Abstract
Background
The United States Centers for Disease Control and Prevention estimates that approximately 60,000 US youth are living with HIV. US youth living with HIV (YLWH) have poorer outcomes compared with adults, including lower rates of diagnosis, engagement, retention, and virologic suppression. With Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) support, new trials of youth-centered interventions to improve retention in care and medication adherence among YLWH are underway.
Objective
This study aimed to use a computer simulation model, the Cost-Effectiveness of Preventing AIDS Complications (CEPAC)-Adolescent Model, to evaluate selected ongoing and forthcoming ATN interventions to improve viral load suppression among YLWH and to define the benchmarks for uptake, effectiveness, durability of effect, and cost that will make these interventions clinically beneficial and cost-effective.
Methods
This protocol, ATN 161, establishes the ATN Modeling Core. The Modeling Core leverages extensive data—already collected by successfully completed National Institutes of Health–supported studies—to develop novel approaches for modeling critical components of HIV disease and care in YLWH. As new data emerge from ongoing ATN trials during the award period about the effectiveness of novel interventions, the CEPAC-Adolescent simulation model will serve as a flexible tool to project their long-term clinical impact and cost-effectiveness. The Modeling Core will derive model input parameters and create a model structure that reflects key aspects of HIV acquisition, progression, and treatment in YLWH. The ATN Modeling Core Steering Committee, with guidance from ATN leadership and scientific experts, will select and prioritize specific model-based analyses as well as provide feedback on derivation of model input parameters and model assumptions. Project-specific teams will help frame research questions for model-based analyses as well as provide feedback regarding project-specific inputs, results, sensitivity analyses, and policy conclusions.
Results
This project was funded as of September 2017.
Conclusions
The ATN Modeling Core will provide critical information to guide the scale-up of ATN interventions and the translation of ATN data into policy recommendations for YLWH in the United States.
Keywords: adolescent, costs and cost analysis, health policy, HIV, medication adherence, modeling, retention in care, youth
Introduction
Background
Approximately 60,000 youth are living with HIV in the United States. Youth living with HIV (YLWH) have poorer outcomes than adults living with HIV, including lower rates of diagnosis, engagement, retention, and virologic suppression [1,2]. Established in 2001 by the Maternal and Pediatric Infectious Disease Branch of the Eunice Kennedy Shriver National Institutes of Child Health and Development, the Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) has conducted rigorous evaluations of interventions to improve medication adherence, retention in care, and viral load (VL) suppression among YLWH [3]. ATN is the only national clinical research network that specifically studies adolescents aged 12 to 24 years living with HIV and at risk for acquiring HIV. ATN collaborations have included the United States Centers for Disease Control and Prevention; the Health Resources and Services Administration; the AIDS Clinical Trials Group; the HIV Vaccine Trials Network; the HIV Prevention Trials Network; the International Maternal, Pediatric, and Adolescent AIDS Clinical Trial (IMPAACT) Network; and the Microbicide Trials Network. This ATN began with new National Institutes of Health (NIH) support in 2016 to fund youth-focused projects that aim to reduce risk factors for adolescents at risk of acquiring HIV and to promote behaviors related to adherence and engagement with care for those living with HIV. ATN currently supports 22 protocols [3].
By projecting outcomes beyond the time horizon of traditional studies and thereby permitting estimates of long-term clinical outcomes and cost-effectiveness, computer-based health policy models can add substantial value to clinical trials and observational studies [4]. Projecting such long-term estimates is particularly important for studies among YLWH, for whom the health effects of poor virologic control may not manifest for years or decades [5]. Models can also combine data from multiple sources and compare a wide range of possible interventions, leveraging the extensive data collected within ATN and other studies into timely guideline and policy recommendations [6]. The Cost-effectiveness of Preventing AIDS Complications (CEPAC)-computer simulation models [7] of HIV infection in infants, children, and adults have been used to inform health policy related to HIV prevention [8,9], testing [10-12], and care [13-18], both in the United States and internationally. CEPAC model-based work has been cited in national HIV care guidelines for the United States, Brazil, Chile, Mexico, France, and Colombia, among others, as well as in the World Health Organization (WHO) guidelines [19-23]. For example, a CEPAC-Pediatrics model-based analysis projected that use of lopinavir/ritonavir in children younger than 3 years as first-line antiretroviral therapy (ART) led to longer life-expectancy and was cost-saving compared with first-line use of nevirapine; this analysis helped inform the WHO’s recommendation in 2013 of a lopinavir/ritonavir-based regimen for first-line ART in that age group [16,24]. To date, few HIV modeling or cost-effectiveness studies have been conducted among youth; most have focused on HIV screening and prevention [25-32]. Previous work has not incorporated age- and time-varying changes in adolescent and young adult health-related behavior among YLWH.
Objectives
This protocol will leverage existing data from successfully completed NIH-supported studies (Table 1) to inform the development of novel approaches for modeling critical components of HIV disease and care in YLWH. As ATN investigators study new interventions to improve VL suppression among YLWH, the CEPAC-Adolescent computer simulation model will be developed to define the benchmarks for uptake, effectiveness, durability of effect, and cost that will make these interventions clinically beneficial and cost-effective. In addition, as new data emerge from ongoing ATN trials about the effectiveness of these interventions, the computer simulation model will serve as a flexible tool to project the long-term clinical impact and cost-effectiveness of these interventions. This project will, therefore, provide critical information to guide the scale-up of ATN interventions and the translation of ATN data into policy recommendations for YLWH in the United States.
Table 1.
National Institutes of Health–supported studies from the Adolescent Medicine Trials Network for HIV/AIDS Interventions and the International Maternal, Pediatric, and Adolescent AIDS Clinical Trials Network included in the proposed analysis.
| Study; timea,b | Title | Years | Age at enrollment | N (13-24)c | Populationd |
| ATNe 061 [33-36]; 2.9 years | T-cells in ARTf deintensification | 2007-2010 | 18-24 years | 130 (all) | NPHIVYg |
| ATN 106/086 [37-40]; 1 year | Health status and behavioral risk factors | 2011-2012 | 12-24 years | 2196 (all) | NPHIVY and PHIVYh |
| ATN 125 [41,42]; 1.5 years | Treatment at ATN sites | 2015-2017 | 13-24 years | 922 (all) | NPHIVY |
| Pi1055 [41,43,44]; 1.8 years | Psychiatric conditions in PHIVY | 2005-2006 | 6-17 years | 294 (199) | PHIVY |
| P1066 [45-48]; 1 year | RALj safety, PKk, effectiveness | 2007-2013 | 1 month to 19 years | 126 (71) | PHIVY |
| P1074 [49-51]; 5.3 years | Long-term outcomes | 2009-2014 | 0-24 years | 1236 (all) | NPHIVY and PHIVY |
| P1093 [52,53]; 2 years | Dolutegravir-based ART | 2011-2018 | 1 month to 18 years | 160 (23) | PHIVY |
aMean or median follow-up time.
bMinimum key data for all studies: viral loads, cluster of differentiation 4 (CD4) cell count, ART regimens, opportunistic infections, sexually transmitted infections, pregnancy, and other clinical diagnoses.
cTotal N (n aged 13-24 years): 4904 (4777).
dPopulation: primarily NPHIVY or PHIVY.
eATN: Adolescent Medicine Trials Network for HIV/AIDS Interventions.
fART: antiretroviral therapy.
gNPHIVY: nonperinatally HIV-infected youth.
hPHIVY: perinatally HIV-infected youth.
iP: pediatric.
jRAL: raltegravir.
kPK: pharmacokinetic.
