Abstract
Immune checkpoints have been the subject of a wave of new studies. Among these checkpoints are tytotoxic T-lymphocyte-associated antigen 4, checkpoints programmed death-1 and programmed death-ligand 1; their blockades have been approved by the Food and Drug Administration for therapy of melanoma and other types of cancers. Immunogenomics, which combines the latest nucleic acid sequencing strategy with immunotherapy, provides precise information about genomic alterations (e.g. mutations) and enables a paradigm shift of immune checkpoint therapy from tumor types to molecular signatures. Studying these critical checkpoints in relation to genomic mutations and neoantigens has produced groundbreaking results. This article examines these studies and delves into the relationships between immune checkpoint blockade and tumors harboring certain genomic mutations. Moreover, this article reviews recent studies on resistance to immune checkpoint therapy.
Keywords: CTLA-4, PD-1, PD-L1, immune checkpoint blockade, immunogenomics, MMR deficiency, POLE, somatic, mutation, resistance to checkpoint blockade, neoantigen
Introduction
Although immunotherapy for cancer was launched more than 100 years ago, there had not been much success until the 1st landmark clinical trials reported in 2012 that employed regimes (antibodies), against the immune checkpoints programmed death-1 (PD-1) and programmed death-ligand 1 (PD-L1) [1, 2]. These regimes along with the blockade of cytotoxic T lymphocyte antigen 4 (CTLA-4) have taken center stage in the immunogenomics era and radically changed the outlook of cancer immunotherapy. It is the evasion of host CD4+ and CD8+ T cells that allows tumor cells to become immune tolerant in the tumor microenvironment and escape immune surveillance. Certain types of human cancers harbor numerous genetic and epigenetic alterations that generate neoantigens, which can be recognized by the host immune system [3], and induce upregulation of endogenous immune checkpoints that normally terminate immune responses after antigen activation [4].
Utilizing single-cell and whole-genome exome sequencing approaches along with a clonal approach; a link between genetic mutations in cancer, neoantigens and immunotherapy has been revealed. This immunogenomic strategy has demonstrated that certain patients with somatic mutations exhibit robust responses to immune checkpoint blockade and thus helps fuel breakthroughs in cancer treatment. This review will briefly summarize recent advances in immune checkpoint therapy powered by immunogenomics in cancer.
Immune checkpoints
Immune checkpoints are receptor-based signal cascades that negatively regulate T cells and cause immune tolerance, which allows tumors to evade and escape immune surveillance. The most prominent of these checkpoints are CTLA-4, PD-1 and PD-L1. Blockade of these immune checkpoints with specific monoclonal antibodies has emerged as a revolutionary therapy for cancer (Table 1). Immune checkpoint therapy has been shown to be a promising approach of controlling advanced unresectable melanoma, but it has recently been expanded to treat other malignancies.
Table 1.
Checkpoint blockade, alkylating agent and PARP inhibitor
| Drug | Function | Main interactors |
|---|---|---|
| Ipilimumab | Increases T-cell activation and propagation | CTLA-4 [4–6] |
| Nivolumab | Blocks immune checkpoint receptors | Programmed Cell PD-1 receptors [1, 2, 10] |
| Pembrolizumab | Blocks immune checkpoint receptors | Programmed Cell PD-1 receptors [10, 56, 57] |
| Temozolomide | Alkylating agent used to treat tumors in the CNS | Alkylate or methylate DNA and the 1st line drug for glioblastoma [58–60] |
| Veliparib | Target cancers with DNA- repair defects | PARP [33, 34, 61] |
| Olaparib | Target cancers with DNA repair defects | PARP [33, 34, 62] |
CTLA-4
CTLA-4 is a crucial immune checkpoint that has been extensively studied [4–6]. CTLA-4 is expressed on regulatory T (Treg) cells and on both activated CD4+ and CD8+ T cells. CTLA-4’s ligands are B7-1 and B7-2 (CD80 and CD86), which are expressed on antigen presenting cells (APCs), especially dendritic cells (DCs). Interestingly, CD28 on T cells also share B7-1 and B7-2 as its ligands. As part of the immune response, immature DCs capture and process antigens before evolving into mature DCs that present antigens to CD4+ T cells. When B7-1 and B7-2 bind to CD28 on CD4+ T cells, CD4+ T cells become T helper 1 (Th1) cells and produce interferon gamma (IFN-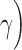 , tumor necrotic factor alpha (TNF-
, tumor necrotic factor alpha (TNF- and interleukin 2 (IL-2), which promote proliferation of CD8+ T cells [7]. However, when the B7 presented by an APC binds to CTLA-4, IFN-
and interleukin 2 (IL-2), which promote proliferation of CD8+ T cells [7]. However, when the B7 presented by an APC binds to CTLA-4, IFN- , TNF-
, TNF- and IL-2 production are reduced and T-cell proliferation is suppressed. When CTLA-4 and CD28 compete to bind with their ligands, B7-1 and B7-2 are much more likely to bind with CTLA-4 because CTLA-4 has a greater affinity to B7-1 and B7-2 than CD28 does (Figure 1) [8].
