Abstract
Aims
Increasing evidence indicates that neuroinflammatory and oxidative stress play two pivotal roles in cognitive impairment after surgery. Honokiol (HNK), as an activator of Sirtuin3 (SIRT3), has potential multiple biological functions. The aim of these experiments is to evaluate the effects of HNK on surgery/anesthesia‐induced cognitive decline in mice.
Methods
Adult C57BL/6 mice received a laparotomy under sevoflurane anesthesia and HNK or SIRT3 inhibitor (3‐TYP) treatment. Cognitive function and locomotor activity of mice were evaluated using fear conditioning test and open field test on postoperative 1 and 3 days. Neuronal apoptosis in CA1 and CA3 area of hippocampus was examined using TUNEL assay. And Western blot was applied to measure the expression of pro‐inflammatory cytokines and SIRT3/SOD2 signaling‐associated proteins in hippocampus. Meanwhile, SIRT3 positive cells were calculated by immunohistochemistry. The mitochondrial membrane potential, malondialdehyde (MDA), and mitochondrial radical oxygen species (mtROS) were detected using standard methods.
Results
Honokiol attenuated surgery‐induced memory loss and neuronal apoptosis, decreased neuroinflammatory response, and ameliorated oxidative damage in hippocampus. Notably, surgery/anesthesia induced an obviously decrease in hippocampal SIRT3 expression, whereas the HNK increased SIRT3 expression and thus decreased the acetylation of superoxide dismutase 2 (SOD2). However, 3‐TYP treatment inhibited the HNK's rescuing effects.
Conclusions
These results suggested that activation of SIRT3 by honokiol may attenuate surgery/anesthesia‐induced cognitive impairment in mice through regulation of oxidative stress and neuroinflammatory in hippocampus.
Keywords: honokiol, neuroinflammation, oxidative stress, postoperative cognitive decline, Sirtuin3
1. INTRODUCTION
Postoperative cognitive decline or delirium is one of the most common complications after surgery, and affects the quality of patients’ daily life which attracts more attention from the medical staff and the public.1, 2 During our life span, surgery, and anesthesia exposure maybe inevitable, so it is an urgent need to elucidate the potential mechanisms and therapeutic target for postoperative cognitive impairment.
Emerging evidence showed that neuroinflammation and oxidative stress are two pivotal roles in pathophysiological process of postoperative cognitive decline and other neurodegenerative disorders such as Parkinson's disease (PD), Alzheimer's disease (AD, and multiple sclerosis (MS).3, 4, 5, 6 An accumulating body of studies indicated that using some anti‐inflammatory or antioxidant interventions, such as anti‐TNF antibody, aspirin‐triggered resolvin D1 (AT‐RvD1) or resveratrol, could inhibit the neuroinflammation and oxidative stress in the models of surgical trauma or neurodegenerative diseases.7, 8, 9 Actually, surgery/anesthesia exposure could alter the normal dynamics of both neuroinflammation and oxidative stress simultaneously, leading to an unhealthy state for the brain and cognitive dysfunction. Ren et.al found that surgery plus isoflurane could induce postoperative delirium and associated biochemical/cellular changes in mice.10 Surgery/anesthesia exposure model is closer to clinic practice. However, the underlying mechanisms of neuroinflammation and oxidative stress on postoperative cognitive impairment remain still unclear.
Sirtuin3(SIRT3)is a class III histone deacetylase (HDAC) and a part of sirtuin gene family predominantly located in mitochondria, which expresses in a variety of tissues and is highly expressed in the brain.11 Recent studies showed that SIRT3 exhibits mighty deacetylase activity and is the key regulator in organ protection under many pathologic states including inflammatory and oxidative stress, acting pivotal roles in development and progression of metabolic diseases including diabetes, neurodegenerative disorders, and other diseases.12, 13, 14, 15 Honokiol (HNK), [2‐(4‐hydroxy‐3‐prop‐2‐enyl‐phenyl)‐4‐prop‐2‐enyl‐phenol], is a bioactive compound obtained from Magnolia grandiflora which possesses multiple properties including anti‐tumor, anti‐arrhythmic, anti‐thrombocytic, anti‐inflammatory, anti‐angiogenesis, anxiolytic, anti‐oxidative activities in vivo and in vitro.16, 17, 18, 19, 20, 21 As a potent reactive oxygen species (ROS) scavenger, it is also reported that HNK can enhance the overexpression of SIRT3 to improve antioxidant activity and mitochondrial energy regulation thereby reducing the levels of Aβ and sAPPβ in Chinese Hamster Ovarian (CHO) cells.22 Additionally, Xian et al suggested that HNK could improve learning and memory impairments induced by scopolamine in mice and attenuate the concentration of prostaglandin E2 and cyclooxygenase‐2 level and the neuroinflammatory processes.23
Therefore, the primary aims of our studies are to investigate the neuroprotection of honokiol in mice following surgery/anesthesia and role of SIRT3 signaling pathway in neuroinflammatory process and oxidative stress.
2. MATERIALS AND METHODS
2.1. Animals
Adult female C57BL/6 J mice (4 months old, weighing 20‐25 g) were purchased from the Beijing Vital River Laboratory. All mice were housed under a 12‐h/12‐h light/dark cycle at 25°C, 60% ± 10% humidity with free access to food and water. All the animals were handled carefully according Animal Ethics Committee of Wuhan University Zhongnan Hospital (Hubei, China). Before the initiation of experiments, all mice were familiar with the laboratory environment for at least 7 days.
2.2. Experimental group and treatment
All mice were randomly divided into the following groups (n = 12 per group): (a) control group; (b) surgery+vehicle group: mice were pretreated with vehicle [DMSO:PBS (1:1)] of HNK for 1 week and underwent surgical operation; (c) surgery+HNK group: mice were pretreated with HNK (10 mg/kg, ip) for 1 week and underwent surgical operation; (d) surgery+3‐TYP group: mice were pretreated with 3‐TYP (ip at a dose of 50 mg/kg every 2 days for a total of three times) and underwent surgical operation; (e) surgery+HNK+3‐TYP group: mice were pretreated with HNK (10 mg/kg, ip) for 1 week and 3‐TYP ip at a dose of 50 mg/kg every 2 days for a total of three times and then underwent surgical operation. (f) control+HNK group: mice were pretreated with HNK (10 mg/kg, ip) for 1 week and did not receive surgical operation; (g) control+3‐TYP group: mice were pretreated with 3‐TYP (ip at a dose of 50 mg/kg every 2 days for a total of three times) and did not receive surgical operation. HNK and 3‐TYP were purchased from Selleck Chemicals. All mice received fear conditioning training 1 day prior to surgical operation. On postoperative 1 day and 3 days, the open‐field test (OFT) was performed 15 minutes before each test phase of fear conditioning. And then mice were carried out the fear conditioning test (FCT). The animals were sacrificed for neurochemical evaluations 1 hour after all behavior tests. The detailed study plan and schematic diagram of experimental timeline is graphically described in Figure 1.
Figure 1.