Methods
Adolescent Medicine Trials Network for HIV/AIDS Interventions Structure and Establishment of the Modeling Core
The ATN structure consists of 3 ATN research program projects (U19s) and a Coordinating Center (U24; Figure 1). Each of the 3 ATN research program projects (U19) has a well-defined research focus supported by core infrastructures as well as participant recruitment and enrollment capacity. These research program projects are as follows:
Figure 1.
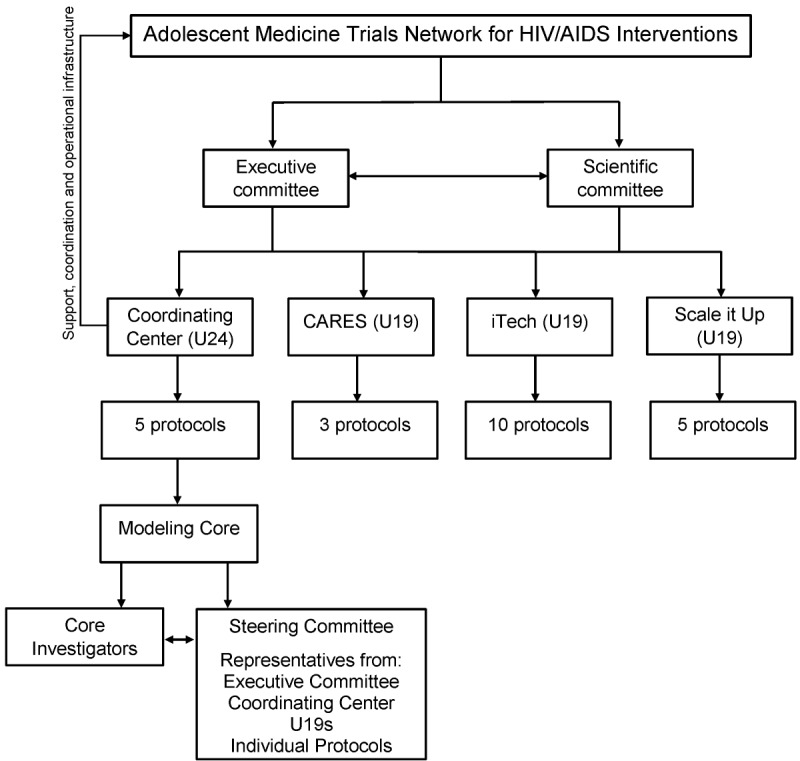
Organizational structure of the Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN).
Comprehensive Adolescent Research and Engagement Studies [54], a comprehensive community-based project that aims to optimize the HIV prevention and treatment continuum for at-risk and acutely infected youth as well as youth with established HIV infection.
iTech [55], a research program that aims to impact the HIV epidemic by conducting innovative, interdisciplinary research using technology-based interventions across the HIV prevention and care continuum for adolescents and young adults.
Scale it Up [56], a research program that aims to assess and enhance the real-world effectiveness, implementation, and scalability of theoretically based and developmentally tailored interventions focused on improving HIV treatment and prevention self-management for youth.
Each research program project (U19) supports several individual protocols [54-56]. The ATN Coordinating Center (U24) is located at the University of North Carolina at Chapel Hill. The Coordinating Center provides support, coordination, and operational infrastructure to ATN. The Coordinating Center also supports several stand-alone protocols such as “A Triggered, Escalating, Real-Time Adherence Intervention,” which uses electronic-dose monitoring to inform an adherence intervention for youth without virologic suppression. The Coordinating Center also supports the Modeling Core.
The Modeling Core has established a Modeling Core Steering Committee that will meet regularly and include Modeling Core investigators, at least 1 principal investigator or liaison from each of the 3 ATN research program projects (U19s) and the ATN Coordinating Center, protocol chairs or representatives from stand-alone trials for which modeling is planned, and additional interested ATN investigators.
ATN investigators in the Modeling Core Steering Committee will provide feedback on the derivation of data inputs, design of new model structure within the CEPAC-Adolescent model, and selection of policy analyses to perform. Once specific ATN studies are identified as potential candidates for modeling analyses, the Modeling Core investigators will work with relevant protocol teams to ensure that data likely to be useful for later modeling are collected prospectively in each study.
After the Modeling Core Steering Committee has determined which policy analyses will be performed, project teams will be assembled for each analysis. Each project team will include Modeling Core investigators and the protocol chair or a representative from the trial being analyzed. Project team members have expertise in multiple relevant areas including epidemiology, health services research, economics, intervention science, implementation science, behavioral science, clinical trials development, and the clinical care of YLWH. Project teams will help develop the research question, identify any additional structural simulation model modifications, provide input on needed data parameters (eg, help identify potential issues of population mismatch for parameters derived from different sources), and review preliminary model results (eg, for face validity and identifying key sensitivity analyses). Abstracts, presentations, and manuscripts presenting model results will be reviewed in accordance with the ATN publications policy.
Scientific Objectives’ Overview
Objective 1
Objective 1 was to determine rates of key clinical events for YLWH engaged in care stratified by age, CD4 cell count, and antiretroviral (ARV) and VL status in completed and ongoing NIH-supported studies. Using ClinicalTrials.gov [57], studies were reviewed that included YLWH aged 13 to 24 years at the US sites, and collected data related to CD4 cell count, VL, and ART regimens as well as clinical event data during the era of modern ART. Selected studies were conducted within the 2 largest NIH-sponsored national networks supporting clinical trials and observational studies in youth affected by HIV—the ATN and the IMPAACT Network. Incidence rates of opportunistic infections; HIV and non-HIV events (Textbox 1); and mortality based on age, sex, patterns of CD4 count and VL, and ARV use among YLWH will be evaluated in completed and ongoing NIH-sponsored studies (Table 1) in accordance with individual data use agreements.
Categories of key outcomes (specific events within each listed category will also be analyzed separately).
Categories of key outcomes:
Centers for Disease Control and Prevention (CDC) HIV clinical diagnoses (CDC-A, B, and C)
Severe or life-threatening, non-HIV–related diagnoses (eg, pneumococcal events)
Chronic non-HIV-related diagnoses (eg, cardiac and renal disease and malignancy)
Medication toxicity (division of AIDS ≥Grade 2)
Psychiatric events
Sexually transmitted infections
Pregnancy or pregnancy outcomes
Death
Objective 2
Objective 2 was to develop the CEPAC-Adolescent model—a simulation model to reflect unique characteristics of YLWH.
The CEPAC-Adolescent simulation model will be developed to reflect the unique characteristics of YLWH. The foundational inputs of the expanded model will be populated with estimates from completed and ongoing NIH-supported studies (Table 1) derived in Objective 1 as well as other published sources. As new data emerge from the ATN or other sources related to clinical events, resource utilization, and specific interventions, model inputs will be updated.
Objective 3
Objective 3 was to use the simulation model to project the clinical impact, cost, and cost-effectiveness of selected interventions evaluated in ATN.
The Modeling Core Steering Committee will work with the ATN Executive Committee and the ATN External Scientific Panel to prioritize ATN studies for model-based analyses, based on data availability and the most relevant questions in health care policy for YLWH each year. The Modeling Core Steering Committee functions will include activities such as providing feedback on the costing perspectives to be used, the primary outcome to be modeled, secondary outcome measures to be included, and the types of economic estimates to be derived from the model.