and IL-2 production are reduced and T-cell proliferation is suppressed. When CTLA-4 and CD28 compete to bind with their ligands, B7-1 and B7-2 are much more likely to bind with CTLA-4 because CTLA-4 has a greater affinity to B7-1 and B7-2 than CD28 does (Figure 1) [8].
Figure 1.

Impaired immunorecognition of tumor cells by CTLA-4 and PD-1/PD-L1. (A) Both CD28 and CTLA-4 bind to their cognate ligands B7 on APCs, on which the major histocompatibility complex (MHC) II present tumor antigen peptides to T-cell receptor (TCR) complex inducing T cell cytotoxicity activity. The CD28 binding triggers but the CTLA-4 binding suppresses T-cell activation and subsequent anti-tumor immunity. However, CTLA-4 has much higher binding affinity to B7 than that of CD28 resulting in a defect in tumor antigen presentation to T cells. CTLA-4 blockade interrupts interaction of CTLA-4 with B7 restoring antigen presentation capacity of APCs and T-cell activation. (B) PD-L1 expressed on tumors interacts with PD-1 on T cells and blocks T cells’ attack on them. PD-L1 or PD-1 blockade inhibits their interaction thus relieving suppression of PD-1 and PD-L1 and leading to killing tumor cells by armed cytotoxic T cells. Note: +, stimulatory response;  inhibitory response.
inhibitory response.
In the tumor microenvironment, tumors produce factors that suppress CD4+ T cells mainly through the upregulation of CTLA-4. The blockade of CTLA-4 has become a great immunotherapy strategy for cancers. Ipilimumab, used as an anti-CTLA-4 drug, was the 1st checkpoint antibody to be approved by Food and Drug Administration (FDA) for treatment of melanoma [9]. Anti-CTLA-4 has been used in the treatment of many carcinomas beyond melanoma. These include cancers of the bladder, blood, colon and rectum, lung, ovary, urothelial tract and uterus.
PD-1/PD-L1
PD-1/PD-L1 is an important pair of immune checkpoints [1, 2, 10]. PD-1 belongs to the same family of receptors as CTLA-4 and CD28. It is inducibly expressed on B cells, DCs, monocytes, natural killer cells and CD4+ and CD8+ T cells. Recently, PD-1 has also been found on tumor-associated macrophages (TAMs) [11]. PD-1 binds to its ligands, PD-L1 and PD-L2, which are not only expressed on APCs but also on the exterior of tumor cells allowing these cells to evade immune surveillance (Figure 1). When PD-L1/2 bind to PD-1, T cells are prevented from effectively recognizing and killing tumor cells and TAMs are not able to exert their phagocytosis function thus enhancing tumor immunity [11]. Therefore, the PD-1 and PD-L1 checkpoints are great targets for cancer immunotherapy, and their blockades have shown dynamic and durable responses in a series of malignancies [12, 13]. Recent data indicate that patients harboring tumors that overexpress PD-L1 react better to anti-PD-1-directed therapy. For example, Topalian et al. reported that 36% of patients with different solid tumors that expressed PD-L1 showed an objective response to nivolumab, a monoclonal antibody that blocks PD-1 receptors. However, none of the patients with PD-L1-negative tumors demonstrated a response [14].
Somatic mutations, neoantigens and immune checkpoint therapy
Recent advances in next generation sequencing combined with single-cell and whole-genome exome sequencing have enabled the diagnosis and treatment of cancers at the cell and molecular levels. Cancer cells can acquire somatic mutations that encode nonself immune antigens, also called neoantigens, which can be recognized by the host immune system (Figure 2) [3]. Because mutational burdens are the engines of neoantigens, if tumors accumulate a large amount of somatic mutations before or after chemotherapy, the surrounding immune cells may either become very reactive and finally get exhausted or become immune tolerant due to overexpression of immune checkpoints. In any case, tumor cells can now proliferate, grow, invade or metastasize to distal organs. Conversely, tumors with these somatic mutations can be susceptible to immune checkpoint blockades, thus becoming better targets for immunotherapy.
Figure 2.

MMR deficiency causes high somatic mutation load and neoantigen expression.