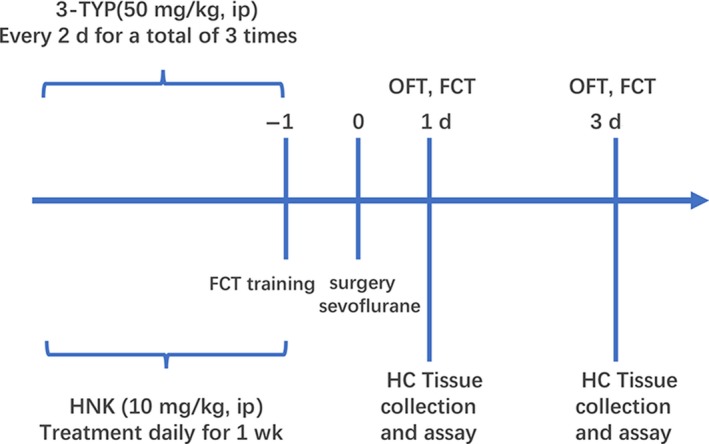
The study plan and schematic diagram of experimental timeline. HNK, honokiol; FCT, fear conditioning test; OFT, open field test; HC, hippocampus
2.3. Anesthesia and surgery
The surgical operation was a laparotomy under general anesthesia. Briefly, animals were inducted with 5% sevoflurane and maintained with 3% sevoflurane carried by 5 L/min oxygen. Using an anesthesia gas monitor (Draeger Medical GmbH, Lübeck, Germany), the concentration levels of sevoflurane and carbon dioxide were continuously observed to avoid carbon dioxide retention and deep anesthesia. During the operation, mice kept spontaneous respiration. A 3‐cm midline abdominal incision was made, and the abdominal organs were explored gently with sterilize gauze. Then the incision was closed by 9/0 Prolene sutures (Ethicon, New Brunswick, NJ, USA). About 0.2% lidocaine solution was applied for postoperative analgesia. All processes lasted approximately 40 minutes. And the rats' body temperature kept at ~37˚C during the surgery using a heat pad. Mice without surgical operation served as the controls.
2.4. Open field test
The open field test (OFT) is a classical method to investigate exploratory behaviors and spontaneous motor activity. The OFT was carried out in a quiet and dim light environment. Each mouse was gently put into the new experimental environment and familiarizes themselves with circumstance for 10 minutes before the test was begun. Utilizing the Any‐Maze animal tracking system software (Xinruan, Shanghai, China), the activities of the mouse traveled in open‐field chamber for 5 minutes were automatically recorded. The total distance travelled and the amount of time spent in the center of the arena were calculated by the software. After each test session, the walls and floor of chamber were swept with 75% ethanol to avoid the interference between trials.
2.5. Contextual fear conditioning test
Based on a previously published model, this contextual Fear conditioning test (FCT) includes two parts: a training phase at 1 day before surgical operation and a test phase on postoperative 1 and 3 days.
In training phase, mice received fear conditioning to establish the long‐term memory. Each animal was allowed to adapt to the conditioning chamber (context) for 120 seconds, followed by six cycles of conditional‐unconditional stimuli. A cycle of conditional‐unconditional stimuli was then applied as a 20 seconds, 80 dB tone (conditional stimuli)—30 seconds delay—5 seconds, 0.75 mA electrical foot shock (unconditional stimuli). The cycles of conditional‐unconditional stimuli were separated by random intervals from 45 to 60 seconds.
The context test, which represents hippocampal‐dependent memory, is the major part of the test phase of the FCT. At postoperative 1 and 3 days, all mice were returned into the original conditioning chamber for 5 minutes, where no tone and no shock were released. The percentage of freezing time (not moving) was captured and collected by Any‐Maze software (Xinruan, Shanghai, China).
2.6. Apoptosis detection in hippocampus
After behavioral tests, mice were anesthetized with pentobarbital sodium (50 mg/kg) and perfused transcardially with 40 mL normal saline, followed by 4% paraformaldehyde. Then, the fixed brain was postfixed in 4% paraformaldehyde overnight at 4°C and embedded in paraffin. Neuron apoptosis was evaluated in hippocampus sections using the terminal dUTP nick‐end labeling (TUNEL) of fragmented nuclei assay. The paraffin‐embedded sections of brain tissues of different groups were processed in according to the manufacturer's instructions (Roche, Mannheim, Germany). Apoptotic cell number in hippocampal CA1 and CA3 was assessed by counting the number of TUNEL‐positive apoptotic cells in three fields per slide randomly at 200x magnification.
2.7. Immunohistochemistry
Hippocampus tissues sections from the brain were collected, and SIRT3 immunoreactivity was evaluated by immunohistochemical assay on formalin‐fixed paraffin‐embedded sections. Hippocampus tissue histology was assessed by immunohistochemistry with primary antibodies against SIRT3 under light microscopy. Three different sections from each group were examined at least.
2.8. MDA assay
The malondialdehyde (MDA) level was detected using commercially available MDA kits following the manufacturer's instructions (Jiancheng, Nanjing, China).
2.9. Western blot analysis
Hippocampal tissues were acquired, and protein extractions were obtained after behavior tests on postoperative 1 and 3 days. Total proteins were separated by SDS‐PAGE after denaturation and transferred onto polyvinylidenedifluoride (PVDF) membranes. After blocking with 5% skim milk, the membranes were incubated with rabbit anti‐mouse monoclonal antibodies against TNF‐α, IL‐1β (1:500; Abcam, Cambridge, UK),SIRT3 (1:1000; Abcam), MCP‐1(1:500; affbiotech, Cambridge, UK), cytochrome C (1:2000; Abcam), Ac‐SOD2 (1:500; Abcam), SOD2 (1:1000; Abcam) and GAPDH(1:400, abcam) overnight at 4°C with shaking. Then, the membranes were washed in TBST and exposed to the corresponding secondary horseradish peroxidase‐conjugated goat anti‐rabbit IgG (1:2000, Santa Cruz, CA, USA) for 1.5 hour at room temperature. After washed three times, the membranes were detected using an enhanced chemiluminescence reagent kit (Aspen, Wuhan, China). All bands were scanned, and the gray values were determined was analyzed using image analysis software (AlphaEaseFC software; Alpha Innotech, Saint leonardo, CA, USA) and normalized to GAPDH.
2.10. Isolation of mitochondria from hippocampus
Utilizing a tissue mitochondria isolation kit (Beyotime, China), the intact mitochondria of hippocampal region were isolated from fresh brain. Briefly, after homogenization of hippocampus tissue in ice‐cold buffer provided in the kit, the homogenate was centrifuged at 6000 g at 4°C for 5 minutes. The collected supernatant was further centrifuged at 11 000 g at 4°C for 10 minutes to obtain a mitochondrial pellet. Then, the pellet was collected as mitochondria and suspended in mitochondrial storage fluid provided in the kit.
2.11. Mitochondrial membrane potential and mitochondrial ROS assay
According to the manufacturer's instructions, changes in mitochondrial membrane potential (ΔΨm) were measured using a JC‐1 mitochondrial membrane potential assay kit (Beyotime, China). At brief, isolated mitochondria were suspended in 0.5 mL medium containing 5 mmol/L JC‐1. All Samples were estimated using an automatic microplate reader (Tecan SPARK, Switzerland) at time scan method. The intensities of green (excitation/emission wave length = 490/530 nm) and red (excitation/emission wave length = 525/590 nm) fluorescence were estimated in each sample and reflected a surrogate marker of loss of mitochondrial ΔΨm. Using a ROS assay kit (Genmed Scientifics, Shanghai, China), mitochondrial ROS was detected in each sample.
