Summary
Stem cell transplantation, especially treatment with bone marrow mesenchymal stem cells (BMSCs), has been considered a promising therapy for the locomotor and neurological recovery of spinal cord injury (SCI) patients. However, the clinical benefits of BMSCs transplantation remain limited because of the considerably low viability and inhibitory microenvironment. In our research, low‐intensity pulsed ultrasound (LIPUS), which has been widely applied to clinical applications and fundamental research, was employed to improve the properties of BMSCs. The most suitable intensity of LIPUS stimulation was determined. Furthermore, the optimized BMSCs were transplanted into the epicenter of injured spinal cord in rats, which were randomized into four groups: (a) Sham group (n = 10), rats received laminectomy only and the spinal cord remained intact. (b) Injury group (n = 10), rats with contused spinal cord subjected to the microinjection of PBS solution. (c) BMSCs transplantation group (n = 10), rats with contused spinal cord were injected with BMSCs without any priming. (d) LIPUS‐BMSCs transplantation group (n = 10), BMSCs stimulated with LIPUS were injected at the injured epicenter after contusion. Rats were then subjected to behavioral tests, immunohistochemistry, and histological observation. It was found that BMSCs stimulated with LIPUS obtained higher cell viability, migration, and neurotrophic factors expression in vitro. The rate of apoptosis remained constant. After transplantation of BMSCs and LIPUS‐BMSCs postinjury, locomotor function was significantly improved in LIPUS‐BMSCs transplantation group with higher level of brain‐derived neurotrophic factor (BDNF) and nerve growth factor (NGF) in the epicenter, and the expression of neurotrophic receptor was also enhanced. Histological observation demonstrated reduced cavity formation in LIPUS‐BMSCs transplantation group when comparing with other groups. The results suggested LIPUS can improve BMSCs viability and neurotrophic factors expression in vitro, and transplantation of LIPUS‐BMSCs could promote better functional recovery, indicating possible clinical application for the treatment of SCI.
Keywords: bone marrow mesenchymal stem cells, low‐intensity pulsed ultrasound, spinal cord injury, stem cell transplantation
1. INTRODUCTION
Spinal cord injury (SCI) is broadly acknowledged as a crucial issue in clinical and experimental research due to its sudden yet unforeseeable nature and the extremely limited regenerative capability of the injured spinal cord, which could cause considerable losses to both individuals and the society. Recent estimates have stated that the annual incidence of SCI is around 17 000 new SCI cases in the United States.1 In a global context, the incidence of SCI in 2007 was estimated to be 23 cases per million population or 179 312 cases per annum.2 The pathology of SCI can be characterized by two phases of lesions: the primary lesion involves mechanical damage of the spinal cord relevant to hemorrhage, electrolyte flow and the release of lysosomes and other cellular components. The secondary lesion includes edema, ischemia, inflammation reactions, ionic imbalance (eg, intracellular calcium), excitotoxicity, caspase and calpain activation, neurotransmitter accumulation, and apoptosis.3, 4 The subacute stage of SCI is known to be deleterious for axonal regeneration and functional recovery. This indicates that spontaneous recovery occurs in a limited time window. To date, no current treatments can substantially address the issues associated with injured spinal cords.
Stem cell‐based therapy, especially BMSCs, has yielded encouraging results.5, 6, 7, 8 BMSCs transplantation therapy is promising in mitigating the extent of SCI, not only due to a favorable ethical profile and safety, but also due to providing beneficial effects on various postinjury aspects: inflammation, apoptosis, axonal regrowth, angiogenesis, tissue sparing, astroglial scar, and motor recovery.9, 10, 11, 12 It is well known that BMSCs secrete a large variety of molecules and many studies have shown beneficial impacts from these factors such as brain‐derived neurotrophic factor (BDNF), nerve growth factor (NGF), vascular endothelial growth factor (VEGF), insulin‐like growth factor (IGF), epidermal growth factor (EGF), and more.13, 14, 15, 16, 17, 18, 19
Previously, neurotrophin and physical stimulation were commonly employed to enhance the effect of BMSCs. Specifically, low‐intensity pulsed ultrasound (LIPUS), as a physical stimulation, appeared to be an effective assistant method. Over the past few decades, the medical use of ultrasound has been extended beyond imaging and diagnosis toward therapeutic applications. LIPUS exposure has been shown to increase proliferation in BMSCs.20, 21 Studies of Lv et al showed that LIPUS and induced pluripotent stem cells‐derived neural crest stem cells (iPSCs‐NCSCs) could promote the regeneration and reconstruction of rat transected sciatic nerve, and LIPUS could enhance the viability and proliferation of iPSCs‐NCSCs.22 Tsuang et al demonstrated that intervention with low‐intensity pulsed ultrasound could promote Schwann cell proliferation and prevent cell death.23 In this study, we investigate how LIPUS affect BMSCs in vitro and then transplant the optimized BMSCs to treat rats with SCI.
2. MATERIALS AND METHODS
2.1. Animals and ethics statement
Ten adult Wistar rats (female, 100‐120 g) were prepared for BMSCs extraction, and another 40 adult Wistar rats (female, 250 ± 10 g) were obtained for in vivo experiments. These rats all came from the Radiation Study Institute‐Animal Center, Tianjin, People's Republic of China. The rats were provided with water and food freely, and were kept on a 12 hours light/dark cycle in a humidity and temperature‐controlled animal facility. All the animal experimental procedures were executed according to the National Guidelines for Experimental Animal Welfare (Ministry of Science and Technology of People's Republic of China, 2006) and approved by the Animal Ethical and Welfare Committee (AEWC) in Tianjin Medical University (Number of Animal use permit: TMUaMEC2017006).
2.2. Isolation and culture of BMSCs
Female Wistar rats, weighing around 100‐120 g, were sacrificed by cervical vertebra luxation after being anesthetized. Rat BMSCs were isolated from the bone marrow of bilateral tibial and femoral bones, then purified and passaged by attachment method. Femurs and tibias were separated and both ends of each femur or tibia were cut. Pooled cells from different donors were cultured in T‐75 cell culture flask, with a cell concentration of 1 × 105/mL, using complete media composed of DMEM‐F12 (Gibco, USA), supplemented with fetal bovine serum (FBS) (10%, v/v, Solarbio Co., Beijing, China), penicillin: streptomycin (100 μL/100 mL) (Solarbio Co., Beijing, China). Cells were incubated under standard cell culture conditions with 5% CO2, at 37°C and 95% relative humidity. The medium was changed every three days, and BMSCs were passaged when 80%‐90% confluency was reached. BMSC identity was confirmed on the basis of morphological criteria, plastic adherence, and specific surface antigen expression: CD29(+), CD90(+), CD34(−), CD45(−).
2.3. Experimental equipment
The LIPUS exposure consisted of a power supply, a function generator, an amplification module, and a transducer. The central frequency of transducer is 1 MHz, whose outside diameter is 10 mm. The distance from the surface of the transducer to the cell layer was 5 mm, which was fixed and kept constant throughout all experiments. The schematic representation of LIPUS exposure process is shown in Figure 1A. According to various voltage and frequency, the acoustic intensity, which was used to stimulate BMSCs, was measured by Hangzhou Applied Acoustics Research Institute.
Figure 1.
