Abstract
Cancer is a global burden. In low- and middle-income countries around 70% of deaths are due to cancer. For a number of years natural products have been a good source of agents for combatting cancer and plants have played a huge role in anti-cancer product development. For many centuries, indigenous cultures around the world have used traditional herbal medicine to treat a myriad of diseases including cancer. In Sri Lanka, a number of plants have been reported to have anti-cancer properties and some of the commonly used plants are described in this review with an account of their compounds and modes of action. Only a small number of the plants in Sri Lanka have been tested for their bioactivity and more research is required to determine their medicinal activity with the aim of developing novel drugs to fight this disease.
Keywords: Plants with anti-cancer properties, Traditional medicine, Cancer
1. Introduction
Currently, one in six deaths are due to cancer worldwide and around 70% of deaths from cancer occur in low- and middle-income countries (Organization, 2018). This could be due to behavioural and dietary risks such as physical inactivity, smoking, use of alcohol and having an unhealthy diet low in fruit and vegetables. Additional factors contributing to the high incidence rate are an aging population, and exposure to certain chemicals, metals and infectious agents (Jemal et al., 2011, Iqbal et al., 2017). This makes cancer an important health problem which requires effective prevention and treatment measures.
Among natural products plants have played a key role in treating a number of diseases including cancer. The sheer variety and number of plants with medicinal properties around the world is quite astonishing. It is estimated that around 70,000 plant species, from lichens to towering trees, have been used at one time or another for medicinal purposes. Ancient cultures respected the curative powers of healing plants, illustrated by findings from the excavation of a 60,000 year old burial site in Iraq. Eight different medicinal plants were found at this site; the inclusion of the plants in the tomb suggests that they had supernatural significance and medicinal value (Pan et al., 2013). Excavations in Sri Lanka have shown that the Balangoda man used plants for medicinal purposes, about 30,000 years ago (Perera, 2004). Extensive investigations around the globe have revealed that medicinal plants were used by humans in the prehistoric era and that crude extracts or pure molecules isolated from medicinal plants represent the most ancient mode of medications.
Plant-derived agents have played a vital role in the treatment of cancer. Hartwell, in his publications reported over 3000 species that possess anti-cancer properties (Graham et al., 2000). The search for anti-cancer agents from plants sources started in the early 1950s and over the years medicinal plants have been exploited as an initial point for the synthesis of new compounds for cancer with different structural parameters in the synthetic, combinatorial and biotechnological sciences. Over 60% of currently used anti-cancer agents are derived from natural sources such as plants (Khan, 2014, Cragg and Newman, 2005). The curative properties originate in different parts of these plants, due to the presence of an array of low-molecular-mass substances known as secondary metabolites. These secondary metabolites are distinct from the components of primary metabolism, as they are not involved in the general metabolism but are used against microbial attacks or animal predation (Dixon, 2001, Prajapati et al., 2007). Examples of secondary metabolites in plants are flavonoids and phenolics, terpenoids, alkaloids and sulphur-containing compounds. Related plant species may produce related chemicals, for instance compounds that have anti-mutagenic and anti-cancer properties may play significant roles in either inhibiting or activating signal transduction pathways in living cells (Verpoorte, 1998).
2. Plant-derived anti-cancer agents in clinical use
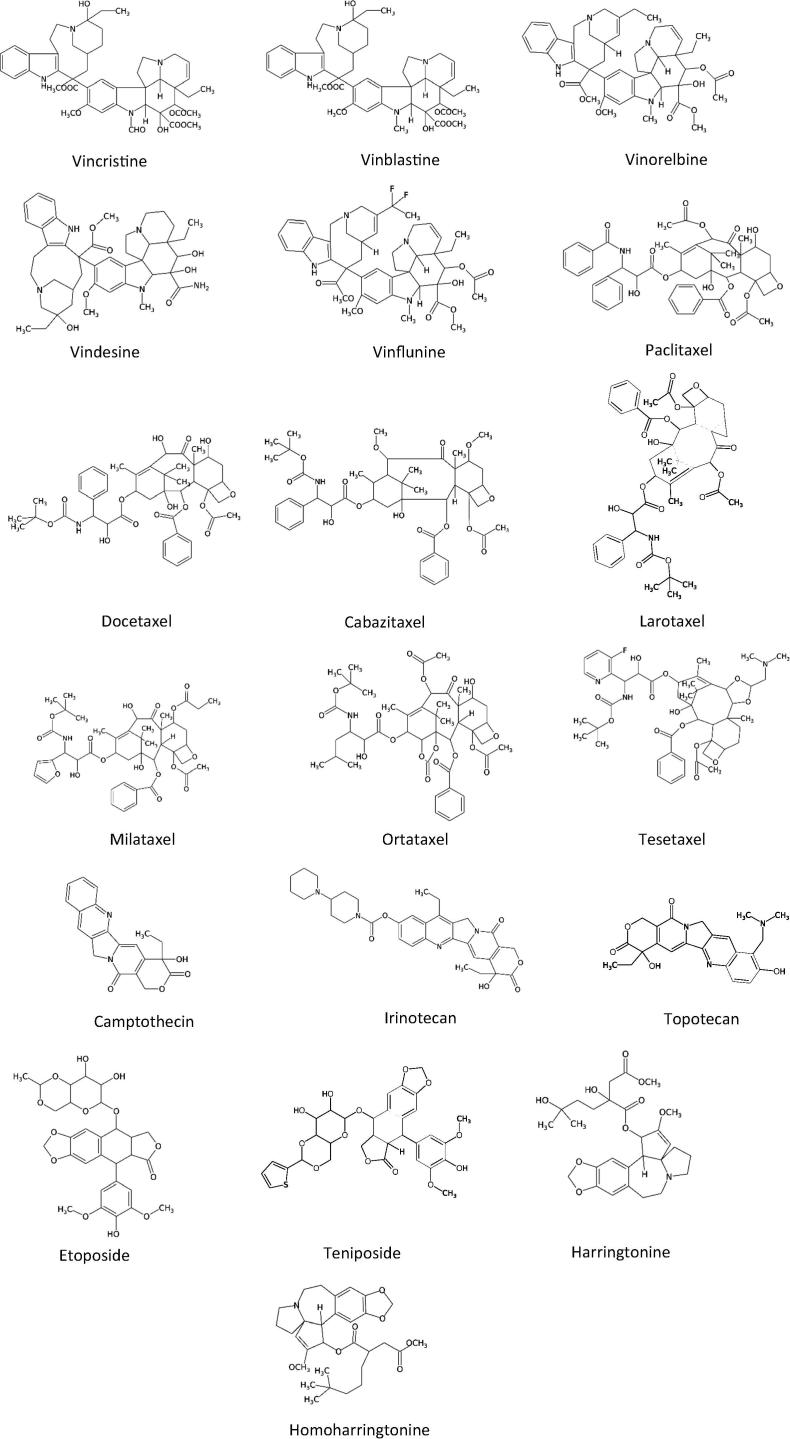
Vinca alkaloids, are one of oldest class of agent used to treat cancer and are the second-most commonly used agents in the clinic. They were first developed in the 1950s by Canadian scientists Robert Noble and Charles Beer. These alkaloids were isolated from Catharanthus roseus (Apocynaceae) and were given to patients with breast cancer, Hodgkin’s lymphoma, leukaemia, testicular cancer and lung cancer (Cragg and Newman, 2005, Mann, 2002). The two main vinca alkaloids are vincristine and vinblastine and a few structural analogues such as vinorelbine, vindesine and vinflunine (Fig. 1) have been developed (Iqbal et al., 2017, Mann, 2002). Vinblastine, vincristine and vinorelbine are approved for use in the USA and vinflunine was approved in 2008 in Europe. The main mechanism of action of these agents is that they bind to tubulin and disrupt the function of microtubules, particularly those comprising the mitotic spindle apparatus, by arresting metaphase of the cell cycle (Moudi et al., 2013).
Fig. 1.
Chemical structures of anti-cancer drugs derived from plants that are in clinical use.