2.12. Statistical analysis
All data are expressed as mean±standard error of the mean (SEM). Statistical analysis of differences between groups was performed by using one‐way ANOVA followed by a SNK test for post hoc comparisons. All analyses were performed by utilizing GraphPad Prism 5 (GraphPad, San Diego, CA), P < 0.05 were considered to be statistically significant.
3. RESULTS
3.1. The outcomes of open field test and fear conditioning test
Based on our pre‐experiments, we found that HNK or 3‐TYP pretreatment alone did not change the cognitive function of the control mice. Furthermore, HNK or 3‐TYP did not significantly influence markers of neuroinflammation and oxidative stress compared with those results of the control group (As shown in Figures [Link], [Link], [Link]).
Previous studies showed that surgery/anesthesia could lead to behavioral and cognitive disability at early stage in mice.24, 25 To evaluate whether HNK ip can suppress the memory and cognitive damage in surgical stressed mice, OFT and contextual FCT were performed as described above. 3‐TYP, a selective SIRT3 inhibitor, was utilized to assay the necessity of the SIRT3 signaling pathway in neuroprotection of HNK.
As shown in Figure 2C,F, surgery/anesthesia, HNK and/or 3‐TYP treatment did not cause a remarkably decrease in total distance during 5 minutes of exploration in open field, indicating that locomotor activity of mice was not affected by above treatment (P > 0.05).
Figure 2.
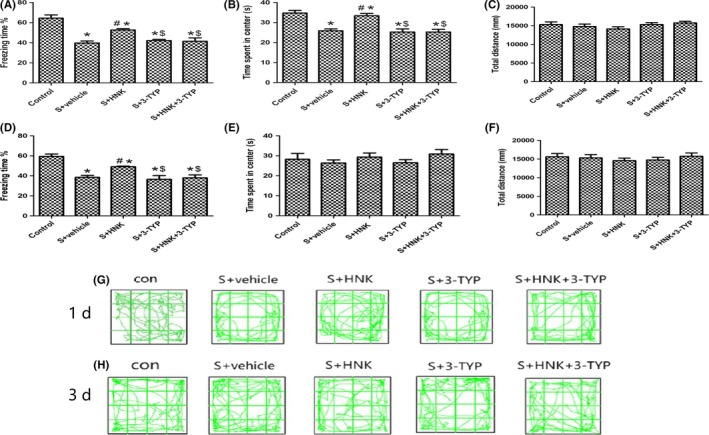
Effects of HNK or 3‐TYP on behavior changes of mice induced by surgery/anesthesia. (A, D) The percentage of freezing time during 5 min in context test (test phase of the FCT) at postoperative 1 and 3 d, respectively; (B, E) time spent in the center of the open field during 5 min of open field exploration at postoperative 1 and 3 d, respectively; (C, F) total distance traveled during 5 min of open field exploration at postoperative 1 and 3 days, respectively; (G, H) the movement tracks showing 5 min of open field exploration by the mice at postoperative 1 and 3 d, respectively. The data are presented as the mean ±standard error of the mean for each group (n = 6 per group). *P < 0.05 vs the control group; #P < 0.05 vs the surgery+vehicle group; $P < 0.05 vs the honokiol group
However, on 1 day after surgery, compared with control group, surgery decreased the time traveled by mice in the center of the open field, and honokiol reversed this situation, indicating a potential anti‐anxiety property of honokiol treatment (P > 0.05), and this situation did not exist at postoperative 3 day (Figure 2B,E).
FCT were employed after each test phase of the open field tests on postoperative 1 and 3 days. In the training phase of fear conditioning, the freezing time had no obviously differences among the groups, showing that the baseline learning and memory properties among these groups were related equal.
As shown in Figure 2A,D, we identified that surgery could notably decrease the freezing time compared with control group on postoperative 1 and 3 days (P < 0.05). HNK treatment enhanced the recovery of learning and memory, as confirmed by increased freezing time in the HNK‐treated group compared with surgery group. Treatment with 3‐TYP removed the neuroprotection of HNK by reducing the freezing time in context fear test on 1 and 3 days after surgery operation (P < 0.05).
3.2. Neuronal apoptosis of hippocampus
To investigate the neuronal damaged situation and the potential mechanism of cognitive decline induced by surgical stress, mice killed at 1 day postoperation and the neuronal apoptosis in the hippocampal CA1 and CA3 region were observed. As shown in Figure 3, surgical trauma caused a noticeable increase in neuronal apoptosis in CA1 and CA3 of hippocampus on postoperative 1 day compared with the control group (P < 0.05). And treatment with HNK markedly suppressed the increase in apoptotic index caused by surgical trauma (P < 0.05). Additionally, intraperitoneal injection of 3‐TYP can increased the neuronal apoptosis in CA1 and CA3 areas of hippocampus on postoperative 1 day compared with surgery+HNK group and eradicated the neuroprotection of HNK (P < 0.05).
Figure 3.
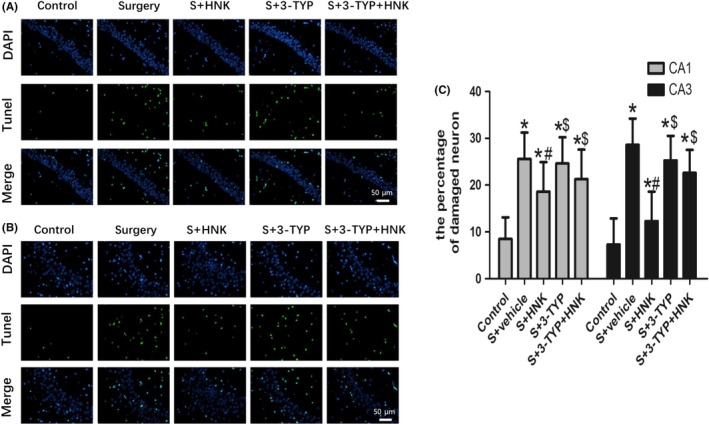
Effects of HNK or 3‐TYP on surgery/anesthesia‐induced neuronal apoptosis (A, B) Representative images of neuronal apoptosis in CA1 and CA3 areas of the hippocampus at postoperative 1 d. Original magnification=×200, scale bar = 50 μm. The apoptotic cells were detected by TUNEL (green), and the nuclei were detected by DAPI (blue). (C) The percentage of damaged neuron. The data are presented as the mean ±standard error of the mean for each group (n = 3 per group). *P < 0.05 vs the control group; #P < 0.05 vs the surgery+vehicle group; $P < 0.05 vs the honokiol group
3.3. Inflammatory response and microglia activation in hippocampus
As shown in Figures 4 and 5, the effects of surgery/anesthesia, honokiol, and/or 3‐TYP on neuroinflammation in surgery‐treated mice were examined on postoperative 1 and 3 days. TNF‐α, IL‐1β, and MCP‐1, known as pro‐inflammatory cytokines, play an essential role in neuroinflammation. And Iba‐1, a microglial marker, represents the activation of microglia. Compared with control group, surgery/anesthesia induced a significant increase expression levels of TNF‐α, IL‐1β, and MCP‐1 in hippocampus region (P < 0.05). Besides, surgery/anesthesia treatment increased the expression of Iba‐1 in hippocampus, suggesting that microglia were activated following surgical trauma (P < 0.05). The results also indicated a significant reduction in pro‐inflammatory cytokines expression in HNK group compared to surgery+vehicle group. Significant reduction in Iba‐1 expression was found in hippocampus region, indicating that HNK treatment can also ameliorate the activation of microglia compared to surgery+vehicle group (P < 0.05). On 1 and 3 days after surgery, HNK‐induced reductions in TNF‐α,IL‐1β, and MCP‐1 expression were eliminated by 3‐TYP treatment (P < 0.05). Besides, the protective effects of HNK on the activation of microglia in hippocampus, as confirmed by the reduced expression of Iba‐1, were abolished by 3‐TYP (P < 0.05).
