Abstract
The aim of the present study was to measure the expression of microRNA (miR)-146a in liver tissues, peripheral blood mononuclear cells (PMBC) and serum from patients with Hepatitis B and either liver fibrosis or cirrhosis, as well as to determine the regulatory mechanism of miR-146a. A total of 36 patients with Hepatitis B and liver fibrosis and 25 patients with hepatitis B and liver cirrhosis admitted to Linyi People's Hospital (Shandong, China) between June 2012 and February 2016 were included in the present study. Reverse transcription-quantitative polymerase chain reaction was performed to determine the expression of miR-146a and interleukin (IL)-6 mRNA in the liver tissue, PBMCs and serum. Western blotting was used to assess the expression of IL-6 in liver tissues and PBMCs. An enzyme-linked immunosorbent assay was conducted to measure IL-6 levels in serum. To identify the direct interaction between IL-6 and miR-146a, a dual luciferase reporter assay was performed. IL-6 mRNA expression in liver tissues, PBMCs and serum from patients with liver cirrhosis was significantly higher than that from patients with liver fibrosis (P<0.05). Furthermore, IL-6 expression in liver tissues and PBMCs from patients with liver cirrhosis was enhanced and levels of IL-6 protein in the serum of patients with liver cirrhosis were significantly elevated compared with patients with liver fibrosis (P<0.05). By contrast, levels of miR-146a in liver tissues, PBMCs and serum from patients with liver cirrhosis were significantly downregulated (P<0.05) compared with patients with liver fibrosis. miR-146a regulated the expression of IL-6 by binding to its 3′-untranslated region. Thus, in the transformation from liver fibrosis to cirrhosis, the upregulation of IL-6 in liver tissues, PBMCs and serum may be associated with the downregulation of miR-146a. miR-146a directly targets IL-6, which may regulate the occurrence and immune responses of Hepatitis B.
Keywords: microRNA-146a, hepatitis B, fibrosis, interleukin-6, cirrhosis
Introduction
Liver fibrosis is usually observed in chronic liver diseases with different causes and its morphology is characterized by the deposition of a large amount of extracellular matrix in liver tissues (1). Liver fibrosis may progress into cirrhosis that threatens the health and life of patients (2). Chronic Hepatitis B can cause liver inflammation and fibrosis. If not detected promptly and treated, patients can develop cirrhosis (3). Decompensated cirrhosis has poor prognosis and can easily develop into hepatocellular carcinoma, a serious and life-threatening complication of cirrhosis (4,5). A previous prospective study determined that the annual rate of conversion from chronic Hepatitis B into liver cirrhosis is 2.1% (6). A follow-up study of HBeAg-negative patients with chronic Hepatitis B over an average of 9 years (1–18.4 years) demonstrated that the percentage of patients who developed liver cirrhosis was 23% (7,8). Interferon and nucleotide analogues are currently used in the treatment of Hepatitis B virus (9). In addition to antiviral therapy, the treatment of hepatic fibrosis and cirrhosis following Hepatitis B infection needs to strengthen hepatocyte regeneration, inhibit inflammation and reduce the risk of further complications (10,11). Therefore, it is important to investigate the mechanism of the transformation from liver fibrosis to cirrhosis at the molecular level in order to develop novel treatments for Hepatitis B.
MicroRNA (miR) molecules are a type of non-encoding small RNA molecule that are 18–22 nucleotides long and can regulate the expression of proteins at the mRNA level (12–14). The processes of liver fibrosis and cirrhosis are accompanied by alterations in the expression of different miRs and proteins, suggesting that miR may serve important roles in regulating the expression of proteins associated with liver fibrosis and cirrhosis (15,16). Hepatitis B may also induce inflammation to a certain extent (17,18), which, in turn, may lead to the development of liver cancer (19,20). Previous studies have demonstrated that the inflammatory factor interleukin (IL)-6 serves a key role in the onset of hepatitis B (21,22). However, the regulation of liver fibrosis and cirrhosis by IL-6 and the mechanism by which miRs regulate IL-6 remain unclear. In the present study, the expression of IL-6 mRNA and protein in patients with liver fibrosis and cirrhosis was assessed and the association between miR-146a and IL-6 was evaluated.