Design for Objective 1
The design for Objective 1 was to determine rates of key clinical events for YLWH engaged in care stratified by age, CD4 cell count, and ARV and VL status in completed and ongoing NIH-supported studies.
Incidence rates of key clinical events (Textbox 1) will be described based on current age, sex, current CD4, current ARV use, and VL as well as mode of HIV acquisition (perinatally HIV-infected youth [PHIVY] or nonperinatally HIV-infected youth [NPHIVY]) [58]. These data will permit assigning risks of clinical events to simulated patients in the simulation model developed in Objective 2.
Population and Data Sources
Formal requests were approved to analyze data from 4800 YLWH in completed NIH-supported studies after appropriate data use agreement and network approvals were secured (Table 1). These studies include observational studies, nonrandomized interventions, and a randomized trial. All include youth aged 13 to 24 years at study entry. The primary focus of each study ranged widely, from determining the safety and efficacy of ARV medications to evaluating clinical, immunological, and psychiatric outcomes. All included a minimum set of key outcomes needed for this analysis, and all clinical events were recorded using comparable diagnostic codes. Protocols and data collection forms from all studies will be reviewed to understand how data can be harmonized among studies, as has been done in previous analyses [58]. Resource use input parameters will be derived from adolescent intervention or trial-specific data where available, as in previous work [29,59]. New data emerging from ongoing studies will be integrated into the model.
Data Management
Data analysis concept sheets and data use agreements have been approved for these analyses by individual networks as well as through the Eunice Kennedy Shriver National Institute of Child Health and Development Data and Specimen Hub repository [60]. Data will be cleaned (when applicable), harmonized, and safely stored at the Center for Biostatistics in AIDS Research at the Harvard TH Chan School of Public Health, which is compliant with federal regulations governing information security.
Outcomes
Clinical events that impact short- and long-term (lifetime) outcomes and health care costs, such as the occurrence of specific opportunistic infections, non-AIDS-defining illnesses, sexually transmitted infections, pregnancy, and psychiatric events, within the categories listed in Textbox 1 will be analyzed.
Statistical Analysis
Incidence rates of each outcome will be estimated, stratified by mode of HIV acquisition and the combination of time-varying age (7-12, 13-17, 18-24, and 25-30 years), CD4 cell count (<200, 200-499, and ≥500/µL), and VL and ARV status, as in previous work [58]. The VL or ARV status will be categorized as follows: (1) suppressive ARVs—VL less than 400 copies/mL and any prescribed ARVs, (2) nonsuppressive ARVs—VL 400 copies/mL or more and prescribed ARVs expected to be suppressive, and (3) no ARVs—VL 400 copies/mL or more and no prescribed ARVs [58]. Linear interpolation between CD4 cell counts and log10-transformed VL will be used to estimate dates when strata thresholds are crossed. These estimated dates will allow us to determine baseline strata and calculate total person-time contributed to each stratum.
As in previous work, trends in incidence rates of outcomes across ordinal age, CD4 cell count, and VL/ARV categories, stratified by mode of HIV acquisition, will be assessed using Poisson regression models, accounting for within-subject correlation with robust SEs [61]. The hypothesis that higher rates of clinical events will be associated with person-time spent with lower CD4 counts, older age, and at higher VL will be examined [58]. VL of 400 copies/mL or more was selected based on historic lower levels of detection for assays used during the study period [58].
We will also advance approaches to describe and predict the trajectories of CD4, VL, and care engagement over time. Locally weighted smoothing plots will be used to obtain a graphical summary of CD4 and VL trajectories over time by mode of HIV acquisition and baseline age [62]. On the basis of visual inspection, linear regression or piecewise linear regression models will be fitted to obtain slope parameters for CD4 and VL over follow-up time among subjects with at least 2 available measures. Baseline covariates such as mode of HIV acquisition, age, CD4 count, VL, and ART regimen will be added to these regression models to assess association with observed CD4 and VL trajectories. To determine whether there are any important differences between subjects with longitudinal CD4 and VL data and those missing such data, baseline characteristics will be compared between these 2 populations.
If there are sufficient numbers of YLWH who miss visits or are lost to follow-up, these will also be used to identify patterns of care followed by distinct subgroups such as those who are in care, those who are care interrupters, and those who are not in care. Latent trajectory groups will be identified from the study data with group-based trajectory modeling [63,64]. After we identify groups of participants following similar trajectories, in a secondary analysis, we will assess associations between baseline characteristics of study participants and membership in particular trajectory groups. In the CEPAC-Adolescent model, these attributes will be used to account for heterogeneity in care engagement.
Design for Objective 2
The design for Objective 2 was to develop a simulation model to reflect the unique characteristics of YLWH.
Current Model Structure
The CEPAC-Adult and -Pediatric models are Monte Carlo, state-transition models of HIV disease and treatment [7,8,17,18,29,65]. The models simulate people living with HIV with user-specified characteristics including age, CD4 cell count, VL, and treatment history, from model entry until death. In the absence of effective ART, CD4 cell counts decline monthly; with VL suppression, CD4 cell counts rise at user-specified rates. In each month, modeled patients face risks of key clinical events, such as opportunistic infections, other illnesses, and mortality, determined by current age and current CD4 cell count. Patients can also initiate or continue ARVs, with subsequent VL suppression or virologic failure, and can be lost to follow-up. Onward transmission risk is determined by pooled cohort VL levels using rates derived from published estimates (eg, 2.06/100PY transmission with HIV RNA of 3000-10,000 copies/mL) from adolescents, where available [66,67].
Patients are simulated within the model one at a time; the model tracks their clinical course, from time of entry into the model until death. Upon a patient’s death, the model records summary statistics, and a new patient then enters the model. This continues until the last patient in a cohort dies and exits the model, at which time the model tallies clinical events, durations spent in each health state, monthly life and quality-adjusted life expectancies, and costs. State transitions are stochastic and determined by the Mersenne Twister random number generator algorithm [68] that was adapted for use in C++, the programming language of CEPAC. When finished running, model output can be extracted and analyzed by the user, as shown in Figure 2, which traces a simulated patient’s CD4 cell count and HIV RNA over the course of several important clinical events.
Figure 2.
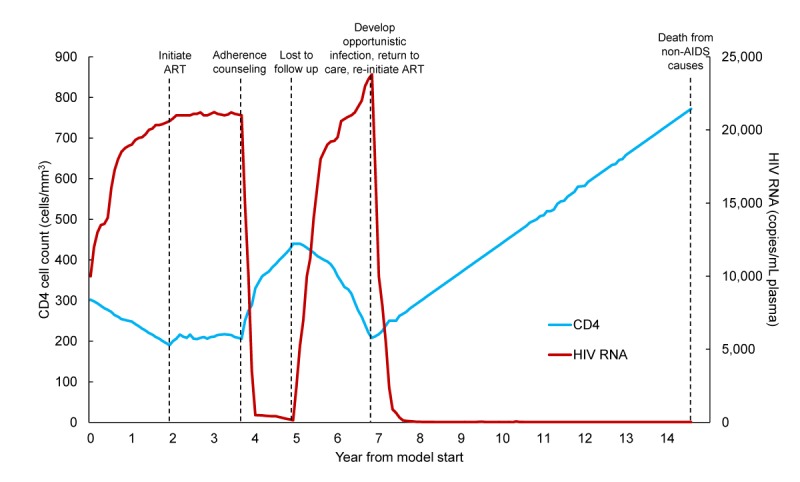
Sample simulated patient trace. CD4 cell count (cells/mm3) is presented on the vertical left-most axis and in the blue line. HIV RNA (copies/mL) is presented on the vertical right-most axis and in the red line. The horizontal axis shows years from model start. The dashed lines mark key clinical events for a simulated patient: initiating ART but failing to suppress HIV RNA and without improvement in CD4 cell count; receiving adherence counseling leading to HIV RNA suppression and improvement in CD4 cell count; becoming lost to follow up with subsequent rise in HIV RNA and decline in CD4 cell count; developing an opportunistic infection resulting in returning to care and reinitiating ART with subsequent HIV RNA suppression and improvement in CD4 cell count; and eventual death from non-AIDS-related causes. ART: antiretroviral therapy.