Intrinsic or extrinsic factors cause DNA damages, which are immediately repaired by DNA repair machineries. DNA MMR deficiency leads to the failure of DNA repair and subsequent accumulation of somatic mutations and tumorigenesis. Intriguingly, the accumulated somatic mutations can induce expression of neoantigens that can be recognized by the host immune system, and upregulation of CTLA-4, PD-1 and PD-L1, which are targets of immune checkpoint therapy. Thus, high levels of somatic mutation load, neoantigen expression and immune checkpoint upregulation can be used as great indicators for immune checkpoint therapy.
DNA mismatch repair, somatic mutation, neoantigens and checkpoint therapy
DNA mismatch repair (MMR) is a critical pathway that is able to correct DNA mismatches during DNA replication and prevents mutations from becoming permanent in dividing cells [15]. MutS homolog 2 (MSH2), MSH6, mutL homolog 1 (MLH1) and PMS1 homolog 2 (PMS2) are MMR proteins that are involved in DNA repair and the loss of these proteins can result in frameshift mutations and the creation of novel microsatellite fragments [16]. Microsatellite instability (MSI) is thus used as a phenotypic evidence that the MMR of a cell is deficient. MSI-high (MSI-H) leads to an accumulation of somatic mutations in tumor cells. MSI colorectal cancer (CRC) accounts for approximately 15% of sporadic CRC [17]. It has been postulated that MSI CRC displays an active Th1/CTL immune microenvironment because of the recognition of many tumor neoantigens [17]. In addition, MSI CRC tumors have a high level of expression of the checkpoint molecules PD-1, PD-L1 and CTLA-4 [18]. It has been suggested that somatic mutations associated with MMR deficiency are strongly correlated with a heightened response to treatment, such as pembrolizumab (an antibody that targets the PD-1 checkpoint), in CRCs [14]. On the other hand, patients with CRC in the study with proficient MMR had no response to pembrolizumab. Recently, pembrolizumab has been approved by FDA to treat MSI-H tumors [12].
Although the inactivation of MMR within human cells has primarily been linked with CRCs, MMR deficiency has been identified in cancers of the urothelial tract, uterus, biliary tract, stomach, pancreas, ovary, prostate and small intestine. MMR deficiency is also associated with high checkpoint expression and a better response to checkpoint blockades. For example, a patient with high-grade urothelial cancer of the renal pelvis was found to have a high load of mutations along with the MMR-deficient phenotype due to the absence of MSH2 and MSH6 [19]. An elevated expression level of PD-1 was detected, but the patient experienced complete remission and had no severe side effects from the PD-L1 inhibition treatment. Another study compared PD-L1 expression in MMR-deficient endometrial carcinomas (ECs), which included Lynch Syndrome-associated and MLH hypermethylation cases, with MMR-proficient cases [20]. Overall, it was found that 53% of MMR-deficient ECs showed a high expression of PD-L1. Among MMR-deficient ECs, 70% of Lynch Syndrome-associated ECs and 30% of MLH hypermethylation-associated ECs exhibited a high expression of PD-L1. In contrast, only 10% of MMR-proficient ECs expressed very low amounts of PD-L1. These findings suggest that MMR-deficient ECs associated with Lynch Syndrome are great targets for the immune checkpoint therapy.
However, MMR deficiency in some cancers may not lead to upregulation of immune checkpoints. Instead, its resultant somatic mutations generate a large amount of neoantigens, which can incorporate into human leukocyte antigens (HLAs) and make tumors susceptible to checkpoint blockades. Czink et al. brought to a light an intriguing case that involved a woman who bore a tumor that was MMR deficient and exhibited MSI [21]. Although the woman was not treated until an advanced stage of disease, she showed a robust and lasting response to pembrolizumab. What makes this situation unique is that the tumor did not display common features that are predictive of responsiveness to immunotherapy such as high expression levels of immune checkpoints. Elevated levels of expression of the HLA classes I and II antigens were identified in the patient, which led the researchers to put forward the notion of using MSI status and HLA classes I and II antigen expression in tumors to determine eligibility for immune checkpoint blockade even when traditional predictive markers are not detectable.