Another important class of anti-cancer agents are the taxanes. Paclitaxel (Taxol) (Fig. 1), derived from extracts of the Pacific yew tree Taxus brevifolia, is given to patients with breast, ovarian, lung, head and neck, oesophageal, prostate and bladder cancers. This compound acts by binding to microtubules and enhancing tubulin polymerization, leading to microtubule stabilization, cell cycle arrest and aberrant mitosis. Docetaxel (Fig. 1) is a semisynthetic taxane, derived from extracts of European yew tree Taxus baccata. This agent is similar to paclitaxel with a related mechanism of action, but better solubility in water. Docetaxel is effective in breast, ovarian, head and neck, lung, gastric and bladder cancers (Fu et al., 2009). Several different analogues of taxanes have been clinically evaluated. A nanoparticle albumin-bound form of paclitaxel (abraxane), having greatly reduced systemic toxicity, was approved in 2005 for the treatment of metastatic breast cancer. Another analogue, cabazitaxel (Fig. 1), was approved in 2010 for the treatment of metastatic prostate cancer. Further, larotaxel, milataxel, ortataxel, and tesetaxel (Fig. 1) are currently under clinical evaluation (Iqbal et al., 2017, Yared and Tkaczuk, 2012, Ojima et al., 2016).
Camptothecin (Fig. 1) a quinoline alkaloid, was obtained in 1966 from the stem of Camptotheca acuminate, a Chinese ornamental tree. It binds to topoisomerase I, allowing DNA cleavage but inhibiting subsequent ligation which results in DNA strand breaks. This agent proved unsuccessful in the clinic due to severe bladder toxicity but extensive research led to the discoveries of irinotecan and topotecan (Fig. 1) which are derivatives of camptothecin. Irinotecan is approved for treating colorectal cancer and topotecan is approved for the treatment of ovarian, cervical and small cell lung cancers (Cragg and Newman, 2005, Liu et al., 2006).
Etoposide, a semisynthetic derivative of Podophyllum peltatum, was first synthesized in 1966 and was approved for cancer therapy in 1983. This agent targets topoisomerase II and forms a complex with topoisomerase II and DNA. The complex induces breaks in double-stranded DNA and prevents repair by topoisomerase II binding. Etoposide is used to treat Hodgkin’s and non-Hodgkin’s lymphomas, lung, gastric, breast, and testicular cancers (Montecucco et al., 2015, Hande, 1998). A number of novel etoposide derivatives are undergoing clinical evaluation (Zhang et al., 2017, Gentry et al., 2011). Teniposide, another semisynthetic derivative of Podophyllum peltatum, has a similar mechanism of action to etoposide but is insoluble in water (Clark and Slevin, 1987).
Harringtonine and Homoharringtonine (Fig. 1) are also plant-derived agents with anti-leukaemic properties. They are isolated from Cephalotaxus harringtonia (harringtonine was first isolated in 1963). These agents inhibit protein translation by preventing the initial elongation step of protein synthesis via an interaction with the ribosomal A-site. A racemic mixture of these two agents has been given to patients with acute myelogenous leukaemia and chronic myelogenous leukaemia in China and in 2012 homoharringtonine was approved in USA for the treatment of chronic myeloid leukaemia (Cragg and Newman, 2005, Moirangthem et al., 2014, Lü and Wang, 2014).
3. Sri Lankan plants that have anti-cancer properties
Sri Lanka has a rich history of traditional medicine practice dating back many centuries. This practice is a mixture of the Sri Lankan indigenous medicine system “deshiya chikitsa” ayurveda and siddha systems, introduced by India, and the unani system that originated in Greece and introduced by the Arabs to Sri Lanka. Currently, around 70% of the population of the country uses this traditional medicine system (Jayasinghe et al., 2017), which is popular for treating tumours both benign and malignant. The Sri Lankan herbal preparations or traditional medicine formulae used against cancer typically contain extracts from more than one plant species; very rarely is an extract of just a single plant is given to patients having cancer, unless it is very potent. A single plant may contain one or more active compounds, working together synergistically with compounds of other plants to offer a combinatorial approach that would deliver an enhanced therapeutic effect. This combinatorial approach may also overcome resistance by reducing the activity of cross talk of signalling pathways activated in cancer. Sri Lanka which is a biologically diverse country, is home to around 1430 medicinal plants (Russell-Smitha et al., 2006), giving rise to numerous herbal preparations for cancer. The primary mode of administration of such preparations is oral. Various parts of the plant (leaves, bark, stem, roots, flowers, seeds) are used in these medicinal preparations. Further, a specific plant may have various morphotypes in different localities in the country, where one particular type would be widely used therapeutically (Dharmadasa et al., 2012). Ten terrestrial plant species commonly found in anti-cancer herbal preparations of the traditional medicine system of Sri Lanka are described below.
3.1. Zingiber officinale
Zingiber officinale (belongs to the family Zingiberaceae), commonly known as ginger or "inguru" (in Sinhalese), or "ingi" (in Tamil), is widely used in anti-cancer traditional medicine preparations for gastrointestinal, liver and oesophageal cancers (Singh, 2007). The rhizome is used in medicinal preparations and it is mostly used in poly herbal preparations. It is commonly used as a culinary ingredient in Sri Lankan cuisine (Williamson, 2002, Resorts, 2017). Many active ingredients are present in this plant, including gingerols, which are converted to shogaols, paradols and zingerone (Rahmani et al., 2014). Zingiber officinale exerts its anti-cancer effects via multiple pathways. For instance, an extract of Zingiber officinale was found to significantly reduce the expression of NFĸB through suppression of proinflammatory TNFα in liver cancer induced rats (Habib et al., 2008). NFĸB is a transcription factor that plays a role in biological processes such as inflammation, cell growth and survival, and TNFα is a cytokine which exerts its biological functions through activating NFĸB (Lin, 2008). Another study showed that when gingerol was given to male F344 rats at a concentration of 0.02% for around 3 weeks, azoxymethane-induced intestinal cancer was significantly suppressed (Singh, 2007). Research has suggested that gingerol in ginger could be an effective chemo-preventive and/or chemotherapeutic agent for colorectal cancers. For example, ginger showed significant efficacy in mice when they were fed with ginger before and after tumour cells were injected, and also when ginger was fed after tumours had grown to a particular size (Singh, 2007). Gingerol, has demonstrated anti-angiogenic activity in vitro and in vivo, suggesting that it might have a possible role in preventing metastasis as well (Kim et al., 2005). Other than for cancer, Zingiber officinale is often used as an antibacterial agent for infections, it is also used for nausea and diarrhoea, loss of appetite, and for inflammation (Resorts, 2017).
3.2. Curcuma longa
The rhizome of Cucurma longa (Zingiberaceae) is commonly used in poly-herbal preparations, and is used to treat a wide range of cancers. This plant, known as turmeric or "kaha" in the Sinhalese langauge and "manchal" in Tamil. It is regularly used as a spice and flavouring agent in Sri Lankan cuisine (Williamson, 2002, Resorts, 2017). Its major active chemical constituent is curcumin. Scientific evidence demonstrates that this plant is able to inhibit variety of cancers such as colon, hepatocellular, breast, renal, prostate cancers, T cell leukaemia, and B cell lymphoma (Aggarwal et al., 2003). According to reports the mechanisms by which curcumin exerts its bioactivity are anti-oxidation, downregulation of the COX-2 enzyme and by decereasing levels of DNA adducts. One study showed that curcumin is able to inhibit, colo 205 adenocarcinoma (colon cancer) cells and induce apoptosis via caspase-3 activity (Chin-Cheng et al., 2006). The same study showed that curcumin was able to increase the levels of reactive oxygen species and Ca2+; leading to apoptosis of cells. Another study found that a mixture of Curcuma longa, Zingiber officinale and Allium sativum together with tamoxifen induced apoptosis in oestrogen receptor positive MCF-7 and ZR-75 breast cancer cell lines; tamoxifen is an oestrogen antagonist in breast cancer (Vemuri et al., 2017). Garcea et al. investigated curcumin levels in the colon and rectum in 12 patients with colorectal cancer at different stages, where patients had been assigned to varying doses with the highest being 3.6 g of curcumin per day for 7 days prior to surgery. Administration of capsules containing 3.6 g of curcumin decreased M1G levels from 4.8 ± 2.9 adducts per 107 nucleotides in malignant colorectal tissue to 2.0 ± 1.8 adducts per 107 nucleotides (Bar-Sela et al., 2010, Garcea et al., 2005). DNA damage is important in the aetiology of many cancers and damage may be reflected by exocyclic DNA adducts such as M1G (Sharma et al., 2001). Further, Curcuma longa has been clinically evaluated as a chemo-preventive agent, especially for colorectal cancer, and this effect may be due to its ability to compete with aryl hydrocarbons for both AhR and CYP1A1 sites. Activation of AhR results in the induction of CYP1A1 gene, which encodes for enzymes that metabolizes human carcinogens which results in cancer initiation (Aggarwal et al., 2003). In another study, histological improvement of premalignant lesions of various cancers such as intestinal metaplasia of the stomach, superficial bladder carcinoma, uterine cervical intraepithilial neoplasia was observed when 1–8 g of curcumin were given daily to patients for 3 months (Bar-Sela et al., 2010). Other than its anti-cancer uses, Curcuma longa is commonly used as an antibacterial agent and for diabetes mellitus, wounds and skin diseases (Resorts, 2017).