Adolescent-specific patterns of medication adherence and care engagement will be simulated based on work in Objective 1. Currently, in the CEPAC models, clinical event risk, retention in care, and adherence vary between individual people, but do not vary over time or with changes in development or life events. The new model structure will be developed to reflect age- and time-varying adolescent- and young adult-specific aspects of HIV disease progression and care for YLWH based on these patterns. The model structure will account for heterogeneity—the specific additions to the model structure will be informed by the data generated through activities conducted as a part of Objective 1. Additional details of the existing CEPAC models, including flowcharts, a user guide, and sample patient traces can be found on the CEPAC website [7].
Translating Objective 1 Data Into Model Inputs
Incidence rates of clinical events (Textbox 1, Objective 1) will be converted into monthly event probabilities. During each patient-month for which a specific set of characteristics apply (age, CD4, and ARV/VL category), these data will be used to assign a modeled risk of each key clinical event over the next 30-day period.
Resource utilization and cost data related to HIV care, ART, and the occurrence of acute events will be derived from adolescent-specific literature when available and otherwise will be derived from adult literature and varied in sensitivity analyses, as in previous work [29]. When specific interventions and studies are identified as candidates for model-based analyses, the Modeling Core will work with study teams to collect the data necessary for future model-based analyses in real time (eg, time and motion studies, activity logs, and costs of personnel and supplies).
Model Validation and Approach to Uncertainty
The model will be internally validated, assessing the accuracy of the model structure by comparing model output (opportunistic infections, viral suppression probability, and survival) with the empiric data in the studies from which model input parameters were derived [69]. As the model projects outcomes over lifetime horizons, the longer-term model results cannot be compared with empiric data; however, as ATN-studied interventions become more widely implemented over time, past model results will be compared with newly available data. The model will next be calibrated to data from the literature and the studies in Objectives 1 and 2 to reflect current populations of YLWH and treatment strategies. One-way, multiway, and probabilistic sensitivity analyses will be conducted, following international guidelines to address uncertainty in data inputs for the model [70,71]. This involves varying single and multiple parameters over wide ranges and reassessing all clinical results and cost-effectiveness outcomes. Sensitivity analyses can inform the potential impact of strategies in scenarios that more closely resemble programmatic rather than trial settings.
Design for Objective 3
The design for Objective 3 was to use the computer simulation model to project the clinical impact, costs, and cost-effectiveness of interventions evaluated in ATN.
Clinical data not specific to ATN interventions will be from Objective 1, reflecting key components of disease progression and treatment for youth with and without VL suppression. Intervention-specific data will be derived from the ATN studies selected for model-based analyses; for ATN interventions, effectiveness, duration of effect, and intervention cost will be parameterized based on data from each modeled ATN trial. Model outcomes will include short-term survival and costs (calibrated to trial results) as well as projected long-term survival and costs, including life expectancy and lifetime per-person costs, and transmissions averted. To compare interventions, incremental cost-effectiveness ratios (difference in lifetime costs divided by the difference in life expectancy, in dollars per year-of-life saved) will be calculated and compared with commonly used thresholds for the United States [72]. Adolescents comprise only a small fraction of participants in HIV-specific health-related quality of life studies, and emerging data suggest that youth may attach different values to specific health states compared with adults [73-77]. Moreover, one study found that, in general, adults place less weight on impairments in mental health (eg, being worried, sad, or annoyed) and more weight on moderate to severe levels of pain, relative to adolescents [75]. In general, values attached to identical health states are typically lower for younger people in comparison with adults of all ages and may depend on the elicitation method utilized [74]. Where available, adolescent-specific utility weights will be incorporated, and the impact of utility weights on policy conclusions will be examined in sensitivity analyses, as in previous work [29].
Our work in Objective 3 will have the following 4 key areas of emphasis:
Work with trial teams to develop study protocols, ensuring collection of data needed for modeling.
Conduct pretrial modeling analyses to inform study design and establish benchmarks for interpretation of trial results. For example, to inform study design, detailed simulations of disease progression and clinical events in youth can provide additional input into sample size calculations, and model-based projections can inform study protocol elements such as frequency of study visits and maximal permitted turnaround time for return of diagnostic test results. To establish benchmarks for interpretation, model-based projections under a range of assumptions about efficacy and cost can be used to identify critical thresholds for key study outcomes: how effective and durable would an intervention need to be for that intervention to add benefit to current practice? For any given efficacy and effect duration, at what cost and level of uptake would the intervention be cost-effective?
Conduct modeling analyses alongside trials to evaluate the potential clinical impact and cost-effectiveness of trial interventions when implemented at scale for YLWH in the United States.
Identify key parameters that may influence policy conclusions such as the cost of electronic dose monitoring bottles or duration of improved adherence after incentives. If data on these key parameters are lacking, this limitation can help identify new research priorities. The Modeling Core will work with ATN leadership to ensure this feedback informs the ATN strategic planning.
Role of the Funding Sources
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Results
This project was funded as of September 2017.
Discussion
The planned data analyses and model development in Objectives 1 and 2 will position the ATN Modeling Core to evaluate a wide range of new ATN studies and other emerging data. Future work may include new therapies that are likely to be studied in the near future among YLWH, for example, long-acting ART [78]. The Modeling Core also collaborates with the ATN Data Harmonization Working Group to standardize the collection of resource utilization and cost data across all active ATN studies [79].
Strengths and Limitations
This protocol has several limitations inherent to model-based analyses. First, many models necessarily use short-term data to project across longer-term horizons. This extrapolation requires assumptions about whether and how trial-derived clinical risks and costs will change over time. However, when these assumptions are clearly described, examined rigorously in sensitivity analyses, and interpreted appropriately, this ability of models to leverage short-term data into longer-term policy recommendations is one of the key strengths of model-based approaches [4,69]. Second, research participants in Objective 1 studies may not be representative of the larger population of YLWH in the United States. However, these studies remain among the best sources of data for YLWH in the United States. Study-derived risks will be varied widely in model-based sensitivity analyses to examine the potential impact of variations in these results.
Conclusions
In summary, a Modeling Core has been established within ATN 161. A computer simulation model reflecting disease progression, care and treatment outcomes, and HIV transmission among adolescents and young adults will be developed. YLWH are a growing and vulnerable population in the United States, in whom lack of VL suppression contributes to poor clinical outcomes for individual patients, increases health care costs, and drives the ongoing HIV epidemic. Existing data from completed and ongoing NIH-supported studies will be leveraged to develop the adolescent-specific model. The Modeling Core Steering Committee will work closely with ATN leadership and investigators to design and conduct model-based analyses, addressing critical questions about HIV care among YLWH that cannot be fully answered by trials and cohort studies. The Modeling Core will also build a foundation to inform the design of new studies of interventions across ATN and to evaluate the clinical impact and cost-effectiveness of those interventions, directly translating the work of ATN into critical policy recommendations for YLWH in the United States.