DNA polymerase epsilon and PD-1 checkpoint therapy
In addition to MMR deficiency, alterations of the polymerase epsilon (POLE) gene, which encodes the catalytic subunit of DNA replicase, also cause a hypermutated phenotype in CRC and ECs [22–24]. POLE is responsible for chromosomal DNA replication and DNA repair. POLE gene mutations were identified in less than 2% of CRC patients [25], and they occurred in 6–12% of patients with ECs [26]. Interestingly, POLE mutations mainly occurred in male CRC patients [25]. Importantly, the presence of POLE mutations led to better recurrence-free and disease-free survival compared to MSI-proficient tumors. A significant reduction in recurrence was observed in patients with stage II disease, indicating that POLE mutations can be a biomarker for CRC survival and recurrence. Moreover, POLE-mutant CRC had increased CD8+ lymphocyte infiltration and expression of cytotoxic T-cell markers and effector cytokines, similar to that identified in immunogenic MMR-deficient cancers. Furthermore, the effector cytokines such as IFNγ, CXCL9 and CXCL10, as well as immune checkpoints PD-1 and PD-L1 were upregulated in POLE-mutant CRC. Although there are no exact case reports regarding immune checkpoint therapy in CRC patients with POLE mutations, there was a case report on ECs with POLE mutation [23]. A patient with ECs harboring a POLE mutation had an exceptional response to anti-PD-1 therapy. A large amount of somatic mutations was identified by whole exome sequencing. Similar to CRC with POLE mutations, POLE-mutant ECs exhibited significantly high levels of CD8+ T cells, T follicular helper cells, M1 macrophages and natural killer cells. Together, these findings strongly suggest that CRC and EC patients with POLE mutations can be subjected to immune checkpoint therapy.
Recently, POLE mutations have been discovered in lung carcinoma [27]. These patients with lung cancer showed similar immune profiles to those who had CRC and ECs. In POLE-mutant lung tumors, significant increased infiltration of CD4+, CD8+ T cells, Th1 cells, Th17 cells, natural killer cells and DCs was observed. Neoantigens were upregulated. Interestingly, POLE mutations were associated with gene mutations in the type I interferon pathway. Nevertheless, all of these findings suggest that POLE gene mutations are associated with a favorable outcome of lung cancer and are excellent biomarkers for immune checkpoint therapy.
Besides CRC, ECs and lung cancer, POLE mutations are also identified in glioblastoma and glioma. About 78% high-graded pediatric gliomas harbored mutations in MSH6, MSH2, MLH1, PMS2, POLE and POLD1 genes [28]. There was a case report on a patient with glioblastoma whose POLE gene was hypermutated [29]. Analysis of the subclones of the tumor led to the discovery of mutational signatures that are related to DNA-repair defects. A high neoantigen load was detected in all tumors, which is consistent with the high mutation burden. The patient was treated with temozolomide (a chemotherapy drug used to treat brain cancer) followed by pembrolizumab (Table 1). After treatment with pembrolizumab, there were infiltrated lymphocytes in the central nervous system (CNS) indicating active immunosurveillance.
BRCA1 and BRCA2 mutations and checkpoint therapy
Breast cancer 1 (BRCA1) and 2 (BRCA2) are both tumor suppressor genes, which produce proteins that aid in repairing DNA double-strand breaks via the homologous-recombination repair pathway [30]. BRCA1/2-driven tumors are most likely to form when normal cells only have one copy of the gene (heterozygous) but become cancerous by the somatic loss of the BRCA allele that remains [31]. BRCA1/2 mutations increase the risk of breast and ovarian cancers and constitute 20–25% of hereditary breast cancers [32]. Moreover, breast cancers, especially triple-negative breast cancers (TNBCs), arising from BRCA1/2 mutations typically show a poor prognosis. Although (ADP-ribose) polymerase (PARP) inhibitors (e.g. veliparib and olaparib) [33, 34] (Table 1), which cause severe DNA damage and subsequent apoptosis, showed efficacy in a small portion of patients; high rates of relapse and drug resistance have been observed. Thus, there is an urgent need to pave new therapeutic avenues for BRCA1/2-mutated cancers. Recent studies suggested that BRCA1- or BRCA2-mutant TNBCs are associated with a high burden of mutations and noticeable immune cell infiltrations including T lymphocytes [35, 36]. Abundant PD-L1 expression was not only detected in the infiltrated immune cells, but it was also expressed in tumor cells. The feature, of which high mutation load leads to high expression levels of immune checkpoints, provides a rationale for the usage of PD-L1 blockade. Indeed, in the MMTVcre/BRCA1fox/floxp53+/− mouse model that develops human BRCA1-mutated TNBCs-like triple-negative mammary tumors, PD-L1 was abundantly expressed, and combined PD-1 and CTLA-4 blockade efficiently induced a strong immune response and attenuated tumor growth [35].
While PARP blockade has limited efficacy in TNBCs with BRCA1/2 mutations, it may exhibit a synergistic effect when combined with immune checkpoint blockades. In a BRCA1-deficient ovarian cancer mouse model, it was found that CTLA-4 blockade markedly increased CD8+ T cell activity and secretion of IFNγ [37]. PARP inhibition clearly augmented Th1 response leading to increased production of IFNγ and TNFα in the tumor microenvironment leading to lasting effects on T cells and enhanced anti-tumor immunity. As a consequence, the lifespan of the mice was extended.
