Abstract
Clostridium difficile infection (CDI) is a potentially deadly cause of diarrhea that is virtually always connected to healthcare system exposures, both inpatient and outpatient. Once a disease mainly of hospitals, 75% of CDI cases are now diagnosed outside of hospitals. However, the diagnosis location may not reflect where C. difficile spores were acquired or antibiotic exposure occurred. Changing epidemiology and increasing awareness about the role of every segment of the healthcare system in mediating this disease makes it clear that reducing its burden will also require active participation from all US healthcare professionals.
Keywords: Clostridium difficile, CDI, antibiotic stewardship, healthcare-associated infection
Clostridium difficile infection (CDI) is a potentially serious, life-threatening cause of diarrhea.1 Although CDI is often associated with hospital stays, community-onset infections are now more common than hospital-onset infections.2 While nearly half of community-onset infections are termed community-associated, data indicate that most of these patients (82%) had an outpatient healthcare visit within 12 weeks of testing positive for Clostridium difficile (C. difficile).2,3
These findings indicate that healthcare professionals across all healthcare settings play a critical role in preventing the spread of C. difficile in their daily interaction with patients. Limiting the impact of CDI requires two specific actions by healthcare professionals: enacting protocols that reduce patient exposure to C. difficile spores and practicing effective antibiotic stewardship,1 that is, using antibiotics only when necessary and indicated.
Antibiotics cause vulnerability to CDI by disrupting the normal colonic microbiota and providing an environment where C. difficile spores can germinate in the small intestine and then pass through the large intestine where they multiply and produce diarrhea-causing toxins. C. difficile spores, which are transmitted via the fecal-oral route, can persist on any surface, device or material that becomes contaminated.1 These reservoirs of infection set the stage for transfer of the spores to patients, literally, at the hands of healthcare professionals.
HOST DEFENSE AND RISK FACTORS FOR CDI
There are several important host defenses for CDI. First is intact (undisturbed) lower intestinal microbiota. There has been some suggestion that the appendix may be important to maintaining microbiota balance.4 Infants appear to have a natural defense against CDI. They often become colonized with C. difficile bacteria but do not develop infection; animal data suggest a relative lack of toxin receptors during microbiota establishment in the first year of life may be protective.5 Many adults have antibodies to C. difficile toxins that are boosted after colonization or natural infection.6 Demonstration that administering monoclonal antibodies protects against CDI recurrence in humans is strong evidence for an important role of humoral immunity in host defense.7 The association of proton pump inhibitors with CDI could suggest a host defense role for stomach acid;8 however, C. difficile spores are relatively acid resistant9 and thus this association may instead reflect the impact of proton pump inhibitors on the intestinal microbiota.10,11
Prior antibiotic treatment is the single most important risk factor for CDI.1 Other factors include older age, immunosuppressant therapy, inflammatory bowel disease, and tube feeds. Tube feeds may increase the risk of CDI because these patients require more “hands on” time from healthcare professionals, increasing the chance of spore transmission and ingestion. Alternatively or additionally there may be important alteration of the intestinal microbiota affected by tube feeding. Proximity to infected patients increases risk of infection, which is why hospitals and nursing homes have historically been viewed as the most important vectors in CDI epidemiology. This view is expanding as we learn more about the role of other settings in C. difficile transmission.
EPIDEMIOLOGIC CLASSIFICATION OF CDI CASES
CDI is no longer associated only (or even predominantly) with inpatient hospital stays. Recognition of the role of non-hospital settings in C. difficile transmission has led to changes in epidemiologic classification categories used for disease surveillance. Classification depends on the patient’s healthcare exposure in the 12 weeks prior to symptom onset and/or diagnosis and is divided broadly into community-associated or healthcare-associated disease. Cases are further classified by place of onset (Figure 1).2,12,13
Figure 1.