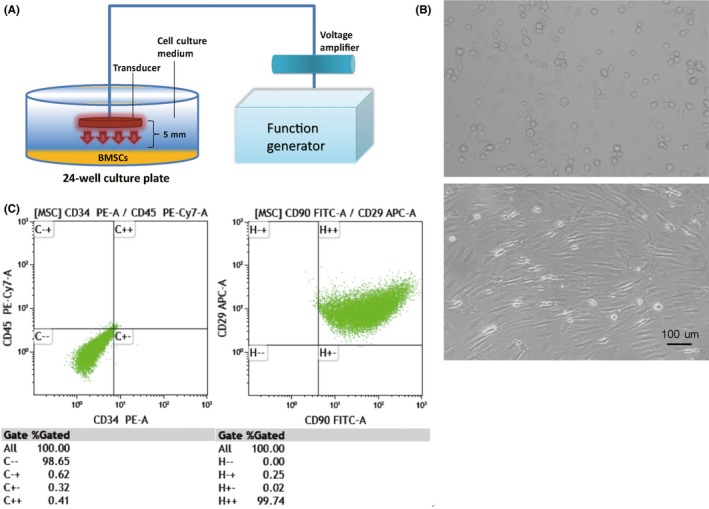
A, LIPUS (low‐intensity pulsed ultrasound) stimulation process. B, Morphology and characterization of bone marrow mesenchymal stem cells (BMSCs). BMSCs are spindle‐shaped with fibroblast‐like process. Light microscopy, scale bar = 100 μm. C, BMSC identity is confirmed based on morphological criteria, plastic adherence, and specific surface antigen expression: CD29(+), CD90(+), CD34(−), CD45(−)
2.4. LIPUS stimulation
BMSCs were seeded at a density of 1 × 105 per well in 24‐well plates 3 days before LIPUS experiments to permit cell attachment to the plates. Prior to ultrasound exposure, the medium was rinsed with phosphate‐buffered saline (PBS) (Solarbio Co., Beijing, China). LIPUS was used to stimulate the BMSCs after adding 1 mL of medium to each well. To determine the optimal LIPUS intensity, cells were exposed by pulsed ultrasound with different intensities (10, 30, 50, 70 mW/cm2, 3 min/d, 3 days). The control group underwent the same submersion without ultrasound stimulation. After LIPUS stimulation for predetermined parameters, samples were rinsed again using PBS.
2.5. Determination of optimal LIPUS parameters
After LIPUS stimulation, the cells were lifted with 0.25% trypsin‐EDTA solution (Solarbio Co., Beijing, China). Cells were seeded at a density of 1 × 104 per well (100 μL) in 96‐well plates, while a blank well was kept for medium without cells in every plate. Cell proliferation was quantified 24 hours later by cell counting kit‐8 (CCK‐8) following the manufacturer's protocol (BestBio, China). 10 μL CCK‐8 solution was added in each well, and the complete media was incubated for 2 hours before determination. The final optical density (OD) was measured at a wavelength of 450 nm to estimate cell proliferation in the different groups. The experiment was repeated eight independent times. BMSCs stimulated by LIPUS with the best parameter were set as the experimental group, to be compared with the control group in other tests.
2.6. Flowcytometric analysis of culture‐expanded BMSCs
To characterize the cell markers of culture‐expanded cells after LIPUS stimulation, monolayer adherent cells (Passage 3) were trypsinized and stained with monoclonal antibodies specific for BMSCs as follows: anti‐CD90‐phycoerythrin (PE), anti‐CD29‐fluorescein isothiocyanate (FITC), anti‐CD34‐FITC, and anti‐CD45‐FITC. Flowcytometric analyses were performed using PAS flowcytometry (Partec GmbH, Germany).
2.7. Trophic factor examination
Cells were harvested using RIPA lysis buffer (Beyotime Institute of Biotechnology, China). Equal amounts of protein (50 μg) were loaded onto a gel for 8% sodium dodecyl sulfate‐polyacrylamide gel (SDS‐PAGE) and followed by electrophoresis for 1 hour at 150 V. The separated proteins were transferred to a nitrocellulose membrane for 2 hours at 350 mA and were subsequently incubated with primary antibodies overnight at 4°C. Rabbit anti‐neurotrophin‐3 polyclonal primary antibody (Abcam, 1:10 000), rabbit anti‐NGF monoclonal primary antibody (Abcam, 1:1000), and rabbit anti‐BDNF polyclonal antibody (Bioworld, 1:1000) were applied to detect, NGF and BDNF protein expression 48 hours posttransduction. After washing, the membranes were treated with conjugated secondary antibody, Cy3 labeled goat anti‐rabbit IgG(H+L) (1:5000, Beyotime Institute of Biotechnology, China) for 2 hours at room temperature, and then washed repeatedly. The membranes were developed using an enhanced chemiluminescence (ECL) detection system to transfer to film. GAPDH was used as a loading control.
2.8. Population doubling time (PDT) of BMSCs
The populations of BMSCs in each group from passage 3 were trypsinized from the culture disks before plating into the 24‐well plates at a density of 3 × 103/well. Each group of BMSCs was seeded in 12 wells. Three days later, the cells were harvested with trypsin/EDTA and counted using a hemocytometer. The mean value of the cell number counts was calculated from 12 wells in each group and the mean population doubling time was obtained for each group according to the following formula: population doubling time = T × lg2/(lgNt − lgN0), where T is the culture time, N0 is the initial cell number, and Nt is the harvested cell number.
2.9. Cell apoptosis
Apoptosis was evaluated with the TUNEL method, according to the manufacturer's protocol (Roche). Air‐dried BMSCs were fixed with freshly prepared paraformaldehyde for 1 hour at room temperature. The cells were rinsed with PBS before and after they were incubated with Triton X‐100 on ice for 2 minutes. Then, the BMSCs with their peripheral area were dried again. The TUNEL reaction mixture was prepared immediately (Enzyme solution: Label Solution = 1:9) and then added on the cells (50 μL each well). Then, BMSCs samples were incubated in a humidified atmosphere in the dark for 60 minutes at 37°C. PBS was used to rinse the slide. Label Solution was used alone for negative controls, while fixed and permeabilized cells were incubated with micrococcal nuclease for 10 minutes at room temperature for positive controls. Photomicrographs of 20 random fields per experimental condition were taken (Olympus AX70) at 40× magnification.
2.10. Transwell migration assay
Cell invasion ability was assessed using a 24‐well transwell chamber (6.5‐mm Transwell® with 8.0 µm pore polyester membrane insert, product #3464, Corning Costar, New York, NY, USA) covered with Matrigel (BD Biosciences, San Diego, CA, USA) as described in the manufacturers’ protocol. Cells were treated as indicated and serum‐starved for 24 hours. Approximately 2 × 104 cells were resuspended in 500 μL FBS‐free medium and seeded into the upper wells. The lower wells were added with 500 μl of 20% FBS medium. After 24‐hours incubation, cells on the upper surface of the filter were removed with a cotton swab. The remaining cells in the lower chambers were washed with PBS, fixed with methanol, and stained with 0.1% crystal violet. The invaded cells were photographed and the cell number was counted from at least 10 random microscopic central fields.
2.11. Statistical analysis
The data collected in the present study are presented as mean standard deviation (mean SD) and analyzed by one‐way repeated measures ANOVA, followed by post hoc test for least significant difference (LSD) to determine differences between two groups. Statistical significance was defined as P < 0.05.
2.12. Establishment of SCI model
Forty female Wistar rats, weighing 250 ± 10 g, were randomized into four groups as follows: Sham group (n = 10), in which rats received laminectomy only and the spinal cord remained intact. Injury group (n = 10), in which rats with contused spinal cord subjected to the microinjection of PBS solution. BMSCs transplantation group (n = 10), in which rats with contused spinal cord were injected with BMSCs without any priming. LIPUS‐BMSCs transplantation group (n = 10), in which BMSCs stimulated with LIPUS were injected at the injured epicenter after contusion. Each experimental rat was anesthetized through intraperitoneal injection with 10% chloral hydrate (0.33 mL/100 g). Skin preparation was performed at the operative region, and iodophor was chosen as the sterilization method. The vertebral plate, with spinous process, was removed with a micro‐rongeur (Aesculap AG, Tuttlingen, Germany) at T10 to unfold the dura. The model of SCI (T10 plane) was created using NYU impactor machine (WM Keck Center for Collaborative Neuroscience, Piscataway Township, NJ, USA), with 10 g weight and 50 mm height. In each SCI model, the rat's two hindlimbs twitched, with the tail wagged involuntarily, which was in accordance with the standards of the SCI model. After the operation, the rats were put in incubation chambers with appropriate humidity and temperature until they awoke. After that, the rats were transferred to individual cages and bladder evacuation was implemented every day, until the rats gained autourination function.
