Summary
Aims
In this study, we examined the expression of GINS2 in glioma and determined its role in glioma development.
Methods
The protein expression of GINS2 was assessed in 120 human glioma samples via immunohistochemistry. Then, we suppressed the expression of GINS2 in glioma cell strains U87 and U251 using a short hairpin RNA lentiviral vector. In addition, RNA sequencing and bioinformatics analysis were performed on glioma cells before and after GINS2 knockdown. Subsequent co‐immunoprecipitation and western blot experiments indicated possible downstream regulatory molecules.
Results
The present results showed that GINS2 can accelerate the growth of glioma cells, whereas the suppression of GINS2 expression decreased the proliferation and tumorigenicity of glioma cells. Mechanism research experiments proved that GINS2 can block the cell cycle by regulating certain downstream molecules, such as MCM2, ATM, and CHEK2.
Conclusion
GINS2 is closely related to the occurrence and development of glioma, and is likely to become a prognostic marker for glioma patients, as well as a potential therapeutic target in the treatment of glioma.
Keywords: cell cycle, cell proliferation, digital gene expression profiling, GINS2, human gliomas
1. INTRODUCTION
Glioma, the most common primary intracranial malignant tumor among adults, accounts for about one‐third of central nervous system tumors.1, 2, 3 According to WHO guidelines, glioma can be graded as level I‐IV, with I and II being labeled “low grade,” while III and IV are considered “high grade.” In most cases, patients with high‐grade glioma experience relapse several months after the initial treatment, despite the use of comprehensive therapies such as resection, radiotherapy, and chemotherapy.4, 5 It is estimated that the 5‐year survival rate of patients with glioma is lower than 30%, whereas the median survival time of those with level IV gliomas is only 12‐18 months.6, 7, 8 The occurrence and development of glioma involve multiple factors and steps. Advances in molecular biology have enabled researchers to analyze the functions of various genes and proteins in glioma, laying the foundation for the study of glioma pathogenesis, gradation and classification, biological targeted therapy, and prognosis. In recent years, researchers have identified various genes9, 10, 11, 12 closely related to glioma, some of which participate in glioma formation by promoting tumor cell growth, inhibiting apoptosis, and facilitating tumor infiltration. However, the exact molecular mechanism underlying the initiation and development of glioma remains unclear. Therefore, further research on pathogenesis‐related genes and their potential as therapeutic targets is of great clinical significance.
GINS2, a member of the GINS DNA replication complex, is a gene located on chromosome 16q24, with a coding relative molecular weight of 21 000 Da and mRNA length of 1196 bp. The structure of GINS2 is a replicative helicase, which opens the double strands before replication and plays an important role in the initiation of DNA replication. The DNA replication complex consisting of Sld5, Psf1, Psf2, and Psf3 is called GINS for short. Studies have shown that certain DNA replication proteins play different roles in different cells. For example, GINS exerts an effect on the stages and number of centrosome duplications during disease initiation, thus affecting chromosome segregation.13 Furthermore, recent studies indicate that GINS is involved in cancer initiation. For instance, GINS members are overexpressed in invasive melanoma,14 and GINS1 is regarded as the target of estrogen in the human breast cancer cell line MCF‐7.15 Besides, GINS2 overexpression is closely related to the occurrence and development of multiple tumors. For example, in cholangiocarcinoma tissues, GINS2 gene expression is remarkably upregulated.16 Moreover, Liu et al17 found that lung cancer tissues overexpress GIN2, which is connected to lung cancer metastasis. Apart from that, GINS2 can improve the invasiveness of breast cancer,18 enhance the proliferation of leukemia cells19 and is related to the early development of cervical cancer.20 Nevertheless, the expression and possible role of GINS2 in glioma are still unclear.
In the present study, the expression of GINS2 in glioma was detected for the first time, revealing a positive correlation between GINS2 expression level and the pathological grade of glioma. Meanwhile, the suppression of GINS2 expression in U251 and U87 cells via RNA interference decreased the proliferation and clonality of glioma cells, in both in vitro and in vivo experiments. In addition, the possible regulatory mechanism by which GINS2 promotes glioma cell growth was analyzed. Obtained results suggest that GINS2, which is closely related to the occurrence and development of glioma, is likely to become a prognostic marker for glioma patients and potential therapeutic target in the treatment of glioma.
2. MATERIALS AND METHODS
2.1. Collection and immunohistochemical analysis of human glioma samples
The 120 glioma samples analyzed in this study were provided by the Neurosurgery Department of Southern Medical University. Legally effective informed consent was obtained from all patients prior to sample collection. An ethics committee approval for conducting this study was also obtained. All samples were pathologically proven to be glioma and included 18 cases of grade I glioma, 35 of grade II, 35 of grade III, 32 of grade IV (WHO grading standard). GINS2 immunohistochemical staining was performed on all glioma samples, according to a previously described protocol.20 The results were jointly determined by two double‐blinded pathologists in accordance with the following guidelines21: <5% (the ratio of positive tumor cells), negative; 6%‐100%, positive. The relationship between GINS2 expression and glioma grade was analyzed via the SPSS 20.0 software, and the “H‐test” (P < 0.05) was used to determine statistical significance.
2.2. Construction of a GINS2‐shRNA expression vector
Based on the GINS2 gene, in accordance with the RNA interference sequence, we designed multiple 19‐21nt RNA interfering target sequences. After evaluation and assessment by the siRNA Selection Program (Whitehead Institute), the following sequence was chosen as interference target: GATTAACCTGAAACAAAGA. The sequence of scrambled shRNA, ie, the negative control, was: TTCTCCGAACGTGTCACGT. Subsequently, we designed a shRNA interfering sequence in line with the target sequence chosen, adding restriction enzyme cutting sites on both ends to complete vector construction. In addition, the TTTTT terminal signal was added to the 3′ end of the plus strand and a terminal signal complementary sequence was added to the 5′ end of the antisense strand. The obtained compounded single‐stranded DNA oligo dry powder was then dissolved in annealing buffer (100 mol/L) and heated in a water bath for 15 minutes at 90℃. Double strands with sticky ends were finally obtained after cooling the solution to room temperature.
The annealed double‐stranded DNA was then connected with pLVX‐shRNA2 (Novagen, Madison, WI, USA) that had been subjected to enzyme digestion using BamH I and EcoR I via T4 ligase for 16 hours, at 14‐16℃. The obtained recombinant vector was used to transform the competence of Escherichia coli, which was then cultured with Amp at 37℃ for 16 hours. Lastly, the construction was verified by sequencing analysis of a selected monoclonal colony.
2.3. Cell culture and the establishment of a GINS2‐shRNA stable cell line
Human glioma cell lines U87 and U251 were purchased from the Chinese Academy of Sciences (Shanghai, China). Cells were initially cultured at 37℃, 5% CO2 under saturated humidity, in Gibco high‐glucose solution with 10% fetal bovine serum and passaged. Cells in logarithmic phase were excluded for experiment. Then, these cells were seeded on culture dishes with a specific density (2 × 105). On the second day, the medium was discarded and replaced with medium containing 8 µg/mL polybrene. Lentivirus suspension was then added to the cell culture, and the culture medium was refreshed daily. After 96 hours, the expression efficiency was observed with a fluorescence microscope. A monoclonal colony was then selected, and the cells were suspended in solution, diluted 1/10 μL. Subsequently, cells were seeded into 48‐well plates to enable proliferation. Finally, the expression of the target gene was assessed via RT‐PCR, and the stable transfection cells were chosen to develop the GINS2‐shRNA cell line.
