Summary
Aims
To evaluate the immunogenicity and safety of a seasonal influenza vaccine in a cohort of multiple sclerosis (MS) patients receiving different immunomodulating/immunosuppressive therapies and assess predictors of immune response.
Methods
A prospective, multicenter, non‐randomized observational study including 108 patients receiving a trivalent seasonal influenza vaccination was conducted. Influenza‐specific antibody titers (H1N1, H3N2, and influenza B) were measured to evaluate rates of seroprotection and seroconversion/significant titer increase. Univariable and multivariable analyses were performed to identify prognostic factors of vaccination outcomes.
Results
Regarding the whole cohort, seroprotection rates >70% were achieved for each influenza strain. Interferon‐treated patients reached high seroprotection rates (>84%). Good seroprotection rates were seen in patients treated with glatiramer acetate. In particular for H3N2, response rates were low in natalizumab‐treated patients and in the small subgroup of fingolimod‐treated patients. Patients with a previous disease‐modifying therapy and a longer disease duration were less likely to respond sufficiently. No severe adverse events were reported. MS disease activity was not increased after a one‐year follow‐up period.
Conclusion
Vaccination led to good immunogenicity, especially in MS patients treated with interferons and glatiramer acetate. At least for the H1N1 strain, rates of seroprotection and seroconversion/significant titer increase were high (>70% and >60%, respectively) for all therapeutic subgroups. Patients with a longer duration of the disease are exposed to an increased risk of insufficient immune response to vaccination.
Keywords: disease‐modifying therapy, immunogenicity, immunomodulation, influenza vaccine, multiple sclerosis
1. INTRODUCTION
Multiple sclerosis (MS) is a chronic immune‐mediated disease of the central nervous system (CNS) with heterogeneous clinical manifestations.1, 2 The underlying inflammatory process is triggered by the adaptive immune system and leads to the formation of demyelinating lesions in the gray and white matter and axonal damage.3, 4
The disease usually begins as relapsing‐remitting multiple sclerosis (RRMS), characterized by deficits arising from relapses which are remitting completely or incompletely. Over time, this course often evolves to a secondary progressive phenotype (SPMS) where disability accumulates progressively.5, 6 The less frequent primary progressive course of disease (PPMS) is defined by a steady accumulation of disability from disease onset without unequivocal recovery.7 Disease‐modifying therapies (DMT) target different immunological pathways and act as immunomodulators or immunosuppressants.8, 9
Infections in patients with MS are accompanied by an increased risk of disease exacerbation. Relapses associated with infections more often lead to a prolonged neurological deficit or sustained deterioration than relapses without such an association.10 MS patients are at a higher risk of hospitalization due to infections11, 12 and also have a higher mortality rate associated with infections than people without MS.11 The increase in mortality of MS patients during winter months is associated with pneumonia.13
Vaccination is an effective tool to reduce infection‐associated morbidity and mortality. In the past, its use in MS was constrained by safety concerns.14, 15 Retrospective and prospective studies so far show a complex situation: while yellow fever vaccine may lead to increased MS activity, tetanus and diphtheria vaccination do not generally influence disease manifestation.16, 17, 18 With the exception of a small case series,19 multiple studies reported no increased relapse rates after vaccination against the influenza strain A/California/07/2009 (H1N1), which has been circulating since 2009.17, 20, 21, 22, 23, 24
Inactivated influenza vaccines are thus considered safe and are recommended in national guidelines.16, 18, 25 The response to influenza vaccination in MS patients has been evaluated for some of the DMT in controlled settings.26, 27, 28, 29, 30, 31 Only recently the important question of response to vaccination in comparison across different therapies in a real‐life setting was raised and first results were published.32
The main aim of our study was to evaluate the immunogenicity and safety of a seasonal influenza vaccine in a real‐life cohort of MS patients treated with different DMT. Long‐term disease activity before and after vaccination, safety aspects, and predictors of immune response were assessed.
2. METHODS
2.1. Subjects and study procedures
We conducted a prospective, multicenter, non‐randomized observational study. The participating study sites were one university hospital delivering outpatient care and 27 specialized outpatient care centers in Germany.
The study included patients with MS, aged 18‐70 years, who were treated with a DMT for at least 6 months and had an indication for a seasonal influenza vaccination according to the German national recommendations by the Standing Committee on Vaccination.25 Criteria for exclusion were a current MS relapse or an unstable course of disease, febrile infections (fever above 38°C within the two weeks before vaccination), and other contraindications against the vaccine.
All patients who chose to receive a seasonal influenza vaccine on a routine basis were offered to participate in this study. Written informed consent was obtained. A single dose of an inactivated influenza vaccine (seasons 2010/2011 and 2011/2012) was injected according to the manufacturer's specification. In both seasons, the trivalent vaccine contained the same strains: A/California/7/2009 (H1N1), A/Perth/16/2009 (H3N2), and B/Brisbane/60/2008, as recommended by the WHO.33, 34 The study protocol did not influence the choice of the brand of the vaccine used.
The following data were ascertained at baseline: age, sex, date of first symptoms attributed to MS and date of diagnosis, clinical course, current score in the Expanded Disability Status Scale (EDSS) and 3, 6, 12, and 24 months before vaccination, relapse events in the last and second to last year before vaccination to evaluate disease activity, currently and previously prescribed DMT as well as other medical conditions and therapies.
Before and 4 weeks after vaccination, a sample of approximately 10 mL whole blood was obtained. The samples were sent to a central laboratory and centrifuged upon arrival, with the collected sera stored at −70°C.
The influenza‐specific antibody titer measurements were carried out by the national reference laboratory for influenza at the Robert‐Koch‐Institut (Berlin) using a hemagglutination inhibition assay (HIA) as described before.35 The given HI titer is the highest serum dilution blocking hemagglutination. Every pair of samples was analyzed twice for each influenza strain. Geometric mean titers (GMT) were calculated. For subsequent calculations, HI titers <10 (below cut‐off) were set to 5.36
The immune response was evaluated using the criteria of the European Committee for Medicinal Products for Human Use.36 These consist of three assessments for every virus strain, where at least one of these assessments should be positive in both age groups (Table 1). For the evaluation of immune response, we chose not to categorize subjects by age, as only a few patients over 60 years (n = 4) participated.
Table 1.
Criteria for influenza vaccine immunogenicity by the European Committee for Medicinal Products for Human Use36
| Assessment | Age 18‐60 y | Age >60 y |
|---|---|---|
| Proportion of subjects achieving seroconversion or a significant increase in anti‐HI antibody titera | >40% | >30% |
| Mean geometric titer increase | >2.5 | >2.0 |
| Proportion of subjects achieving an HI titer ≥ 40 | >70% | >60% |
HI, hemagglutination inhibition.