Figure 4.
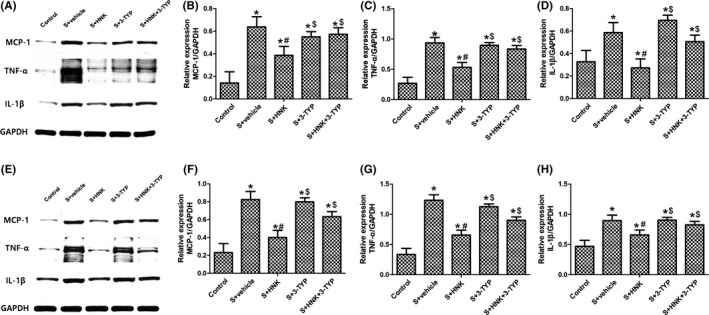
Effects of HNK or 3‐TYP on surgery/anesthesia‐induced an increased expression of pro‐inflammatory cytokines in hippocampus. (A, E) Representative blots at postoperative 1 and 3 d, respectively. (B, F) MCP‐1 relative expression. (C, G) TNF‐α relative expression. (D, H) IL‐1β relative expression. The data are presented as the mean ±standard error of the mean for each group (n = 6 per group). *P < 0.05 vs the control group; #P < 0.05 vs the surgery+vehicle group; $P < 0.05 vs the honokiol group
Figure 5.
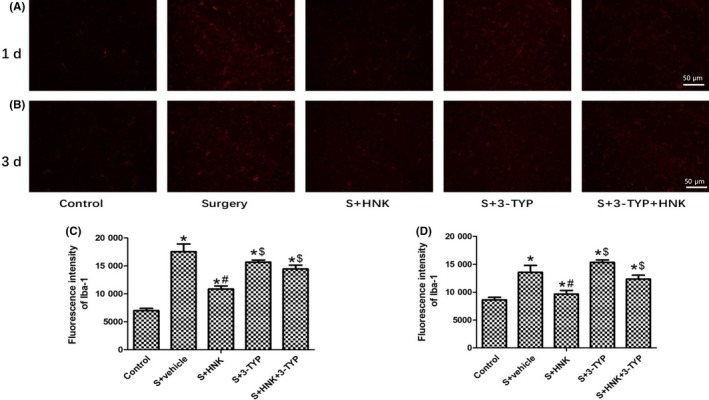
Effects of HNK or 3‐TYP on surgery/anesthesia‐induced an increased expression of Iba‐1 in hippocampus. (A, B) Representative immunofluorescence images show the expression of Iba‐1 (red pixels) in the hippocampal CA1 area of mice 1 and 3 d after surgery. Original magnification=×200, scale bar = 50 μm. (C, D) Quantitative analyses of the immunofluorescence images at postoperative 1 and 3 d, respectively. The data are presented as the mean ±standard error of the mean for each group (n = 3 per group). *P < 0.05 vs the control group; #P < 0.05 vs the surgery+vehicle group; $P < 0.05 vs the honokiol group
3.4. Oxidative stress and mitochondrial impairment
Oxidative stress and mitochondrial impairment were also critical factors of postoperative cognitive decline. So we examined related indicators in hippocampus of mice who received above treatment. As shown in Figure 6, compared with control group, surgical trauma‐induced oxidative stress in hippocampus was accompanied with increased mitochondrial ROS and MDA content and decreased mitochondrial membrane potential (ΔΨm) (P < 0.05). And these changes were reversed by HNK treatment, suggesting its anti‐oxidative capacity (P < 0.05). Cytochrome C is a critical component of the electron transport chain. The increased level of cytochrome C is the result of releasing Cytochrome C from mitochondria to the cytosol, indicating that the integrity of mitochondria is damaged. Our data demonstrated that surgery/anesthesia treatment significantly increased Cytochrome C expression in cytosol (P < 0.05). Compared with the surgery+vehicle group, HNK reduced Cytochrome C expression, indicating that HNK presents therapeutic effects in maintaining the integrity of mitochondria (P < 0.05). However, HNK‐induced reductions in mtROS, cytochrome C, and MDA content were largely eradicated by 3‐TYP treatment at postoperative 1 day (P < 0.05). The increased anti‐oxidative capacity (the level of mitochondrial membrane potential) induced by HNK was also inhibited by 3‐TYP treatment.
Figure 6.
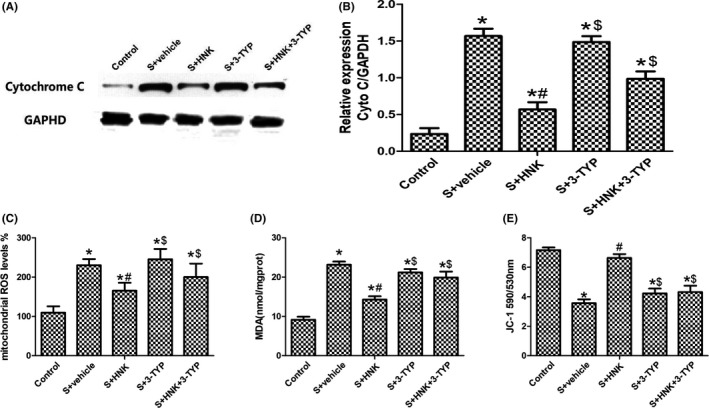
Effects of HNK or 3‐TYP on mitochondrial dysfunction after surgical trauma at postoperative 1 day. (A, B) Representative blots and cytochrome C relative expression. (C) hippocampal MDA content. (D) mitochondrial ROS levels in hippocampus. (E) mitochondrial membrane potential in hippocampus. The data are presented as the mean ±standard error of the mean for each group (n = 6 per group). *P < 0.05 vs the control group; #P < 0.05 vs the surgery+vehicle group. $P < 0.05 vs the honokiol group
3.5. Effects of honokiol on SIRT3/SOD2 signaling pathway
The results of the immunohistochemical assay at postoperative 1 and 3 days showed that SIRT3 immunoreactivity had significantly difference among different groups. Compared to control group, cells positively immunostained for SIRT3 were markedly downregulated in the surgery/anesthesia group (P < 0.05, Figure 7). HNK treatment improved the positive cells number compared with surgery group. And 3‐TYP treatment eliminated this phenomenon.
Figure 7.