Acknowledgments
The authors gratefully acknowledge Dr Sonia Lee for her contributions to the Modeling Core activities as well as the members of the ATN Coordinating Center for their support in the administrative activities in the Modeling Core. The authors also thank Madeline Stern for assistance with formatting and proofreading. This study was funded by U24HD089880-02S1 (principal investigator: Carpenter).
Abbreviations
- ART
antiretroviral therapy
- ARV
antiretroviral
- ATN
Adolescent Medicine Trials Network for HIV/AIDS Interventions
- CDC
Centers for Disease Control and Prevention
- CD4
cluster of differentiation 4
- CEPAC
Cost-Effectiveness of Preventing AIDS Complications
- IMPAACT
International Maternal, Pediatric, and Adolescent AIDS Clinical Trial
- NIH
National Institutes of Health
- NPHIVY
nonperinatally HIV-infected youth
- PHIVY
perinatally HIV-infected youth
- VL
viral load
- WHO
World Health Organization
- YLWH
youth living with HIV
Footnotes
Authors' Contributions: All authors contributed substantively to this manuscript in the following ways: AMN, KP, and ALC contributed to the study design; AMN, KP, and ALC contributed to methods and data analysis plan; AMN and ALC contributed to drafting the manuscript; all authors contributed to critical revision of the manuscript (all authors); and all authors provided final approval of the submitted version.
Conflicts of Interest: None declared.
References
- 1.Zanoni BC, Mayer KH. The adolescent and young adult HIV cascade of care in the United States: exaggerated health disparities. AIDS Patient Care STDS. 2014 Mar;28(3):128–35. doi: 10.1089/apc.2013.0345. http://europepmc.org/abstract/MED/24601734 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.HIV Research Network Hospital and outpatient health services utilization among HIV-infected patients in care in 1999. J Acquir Immune Defic Syndr. 2002 May 01;30(1):21–6. doi: 10.1097/00126334-200205010-00003. [DOI] [PubMed] [Google Scholar]
- 3.Adolescent Medicine Trials Network for HIV/AIDS Interventions. [2018-09-05]. https://atnweb.org/atnweb/
- 4.Hunink M, Glasziou P, Siegel J, Weeks J, Pliskin J, Elstein A, Weinstein M. Decision Making in Health and Medicine: Integrating Evidence and Values. Cambridge: Cambridge University Press; 2003. [Google Scholar]
- 5.Reisner SL, Mimiaga MJ, Skeer M, Perkovich B, Johnson CV, Safren SA. A review of HIV antiretroviral adherence and intervention studies among HIV-infected youth. Top HIV Med. 2009;17(1):14–25. http://www.iasusa.org/sites/default/files/tam/17-1-14.pdf . [PMC free article] [PubMed] [Google Scholar]
- 6.Ciaranello AL, Sohn AH, Collins IJ, Rothery C, Abrams EJ, Woods B, Pei P, Penazzato M, Mahy M. Simulation modeling and metamodeling to inform national and international HIV policies for children and adolescents. J Acquir Immune Defic Syndrome. 2018 Aug;78(1):S49–S57. doi: 10.1097/QAI.0000000000001749. https://www-ncbi-nlm-nih-gov.ezp-prod1.hul.harvard.edu/pubmed/29994920 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medical Practice Evaluation Center. [2018-10-16]. Cost-effectiveness of Preventing AIDS Complications Model https://www.massgeneral.org/mpec/cepac/
- 8.Walensky RP, Ross EL, Kumarasamy N, Wood R, Noubary F, Paltiel AD, Nakamura YM, Godbole SV, Panchia R, Sanne I, Weinstein MC, Losina E, Mayer KH, Chen YQ, Wang L, McCauley M, Gamble T, Seage GR, Cohen MS, Freedberg KA. Cost-effectiveness of HIV treatment as prevention in serodiscordant couples. N Engl J Med. 2013 Oct 31;369(18):1715–25. doi: 10.1056/NEJMsa1214720. http://europepmc.org/abstract/MED/24171517 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciaranello A, Perez F, Maruva M, Chu J, Englesmann B, Keatinge J, Walensky R, Mushavi A, Mugwagwa R, Dabis F, Freedberg KA, CEPAC-International Investigators WHO 2010 Guidelines for prevention of mother-to-child transmission in Zimbabwe: Modeling clinical outcomes in infants and mothers. PLoS One. 2011;6(6):e20224. doi: 10.1371/journal.pone.0020224. https://www-ncbi-nlm-nih-gov.ezp-prod1.hul.harvard.edu/pubmed/21655097 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paltiel AD, Weinstein MC, Kimmel AD, Seage GR, Losina E, Zhang H, Freedberg KA, Walensky RP. Expanded screening for HIV in the United States--an analysis of cost-effectiveness. N Engl J Med. 2005 Feb 10;352(6):586–95. doi: 10.1056/NEJMsa042088.352/6/586 [DOI] [PubMed] [Google Scholar]
- 11.Dunning L, Francke JA, Mallampati D, MacLean RL, Penazzato M, Hou T, Myer L, Abrams EJ, Walensky RP, Leroy V, Freedberg KA, Ciaranello A. The value of confirmatory testing in early infant HIV diagnosis programmes in South Africa: a cost-effectiveness analysis. PLoS Med. 2017 Nov;14(11):e1002446. doi: 10.1371/journal.pmed.1002446. http://dx.plos.org/10.1371/journal.pmed.1002446 .PMEDICINE-D-16-03198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunning L, Kroon M, Fourie L, Ciaranello A, Myer L. Impact of birth HIV-PCR testing on the uptake of follow-up early infant diagnosis services in Cape Town, South Africa. Pediatr Infect Dis J. 2017 Dec;36(12):1159–64. doi: 10.1097/INF.0000000000001677. https://www-ncbi-nlm-nih-gov.ezp-prod1.hul.harvard.edu/pmc/articles/PMC5926182/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freedberg KA, Losina E, Weinstein MC, Paltiel AD, Cohen CJ, Seage GR, Craven DE, Zhang H, Kimmel AD, Goldie SJ. The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med. 2001 Mar 15;344(11):824–31. doi: 10.1056/NEJM200103153441108. [DOI] [PubMed] [Google Scholar]
- 14.Freedberg KA, Scharfstein JA, Seage GR, Losina E, Weinstein MC, Craven DE, Paltiel AD. The cost-effectiveness of preventing AIDS-related opportunistic infections. J Am Med Assoc. 1998 Jan 14;279(2):130–6. doi: 10.1001/jama.279.2.130.joc71368 [DOI] [PubMed] [Google Scholar]
- 15.Ciaranello A, Leroy V, Rusibamayila A, Freedberg KA, Shapiro R, Engelsmann B, Lockman S, Dabis F, Walensky RP. Individualizing the World Health Organization public health approach to infant feeding guidelines: optimal breastfeeding duration to maximize infant HIV-free survival. AIDS. 2014;28(Suppl 3):S287–99. doi: 10.1097/QAD.0000000000000337. https://www-ncbi-nlm-nih-gov.ezp-prod1.hul.harvard.edu/pmc/articles/PMC4098721/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciaranello AL, Doherty K, Penazzato M, Lindsey JC, Harrison L, Kelly K, Walensky RP, Essajee S, Losina E, Muhe L, Wools-Kaloustian K, Ayaya S, Weinstein MC, Palumbo P, Freedberg KA. Cost-effectiveness of first-line antiretroviral therapy for HIV-infected African children less than 3 years of age. AIDS. 2015 Jun 19;29(10):1247–59. doi: 10.1097/QAD.0000000000000672. http://europepmc.org/abstract/MED/25870982 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freedberg KA, Hirschhorn LR, Schackman BR, Wolf LL, Martin LA, Weinstein MC, Goldin S, Paltiel AD, Katz C, Goldie SJ, Losina E. Cost-effectiveness of an intervention to improve adherence to antiretroviral therapy in HIV-infected patients. J Acquir Immune Defic Syndr. 2006 Dec 01;43:S113–118. doi: 10.1097/01.qai.0000248334.52072.25.00126334-200612011-00015 [DOI] [PubMed] [Google Scholar]
- 18.Goldie SJ, Paltiel AD, Weinstein MC, Losina E, Seage GR, Kimmel AD, Walensky RP, Sax PE, Freedberg KA. Projecting the cost-effectiveness of adherence interventions in persons with human immunodeficiency virus infection. Am J Med. 2003 Dec 01;115(8):632–41. doi: 10.1016/j.amjmed.2003.07.00.S0002934303005114 [DOI] [PubMed] [Google Scholar]
- 19.Department of STIs, AIDS and Viral Hepatitis Ministry of Health Ministry of Health. 2015. [2019-03-13]. Protocolo Clínico e Diretrizes Terapêuticas para Manejo da Infecção pelo HIV em Adultos Brazil http://www.sierj.org.br/artigos/protocolofinal_31_7_2015_pdf_31327.pdf .