Either a pre‐vaccination HI titer <1:10 and a post‐vaccination HI titer ≥ 1:40 or a pre‐vaccination HI titer ≥ 1:10 and a minimum fourfold rise in post‐vaccination HI antibody titer.
Subjects were followed for one year after immunization, with follow‐up visits after 1, 3, 6, and 12 months. After four weeks, local and systemic adverse events (AE) were registered. The severity of AE was graded as follows: “mild” (no limitation of day‐to‐day activities), “moderate” (minor limitations), and “severe” (inability to accomplish day‐to‐day activities). A causal relationship between vaccination and AE was evaluated by the investigator. No association was assumed if symptoms existed before vaccination, began long after vaccination, or if there was proof of another etiology.
The activity of MS (relapses and progression of disability in the EDSS), changes of DMT, new medical conditions, and changes in concurrent medication were registered during follow‐up visits after 3, 6, and 12 months.
The study has been carried out in accordance with the Declaration of Helsinki. Institutional review boards and ethics committees at participating study sites approved the protocol. The trial is registered at ClinicalTrials.gov (NCT02275741).
2.2. Statistical analysis
Analyses were conducted in the per‐protocol population: all patients who entered the study, received influenza vaccination, and had available antibody titer results at days 0 and 28.
The individual response to immunization was evaluated using two scenarios: (a) seroprotection (sufficient/insufficient) and (b) seroconversion/significant titer increase (yes/no). Both scenarios were analyzed for the particular influenza strains and for all tested strains combined. The combined analysis addresses seroprotection and seroconversion/significant titer increase, respectively, among all three tested strains, as a clinically important outcome.
Patients were compared by univariable and multivariable analyses to identify predictors of seroprotection and seroconversion/significant titer increase among the baseline parameters. Welch's t‐test, Fisher's exact test, chi‐squared test, McNemar's test, and Mann‐Whitney U test were used when appropriate. Nominal two‐tailed P values <0.05 were considered statistically significant. Binomial logistic regression was performed to evaluate the effects of baseline variables on the likelihood of the outcome. The variables entered the regression models using forward selection (based on likelihood ratio) statistics. A two‐way ANOVA was performed for main and interaction effects of DMT and relapse events before and after vaccination. All analyses were performed using SPSS 23.
3. RESULTS
3.1. Study subjects
In total, 108 patients with MS were included and vaccinated against influenza. Complete blood samples (sample before vaccination and sample four weeks after vaccination, median 31 days) were available from 102 patients (per‐protocol population). The baseline characteristics of the study population are summarized in Table 2.
Table 2.
Demographic and clinical characteristics of the per‐protocol population
| Clinical characteristic | Value |
|---|---|
| Age (mean ± SD) | 42.4 ± 10.2 y |
| Time since MS diagnosis (mean ± SD) | 8.3 ± 7.0 y |
| Time since onset of MS‐attributed symptoms (mean ± SD) | 10.6 ± 8.2 y |
| Sex | |
| Female | 77 (75.5%) |
| Male | 25 (24.5%) |
| Clinical course | |
| Relapsing‐remitting (RRMS) | 94 (92.2%) |
| Secondary progressive (SPMS) | 5 (4.9%) |
| Primary progressive (PPMS) | 3 (2.9%) |
| Current DMT | |
| Interferons | 45 (44.1%) |
| Glatiramer acetate | 26 (25.5%) |
| Natalizumab | 14 (13.7%) |
| Fingolimod | 6 (5.9%) |
| Other | 11 (10.8%) |
| Duration of current DMT (mean ± SD) | 32.6 ± 27.4 mo |
| Previous DMT | |
| Yes | 50 (49.0%) |
| No | 50 (49.0%) |
| Unknown | 2 (2.0%) |
| EDSS | |
| 12 mo prior to vaccination (mean ± SD) | 2.16 (±1.86) |
| 6 mo prior to vaccination (mean ± SD) | 2.17 (±1.87) |
| 3 mo prior to vaccination (mean ± SD) | 2.27 (±1.89) |
| At vaccination day (mean ± SD) | 2.32 (±1.88) |
| Relapse rate | |
| Month 24‐13 prior to vaccination | 0.7 relapses/patient/y |
| Month 12‐0 prior to vaccination | 0.5 relapses/patient/y |
The per‐protocol population comprised 102 subjects (± indicates standard deviation).
DMT, disease‐modifying therapy; EDSS, Expanded Disability Status Scale; MS, multiple sclerosis; PPMS, primary progressive multiple sclerosis; RRMS, relapsing‐remitting multiple sclerosis; SD, standard deviation; SPMS, secondary progressive multiple sclerosis.
The majority of included subjects were female (75.5%), and most of the patients were affected by RRMS (92.2%). The mean (± standard deviation) EDSS score at the vaccination day was 2.32 (±1.88). In the year prior to vaccination, the participants experienced 0.50 relapses on average. There was no statistically significant difference for baseline variables when comparing male with female study participants.
Disease‐modifying therapies used were interferons (IFN, 44.1%), glatiramer acetate (25.5%), natalizumab (13.7%), fingolimod (5.9%), and others (10.8%); among these: glucocorticosteroids (4.9%), mitoxantrone (2.0%), intravenous immunoglobulins (2.0%), teriflunomide (1.0%), and fumaric acid esters (1.0%).
3.2. Immunogenicity
Protective antibody titers against H1N1 prior to vaccination were detectable in 20.6% of the subjects, against H3N2 in 22.5% and against the B strain in 43.1%. 6.8% of the participants had protective antibody titers for all three strains before vaccination.
After vaccination, protective antibody titers were detectable in more than 70% of the patients for every individual influenza strain (H1N1 85.3%, H3N2 72.5%, and B strain 80.4%) and in 56.9% of the participants for all three strains. The antibody responses are summarized in Table 3. In the following, differences in the rates of seroprotection and seroconversion/significant titer increase four weeks after vaccination are outlined between patient groups.
Table 3.