3.3. Hemidesmus indicus
Hemidesmus indicus (Apocynaceae), commonly known as Indian sarsaparilla or "iramusu" in Sinhalese and "nannari" in Tamil, is used in poly-herbal preparations aimed against cancer; the root is mostly used in traditional medicine preparations (Turrini et al., 2018). The leaves are used to make gruel or herbal drinks in Sri Lanka. This plant is mostly given for liver, uterine and breast cancers and leukaemia. The root of this plant contains ledol, nerolidol, caryophyllene, camphor, borneol, dehydrolupanyl-3 acetate, dehydrolupeol acetate, lupeol, dodecanoic acid, hexadecanoic acid, hemidesminin, hemidesmin-1 and 2 compounds; out of the above compounds, caryophyllene has shown to have anti-cancer properties in literature and this could be one of the compounds responsible for the cytotoxic properties of Hemidesmus indicus (Rajan et al., 2011, Nagat et al., 2016, Das and Singh Bisht, 2013, Gao et al., 2018). Decoctions of Hemidesmus indicus have demonstrated a cytotoxic effect in HepG2 hepatocellular carcinoma cells (IC50 33.52 ± 0.13 μg/ml) while a hydroalcoholic extract has shown activity against HepG2 (IC50 34.50 ± 0.14 μg/ml), LoVo (colon cancer) (IC50 29.84 ± 0.24 μg/ml), and Jurkat (leukaemia) (IC50 63.79 ± 7.97 μg/ml) cell lines (Statti et al., 2015). A decoction of Hemidesmus indicus was shown to induce an immunogenic type of cell death in DLD1 (colon cancer) cells, stimulating an upregulation of CD83 that caused dendritic cell maturation. Thus, this plant has the potential to act as an adjuvant agent by enhancing an immunogenic response in cancer (Turrini et al., 2018). In other studies, a poly-herbal formulation of Nigella sativa seeds, Hemidesmus indicus roots and Smilax glabra rhizomes induced apoptosis in HepG2 cells through activation of caspases 3 and 9 (Ira Thabrew et al., 2005, Samarakoon et al., 2012). A group of reseachers reported that long-term treatment (around 16 months) with this poly-herbal decoction inhibited diethylnitrosamine-induced glutathione S-transferase P expression in rat liver. Further, the same decoction inhibited carcinogen-mediated development of overt tumours along with histopathological changes that were leading to tumour development, possibly be due to a significant reduction of angiogenesis that was observed (Iddamaldeniya et al., 2006). Galhena et al, demonstrated that the above decoction can protect against the cytogenetic damage mediated by bleomycin in human peripheral blood lymphocytes compared with cells that were treated only with bleomycin. Bleomycin is an anti-cancer drug which is shown to mediate DNA double-strand breaks in both cancer cells and normal cells. Thus, this decoction of Hemidesmus indicus could be used in cancer management (Galhena et al., 2017). Hemidesmus indicus is also used as a blood purifier and for skin diseases and gastric aliments (Resorts, 2017, Statti et al., 2015).
3.4. Munronia pinnata
Munronia pinnata, a member of the Meliacea family, is used in poly-herbal formulations and is also given as a single extract to cancer patients. In Sri Lanka this plant is commonly known as "bin kohomba" in Sinhalese (Dharmadasa et al., 2012), and is mostly given for lung and brain cancers. Leaves, roots or the whole plant are used for medicinal purposes. The demand for this herb is very high in Sri Lanka and as a result it is very expensive to obtain. There is also a threat of extinction of Munronia pinnata due to insufficient supply to meet the high demand. Thus, conservation of this rare and valuable plant is important (Napagoda et al., 2014). A study has revealed that this plant contains β-caryophyllene, caryophyllene oxide and ganoderiol F as active compounds. There is limited research conducted on the anti-cancer properties of Munronia pinnata. However, the compounds found in this plant have demonstrated cytotoxic activity in various studies and might be responsible for the plant’s anti-cancer properties (Napagoda et al., 2014). For instance, ganoderiol F has shown remarkable inhibitory effects against LLC (mouse Lewis lung cancer), T47D (breast cancer), S-180 and Meth-A (mouse sarcoma) cell lines. The same compound demonstrated remarkable inhibitory effect on the growth of Lewis lung carcinoma tumours in mice (Gao et al., 2006). Further, β-caryophyllene, has been shown to inhibit HCT-116 and HT-29 (colon cancer) cell lines and PANC-1 (pancreatic cancer) cells. Caryophyllene oxide has been shown to inhibit SNU-1, SNU-16 (stomach cancer) cell lines and Hela (cervical cancer) cells. These compounds may exert their action by inhibiting PI3K/AKT/mTOR and STAT3 signalling pathways which are responsible in cell survival and proliferation of cancer cells (Fidyt et al., 2016). Further studies have shown that these compounds are able to enhance the efficacy of paclitaxel and doxorubicin. For instance, researchers have found that β-caryophyllene enhanced the activity of paclitaxel in MCF-7, DLD-1 and L-929 (murine fibroblast) cells by about 10-fold, and the most potent results were observed in DLD-1 cells: paclitaxel IC50 0.43 ± 0.09 μg/ml, and β-caryophyllene + paclitaxel IC50 0.04 ± 0.01 μg/ml (Fidyt et al., 2016). This plant is also used to treat inflammation, malaria and haemorrhoids (Dharmadasa et al., 2012, Resorts, 2017).
3.5. Smilax zeylanica
Smilax zeylanica (Smilacaceae), commonly known as kumarika in English and "kabarossa” in Sinhalese, is used as a single agent and also as an ingredient of poly-herbal formulations (Resorts, 2017). It is used to treat a wide variety of cancers in Sri Lanka and mainly the root is used for medicinal purposes. Smilax zeylanica root contains diosgenin, smilagenin and β-sitosterol as active compounds (Murali et al., 2011). A study performed in male swiss albino mice showed that Smilax zeylanica leaf extract (400 mg/kg daily) suppressed benzo[a]pyrene-induced lung carcinoma by decreasing the number of nodules in the lung (1.33 ± 0.22 nodules in treated mice compared with 12.50 ± 1.23 in untreated animals; p < 0.001), accompanied by significant weight gain during the experimental period (Rajesh and Perumal, 2013). In another study, a petroleum ether extract of the stem demonstrated an IC50 value of 15.49 ± 1.18 μg/ml, close to the IC50 of the positive control tamoxifen (IC50 5.31 ± 0.38 μg/ml), in MCF-7 cancer cells illustrating strong cytotoxic potential (Uddin et al., 2015). Interestingly reports have illustrated that diosgenin which is a compound of this plant has a unique structural similarity to oestrogen and is able to inhibit proliferation of MCF-7 and MDA-MB 231 (breast cancer) cells by upregulation of p53 tumour suppressor gene and down regulation of Bcl2 which promotes cell survival (Sethi et al., 2018). Furthermore, the cytotoxicity of this plant could also be due to its high anti-oxidant activity as it contains a good amount of phenolics, flavonoids and tannins. It has been shown that anti-oxidants are able to protect living cells from DNA damage and lipid peroxidation caused by reactive oxygen species which could initiate cancer (Uddin et al., 2015). As an illustration, a methanol extract of the root had an IC50 of 3.00 ± 0.03 μg/ml, which was better than the anti-oxidant activity of ascorbic acid (standard) IC50 4.25 ± 0.29 μg/ml (Murali et al., 2011). However, more research is warranted to elucidate the actual mechanism of action of this plant (Murali et al., 2011, Uddin et al., 2015). Smilax zeylanica is also used to treat abscesses, wounds, inflammation, epilepsy and skin disorders (Rajesh and Perumal, 2013).