- 20.Ministerio de Salud . [Ministry of Health] Santiago: Chile División de prevención y control de enfermedades; 2013. [Clinical guide AUGE: Acquired HIV / AIDS immunodeficiency syndrome] [Google Scholar]
- 21.Ramirez LE, Bastos EH. [Antiretroviral management guide for people with HIV. 6 edition] Mexico: Centro Nacional para la Prevención y el Control del VIH y Sida; 2015. [Google Scholar]
- 22.Hoen B, Bonnet F, Delaugerre C, Delobel P, Goujard C, L'Hénaff M, Persiaux R, Rey D, Rouzioux C, Taburet A, Morlat P, 2013 French HIV expert group French 2013 guidelines for antiretroviral therapy of HIV-1 infection in adults. J Int AIDS Soc. 2014;17(1):19034. doi: 10.7448/IAS.17.1.19034.19034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ministerio de Salud y Protección Social DSYPS . [Clinical practice guideline (CPG) based on scientific evidence for the care of HIV / AIDS infection in adolescents (13 years of age or older) and adults] Bogotá, Colombia: Ministerio de Salud y Protección Social y Fondo de Población de las Naciones Unidas; 2014. [Google Scholar]
- 24.Consolidated Guidelines on the Use of Antiretrovirals for the Prevention of HIV Infection. Geneva: World Health Organization; 2013. Chapter 7: Clinical guidance across the continuum of care: antiretroviral therapy; pp. 91–154. [Google Scholar]
- 25.Pinkerton SD, Holtgrave DR, Jemmott JB. Economic evaluation of HIV risk reduction intervention in African-American male adolescents. J Acquir Immune Defic Syndr. 2000 Oct 01;25(2):164–72. doi: 10.1097/00126334-200010010-00011·. [DOI] [PubMed] [Google Scholar]
- 26.Tao G, Remafedi G. Economic evaluation of an HIV prevention intervention for gay and bisexual male adolescents. J Acquir Immune Defic Syndr Hum Retrovirol. 1998 Jan 01;17(1):83–90. doi: 10.1097/00042560-199801010-00013. [DOI] [PubMed] [Google Scholar]
- 27.Kahn JG, Kegeles SM, Hays R, Beltzer N. Cost-effectiveness of the Mpowerment Project, a community-level intervention for young gay men. J Acquir Immune Defic Syndr. 2001 Aug 15;27(5):482–91. doi: 10.1097/00126334-200108150-00010. [DOI] [PubMed] [Google Scholar]
- 28.Moodley N, Gray G, Bertram M. The case for adolescent HIV vaccination in South Africa: a cost-effectiveness analysis. Medicine (Baltimore) 2016 Jan;95(4):e2528. doi: 10.1097/MD.0000000000002528. http://Insights.ovid.com/pubmed?pmid=26825890 .00005792-201601250-00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neilan AM, Dunville R, Ocfemia MC, Salomon JA, Francke JA, Bulteel AJ, Wang LY, Hsu KK, DiNenno EA, Walensky RP, Parker RA, Freedberg KA, Ciaranello AL. The optimal age for screening adolescents and young adults without identified risk factors for HIV. J Adolesc Health. 2018 Jan;62(1):22–28. doi: 10.1016/j.jadohealth.2017.08.028. https://linkinghub.elsevier.com/retrieve/pii/S1054-139X(17)30475-5 .S1054-139X(17)30475-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamilton DT, Goodreau SM, Jenness SM, Sullivan PS, Wang LY, Dunville RL, Barrios LC, Rosenberg ES. Potential impact of HIV preexposure prophylaxis among black and white adolescent sexual minority males. Am J Public Health. 2018 Nov;108(S4):S284–91. doi: 10.2105/AJPH.2018.304471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodreau SM, Hamilton DT, Jenness SM, Sullivan PS, Valencia RK, Wang LY, Dunville RL, Barrios LC, Rosenberg ES. Targeting human immunodeficiency virus pre-exposure prophylaxis to adolescent sexual minority males in higher prevalence areas of the United States: a modeling study. J Adolesc Health. 2018 Mar;62(3):311–9. doi: 10.1016/j.jadohealth.2017.09.023. http://europepmc.org/abstract/MED/29248392 .S1054-139X(17)30490-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee MB, Leibowitz A, Rotheram-Borus MJ. Cost-effectiveness of a behavioral intervention for seropositive youth. AIDS Educ Prev. 2005 Apr;17(2):105–18. doi: 10.1521/aeap.17.3.105.62906. [DOI] [PubMed] [Google Scholar]
- 33.Agwu AL, Bethel J, Hightow-Weidman LB, Sleasman JW, Wilson CM, Rudy B, Kapogiannis BG, ATN 061 Team and the Adolescent Medicine Trials Network for HIV/AIDS Interventions Substantial multiclass transmitted drug resistance and drug-relevant polymorphisms among treatment-naïve behaviorally HIV-infected youth. AIDS Patient Care STDS. 2012 Apr;26(4):193–6. doi: 10.1089/apc.2011.0420. http://europepmc.org/abstract/MED/22563607 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudy BJ, Lindsey JC, Flynn PM, Bosch RJ, Wilson CM, Hughes ME, Douglas SD, Pediatric AIDS Clinical Trials Group 381 Study Team Immune reconstitution and predictors of virologic failure in adolescents infected through risk behaviors and initiating HAART: week 60 results from the PACTG 381 cohort. AIDS Res Hum Retroviruses. 2006 Mar;22(3):213–21. doi: 10.1089/aid.2006.22.213. [DOI] [PubMed] [Google Scholar]
- 35.Mullins TL, Li SX, Bethel J, Goodenow MM, Hudey S, Sleasman JW, Adolescent Medicine Trials Network for HIV/AIDS Interventions Sexually transmitted infections and immune activation among HIV-infected but virally suppressed youth on antiretroviral therapy. J Clin Virol. 2018 May;102:7–11. doi: 10.1016/j.jcv.2018.02.001.S1386-6532(18)30034-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Institutes of Health. 2017. [2018-09-27]. Preservation and expansion of T-cell subsets following HAART de-intensification to atazanavir/ritonavir (ATV/r) https://clinicaltrials.gov/ct2/show/NCT00491556 .