2.4. Quantitative real‐time PCR
After collecting 107 cells, mRNA was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and cDNA was synthetized with the PrimeScript II 1st Strand cDNA Synthesis Kit (TaKaRa, Tokyo, Japan). Subsequently, the nucleotide sequence was verified using the NCBI database, according to the respective species and the name of the target gene. Real‐time PCR primers were designed using Primer 3.0 (Sangon, Shanghai). GINS2 primers were as follows: 5′‐AGACAGAAATGTCGCCTGCT‐3′ (GINS2 forward), 5′‐AGCAGACACTCGGAGTTTGG‐3′ (GINS2 reverse), 5′‐AACGGATTTGGTCGTATTGGG‐3′ (GAPDH forward), and 5′‐CCTGGAAGATGGTGATGGGAT‐3′ (GAPDH reverse). Each reaction system included 2× Ex SYBR Taq 10.0 μL, 10 μmol/L PCR forward primer 0.8 μL, 10 μmol/L PCR reverse primer 0.8 μL, cDNA template 2 μL, and dH2O 6.4 μL. Finally, PCR was performed with the Bio‐Rad CFX96 Real‐Time PCR, and the melting curve was generated. qPCR Ct values were analyzed via the 2−ΔΔCt method.
2.5. Cell cycle analysis
Cells were collected into 2.0‐mL tubes, washed three times with pre‐cooled 1× PBS, and centrifuged at 80 g. After discarding the supernatant, cells were re‐suspended in 0.1 mL 1× PBS and incubated with 1.9 mL 75% pre‐cooled ethanol, overnight, at −20℃. The solution was then centrifuged, and the resulting supernatant was discarded. To remove residual ethanol, cells were then washed at least three times. Subsequently, cells were resuspended in 0.1 mL RNase A (0.1 mg/mL) and incubated at 37℃, for 30 minutes, in a water bath. Lastly, 0.4 mL PI was added to the suspension, and the cells were incubated at ambient temperature. The cell cycle was analyzed by flow cytometry (FCM).
2.6. Colony‐forming assay
Stable cells at the logarithmic growth phase were assimilated with 0.25% trypsin, and the cell density was adjusted to 1 × 106 cells/L by using DMEM culture solution containing 20% fetal bovine serum. The single‐cell suspension was thoroughly mixed and incubated (about 1000 cells/well) at 37℃ and 5% CO2 for 10‐14 days. Subsequently, the culture medium was removed, and the cells were washed with PBS for 2‐3 times, then fixed with 500 μL paraformaldehyde for 15 minutes, and stained with crystal violet for 15 minutes. After discarding the crystal violet, the cells were again washed with PBS for 2‐3 times. Lastly, the number of cell clones was counted using an inverted microscope, and the rate of colony formation was calculated.
2.7. Apoptosis assay
To assess apoptosis, the concentration of cells to be measured was adjusted to about 5 × 105/mL. Subsequently, 1 mL cells were centrifuged at 1000 rpm, at 4℃, for 5 minutes, and the resulting supernatant was discarded. The pellet was then resuspended in 1 mL cold PBS. The suspension was then diluted to 1 × 106 cells/mL with 250 μL binding buffer (pre‐diluted 1:3 with deionized water). After that, 100 μL cell suspension was mixed with 5 μL Annexin V/FITC and 10 μL PI solution (20 μg/mL), and then incubated for 15 minutes in the dark, at room temperature.
2.8. Cell proliferation assay
The proliferation ability of glioma cell lines in the Scr‐shRNA and GINS2‐shRNA groups was assessed via Cell Counting Kit‐8 (Beyotime, Shanghai, China). Single cells were collected in the logarithmic phase. Then, cells were suspended and seeded in 96‐well plates at a density of 2000 cells/well (10 μL/well), followed by overnight incubation under 5% CO2, at 37℃. Virus‐concentrated solution and polybrene were added 1, 2, and 3 days respectively, after cell adhesion, for further culturing. Lastly, cells were incubated with CCK8 solution (10 μL/well) for 3 hours, and the absorbance was then measured at 450 nm with a microplate reader.
2.9. EdU proliferation assay
After cell digestion and centrifugation, a single‐cell suspension was prepared with serum culture medium. Subsequently, cells were plated into 96‐well plates at a density of 5 × 104 cells/well for further culturing. EdU solution diluted 1:1000 with culture medium was then added to each well (100 μL/well). After a 2‐hour incubation period, cells were collected and fixed with 4% formaldehyde. Then, Apollo and Hoechst33342 solutions were added for staining, and images were captured and analyzed using a fluorescence microscope.
2.10. Establishment of a mouse model of glioma & bioluminescence imaging
Overall, 12 BALB/c‐nu/nu nude female mice (3‐4 weeks, 12 ± 2 g) were raised in a specific pathogen‐free (SPF) environment. A stable cell strain was obtained after trypsinization, and then, the cells were washed twice with RMPI 1640 solution (serum‐free). The density of the cells was adjusted to 1 × 107/0.1 mL prior to being placed on ice. Having been disinfected with alcohol, the mice were injected subcutaneously with 0.1 mL cell solution at the dorsal part of the right forelimb (where the skin is thicker). The subcutaneous inoculation was conducted under strict sterilization, within half an hour after the cells harvest. Subsequently, the mice continued to be raised under SPF for 3‐4 weeks, being carefully observed and weighed every other day. Bioluminescence imaging was conducted under IVIS on the 1st, 3rd, 7th, 14th, and 21st day, respectively, following the cell injection.
2.11. RNA sequencing and data analysis
The standard extraction method was used to extract RNA from U87 cells transfected with lentivirus‐mediated Scr‐shRNA (n = 3) or GINS2‐shRNA (n = 3), and then, the RNA samples were subjected to strict quality control. After monitoring RNA degradation and contamination, sample purity was tested using the NanoPhotometer spectrophotometer (IMPLEN, Calabasas, CA, USA). RNA concentration was precisely quantitated in Qubit 2.0 Fluorometer (Life Technologies, Gaithersburg, CA, USA). RNA integrity was assessed using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). Samples containing 3 µg RNA were used as input material for RNA preparation. Sequencing libraries were generated using NEBNext UltraTM RNA Library Prep Kit for Illumina (NEB, Beverly, MA, USA), according to the manufacturer’s instructions. Subsequently, Illumina HiSeq sequencing was performed as described previously.22, 23
The obtained images were converted into sequenced reads by CASAVA base identification and stored in FASTQ format. All subsequent analyses were based on the high‐quality data. Differential expression analysis of two conditions was performed using the DESeq2 R package (1.10.1). Genes with an adjusted P‐value <0.05, identified by DESeq2, were considered differentially expressed. The clusterProfiler R package was used to test the statistical enrichment of differentially expressed genes in the KEGG pathways. Lastly, the cell cycle‐associated signaling pathway was imported into Cytoscape to construct a visual molecular interaction network.