Serum antibody titers and titer changes following seasonal influenza vaccination
| A(H1N1)‐California | A(H3N2)‐Perth | B‐Brisbane | All strainsa | |
|---|---|---|---|---|
| Before vaccination (baseline) | ||||
| Seroprotection, n (%) | 21 (20.6%) | 23 (22.5%) | 44 (43.1%) | 7 (6.8%) |
| GMT | 11 | 11 | 22 | ‐ |
| After vaccination (day 28) | ||||
| GMT | 125 | 55 | 63 | ‐ |
| GMT increase | 11.2 | 4.9 | 2.9 | ‐ |
| Seroprotection, n (%) | 87 (85.3%) | 74 (72.5%) | 82 (80.4%) | 58 (56.9%) |
| Seroconversion or significant titer increase (all subjects), n (%) | 71 (69.6%) | 54 (52.9%) | 39 (38.2%) | 28 (27.5%) |
| Seroconversion, n (%) | 47/59 (79.7%) | 30/57 (52.6%) | 11/17 (64.7%) | ‐ |
| Significant titer increase, n (%) | 24/43 (55.8%) | 24/45 (53.3%) | 28/85 (32.9%) | ‐ |
GMT, geometric mean titer; GMT increase: GMT ratio post‐vaccination/pre‐vaccination; seroprotection: proportion of subjects with antibody titers ≥40 at baseline or after vaccination; seroconversion: proportion of subjects with antibody titers at baseline <10 and ≥40 after vaccination; significant titer increase: proportion of subjects with antibody titers at baseline >10 and ≥4‐fold titer increase after vaccination.
Subjects who achieved this criterion in all three strains.
3.3. Seroprotection by therapeutic regimen
Heterogeneous seroprotection rates concerning the individual influenza strains were found within the therapeutic subgroups (Table 4). While there were no significant differences in protection rates against H1N1 among different DMT, the protection rates against H3N2 differed significantly (chi‐squared test, P < 0.001), with a seroprotection rate in natalizumab‐treated patients (n = 14) of 28.6%. The seroprotection rate against the B strain was also lowest (57.1%) in those patients treated with natalizumab.
Table 4.
Seroprotection for influenza strains H1N1, H3N2, and B and combined for all strains after vaccination
| n | Seroprotection (H1N1) | P | Seroprotection (H3N2) | P | Seroprotection (B) | P | Seroprotection (all strains) | P | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Insufficient (n = 15) | Sufficient (n = 87) | Insufficient (n = 28) | Sufficient (n = 74) | Insufficient (n = 20) | Sufficient (n = 82) | Insufficient (n = 44) | Sufficient (n = 58) | ||||||
| Mean age, y, (±SD) | 46.5 (10.4) | 41.7 (10.1) | 0.114a | 42.1 (10.7) | 42.5 (10.1) | 0.866 | 38.9 (10.5) | 43.3 (10.1) | 0.101 | 43.5 (10.7) | 41.7 (9.9) | 0.387 | |
| Sex, n (%) | |||||||||||||
| Female | 77 | 10 (13.0) | 67 (87.0) | 0.515b | 17 (22.1) | 60 (77.9) | 0.041 | 12 (15.6) | 65 (84.4) | 0.087 | 28 (36.4) | 49 (63.6) | 0.020 |
| Male | 25 | 5 (20.0) | 20 (80.0) | 11 (44.0) | 14 (56.0) | 8 (32.0) | 17 (68.0) | 16 (64.0) | 9 (36.0) | ||||
| Current DMT, n (%) | |||||||||||||
| Interferons | 45 | 7 (15.6) | 38 (84.4) | 0.449c | 4 (8.9) | 41 (91.1) | <0.001 | 5 (11.1) | 40 (88.9) | 0.108 | 12 (26.7) | 33 (73.3) | 0.002 |
| Glatiramer acetate | 26 | 3 (11.5) | 23 (88.5) | 7 (26.9) | 19 (73.1) | 5 (19.2) | 21 (80.8) | 11 (42.3) | 15 (57.7) | ||||
| Natalizumab | 14 | 4 (28.6) | 10 (71.4) | 10 (71.4) | 4 (28.6) | 6 (42.9) | 8 (57.1) | 12 (85.7) | 2 (14.3) | ||||
| Fingolimod | 6 | 0 (0.0) | 6 (100.0) | 4 (66.7) | 2 (33.3) | 2 (33.3) | 4 (66.7) | 4 (66.7) | 2 (33.3) | ||||
| Others | 11 | 1 (9.1) | 10 (90.9) | 3 (27.3) | 8 (72.7) | 2 (18.2) | 9 (81.8) | 5 (45.5) | 6 (54.5) | ||||
| Median duration of current DMT, mo (range) | 30 (6‐101) | 25 (6‐124) | 0.568d | 22 (6‐83) | 28 (6‐124) | 0.714 | 27 (6‐124) | 24 (6‐107) | 0.335 | 25 (6‐124) | 26 (6‐107) | 0.632 | |
| Previous DMT, n (%) | |||||||||||||
| Yes | 50 | 10 (20.0) | 40 (80.0) | 0.262c | 21 (42.0) | 29 (58.0) | 0.003 | 13 (26.0) | 37 (74.0) | 0.125 | 29 (58.0) | 21 (42.0) | 0.004 |
| No | 50 | 5 (10.0) | 45 (90.0) | 7 (14.0) | 43 (86.0) | 6 (12.0) | 44 (88.0) | 14 (28.0) | 36 (72.0) | ||||
| Not reported | 2 | 0 (0.0) | 2 (100.0) | 0 (0.0) | 2 (100.0) | 1 (50.0) | 1 (50.0) | 1 (50.0) | 1 (50.0) | ||||
| Median disease duration, y (range) | 11 (1‐30) | 6 (0‐33) | 0.110d | 9 (1‐25) | 6 (0‐33) | 0.151 | 7 (1‐21) | 6 (0‐33) | 0.507 | 9 (1‐30) | 5 (0‐33) | 0.032 | |
| Course of MS, n (%) | |||||||||||||
| RRMS | 94 | 14 (14.9) | 80 (85.1) | 0.729c | 27 (28.7) | 67 (71.3) | 0.509 | 18 (19.1) | 76 (80.9) | 0.830 | 41 (43.6) | 53 (56.4) | 0.929 |
| SPMS | 5 | 1 (20.0) | 4 (80.0) | 1 (20.0) | 4 (80.0) | 1 (20.0) | 4 (80.0) | 2 (40.0) | 3 (60.0) | ||||
| PPMS | 3 | 0 (0.0) | 3 (100.0) | 0 (0.0) | 3 (100.0) | 1 (33.3) | 2 (66.7) | 1 (33.3) | 2 (66.7) | ||||
| Mean EDSS at vaccination (SD) | 2.23 (1.69) | 2.34 (1.93) | 0.832a | 2.56 (1.40) | 2.23 (2.02) | 0.387 | 2.55 (1.67) | 2.26 (1.94) | 0.521 | 2.48 (1.58) | 2.02 (2.08) | 0.442 | |
| Relapses year 1 before vaccination, mean (SD) | 0.86 (1.35) | 0.44 (0.62) | 0.276a | 0.59 (1.05) | 0.46 (0.65) | 0.558 | 0.42 (0.61) | 0.52 (0.81) | 0.561 | 0.54 (0.92) | 0.47 (0.66) | 0.710 | |
| Relapses year 2 before vaccination, mean (SD) | 0.71 (0.73) | 0.69 (0.85) | 0.943a | 0.67 (0.83) | 0.71 (0.83) | 0.802 | 0.61 (0.78) | 0.72 (0.85) | 0.597 | 0.68 (0.80) | 0.72 (0.86) | 0.795 | |
DMT, disease‐modifying therapy; EDSS, Expanded Disability Status Scale; PPMS, primary progressive multiple sclerosis; RRMS, relapsing‐remitting multiple sclerosis; SD, standard deviation; SPMS, secondary progressive multiple sclerosis.