- 37.Kahana SY, Fernandez MI, Wilson PA, Bauermeister JA, Lee S, Wilson CM, Hightow-Weidman LB. Rates and correlates of antiretroviral therapy use and virologic suppression among perinatally and behaviorally HIV-infected youth linked to care in the United States. J Acquir Immune Defic Syndr. 2015 Feb 01;68(2):169–77. doi: 10.1097/QAI.0000000000000408. http://europepmc.org/abstract/MED/25590270 .00126334-201502010-00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brick LA, Nugent NR, Kahana SY, Bruce D, Tanney MR, Fernández MI, Bauermeister JA. Interaction effects of neighborhood disadvantage and individual social support on frequency of alcohol use in youth living with HIV. Am J Community Psychol. 2018 Jun;61(3-4):276–84. doi: 10.1002/ajcp.12227. [DOI] [PubMed] [Google Scholar]
- 39.National Institutes of Health. 2017. [2018-09-27]. Network-wide assessment of current health status and behavioral risk factors https://clinicaltrials.gov/ct2/show/NCT01009827 .
- 40.National Institutes of Health. 2017. [2018-09-27]. Managerial Database II https://clinicaltrials.gov/ct2/show/NCT01322217 .
- 41.National Institutes of Health. 2017. [2018-09-26]. PHASES: Provision of HIV treatment at ATN sites: An evaluation for stakeholders https://clinicaltrials.gov/ct2/show/NCT02438592 .
- 42.Lally MA, van den Berg JJ, Westfall AO, Rudy BJ, Hosek SG, Fortenberry JD, Monte D, Tanney MR, McFarland EJ, Xu J, Kapogiannis BG, Wilson CM, Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) HIV continuum of care for youth in the United States. J Acquir Immune Defic Syndr. 2018 Dec 01;77(1):110–117. doi: 10.1097/QAI.0000000000001563. http://europepmc.org/abstract/MED/28991884 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kacanek D, Angelidou K, Williams PL, Chernoff M, Gadow KD, Nachman S, International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) P1055 Study Team Psychiatric symptoms and antiretroviral nonadherence in US youth with perinatal HIV: a longitudinal study. AIDS. 2015 Jun 19;29(10):1227–37. doi: 10.1097/QAD.0000000000000697. http://europepmc.org/abstract/MED/26035322 .00002030-201506190-00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Institutes of Health. 2011. [2018-09-27]. Psychiatric problems in children and adolescents infected with HIV at birth https://clinicaltrials.gov/ct2/show/NCT00100542 .
- 45.Nachman S, Alvero C, Acosta EP, Teppler H, Homony B, Graham B, Fenton T, Xu X, Rizk ML, Spector SA, Frenkel LM, Worrell C, Handelsman E, Wiznia A. Pharmacokinetics and 48-Week Safety and Efficacy of Raltegravir for Oral Suspension in Human Immunodeficiency Virus Type-1-Infected Children 4 Weeks to 2 Years of Age. J Pediatric Infect Dis Soc. 2015 Dec;4(4):e76–83. doi: 10.1093/jpids/piu146. http://europepmc.org/abstract/MED/26582887 .piu146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nachman S, Zheng N, Acosta EP, Teppler H, Homony B, Graham B, Fenton T, Xu X, Wenning L, Spector SA, Frenkel LM, Alvero C, Worrell C, Handelsman E, Wiznia A, International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) P1066 Study Team Pharmacokinetics, safety, and 48-week efficacy of oral raltegravir in HIV-1-infected children aged 2 through 18 years. Clin Infect Dis. 2014 Feb;58(3):413–22. doi: 10.1093/cid/cit696. http://europepmc.org/abstract/MED/24145879 .cit696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tuluc F, Spitsin S, Tustin NB, Murray JB, Tustin R, Schankel LA, Wiznia A, Nachman S, Douglas SD. Decreased PD-1 expression on CD8 lymphocyte subsets and increase in CD8 Tscm cells in children with HIV receiving raltegravir. AIDS Res Hum Retroviruses. 2017 Dec;33(2):133–42. doi: 10.1089/AID.2016.0108. http://europepmc.org/abstract/MED/27615375 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.National Institutes of Health. 2018. [2018-09-27]. Safety and Pharmacokinetics (PK) of Raltegravir in HIV (Human Immunodeficiency Virus)-Infected Children and Adolescents https://clinicaltrials.gov/ct2/show/NCT00485264 .
- 49.Patel K, Hernán MA, Williams PL, Seeger JD, McIntosh K, Van Dyke RB, Seage GR, Pediatric AIDS Clinical Trials Group 219/219C Study Team Long-term effectiveness of highly active antiretroviral therapy on the survival of children and adolescents with HIV infection: a 10-year follow-up study. Clin Infect Dis. 2008 Feb 15;46(4):507–15. doi: 10.1086/526524. [DOI] [PubMed] [Google Scholar]
- 50.Camacho-Gonzalez AF, Chernoff MC, Williams PL, Chahroudi A, Oleske JM, Traite S, Chakraborty R, Purswani MU, Abzug MJ, International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) P1074 Study Team Sexually transmitted infections in youth with controlled and uncontrolled Human Immunodeficiency Virus infection. J Pediatric Infect Dis Soc. 2017 Sep 01;6(3):e22–29. doi: 10.1093/jpids/piw039. http://europepmc.org/abstract/MED/27440505 .piw039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.National Institutes of Health. 2015. [2018-09-27]. IMPAACT P1074: Long-Term Outcomes in HIV-Infected Infants, Children, and Adolescents https://clinicaltrials.gov/ct2/show/results/NCT01061164 .
- 52.National Institutes of Health. 2017. [2018-09-26]. Safety of and Immune Response to Dolutegravir in HIV-1 Infected Infants, Children, and Adolescents https://clinicaltrials.gov/ct2/show/NCT01302847 .