3.6. Tinospora cordifolia
The stem of Tinospora cordifolia which belongs to the family Menispermaceae is used in medicinal preparations for cancer. Commonly known as heart-leaved moonseed (in English) or “rasakinda” (in Sinhalese) / "chintil" (in Tamil) (Resorts, 2017). It is mostly used in poly-herbal formulations and is frequently given to leukaemia patients in Sri Lanka. The active compounds of this plant are berberine, choline, tembetarine, tetrahydropalmatine, β-sitosterol, giloinsterol, furanolactone, 18-norclerodane glucoside, tinosporin, palmatine, magnoflorine, tinocordiside and cordifolioside A (Saha and Ghosh, 2012, Sinha et al., 2004, Patel et al., 2013). A 50% ethanolic extract of Tinospora cordifolia stem was found to reduce cell proliferation in C6 rat glioma cells (at 350 µg/ml), accompanied by senescence. The extract also showed anti-migratory and anti-invasive potential, with downregulation of the neural cell adhesion molecule. Further, the extract was able to inhibit cell cycle progression in C6 cells in gap1 and gap2/mitosis phases of the cell cycle, probably due to suppression of cyclin D1, and induce apoptosis in treated cells by reducing expression of anti-apoptotic protein Bcl-xL (Mishra and Kaur, 2013). Another study has shown that this plant has a radioprotective role, which could be due to cordifolioside A, a compound found in this plant that has shown in vivo radioprotective effects (Patel et al., 2013). Rao et, al, demonstrated that treatment of Hela cancer cells with ∼1 µg/ml Tinospora cordifolia dichloromethane extract before exposure to 2 Gy γ-radiation caused a significant decline in cell viability compared with irradiation of Hela cells with different doses of γ-radiation only. Further, treatment of Hela cells with various concentrations of the plant extract caused a significant decline in cell viability after exposure to 1–4 Gy γ-radiation, demonstrating that the cytotoxicity effect of γ-radiation was increased (Rao and Rao, 2010). Tinospora cordifolia has subdued diethylnitrosamine-induced hepatocellular carcinoma in male Wistar albino rats by increasing anti-oxidant activity via superoxide dismutase and catalase enzymes; the activity of hepatic markers such as serum glutamic oxaloacetic transaminase and serum glutamic pyruvate transaminase enzymes reverted to normal levels, confirming the plant’s hepatoprotective properties (Dhanasekaran et al., 2009). Other than in cancer, this plant is used in Sri Lankan traditional medicine practice in a variety of diseases such as jaundice, skin diseases, fever, malaria, chronic diarrhoea, diabetes mellitus, snake bites, inflammation and dysentery (Resorts, 2017, Saha and Ghosh, 2012).
3.7. Adenanthera pavonina
Adenanthera pavonina, is known as red lucky seed in English or “madatiya” in Sinhalese / "anaikuntumani" in Tamil (Resorts, 2017). This plant is used as a single agent and also in poly-herbal formulations and the bark is used mainly in medicinal preparations. This member of the Leguminosae family is mostly given for leukaemia and lymphoma. A number of active compounds have been isolated from this plant, including arginine, cysteine, arachidonic acid, β-sitosterol, dulcitol, echinocystic acid, glutamic acid, lignoceric acid, oxalic acid, malonic acid and octacosanol (Mujahid et al., 2016, Bhadran et al., 2017). Researchers have reported that a combination of equal proportions of Adenanthera pavonina and Thespesia populnea bark in the form of a decoction showed anti-proliferative effects (inhibition of cell survival: 78.54% at 160 µg/ml of decoction); with the induction of apoptosis in HEp-2 cells (cervical cancer), and that the same combination showed high anti-oxidant activity as well (Lindamulage and Soysa, 2016, Silva and Soysa, 2011). Another study showed that an ethanolic extract of Adenanthera pavonina seeds inhibited cell growth in HCT-8 (colon cancer) and HL-60 (leukaemia) cell lines (Ferreira et al., 2011). Further studies have shown that a methanol stem extract of Adenanthera pavonina caused cytotoxic activity in Hela cells (IC50 39.89 ± 0.11 μg/ml) and HCT-116 cells (IC50 25.86 ± 0.21 μg/ml). These investigations have shown that this plant has high anti-oxidant activity and acts as a free radical scavenger. Studies have also shown that this plant is able to arrest the gap2/mitosis phase in the cell cycle in cancer cells along with apoptotic induction (Silva and Soysa, 2011, Bhadran et al., 2017). Moreover, Kumar et al, revealed that when male Swiss albino mice were induced with Dalton's ascitic lymphoma, Adenanthera pavonina stem extract (250 mg/kg daily) restored the haemoglobin and red blood cell content to near normal levels, along with a reduction of viable cancer cells compared to control; certain chemotherapy drugs do reduce blood cell count levels, and herbal preparations of this plant may help as an additive in such conditions (Arihara Siva Kumar et al., 2017). Other than for cancer, this plant is used to treat inflammation, bacterial infections, diarrhoea and depression (Resorts, 2017, Mujahid et al., 2016).
3.8. Thespesia populnea
Thespesia populnea (Malvaceae), commonly known as tulip tree or “gansuriya” (Sinhalese) / "kavarachu" (Tamil) (Resorts, 2017), is used as a single agent and in combination with other plants as a ploy herbal formulation in the Sri Lankan traditional medicine practice. It is often given to patients with leukaemia and lymphoma. Some of the active chemical compounds isolated from this plant are mansonone C, D, E, G, H and S, populene A-H, kaempferol, kaempferol 3-glucoside, quercetin, quercetin 3-glucoside, rutin, nonacosane, lupenone, myricyl alcohol, lupeol, β-sitosterol, gossypol and thespone (Phanse et al., 2013, Boonsri et al., 2008). A methanol leaf extract of this plant was found to inhibit B16-F10 melanoma solid tumour development in mice; the tumour volume was reduced (1.46 ± 1.19 mm3) significantly compared with control (2.31 ± 1.26 mm3) treatment (Mika and Guruvayoorappan, 2012). Further, the extract reduced glutathione levels in tumour cells (15.6 ± 0.6 nmol/mg protein to 9.2 ± 0.2 nmol/mg protein) as well as serum γ-glutamyltransferase (γ-glutamyl transpeptidase) levels (142.8 ± 2.3 nmol p-nitroaniline/ml to 52.9 ± 1.2 nmol p-nitroaniline/ml) in tumour-bearing mice, resulting in apoptosis induction. γ-glutamyltransferase is a membrane-bound enzyme involved in the metabolism of glutathione and plays an active role in neoplastic transformation. Glutathione protects cancer cells against free radicals and regulates the sensitivity of cells to radiation and drug-induced cytotoxicity. The same study also showed that treatment with Thespesia populnea considerably increased blood count levels such as white blood cell count and haemoglobin content compared to control animals (Mika and Guruvayoorappan, 2012, Jose et al., 2006, Corti et al., 2010). Another study showed that hexane and chloroform leaf extracts of Thespesia populnea demonstrated anti-proliferative activity against murine lymphoid cancer cells such as Ehrlich ascites (IC50 hexane: 38.94 µg/ml; chloroform: 41.32 µg/ml) and Dalton’s lymphoma ascites (IC50 hexane: 32.85 µg/ml; chloroform:18.55 µg/ml) cells (Chandran et al., 2016). Further, compounds isolated from a dichloromethane stem extract of Thespesia populnea have shown inhibitory activity in cancer cell lines and the highest inhibitory effects were shown by the compound, mansonone E; MCF-7 (IC50 0.05 µg/ml), Hela (IC50 0.55 µg/ml) and HT-29 (IC50 0.18 µg/ml). Further, mansonone D and populene D possessed strong inhibitory activity against MCF-7 and Hela cells respectively, whereas populene C exhibited moderate inhibitory activity against all cell lines tested (Boonsri et al., 2008). Some of the cytotoxic activity of this plant might be due to its high anti-oxidant activity in quenching free radicals, but more research is needed to determine the actual mechanism of action (Silva and Soysa, 2011). This plant is also used for inflammation, piles, boils, ulcers, bacterial infections and diarrhoea (Resorts, 2017, Phanse et al., 2013).