Materials and methods
Patient samples
A total of 36 patients with Hepatitis B and liver fibrosis and 25 patients with Hepatitis B and liver cirrhosis admitted to the Linyi People's Hospital (Linyi, China) between June 2012 and February 2016 were included in the present study. Among the 36 patients with liver fibrosis, 20 were male and 16 were female, and they had a median age of 41 years (age range, 20–61 years). Among the 25 patients with liver cirrhosis, 15 were male and 10 were female, and they had a median age of 43 years (age range, 18–65 years). All patients were diagnosed with liver fibrosis or cirrhosis according to the guidelines for the prevention and treatment of chronic Hepatitis B (23). The levels of alanine aminotransferase and aspartate aminotransferase were determined in patients with liver fibrosis using an automatic biochemical analyzer (Beckman Coulter, Inc., Brea, CA, USA). The normal concentrations in the blood for alanine aminotransferase and aspartate aminotransferase levels were 0–40 and 5–40 U/l, respectively. The levels of alanine aminotransferase and aspartate aminotransferase in patients with liver fibrosis were 168.6±39.6 and 146.2±28.5 U/l, respectively. The levels of alanine aminotransferase and aspartate aminotransferase in patients with liver cirrhosis were 182.8±51.9 and 161.2±39.3 U/l, respectively. Patients with immune-related diseases or immune diseases, including diabetes and cancer, were excluded from the current study. Liver biopsies (1.0–1.5 cm in length) and peripheral blood samples (20 ml) were collected from each patient. Blood serum was isolated from peripheral blood following centrifugation at 400 × g for 10 min at 4°C. To obtain peripheral blood mononuclear cells (PMBCs), a mixture of heparin-anticoagulant venous blood and equal amount of serum-free Iscove's modified Dulbecco's medium (1:1 v/v) was added gently to the lymphocyte separation medium before centrifugation at 400 × g for 30 min at 4°C. After centrifugation, the middle layer was aspirated and mixed with 5 volumes of Hanks solution before centrifugation at 300 × g for 10 min at 4°C, washed twice and cell density was adjusted to 1×106 cells/ml. Cells were seeded into six-well plates at a density of 3×106 cells/well and incubated at 37°C in a 5% CO2-humidified incubator for 1–2 h. The cells that attached after 1–2 h incubation were PBMCs. All procedures were approved by the Ethics Committee of Linyi People's Hospital and written informed consent was obtained from all patients or their families.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Prior to total RNA extraction, tissue samples (100 mg) were ground into powder using liquid nitrogen. Total RNA was extracted using 1 ml TRIzol® (10606ES60; Shanghai Yeasen Biotechnology, Co., Ltd., Shanghai, China). Following lysis, miRNA was isolated using the miRcute miRNA isolation kit (Tiangen Biotech Co., Ltd., Beijing, China). The purity of RNA was determined at A260/A280 using an ultraviolet spectrophotometer (NanoDrop ND1000; Thermo Fisher Scientific, Inc., Wilmington, DE, USA). The TIANScript II cDNA First Chain Synthesis kit (KR107; Tiangen, Biotech, Co., Ltd., Beijing, China) was used to reverse transcribe 1 µg RNA into cDNA, which was stored at −20°C. The primer sequences used were as follows: IL-6 forward, 5′-GGCACTGGCAGAAAACAACC-3′ and reverse, 5′-GCAAGTCTCCTCATTGAATCC-3′; GAPDH, forward, 5′-GGGAAACTGCGGCGTGAT-3′ and reverse, 5′-AAAGGTGGAGGAGTGGGT-3′. The PCR reaction mixture (20 µl) contained 10 µl SuperReal PreMix SYBR-Green (Tiangen Biotech Co., Ltd.), 0.5 µl upstream primer, 0.5 µl downstream primer, 2 µl cDNA and 7 µl ddH2O. The following thermocyclic conditions were used for the qPCR: Initial denaturation at 95°C for 30 sec; 45 cycles of 95°C for 5 sec and 57°C for 30 sec. The qPCR was performed on an iQ5 Real-Time PCR Detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The 2−ΔΔCq method was used to calculate the relative expression of IL-6 mRNA against the reference gene GAPDH (24). For miR-146a, the primer sequences were as follows: miR-146a forward, 5′-CGGCGGTGAGAACTGAATTCCA-3′ and reverse, 5′-GTGCAGGGTCCGAGGT-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′. The following thermocyclic conditions were used for the qPCR: Initial denaturation at 95°C for 5 min; 40 cycles of denaturation at 95°C for 10 sec, 60°C for 20 sec and 70°C for 10 sec. The qPCR was performed as above and the 2−ΔΔCq method was used to calculate the relative expression of miR-146a against the reference gene U6.
