:393‐398. [DOI] [PubMed] [Google Scholar]
- 7. de Vos CC, Rajan V, Steenbergen W, van der Aa HE, Buschman HP. Effect and safety of spinal cord stimulation for treatment of chronic pain caused by diabetic neuropathy. J Diabetes Complicat. 2009;23:40‐45. [DOI] [PubMed] [Google Scholar]
- 8. Pluijms WA, Slangen R, Bakkers M, et al. Pain relief and quality‐of‐life improvement after spinal cord stimulation in painful diabetic polyneuropathy: a pilot study. Br J Anaesth. 2012;109:623‐629. [DOI] [PubMed] [Google Scholar]
- 9. Slangen R, Pluijms WA, Faber CG, Dirksen CD, Kessels A, Van Kleef M. Sustained effect of spinal cord stimulation on pain and quality of life in painful diabetic peripheral neuropathy. Br J Anaesth. 2013;111:1030‐1031. [DOI] [PubMed] [Google Scholar]
- 10. Slangen R, Schaper NC, Faber CG, et al. Spinal cord stimulation and pain relief in painful diabetic peripheral neuropathy: a prospective two‐center randomized controlled trial. Diabetes Care. 2014;37:3016‐3024. [DOI] [PubMed] [Google Scholar]
- 11. De Vos CC, Meier K, Zaalberg PB, et al. Spinal cord stimulation in patients with painful diabetic neuropathy: A multicentre randomized clinical trial. Pain. 2014;155:2426‐2431. [DOI] [PubMed] [Google Scholar]
- 12. Van Beek M, Slangen R, Schaper NC, et al. Sustained treatment effect of spinal cord stimulation in painful diabetic peripheral Neuropathy: 24‐month follow‐up of a prospective two‐center randomized controlled trial. Diabetes Care. 2015;38:e132–e134. [DOI] [PubMed] [Google Scholar]
- 13. Liem L, Russo M, Huygen F, et al. A multicenter, prospective trial to assess the safety and performance of the spinal modulation dorsal root ganglion neurostimulator system in the treatment of chronic pain. Neuromodulation. 2013;471–482. [DOI] [PubMed] [Google Scholar]
- 14. Liem L, Russo M, Huygen F, et al. One‐year outcomes of spinal cord stimulation of the dorsal root ganglion in the treatment of chronic neuropathic pain. Neuromodulation. 2015;18:41‐49. [DOI] [PubMed] [Google Scholar]
- 15. Eldabe S, Espinet A, Wahlstedt A et al. Retrospective case series on the treatment of painful diabetic peripheral neuropathy with dorsal root ganglion stimulation. Neuromodulation. 2018; 10.1111/ner.12767. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16. Galer BS, Gianas A, Jensen MP. Painful diabetic polyneuropathy: epidemiology, pain description, and quality of life. Diabetes Res Clin Pract. 2000;47:123‐128. [DOI] [PubMed] [Google Scholar]
- 17. Pluijms WA, vanKleef M, Honig WM, Janssen SP, Joosten EA. The effect of spinal cord stimulation frequency in experimental painful diabetic polyneuropathy. Eur J Pain. 2013;17:1338‐1346. [DOI] [PubMed] [Google Scholar]
- 18. Pan B, Yu H, Fischer GJ, Kramer JM, Hogan QH. Dorsal root ganglionic field stimulation relieves spontaneous and induced neuropathic pain in rats. J Pain. 2016;17:1349‐1358. [DOI] [PubMed] [Google Scholar]
- 19. Calcutt NA. Modeling diabetic sensory neuropathy in rats. Methods Mol Med. 2004;99:55‐65. [DOI] [PubMed] [Google Scholar]
- 20. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55‐63. [DOI] [PubMed] [Google Scholar]