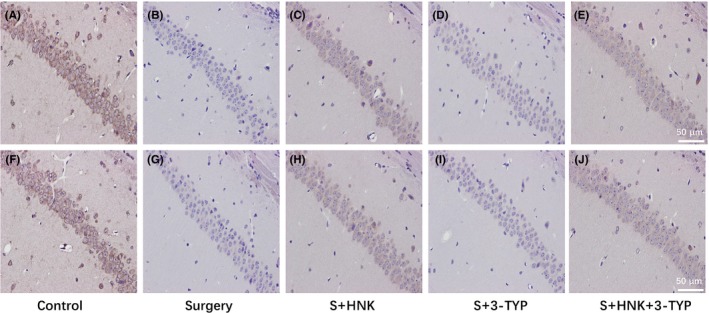
Immunohistochemical staining for SIRT3 on hippocampus sections. (A‐E) Representative immunohistochemical staining show the expression of SIRT3 in the hippocampal of mice 1 d after surgery. Original magnification=×200, scale bar = 50 μm; (F‐I) Representative immunohistochemical staining show the expression of SIRT3 in the hippocampal of mice 3 d after surgery. Original magnification=×200, scale bar=50 μm, (n = 3 per group)
As shown in Figure 8, compared with the control group, surgical trauma blunted the expression level of SIRT3 protein and upregulated the acetylation of SOD2, an important role in maintaining redox homeostasis (P < 0.05). Compared with surgery+vehicle group, HNK treatment increased the SIRT3 expression and decreased the Ac‐SOD2 at postoperative 1 and 3 days (P < 0.05). However, 3‐TYP treatment attenuated the SIRT3 expression and increased the acetylation of SOD2 compared with that in the surgery+HNK group at postoperative 1 and 3 days (P < 0.05).
Figure 8.
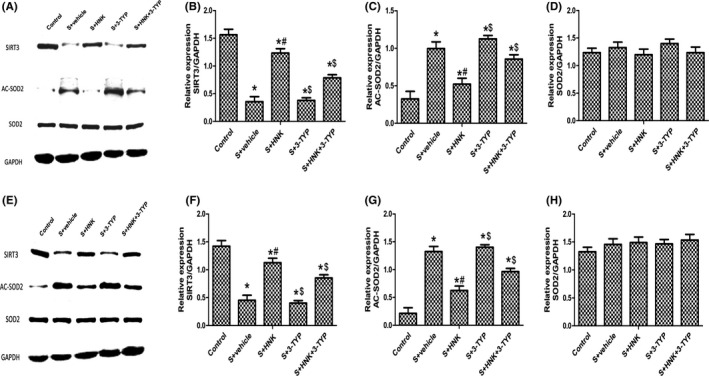
Effects of HNK or 3‐TYP on SIRT3/SOD2 signaling pathway in surgery/anesthesia‐induced mice. (A, E) Representative blots at postoperative 1 and 3 d. (b, f) SIRT3 relative expression. (C, G) Ac‐SOD2 relative expression. (D, G) SOD2 relative expression. The data are presented as the mean ±standard error of the mean for each group (n = 6 per group). $P < 0.05 vs the surgery+HNK group; #P < 0.05 vs the surgery+vehicle group
4. DISCUSSION
The aim of the current study was to explore the neuroprotective role and potential mechanism of HNK, an activator of SIRT3, in suppressing surgery/anesthesia‐induced cognitive deficit, neuroinflammation and oxidative stress in adult mice. In our experiments, pretreatment HNK ip for7 days could provide neuroprotective effects on hippocampus of mice, as confirmed by increased postoperative cognitive recovery, reduced neuronal apoptosis, suppressed neuroinflammatory cytokines, and blunted oxidative stress in hippocampal tissue. Interestingly, on 1 day after surgery, surgery decreased the time traveled by mice in the center of the open field, and honokiol reversed this situation, indicating a potential anti‐anxiety property of honokiol treatment. Several studies found that honokiol also possessed the anti‐anxiety ability.26 In present study, consideration of pain and nociceptive response, anxiety‐like behavior may more obvious at postoperative 1 day. Honokiol could cause anxiolytic action and ameliorate this situation. At postoperative 3 days, these nociceptive response and incision pain may have been released, so these anxiety‐like behaviors could not be observed. As far as we know, this was first evaluation of the effects of HNK on cognitive recovery in surgical mice. The findings also demonstrated that the potential neuroprotective effects of HNK are involving in activating the SIRT3 signaling pathway at least partially, as the selective SIRT3 inhibitor, 3TYP, notably eradicated these protective effects of HNK on surgical trauma.
Although cognitive decline after surgery was widely emphasized by clinicians and researchers and several interventions seemed to do work, its underlying mechanisms and specific therapeutic target remained unknown and should be studies further. Considerable mechanisms are implicated in the etiology of postoperative cognitive impairment including oxidative stress and neuroinflammation, which contribute to the loss of neurons.27, 28, 29, 30 HNK, due to its multiple biological functions, has been demonstrated effectively to ameliorate brain injury, cardiomyopathy, and suppress tumor. In these researches, HNK owned a wealth of approaches by which it buffers inflammatory and oxidative damage, such as JAK/STAT, sirtuins, Stat3/Zeb1/E‐cadherin axis, amyloidogenic pathway, and mitochondrial dynamics.31, 32, 33, 34, 35 Meanwhile, HNK can activate the expression of SIRT3 and sirtuins, which confer protection against many pathological conditions including ischemic injury, cancer, and aging.36, 37 Thereby, the relationships among cognitive deficit, neuroinflammation, oxidative stress, and SIRT3 expression in adult mice subjected to surgery/anesthesia were focused in recently.
Neuroinflammation, especially characterized by activation of microglia, is supposed to be a critical mediator of progressive secondary injury after surgical trauma and other neurodegenerative conditions.38, 39, 40 Under surgery/anesthesia or other pathological conditions, activated microglia released a series of inflammatory, such as IL‐1β, TNF‐α, and MCP‐1. A mass of inflammatory cytokines in hippocampus following surgical trauma caused learning and memory, which could be observed in our studies and were consistent with other's related studies suggesting a strong correlation between neuroinflammation and postoperative cognitive impairment.