2.13. Cell transplantation
One week after the day of injury, all rats with SCI were assessed for locomotion and excluded if one hindlimb joint could move before transplantation. Rats were then anesthetized and immobilized in a stereotaxic frame. All surgical procedures were performed under sterile conditions. The injured cords were exposed, and fluid from the cavity was aspirated before cell grafting. PBS (10 μL) or a total of 5 × 105 cells (10 μL) was injected into the epicenter of the SCI (with a needle at an angle of 45° rostral and caudal to the injury site and at an angle of 90° at the center of the injury site). Following surgery, the rats received intensive care for 2 weeks until spontaneous bladder function was recovered, which was indicated by overfilling of the bladder.
2.14. Behavioral observation
Basso‐Beattie‐Bresnahan (BBB) score was used to assess the functional recovery. This is an open‐field locomotor evaluation test, with a scale of 21 points. The scale was set from 0 points representing no movement of the hindlimbs to 21 points being normal movement. Each rat was put in an open field individually with a skid‐proof ground. The test was carried out by two independent observers blind to the animals' grouping. The examination time lasted no less than 4 minutes. If the rat stopped moving for more than 1 minutes, it was put in the open field again for retesting. The test was performed before cell transplantation and at days, 1, 7, 14, 21, 28, 35, 42, 49, 56, after the operation until all the rats were sacrificed.
2.15. Immunofluorescent staining
Indirect immunofluorescent staining was performed on the population of all four groups on day 56 after SCI. Briefly, cells were washed twice with PBS for 2 minutes and fixed with 4% paraformaldehyde at room temperature (RT) for 12 minutes, followed by three washes of TPBS (0.05% Tween‐20 in PBS). Subsequently, cells were permeabilized with 0.2% Triton X‐100 for 10 minutes, and nonspecific sites were blocked with 1% BSA in TPBS at RT for 1 hour. Primary antibodies used were as follows: rabbit anti‐NF‐200 (Abcam, Cat No: ab18207, 1:200) and rabbit anti‐GFAP (Abcam, Cat No. ab7260, 1:1000). Secondary antibody was goat anti‐rabbit IgG FITC conjugated (Sigma, Cat No. F1262, 1:100).
2.16. ELISA analysis
Dissected tissues were quick‐frozen in liquid nitrogen and stored at −80°C until required for analysis. Tissue was homogenized in ice cold 0.9% NaCl containing a cocktail of protease inhibitors (Roche Applied Science, Upper Bavaria, Germany), centrifuged at 12 000 × g for 10 minutes at 4°C. BDNF and NF‐200 concentrations were measured in duplicate by using BDNF ELISA kit (GenWay Biotech, San Diego, CA, USA) according to the manufacturer's instructions, and results were normalized to total protein content.
2.17. HE staining
Rats were sacrificed and perfused with normal saline solution (0.9% NaCl) and then with 4% paraformaldehyde on day 56 after SCI. The T10 spinal cord segment containing the injury epicenter (1 cm = 0.5 cm either side from the injury epicenter) was dissected, cryoprotected in 30% sucrose in 4% PBS‐buffered paraformaldehyde overnight at 4°C, frozen in isopentane (2‐methylbutane) at −56°C and then stored at −80°C. The degree of tissue damage following injury was observed by two independent investigators blinded to the experiment using sagittal sections of the spinal cord (5 μm) stained with a standard H&E staining procedure.
3. RESULTS
3.1. Morphological and phenotypic characterization of BMSCs
BMSCs were cultured to 3rd passage (P3) (50 mW/cm2, 3 min/d for 3 days), then expanded in plastic dishes in vitro. The culture‐expanded cells had uniform morphology and cells with fibroblast‐like morphology were observed (Figure 1B). Phenotypic characterization confirmed that the cells are BMSCs before LIPUS stimulation. P3 BMSCs expressed surface antigens CD29 and CD90 (98.65%), but did not express CD45 and CD34 (99.74%). These results show that the isolated cells have the basic properties of BMSCs (Figure 1C).
3.2. Increased cell proliferation of BMSCs after LIPUS stimulation
To determine the best LIPUS intensity, a CCK‐8 assay was used to determine its effect on proliferation of BMSCs. The un‐stimulated group was set as the control group, and in the experimental groups, BMSCs were stimulated with LIPUS in 10, 30, 50, and 70 mW/cm2. The absorbance of the collected medium was measured at 450 nm. All the absorbance rates are expressed as percent of the absorbance rate of control group (without LIPUS stimulation), which was set as 100%. Data were obtained from 3 different cultures and are expressed as mean ±SD OD values in the experimental groups (10, 30, 50, 70 mW/cm2) were significantly higher than that in control group. Meanwhile, OD values in the 50 mW/cm2 group were significantly higher than those in other groups (one‐way ANOVA, P < 0.05) (Figure 2A). These data indicated that LIPUS can promote the proliferation capacity of BMSCs. From this, we discerned that 50 mW/cm2 was the best choice and was used for all other experiments. To prove the effect of promoting proliferation by LIPUS compared to the control group, the population doubling time of BMSCs in each group from P3 was investigated (Figure 2B), BMSCs in LIPUS group showed shorter doubling time compared to control group (1.522 ± 0.048 vs 1.695 ± 0.059, P < 0.05), which indicates LIPUS can effectively promote proliferation of BMSCs.
Figure 2.
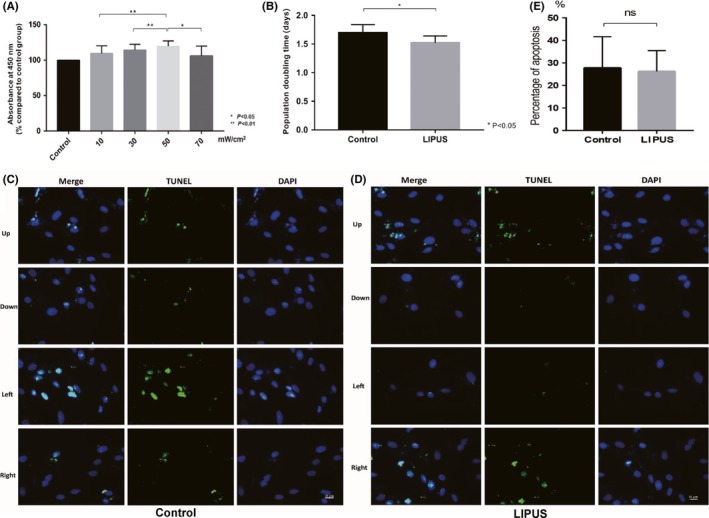
A, Proliferation rate of bone marrow mesenchymal stem cells (BMSCs) stimulated with LIPUS in 10, 30, 50, 70 mW/cm2, respectively, was analyzed using the Cell Counting Kit‐8 (CCK‐8). After the incubation with CKK‐8 solution, the absorbance of the collected medium was measured at 450 nm. All the absorbance rates are expressed as percent of the absorbance rate of control group (without LIPUS stimulation), which was set as 100%. Data were obtained from three different cultures and are expressed as mean ± SD BMSCs cell culture obtained the highest level of proliferation rate under 50 mW/cm2 LIPUS stimulation for 3 days, 3 minutes per day (P < 0.01 compared with 30 mW/cm2 LIPUS stimulation group and 10 mW/cm2 LIPUS stimulation group; P < 0.05 compared with 70 mW/cm2 LIPUS stimulation group). B, Population doubling time of BMSCs in each group. Doubling time at passage 3 was compared between LIPUS group and control group, values are expressed as means ± SEM; n = 12 per group, P < 0.05. C‐E, TUNEL analysis of BMSCs in control group and LIPUS group. Four sets of pictures in each control group (C) and LIPUS group (D) were analyzed. Every set included three pictures: one for merged picture, green represented the apoptotic cells, while blue stands for all cells. No significant difference was found between control group and experimental group, P > 0.05 (E)
3.3. Effect of LIPUS stimulation upon BMSCs
After BMSCs were stimulated by LIPUS, apoptosis of BMSCs in control group and LIPUS group was examined using TUNEL staining. In each group, four fixed sites (up, down, left, and right) were photographed and used for calculating the cell apoptosis (Figure 2C and D). We found that LIPUS stimulation did not significantly increase the apoptosis of BMSCs (Figure 2E; P > 0.05).