Western blotting
Total protein was extracted from tissues and PBMCs using radioimmunoprecipitation assay lysis buffer (600 µl; 50 mM Tris-base, 1 mM EDTA, 150 mM NaCl, 0.1% sodium dodecyl sulfate, 1% Triton X-100, 1% sodium deoxycholate; Beyotime Institute of Biotechnology, Shanghai, China) according to the manufacturer's protocol. Following lysis for 50 min on ice, the mixture was centrifuged at 13,400 × g and 4°C for 5 min. The supernatant was used to determine protein concentration using a bicinchoninic acid protein concentration determination kit [RTP7102; Real-Times (Beijing) Biotechnology Co., Ltd., Beijing, China]. Protein samples (20 µg) were then mixed with sodium dodecyl sulfate loading buffer prior to denaturation in a boiling water bath for 5 min. Afterwards, 50 µg protein/lane was separated via SDS-PAGE on a 10% gel. Resolved proteins were then transferred to polyvinylidene difluoride membranes on ice (100 V, 2 h) and blocked with 5% skimmed milk at room temperature for 1 h. Subsequently, membranes were incubated with rabbit anti-human IL-6 polyclonal primary antibody (1:1,000; ab6672) and rabbit anti-human β-actin primary antibody (1:5,000; ab129348; both Abcam, Cambridge, UK) at 4°C overnight. Following three extensive washes with phosphate-buffered saline with Tween 20 for 15 min each time, membranes were incubated with goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:3,000; ab6721; Abcam) for 1 h at room temperature prior to three washes with phosphate-buffered saline with Tween-20 for 15 min each time. Subsequently, the membrane was developed using an enhanced chemiluminescence detection kit (ab65623; Abcam) for imaging. Image lab v3.0 software (Bio-Rad Laboratories, Inc.) was used to acquire and analyze imaging signals. The relative content of IL-6 protein was expressed as the IL-6/β-actin ratio.
Enzyme-linked immunosorbent assay (ELISA)
Serum samples were tested using an IL-6 ELISA kit (ab178013; Abcam). In microplates, standards (50 µl), samples (10 µl sample liquid and 40 µl diluent) and blank were added to predefined wells. In the wells for standards and samples, horseradish peroxidase-labeled conjugates (100 µl) were added prior to sealing the plates for incubation at 37°C for 1 h. Following five washes of the plates, substrates A (50 µl) and B (50 µl) were added to each well. Following incubation at 37°C for 15 min, stop solution (50 µl) was added to each well, and the absorbance of each well was measured at 450 nm within 15 min.
Dual luciferase reporter assay
Bioinformatic predictions are a useful tool to use to evaluate miR function. To determine the regulation of IL-6 in liver fibrosis and cirrhosis, miRanda (www.microrna.org/rnicrorna/home.do), TargetScan (www.targetscan.org), PiTa (genie.weizmann.ac.il/pubs/mir07/mir07_data.html), RNAhybrid (bibiserv.techfak.uni-bielefeld.de/rnahybrid) and PICTA (pictar.mdc-berlin.de) were used to predict the miR molecules that regulate IL-6 and indicated that miR-146a may regulate IL-6 (Fig. 1). According to the bioinformatic results, wild-type (WT) and mutant seed regions of miR-146a in the 3′-untranslated region (UTR) of IL-6 were chemically synthesized in vitro, SpeI and HindIII restriction sites were added, and the WT and mutant miR-146a were cloned into pMIR-REPORT luciferase reporter plasmids (Ambion; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Plasmids (0.8 µg) with WT or mutant 3′-UTR DNA sequences were co-transfected with 100 nM agomiR-146a (Sangon Biotech, Shanghai, China) into 293T cells were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Following 24 h cultivation, cells were lysed using a dual luciferase reporter assay kit (Promega Corporation, Madison, WI, USA) according to the manufacturer's protocol and fluorescence intensity was measured using a GloMax 20/20 luminometer (Promega Corporation). Renilla fluorescence activity was used as internal reference to measure the fluorescence values of each group of cells.