Surgery plus anesthesia might induce oxidative stress in hippocampus which led to biochemical/cellular and behavioral changes and associated with postoperative cognitive decline or postoperative delirium.41, 42, 43 Mitochondrion is recognized to be the major source of intracellular ROS and plays a fundamental role in redox modulation.44 Increased ROS can be generated by an imbalance between the production of oxygen containing free radicals (ROS, superoxide anion, hydroxyl radicals, and hydrogen peroxide) and their elimination.45, 46
Overproduction of ROS leads to deficits in mitochondrial membrane integrity, damage to the mitochondrial respiratory chain and lipid peroxidation. Furthermore, injured mitochondria are dysfunctional and further generate free radicals, thus leading a “vicious cycle”.47, 48, 49 In our studies, exploratory laparotomy disrupted the mitochondrial redox homeostasis and broke the mitochondrial membrane integrity and increased lipid peroxidation in hippocampus, as confirmed by increased mtROS and MDA content, decreased mitochondrial membrane potential (ΔΨm), and increased level of cytochrome C from mitochondria to the cytosol. These results can also be observed in a large number of brain disorders, including stroke, traumatic brain injury, psychiatric disorders, and sepsis‐associated encephalopathy as well as AD, PD, and other neurodegenerative diseases.50, 51, 52
SIRT3 is the one member of the sirtuin family that exhibits deacetylase activity and is mainly localized to the mitochondria. Regarding to oxidative stress, SIRT3 mainly regulates mitochondrial ROS levels by deacetylating SOD2 and has a positive role in several neurological disorders, including AD, PD and Huntington's disease.53, 54, 55, 56 And with the downregulated of oxidative stress, the inflammatory and related cytokines can also be normalized. For example, bitter melon (BM) supplementation could significantly ameliorate brain oxidative stress induced by high fat diet (HFD) with a concomitant reduction in FoxO, normalization of SIRT1 protein expression, and upregulation of SIRT3 mRNA expression. Furthermore, mice fed HFD with BM could also normalize the levels of plasma antioxidant enzymes and pro‐inflammatory cytokines as compared to HFD‐fed mice.57 Microglia‐induced oxidative stress and neuron stem cell dysfunction can be attenuated by SIRT3 overexpression through mitochondrial apoptosis pathway, including the decreased mitochondrial permeability transition pore (mPTP) opening, reduced mitochondrial cytochrome C release to cytoplasm, declined Bax/Bcl‐2 ratio and inhibition of caspase‐3/9 activity.11 Moreover, HNK, as an activator of SIRT3, can also ameliorate kidney injury induced by hypertension via activating SIRT3/KLF15 signaling pathway.58 Besides, utilizing the Sirt3‐knockout mice and the overexpression of SIRT3 by lentivirus transfection, other studies showed that SIRT3 can protect against cardiac fibrosis by inhibiting myofibroblasts trans‐differentiation via regulation of the STAT3‐NFATc2 signaling pathway.59 Formyl peptide receptor 1 (FPR1) mediates neutrophil activation induced by bacterial and mitochondrial N‐formyl peptides and may a promising therapeutic target for drugs to treat septic or sterile inflammatory diseases. As shown in the study by Liu et al, HNK attenuated formyl peptide‐induced human neutrophil activation by blocking FRP1.18 An array of studies supported that 3‐TYP is a high selective inhibitor can reverse the activation of SIRT3 and aggravate organ (heart, small intestine) injury.60, 61 For SIRT3‐SOD2‐mROS‐dependent autophagy, 3‐TYP can also block the melatonin‐mediated suppression of autophagy by inhibiting SIRT3‐SOD2 signaling and delete the hepatoprotective effect of melatonin on mitochondrial‐derived O2•−‐stimulated autophagic cell death.62 In our studies, through pretreatment with 3‐TYP, we found that the anti‐oxidative and anti‐inflammatory capacity in brain provided by HNK can also abolished by 3‐TYP at different degree, which were similar with above studies. These data demonstrated that SIRT3 signaling pathway, at least partially, involves in the neuroprotection of HNK surgery/anesthesia induced mice.
There are some limitations in our studies. First, we just performed the animal experiments of HNK on neuroinflammation and oxidative stress and did not verify the outcome in vitro. Nevertheless, Dennis et.al had demonstrated that magnolol and honokiol can ameliorate oxidative and inflammatory responses in neurons and microglial cells through suppressing IFNγ‐induced p‐ERK1/2 and its downstream pathway at least in part.63 So, we had reason to believe that HNK could improve the behavior outcomes, neuroinflammation, and oxidative stress of surgery/anesthesia mice. Considered the rigor of the studies, future experiments should explore the potential effects of SIRT3 signaling pathway on LPS‐induced inflammatory response in microglia and astrocyte. Second, in current studies, our just tested the surgery/anesthesia‐induced neuroinflammatory and oxidative stress in hippocampus of mice, but other region in brain (such as prefrontal cortex) and systemic inflammation was not investigated. What's more, several studies showed that SIRT3 could activate autophagy through the LKB1‐AMPK‐mTOR pathway and then protect rotenone‐induced injury in SH‐SY5Y cells.64 In addition, SIRT3 can directly blunt the assembly and activation of NLRP3 inflammasome.65 And these downstream moleculars of SIRT3/SOD2 pathway and the changes of mitochondrial dynamic that may influence the cognitive levels were valued to be investigated in the future. Third, we just observed the cognitive outcomes at postoperative 1 and 3 days. The related index at postoperative 7 or 14 days is required further investigation and may more valuable. Forth, because of lack of the SIRT3 knockout mice, we cannot make sure that HNK only activate the SIRT3 pathway; however, many studies had shown its capacity of upregulated SIRT3. Additionally, on the account of the expensive cost and long cultivation cycles, we employed only adult female mice in our studies and eliminated the possible interspecies differences.
5. CONCLUSIONS
Taken together, our result indicated that HNK effectively ameliorates surgery/anesthesia‐induced cognitive declinet in mice through activating SIRT3 regulation of mitochondrial dysfunction and neuroinflammatory. The proposed mechanisms of HNK neuroprotection are summarized in Figure 9.
Figure 9.
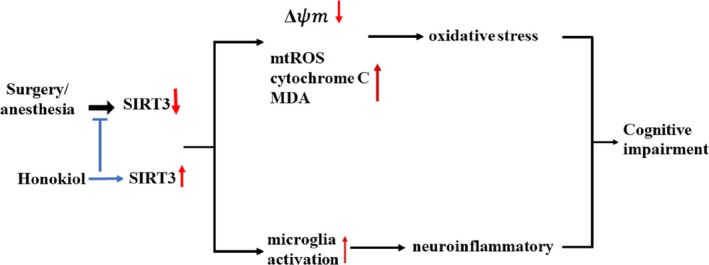
Proposed schematic diagram illustrating HNK neuroprotection against surgery/anesthesia‐induced cognitive impairment. Surgery/anesthesia treatment downregulates SIRT3 in the hippocampus leading to decreased levels of ΔΨm, and increased levels of mtROS, cytochrome C and MDA, all contributing to mitochondrial dysfunction. Meanwhile, microglia were activated and neuroinflammation was enhanced. Activation of Sirt3 by HNK reversed these two processes above
ETHICS APPROVAL
The experimental protocol was approved by the Animal Ethics Committee of Zhongnan Hospital of Wuhan University, Hubei, China, and all experiments were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
We thank the Central Laboratory (Renmin Hospital of Wuhan University) for the equipment and excellent technical assistance.
Ye J‐S, Chen L, Lu Y‐Y, Lei S‐Q, Peng M, Xia Z‐Y. SIRT3 activator honokiol ameliorates surgery/anesthesia‐induced cognitive decline in mice through anti‐oxidative stress and anti‐inflammatory in hippocampus. CNS Neurosci Ther. 2019;25:355–366. 10.1111/cns.13053
Funding information
This study was supported by the National Natural Science Foundation of China (Grant No. 81471844 and 81671891).