2.12. Co‐IP
For this experiment, 1 × 107 cells were collected, rinsed three times with precooled 1× PBS, and lysed with 1 mL lysis buffer, which was followed by the addition of protein‐A/G agarose beads. After 10‐30 minutes of pretreatment, the lysis buffer (with protease inhibitor) was divided into Input, IP, and IgG (1:4:2). IP and IgG were incubated with target antibody and IgG antibody, respectively, at 4℃, on a rotator, for 12‐18 hours. Then, the cells were rinsed three times with precooled washing buffer. After that, the solution was boiled for 10 minutes, followed by addition of loading buffer and centrifugation. Supernatants were thereby acquired, and the protein samples were subjected to PAGE electrophoresis and silver staining.
2.13. Western blot analysis
Single cells were prepared by grinding tissue sample with liquid nitrogen, while 5 × 106 cultured cells with PMSF were lysed on ice. Next, 40‐60 μg protein was loaded onto SDS‐PAGE gels for thermal denaturation and then transferred onto PVDF membranes. The membranes were sealed with TBST containing 5% skimmed milk for 60 minutes, followed by overnight primary antibody incubation. Subsequently, membranes were incubated with secondary antibody (tagged by HRP) for 1 hour, at room temperature. Finally, BeyoECLPlus was added, and images were captured on X‐ray film for processing. Results were then quantified via densitometry, for comparisons between the different groups.
3. RESULTS
3.1. Expression of GINS2 positively correlated with glioma grade
To determine the role of GINS2 in glioma development, the protein expression of GINS2 was assessed via immunohistochemistry in samples of glioma with different grades. The results showed that GINS2 protein expression in glioma increases with the pathological grade (Figure 1). It should be noted that out of 18 samples of grade I glioma, 1 was positive for GINS2; 15 positive samples were detected among 35 cases of grade II glioma; 23 positives within 35 samples of grade III glioma; and 30 positives within 32 samples of grade IV glioma (Table 1). The differences in positive expression rates among groups were statistically significant.
Figure 1.
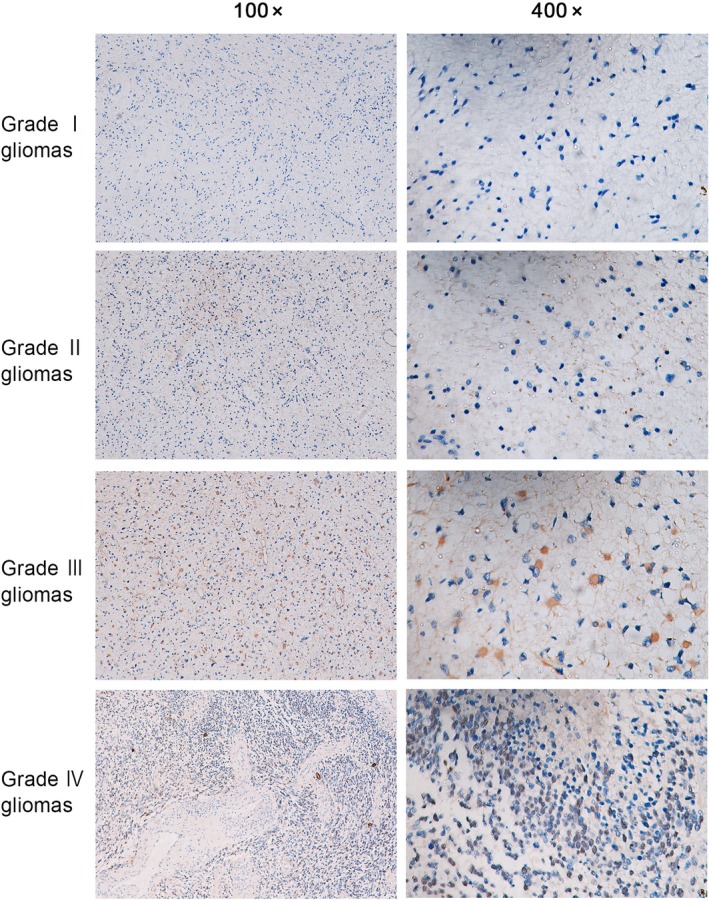
GINS2 protein expression in glioma of different grades
Table 1.
Relationship between GINS2 expression and glioma grade
| WHO grading | N | GINS2 expression | |
|---|---|---|---|
| Positive (n) | % | ||
| Grade I | 18 | 1 | 5.56 |
| Grade II | 35 | 15 | 42.86 |
| Grade III | 35 | 23 | 65.71 |
| Grade IV | 32 | 30 | 93.75 |
3.2. GINS2 expression in glioma cells declined dramatically after interference of shRNA mediated by lentivirus
In U87, U373, A172, and U251 glioma cells, GINS2 is highly expressed. In this study, we established GINS2 stable transfection cell lines with U87 and U251 cells, both of which featured higher expression (Figure 2A).
Figure 2.
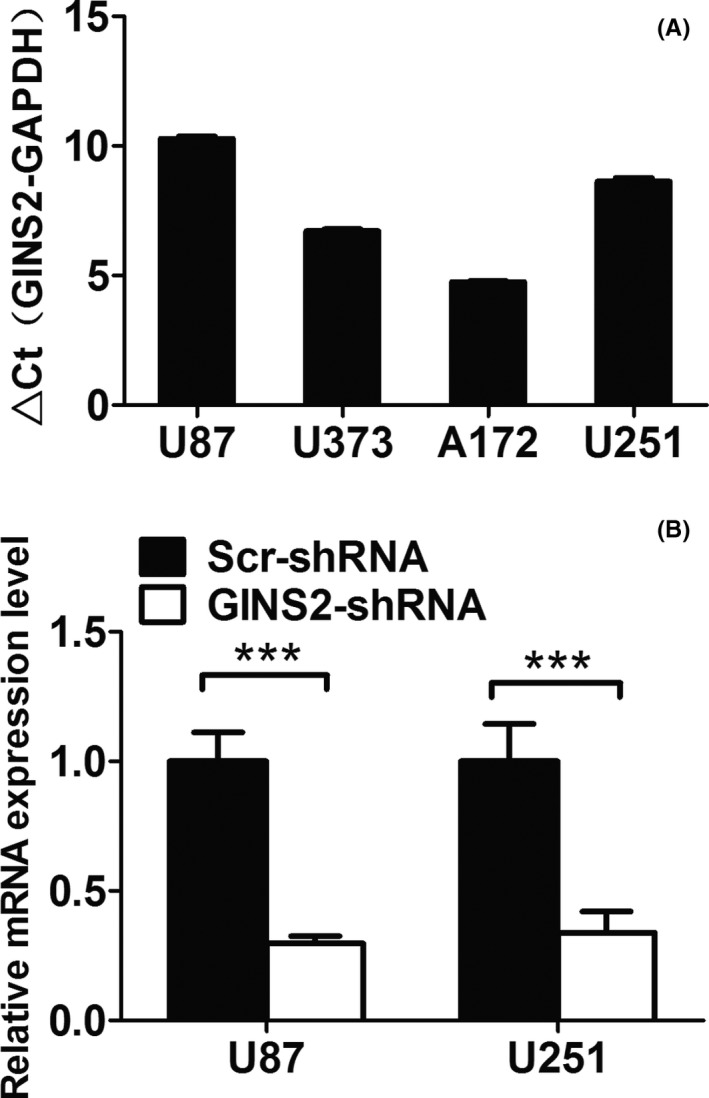
GINS2 expression in glioma cells declined dramatically after interference of shRNA mediated by lentivirus. A, mRNA expression of GINS2 in different cells via real‐time quantitative PCR. B, Real‐time quantitative PCR shows that the expression of GINS2 (mRNA level) in U87 and U251 cells in the control group was inhibited (P < 0.05)
To explore the functions of GINS2 in glioma cells, we suppressed GINS2 expression in glioma cell strains U87 and U251 via lentivirus‐based shRNA. As a result, the mRNA expression of GINS2 in both U87 and U251 cells declined dramatically (Figure 2B).