3.4. Enhanced expression of neurotrophic factors after stimulation with LIPUS in vitro
To examine whether LIPUS stimulation can enhance the ability of BMSCs (P3 cell culture) to produce more neurotrophic factors in vitro, the expression level of BDNF and NGF in both cytosol (by Western blot analysis) and supernatant (by ELISA analysis) of BMSCs before and after LIPUS stimulation were examined. We showed that the expression of BDNF and NGF was significantly upregulated in cytosol (Figure 3A‐C) and supernatant (Figure 3D and E), which may be associated with increased cell viability of BMSCs following LIPUS stimulation.
Figure 3.
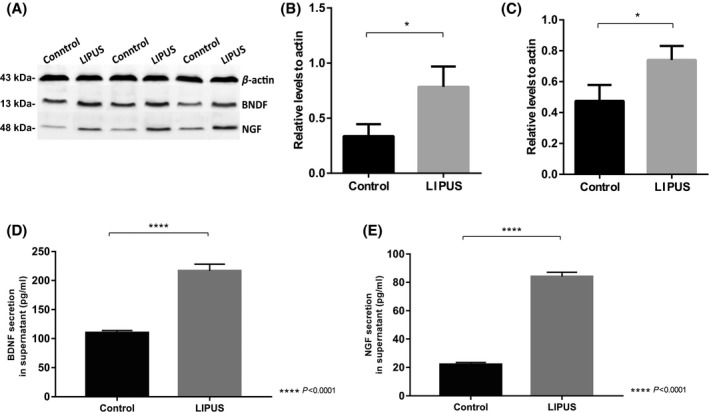
A‐C, Western blotting results show expression of BDNF (B) and NGF (C) in control group and LIPUS stimulation group. Graphs indicate relative band intensities compared with that of β‐actin (A) (n = 3 for each group). The intensity of BDNF (B) and NGF (C) bands in the LIPUS group was significantly higher than those in control group. *P < 0.05, compared to β‐actin. D‐E, Expression of BDNF and NGF in supernatant by ELISA analysis. (D) BDNF secretion in supernatant of LIPUS group was significantly higher than that in control group (P < 0.0001). (E) NGF secretion in supernatant of LIPUS group was significantly higher than that in control group (P < 0.0001)
3.5. Migration and differentiation after LIPUS stimulation
To investigate the migration ability after LIPUS stimulation, transwell assay was employed, the results demonstrated that the migration of BMSCs (Figure 4B) was significantly upregulated compared with BMSCs without treatment (Figure 4A). Notably, as shown in Figure 4C, the number of cells treated with LIPUS that migrated was almost 2‐fold more than control (P < 0.0001), suggesting LIPUS stimulation can effectively enhance the migration ability of BMSCs in vitro. We also examined differentiation of BMSCs following LIPUS stimulation using Western blot. The expression of NSE and GFAP was used to determine neuronal and gliosis differentiation, respectively (Figure 4D). The results suggested that LIPUS stimulation inhibited gliosis differentiation (P < 0.05) (Figure 4E) but did not promote neuronal differentiation (n = 0.057) (Figure 4F).
Figure 4.
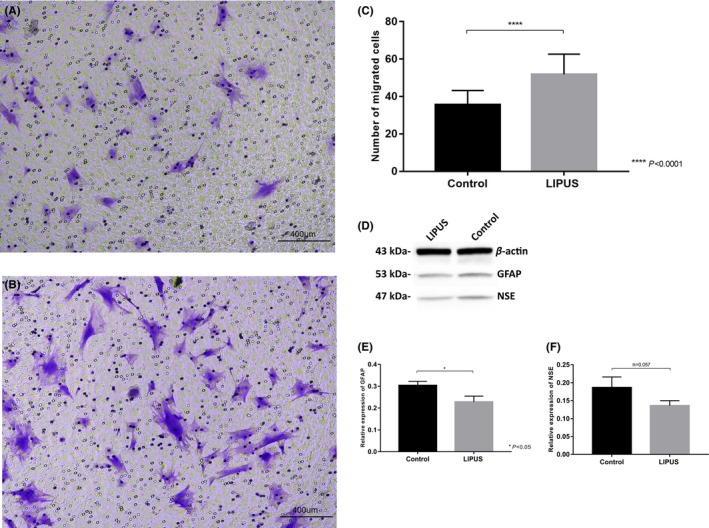
A‐C, Determination of BMSCs migration with the transwell assay. Migration of BMSCs was assessed with transwell assays. Representative images of migrated cells in control group (A) and LIPUS stimulation group (B) stained with crystal violet are shown (scale bar = 400 μm). The number of migrated cells was counted(C). Data are shown as the mean ±SD *P < 0.05, **P < 0.01. D‐F, The expression of NSE and GFAP is determined by Western blot (D), GFAP expression was decreased after LIPUS stimulation (P < 0.05) (E), while the difference of NSE expression between two groups was not significant (P > 0.05) (F)
3.6. Evaluation of locomotor function
The Basso, Beattie, and Bresnahan (BBB) open‐field locomotor test was employed to evaluate hindlimb function recovery after BMSCs and LIPUS‐BMSCs transplantation treatment at 1, 3, 7, 14, 21, 28, 35, 42, 49, and 56 days after cell transplantation. Immediately after SCI operation, rats in each group showed significant loss of motor function of hindlimbs and the BBB score reduced to 0‐1 point. The cell transplantation operation was performed one week after SCI, during the period before cell transplantation. No obvious locomotor function reduction was observed in Sham group, while rats which had undergone SCI presented limited functional recovery (remained at 0‐2 points). After cell transplantation treatment, BBB scores increased with time and starting from 21 days after transplantation, significant differences in locomotor function appeared between BMSC transplantation group and Injury group (BMSC: 5.71 ± 0.95; Injury: 4.14 ± 0.90. P < 0.05); LIPUS‐BMSC transplantation group and Injury group (LIPUS‐BMSC: 6.14 ± 1.35; Injury: 4.14 ± 0.90. P < 0.01). Significant difference between LIPUS‐BMSC transplantation group and BMSC transplantation group was observed from 28 days after transplantation (LIPUS‐BMSC: 9.14 ± 1.07; BMSC: 7.43 ± 0.79. P < 0.05). On the 56th day after transplantation, the BBB score in LIPUS‐BMSC transplantation group (14.57 ± 0.78) was significantly higher than that in BMSC transplantation group (12.29 ± 1.11, P < 0.001). The result of BBB score evaluation (Figure 5J) demonstrated that rats with LIPUS‐BMSCs transplantation treatment exhibit better locomotor functional recovery compared with rats simply treated with BMSCs without LIPUS stimulation. When comparing with Injury only group, both cell transplantation groups showed higher BBB scores, which demonstrate that BMSCs transplantation treatment is a satisfactory strategy for SCI, and LIPUS stimulation upon BMSCs can further enhance the effect of transplantation treatment.
Figure 5.