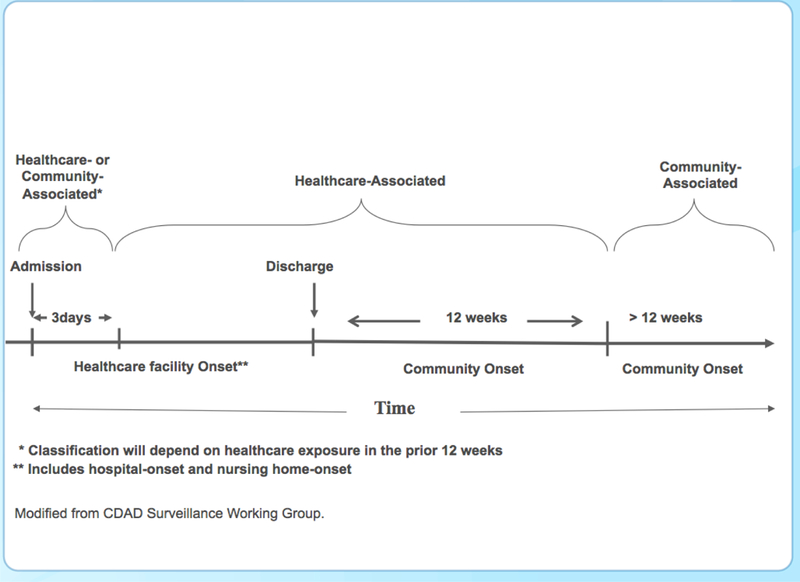
Epidemiologic Classification of CDI Cases Based on the Time of Symptom Onset12
Data from the National Healthcare Safety Network looked at the percentage of laboratory-identified CDIs by hospitalization status (present-on-admission or hospital-onset).1 About half (52%) of the cases diagnosed in hospitals in 2010 were present-on-admission (i.e., diagnosed within the first 3 days), though still largely healthcare related. Although many other patients may have recent inpatient exposures, only about 16% of these cases were among patients recently discharged from the same hospital. These data illustrate the interdependence of hospitals and the community in minimizing CDI while also demonstrating the role of previous hospitalization as an important risk factor for CDI.
CDI BURDEN IN THE US
Based on active population- and laboratory-based surveillance across 10 geographic areas, there were an estimated 453,000 US CDI cases in 2011.2 Approximately two-thirds of all cases [293,300] were categorized as (inpatient) healthcare-associated but only 24% [107,600] hospital onset. A similar percentage had nursing home onset (23%) and a somewhat smaller percentage had post-discharge onset (18%). The remaining approximately one-third of cases [159,700] were community-associated and therefore without overnight inpatient healthcare exposures in the previous 12 weeks.2 By performing interviews in similar community-associated case patients from January 1, 2009 through May 31, 2011 Chitnis and colleagues noted that 82% reported outpatient healthcare exposures, such as visits to a doctor or dentist.3
Lessa et al also reported 29,000 deaths within 30 days of CDI diagnosis and, based on other estimates from the literature, at least half were likely attributable to C. difficile.2 The morbidity and financial impact of CDI is also considerable. There are a reported 83,000 annual recurrences within 8 weeks of the initial case. In their systematic review, Kwon et al looked at the colectomy rates as a measure of the severity of CDI.14 The rate of colectomies began to increase dramatically in 2000 and has been reported as high as 6.2% in epidemic periods. They also reported that CDI extends inpatient hospital stays by 2.3 to 12 days and increases the financial burden by $2,454 to $27,160 per case.
EMERGING TOXIN GENE-VARIANT STRAIN OF C. DIFFICILE
Although US CDI rates have plateaued over the past five years, incidence increased dramatically from 2000 to 2010 such that CDI has become the most common cause of healthcare associated infections in US hospitals.1,15–17 Much of this increase was likely due to emergence of an epidemic toxin gene-variant strain, NAP1/ribotype 027.18–21 Although previously uncommon, this strain is now epidemic in the US.
The NAP1/027 strain is more resistant to fluoroquinolone antibiotics and more virulent than other strains.18 In a case-control study, the NAP1 strain was associated with greater odds of severe disease than other strains (adjusted odds ratio [AOR], 1.74; 95% CI 1.36–2.22), severe outcome (AOR, 1.66; 95% CI 1.09–2.54), and death within 14 days (AOR, 2.12; 95% CI, 1.22–3.68).22 NAP1/027, NAP4/014/020, and NAP11/106 comprise the three most common causes of infection in both community-and healthcare-associated CDI in the US.2 NAP4/014/020 is the second most prevalent strain in the US and is endemic in Europe.2,21 NAP11/106, which has been associated with outbreaks in Europe, is resistant to erythromycin and fluoroquinolones.
KEY COMPONENTS OF CDI PREVENTION
In its 2012 Vital Signs report, the Centers for Disease Control and Prevention highlighted CDI and reviewed 6 key components to prevention (Table 1).1 The report emphasized the importance of antibiotic stewardship along with infection control applied across different healthcare settings in order to achieve major reductions in CDI. Antibiotic stewardship is now on the forefront of CDI prevention efforts due to growing recognition that it is essential for meaningful reduction in disease rates.23
Table 1.
Six Key Components of C. difficile Infection Prevention Efforts1
| ➢ Prescribe and use antibiotics carefully |
| ➢ Focus on early and reliable diagnosis |
| ➢ Isolate patients immediately |
| ➢ Wear gloves and gowns for all contact with patient and patient-care environment |
| ➢ Assure adequate cleaning of the patient-care environment; augment with EPA-registered C. difficile sporicidal disinfectant |
| ➢ Notify facilities upon patient transfer |
The Vital Signs report emphasized measures to reduce transmission of C. difficile spores (i.e., reducing patient exposure).1,24 This includes early and reliable diagnosis of CDI patients followed by immediate isolation and implementation of Contact Precautions, adequate cleaning of the patient care environment, augmented by use of an EPA-registered C. difficile sporicidal disinfectant (Table 2), and communication between facilities of prior, current, or suspected CDI upon patient transfer. Interfacility communication is key to addressing regional prevention of CDI since patient exposures in one facility can impact infections in other settings.