3.3. Proliferation of glioma cells declined remarkably after the suppression of GINS2 expression
Cell proliferation is one of the core hallmarks of tumors.24 In this study, we tested the proliferation of glioma cell strains U87 and U251 via CCK‐8 assay after inhibiting GINS2 expression (Figure 3A). The normalized optical density value of U87 cells was 0.81 ± 0.06, which was significantly lower than that of the control group. The same result was obtained for the U251 group, and the difference was statistically significant.
Figure 3.
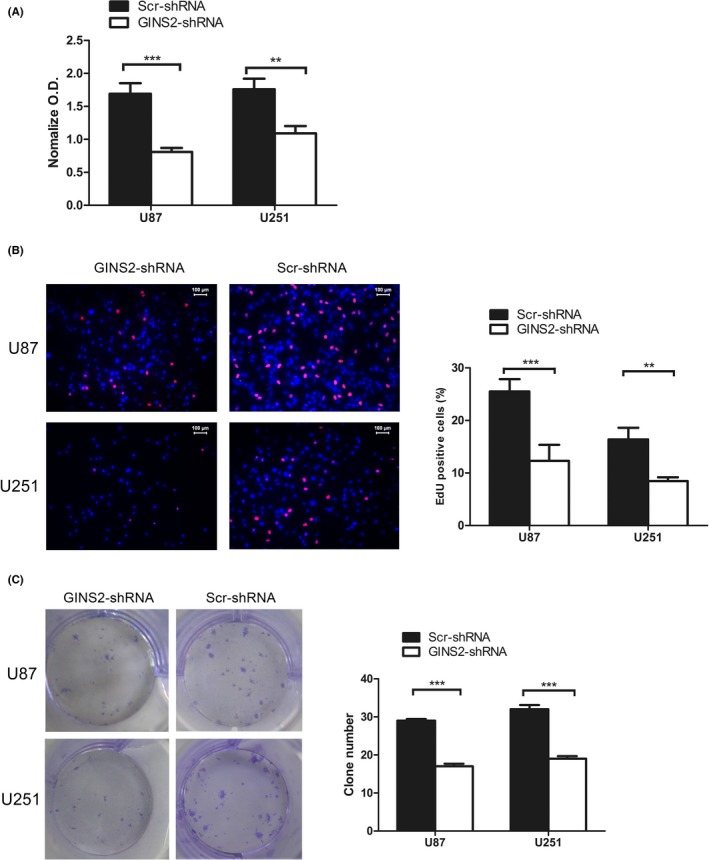
Proliferation of glioma cells U87 and U251 significantly declined after suppressing GINS2 expression. A, CCK‐8 showed that the proliferation of U87 and U251 cells was reduced after GINS2‐shRNA transfection, compared with the Scr‐shRNA group. Absorbency was assessed at 450 nm. Results are presented as mean ± SD of three separate experiments (*P < 0.05). B, EDU showed that the proliferation of U87 and U251 cells was reduced after GINS2‐shRNA transfection, compared with the Scr‐shRNA group. C, Clone formation assay demonstrated that the cloning ability of U87 and U251 cells declined notably after GINS2‐shRNA transfection. The results represent mean ± SD of three separate experiments (*P < 0.05)
Direct measurement of DNA synthesis is one of the most accurate ways of assessing cell proliferation. To further confirm the impact of suppressing GINS2 on glioma cell proliferation, we detected DNA activity through the EdU infiltration experiment (Figure 3B). The results demonstrated that, compared with the control group, DNA replication activity of U87 and U251 cells declined remarkably after the downregulation of GINS2 expression, and the differences were statistically significant.
3.4. Clonality of glioma cells declined after suppression of GINS2 expression
Colony‐forming assay is one of the most effective ways to detect the proliferation ability of single cells. For this purpose, we analyzed changes in the biological traits of glioma cell lines U87 and U251 after transfection of lentivirus expressing GINS2‐ or Scr‐shRNA (Figure 3C). The results showed that, compared with Scr‐shRNA, the clonality of U251 and U87 cells decreased notably after GINS2‐shRNA transfection.
3.5. Glioma cell cycle was arrested at G1 phase after GINS2 suppression
To grasp the impact of the GINS2 gene on glioma cell cycle, we evaluated the glioma cell cycle via FCM after the knockdown of GINS2. The average value and standard deviation were calculated for different phases of the cell cycle in each group, and the results were statistically analyzed. Compared with the control group, the ratio of G1 phase U87 cells increased from 49.91% to 65.31%, while the proportion of S phase cells decreased from 23.09% to 9.74%. In U251 cells, the ratio of G1 phase cells rose from 50.14% to 68.96%, while that of S phase cells decreased from 26.33% to 11.64%. These results suggest that the downregulation of GINS2 expression can arrest glioma U87 and U251 cells in the G1 phase (Figure 4A).
Figure 4.
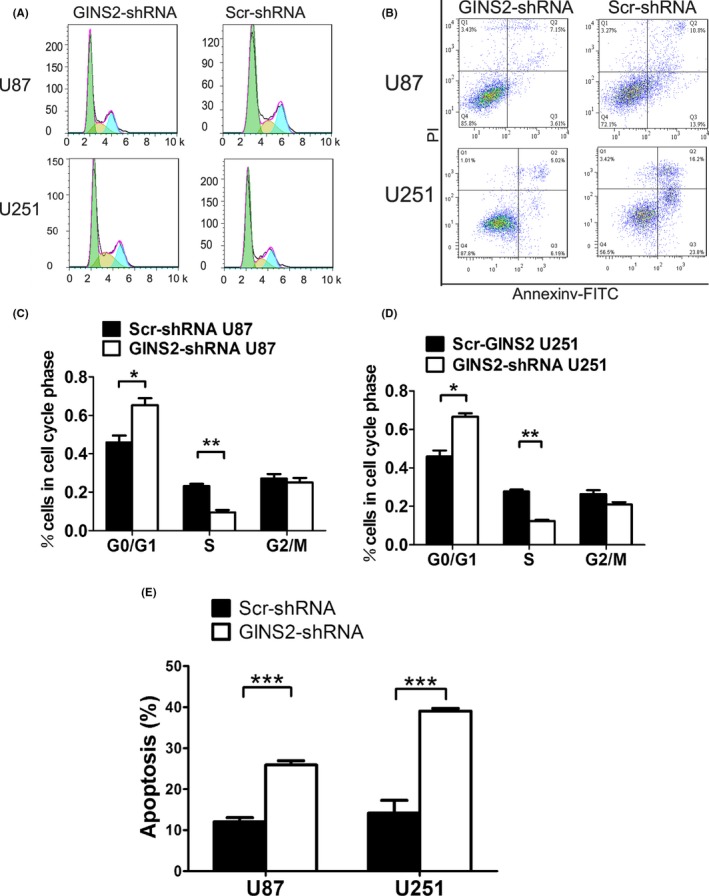
Effect of GINS2‐shRNA and Scr‐shRNA transfection on cell cycle and apoptosis of U87 and U251 glioma cells, assessed via FCM. A, Period distribution of U87 and U251 cells after GINS2‐shRNA transfection. B, Apoptosis by Annexin V staining of U87 and U251 cells after GINS2‐shRNA transfection. C‐D, Ratio analysis of U87, U251, G0/G1 phase, S phase, and G2/M phase cells after GINS2‐shRNA and Scr‐shRNA transfection. The results represent mean ± SD of three separate experiments (*P < 0.05). E, Apoptosis rate of U87 and U251 cells after GINS2‐shRNA and Scr‐shRNA transfection. The results represent mean ± SD of three separate experiments (*P < 0.05)
3.6. Apoptosis rate of glioma cells increased after GINS2 suppression
Apoptosis is another significant factor influencing tumor growth.25, 26, 27 Herein, we detected the glioma cell apoptosis by FCM after the knockdown of GINS2. The results indicated that, compared with Scr‐shRNA, the apoptosis rate of U251 cells rose remarkably from 14.17 to 39.09 after transfection of GINS2‐shRNA; meanwhile, the apoptosis rate of U87 cells increased only slightly (Figure 4B).