Welch's t test.
Fisher's exact test.
chi‐squared test.
Mann‐Whitney U test; P < 0.05 indicated in bold.
Considering all strains (ie, seroprotection of an individual patient for all three influenza strains), the seroprotection rates differed by the DMT (chi‐squared test, P = 0.002). In comparison to other treatments, seroprotection was highest in IFN‐treated patients (Figure 1).
Figure 1.
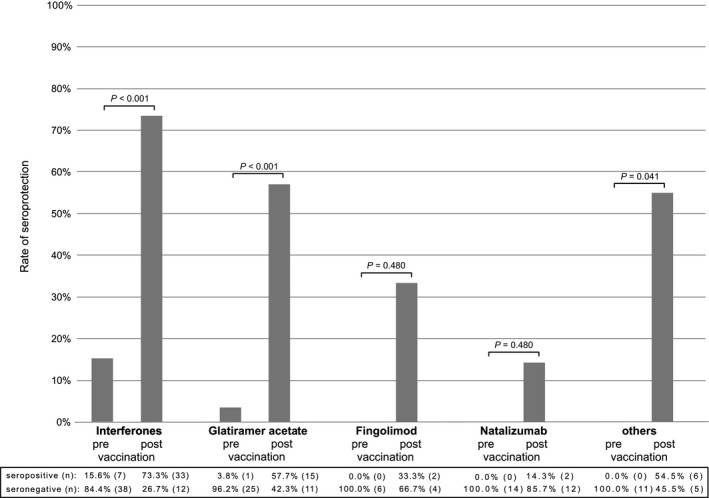
Seroprotection rates against all three influenza strains (H1N1, N3N2, and B) before and after vaccination stratified by disease‐modifying drug
3.4. Seroprotection by other clinico‐demographic variables
H3N2 was associated with higher seroprotection rates in women compared to men (77.9% vs 56.0%, Fisher's exact test, P = 0.041) as well as higher seroprotection rates in patients who have not been treated with other DMT before their current therapy versus those who already switched their DMT (86.0% vs 58.0%, chi‐squared test, P = 0.003).
Considering all strains (ie, seroprotection of an individual patient against all three influenza strains in combination), seroprotection after vaccination was associated with a shorter duration of disease (median of 5 years) and lack of seroprotection was associated with a longer duration of disease (median of 9 years, Mann‐Whitney U test, U = 958.5, P = 0.032). Moreover, in men, seroprotection against all three strains was significantly less frequent than in women (36.0% vs 63.6%, Fisher's exact test, P = 0.020). Higher seroprotection rates were also seen in patients who have not been treated with other DMT before their current therapy versus those who switched their DMT in the past (72.0% vs 42.0%, chi‐squared test, P = 0.004).
3.5. Seroconversion or significant titer increase
The results of the total study population concerning seroconversion, reflecting an adequate immune response, showed rates >40% for H1N1 (69.6%) and H3N2 (52.9%), whereas this rate was 38.2% for B‐Brisbane (Table 3). The GMT increase was 11.2, 4.9, and 2.9, respectively, among the three influenza strains. Seroconversion or a significant titer increase in all three strains was observed in 27.5% of all subjects. Statistically significant differences in the duration of the disease were found in the group comparisons: adequate titer movements in all three strains were associated with a shorter duration of the disease (median of 3 years) and insufficient titer movements were associated with a longer duration of the disease (median of 8 years, Mann‐Whitney U test, U = 721.0, P = 0.018). Similar differences in duration of disease were also seen when analyzing H1N1 (U = 803.0, P = 0.030) and the B strain (U = 818.5, P = 0.005) separately. Male sex was associated with less frequent adequate antibody titer movements against H3N2 than female sex (32.0% vs 59.7%, Fisher's exact test, P = 0.021, Table 5). Significant anti‐H3N2 titer rises were also less common in patients who have been treated with other DMT before their current therapy versus those who did not switch their DMT in the past (40.0% vs 64.0%, chi‐squared test, P = 0.027).
Table 5.