REFERENCES
- 1. Li XM, Su F, Ji MH, et al. Disruption of hippocampal neuregulin 1‐ErbB4 signaling contributes to the hippocampus‐dependent cognitive impairment induced by isoflurane in aged mice. Anesthesiology. 2014;121(1):79‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cristina Z, Michela B, Agostino B, et al. Post‐operative cognitive decline (POCD) after gynaecologic surgery: current opinions and future applications. Arch Gynecol Obstet. 2018;287(3):551‐554. [DOI] [PubMed] [Google Scholar]
- 3. Buendia I, Michalska P, Navarro E, Gameiro I, Egea J, León R. Nrf2‐ARE pathway: An emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol Ther. 2016;157:84‐104. [DOI] [PubMed] [Google Scholar]
- 4. Sarkar S, Malovic E, Harishchandra DS, et al. Mitochondrial impairment in microglia amplifies NLRP3 inflammasome proinflammatory signaling in cell culture and animal models of Parkinson's disease. NPJ Parkinsons Dis. 2017;3:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jung UJ, Kim SR. Beneficial effects of flavonoids against parkinson's disease. J Med Food. 2018;21(5):421‐432. [DOI] [PubMed] [Google Scholar]
- 6. Wang J, Li L, Wang Z, et al. Supplementation of lycopene attenuates lipopolysaccharide‐induced amyloidogenesis and cognitive impairments via mediating neuroinflammation and oxidative stress. J Nutr Biochem. 2018;56:16‐25. [DOI] [PubMed] [Google Scholar]
- 7. Terrando N, Monaco C, Ma D, Foxwell BM, Feldmann M, Maze M. Tumor necrosis factor‐alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A. 2010;107(47):20518‐20522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Terrando N, Gómez‐Galán M, Yang T, et al. Aspirin‐triggered resolvin D1 prevents surgery‐induced cognitive decline. FASEB J. 2013;27(9):3564‐3571. [DOI] [PubMed] [Google Scholar]
- 9. Bonsack F, Alleyne CH, Sukumari‐Ramesh S. Resveratrol attenuates neurodegeneration and improves neurological outcomes after intracerebral hemorrhage in mice. Front Cell Neurosci. 2017;11:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ren Q, Peng M, Dong Y, et al. Surgery plus anesthesia induces loss of attention in mice. Front Cell Neurosci. 2015;9:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jiang DQ, Wang Y, Li MX, Ma YJ, Wang Y. SIRT3 in neural stem cells attenuates microglia activation‐induced oxidative stress injury through mitochondrial pathway. Front Cell Neurosci. 2017;11:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu X, Zhang L, Wang P, et al. Sirt3‐dependent deacetylation of SOD2 plays a protective role against oxidative stress in oocytes from diabetic mice. Cell Cycle. 2017;16(13):1302‐1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sun F, Si Y, Bao H, et al. Regulation of sirtuin 3‐mediated deacetylation of cyclophilin d attenuated cognitive dysfunction induced by sepsis‐associated encephalopathy in mice. Cell Mol Neurobiol. 2017;37(8):1457‐1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li R, Quan Y, Xia W. SIRT3 inhibits prostate cancer metastasis through regulation of FOXO3A by suppressing Wnt/β‐catenin pathway. Exp Cell Res. 2018;364(2):143‐151. [DOI] [PubMed] [Google Scholar]
- 15. Yang X, Geng KY, Zhang YS, et al. Sirt3 deficiency impairs neurovascular recovery in ischemic stroke[J]. CNS Neurosci Ther. 2018; 10.1111/cns.12853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qu WM, Yue XF, Sun Y, et al. Honokiol promotes non‐rapid eye movement sleep via the benzodiazepine site of the GABA(A) receptor in mice. Br J Pharmacol. 2012;167(3):587‐598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chicca A, Gachet MS, Petrucci V, Schuehly W, Charles RP, Gertsch J. 4'‐O‐methylhonokiol increases levels of 2‐arachidonoyl glycerol in mouse brain via selective inhibition of its COX‐2‐mediated oxygenation. J Neuroinflammation. 2015;12:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu FC, Yu HP, Syu YT, et al. Honokiol suppresses formyl peptide‐induced human neutrophil activation by blocking formyl peptide receptor 1. Sci Rep. 2017;7(1):6718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pillai VB, Kanwal A, Fang YH, et al. Honokiol, an activator of Sirtuin‐3 (SIRT3) preserves mitochondria and protects the heart from doxorubicin‐induced cardiomyopathy in mice. Oncotarget. 2017;8(21):34082‐34098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang M, Li Y, Ni C, Song G. Honokiol attenuates oligomeric amyloid β1‐42‐induced alzheimer's disease in mice through attenuating mitochondrial apoptosis and inhibiting the nuclear factor kappa‐B signaling pathway. Cell Physiol Biochem. 2017;43(1):69‐81. [DOI] [PubMed] [Google Scholar]
- 21. Li Z, Dong H, Li M, et al. Honokiol induces autophagy and apoptosis of osteosarcoma through PI3K/Akt/mTOR signaling pathway. Mol Med Rep. 2018;17(2):2719‐2723. [DOI] [PubMed] [Google Scholar]
- 22. Ramesh S, Govindarajulu M, Lynd T, et al. SIRT3 activator Honokiol attenuates β‐Amyloid by modulating amyloidogenic pathway. PLoS One. 2018;13(1):e0190350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xian YF, Ip SP, Mao QQ, et al. Honokiol improves learning and memory impairments induced by scopolamine in mice. Eur J Pharmacol. 2015;760:88‐95. [DOI] [PubMed] [Google Scholar]
- 24. Sun L, Dong R, Xu X, Yang X, Peng M. Activation of cannabinoid receptor type 2 attenuates surgery‐induced cognitive impairment in mice through anti‐inflammatory activity. J Neuroinflammation. 2017;14(1):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zheng B, Lai R, Li J, Zuo Z. Critical role of P2X7 receptors in the neuroinflammation and cognitive dysfunction after surgery. BrainBehav Immun. 2017;61:365‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ku TH, Lee YJ, Wang SJ, Fan CH, Tien LT. Effect of honokiol on activity of GAD(65) and GAD(67) in the cortex and hippocampus of mice. Phytomedicine. 2011;18(13):1126‐1129. [DOI] [PubMed] [Google Scholar]
- 27. Twaroski DM, Yan Y, Zaja I, Clark E, Bosnjak ZJ, Bai X. Altered mitochondrial dynamics contributes to propofol‐induced cell death in human stem cell‐derived neurons. Anesthesiology. 2015;123(5):1067‐1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li Z, Mo N, Li L, et al. Surgery‐induced hippocampal angiotensin II elevation causes blood‐brain barrier disruption via MMP/TIMP in aged rats. Front Cell Neurosci. 2016;10:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Makhani SS, Kim FY, Liu Y, et al. Cognitive impairment and overall survival in frail surgical patients. J Am Coll Surg. 2017;225(5):590‐600. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang Z, Ji M, Liao Y, Yang J, Gao J. Endotoxin tolerance induced by lipopolysaccharide preconditioning protects against surgery‑induced cognitive impairment in aging mice. Mol Med Rep. 2018;17:3845–3852. [DOI] [PubMed] [Google Scholar]
- 31. Chen CM, Liu SH, Lin‐Shiau SY. Honokiol, a neuroprotectant against mouse cerebral ischaemia, mediated by preserving Na+, K+‐ATPase activity and mitochondrial functions. Basic Clin Pharmacol Toxicol. 2007;101(2):108‐116. [DOI] [PubMed] [Google Scholar]