3.7. Suppression of GINS2 expression lowered the tumorigenicity of glioma cells
To confirm the inhibitory effect of GINS2 knockdown on glioma growth, we conducted subcutaneous tumorigenicity experiments on 12 BALB/c‐nu/nu nude mice. After being injected with U87 or U251 cells, all mice experienced nodule formation and were killed 21 days later. In addition, all mice underwent bioluminescence imaging on IVIS, on the 1st, 3rd, 7th, 14th, and 21st day of the experiment. The size and weight of the tumor were measured upon collection. The results showed that, compared with the negative control group, the interference group featured a lower tumorigenicity level (Figure 5).
Figure 5.
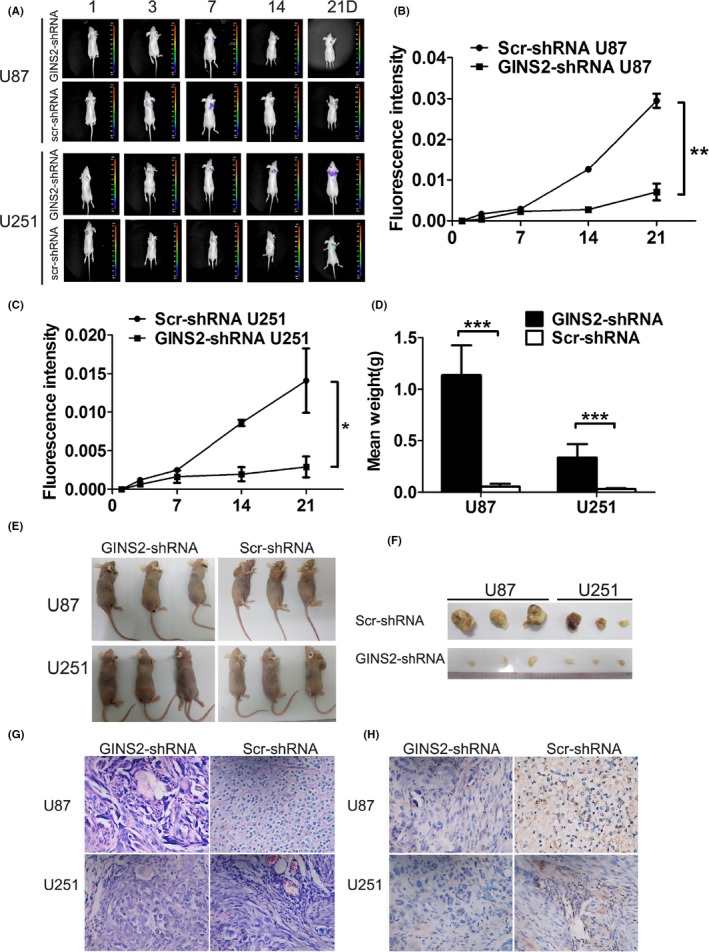
Growth of U87 and U251 cells after GINS2 suppression in vivo. A, B, C, Fluorescence imaging indicated that tumor formation of the GINS2‐shRNA group was reduced compared with the Scr‐shRNA group. E, Tumor formation at 3 wk after subcutaneous implantation of glioma cells in nude mice (GINS2‐shRNA group vs Scr‐shRNA group). D, F, Comparison of tumor weight between the GINS2‐shRNA group and the Scr‐shRNA group. G‐H, Expression of GINS2 in subcutaneous tumors of nude mice via HE staining and immunohistochemistry. The results represent mean ± SD of three separate experiments (*P < 0.05).
3.8. GINS2 inhibition affected key signaling pathways mediating glioma growth
The abovementioned experimental results indicate that GINS2 plays a significant role in the proliferation and growth of glioma cell strains U87 and U251. However, the exact underlying mechanism is yet to be determined. Here, we transfected U87 cells with GINS2‐shRNA and then determined their genome‐wide expression profile via high‐throughput sequencing, obtaining a total of 12 429 differentially expressed genes (P < 0.05 and absolute fold change >2). Of these, 5336 genes were upregulated and 7093 genes were downregulated (Figure 6A‐B).
Figure 6.