Seroconversion or significant titer increase for influenza strains H1N1, H3N2, and B and combined for all strains after vaccination
| n | Seroconversion or significant titer increase (H1N1) | Seroconversion or significant titer increase (H3N2) | Seroconversion or significant titer increase (B) | Seroconversion or significant titer increase (all strains) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No (n = 31) | Yes (n = 71) | P | No (n = 48) | Yes (n = 54) | P | No (n = 63) | Yes (n = 39) | P | No (n = 74) | Yes (n = 28) | P | ||
| Mean age, y (SD) | 44.5 (10.5) | 41.5 (10.1) | 0.182a | 41.6 (9.8) | 43.2 (10.6) | 0.419 | 42.4 (9.8) | 42.5 (11.1) | 0.982 | 42.8 (9.9) | 41.5 (11.3) | 0.613 | |
| Sex, n (%) | |||||||||||||
| Female | 77 | 24 (31.2) | 53 (68.8) | 1.000b | 31 (40.3) | 46 (59.7) | 0.021 | 45 (58.4) | 32 (41.6) | 0.248 | 53 (68.8) | 24 (31.2) | 0.198 |
| Male | 25 | 7 (28.0) | 18 (72.0) | 17 (68.0) | 8 (32.0) | 18 (72.0) | 7 (28.0) | 21 (84.0) | 4 (16.0) | ||||
| Current DMT, n (%) | |||||||||||||
| Interferons | 45 | 15 (33.3) | 30 (66.7) | 0.858c | 17 (37.8) | 28 (62.2) | 0.081 | 28 (62.2) | 17 (37.8) | 0.187 | 32 (71.1) | 13 (28.9) | 0.354 |
| Glatiramer acetate | 26 | 7 (26.9) | 19 (73.1) | 11 (42.3) | 15 (57.7) | 12 (46.2) | 14 (53.8) | 17 (65.4) | 9 (34.6) | ||||
| Natalizumab | 14 | 5 (35.7) | 9 (64.3) | 11 (78.6) | 3 (21.4) | 12 (85.7) | 2 (14.3) | 13 (92.9) | 1 (7.1) | ||||
| Fingolimod | 6 | 2 (33.3) | 4 (66.7) | 4 (66.7) | 2 (33.3) | 4 (66.7) | 2 (33.3) | 5 (83.3) | 1 (16.7) | ||||
| Others | 11 | 2 (18.2) | 9 (81.8) | 5 (45.5) | 6 (54.5) | 7 (63.6) | 4 (36.4) | 7 (63.6) | 4 (36.4) | ||||
| Median duration of current DMT, mo (range) | 25 (6‐101) | 25 (6‐124) | 0.937d | 22 (6‐83) | 30 (6‐124) | 0.167 | 29 (6‐124) | 21 (6‐99) | 0.101 | 25 (6‐124) | 24 (6‐99) | 0.765 | |
| Previous DMT | |||||||||||||
| Yes | 50 | 19 (38.0) | 31 (62.0) | 0.194c | 30 (60.0) | 20 (40.0) | 0.027 | 35 (70.0) | 15 (30.0) | 0.149 | 40 (80.0) | 10 (20.0) | 0.176 |
| No | 50 | 12 (24.0) | 38 (76.0) | 18 (36.0) | 32 (64.0) | 27 (54.0) | 23 (46.0) | 33 (66.0) | 17 (44.0) | ||||
| Not reported | 2 | 0 (0.0) | 2 (100.0) | 0 (0.0) | 2 (100.0) | 1 (50.0) | 1 (50.0) | 1 (50.0) | 1 (50.0) | ||||
| Median disease duration, y (range) | 8 (0‐30) | 5 (0‐33) | 0.030 d | 8 (0‐33) | 6 (0‐6) | 0.399 | 8 (0‐33) | 4 (0‐19) | 0.005 | 8 (0‐33) | 3 (0‐19) | 0.018 | |
| Course of disease, n (%) | |||||||||||||
| RRMS | 94 | 30 (31.9) | 64 (68.1) | 0.434c | 45 (47.9) | 49 (52.1) | 0.839 | 59 (62.8) | 35 (37.2) | 0.585 | 70 (74.5) | 24 (25.5) | 0.236 |
| SPMS | 5 | 1 (20.0) | 4 (80.0) | 2 (40.0) | 3 (60.0) | 2 (40.0) | 3 (60.0) | 2 (40.0) | 3 (60.0) | ||||
| PPMS | 3 | 0 (0.0) | 3 (100.0) | 1 (33.3) | 2 (66.7) | 2 (66.7) | 1 (33.3) | 2 (66.7) | 1 (33.3) | ||||
| Mean EDSS at vaccination (SD) | 1.98 (1.47) | 2.47 (2.03) | 0.240a | 2.59 (1.65) | 2.09 (2.05) | 0.190 | 2.36 (1.79) | 2.26 (2.04) | 0.789 | 2.36 (1.71) | 2.21 (2.30) | 0.757 | |
| Relapses year 1 before vaccination, mean (SD) | 0.53 (1.04) | 0.48 (0.63) | 0.192a | 0.54 (0.91) | 0.46 (0.64) | 0.612 | 0.46 (0.87) | 0.55 (0.60) | 0.565 | 0.48 (0.83) | 0.53 (0.64) | 0.749 | |
| Relapses year 2 before vaccination, mean (SD) | 0.63 (0.76) | 0.73 (0.86) | 0.186a | 0.69 (0.81) | 0.70 (0.85) | 0.952 | 0.67 (0.84) | 0.74 (0.83) | 0.735 | 0.69 (0.81) | 0.71 (0.89) | 0.965 | |
DMT, disease‐modifying therapy; EDSS, Expanded Disability Status Scale; PPMS, primary progressive multiple sclerosis; RRMS, relapsing‐remitting multiple sclerosis; SD, standard deviation; SPMS, secondary progressive multiple sclerosis.
Welch's t test.
Fisher's exact test.
Chi‐squared test.
Mann‐Whitney U test; P < 0.05 indicated in bold.
3.6. Predictors of response
A previous DMT was a negative predictor for obtaining a protective titer against H1N1 (P = 0.028, OR = 0.165). All other independent variables did not significantly improve the prediction. In the case of protective titers against H3N2, the logistic regression model indicated that pre‐treatment is again a negative predictor (P = 0.009, OR = 0.221) and also that the current DMT has a significant impact on the outcome (P = 0.024, OR = 1.773). Regarding protective titers against the B strain, no variable contributed significantly to the model. When looking at protective antibody titers against all three influenza strains, previous DMT (P = 0.002, OR = 0.205) was the only predictive variable included in the resulting model.
A longer duration of disease was a negative predictor for seroconversion or significant titer increase in the case of H1N1 (P = 0.006, OR = 0.899). In the case of H3N2, the model indicated that a previous DMT (P = 0.033, OR = 0.352) and male sex (P = 0.012, OR = 0.217) are negative predictors for seroconversion or significant titer increase. Regarding seroconversion or significant titer increase after immunization against the B strain, the logistic regression included disease duration (P = 0.009, OR = 0.892) as the predictive variable. When looking at all influenza strains, again a longer disease duration (P = 0.040, OR = 0.910) was associated with an insufficient response to the vaccine.
Thus, a previous DMT and a long duration of disease were in general the best predictors for insufficient seroprotection and seroconversion, respectively, whereas current DMT and age were less informative in this modelling.
3.7. Disease activity
The annual relapse rate in the year before vaccination was 0.50 relapses per patient (data for 98/102 patients) and in the year following vaccination 0.41 relapses per patient (data for 69/102 patients). The difference in relapse rates before and after vaccination when adjusted for therapy was not statistically significant (two‐way ANOVA, P = 0.807). The mean EDSS score one‐year after vaccination was 2.26 (±1.76, data for 67 of 102 patients) compared to the mean EDSS score at the vaccination day of 2.32 (±1.88).
3.8. Safety
Follow‐up information on AE was available for 101 subjects (99.0%). During the first month after vaccination, 9/101 (8.9%) of the subjects reported local and 15/101 (14.9%) systemic AE. In total, 18/101 (17.6%) of the subjects were affected by local and/or systemic AE (six subjects reported both, resulting in 30 reported AE).
Reported local AE were mild or moderate pain, redness, or swelling. Mild systemic AE were reported by 9 of 15 subjects (60.0%) and moderate systemic AE following vaccination were reported by 6 of 15 subjects (40.0%). There was no severe local or systemic AE.