Table 2.
Agents with C. difficile EPA Sporicidal Claim24
| Primary Registered Product Name |
| Activate 5.25% Institutional Bleach |
| Austin A-1 Ultra Disinfecting Bleach |
| Austin’s A-1 Concentrated Bleach 8.25% |
| Bath and Tile Disinfecting Cleaner |
| Bleach Rite Disinfecting Spray with Bleach |
| Buster |
| Clorox HW |
| Cppc Ultra Bleach 2 |
| Cppc Tsunami |
| Crockett |
| Csp-3002-3 |
| Dispatch Hospital Cleaner Disinfectant with Bleach |
| Dispatch Hospital Cleaner Disinfectant Towels with Bleach |
| FFATH |
| Geronimo 160a |
| Haste-SSD-Component B |
| Haste-SSD-Component A |
| Hype-Wipe Disinfecting Towel with Bleach |
| Kimtech Germicidal Wipe |
| Klorsept |
| Lysol Brand Disinfectant Bleach Plus |
| Maguard 5626 |
| Massasoit A |
| Metacomet 160B |
| Osceola 160C |
| Pdi sani-cloth bleach wipes |
| Peridox Rtu T |
| Puma |
| Pure bright germicidal ultra bleach |
| Pure bright germicidal 160 bleach |
| Sanosil Halomist |
| Steriplex SD Part A |
| Steriplex SD Activator (Part B) |
| Super-chlor |
| Tecumseh B |
| Tubbs |
| Virasept |
| Wampatuck C |
There is also growing concern about the possible association between use of gastric acid suppression drugs, particularly proton pump inhibitors (PPIs), and development of CDI, although data are limited by heterogeneity of studies, low quality of evidence, and conflicting results among several studies.25,26 The US Food and Drug Administration issued a 2012 warning about the possible association,8 but while some studies have shown that PPIs are independently associated with the risk of CDI,11,27,28 others have not.29,30 The association may be confounded by the fact that PPIs themselves can cause diarrhea and predispose patients to increased testing, leading to more detection of asymptomatic carriage. In addition, potential bias in studies showing an association as a result of sicker patients being at greater risk for CDI and being more likely to receive PPIs has been suggested.30
Hospital and Community-Wide Antibiotic Stewardship Efforts Needed
Antibiotic exposure has lasting impact on the microbiome and is the single most important risk factor for CDI. Of 84 patients diagnosed with CDI after recent hospital discharge, 83 (99%) had received antibiotics within the previous 90 days, either in the inpatient setting, outpatient setting, or both.31 The odds of CDI increase during antibiotic therapy and in the 3 months following are highest while on antibiotic therapy and in the first month after completion (OR 6.7–10.4).32 The lasting impact of antibiotics likely accounts for the high level of community-onset (post-discharge) CDI.32
Antibiotic stewardship is highlighted in a 2014 CDC MMWR Vital Signs which lays out seven core elements critical to the success of hospital antibiotic stewardship programs.23 The Society for Healthcare Epidemiology of America and Infectious Diseases Society of America 2014 Practice Recommendation on preventing CDI in acute care hospitals33 emphasizes appropriate use of antibiotics as a basic practice for prevention of CDI for all hospitals, including avoiding patient exposure to unnecessary antibiotics and selecting antibiotics associated with lower risk of CDI when possible. Antibiotics predisposing to CDI include fluoroquinolones, 3rd and 4th generation cephalosporins, ampicillin, and clindamycin.33
Fluoroquinolones and cephalosporins in particular are commonly misused for presumed respiratory and urinary tract infections (e.g., misdiagnosis of asymptomatic bacteriuria as a urinary tract infection).34 Appropriate use of these antibiotics is critical to reducing CDI incidence. Since use of these antibiotics extends into community practices, stewardship efforts should also extend into the community.
Chitnis et al report that 64% of patients with community-associated CDI were taking antibiotics within 12 weeks of having a positive stool test.3 The most common reasons for taking antibiotics were ear, sinus, or upper respiratory tract infection (34.7%), dental cleaning or oral surgery (15.1%), urinary tract infection (9.3%), skin infection (7.5%), and bronchitis or pneumonia (7.5%). Roughly the same proportion of patients received cephalosporins (23.6%), beta-lactam or beta-lactamase inhibitors (23%), penicillins (22.7%) and fluoroquinolones (22%), with a smaller percentage receiving clindamycin (18.9%).3
Also of note is that nearly 28% of all patients with CDI in this study reported recent PPI use and just over 31% of the CDI patients with no prior antibiotic exposure received PPIs. The large proportion of patients with PPI exposure adds to the growing concern of a potential causal relationship between PPIs and CDI.3
Antibiotic Stewardship: Role for HCPs and Other Stakeholders
The CDC’s Vital Signs program recommends actions by a wide range of stakeholders to improve antibiotic stewardship and reduce CDI incidence (Table 3).35 The authors urge readers to examine Table 3 carefully to identify actions they can take.