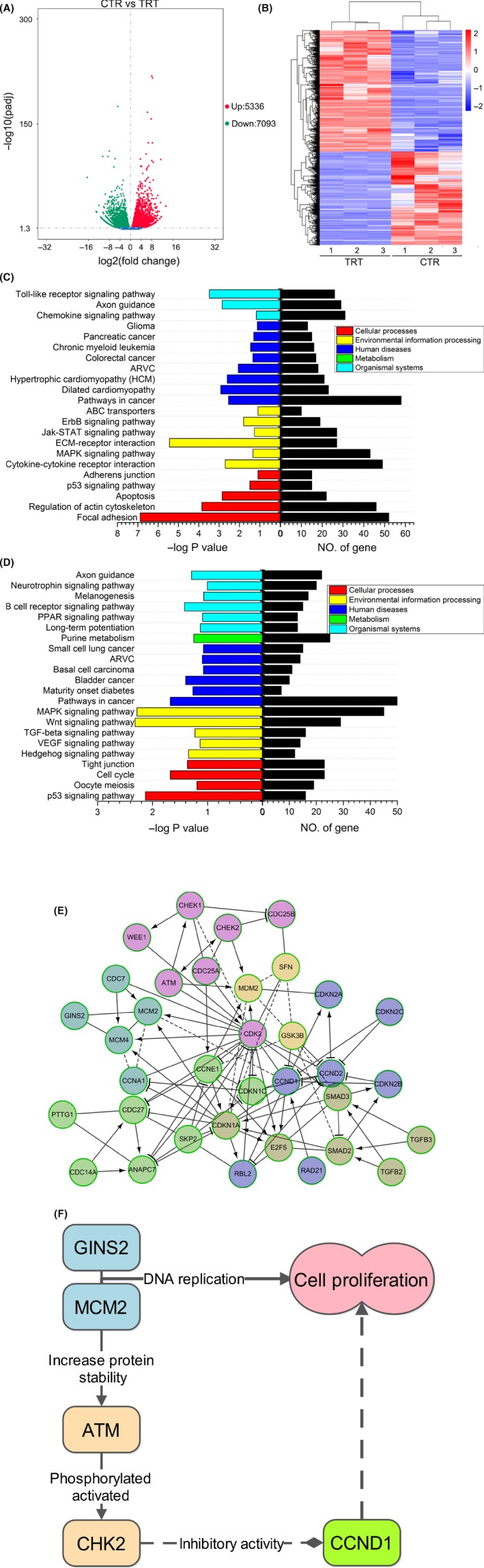
Genome‐wide sequencing profile of glioma cells after GINS2 suppression. A, Volcano plot of differentially expressed genes. The red part represents upregulated genes, whereas the green stands for downregulated ones. Scr‐shRNA vs GINS2‐shRNA comparison: the total number of differentially expressed genes was 12 429, of which 5336 were upregulated and 7093 were downregulated. B, Differences in gene cluster heat map. The horizontal axis reveals the clustering results of samples, while the vertical axis shows the clustering results of differentially expressed genes. Red is indicative of high expression, while the blue part means low expression (∣fold change∣ > 2, P < 0.05). C, D, KEGG enrichment histogram. C gene up‐pathway, D gene down‐pathway.The left reveals the gene enrichment in different pathways. Horizontal axis on the left is a logarithmic function based on P‐value, representing the enrichment degree; different colors represent different biological functions. The right reveals the number of enriched genes in relevant pathways. E, Networks were constructed for differentially expressed genes after GINS2 suppression by Cytoscape Reactome FI plugin. Solid arrow in the figure represents a molecular interaction or relation, and dotted line means indirect link or unknown reaction. F, GINS2 regulates downstream molecules in cell cycle‐associated signal pathways.
Though the KEGG method, we found that genes with different expressions mainly gathered in 57 signaling pathways (based on a P < 0.05 threshold), including the MAPK signaling pathway, Wnt signaling pathway, P53 signaling pathway, cell cycle signaling pathway, etc. These pathways play important roles in the development and progression of tumors (Figure 6C‐F).
3.9. Suppression of GINS2 can decrease glioma cell proliferation by arresting the cell cycle
To determine the possible mechanism by which GINS2 inhibits glioma growth, key molecules in signaling pathways regulating the cell cycle were assessed. The GINS2 gene and MCM2 molecule jointly participate in the process of DNA replication by forming the “minimum complex.”28, 29 The detection of MCM2 and ATM proteins in the Input group revealed normal expression. The detection of MCM2 protein in the IP group suggested that Co‐IP acquired MCM2, the bait protein; the detection of ATM protein highlighted the interaction between ATM and MCM2 (Figure 7A). Additionally, we observed that the MCM2 and ATM proteins are mainly located in the nucleus via laser confocal microscopy (Figure 7B). So, the Co‐IP experiment demonstrated that ATM interacts with MCM2. In addition, the downregulation of ATM, CHEK2, and CCND1, important signaling molecules in controlling the cell cycle, can lead to cell cycle arrest. WB showed that when the expression of GINS2 was inhibited, the expressions of MCM2, ATM, CHEK2, p‐CHEK2, and CCND1 declined, as did the proliferative ability of glioma cells (Figure 7C‐D). Collectively, these results indicate that GINS2 facilitates glioma cell proliferation by regulating the signaling pathways of ATM molecules through chromatin‐gathered MCM2.
Figure 7.
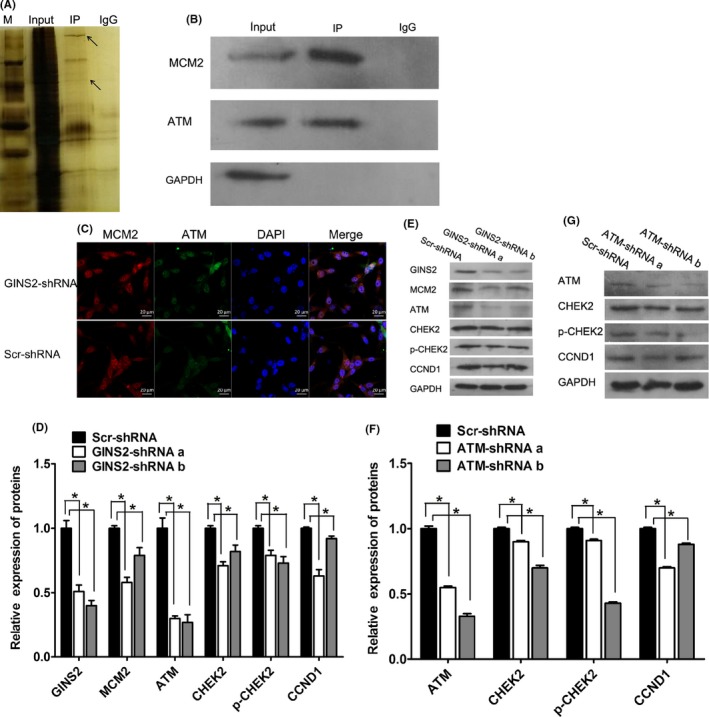
GINS2 suppression can inhibit glioma cell proliferation by regulating gene expression related to cell cycle pathways. A, B, Interaction between MCM2 and ATM by Co‐IP. A, silver staining picture. The two arrows indicate the bait protein MCM2 and the captured protein ATM; the upper arrow is ATM, and the lower one is MCM2. B, Western blot X‐ray plate. MCM2 and ATM protein levels in the Input group suggest normal expression. The detection of MCM2 protein in the IP group suggested that Co‐IP acquired MCM2, the bait protein, and the detection of ATM indicated the interaction between ATM and MCM2. C, IF double standard experiment. Red fluorescence is ATM; green fluorescence is MCM2; blue is the DAPI counterstaining. Confocal laser scanning microscope, CLSM. D, E, After suppressing GINS2 expression in human U87 glioma cells, the protein levels of MCM2, CHEK2, p‐CHEK2, CCND1, etc, declined, as shown by WB analysis. F, G, After suppressing ATM expression in human U87 glioma cells, the protein levels of CHEK2, p‐CHEK2, CCND1, etc, declined by WB analysis. The results represent mean ± SD of three separate experiments (*P < 0.05)
4. DISCUSSION
GINS2 upregulation is closely related to the initiation and progression of multiple tumors. For example, in lung adenocarcinoma, the expression of GINS2 correlates with TNM stages.17 Studies have also shown that the expression of GINS2 in human breast cancer cells and early cervical cancer cells is upregulated, promoting tumor development.18, 20 However, to date, the role of GINS2 in glioma remains unclear. Given its proven involvement in tumor development, it is necessary to perform an in‐depth study on the effect of GINS2 overexpression on the biological behavior of glioma, as well as to elucidate the specific regulatory mechanism underlying glioma cell growth. General classification of glioma includes 4 levels (grade I, II, III, and IV), determined based on malignant degree.30 In this study, the expression of GINS2 is positively correlated with glioma grade, indicating that GINS2 is likely to play an important role in the development of glioma.