Epidermal growth factor mutations, non-small cell lung cancers and immune checkpoint therapy
Epidermal growth factor (EGFR) is one of the receptor tyrosine kinases and plays a critical role in pathophysiology [38]. EGFR mutations have been identified in many cancers, especially, non-small cell lung cancers (NSCLCs). These mutations cause the hyper-activation of EGFR and subsequent transformation, malignancy and metastasis. Also, overactive EGFR can cause activation of Akt and mitogen-activated protein kinases [24, 38, 39], which are critical for tumor growth and metastasis, and immune suppression by enhancing Treg activation [40, 41]. Since conventional chemotherapies have limited clinical efficacy, target therapy using EGFR inhibitors and immunotherapy especially immune checkpoint therapy, have become choices for new therapeutic avenues. However, NSLCs can acquire resistance to EGFR inhibitors such as tyrosine kinase inhibitors [42]. Thus, checkpoint therapy has recently drawn more attention.
Based on two early clinical trials which showed that PD-1 and PD-L1 blockades induced favorable tumor regression and prolonged disease stabilization in 20–25% of cancers including NSCLCs [1, 2], a study investigated the relationship between the effects of PD-1 on the tumor microenvironments in EGFR-driven lung cancers [43]. It was shown that EGFR activation induced PD-L1 expression in human bronchial epithelial cells and reduced the CD8+ T cell/Treg cell ratio leading to an immune suppressive tumor microenvironment. Blockade of PD-1 slowed down the tumor growth and significantly enhanced survival. Cellular analysis revealed that PD-1 blockade activated T cells and stimulated their effector activity. A later study examined the association of PD-L1 expression with the EGFR mutation [44]. It was found that high PD-L1 expression was associated with EGFR mutations in resected human NSCLC tumor tissues. Interestingly, this study also suggested that higher levels of PD-L1 were found in women than in men, indicating a sexual preference [44]. In addition, this study suggested that high PD-L1 expression was significantly associated with poor survival of NSCLC patients. Supporting this notion was a related study, which examined the association of PD-L1 and EGFR status in patients with advanced NSCLC [45]. It was found that high PD-L1 expression was associated with EGFR mutations and that high PD-L1 expression was significantly associated with poor survival of patients with wild-type EGFR. Conversely, two studies suggested that high PD-L1 expression was significantly correlated with a favorable survival rate in stage I patients with lung squamous cell cancer [46] or in the early stages of NSCLCs [47]. The discrepancy here might be caused by EGFR statuses, lung cancer stages or the sizes of cohorts. Nevertheless, the induction of PD-L1 expression by EGFR activation was also examined in an in vitro study using human NSCLC cell lines. EGFR mutation not only stimulated EGFR activation but also induced PD-L1 expression through extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) activation [48].
Resistance to immune checkpoint blockade
Despite success of immune checkpoint therapy in a specific subset of advanced cancers, the common feature is that many patients with cancer do not respond to checkpoint blockade. It is understood that this unresponsiveness is due to tumors with low or undetectable expression of checkpoints or due to the lack of tumor-infiltrating lymphocytes in the tumor microenvironment. However, the fact is that certain tumors with high levels of PD-1 do not respond to checkpoint blockade [49]. One of the resistance mechanisms is that in certain tumors beta-2-microglobulin (B2M), which is a key factor for assembling all MHC class I or HLA class I complex and is essential for antigen presentation [50], is mutated or deleted [49] (Figure 3). Moreover, analysis of the biopsies of the same melanoma patient cohorts revealed that loss of heterozygosity (LOH) in B2M was 3-fold enriched in 30% nonresponders compared to 10% responder. These abnormalities of B2M led to defects in antigen presentation and IFNγ pathway activation resulting in non-responsiveness to PD-1 or CTLA-4 blockade. Another mechanism is that tumors harbor point mutations in the genes of Janus kinase 1 (JAK1) and 2 (JAK2), of which their encoding proteins are essential for IFNγ signaling and CD8+ T cell activation [51] (Figure 3). Moreover, a recent study conducted in an inducible melanoma animal model suggested that the resistance to immune checkpoint therapy largely depends on the tumor microenvironment. In this microenvironment, tumor-infiltrated lymphocytes underwent apoptosis triggered by the Fas-ligand, which was released by myeloid-derived suppressor cells [49].
Figure 3.

Potential mechanisms of checkpoint resistance.