- 32. Avtanski DB, Nagalingam A, Bonner MY, Arbiser JL, Saxena NK, Sharma D. Honokiol inhibits epithelial‐mesenchymal transition in breast cancer cells by targeting signal transducer and activator of transcription 3/Zeb1/E‐cadherin axis. Mol Oncol. 2014;8(3):565‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pillai VB, Samant S, Sundaresan NR, et al. Honokiol blocks and reverses cardiac hypertrophy in mice by activating mitochondrial Sirt3. Nat Commun. 2015;6:6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Akamata K, Wei J, Bhattacharyya M, et al. SIRT3 is attenuated in systemic sclerosis skin and lungs, and its pharmacologic activation mitigates organ fibrosis. Oncotarget. 2016;7(43):69321‐69336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang JS, Yao CJ, Chuang SE, et al. Honokiol inhibits sphere formation and xenograft growth of oral cancer side population cells accompanied with JAK/STAT signaling pathway suppression and apoptosis induction. BMC Cancer. 2016;16:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lu Y, Wang YD, Wang XY, Chen H, Cai ZJ, Xiang MX. SIRT3 in cardiovascular diseases: Emerging roles and therapeutic implications. Int J Cardiol. 2016;220:700‐705. [DOI] [PubMed] [Google Scholar]
- 37. Luo LX, Li Y, Liu ZQ, et al. Honokiol induces apoptosis, G1 arrest, and autophagy in KRAS mutant lung cancer cells. Front Pharmacol. 2017;8:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Peng M, Ye JS, Wang YL, Chen C, Wang CY. Posttreatment with propofol attenuates lipopolysaccharide‐induced up‐regulation of inflammatory molecules in primary microglia. Inflamm Res. 2014;63(5):411‐418. [DOI] [PubMed] [Google Scholar]
- 39. Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nat Med. 2017;23(9):1018‐1027. [DOI] [PubMed] [Google Scholar]
- 40. Spangenberg EE, Green KN. Inflammation in Alzheimer's disease: Lessons learned from microglia‐depletion models. Brain Behav Immun. 2017;61:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lv X, Yan J, Jiang J, Zhou X, Lu Y, Jiang H. MicroRNA‐27a‐3p suppression of peroxisome proliferator‐activated receptor‐γ contributes to cognitive impairments resulting from sevoflurane treatment. J Neurochem. 2017;143(3):306‐319. [DOI] [PubMed] [Google Scholar]
- 42. Wu J, Hao S, Sun XR, et al. Elamipretide (SS‐31) ameliorates isoflurane‐induced long‐term impairments of mitochondrial morphogenesis and cognition in developing rats. Front Cell Neurosci. 2017;11:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhao WX, Zhang JH, Cao JB, et al. Acetaminophen attenuates lipopolysaccharide‐induced cognitive impairment through antioxidant activity. J Neuroinflammation. 2017;14(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Huang HF, Guo F, Cao YZ, Shi W, Xia Q. Neuroprotection by manganese superoxide dismutase (MnSOD) mimics: antioxidant effect and oxidative stress regulation in acute experimental stroke. CNS Neurosci Ther. 2012;18(10):811‐818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Han Y, Xu X, Tang C, et al. Reactive oxygen species promote tubular injury in diabetic nephropathy: The role of the mitochondrial ros‐txnip‐nlrp3 biological axis. Redox Biol. 2018;16:32‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Volobueva AS, Melnichenko AA, Grechko AV, Orekhov AN. Mitochondrial genome variability: the effect on cellular functional activity. Ther Clin Risk Manag. 2018;14:237‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kumari S, Badana AK, Murali Mohan G, Shailender G, Malla R. Reactive oxygen species: a key constituent in cancer survival. Biomark Insights. 2018;13:1177271918755391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Teixeira J, Deus CM, Borges F, Oliveira PJ. Mitochondria: Targeting mitochondrial reactive oxygen species with mitochondriotropic polyphenolic‐based antioxidants. Int J Biochem Cell Biol. 2018;97:98‐103. [DOI] [PubMed] [Google Scholar]
- 49. Cheng KY, Guo F, Lu JQ, et al. MnTM‐4‐PyP modulates endogenous antioxidant responses and protects primary cortical neurons against oxidative stress. CNS Neurosci Ther. 2015;21(5):435‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bartolome F, de la Cueva M, Pascual C, et al. Amyloid β‐induced impairments on mitochondrial dynamics, hippocampal neurogenesis, and memory are restored by phosphodiesterase 7 inhibition. Alzheimers Res Ther. 2018;10(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ghasemi M, Mayasi Y, Hannoun A, Eslami SM, Carandang R. Nitric oxide and mitochondrial function in neurological diseases. Neuroscience. 2018;376:48‐71. [DOI] [PubMed] [Google Scholar]
- 52. Song Y, Du Y, Zou W, Luo Y, Zhang X, Fu J. Involvement of impaired autophagy and mitophagy in Neuro‐2a cell damage under hypoxic and/or high‐glucose conditions. Sci Rep. 2018;8(1):3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Anamika, Khanna A, Acharjee P, Acharjee A, Trigun SK. Mitochondrial SIRT3 and neurodegenerative brain disorders. J Chem Neuroanat. 2017; 10.1016/j.jchemneu.2017.11.009 [DOI] [PubMed] [Google Scholar]
- 54. Buck E, Bayer H, Lindenberg KS, et al. Comparison of sirtuin 3 levels in als and huntington's disease‐differential effects in human tissue samples vs. transgenic mouse models. Front Mol Neurosci. 2017;10:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Salvatori I, Valle C, Ferri A, Carrì MT. SIRT3 and mitochondrial metabolism in neurodegenerative diseases. Neurochem Int. 2017;109:184‐192. [DOI] [PubMed] [Google Scholar]
- 56. Shi H, Deng HX, Gius D, Schumacker PT, Surmeier DJ, Ma YC. Sirt3 protects dopaminergic neurons from mitochondrial oxidative stress. Hum Mol Genet. 2017;26(10):1915‐1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nerurkar PV, Johns LM, Buesa LM, et al. Momordica charantia (bitter melon) attenuates high‐fat diet‐associated oxidative stress and neuroinflammation. J Neuroinflammation. 2011;8:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Li N, Zhang J, Yan X, et al. SIRT3‐KLF15 signaling ameliorates kidney injury induced by hypertension. Oncotarget. 2017;8(24):39592‐39604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Guo X, Yan F, Li J, Zhang C, Bu P. SIRT3 attenuates AngII‐induced cardiac fibrosis by inhibiting myofibroblasts transdifferentiation via STAT3‐NFATc2 pathway. Am J Transl Res. 2017;9(7):3258‐3269. [PMC free article] [PubMed] [Google Scholar]
- 60. Zeng Z, Yang Y, Dai X, et al. Polydatin ameliorates injury to the small intestine induced by hemorrhagic shock via SIRT3 activation‐mediated mitochondrial protection. Expert Opin Ther Targets. 2016;20(6):645‐652. [DOI] [PubMed] [Google Scholar]
- 61. Zhai M, Li B, Duan W, et al. Melatonin ameliorates myocardial ischemia reperfusion injury through SIRT3‐dependent regulation of oxidative stress and apoptosis. J Pineal Res. 2017;63(2):e12419. [DOI] [PubMed] [Google Scholar]
- 62. Pi H, Xu S, Reiter RJ, et al. SIRT3‐SOD2‐mROS‐dependent autophagy in cadmium‐induced hepatotoxicity and salvage by melatonin. Autophagy. 2015;11(7):1037‐1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chuang DY, Chan MH, Zong Y, et al. Magnolia polyphenols attenuate oxidative and inflammatory responses in neurons and microglial cells. J Neuroinflammation. 2013;10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang M, Deng YN, Zhang JY, et al. SIRT3 Protects Rotenone‐induced Injury in SH‐SY5Y Cells by Promoting Autophagy through the LKB1‐AMPK‐mTOR Pathway. Aging Dis. 2018;9(2):273‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Traba J, Geiger SS, Kwarteng‐Siaw M, et al. Prolonged fasting suppresses mitochondrial NLRP3 inflammasome assembly and activation via SIRT3‐mediated activation of superoxide dismutase 2. J Biol Chem. 2017;292(29):12153‐12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