To date, studies highlighting the relationship between GINS2 expression and tumor progression have mainly focused on tumor cell growth and the cell cycle. Zhang et al19 discovered that the silencing of GINS2 gene can reduce the proliferation of acute promyelocytic leukemia cells and induce apoptosis, which agrees with the present results in glioma cells. After the construction of an RNA interference vector and subsequent transfection into U87 and U251 cells, we found that the knockdown of GINS2 can impair the growth of these cells, leading to an obvious increase in apoptotic rate, as well as a substantial decline in cell clonality. Moreover, obtained data also suggested that GINS2 silencing impaired the subcutaneous tumor formation capacity of U87 and U251 glioma cells. FCM after shRNA‐GINS2 transfection showed that the proportion of U87 and U251 cells in the S stage declined dramatically, while the ratio of cells in the G0/G1 stage increased, suggesting cell cycle arrest at G1. This shows that the knockdown of GINS2 disrupts the transition process of glioma cells at the G1/S stage, thereby suppressing tumor cell proliferation. Previous studies showed that GINS2 is located in the nucleus and acts in concert with other proteins.31 Some studies32 have indicated that GINS2 is likely to influence the growth and invasiveness of tumors by regulating the expression of matrix metallopeptidase 9. Proteins such as ATM and CHEK2 are also thought to influence the GINS2 regulation of the tumor cell cycle.19 A recent study showed that the amplification pattern of GINS2 also plays a significant part in the differentiation of neural stem cells into glioma cells.33 Although we proved the vital role of GINS2 in vitro using two glioma cell lines, U87 and U251, it remains unclear how GINS2 influences glioma cell growth.
To study the mechanism underlying the GINS2 regulation of glioma growth, we examined the whole‐gene expression profile of U87 cells after lentivirus‐based shRNA transfection. Analysis revealed 12 429 differentially expressed genes (including 7093 downregulated genes and 5336 upregulated genes). After KEGG pathway enrichment analysis of obtained digital gene expression profiling data, multiple pathways related to the development of tumors were identified. For example, the MAPK signaling pathway, which mediates a series of physiological activities such as cell growth, development, differentiation, and apoptosis, is an important signaling pathway involved in carcinogenesis.34, 35 The Wnt signaling pathway, related to the occurrence, proliferation, invasion, and transference of tumors, has great potential as both a marker for tumor diagnosis and target for cancer treatments.36, 37 Besides, other pathways, such as the P53 signaling pathway, are also highly connected with tumor development.38, 39 Therefore, in order to verify these bioinformatics analysis results, we further assessed relevant cell cycle signaling pathways to determine their connection with GINS2. Generally, the GINS2 gene and MCM2 molecule jointly participate in the DNA replication process as a “minimum complex”.31, 40 High expression of GINS2 in tumor cells is bound to gather MCM2 molecules to chromatin. As a significant upstream signaling molecule controlling the cell cycle, ATM can phosphorylate and activate CHEK2, CCND1, etc, blocking the cell cycle during DNA duplication or during exposure to other external factors. ATM can also phosphorylate and activate TP53, triggering procedural cell death. Reports have shown that the suppression of ATM in glioma cells41, 42, 43, 44, 45 can not only reduce tumor cell growth by regulating cellular proliferation and apoptosis, but can also improve the sensitivity of glioma cells to radiotherapy and chemotherapy. Using laser confocal microscopy, we highlighted the interaction between MCM2 and ATM. Meanwhile, knockdown of GINS2 in U87 cells led to a decline in MCM2 expression, which further caused a decrease in the expression of ATM, CHEK2, p‐CHEK2, and CCND1, as well as in the proliferative ability of glioma cells. Similar to the influence of interfered ATM on glioma cells, the results above indicate that GINS2 in glioma can ultimately facilitate tumor cell growth by regulating the expression of certain genes that mediate cell cycle pathways.
In summary, our study revealed that the protein expression level of GINS2, which facilitates tumor growth in glioma patients, positively correlates with glioma grade. Mechanism research showed that GINS2 likely enhances glioma cell growth by regulating MCM2, ATM, CHEK2, and CCND1 and subsequently affecting the cell cycle. Therefore, GINS2 should be further investigated as a glioma prognostic marker and novel therapeutic target.
5. CONCLUSIONS
This is the first report on the role of GINS2 in glioma, which shows that GINS2 expression positively correlates with glioma grade. Moreover, obtained results indicated that the suppression of GINS2 can decrease the proliferation and tumorigenicity of glioma cells, while inducing apoptosis and cell cycle arrest. RNA sequencing and bioinformatics analysis on glioma cells before and after GINS2 knockdown revealed that multiple cancer‐related signaling pathways might participate in the GINS2 regulation of glioma growth. Co‐IP and WB experiments proved that GINS2 can block the cell cycle by regulating downstream molecules such as MCM2, ATM, and CHEK2, thereby suppressing tumor growth. This research enhances our understanding of the glioma development mechanism and might contribute to the establishment of new therapeutic strategies.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by Grants from the Medical Scientific Research Foundation of Guangdong Province (No. A2015532), Science and Technology Planning Project of Guangdong Province (No. 2014A020212727)
Shen Y‐L, Li H‐Z, Hu Y‐W, Zheng L, Wang Q. Loss of GINS2 inhibits cell proliferation and tumorigenesis in human gliomas. CNS Neurosci Ther. 2019;25:273–287. 10.1111/cns.13064
REFERENCES
- 1. Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the united states in 2006–2010. Neuro‐Oncol. 2013;15:1‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goodenberger ML, Jenkins RB. Genetics of adult glioma. Cancer Genet. 2012;11:613‐621. [DOI] [PubMed] [Google Scholar]
- 3. Omuro A, DeAngelisL M. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310:1842‐1850. [DOI] [PubMed] [Google Scholar]
- 4. Bie L, Zhao G, Cheng P, et al. The accuracy of survival time prediction for patients with glioma is improved by measuring mitotic spindle checkpoint gene expression. PloS One. 2011;6:e25631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gladson CL, Prayson RA, Liu W. The pathobiology of glioma tumors. Annu Rev Pathol. 2010;5:33‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grossman SA, Ye X, Piantadosi S, et al. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res. 2010;16:2443‐2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987‐996. [DOI] [PubMed] [Google Scholar]