(A) Mutation, deletion and heterozygosity (LOH) in beta-2-microglobulin gene of cancer patients result in the failure of assembling MHC or HLA class complexes and reduction of their neoantigen presentation capacity to TCR complex as well as a great decrease in clinical efficacy of immune checkpoint blockade. (B) Mutations in Janus kinase 1 ( JAK1) gene or JAK2 gene lead to the failure of transducing IFNγ signaling to their downstream events including signal transducer and activator of transcription 1 and anti-tumor immunity of IFNγ.
In clinic practice, it is often found that patients who originally responded well to checkpoints blockade acquired resistance to the therapy. One of the potential mechanisms is that after responding to checkpoint blockade, somatic mutations in tumors were markedly reduced leading to a striking decrease in neoantigen production [52].
Closing remarks and future directions
The combination of immune checkpoint therapy with immuno-genomics has revolutionarily shaped immunotherapy framework for many advanced cancers and allowed for a paradigm shift of immune checkpoint therapy from tumor types to molecular signatures. However, the major limitation of mono-therapy is the presence of primary resistance or acquired resistance in a certain group of patients who previously responded to the therapy. In this review, we deliberated on CTLA-4, PD-1 and PD-L1 and their blockade but had limited space to touch on other immune checkpoints such as T-cell immunoglobulin mucin 3 [53] and lymphocyte activation 3 [18]. We also discussed several potential mechanisms involving this resistance. Future studies, in addition to what has been discussed in this article, need to elucidate on whether immune metabolomics and microbial status in the gut as well as signaling pathway networks associated with tumor-intrinsic and extrinsic factors play a profound role in the success of immune checkpoint therapy. Moreover, to achieve greater success with checkpoint therapy, combination therapy or synergic therapy will need to be implemented in order to reduce non-responsiveness and improve clinical efficacy. Furthermore, there is an urgent need for optimal management of treatment-related side effects such as autoimmunity and tumor hyperprogression in some patients who have been treated with immune checkpoint blockade [54, 55].
Key Points
CTLA-4, PD-1 and PD-L1 are prominent immune checkpoints that lead to immune tolerance in cancer patients.
MMR deficiency leads to defective DNA repair, generation of microsatellite fragments and accumulation of somatic mutations.
High somatic mutation burden is the engine of neoantigens, which can be recognized by the host immune system and can also induce expression of immune checkpoints.
MMR deficiency, MSI, POLE mutations, high somatic mutation load and high neoantigen burden are excellent indicators for immune checkpoint therapy
Mutations, deletions and LOH in the B2M gene, mutations in the genes encoding JAKs or apoptosis of CD4+ and CD8+ T cells lead to resistance to immune checkpoint therapy.
Funding
This article was supported in part by National Institute of Health/National Cancer Institute grant U01CA188387-01 to Jia and Chu and University of Hawaii Pilot Awards and Star-up fund to Chu.
REFERENCES
- 1. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366(26):2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366(26):2455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sjoblom T, Jones S, Wood LD, et al. The consensus coding sequences of human breast and colorectal cancers. Science 2006;314(5797):268–74. [DOI] [PubMed] [Google Scholar]
- 4. Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364(26):2517–26. [DOI] [PubMed] [Google Scholar]
- 5. Maker AV, Phan GQ, Attia P, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol 2005;12(12):1005–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weber J. Review: anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. Oncologist 2007;12(7):864–72. [DOI] [PubMed] [Google Scholar]
- 7. Luckheeram RV, Zhou R, Verma AD et al. CD4(+)T cells: differentiation and functions. Clin Dev Immunol 2012;2012:925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pentcheva-Hoang T, Egen JG, Wojnoonski K, Allison JP. B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity 2004;21(3):401–13. [DOI] [PubMed] [Google Scholar]
- 9. Chuk MK, Chang JT, Theoret MR, et al. FDA approval summary: accelerated approval of pembrolizumab for second-line treatment of metastatic melanoma. Clin Cancer Res 2017;23(19):5666–70. [DOI] [PubMed] [Google Scholar]
- 10. Izzedine H, Mateus C, Boutros C, et al. Renal effects of immune checkpoint inhibitors. Nephrol Dial Transplant 2017;32(6):936–42. [DOI] [PubMed] [Google Scholar]
- 11. Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 2017;545(7655):495–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chang L, Chang M, Chang HM et al. Microsatellite instability: a predictive biomarker for cancer immunotherapy. Appl Immunohistochem Mol Morphol 2018;26(2):e15–e21. [DOI] [PubMed] [Google Scholar]