Figure 1.
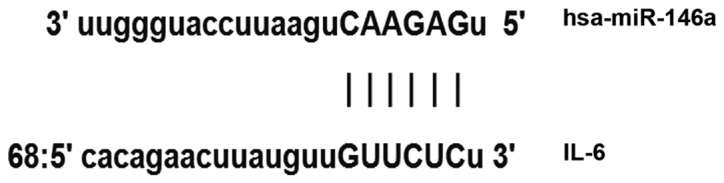
Bioinformatics prediction of direct interactions between miR-146a and IL-6. miR, microRNA, IL, interleukin.
Statistical analysis
The results were analyzed using SPSS 18.0 statistical software (SPSS, Inc., Chicago, IL, USA). The data are presented as the mean ± standard deviation and were tested for normality. Multigroup measurement data were analyzed using one-way analysis of variance. In the case of homogeneity of variance, Least Significant Difference and Student-Newman-Keuls methods were used; in the case of heterogeneity of variance, Tamhane's T2 or Dunnett's T3 method were used. P<0.05 indicated a statistically significant difference.
Results
IL-6 mRNA expression in liver tissues, PBMCs and serum from patients with liver cirrhosis is higher than that from patients with liver fibrosis
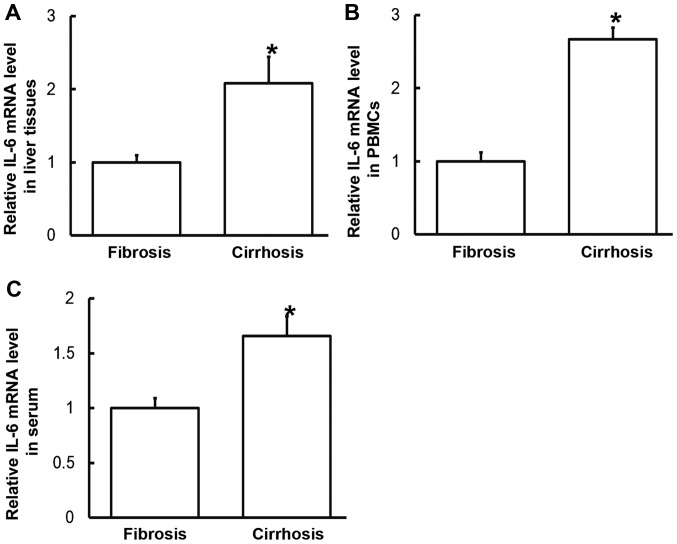
To measure the expression of IL-6 mRNA in the different samples, RT-qPCR was performed. The data indicated that levels of IL-6 mRNA in liver tissues, PBMCs and serum from patients with liver cirrhosis were significantly increased compared with those from patients with liver fibrosis (P<0.05; Fig. 2). These results suggest that the expression of IL-6 mRNA in patients with liver cirrhosis is higher than in patients with liver fibrosis.
Figure 2.
Expression of IL-6 mRNA in (A) liver tissues, (B) PBMCs and (C) serum of patients with hepatitis B and liver fibrosis or cirrhosis. Reverse transcription-quantitative polymerase chain reaction was used to determine mRNA expression. Data are presented as the mean + standard deviation. *P<0.05 vs. patients with liver fibrosis. PBMC, peripheral blood mononuclear cells; IL, interleukin.