- 8. Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ. Exciting new advances in neuro‐oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60:166‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rohle D, Popovici‐Muller J, Palaskas N, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science. 2013;340:626‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun Y, Zhang W, Chen D, et al. A glioma classification scheme based on coexpression modules of EGFR and PDGFRA. Proc Natl Acad Sci U S A. 2014;111:3538‐3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cancer Genome Atlas Research Network , Brat DJ, Verhaak RG, et al. Comprehensive, integrative genomic analysis of diffuse lower‐grade gliomas. N Engl J Med. 2015;372:2481‐2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hayashi R, Arauchi T, Tategu M, Goto Y, Yoshida K. A combined computational and experimental study on the structure‐regulation relationships of putative mammalian DNA replication initiator GINS. Genomics Proteomics Bioinformatics. 2006;4:156‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ryu B, Kim DS, Deluca AM, Alani RM. Comprehensive expression profiling of tumor cell lines identifies molecular signatures of melanoma progression. PLoS One. 2007;2:e594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nakahara I, Miyamoto M, Shibata T, et al. Up‐regulation of PSF1 promotes the growth of breast cancer cells. Genes Cells. 2010;15:1015‐1024. [DOI] [PubMed] [Google Scholar]
- 16. Obama K, Ura K, Satoh S, Nakamura Y, Furukawa Y. Up‐regulation of PSF2, a member of the GINS multiprotein complex, in intrahepatic cholangiocarcinoma. Oncol Rep. 2005;14:701‐706. [PubMed] [Google Scholar]
- 17. Liu M, Pan H, Zhang F, et al. Identification of TNM stage‐specific genes in lung adenocarcinoma by genome‐wide expression profiling. Oncol Lett. 2013;6:763‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zheng M, Zhou Y, Yang X, et al. High GINS2 transcript level predicts poor prognosisand correlates with high histological grade and endocrine therapy resistance through mammary cancer stem cells in breast cancer patients. Breast Cancer Res Treat. 2014;148:423‐436. [DOI] [PubMed] [Google Scholar]
- 19. Zhang X, Zhong L, Liu BZ, Gao YJ, Gao YM, Hu XX. Effect of GINS2 on proliferation and apoptosis in leukemic cell line. Int J Med Sci. 2013;10:1795‐1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ouyang F, Liu J, Xia M, et al. GINS2 is a novel prognostic biomarker and promotes tumor progression in early‐stage cervical cancer. Oncol Rep. 2017;37:2652‐2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Diao J, Wu C, Zhang J, et al. Loss of diacylglycerol kinase‐Ζ inhibits cell proliferation and survival in human gliomas. Mol Neurobiol. 2016;53:5425‐5435. [DOI] [PubMed] [Google Scholar]
- 22. Wu H, Wei L, Fan F, et al. Integration of Hippo signalling and the unfolded protein response to restrain liver overgrowth and tumorigenesis. Nat Commun. 2015;6:6239‐6254. [DOI] [PubMed] [Google Scholar]
- 23. Duan Q, Xiao Y, Zhu L, et al. BET bromodomain is a novel regulator of TAZ and its activity. Biochim Biophys Acta. 2016;1859:1527‐1537. [DOI] [PubMed] [Google Scholar]
- 24. Markert EK, Vazquez A. Mathematical models of cancer metabolism. Cancer Metab. 2015;3:14‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Enderling H, Hahnfeldt P. Cancer stem cells in solid tumors: is ‘evading apoptosis’ a hallmark of cancer? Prog Biophys Mol Biol. 2011;106:391‐399. [DOI] [PubMed] [Google Scholar]
- 26. Fernald K, Kurokawa M. Evading apoptosis in cancer. Trends Cell Biol. 2013;23:620‐633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646‐674. [DOI] [PubMed] [Google Scholar]
- 28. Nagata M, Ishino S, Yamagami T, et al. The Cdc45/RecJ‐like protein forms a complex with GINS and MCM, and is important for DNA replication in Thermococcus kodakarensis. Nucleic Acids Res. 2017;45:10693‐10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu Y, Gristwood T, Hodgson B, Trinidad JC, Albers SV, Bell SD. Archaeal orthologs of Cdc45 and GINS form a stable complex that stimulates the helicase activity of MCM. Proc Natl Acad Sci U S A. 2016;113:13390‐13395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goodenberger ML, Jenkins RB. Genetics of adult glioma. Cancer Genet. 2012;205:613‐621. [DOI] [PubMed] [Google Scholar]
- 31. Takaya J, Kusunoki S, Ishimi Y. Protein interaction and cellular localization of human CDC45. J Biochem. 2013;153:381‐388. [DOI] [PubMed] [Google Scholar]
- 32. Peng L, Song Z, Chen D, et al. GINS2 regulates matrix metallopeptidase 9 expression and cancer stem cell property in human triple negative Breast cancer. Biomed Pharmacother. 2016;84:1568‐1574. [DOI] [PubMed] [Google Scholar]
- 33. Fischer U, Kim E, Keller A, Meese E. Specific amplifications and copy number decreases during human neural stem cells differentiation towards astrocytes, neurons and oligodendrocytes. Oncotarget. 2017;8:25872‐25884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lin L, Cai J, Jiang C. Recent advances in targeted therapy for glioma. Curr Med Chem. 2017;24:1365‐1381. [DOI] [PubMed] [Google Scholar]
- 35. Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res. 2015;35:600‐604. [DOI] [PubMed] [Google Scholar]
- 36. Zhang L, Liu H, Mu X, Cui J, Peng Z. Dysregulation of Fra1 expression by Wnt/β‐catenin signalling promotes glioma aggressiveness through epithelial‐mesenchymal transition. Biosci Rep. 2017;37:pii:BSR20160643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wickström M, Dyberg C, Milosevic J, et al. Wnt/β‐catenin pathway regulates MGMT gene expression in cancer and inhibition of Wnt signalling prevents chemoresistance. Nat Commun. 2015;6:8904‐8914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yu G, Li N, Zhao Y, Wang W, Feng XL. Salidroside induces apoptosis in human ovarian cancer SKOV3 and A2780 cells through the p53 signaling pathway. Oncol Lett. 2018;15:6513‐6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tu W, Zhang Q, Liu Y, et al. Fluoride induces apoptosis via inhibiting SIRT1 activity to activate mitochondrial p53 pathway in human neuroblastoma SH‐SY5Y cells. Toxicol Appl Pharmacol. 2018;347:60‐69. [DOI] [PubMed] [Google Scholar]
- 40. Kang YH, Galal WC, Farina A, Tappin I, Hurwitz J. Properties of the human Cdc45/Mcm2‐7/GINS helicase complex and its action with DNA polymerase epsilon in rolling circle DNA synthesis. Proc Natl Acad Sci U S A. 2012;109:6042‐6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Han B, Cai J, Gao W, et al. Loss of ATRX suppresses ATM dependent DNA damage repair by modulating H3K9me3 to enhance temozolomide sensitivity in glioma. Cancer Lett. 2018;419:280‐290. [DOI] [PubMed] [Google Scholar]
- 42. Yang CH, Wang Y, Sims M, et al. MicroRNA203a suppresses glioma tumorigenesis through an ATM‐dependent interferon response pathway. Oncotarget. 2017;8:112980‐112991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li Y, Li L, Wu Z, et al. Silencing of ATM expression by siRNA technique contributes to glioma stem cell radiosensitivity in vitro and in vivo. Oncol Rep. 2017;38:325‐335. [DOI] [PubMed] [Google Scholar]
- 44. Blake SM, Stricker SH, Halavach H, et al. Inactivation of the ATMIN/ATM pathway protects against glioblastoma formation. Elife. 2016;5:e08711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhan XH, Xu QY, Tian R, et al. MicroRNA16 regulates glioma cell proliferation, apoptosis and invasion by targeting Wip1‐ATM‐p53 feedback loop. Oncotarget. 2017;8:54788‐54798. [DOI] [PMC free article] [PubMed] [Google Scholar]