3.9. Phyllanthus emblica
The fruit of Phyllanthus emblica (Phyllanthaceae) is used in medicinal preparations, usually in combination with other herbs. This plant, commonly known as gooseberry in English. It is called “nelli” in Sinhalese or "topu-nelli" in Tamil (Resorts, 2017). This plant is mostly given for throat and lung cancers in Sri Lanka, and is often consumed as a fruit or as fruit juice. Active compounds found in the fruit of this plant include geraniin, isocorilagin, ellagic acid, tannic acid, ascorbic acid, chebulagic acid, gallic acid, corilagin, pyrogallol, quercetin, quercetin 3-β-D-glucopyranoside, kaempferol, and kaempferol 3-β-D-glucopyranoside (Zhao et al., 2014, Liu et al., 2012, De et al., 2013). An aqueous decoction of Phyllanthus emblica fruit has demonstrated inhibitory activity in A549 (lung), HepG2, Hela, MDA-MB 231, SKOV-3 (ovarian) and SW620 (colon) cancer cell lines, and the most potent activity was observed against the Hela cell line (GI50 46.30 ± 6.30 μg/ml). However, the extract was not toxic to MRC-5 (non-transformed lung fibroblast) cells (GI50 > 400 µg/ml). This extract caused induction of caspase 3/7 and caspase 8 and upregulation of Fas protein in Hela cells, resulting in apoptosis by activating the death receptor Fas/caspase-8-dependent apoptosis pathway that may lead to inhibiting the biological activity of NFĸB (Ngamkitidechakul et al., 2010). The anti-tumour promoting activity of the fruit extract was evaluated by a 7,12-dimethylbenz[a]anthracene (DMBA)/12-otetradecanoylphorbol-13-acetate (TPA)-induced skin tumourigenesis mouse model. When mice were treated with DMBA, TPA and the extract (4 mg), both tumour numbers and volumes had significantly reduced to >50% over a 20 week period (p < 0.01). This plant has shown to have high levels of anti-oxidant activity by compounds, such as ellagic acid, gallic acid and tannic acid. These compounds have demonstrated to protect against skin tumour promotion by TPA via inhibition of ornithine decarboxylase activity and hydrogen peroxide production, thus enhancing anti-cancer activity (Ngamkitidechakul et al., 2010). An aqueous solution of Phyllanthus emblica (400 µg/ml) was found to inhibit the growth of OVCAR3 cells (ovarian); the same solution (100 mg/kg daily) inhibited mouse ovarian xenograft tumours significantly reducing the expression of angiogenic gene hypoxia-inducible factor 1α and by autophagy. Further, the Phyllanthus emblica solution (300 µg/ml) has acted synergistically with cisplatin (1–10 µg/ml) (a first-line chemotherapeutic drug given for ovarian cancer) to reduce OVCAR3 cell proliferation (De et al., 2013). This plant is also given for diabetes mellitus, mental disorders, abdominal diseases and skin diseases (Resorts, 2017).
3.10. Boerhavia diffusa
The root of Boerhavia diffusa (Nyctaginaceae), commonly known as hog weed or “pita sudu sarana” in Sinhalese / "karichcharani" in Tamil (Resorts, 2017), is used in traditional Sri Lankan medicine preparations. It is used in poly-herbal formulae and is mainly given for gastric and liver cancers in Sri Lanka. The leaves of this plant are also consumed in Sri Lankan cuisine. Some of the active chemical compounds of this plant are borhaavone, boeravinone A-J, punarnavoside, quercetin, kaempferol, 3,4-dihydroxy-5-methoxycinnamoyl-rhamnoside, β-ecdysone, boeradiffusene, triacont-24-en-1-oic acid and eupalitin-3-O-β-D-galactopyranoside (Mishra et al., 2014). A methanol: chloroform fraction (300 μg/ml) of an ethanolic extract of Boerhavia diffusa root was found to have anti-proliferative effects on Hela cancer cells (85% cell death), with inhibition of the synthesis phase of the cell cycle along with induction of apoptosis by triggering caspase 3/9 (Srivastava et al., 2009). A methanol extract (320 µg/ml) of the whole plant demonstrated anti-proliferative effects in MCF-7 cells (inhibition of 46.8%) with an arrest in gap1 phase in the cell cycle; indicating potential anti-oestrogenic activity of Boerhaavia diffusa against human breast cancer cells (Sreeja, 2009). Administration of an aqueous methanol (3:7) extract of Boerhavia diffusa whole plant was found to be effective in hindering the formation of B16F10 melanoma-induced lung metastases in mice (95% inhibition compared to control), by inhibiting the expression of matrix metalloproteinases 2/9 which are associated with cell invasion and angiogenesis. The treated mice had showed much lower lung collagen hydroxyproline content indicating a reduced fibrosis and a smooth alveolar function. A reduction in the number of lung tumour nodules that are metastatic colonies of melanoma, correlated with the findings. Currently the principal compounds responsible in inhibiting the cascade of event of metastasis are not known. However, the prevention of tumour cell proliferation, which is established from these experiments together with the angiostatic nature of the extract might be contributing to the anti-metastatic property shown by this plant (Leyon et al., 2004). This plant is also used for liver disorders, asthma, skin diseases, snake bites, inflammation and heart diseases (Resorts, 2017, Mishra et al., 2014,).
4. Conclusions
The plants described in this review have a diverse range of medicinal properties including anti-cancer properties. In the Sri Lankan traditional medicine practice, these plants are often used in combination with other plant sources as poly herbal formulae to treat cancer. The combinatorial approach increases the synergetic effect of all plants, improving the effectiveness of the treatment and lessening side effects. Combination therapy also decreases the likelihood of developing resistant cancer cells by targeting multiple signalling pathways often activated in a complex disease such as cancer. Supporting the above statement, some of the plants in Sri Lanka have also shown to have remarkable anti-cancer activity against currently incurable cancers. Therefore, traditional medicine knowledge should be used to discover novel drug leads for cancer. Even though many plants are being used for treatment purposes, there is a lack of scientific evidence to support such use for several of these species. Thus, it is very important that these plants/poly herbal formulae are evaluated in preclinical and clinical studies. Further, utilization of modern biotechnological approaches such as nanotechnology-based drug delivery systems will support the progression of medicinal plant research to its full potential and help to minimize side effects of the drugs developed from these plants. (Wang et al., 2012). Medicinal plants could also possess effective anticancer compounds that may be used as adjuvants to existing chemotherapy to improve efficacy and/or reduce drug-induced toxicity; such as chemotherapy-induced nausea and vomiting to improve patients’ quality of life. Nevertheless, human clinical trials are warranted to verify the clinical utility of these medicinal plants in such treatment, since there could be positive as well as negative outcomes via pharmacodynamic and pharmacokinetic herb-drug ineractions. Furthermore, research has suggested an inverse correlation between human cancers and various dietary constituents. The daily diet should be made up of nutrient rich plant foods, which includes vegetables, fresh fruits, beans, seeds, and whole grains. These foods or nutraceuticals construct a health-promoting, disease-preventing diet with protective substances (Pandey et al., 2013). Sri Lankan cuisine often incorporates gruels, herbal drinks and spices made out of plants; these culinary preparations have enormous health benefits, including chemo-preventive properties, and are effective inhibitors of cancer. Prevention is certainly an attractive cancer management strategy. Thus it might be possible to reduce the process of carcinogenesis with regular use of these plants along with a healthy lifestyle.
Conflicts of interest
None.
Acknowledgments
The authors would like to thank Dr. Nimal Jayathilaka, Ayurveda cancer specialist, Bandaranayaka Memorial Ayurvedic Research Institute, Navinna, Maharagama, Sri Lanka, and Dr. Nishan Jayasundara, Ayurveda physician, Institute of Indigenous Medicine, University of Colombo, Sri Lanka, for the information provided on plants used in the traditional medicine practice in Sri Lanka. The authors also thank Dr. E. J. Laughton for editorial support of the manuscript.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Anchala I. Kuruppu, Email: anchala_k@yahoo.com.
Charitha L. Goonasekara, Email: charithalg@kdu.ac.lk.