Expression of IL-6 protein in liver tissues and PBMCs from patients with liver cirrhosis is enhanced
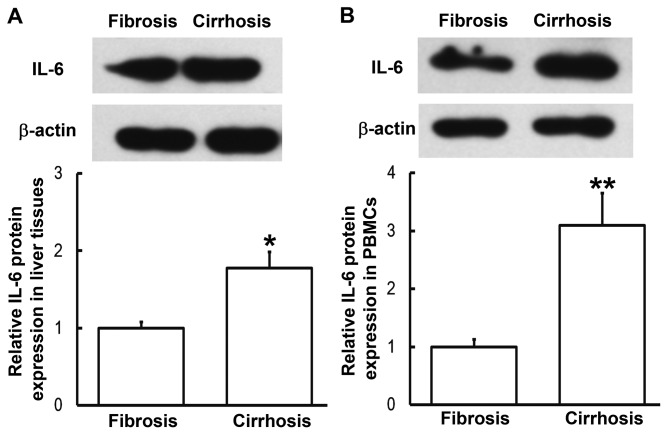
To determine the expression of IL-6 protein in liver tissues and PBMCs, western blotting was performed. Compared with patients with liver fibrosis, the expression of IL-6 in liver tissues and PBMCs from patients with liver cirrhosis was significantly enhanced (P<0.05, P<0.01, respectively; Fig. 3). This was consistent the results from RT-qPCR. These results indicate that IL-6 exerts its regulatory effect on liver cirrhosis at the protein level.
Figure 3.
Expression of IL-6 protein in (A) liver tissues and (B) PBMCs from patients with hepatitis B and liver fibrosis or cirrhosis. Western blotting was used to measure protein expression. Data are presented as the mean + standard deviation. *P<0.05; **P<0.01 vs. patients with liver fibrosis. IL-6, interleukin-6; PBMC, peripheral blood mononuclear cells.
IL-6 protein content in serum from patients with liver cirrhosis is elevated compared with patients with liver fibrosis
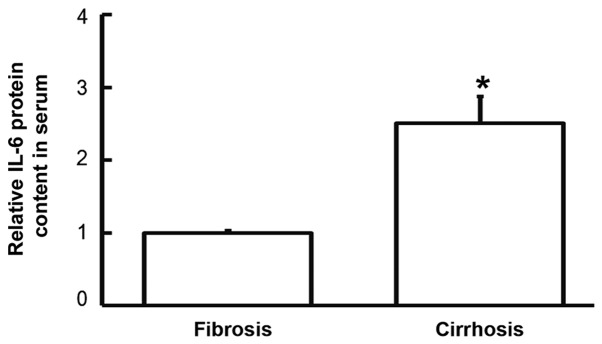
To examine the content of IL-6 protein in the serum, ELISA was employed. The data demonstrated that IL-6 levels in the serum of patients with liver cirrhosis were significantly higher that in patients with liver fibrosis (P<0.05; Fig. 4). This suggests that increased IL-6 protein levels in serum may be released from PBMCs.
Figure 4.
Levels of IL-6 protein in the serum of patients with hepatitis B and liver fibrosis or cirrhosis. ELISA was used to determine IL-6 levels. Data are presented as the mean + standard deviation. Data are presented as the mean + standard deviation. *P<0.05 vs. patients with liver fibrosis. IL-6, interleukin-6.
Altered IL-6 expression may be induced by miR-146a levels
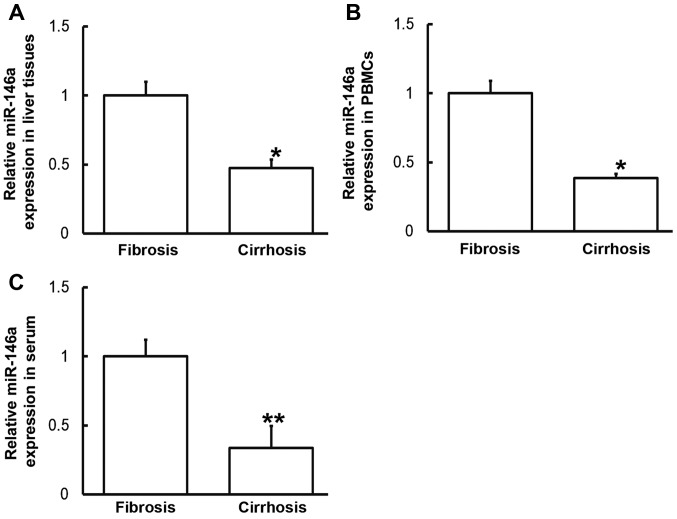
To measure miR-146a levels in various samples, RT-qPCR was performed. Compared with patients with liver fibrosis, miR-146a levels in the liver tissues, PBMCs and serum from patients with liver cirrhosis were significantly downregulated (P<0.05, P<0.05 and P<0.01, respectively; Fig. 5). These results indicate that altered IL-6 expression may be induced by alterations in miR-146a levels.