Six patients treated with glatiramer acetate showed either flu‐like symptoms (n = 3), increase in temperature (n = 2), or nightly sweating (n = 1). Six patients under interferon treatment reported flu‐like symptoms (n = 4), headache (n = 1), or feeling weak (n = 1). Two patients treated with fingolimod developed exanthema. One relapse occurred in a patient with SPMS receiving glucocorticosteroids 19 days after the vaccination. This patient had a post‐vaccination seroconversion for all three influenza strains.
The majority of AE (29 of 30) was associated with the preceding vaccination. Among those, 8 were rated as possibly and 21 were rated as likely related to the vaccination. One patient treated with fingolimod developed an exanthema not associated with the vaccination.
4. CONCLUSION
Our multicenter study showed that after immunization with a seasonal trivalent influenza vaccine, most endpoints for the immunogenicity of individual influenza strains could be met in a heterogeneous group of MS patients: (a) for each strain, >70% of all subjects achieved an anti‐HI titer ≥40, (b) for H1N1 and H3N2 (but not for the B strain), >40% of all subjects achieved a seroconversion or significant increase in antibody titers, and (c) a mean geometric titer increase >2.5 could be noted for each particular strain. The overall results on immunogenicity for the study cohort should, however, not distract from those subgroups where immunization falls short of a sufficient response.
When looking at seroprotection in more detail, patients treated with IFN reached seroprotection rates of >80% for the individual strains (H1N1 84.4%, H3N2 91.1%, B strain 88.9%). Protective antibody titers against all three strains were detectable after vaccination in 73.3% of these subjects. For individual strains, similar results in IFN‐treated patients after seasonal influenza vaccination were previously shown in a smaller exploratory study (n = 17), with rates of seroprotection of 88.2% against H1N1 and H3N2, respectively.29 A single‐strain analysis demonstrated seroprotection rates against H3N2 in patients receiving IFN comparable to those achieved in of healthy controls (93.0% vs 90.9%, n = 86).26 In a study of patients with reduced clinical and radiological MS disease‐activity under IFN therapy, the immune response to influenza vaccination was similar to the one seen in healthy controls.28
An earlier study reported lower rates of seroprotection in patients receiving glatiramer acetate in comparison to healthy controls (H1N1: 58.3% vs 71.2%, H3N2: 41.7% vs 79.5%, n = 12).29 In contrast, our study showed higher seroprotection rates under therapy with glatiramer acetate after seasonal influenza vaccination (H1N1 88.5%, H3N2 73.1%, B strain 80.8%, n = 26).
In patients receiving natalizumab, the antibody response to H3N2 was low compared to H1N1 and the B strain. It remains unclear why seropositivity differs to that extent after vaccination. Previously published results on seasonal influenza vaccination in natalizumab‐treated patients have been contradictory, with a described immunoreaction comparable to that in healthy subjects on the one hand,37 but reduced H3N2 seroprotection rates (50.0% vs 79.5%, n = 8) after vaccination on the other hand.29 The same study demonstrated seroprotection rates against H1N1 comparable to that of healthy controls (75.0% vs 71.2%, n = 8).29
Similar to natalizumab, antibody response to H3N2 in fingolimod‐treated patients was lower than to the other antigens, but this finding is limited by small size of this subgroup (n = 6). A placebo‐controlled study showed that a sufficient immune response to the seasonal influenza vaccine under fingolimod is possible, but the response rate is lower than in untreated control groups.30
A previous study found comparable H1N1 seroprotection rates in 90 MS patients treated with IFN‐beta (88.0%), glatiramer acetate (91.3%), natalizumab (72.7%), or fingolimod (71.4%) 3 months after vaccination.32 In contrast, H3N2 seroprotection rates were lower compared to our study in case of IFN‐beta (44.0% vs 91.1%) and glatiramer acetate (26.1% vs 73.1%) therapy. One explanation for this difference might be that different H3N2 antigen strains were vaccinated (A/Victoria/361/2011 vs A/Perth/16/2009).
In our study, both seroprotection and seroconversion showed consistently higher antibody responses to all strains in patients with no previous switch of DMT. However, these results reached statistical significance only in some subgroups (see Tables 4 and 5). In our cohort, use of previous DMT, which can be interpreted as a sign of a more active disease in the individual patient's history, seems to be a predictor for poor immune response but does not generally implicate failure of vaccination. Similar differences were seen regarding disease duration: overall, patients with a longer disease duration showed reduced antibody responses.
Generally, age, duration of disease, the current DMT, and previous use of a different DMT might influence the vaccine response. However, these factors are not independent from each other and may influence therapeutic decisions. Moreover, immunosenescence alters the immune response without underlying disease as well.38 Our data cannot clearly differentiate between age‐dependent alterations of the immune response and potential disease‐specific factors of the impaired immune response.
Multiple sclerosis disease activity (as measured by relapse rates and EDSS) was not increased during one year after vaccination. Vaccination was well tolerated overall. The rate of local and systemic AE was low compared to influenza vaccine approval studies (17.6% of MS subjects affected by AE vs 64.0% of healthy non‐elderly adults).39 However, the lower rates of AE may be due to reporting differences. This study relied on self‐reporting of events at follow‐up visits, whereas other studies asked subjects to report solicited and unsolicited events following vaccination daily, thereby reducing the risk of underreporting.
When comparing participants by sex, there was no statistically significant difference for baseline variables. The female:male sex ratio of 3:1 is similar to the higher share of women affected by multiple sclerosis.40 A lower antibody response in males compared to females, shown in this study regarding antibody titers against H3N2, is a known feature of influenza vaccines.41
In line with a previous study, this study underlines good seroprotection rates one month after influenza vaccination in a real‐life cohort of MS patients, especially in those treated with interferons. It also demonstrates that an adequate immune reaction and good seroprotection rates can be achieved under glatiramer acetate therapy. For a conclusive assessment of vaccination effects during natalizumab and fingolimod treatment, further studies are necessary since numbers of subjects were small, both in this study and a previous evaluation.32 One must bear in mind that the detected antibody response only serves as a surrogate marker for protection against influenza infection.
This multicenter, not placebo‐controlled study covers data of more than 100 MS patients in a real‐world scenario. To achieve more reliable statistical results, higher numbers of participants are desirable in general. Despite other studies in the context of vaccination and MS, this study contributes prospective results related to MS disease activity and safety data for a follow‐up period of one year after vaccination. Due to the various subgroups of patients treated with different DMT and the use of different antigens for vaccination, an approach to statistical analysis of predictors of antibody response is challenging and complex.