Table 3.
Actions to Reduce CDI Incidence35
| Doctors and nurses can |
| • Prescribe antibiotics carefully. Once culture results are available, check whether the prescribed antibiotics are correct and necessary. |
| • Order a C. difficile test (preferably a nucleic acid test) if the patient has had 3 or more unformed stools within 24 hours. |
| • Be aware of infection rates in their facility or practice, and follow infection control recommendations with every patient. This includes isolating patients who test positive for CDI and wearing gloves and gowns to treat them. |
| Healthcare facility administrators can |
| • Support better testing, tracking, and reporting of infections and prevention efforts. |
| • Make sure cleaning staff follows CDC recommendations, using an EPA-approved, spore-killing disinfectant in rooms where C. difficile patients are treated (see Table 2). |
| • Notify other healthcare facilities about infectious diseases when patients transfer, especially between hospitals and nursing homes. |
| • Participate in a regional C. difficile prevention effort. |
| States and communities can |
| • Encourage healthcare facilities to track and share data using CDC's National Healthcare Safety Network. |
| • Develop regional C. difficile prevention projects with many types of facilities. |
| • Help healthcare facilities in their prevention efforts. |
| • Provide a standardized form for facilities to use during patient transfers, especially between hospitals and nursing homes. |
| Patients can |
| • Take antibiotics only as prescribed by their doctor. |
| • Tell their doctor if they have been on antibiotics and get diarrhea within a few months. |
| • Wash their hands after using the bathroom. |
| • Try to use a separate bathroom if they have diarrhea, or be sure the bathroom is cleaned well if someone with diarrhea has used it. |
Callout Box
Small- and Large-Scale Antibiotic Stewardship Successes
A successful antibiotic stewardship program at a U.K. teaching hospital resulted in significant reductions in CDI incidence in elderly inpatients aged ≥80 years.36 The hospital’s policy targeted replacement of broad-spectrum antibiotics, specifically cephalosporins and amoxicillin/clavulanate, with narrow-spectrum antibiotics such as benzyl penicillin, amoxicillin, and trimethoprim. There was a rapid and sustained move from broad- to narrow-spectrum antibiotics (all other antibiotic use remained unchanged) and a significant fall in CDI associated with the intervention but not in the control outcome, MRSA (CDI incidence rate ratios of 0.35 [0.17, 0.73, p=0.009] compared with MRSA IRR of 0.79 [0.49, 1.29, p=0.32]).36
A national antibiotic stewardship program in the UK provides even more compelling evidence of its potential impact on reducing the burden of CDI. Faced with a CDI epidemic, the UK introduced a national antibiotic stewardship program to limit the use of 2nd and 3rd generation cephalosporins and fluoroquinolones. These antibiotic reductions were met with coincident decreases in hospital CDI rates.37 The target for CDI reduction was actually far exceeded, with a 61% reduction in CDI reports from 36,095 in 2008–2009 to 21,698 from 2010–2011.21
The C. difficile Ribotyping Network (CDRN), established in the UK in 2007, also saw a gradual reduction in the prevalence of ribotype NAP1/027, which is resistant to fluoroquinolones, from 55% of all cases in 2007 to 36% in 2008 and 21% in 2009.21 In addition, the cases that occurred over time were decreasingly associated with both cephalosporins and fluoroquinolones.21End Callout Box
Measures to Reduce Acquisition of C. difficile Spores
Reducing patient acquisition of C. difficile spores requires a multi-faceted approach that includes prompt identification and isolation of infected patients, reducing spore contamination in patient care environments, adhering to Contact Precautions, and effective communication between healthcare facilities.
Hand Hygiene, Gloves, and Contact Precautions
The core hand hygiene recommendations for care of patients with CDI follow CDC and World Health Organization guidelines and call for preferential use of soap and water in outbreak or hyperendemic settings.38 Special approaches to preventing CDI, which have already been adopted by many hospitals, call for hand washing with soap and water before exiting the room of any CDI patient.33 In all cases, measuring compliance is critical to success.
Glove use is most essential to reducing spore transmission.39 C. difficile spores are very difficult to remove from the hands even with proper washing. Therefore, strict adherence to glove use as part of Contact Precautions are essential to any efforts to reduce spore transmission.