- 13. Sunshine J, Taube JM. PD-1/PD-L1 inhibitors. Curr Opin Pharmacol 2015;23:32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372(26):2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Modrich P, Lahue R. Mismatch repair in replication fidelity, genetic recombination, and cancer biology. Annu Rev Biochem 1996;65:101–33. [DOI] [PubMed] [Google Scholar]
- 16. Zhang L. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part II. The utility of microsatellite instability testing. J Mol Diagn 2008;10(4):301–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xiao Y, Freeman GJ. The microsatellite instable subset of colorectal cancer is a particularly good candidate for checkpoint blockade immunotherapy. Cancer Discov 2015;5(1):16–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Llosa NJ, Cruise M, Tam A, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov 2015;5(1):43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Castro MP, Goldstein N. Mismatch repair deficiency associated with complete remission to combination programmed cell death ligand immune therapy in a patient with sporadic urothelial carcinoma: immunotheranostic considerations. J Immunother Cancer 2015;3:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sloan EA, Ring KL, Willis BC, et al. PD-L1 expression in mismatch repair-deficient endometrial carcinomas, including lynch syndrome-associated and MLH1 promoter hypermethylated tumors. Am J Surg Pathol 2017;41(3):326–33. [DOI] [PubMed] [Google Scholar]
- 21. Czink E, Kloor M, Goeppert B, et al. Successful immune checkpoint blockade in a patient with advanced stage microsatellite-unstable biliary tract cancer. Cold Spring Harb Mol Case Stud 2017;3(5):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bourdais R, Rousseau B, Pujals A et al. Polymerase proofreading domain mutations: new opportunities for immunotherapy in hypermutated colorectal cancer beyond MMR deficiency. Crit Rev Oncol Hematol 2017;113:242–8. [DOI] [PubMed] [Google Scholar]
- 23. Mehnert JM, Panda A, Zhong H, et al. Immune activation and response to pembrolizumab in POLE-mutant endometrial cancer. J Clin Invest 2016;126(6):2334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heong V, Ngoi N, Tan DS. Update on immune checkpoint inhibitors in gynecological cancers. J Gynecol Oncol 2017;28(2):e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Domingo E, Freeman-Mills L, Rayner E, et al. Somatic POLE proofreading domain mutation, immune response, and prognosis in colorectal cancer: a retrospective, pooled biomarker study. Lancet Gastroenterol Hepatol 2016;1(3):207–16. [DOI] [PubMed] [Google Scholar]
- 26. Gool IC, Eggink FA, Freeman-Mills L, et al. POLE proofreading mutations elicit an antitumor immune response in endometrial cancer. Clin Cancer Res 2015;21(14):3347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chae YK, Anker JF, Bais P, et al. Mutations in DNA repair genes are associated with increased neo-antigen load and activated T cell infiltration in lung adenocarcinoma. Oncotarget 2018;9(8):7949–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johnson A, Severson E, Gay L, et al. Comprehensive genomic profiling of 282 pediatric low- and high-grade gliomas reveals genomic drivers, tumor mutational burden, and hypermutation signatures. Oncologist 2017;22(12):1478–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johanns TM, Miller CA, Dorward IG, et al. Immunogenomics of hypermutated glioblastoma: a patient with germline POLE deficiency treated with checkpoint blockade immunotherapy. Cancer Discov 2016;6(11):1230–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prakash R, Zhang Y, Feng W, Jasin M. Homologous recombination and human health: the roles of BRCA1, BRCA2, and associated proteins. Cold Spring Harb Perspect Biol 2015;7(4):a016600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iyevleva AG, Imyanitov EN. Cytotoxic and targeted therapy for hereditary cancers. Hered Cancer Clin Pract 2016;14(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Easton DF. How many more breast cancer predisposition genes are there? Breast Cancer Res 1999;1(1):14–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Du Y, Yamaguchi H, Wei Y, et al. Blocking c-Met-mediated PARP1 phosphorylation enhances anti-tumor effects of PARP inhibitors. Nat Med 2016;22(2):194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hassan S, Esch A, Liby T, et al. Pathway-enriched gene signature associated with 53BP1 response to PARP inhibition in triple-negative breast cancer. Mol Cancer Ther 2017;16(12):2892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nolan E, Savas P, Policheni AN, et al. Combined immune checkpoint blockade as a therapeutic strategy for BRCA1-mutated breast cancer. Sci Transl Med 2017;9(393). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Denkert C, Liedtke C, Tutt A, Minckwitz G. Molecular alterations in triple-negative breast cancer-the road to new treatment strategies. Lancet 2017;389(10087):2430–42. [DOI] [PubMed] [Google Scholar]