Irrespective of the therapy, patients with a longer duration of the disease are particularly exposed to the risk of an insufficient immune response to vaccination. Otherwise, seasonal influenza immunization in MS is safe and well tolerated. Seasonal influenza vaccination thus should be attempted to achieve the best possible reductions in vaccination‐preventable morbidity, hospitalization, and mortality.
CONFLICT OF INTEREST
CM reports no conflicts of interest. AW received travel expense compensation and fees for speaking, consulting, serving on advisory boards, and conducting clinical trials from Alexion, Bayer HealthCare, Biogen, Genzyme, Merck, Novartis, Octapharma, Roche, Sanofi, and Teva. ML has received fees for speaker's honoraria and conducting clinical trials from Abbvie, Astellas, GlaxoSmithKline, Gilead, Novartis Vaccines, Janssen, and Roche. MH received speaking fees and travel funds from Bayer HealthCare, Biogen, Novartis, and Teva. BS reports no conflicts of interest. ECR has received speaker's honoraria, travel expense compensation, and fees for conducting clinical trials from Activaero, Bayer, GlaxoSmithKline, Novartis Vaccines, Roche Pharma, and Sanofi Pasteur MSD. UKZ received research support as well as speaking fees and travel funds from Almirall, Bayer HealthCare, Biogen, Merck Serono, Novartis, Roche, Sanofi, and Teva.
AUTHOR CONTRIBUTIONS
All authors reviewed the drafts and approved the final manuscript. CM drafted the manuscript and participated in data acquisition, data analysis, and interpretation of the data. AW and ML participated in the study design, data analysis, and interpretation of the data. ML furthermore contributed logistical assistance to conduct the study. MH participated in data analysis and interpretation of the data. BS conducted the serological analysis. ECR contributed logistical assistance to conduct the study. UKZ was the principal investigator and participated in the study design, data analysis, and interpretation of the data.
ACKNOWLEDGMENTS
This study was financially supported by Bayer HealthCare, Merck Serono, Sanofi‐Aventis, Teva, Biogen Idec, F. Hoffmann‐La Roche, and Novartis. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Contributors of the Vaccination in MS patients study group (VIMPS): M. Steinle (Leutkirch); M. Lang (Ulm); A. Bergmann (Neuburg); U. Nadjafi, and K. Krampfl (Neuötting); L. Hauk‐Westerhoff, and M. Michaelis (Rostock); S. Mastri (Berlin); J. Osterhage, E. Becker, K. Tiel‐Wilck, and R. Puzich (Berlin); B. Brockmeier, and C. Schrey (Berlin); R. Ehret (Berlin); A. Wiborg, and B. Kramer (Neu‐Ulm); U. Kirchhöfer, and U. Bahr (Erfurt); G. Roth (Ostfildern), M. Seiler (Aalen); U. Kausch (Bogen); J. Kirchmeier (Schwäbisch Gmünd); L. Dieterle, W. Maier‐Janson, and J. Kunz (Ravensburg); C. Bischoff, C. Neudert, W. Scheuerer, and V. Arbusow (München); R. Hartmann, and C. Wolf (Eltville); K. Gehring, and D. Krause (Itzehoe); P. Emrich, J. Vogt, and C. Müller‐Habich (Hamburg); K. Tinschert, and B. Schwandt (Jena); M. Freidel (Kaltenkirchen); C. Rieth, J. Saur, and R. Pfister (Neusäß). We thank David P. Yates for proofreading the manuscript.
Metze C, Winkelmann A, Loebermann M, et al. Immunogenicity and predictors of response to a single dose trivalent seasonal influenza vaccine in multiple sclerosis patients receiving disease‐modifying therapies. CNS Neurosci Ther. 2019;25:245–254. 10.1111/cns.13034
REFERENCES
- 1. Zettl UK, Stüve O, Patejdl R. Immune‐mediated CNS diseases: a review on nosological classification and clinical features. Autoimmun Rev. 2012;11(3):167‐173. [DOI] [PubMed] [Google Scholar]
- 2. Hardy TA, Reddel SW, Barnett MH, Palace J, Lucchinetti CF, Weinshenker BG. Atypical inflammatory demyelinating syndromes of the CNS. Lancet Neurol. 2016;15(9):967‐981. [DOI] [PubMed] [Google Scholar]
- 3. Lassmann H. Pathology and disease mechanisms in different stages of multiple sclerosis. J Neurol Sci. 2013;333(1–2):1‐4. [DOI] [PubMed] [Google Scholar]
- 4. Hemmer B, Kerschensteiner M, Korn T. Role of the innate and adaptive immune responses in the course of multiple sclerosis. Lancet Neurol. 2015;14(4):406‐419. [DOI] [PubMed] [Google Scholar]
- 5. Confavreux C, Vukusic S. The clinical course of multiple sclerosis. Handb Clin Neurol. 2014;122:343‐369. [DOI] [PubMed] [Google Scholar]
- 6. Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502‐1517. [DOI] [PubMed] [Google Scholar]
- 7. Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wingerchuk DM, Weinshenker BG. Disease modifying therapies for relapsing multiple sclerosis. BMJ. 2016;354:i3518–i3616. [DOI] [PubMed] [Google Scholar]
- 9. Winkelmann A, Loebermann M, Reisinger EC, Hartung H‐P, Zettl UK. Disease‐modifying therapies and infectious risks in multiple sclerosis. Nat Rev Neurol. 2016;12(4):217‐233. [DOI] [PubMed] [Google Scholar]
- 10. Buljevac D, Flach HZ, Hop W, et al. Prospective study on the relationship between infections and multiple sclerosis exacerbations. Brain. 2002;125(Pt 5):952‐960. [DOI] [PubMed] [Google Scholar]
- 11. Nelson RE, Xie Y, DuVall SL, et al. Multiple Sclerosis and Risk of Infection‐Related Hospitalization and Death in US Veterans. Int J MS Care. 2015;17(5):221‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Montgomery S, Hillert J, Bahmanyar S. Hospital admission due to infections in multiple sclerosis patients. Eur J Neurol. 2013;20(8):1153‐1160. [DOI] [PubMed] [Google Scholar]
- 13. Pellegrino P, Carnovale C, Perrone V, et al. Efficacy of vaccination against influenza in patients with multiple sclerosis: the role of concomitant therapies. Vaccine. 2014;32(37):4730‐4735. [DOI] [PubMed] [Google Scholar]
- 14. Miller AE, Morgante L, Buchwald LY, et al. A multicenter, randomized, double‐blind, placebo‐controlled trial of influenza immunization in multiple sclerosis. Neurology. 1997;48(2):312‐314. [DOI] [PubMed] [Google Scholar]