Patient hand washing should also be emphasized as part of CDI prevention efforts, as patients may self-inoculate with the C. difficile spores if their hands come into contact with surfaces contaminated with the spores. As hand washing is more effective than alcohol hand rub in removing spores,40 frequent patient hand washing should be encouraged, especially after using the bathroom and before eating.
Extending Contact Precautions: Asymptomatic C. difficile Carriage
If CDI rates remain high despite adherence to core recommendations, the duration of Contact Precautions may need to extend beyond resolution of diarrhea symptoms. Patients frequently continue to shed C. difficile spores after resolution of diarrhea and beyond the end of treatment.41 The lowest point of shedding coincides with the end of treatment, but then increases in the first 4 weeks post treatment before declining again.
Environmental Cleaning and Use of Sporicidal Agents
There are limited data suggesting that disinfecting with a 1:10 bleach dilution prepared fresh daily reduces C. difficile transmission, particularly in units with high endemic rates such as bone marrow transplant units.42,43 When considering a switch to a sporicidal agent, facilities may want to begin by focusing on units where C. difficile rates are high, as some sporicidal agents may have adverse effects such as corrosion. A list of EPA-registered agents with sporicidal claims can be found in Table 2 and online at the EPA website where it is updated periodically.24
Another key to effective spore removal is measuring adequacy of cleaning procedures. Carling et al tested 1404 surface objects in 157 rooms in 3 hospitals and found 47% of the objects had been cleaned.44 This cleaning audit, coupled with an educational intervention among cleaning staff led to a sustained improvement in cleaning objects and a greater than 2-fold improvement in cleaning high-touch surfaces that had previously been cleaned less than 85% of the time.
As part of its toolkit on reducing hospital-associated infections, CDC provides a checklist for identifying high-touch surfaces (available at: cdc.gov/hai/pdfs/toolkits/environ-cleaning-eval-toolkit12–2-2010.pdf). The toolkit also reviews methods for assessing the adequacy of cleaning and pros and cons of different systems.
Rutala et al looked at spore count reduction on surfaces from several cleaning methods.45 Wiping alone with a non-sporicidal agent resulted in a 2.90 log reduction in spore count. The addition of a sporidical agent led to a greater reduction (3.70 log reduction). Spraying with a sporicidal disinfectant led to a 3.40 log reduction, but is associated with prolonged drying time lack of debris removal. The investigators concluded that wiping with a sporicidal agent is the best approach.
Interfacility Transfers
The two key factors in CDI burden, antibiotic exposure and spore acquisition, can occur in different settings, making it difficult to attribute cases to a specific facility or segment of the healthcare system. Since patient transfers among a variety of settings (e.g., acute care hospitals, long-term care, nursing homes, home health, etc.) are common, facilities must focus on optimizing communication during these transfers to prevent C. difficile transmission to other facilities.
It is essential that discharging and receiving facilities communicate key information about patients during transfer. This includes whether the patient has or had C. difficile or any other drug-resistant organism. CDC provides a sample Inter-facility Infection Control Transfer Form as part of its online toolkit for reduction of healthcare associated infections. (http://www.cdc.gov/hai/pdfs/toolkits/InfectionControlTransferFormExample1.pdf)
CONCLUSION
Virtually all CDI cases are healthcare-related. The primary causes of CDI—antibiotic exposure and spore acquisition—occur in a variety of settings. CDI onset and diagnosis can likewise happen in a variety of inpatient and outpatient settings. The current epidemiology of CDI necessitates active participation from all segments of the healthcare community in a comprehensive approach to reduce the burden of CDI through effective antibiotic stewardship and active measures to reduce spore transmission.
Self-Assessment Examination
A minimum assessment score of 80% is required.
- Development of CDI usually requires:
- Diagnosis of Irritable Bowel Syndrome (IBS)
- Disruption of the fecal microbiota (typically via exposure to antibiotics)
- Acquisition of the organism via the fecal-oral route
- A & B
- B & C
- What precautions should all healthcare personnel use to help prevent the spread of CDI?
- Wear gloves and gowns when caring for CDI patients
- Careful attention to environmental cleaning of high-touch surfaces
- Notify receiving facilities or units of CDI status (including recently resolved infections) upon transfer
- A & C
- All of the above
- Which of the following is true?
- Once a patient becomes asymptomatic, shedding no longer poses a transmission risk
- The lowest point of shedding usually coincides with the end of treatment
- Shedding is higher in the first four weeks post-treatment than it is at treatment completion
- A & B
- B & C
- Which of the following most accurately approximates the annual US burden of CDI?
- 250,000 cases and 19,000 deaths within 30 days
- 350,000 cases and 19,000 deaths within 30 days
- 350,000 cases and 29,000 deaths within 30 days
- 450,000 cases and 19,000 deaths within 30 days
- 450,000 cases and 29,000 attributable deaths
- Which of the following antibiotics pose the highest risk for CDI?