References
- Aggarwal Bharat B., Kumar A., Bharti A.C. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- Arihara Siva Kumar G., Javvadi R.K., Vinay Kumar K., Manohar Reddy E., Veera Reddy Y., Harshavardhan G., Akbar M.D. Effect of methanolic extract of adenanthera pavonina linn on dalton's ascitic lymphoma. Indian J. Res. Pharm. Biotechnol. 2017;1:138–141. [Google Scholar]
- Bar-Sela G., Epelbaum R., Schaffer M. Curcumin as an anti-cancer agent: review of the gap between basic and clinical applications. Curr. Med. Chem. 2010;17:190–197. doi: 10.2174/092986710790149738. [DOI] [PubMed] [Google Scholar]
- Bhadran Shubha, George S.A., Sudhakar Malla Harini Bp. Screening of bioprotective properties of various plant extracts and gas chromatography-mass spectrometry profiling of adenanthera pavonina stem extract. Asian J. Pharm. Clin. Res. 2017;10:1–8. [Google Scholar]
- Boonsri Sompong, Karalai Chatchanok, Ponglimanont Chanita, Chantrapromma Suchada, Kanjana-opas Akkharawit. Cytotoxic and Antibacterial Sesquiterpenes from Thespesia populnea. J. Nat. Prod. 2008;71:1173–1177. doi: 10.1021/np800055q. [DOI] [PubMed] [Google Scholar]
- Chandran R.P., Manju S., Shaji P.K., Nair G.A., Sukumar B. In vitro cytotoxic activities of leaf extracts of Thespesia Populnea and Hygrophilla Schulli against Dalton’s lymphoma ascites and ehrlich ascites carcinoma cell lines. Austin J. Lung Cancer Res. 2016;1:1007. [Google Scholar]
- Chin-Cheng Su, Lin J.-G., Li Te-Mao, Chung Jing-Gung, Yang Jai-Sing, Ip Siu-Wan, Lin Wen-Chuan, Chen Guang-Wei. Curcumin-induced apoptosis of human colon cancer colo 205 cells through the production of ROS, Ca2+ and the activation of caspase-3. Anticancer Res. 2006;26:4379–4390. [PubMed] [Google Scholar]
- Corti Alessandro, Franzini Maria, Paolicchi Aldo, Pompella Alfonso. Gamma-glutamyltransferase of cancer cells at the crossroads of tumor progression, drug resistance and drug targeting. Anticancer Res. 2010;30:1169–1181. [PubMed] [Google Scholar]
- Das S., Singh Bisht S. The bioactive and therapeutic potential of hemidesmus indicus r. br. (indian sarsaparilla) root. Phytother. Res. 2013;6:791–801. doi: 10.1002/ptr.4788. [DOI] [PubMed] [Google Scholar]
- De Alok, De A., Papasian Chris, Hentges Shane, Banerjee Snigdha, Haque Inamul, Banerjee Sushanta K. Emblica officinalis extract induces autophagy and inhibits human ovarian cancer cell proliferation, angiogenesis, growth of mouse xenograft tumors. PLoS ONE. 2013;8:1–16. doi: 10.1371/journal.pone.0072748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanasekaran Muniyappan, Baskar Arul-Albert, Ignacimuthu Savarimuthu, Agastian Paul, Duraipandiyan Veeramuthu. Chemopreventive potential of Epoxy clerodane diterpene from Tinospora cordifolia against diethylnitrosamine-induced hepatocellular carcinoma. Invest. New Drugs. 2009;27:347–355. doi: 10.1007/s10637-008-9181-9. [DOI] [PubMed] [Google Scholar]
- Dharmadasa R.M., Premakumara G.A.S., Hettiarachchi P.L., Ratnasooriya W.D. Cytotoxcity and in vivo antimalarial activity of aqueous whole plant extract of munronia pinnata (Wall.) Theob. (Meliaceae) in mice. Res. J. Med. Plants. 2012;6:267–273. [Google Scholar]
- Dixon R.A. Natural products and plant disease resistance. Nature. 2001;411:843–847. doi: 10.1038/35081178. [DOI] [PubMed] [Google Scholar]
- Ferreira Paulo Michel P., Farias D.F., Viana Martônio P., Souza Terezinha M., Vasconcelos Ilka M., Soares Bruno M., Pessoa Cláudia, Costa-lotufo Letícia V., Moraes Manoel O., Carvalho Ana.F.U. Study of the antiproliferative potential of seed extracts from Northeastern Brazilian plants. Ann. Brazilian Acad. Sci. 2011;83:1045–1058. doi: 10.1590/s0001-37652011005000017. [DOI] [PubMed] [Google Scholar]
- Fidyt Klaudyna, Fiedorowicz Anna, Strzadała Leon, Szumny Antoni. β-caryophyllene and β-caryophyllene oxide—natural compounds of anticancer and analgesic properties. Cancer Med. 2016;5:3007–3010. doi: 10.1002/cam4.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Li S., Zu Y., Yang G., Yang Z., Luo M., Jiang S., Wink M., Efferth T. Medicinal chemistry of paclitaxel and its analogues. Curr. Med. Chem. 2009;16:3966–3985. doi: 10.2174/092986709789352277. [DOI] [PubMed] [Google Scholar]
- Galhena Bandula Prasanna, Samarakoon S.S.R., Thabrew Myrtle Ira, Paul Solomon.F.D., Perumal Venkatachalam, Mani Chinnadurai. Protective effect of a polyherbal aqueous extract comprised of nigella sativa (seeds), hemidesmus indicus (roots), and smilax glabra (rhizome) on bleomycin induced cytogenetic damage in human lymphocytes. Biomed Res. Int. 2017:1–7. doi: 10.1155/2017/1856713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Jiang-Jing, Hirakawa A., Min Byung Sun, Nakamura Norio, Hattori Masao. In vivo antitumor effects of bitter principles from the antlered form of fruiting bodies of Ganoderma lucidum. J. Nat. Med. 2006;60:42–48. [Google Scholar]
- Gao Xiaoxu, Wei J., Hong Lina, Fan Sanpeng, Gaosheng Hu, Jia Jingming. Comparative analysis of chemical composition, anti-inflammatory activity and antitumor activity in essential oils from siegesbeckia orientalis, S. glabrescens and S. pubescens with an ITS sequence analysis. Molecules. 2018;23:2185. doi: 10.3390/molecules23092185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcea Giuseppe, Berry D.P., Jones Donald J.L., Singh Raj, Dennison Ashley R., Farmer Peter B., Sharma Ricky A., Steward William P., Gescher Andreas J. Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol. Biomark. Prev. 2005;14:120–125. [PubMed] [Google Scholar]
- Gentry Amanda C., Pitts S.L., Jablonsky Michael J., Bailly Christian, Graves David E., Osheroff Neil. Interactions between the etoposide derivative F14512 and human type II topoisomerases: implications for the C4 spermine moiety in promoting enzyme-mediated DNA cleavage. Biochemistry. 2011;50:3240–3249. doi: 10.1021/bi200094z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J.G., Fabricant M.L.Q.D.S., Farnsworth N.R. Plants used against cancer – an extension of the work of jonathan hartwell. J. Ethnopharmacol. 2000;73:347–377. doi: 10.1016/s0378-8741(00)00341-x. [DOI] [PubMed] [Google Scholar]
- Habib Shafina Hanim Mohd, Makpol S., Hamid Noor Aini Abdul, Das Srijit, Ngah Wan Zurinah Wan, Yusof Yasmin-Anum-Mohd. Ginger extract (Zingiber officinale) has anti-cancer and anti-inflammatory effects on ethionine-induced hepatoma rats. Clinics. 2008;63:807–813. doi: 10.1590/S1807-59322008000600017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hande K.R. Etoposide: four decades of development of a topoisomerase II inhibitor. Eur. J. Cancer. 1998;34:1514–1521. doi: 10.1016/s0959-8049(98)00228-7. [DOI] [PubMed] [Google Scholar]
- Iddamaldeniya S.S., Thabrew M.I., Wickramasinghe S.M.D.N., Ratnatunge N., Thammitiyagodage M.G. A long-term investigation of the anti-hepatocarcinogenic potential of an indigenous medicine comprised of Nigella sativa, Hemidesmus indicus and Smilax glabra. J. Carcinogenesis. 2006;5:11. doi: 10.1186/1477-3163-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal Javed, Abbasi B.A., Mahmood Tariq, Kanwal Sobia, Ali Barkat, Shah Sayed Afzal, Khalil Ali Talha. Plant-derived anticancer agents: a green anticancer approach. Asian Pacific J. Trop. Biomed. 2017;7:1129–1150. [Google Scholar]