- 37. Higuchi T, Flies DB, Marjon NA, et al. CTLA-4 blockade synergizes therapeutically with PARP inhibition in BRCA1-deficient ovarian cancer. Cancer Immunol Res 2015;3(11):1257–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cao C, Huang X, Han Y, et al. Galpha(i1) and Galpha(i3) are required for epidermal growth factor-mediated activation of the Akt-mTORC1 pathway. Sci Signal 2009;2(68):ra17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang Z, Dela Cruz R, Ji F, et al. G(i)α proteins exhibit functional differencesin the activation of ERK1/2, Akt and mTORC1 by growth factors in normal and breast cancer cells. Cell Commun Signal 2014;13:12–10, doi: 10.1186/1478-811X-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zaiss DM, Loosdregt J, Gorlani A, et al. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity 2013;38(2):275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jiang R, Tang J, Chen Y, et al. The long noncoding RNA lnc-EGFR stimulates T-regulatory cells differentiation thus promoting hepatocellular carcinoma immune evasion. Nat Commun 2017;8:15129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee DH. Treatments for EGFR-mutant non-small cell lung cancer (NSCLC): the road to a success, paved with failures. Pharmacol Ther 2017;174:1–21. [DOI] [PubMed] [Google Scholar]
- 43. Akbay EA, Koyama S, Carretero J, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 2013;3(12):1355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Azuma K, Ota K, Kawahara A, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol 2014;25(10):1935–40. [DOI] [PubMed] [Google Scholar]
- 45. Tang Y, Fang W, Zhang Y, et al. The association between PD-L1 and EGFR status and the prognostic value of PD-L1 in advanced non-small cell lung cancer patients treated with EGFR-TKIs. Oncotarget 2015;6(16):14209–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yang CY, Lin MW, Chang YL, et al. Programmed cell death-ligand 1 expression is associated with a favourable immune microenvironment and better overall survival in stage I pulmonary squamous cell carcinoma. Eur J Cancer 2016;57:91–103. [DOI] [PubMed] [Google Scholar]
- 47. Cooper WA, Tran T, Vilain RE, et al. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer 2015;89(2):181–8. [DOI] [PubMed] [Google Scholar]
- 48. Chen N, Fang W, Zhan J, et al. Upregulation of PD-L1 by EGFR activation mediates the immune escape in EGFR-driven NSCLC: implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J Thorac Oncol 2015;10(6):910–23. [DOI] [PubMed] [Google Scholar]
- 49. Zhu J, Powis de Tenbossche CG, Cane S, et al. Resistance to cancer immunotherapy mediated by apoptosis of tumor-infiltrating lymphocytes. Nat Commun 2017;8(1):1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hulpke S, Tampe R. The MHC I loading complex: a multitasking machinery in adaptive immunity. Trends Biochem Sci 2013;38(8):412–20. [DOI] [PubMed] [Google Scholar]
- 51. Syn NL, Teng MWL, Mok TSK, Soo RA. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol 2017;18(12):e731–41. [DOI] [PubMed] [Google Scholar]
- 52. Anagnostou V, Smith KN, Forde PM, Niknafs N, et al. Evolution of neoantigen landscape during immune checkpoint blockade in non-small cell lung cancer. Cancer Discov 2017;7(3):264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Anderson AC. Tim-3, a negative regulator of anti-tumor immunity. Curr Opin Immunol 2012;24(2):213–6. [DOI] [PubMed] [Google Scholar]
- 54. June CH, Warshauer JT, Bluestone JA. Is autoimmunity the Achilles heel of cancer immunotherapy? Nat Med 2017;23(5):540–7. [DOI] [PubMed] [Google Scholar]
- 55. Champiat S, Dercle L, Ammari S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res 2017;23(8):1920–8. [DOI] [PubMed] [Google Scholar]
- 56. Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369(2):134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. PD-1 inhibitor becomes breakthrough therapy. Cancer Discov 2013;3(7):OF14. [DOI] [PubMed] [Google Scholar]
- 58. Cai T, Liu Y, Xiao J. Long noncoding RNA MALAT1 knockdown reverses chemoresistance to temozolomide via promoting microRNA-101 in glioblastoma. Cancer Med 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yung WK, Albright RE, Olson J, et al. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer 2000;83(5):588–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Erasimus H, Gobin M, Niclou S, et al. DNA repair mechanisms and their clinical impact in glioblastoma. Mutat Res Rev Mutat Res 2016;769:19–35. [DOI] [PubMed] [Google Scholar]
- 61. Stordal B, Timms K, Farrelly A, et al. BRCA1/2 mutation analysis in 41 ovarian cell lines reveals only one functionally deleterious BRCA1 mutation. Mol Oncol 2013;7(3):567–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 2009;361(2):123–34. [DOI] [PubMed] [Google Scholar]