- Erythromycin, clarithromycin, and azithromycin
- Cephalosporins, fluoroquinolones, and clindamycin
- Trimethoprim/sulfamethoxazole
- Tetracycline
- Amoxicillin, penicillin
Evaluation
Your input is important in improving future publications and identifying areas of need for other educational activities. Please circle the choice that best answers the following:
-
1.
The format was appropriate for the subject matter.
Agree Neutral Disagree
-
2.
This activity supported achievement of the learning objectives.
Agree Neutral Disagree
-
3.
The material was organized clearly for learning to occur.
Agree Neutral Disagree
-
4.
I acquired a new strategy to use in my clinical practice.
Agree Neutral Disagree
-
6.
The activity was objective and free of commercial bias.
Agree Neutral Disagree
-
5.
I would recommend this activity to my colleagues.
Agree Neutral Disagree
-
6.
I spent _________ minutes participating in this activity.
Additional comments and suggested continuing education topics that would be of value to you: ___________________________________________________ ___________________________________________________
You must print legibly and provide all of the information below to obtain credit. Your certificate will be sent to the e-mail address provided below:
Name/Degree________________________________________ Title_______________________________________________
Affiliation __________________________________________
Address ____________________________________________ City_______________________State___________Zip_______
E-Mail _____________________________________________
Telephone __________________________________________
Check the appropriate box:
□ I am an MD or DO and wish to receive 0.5 AMA PRA Category 1 Credits™
□ I wish to receive a certificate of completion
___________________________ ____________________
Signature Date
Acknowledgments
This publication is based on presentations by Dr Gould and Dr McDonald during an NFID webinar held in June 2015. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
This activity is supported by an unrestricted educational grant from Merck & Co., Inc.
Footnotes
TARGET AUDIENCE
Physicians and other healthcare professionals interested in the causes and prevention of Clostridium difficile infection.
EDUCATIONAL OBJECTIVES
Following the educational activity, participants will be able to describe the epidemiology and burden of Clostridium difficile infection (CDI); identify patient populations at risk for CDI; discuss the role of healthcare professionals and the healthcare system in the prevention of CDI; and list core actions all healthcare professionals should implement to prevent CDI.
PARTICIPATION IN THE LEARNING PROCESS
Credit is based on the approximate time it should take to read this publication and complete the assessment and evaluation. A minimum assessment score of 80% is required. Publication date is November 1, 2015. Requests for credit or contact hours must be postmarked no later than May 1, 2016, after which this material is no longer certified for credit.
CONTINUING MEDICAL EDUCATION
The National Foundation for Infectious Diseases (NFID) is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians. NFID designates this enduring material for a maximum of 0.5 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
CME INSTRUCTIONS
To receive credit after reading the publication, complete and return the self-assessment examination, evaluation, and your contact information, via fax to 301–907-0878 or by mail to NFID CME Office, 7201 Wisconsin Ave, Suite 750, Bethesda, MD 20814. No fee is required. Please allow 2 to 4 weeks for processing. Inquiries may be directed to 301–656-0003 or cme@nfid.org.
DISCLOSURE
NFID must ensure balance, independence, objectivity, and scientific rigor in its educational activities. All individuals with control over content are required to disclose any relevant financial interest or other relationship with manufacturer(s) of any product or service discussed in an educational presentation and/or with the commercial supporters of this activity. Disclosure information is reviewed in advance to manage and resolve any conflict of interest, real or apparent, that may affect the balance and scientific integrity of an educational activity.
Marla Dalton, CAE (NFID staff, content reviewer) owns stock, stock options, or bonds from Merck & Co. Inc. Thomas M. File, Jr. MD (author) served as an advisor or consultant for Astellas, Cubist, Durata, GlaxoSmithKline, Nabriva, Pfizer Inc., and Tetraphase and received grant or research support from Cempra and Pfizer Inc. Carolyn V. Gould, MD (author) has no relevant financial relationships to disclose. L. Clifford McDonald, MD (author) has no relevant financial relationships to disclose.
All other authors, planners, and content reviewers have no funding or conflicts of interest to disclose.
Adapted from CDC. Marking Health Care Safer. Stopping C. difficile infections. Available at: http://www.cdc.gov/vitalsigns/hai/stoppingcdifficile/.