Figure 5.
Expression of miR-146a in (A) liver tissues, (B) PBMCs and (C) serum of patients with hepatitis B and liver fibrosis or cirrhosis. Reverse transcription-quantitative polymerase chain reaction was used to determine miR-146a expression. Data are presented as the mean + standard deviation. *P<0.05; **P<0.01 vs. patients with liver fibrosis. PBMC, peripheral blood mononuclear cells; miR, microRNA.
miR-146a regulates the expression of IL-6 by binding to its 3′-UTR
To identify the interaction between miR-146a and IL-6, a dual luciferase reporter assay was performed. The data demonstrated that the fluorescence value of the WT group was significantly lower than that of the NC (P<0.01), whereas that of the mutant group did not differ significantly from the control (P>0.05; Fig. 6). The results suggest that miR-146a regulates the expression of IL-6 by binding to its 3′-UTR.
Figure 6.
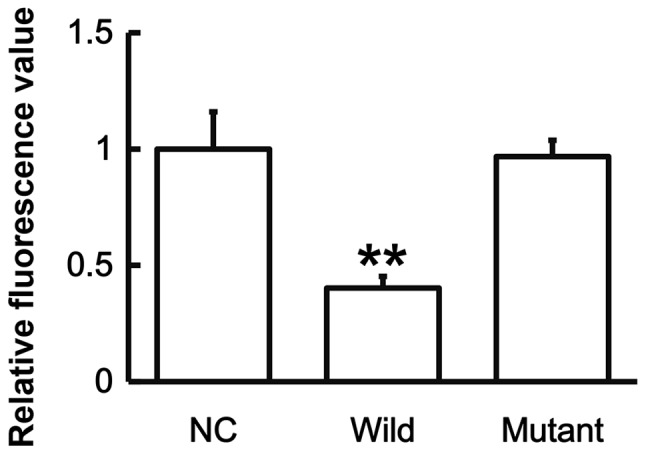
Fluorescence values of HEK293T cells transfected with wild-type or mutant 3′-UTR DNA sequences of IL-6 and agomiR-146a. A dual luciferase reporter assay was used to evaluate the interaction between miR-146a and IL-6. Data are presented as the mean + standard deviation. **P<0.01 vs. NC. 3′-UTR, 3′-untranslated region; IL-6, interleukin-6; NC, negative control; miR, microRNA.
Discussion
IL-6 is an interleukin that serves a variety of functions and is released during immune responses. Bacteria, endotoxin and dust particles may stimulate the production of IL-6 in the body (25). IL-6 serves important roles in immune responses, inflammation, cell differentiation, coagulation and the occurrence and development of tumors (26). The expression of IL-6 is markedly elevated in inflammatory responses caused by injury, trauma, stress and infection (27). In inflammation, IL-6 induces the production of C-reactive protein and fibrinogen in the body, and promotes thrombogenesis (28). Additionally, elevated IL-6 levels induce the onset of inflammatory diseases including rheumatoid arthritis and Crohn's disease by binding to IL-6 receptors (29). In rheumatoid arthritis, IL-6 stimulates the secretion of inflammatory mediators by T and B lymphocytes, promotes the maturation and differentiation of B lymphocytes and enhances the effects of IL-1β and TNF-α (30). In inflammatory responses, IL-6 has chemotaxis on other inflammatory cells including neutral lymphocytes and mononuclear macrophages (31). In the present study, the expression of IL-6 mRNA and protein in liver tissues, PBMCs and serum from patients with liver cirrhosis was higher than in patients with liver fibrosis, suggesting that inflammation may be aggravated during the transformation from liver fibrosis to cirrhosis. Upregulation of IL-6 may be caused by the activation of monocytes and lymphocytes, which can secrete abundant IL-6 factors and produce antigen immune responses (32).