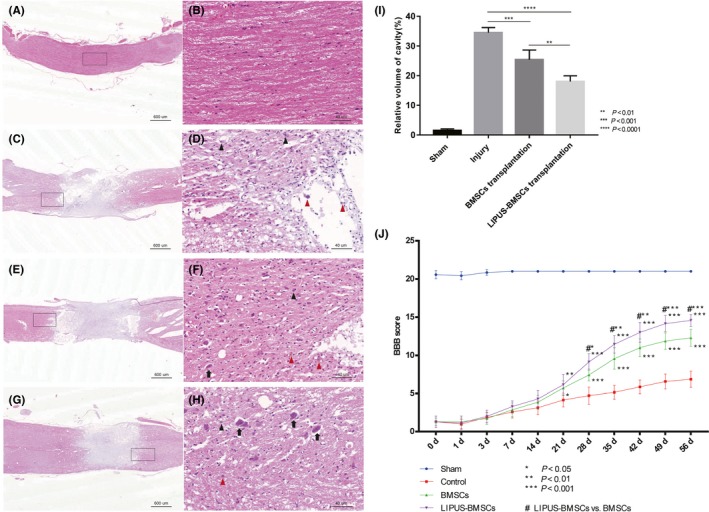
A‐H, Representative photomicrographs of Hematoxylin and eosin staining of spinal cord tissues in Sham group (A, B), Injury group (C, D), BMSCs transplantation group (E,F), LIPUS‐BMSCs transplantation group (G,H), the black boxes in A, C, E, G (scale bar = 600 μm) magnified in B, D, F, H (scale bar = 40 μm). Motor neurons (black arrows) were clearly observed in LIPUS‐BMSCs transplantation group (H); neutrophils (black arrow head) and macrophages (red arrow head) infiltration were observed in Injury group and BMSCs group (D & F). I, Size of lesion cavity is measured from sagittal images of A, C, E, G, and the relative volume of cavity is compared with each other, cavity volume of LIPUS‐BMSCs transplantation group was significantly smaller than BMSCs transplantation group (P < 0.001) and Injury group (P < 0.0001). J, The Basso, Beattie, and Bresnahan (BBB) open‐field locomotor test. Data are reported as mean ± SD in each group. BBB scores range from 0 (complete paralysis) to 21 (normal gait). The results demonstrated that rats in the LIPUS‐BMSCs transplantation group have the best hindlimb functional recovery among all the groups. From the time point of 49 days, the curve in the four groups gradually plateaued. BBB locomotor functional score is given as the mean for all four groups and error bars represent the SD. Analysis was performed using two‐way ANOVA with Bonferroni posttests, *P < 0.05, **P < 0.01, ***P < 0.001 compared with Injury group. #*P < 0.05, #**P < 0.01,#***P < 0.001, compared with BMSCs transplantation group
3.7. Morphology of the lesion site revealed by HE staining
Rats in each group were sacrificed and perfused after 8 weeks of SCI. Tissue in the Sham group remained intact without mass infiltration of neutrophils and macrophages (Figure 5A and B). As for the Injury group (Figure 5C and D), there was disorganization of the lesion site, infiltration of the inflammatory cells, formation of cavity and scar formation at the injury site were confirmed. After cell transplantation treatment with BMSCs and LIPUS‐BMSCs, decreased area of cavity in both groups was observed, especially in LIPUS‐BMSCs transplantation group. In addition to this, we observed smaller area of cavity, less apoptotic neurons, well‐arranged tissues, and motor neurons. Specifically in the LIPUS‐BMSCs transplantation group (Figure 5G and H) and motor neurons were clearly observed, and the number of neutrophils and macrophages was less than other groups. Notably, the volume of cavity in LIPUS‐BMSCs transplantation group was significantly decreased (around 20% of the lesion site) compared to BMSCs transplantation group (P < 0.01) and Injury group (P < 0.0001) (Figure 5I). This result indicated that LIPUS‐BMSCs transplantation can promote better recovery after SCI.
3.8. Transplantation of LIPUS‐BMSCs promoted axon regeneration and reduced reactive gliosis
Previous studies have demonstrated that BMSCs with LIPUS stimulation obtained significantly higher viability, the rate of apoptosis remained steady, and high expression levels of BDNF and NGF in vitro. We investigated whether the transplanted cells can increase the regeneration of axons and inhibit the astroglial activation, after transplantation into the epicenter of SCI. First, we examined axonal growth by immunostaining analysis for NF200 expression and found extensive organized axonal growth in the BMSCs group and LIPUS‐BMSCs group (Figure 6A). The expression area of NF200‐positive nerve fibers in the BMSC group and LIPUS‐BMSCs group was significantly higher than that of Injury group (*P < 0.05 when compared with BMSCs group, **P < 0.01 when compared with LIPUS‐BMSCs group). Quantification of NF‐200 expression showed that the relative expression of NF‐200 in LIPUS‐BMSCs group had the most nerve fibers in the lesion site; however, the difference was not significant after calculation (Figure 6B). This indicated that LIPUS‐BMSCs transplantation may not be more effective compared with BMSCs transplantation with regard to promoting nerve fiber growth. However, this could be the result of the inhibitory microenvironment in the injury site after SCI. GFAP was employed as a marker of astrocyte activation (Figure 6C). We examined tissue sections at the epicenter 56 days after SCI, and the level of immunofluorescence was quantitated using ImageJ software. Overall, there was a 2‐3‐fold increase in GFAP immunoreactivity in Injury group as compared to tissue from sham‐injured mice. GFAP immunoreactivity in BMSCs group and LIPUS‐BMSCs group was significantly lower than the Injury group (*P < 0.05), and GFAP immunoreactivity in LIPUS‐BMSCs group was significantly lower than that of BMSCs group (*P < 0.05) (Figure 6D). The result indicates that both BMSCs transplantation and LIPUS‐BMSCs transplantation can provide help to reduce the reactive gliosis. Furthermore, LIPUS‐BMSCs transplantation can further alleviate the astroglial activation process during the repair after SCI.
Figure 6.
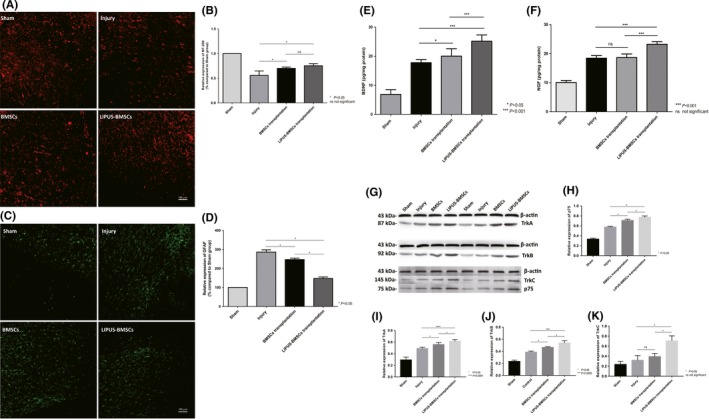
A‐D, Transplantation of BMSCs and LIPUS‐BMSCs harnesses axon regeneration and reduces reactive gliosis. A, Immunofluorescence demonstrated NF‐200 staining in red channel from Sham group, Injury group, BMSCs group, and LIPUS‐BMSCs group on day 56 after SCI, respectively. B, Semiquantification of NF‐200 expression area according to immunostaining (A), expression level was expressed as percentage compared with Sham group (set as 100%). C, Immunofluorescence demonstrated GFAP staining in green channel from Sham group, Injury group, BMSCs group, and LIPUS‐BMSCs group on day 56 after SCI, respectively. D, Semiquantification of GFAP expression area according to immunostaining (C), expression level was expressed as percentage compared with Sham group (set as 100%). *P < 0.05 compared with Injury group, P < 0.05 compared with LIPUS‐BMSCs group. One‐way ANOVA followed by Tukey's post hoc test. ns indicates no significant difference. E‐F, ELISA evaluation of BDNF (E) and NGF (F) expression level in Sham group, Injury group, BMSCs transplantation group, and LIPUS‐BMSCs transplantation group. *** shows significant difference with BMSCs transplantation group and Injury group in P < 0.001 level; ns shows no significant difference was observed compared with Injury group. G‐K, Western blot analysis of neurotrophic factors receptors. Western blotting was performed to examine expression of TrkA, TrkB, TrkC, and p75 proteins. To detect the phosphorylated form of the neurotrophic factors, the corresponding blot was stripped and subsequently stained with an antibody to phosphorylated protein. The notation to the left of each blot indicates the running position of molecular weight standard proteins on the same gel (G). Relative expression of p75 (H), relative expression of TrkA (I), relative expression of TrkB (J), and relative expression of TrkC (K)
3.9. Neurotrophic factors and receptor expression after transplantation treatment
Rats in each group were sacrificed on day 56 after SCI, and tissue biopsies around lesion epicenter (T10 level) were obtained for ELISA analysis to examine the expression of BDNF and NGF. The results of the ELISA analysis (Figure 6E and F) for the LIPUS‐BMSCs transplantation group showed the highest level of BDNF expression in the microenvironmental of injury epicenter compared with BMSCs transplantation group and Injury group (P < 0.001). This is consistent with the Western blot analysis investigating neurotrophic factors expression in vitro. When comparing the BDNF expression level of BMSCs transplantation group and Injury group, increased levels of BDNF expression were observed in BMSCs transplantation group (P < 0.05). This demonstrated that the transplanted BMSCs have the ability to produce more BDNF at the basic of injury level, and the stimulation by LIPUS can further strength the ability of BDNF secretion (Figure 6E). NGF expression levels after transplantation treatment were significantly higher in the LIPUS‐BMSC group compared with BMSCs transplantation group or Injury group. This was also consistent with our previous studies in vitro. However, when comparing NGF expression between BMSCs transplantation group and Injury group, no significant difference was observed (Figure 6F).