- Ira Thabrew M., Mitry R.R., Morsy Mohammed A., Hughes Robin D. Cytotoxic effects of a decoction of Nigella sativa, Hemidesmus indicus and Smilax glabra on human hepatoma HepG2 cells. Life Sci. 2005;77:1319–1330. doi: 10.1016/j.lfs.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Jayasinghe Susanthi, Bandra B.M.R., Wickramasinghe A., Karunaratne D.N., Wijesundara D.S.A., Karunaratne Veranja. The importance of harnessing the rich diversity of Sri Lankan flora for their. Ceylon J. Sci. 2017;46:3–13. [Google Scholar]
- Jemal Ahmedin, Bray Freddie, Center Melissa M., Ferlay Jacques, Ward Elizabeth, Forman David. Global cancer statistics. CA Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Jose Jose M., Ortega Angel, Obrador Elena. Glutathione in cancer biology and therapy. Crit. Rev. Clin. Lab. Sci. 2006;43:143–181. doi: 10.1080/10408360500523878. [DOI] [PubMed] [Google Scholar]
- Khan H. Medicinal plants in light of history: recognized therapeutic modality. Evid.-Based Complement. Alternat. Med. 2014;19:216–219. doi: 10.1177/2156587214533346. [DOI] [PubMed] [Google Scholar]
- Kim Eok-Cheon, Min J.-K., Kim Tae-Yoon, Lee Shin-Jeong, Yang Hyun-Ok, Han Sanghwa, Kim Young-Myeong, Kwon Young-Guen. [6]-Gingerol, a pungent ingredient of ginger, inhibits angiogenesis in vitro and in vivo. Biochem. Biophys. Res. Commun. 2005;335:300–308. doi: 10.1016/j.bbrc.2005.07.076. [DOI] [PubMed] [Google Scholar]
- Leyon P.V., Lini C.C., Kuttan G. Inhibitory effect of Boerhaavia diffusa on experimental metastasis by B16F10 melanoma in C57BL/6 mice. Life Sci. 2004;76:1339–1349. doi: 10.1016/j.lfs.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Lin X.W.A.Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol. Sin. 2008;29:1275–1288. doi: 10.1111/j.1745-7254.2008.00889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindamulage I.K.S., Soysa P. Evaluation of anticancer properties of a decoction containing Adenanthera pavonina L. and Thespesia populnea L. BMC Complement. Alternat. Med. 2016;16:1–8. doi: 10.1186/s12906-016-1053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Leroy, Desai S.D, Li Tsai-Kun, Mao Yong, Sun Mei, Sim Sai-Peng. Mechanism of action of camptothecin. Ann. N. Y. Acad. Sci. 2006;922:1–10. doi: 10.1111/j.1749-6632.2000.tb07020.x. [DOI] [PubMed] [Google Scholar]
- Liu Xiaoli, Zhao M., Kegang Wu, Chai Xianghua, Hongpeng Yu, Tao Zhihua, Wang Jinshui. Immunomodulatory and anticancer activities of phenolics from emblica fruit (Phyllanthus emblica L.) Food Chem. 2012;131:685–690. [Google Scholar]
- Lü S., Wang J. Homoharringtonine and omacetaxine for myeloid hematological malignancies. J. Hematol. Oncol. 2014;7:2. doi: 10.1186/1756-8722-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann J. Natural products in cancer chemotherapy: past, present and future. Nature. 2002;2:143–148. doi: 10.1038/nrc723. [DOI] [PubMed] [Google Scholar]
- Mika D., Guruvayoorappan C. Experimental study on anti-tumor and antiinflammatory effect of Thespesia populnea phytochemical extract in mice models. Immunopharmacol. Immunotoxicol. 2012;35:157–163. doi: 10.3109/08923973.2012.735237. [DOI] [PubMed] [Google Scholar]
- Mishra Shikha, Aeri V., Gaur Praveen Kumar, Jachak Sanjay M. Phytochemical, therapeutic, and ethnopharmacological overview for a traditionally important herb: boerhavia diffusa linn. Biomed Res. Int. 2014:1–19. doi: 10.1155/2014/808302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra R., Kaur G. Aqueous ethanolic extract of tinospora cordifolia as a potential candidate for differentiation based therapy of glioblastomas. PLoS ONE. 2013;8:1–13. doi: 10.1371/journal.pone.0078764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moirangthem Dinesh Singh, Borah J.C, Laishram Surbala, Kalita Mohan Chandra, Talukdar Narayan Chandra. HPLC analysis of harringtonine and homoharringtonine in the needles of cephalotaxus griffithii alkaloid fraction and cytotoxic activity on chronic myelogenous leukaemia K562 cell. Nat. Prod. Res. 2014;28:1503–1506. doi: 10.1080/14786419.2014.913241. [DOI] [PubMed] [Google Scholar]
- Montecucco Alessandra, Zanetta Francesca, Biamonti Giuseppe. Molecular mechanisms of etoposide. Exp. Clin. Sci. 2015;14:95–108. doi: 10.17179/excli2015-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moudi Maryam, Go R., Yien Christina Yong Seok, Nazre Mohd. Vinca alkaloids. Int. J. Prevent. Med. 2013;4:1231–1235. [PMC free article] [PubMed] [Google Scholar]
- Mujahid Md., Ansari V.A., Sirbaiya Anup K., Kumar Ranjan, Usmani Afreen. An insight of pharmacognostic and phytopharmacology study of Adenanthera pavonina. J. Chem. Pharmaceut. Res. 2016;8:586–596. [Google Scholar]
- Murali Anita, Ashok P., Madhavan V. In vitro antioxidant activity and hptlc studies on the roots and rhizomes of Smilax zeylanica l. (smilacaceae) Int. J. Pharm. Pharmaceut. Sci. 2011;3:192–195. [Google Scholar]
- Nagat M., Reena Lawrence E.B., Saani Mariya. Phytochemical screening, antioxidant and antibacterial activity of active compounds from hemidesmus indicus. Int. J. Curr. Pharm. Res. 2016;8:24–27. [Google Scholar]
- Napagoda Mayuri, Gerstmeier J., Koeberle Andreas, Wesely Sandra, Popella Sven, Lorenz Sybille, Scheubert Kerstin, Böcker Sebastian, Svatoš Aleš, Werz Oliver. Munroniapinnata (Wall.)Theob.: unveiling phytochemistry and dualinhibitionof5-lipoxygenase and microsomal prostaglandin E2 synthase (mPGES)-1. J. Ethnopharmacol. 2014;151:882–890. doi: 10.1016/j.jep.2013.11.052. [DOI] [PubMed] [Google Scholar]
- Cragg G.M., Newman D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005;100:72–79. doi: 10.1016/j.jep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Ngamkitidechakul C., Jaijoy K., Hansakul P., Soonthornchareonnon N., Sireeratawong S. Antitumour effects of phyllanthus emblica l.: induction of cancer cell apoptosis and inhibition of in vivo tumour promotion and in vitro invasion of human cancer cells. Phytother. Res. 2010;24:1405–1413. doi: 10.1002/ptr.3127. [DOI] [PubMed] [Google Scholar]
- Ojima Iwao, Lichtenthal B., Lee Siyeon, Wang Changwei, Wang Xin. Taxane anticancer agents: a patent perspective. Expert Opin. Ther. Pat. 2016;26:1–20. doi: 10.1517/13543776.2016.1111872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization, W.H., 2018. Cancer 2018 [cited 2018 05; 15]. Available from: <http://www.who.int/news-room/fact-sheets/detail/cancer>.
- Pan Si-Yuan, Litscher G., Gao Si-Hua, Zhou Shu-Feng, Zhi-Ling Yu, Chen Hou-Qi, Zhang Shuo-Feng, Tang Min-Ke, Sun Jian-Ning, Ko Kam-Ming. Historical perspective of traditional indigenous medical practices: the current renaissance and conservation of herbal resources. Evid.-Based Complement. Alternat. Med. 2013;2014:1–20. doi: 10.1155/2014/525340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey M.M., Rastogi S., Rawat A.K.S. Indian traditional ayurvedic system of medicine and nutritional supplementation. Evid.-Based Complement. Alternat. Med. 2013:1–12. doi: 10.1155/2013/376327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel Arti, Bigoniya Papiya, Singh Chandra Shekhar, Patel Narayan Singh. Radioprotective and cytoprotective activity of Tinospora cordifolia stem enriched extract containing cordifolioside-A. Indian J. Pharmacol. 2013;45:237–243. doi: 10.4103/0253-7613.111919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera, D.L., 2004. Truth and Myth of Green Piracy.