- 21. Beek M, Kleef M, Linderoth B, et al. Spinal cord stimulation in experimental chronic painful diabetic polyneuropathy: Delayed effect of High‐frequency stimulation. Eur J Pain. 2017;21:795‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mills C, Leblond D, Joshi S, et al. Estimating efficacy and drug ED50’s using von frey thresholds: Impact of Weber’s Law and log transformation. J Pain. 2012;13:519‐523. [DOI] [PubMed] [Google Scholar]
- 23. Guastella V, Mick G. Strategies for the diagnosis and treatment of neuropathic pain secondary to diabetic peripheral sensory polyneuropathy. Diabetes Metab. 2009;35:12‐19. [DOI] [PubMed] [Google Scholar]
- 24. Mailis‐Gagnon A, Furlan A, Sandoval J, Taylor R. Spinal cord stimulation in chronic pain. Cochrane Database Syst Rev. 2004;32:11‐21. [DOI] [PubMed] [Google Scholar]
- 25. Linderoth B, Foreman RD. Conventional and novel spinal stimulation algorithms: hypothetical mechanisms of action and comments on outcomes. Neuromodulation. 2017;20:525‐533. [DOI] [PubMed] [Google Scholar]
- 26. Yakhnitsa V, Linderoth B, Meyerson BA. Spinal cord stimulation attenuates dorsal horn neuronal hyperexcitability in a rat model of mononeuropathy. Pain. 1999;79:223‐233. [DOI] [PubMed] [Google Scholar]
- 27. Chang Chien GC, Mekhail N. Alternate intraspinal targets for spinal cord stimulation: a systematic review. Neuromodulation. 2017;20:629‐641. [DOI] [PubMed] [Google Scholar]
- 28. Sapunar D, Kostic S, Banozic A, Puljak L. Dorsal root ganglion ‐ a potential new therapeutic target for neuropathic pain. J Pain Res. 2012;5:31‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McCallum JB, Kwok W‐M, Sapunar D, Fuchs A, Hogan QH. Painful peripheral nerve injury decreases calcium current in axotomized sensory neurons. Anesthesiology. 2006;105:160‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rush AM, Dib‐Hajj SD, Liu S, Cummins TR, Black JA, Waxman SG. A single sodium channel mutation produces hyper‐ or hypoexcitability in different types of neurons. Proc Natl Acad Sci USA. 2006;103:8245‐8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Devor M. Neuropathic pain: what do we do with all these theories? Acta Anaesthesiol Scand. 2001;45:1121‐1127. [DOI] [PubMed] [Google Scholar]
- 32. Miller JP, Eldabe S, Buchser E, Johanek LM, Guan Y, Linderoth B. Parameters of spinal cord stimulation and their role in electrical charge delivery: a review. Neuromodulation. 2016;19:373‐384. [DOI] [PubMed] [Google Scholar]
- 33. Geurts JW, Joosten EA, Van Kleef M. Current status and future perspectives of spinal cord stimulation in treatment of chronic pain. Pain. 2017;158:771‐774. [DOI] [PubMed] [Google Scholar]
- 34. Deer TR, Grigsby E, Weiner RL, Wilcosky B, Kramer JM. A prospective study of dorsal root ganglion stimulation for the relief of chronic pain. Neuromodulation. 2013;16:67‐72. [DOI] [PubMed] [Google Scholar]
- 35. Van Buyten J‐P, Smet I, Liem L, Russo M, Huygen F. Stimulation of dorsal root ganglia for the management of complex regional pain syndrome: a prospective case series. Pain Pract. 2014;15:208‐216. [DOI] [PubMed] [Google Scholar]
- 36. Schu S, Gulve A, Eldabe S, et al. Spinal cord stimulation of the dorsal root ganglion for groin pain‐A retrospective review. Pain Pract. 2014;15:293‐299. [DOI] [PubMed] [Google Scholar]
- 37. Meuwissen K, Gu JW, Zhang TC, Joosten E. Conventional‐SCS vs. Burst‐SCS and the behavioral effect on mechanical hypersensitivity in a rat model of chronic neuropathic pain: effect of amplitude. Neuromodulation. 2018;21:19‐30. [DOI] [PubMed] [Google Scholar]