The expression of neurotrophic factors receptors including TrkA, TrkB, TrkC, and p75 was also examined by Western blot analysis (Figure 6G). The expression of TrkA, TrkB, TrkC, and p75 was all upregulated in LIPUS‐BMSCs transplantation group when comparing with BMSCs transplantation group and Injury group (Figure 6H‐K), which provided support for the function of BDNF, NGF, and other neurotrophic factors.
4. DISCUSSION
Effective treatment strategies for SCI with satisfactory improvement in locomotor and neurological function have always been the focus of the basic research.24 Stem cell transplantation is a potential method for certain neurological diseases as well as a viable treatment for acute SCI. Many kinds of cells have been used in transplantation, including BMSCs, embryonic stem cells, neural stem cells, autologous olfactory ensheathing cells, human umbilical cord blood cells (HUCBCs), and Schwann cells.25, 26, 27, 28 Among these types of cells, BMSCs are the strongest candidates due to the greater availability and weaker immunogenicity.29, 30, 31
Appropriate candidate cells for transplantation treatment require properties including rapidly expansion in culture, capability of long‐term survival, increased cell viability, and close integration in the host tissue. In our research, LIPUS was employed to stimulate BMSCs (P3) to optimized cell culture requirements for transplantation.
LIPUS is a noninvasive and safe form of mechanical energy that can be delivered into biological tissues and cells as acoustic pressure waves,32 which has been widely used for clinical applications including bone fracture healing, rehabilitation treatment of tendon, ligament, and cartilage disorders.33, 34, 35, 36, 37, 38 Furthermore, LIPUS stimulation has been certified and approved by Food and Drug Administration (FDA) in the United States as a safe and effective treatment strategy.39, 40, 41, 42 It was reported by Xu et al that low‐intensity pulsed ultrasound could enhance the cell viability, proliferation, and differentiation of hematopoietic stem/progenitor cells (HSPC) in vitro without changing the percentage of surface antigen expression including CD34+and CD14+.43 Berna et al demonstrated that LIPUS improves the functional properties of cardiac mesoangioblasts in various aspects.44 In this research, Cell Counting Kit‐8 (CCK‐8) was employed to assess cell proliferation after LIPUS stimulation in various parameters. We found that under LIPUS stimulation at the intensity of 50 mW/cm2 (stimulated for 3 days, 3 minutes each day), an optimal cell proliferation rate was obtained. Furthermore, the apoptosis rate following LIPUS stimulation was also examined by TUNEL staining. No significant difference was observed in apoptosis rate between BMSCs cell cultures with and without LIPUS stimulation. Therefore, LIPUS stimulation may be a good strategy for enhancing cell viability in vitro, thus improving efficiency and outcome of stem cell transplantation.
Following stem cell transplantation, a series of unfavorable factors may threaten the survival of transplanted cells and prevent axon regeneration including infiltration of inflammatory cells, release of inflammatory cytokines, and deficiency of neurotrophic factors. Together, all these factors contribute to the formation of an inhibitory microenvironment. It has been shown that neurotrophic factors regulate the imbalanced microenvironment after SCI.45 A variety of important protein molecules was secreted, which play an irreplaceable role by inhibiting the inflammatory response, promoting cell survival, synapses connection, remyelination process, and various pathophysiological procedures. Research showed that a variety of neurotrophic factors contributes to synergistic effect on the postinjury microenvironment and promotion of neurological recovery including BDNF, NT‐3, NGF, VEGF, and the like. In this research, we placed emphasis on the secretion of neurotrophic factors after LIPUS stimulation in vitro and the amount of neurotrophic factors in the lesion site of spinal cord after cell transplantation.46, 47 Among them, BDNF is a protein synthesized in the brain, which is widely distributed in the central nervous system, BDNF can prevent neuronal damage and death; meanwhile, pathological state of neurons can be improved by promoting regeneration and differentiation of injured neurons and transplanted stem cells. NGF can regulate the growth and development of peripheral and central neurons as well as maintaining the survival of transplanted stem cells. NGF is one of the earliest and most thoroughly researched neurotrophic factors, which has a dual biological function of neuronal nutrition and neurite outgrowth promotion. In our in vitro experiments, we observed significant upregulation of BDNF and NGF, in LIPUS‐treated BMSCs cell cultures, revealed by Western blotting analysis. This indicates that LIPUS can promote the secretion of neurotrophic factors in vitro. We also wanted to confirm the cells secrete neurotrophic factors after transplanted to the injured site of SCI. Rats in each group were sacrificed 56 days after cell transplantation, and ELISA analysis of LIPUS‐BMSCs transplantation group showed significantly more BDNF and NGF secretion compared with BMSCs transplantation group, and both groups (LIPUS‐BMSCs transplantation group and BMSCs transplantation group) showed more neurotrophic factors secretion than the control group (SCI without stem cell transplantation). Both in vivo and in vitro experiments demonstrated that BMSCs cell cultures obtained the ability to continuously secrete more neurotrophic factors after LIPUS stimulation.
BMSCs transplantation has proven to be an effective treatment strategy for SCI.48 This study investigated the application of LIPUS stimulation on BMSCs, which increased cell viability, proliferation ability, and secretion of neurotrophic factors. These are all crucial components which contribute to better recovery of locomotor function and alleviation of the astroglial activation process during the repair after SCI. Furthermore, decreased area of the lesion cavity was also observed in LIPUS‐BMSCs transplantation group.
Future directions from this research include investigation into the intrinsic regulatory mechanisms and signaling pathways involved in the functional improvement of LIPUS‐BMSCs in vitro. Notably, the Notch pathway is currently recognized as a signal pathway regulating the process of development and cell maturation by promoting cell proliferation and differentiation in neural stem cells and hematopoietic stem cells.49, 50, 51 Another crucial factor involved in the development of the inflammatory microenvironment at the site of injury is the nuclear factor kappa‐light‐chain‐enhancer of activated B cells (NF‐κB) signaling pathway. NF‐κB regulates the expression of inducible nitric oxide synthase (iNOS), cyclooxygenase 2 (cox‐2), TNF‐α, IL‐1β, IL‐6, IL‐8, IL‐10, and cell adhesion molecule. It has been shown that LIPUS can reduce the activation of RANKL, which act as a receptor activation ligand of the NF‐κB receptor, preventing the activation of NF‐κB signaling pathway.52, 53 Therefore, the effect of LIPUS on microenvironment following SCI may inhibit the inflammatory response by inhibiting the NF‐κB signaling pathway and promote the repair of SCI.54, 55 Meanwhile, remyelination is a crucial step for functional restoration after SCI, growth factors, and neurotrophic factors have been used to boost the endogenous progenitor response to facilitate oligodendrocytes survival and remyelination process.56 According to our present research, BMSCs are mainly served as a neurotrophic factor‐secreting stem cell, and BMSCs stimulated with LIPUS obtained the ability to secrete more neurotrophic factors, which may be beneficial for the remyelination process of oligodendrocyte progenitor cells and oligodendrocytes.