As an important gene regulator, miR molecules participate in various pathophysiological processes, including the proliferation, invasion and metastasis of tumor cells, hypertension, diabetes or atherosclerosis (33,34). The general function of miR molecules is to regulate the expression of their target genes (33,34), however the expression and mechanism of action of miR in Hepatitis B remains unclear. miR-146 was the first miR identified to induce a regulatory effect in the immune response and is associated with a number of diseases. For example, the abnormal expression of miR-146a has been detected in autoimmune diseases, such as rheumatoid arthritis (35,36). Furthermore, clinical and animal models of osteoarthritis suggest that miR-146a is associated with pain in osteoarthritis (37–39). Studies have demonstrated that a number of binding sites of NF-κB exist in the promoter region of miR-146a gene and that lipopolysaccharides, IL-1 and TNF-α all promote miR-146a expression in a NF-κB-dependent manner (40–42). In the current study, bioinformatics predicted that IL-6 may be a direct target gene of miR-146a. The results demonstrated that miR-146a was downregulated and IL-6 was upregulated in liver tissues and PBMCs from patients with liver cirrhosis compared with patients with liver fibrosis, suggesting that the immune system may negatively regulate the targeting of miR-146a on IL-6 by downregulating miR-146a expression. This suggests that miR-146a is not directly correlated with platelet count or serum albumin. Thus, the expression of IL-6 is enhanced and immune responses are generated. Similarly, it was demonstrated that miR-146a is downregulated and IL-6 is upregulated in the serum of patients with liver cirrhosis. This observation indicates that increased levels of IL-6 induced by elevated miR-146a in PBMCs may be released into the serum. Therefore, the levels of miR-146a and IL-6 in the serum indicate the degrees of inflammatory responses and tissue lesions during the transformation from liver fibrosis to cirrhosis. In conclusion, the current study suggests that the decrease of miR-146a expression in the liver tissues, peripheral blood and serum regulates the expression of IL-6, leading to changes in the levels of associated proteins that serve biological roles in the occurrence and development of the disease. The balance between miR-146a and IL-6 expression may determine the occurrence and development of immunity. In addition, miR-146a is stable in the blood, meaning that it may be used for diagnostic purposes.
Acknowledgements
The authors would like to thank Dr Tonglong Xu, the president of the hospital, for his encouragement and advice. In addition, the authors would like to thank Dr Yongli Wei for his contribution to the writing of the manuscript.
Funding
The present study was supported by the Linyi People's Hospital.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author's contributions
ZY and SY designed the study. ZY and YP performed the experiments. ZY and SY analyzed the data. All authors interpreted the results and prepared the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All procedures performed in the current study were approved by the Ethics Committee of Linyi People's Hospital (Linyi, China). Written informed consent was obtained from all patients or their parents, guardians or next of kin.
Patient consent for publication
Written informed consent for publication of any associated data and accompanying images were obtained from all patients or their parents, guardians or next of kin.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Cabre A, Babio N, Lazaro I, Lázaro I, Bulló M, Garcia-Arellano A, Masana L, Salas-Salvadó J. FABP4 predicts atherogenic dyslipidemia development. The PREDIMED study. Atherosclerosis. 2012;222:229–234. doi: 10.1016/j.atherosclerosis.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Tawada A, Kanda T, Imazeki F, Yokosuka O. Prevention of Hepatitis B virus-associated liver diseases by antiviral therapy. Hepatol Int. 2016;10:574–593. doi: 10.1007/s12072-016-9720-y. [DOI] [PubMed] [Google Scholar]