REFERENCES
- 1.CDC. Vital Signs: Preventing Clostridium difficile infections. MMWR Morb Mortal Wkly Rep. 2012;61(09):157–162. [PubMed] [Google Scholar]
- 2.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chitnis AS, Holzbauer SM, Belflower RM, et al. Epidemiology of community-associated Clostridium difficile infection, 2009 though 2001. JAMA Intern Med. 2013;173:1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guinane CM, Tadrous A, Fouhy Y, et al. Microbial composition of human appendices from patients following appendectomy. MBio. 2013;January 15;4(1). pii: e00366–12. doi: 10.1128/mBio.00366-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eglow R, Pothoulakis C, Itzkowitz S, et al. Diminished Clostridium difficile toxin A sensitivity in newborn rabbit ileum is associated with decreased toxin A receptor..J Clin Invest. 1992;90(3):822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly CP, Kyne L The host immune response to Clostridium difficile.J Med Microbiol. 2011;August;60 (Pt 8):1070–9. doi: 10.1099/jmm.0.030015-0. Epub 2011 Mar 17. Review. [DOI] [PubMed] [Google Scholar]
- 7.Lowy I, Molrine DC, Leav B, et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med. 2010;362(3):197–205. [DOI] [PubMed] [Google Scholar]
- 8.US Food and Drug Administration. FDA Drug Safety Communication: Clostridium difficile-associated diarrhea can be associated with stomach acid drugs known as proton pump inhibitors (PPIs). Available at: http://www.fda.gov/drugs/drugsafety/ucm290510.htm. Accessed August 3, 2015.
- 9.Rao A, Jump RL, Pultz NJ, et al. In vitro killing of nosocomial pathogens by acid and acidified nitrate. Antimicrob Agents Chemother. 2006. 50(11):3901–3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freedberg DE, Toussaint NC, Chen SP, et al. Gastroenterology. 2015;July 8 pii: S0016–5085(15)00933–6. doi: 10.1053/j.gastro.2015.06.043. [Epub ahead of print]. [DOI] [Google Scholar]
- 11.Stevens V, Dumyati G, Brown J, et al. Differential risk of Clostridium difficile infection with proton pump inhibitor use by level of antibiotic exposure. Pharmacoepidemiol Drug Saf. 2011;20(10):1035–1042. [DOI] [PubMed] [Google Scholar]
- 12.McDonald LC, Coignard B, Dubberke E, et al. ; the Ad Hoc Clostridium difficile Surveillance Working Group. Recommendations for surveillance of Clostridium difficile-Associated disease. Infect Control Hosp Epidemiol. 2007;28(2):140–145. [DOI] [PubMed] [Google Scholar]
- 13.CDC. National Healthcare Safety Network (NHSN). MDRO & CDI LabID Event Calculator Version 1.0. Available at: http://www.cdc.gov/nhsn/labid-calculator/. Accessed August 15, 2015.
- 14.Kwon JH, Olsen MA, Dubberke ER The morbidity, mortality, and costs associated with Clostridium difficile infection. Infect Dis Clin N Am. 2015;29(1):123–134. [DOI] [PubMed] [Google Scholar]
- 15.Miller BA, Chen LF, Sexton DJ, et al. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile infection and of healthcare-associated infection due to methicillin-resistant Staphylocococcus aureus in community hospitals. Infect Control Hosp Epidemiol. 2011;32(4):387–390. [DOI] [PubMed] [Google Scholar]
- 16.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubberke ER, Olsen MA Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(Suppl 2):S88–S92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353:2433–2441. [DOI] [PubMed] [Google Scholar]
- 19.Stabler RA, Dawson LF, Phua LT, et al. Comparative analysis of BI/NAP1/027 hypervirulent strains reveal novel toxin B-encoding gene (tcdB) sequences. J Med Microbiol. 2008;57(Pt 6):771–775. [DOI] [PubMed] [Google Scholar]
- 20.Akerlund T, Persson I, Unemo M, et al. Increased sporulation rate of epidemic Clostridium difficile type 027/NAP1. J Clin Microbiol. 2008;46(4):1530–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilcox MH, Shetty N, Fawley WN, et al. Changing epidemiology of Clostridium difficile infection following the introduction of a national ribotyping-based surveillance scheme in England. Clin Infect Dis. 2012;55(8):1056–1063. [DOI] [PubMed] [Google Scholar]
- 22.See I, Mu Y, Cohen J, et al. NAP1 strain type predicts outcomes from Clostridium difficile infection. Clin Infect Dis. 2014;58(10):1394–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.CDC. Vital Signs: Improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194–200. [PMC free article] [PubMed] [Google Scholar]
- 24.Environmental Protection Agency. List K: EPA’s registered antimicrobial products effective against Clostridium difficile spores. Available at: http://www.epa.gov/oppad001/list_k_clostridium.pdf. Accessed July 31, 2015.