- Phanse Mohini A., Patil M.J., Abbulu Konde. Review on pharmacological studies of Thespesia populnea linn. Int. J. Pharm. Pharmaceut. Sci. 2013;5:1–5. [Google Scholar]
- Prajapati, Narayan Das, S.S.P., Sharma, Arun K., Kumar, Tarun, 2007. A Handbook of Medicinal Plants: A Complete Source Book.
- Rahmani Arshad H., Al shabrmi Fahad M., Aly Salah M. Active ingredients of ginger as potential candidates in the prevention and treatment of diseases via modulation of biological activities. Int. J. Physiol., Pathophysiol. Pharmacol. 2014;6:125–136. [PMC free article] [PubMed] [Google Scholar]
- Rajan S., Shalini R., Bharathi C., Aruna V., Elgin A., Brindha P. Pharmacognostical and phytochemical studies on hemidesmus indicus root. Int. J. Pharmacognosy Phytochem. Res. 2011;3:74–79. [Google Scholar]
- Rajesh V., Perumal P. Cytoprotective effect of Smilax zeylanica Linn. leaves against Benzo[a]pyrene induced lung cancer with reference to lipid peroxidation and antioxidant system in Swiss albino mice. Oriental Pharm. Exp. Med. 2013;13:1–11. [Google Scholar]
- Rao Shaival K., Rao Priya S. Alteration in the radiosensitivity of HeLa cells by dichloromethane extract of Guduchi (Tinospora cordifolia) Integrative Cancer Therapies. 2010;9:378–384. doi: 10.1177/1534735410387598. [DOI] [PubMed] [Google Scholar]
- Resorts, 2017. Ayurvedic Medicinal Plants of Sri Lanka. 2017 [cited 2018 01-05]; Available from: <http://www.instituteofayurveda.org/plants/>.
- Russell-Smitha Jeremy, Karunaratne N.S., Mahindapala Ranjith. Rapid inventory of wild medicinal plant populations in Sri Lanka. Biol. Conserv. 2006;132:22–32. [Google Scholar]
- Saha S., Ghosh S. Tinospora cordifolia: one plant, many roles. Ancient Sci. Life. 2012;31:151–159. doi: 10.4103/0257-7941.107344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarakoon Sameera R., Thabrew I., Galhena Prasanna B., Tennekoon Kamani H. Modulation of apoptosis in human hepatocellular carcinoma (HepG2 cells) by a standardized herbal decoction of Nigella sativa seeds, Hemidesmus indicus roots and Smilax glabra rhizomes with anti- hepatocarcinogenic effects. BMC Complement. Alternat. Med. 2012;12:25. doi: 10.1186/1472-6882-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi Gautam, Shanmugam Muthu, Warrier Sudha, Merarchi Myriam, Arfuso Frank, Kumar Alan, Bishayee Anupam. Pro-apoptotic and anti-cancer properties of diosgenin: a comprehensive and critical review. Nutrients. 2018;10:645. doi: 10.3390/nu10050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma Ricky A., Ireson C.R., Verschoyle Richard D., Hill Kirsti A., Williams Marion L., Leuratti Chiara, Manson Margaret M., Marnett Lawrence J., Steward William P., Gescher Andreas. Effects of dietary curcumin on glutathione S-transferase and malondialdehyde-DNA adducts in rat liver and colon mucosa: relationship with drug levels. Clin. Cancer Res. 2001;1:1452–1458. [PubMed] [Google Scholar]
- Silva I.K., Soysa P. Evaluation of phytochemical composition and antioxidant capacity of a decoction containing Adenanthera pavonina L. and Thespesia populnea L. Pharmacognosy Mag. 2011;7:193–199. doi: 10.4103/0973-1296.84229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh Y.S.A.M. Cancer preventive properties of ginger: a brief review. Food Chem. Toxicol. 2007;45:683–690. doi: 10.1016/j.fct.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Sinha K., Mishra N.P., Singh J., Khanuja S.P.S. Tinospora cordifolia (Guduchi), a reservoir plant for therapeutic applications: a review. Indian J. Tradit. Knowl. 2004;3:257–270. [Google Scholar]
- Clark P.I., Slevin M.L. The clinical pharmacology of etoposide and teniposide. Clin. Pharmacokinet. 1987;12:223–252. doi: 10.2165/00003088-198712040-00001. [DOI] [PubMed] [Google Scholar]
- Sreeja S.S.A.S. An in vitro study on antiproliferative and antiestrogenic effects of Boerhaavia diffusa L. extracts. J. Ethnopharmacol. 2009;126:221–225. doi: 10.1016/j.jep.2009.08.041. [DOI] [PubMed] [Google Scholar]
- Srivastava Rakhi, Saluja D., Dwarakanath Bilikere S., Chopra Madhu. Inhibition of human cervical cancer cell growth by ethanolic extract of boerhaavia diffusa linn. (punarnava) root. Evid.-Based Complement. Alternat. Med. 2009:1–13. doi: 10.1093/ecam/nep223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statti Giancarlo, Marrelli M., Conforti Filomena, Spagnoletti Antonella, Tacchini Massimo, Fimognari Carmela, Brognara Eleonora, Gambari Roberto, Sacchetti Gianni, Guerrini Alessandra. Inhibition of cancer cell proliferation and antiradical effects of decoction, hydroalcoholic extract, and principal constituents of hemidesmus indicus R Br. Phytother. Res. 2015;29:857–863. doi: 10.1002/ptr.5322. [DOI] [PubMed] [Google Scholar]
- Turrini Eleonora, Catanzaro E., Muraro Manuele G., Governa Valeria, Trella Emanuele, Mele Valentina, Calcabrini Cinzia, Morroni Fabiana, Sita Giulia, Hrelia Patrizia, Tacchini Massimo, Fimognari Carmela. Hemidesmus indicus induces immunogenic death in human colorectal cancer cells. Oncotarget. 2018;9:24443–24456. doi: 10.18632/oncotarget.25325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin Mohammad Nasir, Ahmed T., Pathan Sanzida, Mamun Al-Amin Md., Sohel Rana Md. Antioxidant and cytotoxic activity of stems of Smilax zeylanica in vitro. J. Basic Clin. Physiol. Pharmacol. 2015;26:453–463. doi: 10.1515/jbcpp-2014-0114. [DOI] [PubMed] [Google Scholar]
- Vemuri Satish Kumar, Banala R.R., Subbaiah G.P.V., Srivastava Saurabh Kumar, Gurava Reddy A.V., Malarvili Thekkumalai. Anti-cancer potential of a mix of natural extracts of turmeric, ginger and garlic: a cell-based study. Egyptian Jo. Basic Appl. Sci. 2017;4:332–344. [Google Scholar]
- Verpoorte R. Exploration of nature’s chemodiversity: the role of secondary metabolites as leads in drug development. Drug Discovery Today. 1998;3:232–238. [Google Scholar]
- Wang Chong-Zhi, Calway T., Yuan Chun-Su. Herbal medicines as adjuvants for cancer therapeutics. Am. J. Chin. Med. 2012;40:657–669. doi: 10.1142/S0192415X12500498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson E.M. Elsevier Science; 2002. Major Herbs of Ayurveda. [Google Scholar]
- Yared J.A., Tkaczuk K.H.R. Update on taxane development: new analogs and new formulations. Drug Des., Develop. Therapy. 2012;6:371–384. doi: 10.2147/DDDT.S28997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Xu, Rakesh K.P., Shantharam C.S., Manukumar H.M., Asiri A.M., Marwani H.M., Qin Hua-Li. Podophyllotoxin derivatives as an excellent anticancer aspirant for future chemotherapy: a key current imminent needs. Bioorg. Med. Chem. 2017;26:340–355. doi: 10.1016/j.bmc.2017.11.026. [DOI] [PubMed] [Google Scholar]
- Zhao Tiejun, Sun Q., Marques Maud, Witcher Michael. Anticancer properties of Phyllanthus emblica (Indian Gooseberry) Oxid. Med. Cell. Longevity. 2014;2015:1–7. doi: 10.1155/2015/950890. [DOI] [PMC free article] [PubMed] [Google Scholar]