Additionally, we will also be probing the expression of inflammatory cytokines after LIPUS stimulation, combined with Western blot analysis of the downstream signal cascade of NF‐κB signaling. This will demonstrate the correlation between LIPUS stimulation and inflammatory factor secretion of transplanted stem cells. Finally, the side effect and safety profile of LIPUS‐treated BMSC transplantation should be considered seriously before moving toward clinical applications. This includes further investigation into survival, biodistribution, proliferation and tumorigenicity, and host responses to LIPUS‐BMSCs.
5. CONCLUSION
In conclusion, LIPUS can improve BMSCs viability and neurotrophic factor expression in vitro. Similarly, transplantation of LIPUS‐BMSCs could promote better functional recovery than BMSCs transplantation without stimulation, indicating a translational application for the treatment of SCI.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Ning G‐Z, Song W‐Y, Xu H, et al. Bone marrow mesenchymal stem cells stimulated with low‐intensity pulsed ultrasound: Better choice of transplantation treatment for spinal cord injury. CNS Neurosci Ther. 2019;25:496–508. 10.1111/cns.13071
The first two authors contributed equally to this work.
Funding information
State Key Program of National Natural Science Foundation of China (81330042); Special Program for Sino‐Russian Joint Research Sponsored by the Ministry of Science and Technology, China (2014DFR31210); International Cooperation Program of National Natural Science Foundation of China (81620108018); Program of National Natural Science Foundation of China (81472070); and Program of National Natural Science Foundation of China (81772342).
Contributor Information
Zhi‐Gang Qu, Email: zhigangqu@tust.edu.cn, Email: sqfeng@tmu.edu.cn.
Shi‐Qing Feng, Email: zhigangqu@tust.edu.cn, Email: sqfeng@tmu.edu.cn.
REFERENCES
- 1. Spinal Cord Injury (SCI) . Facts and figures at a glance. J Spinal Cord Med. 2016;39(4):493–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee BB, Cripps RA, Fitzharris M, Wing PC. The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord. 2014;52(2):110–116. [DOI] [PubMed] [Google Scholar]
- 3. Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008;209(2):378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moghaddam A, Child C, Bruckner T, Gerner HJ, Daniel V, Biglari B. Posttraumatic inflammation as a key to neuroregeneration after traumatic spinal cord injury. Int J Mol Sci. 2015;16(4):7900–7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Manley NC, Priest CA, Denham J, Wirth ED 3rd, Lebkowski JS. Human embryonic stem cell‐derived oligodendrocyte progenitor cells: preclinical efficacy and safety in cervical spinal cord injury. Stem Cells Transl Med. 2017;6(10):1917–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Karamouzian S, Nematollahi‐Mahani SN, Nakhaee N, Eskandary H. Clinical safety and primary efficacy of bone marrow mesenchymal cell transplantation in subacute spinal cord injured patients. Clin Neurol Neurosurg. 2012;114(7):935–939. [DOI] [PubMed] [Google Scholar]
- 7. Mendonca MV, Larocca TF, de Freitas Souza BS, et al. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res Ther. 2014;5(6):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Satti HS, Waheed A, Ahmed P, et al. Autologous mesenchymal stromal cell transplantation for spinal cord injury: a phase I pilot study. Cytotherapy. 2016;18(4):518–522. [DOI] [PubMed] [Google Scholar]
- 9. Boido M, Garbossa D, Fontanella M, Ducati A, Vercelli A. Mesenchymal stem cell transplantation reduces glial cyst and improves functional outcome after spinal cord compression. World Neurosurg. 2014;81(1):183–190. [DOI] [PubMed] [Google Scholar]
- 10. Karaoz E, Kabatas S, Duruksu G, et al. Reduction of lesion in injured rat spinal cord and partial functional recovery of motility after bone marrow derived mesenchymal stem cell transplantation. Turk Neurosurg. 2012;22(2):207–217. [DOI] [PubMed] [Google Scholar]
- 11. Nakajima H, Uchida K, Guerrero AR, et al. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J Neurotrauma. 2012;29(8):1614–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Uccelli A, Benvenuto F, Laroni A, Giunti D. Neuroprotective features of mesenchymal stem cells. Best Pract Res Clin Haematol. 2011;24(1):59–64. [DOI] [PubMed] [Google Scholar]
- 13. Hawryluk GW, Mothe A, Wang J, Wang S, Tator C, Fehlings MG. An in vivo characterization of trophic factor production following neural precursor cell or bone marrow stromal cell transplantation for spinal cord injury. Stem Cells Dev. 2012;21(12):2222–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Osborne A, Sanderson J, Martin KR. Neuroprotective effects of human mesenchymal stem cells and platelet‐derived growth factor on human retinal ganglion cells. Stem Cells. 2018;36(1):65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perets N, Segal‐Gavish H, Gothelf Y, et al. Long term beneficial effect of neurotrophic factors‐secreting mesenchymal stem cells transplantation in the BTBR mouse model of autism. Behav Brain Res. 2017;331:254–260. [DOI] [PubMed] [Google Scholar]
- 16. Zhou L, Lin Q, Wang P, et al. Enhanced neuroprotective efficacy of bone marrow mesenchymal stem cells co‐overexpressing BDNF and VEGF in a rat model of cardiac arrest‐induced global cerebral ischemia. Cell Death Dis. 2017;8(5):e2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Timmers L, Lim SK, Hoefer IE, et al. Human mesenchymal stem cell‐conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res. 2011;6(3):206–214. [DOI] [PubMed] [Google Scholar]
- 18. Kim HJ, Lee JH, Kim SH. Therapeutic effects of human mesenchymal stem cells on traumatic brain injury in rats: secretion of neurotrophic factors and inhibition of apoptosis. J Neurotrauma. 2010;27(1):131–138. [DOI] [PubMed] [Google Scholar]
- 19. Togel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007;292(5):F1626–F1635. [DOI] [PubMed] [Google Scholar]
- 20. Lai CH, Chen SC, Chiu LH, et al. Effects of low‐intensity pulsed ultrasound, dexamethasone/TGF‐beta1 and/or BMP‐2 on the transcriptional expression of genes in human mesenchymal stem cells: chondrogenic vs. osteogenic differentiation. Ultrasound Med Biol. 2010;36(6):1022–1033. [DOI] [PubMed] [Google Scholar]
- 21. Uddin SM, Qin YX. Enhancement of osteogenic differentiation and proliferation in human mesenchymal stem cells by a modified low intensity ultrasound stimulation under simulated microgravity. PLoS One. 2013;8(9):e73914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lv Y, Nan P, Chen G, Sha Y, Xia B, Yang L. In vivo repair of rat transected sciatic nerve by low‐intensity pulsed ultrasound and induced pluripotent stem cells‐derived neural crest stem cells. Biotechnol Lett. 2015;37(12):2497–2506. [DOI] [PubMed] [Google Scholar]
- 23. Tsuang YH, Liao LW, Chao YH, et al. Effects of low intensity pulsed ultrasound on rat Schwann cells metabolism. Artif Organs. 2011;35(4):373–383. [DOI] [PubMed] [Google Scholar]
- 24. Garbossa D, Boido M, Fontanella M, Fronda C, Ducati A, Vercelli A. Recent therapeutic strategies for spinal cord injury treatment: possible role of stem cells. Neurosurg Rev. 2012;35(3): 293–311; discussion. [DOI] [PubMed] [Google Scholar]