- 53.Viani RM, Alvero C, Fenton T, Acosta EP, Hazra R, Townley E, Steimers D, Min S, Wiznia A, P1093 Study Team Safety, pharmacokinetics and efficacy of dolutegravir in treatment-experienced HIV-1 infected adolescents: forty-eight-week results from IMPAACT P1093. Pediatr Infect Dis J. 2015 Nov;34(11):1207–13. doi: 10.1097/INF.0000000000000848. http://europepmc.org/abstract/MED/26244832 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nielsen-Saines K, Mitchell K, Kerin T, Fournier J, Kozina L, Andrews B, Cortado R, Bolan R, Flynn R, Rotheram MJ, Abdalian SE, Bryson Y, Adolescent Medicine Trials Network (ATN) CARES Team. Mobilizing Minds Research Group Acute HIV infection in youth: protocol for the Adolescent Trials Network 147 (ATN147) Comprehensive Adolescent Research and Engagement Studies (CARES) study. JMIR Res Protoc. 2019 Jan 16;8(1):e10807. doi: 10.2196/10807. http://www.researchprotocols.org/2019/1/e10807/ v8i1e10807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hightow-Weidman LB, Muessig K, Rosenberg E, Sanchez T, LeGrand S, Gravens L, Sullivan PS. University of North Carolina/Emory Center for Innovative Technology (iTech) for addressing the HIV epidemic among adolescents and young adults in the United States: protocol and rationale for center development. JMIR Res Protoc. 2018 Aug 03;7(8):e10365. doi: 10.2196/10365. http://www.researchprotocols.org/2018/8/e10365/ v7i8e10365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Naar S, Parsons JT, Stanton BF. Adolescent Trials Network for HIV-AIDS scale it up program: protocol for a rational and overview. JMIR Res Protoc. 2019 Feb 01;8(2):e11204. doi: 10.2196/11204. http://www.researchprotocols.org/2019/2/e11204/ v8i2e11204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.ClinicalTrials.gov. National Library of Medicine; [2019-02-05]. https://clinicaltrials.gov/ [Google Scholar]
- 58.Neilan AM, Karalius B, Patel K, Van Dyke RB, Abzug MJ, Agwu AL, Williams PL, Purswani M, Kacanek D, Oleske JM, Burchett SK, Wiznia A, Chernoff M, Seage GR, Ciaranello AL, Pediatric HIV/AIDS Cohort Study and the International Maternal Adolescent Pediatric AIDS Clinical Trials Network Association of risk of viremia, immunosuppression, serious clinical events, and mortality with increasing age in perinatally Human Immunodeficiency Virus-infected youth. JAMA Pediatr. 2017 Dec 01;171(5):450–460. doi: 10.1001/jamapediatrics.2017.0141. http://europepmc.org/abstract/MED/28346597 .2613404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schackman BR, Fleishman JA, Su AE, Berkowitz BK, Moore RD, Walensky RP, Becker JE, Voss C, Paltiel AD, Weinstein MC, Freedberg KA, Gebo KA, Losina E. The lifetime medical cost savings from preventing HIV in the United States. Med Care. 2015 Apr;53(4):293–301. doi: 10.1097/MLR.0000000000000308. http://europepmc.org/abstract/MED/25710311 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.National Institutes of Health. 2018. [2018-09-26]. Data and Specimen Hub (DASH) https://dash.nichd.nih.gov/
- 61.SAS. SAS Institute; [2018-07-12]. Usage Note 24188: Modeling rates and estimating rates and rate ratios (with confidence intervals) http://support.sas.com/kb/24/188.html . [Google Scholar]
- 62.Cleveland W, Devlin S. Locally-weighted regression: An approach to regression analysis by local fitting. J Am Stat Assoc. 1998;33(403):596–610. doi: 10.1080/01621459.1988.10478639. https://pdfs.semanticscholar.org/b81e/61b920b986ff1495af426aad7437c9011d85.pdf . [DOI] [Google Scholar]
- 63.Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res. 2016 Jun 30;29(3):374–93. doi: 10.1177/0049124101029003005. [DOI] [Google Scholar]
- 64.Jones BL, Nagin DS. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociol Methods Res. 2007 May;35(4):542–71. doi: 10.1177/0049124106292364. [DOI] [Google Scholar]
- 65.Ciaranello AL, Morris BL, Walensky RP, Weinstein MC, Ayaya S, Doherty K, Leroy V, Hou T, Desmonde S, Lu Z, Noubary F, Patel K, Ramirez-Avila L, Losina E, Seage GR, Freedberg KA. Validation and calibration of a computer simulation model of pediatric HIV infection. PLoS One. 2013 Dec 13;8(12):e83389. doi: 10.1371/journal.pone.0083389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Neilan A, Bulteel A, Freedberg K, Hosek S, Landovitz R, Wilson C, Walensky R, Ciaranello A. Repeat HIV screening in high-risk youth. Oral Abstract. Society for Adolescent Health and Medicine; March 2018; Seattle, Washington. 2018. https://www.jahonline.org/article/S1054-139X(17)30538-4/fulltext . [Google Scholar]
- 67.Attia S, Egger M, Müller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009 Jul 17;23(11):1397–404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 68.Matsumoto M, Nishimura T. Mersenne twister: a 623-dimensionally equidistributed uniform pseudo-random number generator. ACM Trans Model Comput Simul. 1998 Jan;8(1):3–30. doi: 10.1145/272991.272995. [DOI] [Google Scholar]
- 69.Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB, ISPOR-SMDM Modeling Good Research Practices Task Force Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Med Decis Making. 2012;32(5):733–43. doi: 10.1177/0272989X12454579.32/5/733 [DOI] [PubMed] [Google Scholar]
- 70.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. J Am Med Assoc. 1996 Oct 16;276(15):1253–8. doi: 10.1001/jama.1996.03540150055031. [DOI] [PubMed] [Google Scholar]
- 71.Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD, ISPOR-SMDM Modeling Good Research Practices Task Force Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group-6. Med Decis Making. 2012;32(5):722–732. doi: 10.1177/0272989X12458348.32/5/722 [DOI] [PubMed] [Google Scholar]
- 72.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014 Aug 28;371(9):796–7. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 73.Griebsch I, Coast J, Brown J. Quality-adjusted life-years lack quality in pediatric care: a critical review of published cost-utility studies in child health. Pediatrics. 2005 May;115(5):e600–14. doi: 10.1542/peds.2004-2127.115/5/e600 [DOI] [PubMed] [Google Scholar]
- 74.Ratcliffe J, Chen G, Stevens K, Bradley S, Couzner L, Brazier J, Sawyer M, Roberts R, Huynh E, Flynn T. Valuing child health utility-9D health states with young adults: insights from a time trade off study. Appl Health Econ Health Policy. 2015 Oct;13(5):485–92. doi: 10.1007/s40258-015-0184-3.10.1007/s40258-015-0184-3 [DOI] [PubMed] [Google Scholar]
- 75.Ratcliffe J, Huynh E, Stevens K, Brazier J, Sawyer M, Flynn T. Nothing about us without us? A comparison of adolescent and adult health-state values for the child health utility-9D using profile case best-worst scaling. Health Econ. 2016 Apr;25(4):486–96. doi: 10.1002/hec.3165. [DOI] [PubMed] [Google Scholar]
- 76.Hays RD, Cunningham WE, Sherbourne CD, Wilson IB, Wu AW, Cleary PD, McCaffrey DF, Fleishman JA, Crystal S, Collins R, Eggan F, Shapiro MF, Bozzette SA. Health-related quality of life in patients with human immunodeficiency virus infection in the United States: results from the HIV Cost and Services Utilization Study. Am J Med. 2000 Jun 15;108(9):714–22. doi: 10.1016/s0002-9343(00)00387-9.S0002934300003879 [DOI] [PubMed] [Google Scholar]
- 77.Sherbourne CD, Hays RD, Fleishman JA, Vitiello B, Magruder KM, Bing EG, McCaffrey D, Burnam A, Longshore D, Eggan F, Bozzette SA, Shapiro MF. Impact of psychiatric conditions on health-related quality of life in persons with HIV infection. Am J Psychiatry. 2000 Feb;157(2):248–54. doi: 10.1176/appi.ajp.157.2.248. [DOI] [PubMed] [Google Scholar]
- 78.Edagwa B, McMillan J, Sillman B, Gendelman HE. Long-acting slow effective release antiretroviral therapy. Expert Opin Drug Deliv. 2017 Nov;14(11):1281–91. doi: 10.1080/17425247.2017.1288212. http://europepmc.org/abstract/MED/28128004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McCumber M, Cain D, LeGrand S, Mayer KH, Murphy DA, Psioda MA, Sena AC, Starks TJ, Hudgens M. Adolescent Medicine Trials Network for HIV/AIDS Interventions Data Harmonization: rationale and development of guidelines. JMIR Res Protoc. 2018 Dec 21;7(12):e11207. doi: 10.2196/11207. [DOI] [PMC free article] [PubMed] [Google Scholar]