- 25.Leontiadis GI, Miller MA, Howden CW How much do PPIs contribute to C. difficile infections? Am J Gastroenterol. 2012;107(7):1020–1021. [DOI] [PubMed] [Google Scholar]
- 26.Tleyjeh IM, Bin Abdulhak AA, Riaz M, et al. Association between proton pump inhibitor therapy and Clostridium difficile infection: a contemporary systematic review and meta-analysis. PLoS One. 2012;7(12):e50836. doi: 10.1371/journal.pone.0050836. E pub 2012 Dec 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barletta JF, Sclar DA Proton pump inhibitors increase the risk for hospital-acquired Clostridium difficile infection in critically ill patients. Crit Care. 2014;18(6):714 Doi: 10.1186/s13054-014-0714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buendgens L, Bruensing J, Matthes M, et al. Administration of proton pump inhibitors in critically ill medical patients is associated with increased risk of developing Clostridrium difficile-associated diarrhea.J Crit Care. 2014;29(4):696.e11–2. doi: 10.1016/j.jcrc.2014.03.002. Epub 2014 Mar 7. [DOI] [PubMed] [Google Scholar]
- 29.Pépin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254–1260. [DOI] [PubMed] [Google Scholar]
- 30.Novack L, Kogan S, Gimpelevich L, et al. Acid suppression therapy does not predispose to Clostridium difficile infection: the case of the potential bias. PLos One. 2014;9(10):e110790. doi: 10.1371/journal.pone.0110790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang HT, Krezolek D, Johnson S, et al. Onset of symptoms and time to diagnosis of Clostridium difficile-associated disease following discharge from an acute care hospital. Infect Control Hosp Epidemiol. 2007;28(8):926–931. [DOI] [PubMed] [Google Scholar]
- 32.Hensgens MP, Goorhuis A, Dekkers OM, et al. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother. 2012;67:742–748. [DOI] [PubMed] [Google Scholar]
- 33.Dubberke ER, Carling P, Carrico R, et al. SHEA/IDSA Practice Recommendations. Strategies to prevention Clostridium difficile infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(6):628–645. [DOI] [PubMed] [Google Scholar]
- 34.Shaughnessy MK, Amundson WH, Kuskowski MA, et al. Unnecessary antimicrobial use in patients with current or recent Clostridium difficile infection. Infect Control Hosp Epidemiol. 2013;34(2):109–116. [DOI] [PubMed] [Google Scholar]
- 35.CDC. Vital Signs: Making health care safer. Antibiotic Rx in hospitals: Proceed with caution. Available at: http://www.cdc.gov/vitalsigns/pdf/2014-03-vitalsigns.pdf. Accessed July 31, 15.
- 36.Fowler S, Webber A, Cooper BS, et al. Successful use of feedback to improve antibiotic prescribing and reduce Clostridium difficile infection: a controlled interrupted time series. J Antimicrob Chemother. 2007;59:990–995. [DOI] [PubMed] [Google Scholar]
- 37.Ashiru-Oredope D, Sharland M, Charani E, et al. Improving the quality of antibiotic prescribing in the NHS by developing a new Antimicrobial Stewardship Programme: Start Smart—Then Focus. J Antimicrob Chemother. 2012;67(Suppl 1):i51–i63. [DOI] [PubMed] [Google Scholar]
- 38.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431–455. [DOI] [PubMed] [Google Scholar]
- 39.Johnson S, Gerding DN, Olson MM, et al. Prospective, controlled study of vinyl glove use to interrupt Clostridium difficile nosocomial transmission. Am J Med. 2990;88(2):137–140. [DOI] [PubMed] [Google Scholar]
- 40.Kundrapu S, Sunkesula V, Jury I, et al. A randomized trial of soap and water versus alcohol hand rub for removal of Clostridium difficile spores from hands of patients. Infect Control Hosp Epidemiol. 2014;35(2):204–206. [DOI] [PubMed] [Google Scholar]
- 41.Sethi AK, Al-Nassir WN, Nerandzic MM, et al. Persistence of skin contamination and environmental shedding of Clostridium difficile during and after treatment of C. difficile infection. Infect Control Hosp Epidemiol. 2010;31(1):21–27. [DOI] [PubMed] [Google Scholar]
- 42.Mayfield JL, Leet T, Miller J, et al. Environmental control to reduce transmission of Clostridium difficile. Clin Infect Dis. 2000;31(4):995–1000. [DOI] [PubMed] [Google Scholar]
- 43.Wilcox MH, Fawley WN, Wigglesworth N, et al. Comparison of the effect of detergent versus hypochlorite cleaning on environmental contamination and incidence of Clostridium difficile infection. J Hosp Infect. 2003;54(2):109–114. [DOI] [PubMed] [Google Scholar]
- 44.Carling PC, Briggs JL, Perkins J, et al. Improved cleaning of patient rooms using a new target method. Clin Infect Dis. 2006;42(3):385–388. [DOI] [PubMed] [Google Scholar]
- 45.Rutala WA, Gergen MF, Weber DJ Efficacy of different cleaning and disinfection methods against Clostridium difficile spores: importance of physical removal versus sporicidal inactivation. Infect Control Hosp Epidemiol. 2012;33(12):1255–1258. [DOI] [PubMed] [Google Scholar]