- 25. Gurudutta GU, Satija NK, Singh VK, Verma YK, Gupta P, Tripathi RP. Stem cell therapy: a novel & futuristic treatment modality for disaster injuries. Ind J Med Res. 2012;135(1):15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ruff CA, Wilcox JT, Fehlings MG. Cell‐based transplantation strategies to promote plasticity following spinal cord injury. Exp Neurol. 2012;235(1):78–90. [DOI] [PubMed] [Google Scholar]
- 27. Sandner B, Prang P, Rivera FJ, Aigner L, Blesch A, Weidner N. Neural stem cells for spinal cord repair. Cell Tissue Res. 2012;349(1):349–362. [DOI] [PubMed] [Google Scholar]
- 28. Zhou XH, Ning GZ, Feng SQ, et al. Transplantation of autologous activated schwann cells in the treatment of spinal cord injury: six cases, more than five years of follow‐up. Cell Transplant. 2012;21:S39–S47. [DOI] [PubMed] [Google Scholar]
- 29. Yazdani SO, Pedram M, Hafizi M, et al. A comparison between neurally induced bone marrow derived mesenchymal stem cells and olfactory ensheathing glial cells to repair spinal cord injuries in rat. Tissue Cell. 2012;44(4):205–213. [DOI] [PubMed] [Google Scholar]
- 30. Aggarwal R, Lu JW, Kanji S, et al. Umbilical cord blood‐derived CD34(+) cells reverse osteoporosis in NOD/SCID mice by altering osteoblastic and osteoclastic activities. Plos One. 2012; 7(6):e39365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Urdzikova L, Jendelova P, Glogarova K, Burian M, Hajek M, Sykova E. Transplantation of bone marrow stem cells as well as mobilization by granulocyte‐colony stimulating factor promotes recovery after spinal cord injury in rats. J Neurotrauma. 2006;23(9):1379–1391. [DOI] [PubMed] [Google Scholar]
- 32. Lu HB, Chen C, Qu J, et al. Initiation timing of low‐intensity pulsed ultrasound stimulation for tendon‐bone healing in a rabbit model. Am J Sports Med. 2016;44(10):2706–2715. [DOI] [PubMed] [Google Scholar]
- 33. Padilla F, Puts R, Vico L, Guignandon A, Raum K. Stimulation of bone repair with ultrasound. Therap Ultrasound. 2016;880:385–427. [DOI] [PubMed] [Google Scholar]
- 34. Padilla F, Puts R, Vico L, Raum K. Stimulation of bone repair with ultrasound: a review of the possible mechanic effects. Ultrasonics. 2014;54(5):1125–1145. [DOI] [PubMed] [Google Scholar]
- 35. Salem KH, Schmelz A. Low‐intensity pulsed ultrasound shortens the treatment time in tibial distraction osteogenesis. Int Orthop. 2014;38(7):1477–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Watanabe Y, Matsushita T, Bhandari M, Zdero R, Schemitsch EH. Ultrasound for fracture healing: current evidence. J Orthop Trauma. 2010;24:S56–S61. [DOI] [PubMed] [Google Scholar]
- 37. Gebauer D, Mayr E, Orthner E, Ryaby JP. Low‐intensity pulsed ultrasound: effects on nonunions. Ultrasound Med Biol. 2005;31(10):1391–1402. [DOI] [PubMed] [Google Scholar]
- 38. Tsumaki N, Kakiuchi M, Sasaki J, Ochi T, Yoshikawa H. Low‐intensity pulsed ultrasound accelerates maturation of callus in patients treated with opening‐wedge high tibial osteotomy by hemicallotasis. J Bone Joint Surg Am. 2004;86a(11):2399–2405. [DOI] [PubMed] [Google Scholar]
- 39. Cook SD, Salkeld SL, Patron LP, Doughty ES, Jones DG. The effect of low‐intensity pulsed ultrasound on autologous osteochondral plugs in a canine model. Am J Sports Med. 2008;36(9):1733–1741. [DOI] [PubMed] [Google Scholar]
- 40. Hill GE, Fenwick S, Matthews BJ, Chivers RA, Southgate J. The effect of low‐intensity pulsed ultrasound on repair of epithelial cell monolayers in vitro. Ultrasound Med Biol. 2005;31(12):1701–1706. [DOI] [PubMed] [Google Scholar]
- 41. Fu SC, Shum WT, Hung LK, Wong M, Qin L, Chan KM. Low‐intensity pulsed ultrasound on tendon healing – a study of the effect of treatment duration and treatment initiation. Am J Sports Med. 2008;36(9):1742–1749. [DOI] [PubMed] [Google Scholar]
- 42. Cook SD, Salkeld SL, Popich‐Patron LS, Ryaby JP, Jones DG, Barrack RL. Improved cartilage repair after treatment with low‐intensity pulsed ultrasound. Clin Orthop Relat Res. 2001;391:S231–S243. [DOI] [PubMed] [Google Scholar]
- 43. Xu P, Gul‐Uludag H, Ang WT, et al. Low‐intensity pulsed ultrasound‐mediated stimulation of hematopoietic stem/progenitor cell viability, proliferation and differentiation in vitro. Biotechnol Lett. 2012;34(10):1965–1973. [DOI] [PubMed] [Google Scholar]
- 44. Bernal A, Perez LM, De Lucas B, et al. Low‐intensity pulsed ultrasound improves the functional properties of cardiac mesoangioblasts. Stem Cell Rev. 2015;11(6):852–865. [DOI] [PubMed] [Google Scholar]
- 45. Zhao H, Cheng L, Du X, et al. Transplantation of cerebral dopamine neurotrophic factor transducted BMSCs in contusion spinal cord injury of rats: promotion of nerve regeneration by alleviating neuroinflammation. Mol Neurobiol. 2016;53(1):187–199. [DOI] [PubMed] [Google Scholar]
- 46. Nakao J, Fujii Y, Kusuyama J, et al. Low‐intensity pulsed ultrasound (LIPUS) inhibits LPS‐induced inflammatory responses of osteoblasts through TLR4‐MyD88 dissociation. Bone. 2014;58:17–25. [DOI] [PubMed] [Google Scholar]
- 47. Hsieh YL. Peripheral therapeutic ultrasound stimulation alters the distribution of spinal c‐Fos immunoreactivity induced by early or late phase of inflammation. Ultrasound Med Biol. 2008;34(3):475–486. [DOI] [PubMed] [Google Scholar]
- 48. Wang L, Wang Q, Zhang XM. Progress on bone marrow mesenchymal stem cells transplantation for spinal cord injury. Zhongguo Gu Shang. 2014;27(5):437–440. [PubMed] [Google Scholar]
- 49. van Nes J, Chan A, van Groningen T, van Sluis P, Koster J, Versteeg R. A NOTCH3 transcriptional module induces cell motility in neuroblastoma. Clin Cancer Res. 2013;19(13):3485–3494. [DOI] [PubMed] [Google Scholar]
- 50. Kim JH, Thimmulappa RK, Kumar V, et al. NRF2‐mediated Notch pathway activation enhances hematopoietic reconstitution following myelosuppressive radiation. J Clin Invest. 2014;124(2):730–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yao LL, Cao Q, Wu CY, Kaur C, Hao AJ, Ling EA. Notch signaling in the central nervous system with special reference to its expression in microglia. CNS & Neurol Disord Drug Targets. 2013;12(6):807–814. [DOI] [PubMed] [Google Scholar]
- 52. Bracchi‐Ricard V, Lambertsen KL, Ricard J, et al. Inhibition of astroglial NF‐kappaB enhances oligodendrogenesis following spinal cord injury. J Neuroinflammation. 2013;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lu M, Wang SY, Han X, Lv DC. Butein inhibits NF‐kappa B activation and reduces infiltration of inflammatory cells and apoptosis after spinal cord injury in rats. Neurosci Lett. 2013;542:87–91. [DOI] [PubMed] [Google Scholar]
- 54. Ishikawa C, Senba M, Mori N. Butein inhibits NF‐kappa B, AP‐1 and Akt activation in adult T‐cell leukemia/lymphoma. Int J Oncol. 2017;51(2):633–643. [DOI] [PubMed] [Google Scholar]
- 55. Kumar AA, Kumar SR, Narayanan R, Arul K, Baskaran M. Autologous bone marrow derived mononuclear cell therapy for spinal cord injury: a phase I/II clinical safety and primary efficacy data. Exp Clin Transplant. 2009;7(4):241–248. [PubMed] [Google Scholar]
- 56. Zhao T, Yan W, Xu K, Qi Y, Dai X, Shi Z. Combined treatment with platelet‐rich plasma and brain‐derived neurotrophic factor‐overexpressing bone marrow stromal cells supports axonal remyelination in a rat spinal cord hemi‐section model. Cytotherapy. 2013;15(7):792–804. [DOI] [PubMed] [Google Scholar]