- 15. Confavreux C, Suissa S, Saddier P, Bourdès V, Vukusic S. Vaccines in multiple sclerosis study group. vaccinations and the risk of relapse in multiple sclerosis. N Engl J Med. 2001;344(5):319‐326. [DOI] [PubMed] [Google Scholar]
- 16. Loebermann M, Winkelmann A, Hartung H‐P, Hengel H, Reisinger EC, Zettl UK. Vaccination against infection in patients with multiple sclerosis. Nat Rev Neurol. 2011;8(3):143‐151. [DOI] [PubMed] [Google Scholar]
- 17. Mailand MT, Frederiksen JL. Vaccines and multiple sclerosis: a systematic review. J Neurol. 2016;264(6):1035‐1050. [DOI] [PubMed] [Google Scholar]
- 18. Farez MF, Correale J. Immunizations and risk of multiple sclerosis: systematic review and meta‐analysis. J Neurol. 2011;258(7):1197‐1206. [DOI] [PubMed] [Google Scholar]
- 19. McNicholas N, Chataway J. Relapse risk in patients with multiple sclerosis after H1N1 vaccination, with or without seasonal influenza vaccination. J Neurol. 2011;258(8):1545‐1547. [DOI] [PubMed] [Google Scholar]
- 20. Farez MF, Ysrraelit MC, Fiol M, Correale J. H1N1 vaccination does not increase risk of relapse in multiple sclerosis: a self‐controlled case‐series study. Mult Scler. 2012;18(2):254‐256. [DOI] [PubMed] [Google Scholar]
- 21. Auriel E, Gadoth A, Regev K, Karni A. Seasonal and H1N1v influenza vaccines in MS: safety and compliance. J Neurol Sci. 2012;314(1–2):102‐103. [DOI] [PubMed] [Google Scholar]
- 22. Bardage C, Persson I, Ortqvist A, Bergman U, Ludvigsson JF, Granath F. Neurological and autoimmune disorders after vaccination against pandemic influenza A (H1N1) with a monovalent adjuvanted vaccine: population based cohort study in Stockholm. Sweden. BMJ. 2011;343:d5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Williams SE, Pahud BA, Vellozzi C, et al. Causality assessment of serious neurologic adverse events following 2009 H1N1 vaccination. Vaccine. 2011;29(46):8302‐8308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee GM, Greene SK, Weintraub ES, et al. H1N1 and seasonal influenza vaccine safety in the vaccine safety datalink project. Am J Prev Med. 2011;41(2):121‐128. [DOI] [PubMed] [Google Scholar]
- 25. Robert‐Koch‐Institut . Empfehlungen der Ständigen Impfkommission (STIKO) am Robert Koch‐Institut – 2016/2017. August 2016. Epidemiol Bull. 2016;34:301‐338. [Google Scholar]
- 26. Schwid SR, Decker MD, Lopez‐Bresnahan M, Rebif‐Influenza Vaccine Study Investigators . Immune response to influenza vaccine is maintained in patients with multiple sclerosis receiving interferon beta‐1a. Neurology. 2005;65(12):1964‐1966. [DOI] [PubMed] [Google Scholar]
- 27. Bar‐Or A, Freedman MS, Kremenchutzky M, et al. Teriflunomide effect on immune response to influenza vaccine in patients with multiple sclerosis. Neurology. 2013;81(6):552‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mehling M, Fritz S, Hafner P, et al. Preserved antigen‐specific immune response in patients with multiple sclerosis responding to IFNβ‐therapy. PLoS ONE. 2013;8(11):e78532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Olberg HK, Cox RJ, Nostbakken JK, Aarseth JH, Vedeler CA, Myhr K‐M. Immunotherapies influence the influenza vaccination response in multiple sclerosis patients: an explorative study. Mult Scler. 2014;20(8):1074‐1080. [DOI] [PubMed] [Google Scholar]
- 30. Kappos L, Mehling M, Arroyo R, et al. Randomized trial of vaccination in fingolimod‐treated patients with multiple sclerosis. Neurology. 2015;84(9):872‐879. [DOI] [PubMed] [Google Scholar]
- 31. Lin YC, Winokur P, Blake A, et al. Patients with MS under daclizumab therapy mount normal immune responses to influenza vaccination. Neurol Neuroimmunol Neuroinflamm. 2016;3(1):e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Olberg HK, Eide GE, Cox RJ, et al. Antibody response to seasonal influenza vaccination in patients with multiple sclerosis receiving immunomodulatory therapy. Eur J Neurol. 2018;25(3):527‐534. [DOI] [PubMed] [Google Scholar]
- 33. Recommended viruses for influenza vaccines for use in the 2010–2011 northern hemisphere influenza season. Wkly Epidemiol Rec. 2010;85(10):81‐92. [PubMed] [Google Scholar]
- 34. Recommended composition of influenza virus vaccines for use in the 2011–2012 northern hemisphere influenza season. Wkly Epidemiol Rec. 2011;86(10):86‐90. [PubMed] [Google Scholar]
- 35. Meyer S, Adam M, Schweiger B, et al. Antibody response after a single dose of an AS03‐adjuvanted split‐virion influenza A (H1N1) vaccine in heart transplant recipients. Transplantation. 2011;91(9):1031‐1035. [DOI] [PubMed] [Google Scholar]
- 36. Committee for proprietary medicinal products (CPMP) . Note for guidance on harmonisation of requirements for Influenza vaccines; 1997. http://www.ema.europa.eu/docs/en_GB/document_library/ Scientific_guideline/2009/09/WC500003945.pdf. Accessed March 17, 2018.
- 37. Vågberg M, Kumlin U, Svenningsson A. Humoral immune response to influenza vaccine in natalizumab‐treated MS patients. Neurol Res. 2012;34(7):730‐733. [DOI] [PubMed] [Google Scholar]
- 38. Pawelec G. Age and immunity: what is "immunosenescence"? Exp Gerontol. 2018;105:4‐9. [DOI] [PubMed] [Google Scholar]
- 39. Loebermann M, Anders G, Brestrich G, et al. Safety and immunogenicity of a trivalent single dose seasonal influenza vaccine containing pandemic A(H1N1) antigen in younger and elderly subjects: A phase III open‐label single‐arm study. Vaccine. 2011;29(6):1228‐1234. [DOI] [PubMed] [Google Scholar]
- 40. Koch‐Henriksen N, Sørensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 2010;9(5):520‐532. [DOI] [PubMed] [Google Scholar]
- 41. Klein SL, Pekosz A. Sex‐based biology and the rational design of influenza vaccination strategies. J Infect Dis. 2014;209(suppl 3):S114‐S119. [DOI] [PMC free article] [PubMed] [Google Scholar]