- 3.de Franchis R, Hadengue A, Lau G, Lavanchy D, Lok A, McIntyre N, Mele A, Paumgartner G, Pietrangelo A, Rodés J, et al. EASL international consensus conference on Hepatitis B. 13–14 September, 2002 Geneva, Switzerland. Consensus statement (long version) J Hepatol. 2003;39(Suppl 1):S3–S25. [PubMed] [Google Scholar]
- 4.Chen YC, Chu CM, Yeh CT, Liaw YF. Natural course following the onset of cirrhosis in patients with chronic Hepatitis B: A long-term follow-up study. Hepatol Int. 2007;1:267–273. doi: 10.1007/s12072-007-5001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu CM, Liaw YF. Hepatitis B virus-related cirrhosis: Natural history and treatment. Semin Liver Dis. 2006;26:142–152. doi: 10.1055/s-2006-939752. [DOI] [PubMed] [Google Scholar]
- 6.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI200524282C1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinzani M. Liver fibrosis. Springer Semin Immunopathol. 1999;21:475–490. doi: 10.1007/s002810000037. [DOI] [PubMed] [Google Scholar]
- 8.Safadi R, Friedman SL. Hepatic fibrosis-role of hepatic stellate cell activation. MedGenMed. 2002;4:27. [PubMed] [Google Scholar]
- 9.Trepo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384:2053–2063. doi: 10.1016/S0140-6736(14)60220-8. [DOI] [PubMed] [Google Scholar]
- 10.Sun M, Kisseleva T. Reversibility of liver fibrosis. Clin Res Hepatol Gastroenterol. 2015;39(Suppl 1):S60–S63. doi: 10.1016/j.clinre.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calvaruso V, Craxi A. Regression of fibrosis after HBV antiviral therapy. Is cirrhosis reversible? Liver Int. 2014;34(Suppl 1):S85–S90. doi: 10.1111/liv.12395. [DOI] [PubMed] [Google Scholar]
- 12.Jiang XI, Luo Y, Zhao S, Chen Q, Jiang C, Dai Y, Chen Y, Cao Z. Clinical significance and expression of microRNA in diabetic patients with erectile dysfunction. Exp Ther Med. 2015;10:213–218. doi: 10.3892/etm.2015.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jia W, Wu Y, Zhang Q, Gao GE, Zhang C, Xiang Y. Expression profile of circulating microRNAs as a promising fingerprint for cervical cancer diagnosis and monitoring. Mol Clin Oncol. 2015;3:851–858. doi: 10.3892/mco.2015.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graziano A, Lo Monte G, Piva I, Caserta D, Karner M, Engl B, Marci R. Diagnostic findings in adenomyosis: A pictorial review on the major concerns. Eur Rev Med Pharmacol Sci. 2015;19:1146–1154. [PubMed] [Google Scholar]
- 15.Hayes CN, Chayama K. MicroRNAs as biomarkers for liver disease and hepatocellular carcinoma. Int J Mol Sci. 2016;17:280. doi: 10.3390/ijms17030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ichikawa Y, Joshita S, Umemura T, Shobugawa Y, Usami Y, Shibata S, Yamazaki T, Fujimori N, Komatsu M, Matsumoto A, Tanaka E. Serum Wisteria floribunda agglutinin-positive human Mac-2 binding protein may predict liver fibrosis and progression to hepatocellular carcinoma in patients with chronic hepatitis B virus infection. Hepatol Res. 2017;47:226–233. doi: 10.1111/hepr.12712. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Cheng LS, Wu SD, Wang SQ, Li L, She WM, Li J, Wang JY, Jiang W. IL-10-producing regulatory B-cells suppressed effector T-cells but enhanced regulatory T-cells in chronic HBV infection. Clin Sci (Lond) 2016;130:907–919. doi: 10.1042/CS20160069. [DOI] [PubMed] [Google Scholar]
- 18.MacParland SA, Ma XZ, Chen L, Khattar R, Cherepanov V, Selzner M, Feld JJ, Selzner N, McGilvray ID. Lipopolysaccharide and tumor necrosis factor alpha inhibit interferon signaling in hepatocytes by increasing ubiquitin-like protease 18 (USP18) expression. J Virol. 2016;90:5549–5560. doi: 10.1128/JVI.02557-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang TS, Chen CL, Wu YC, Liu JJ, Kuo YC, Lee KF, Lin SY, Lin SE, Tung SY, Kuo LM, et al. Inflammation promotes expression of stemness-related properties in HBV-related hepatocellular carcinoma. PLoS One. 2016;11:e0149897. doi: 10.1371/journal.pone.0149897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li C, Deng M, Hu J, Li X, Chen L, Ju Y, Hao J, Meng S. Chronic inflammation contributes to the development of hepatocellular carcinoma by decreasing miR-122 levels. Oncotarget. 2016;7:17021–17034. doi: 10.18632/oncotarget.7740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang L, Lan T, Wu L, Li C, Yuan Y, Liu Z. The association between three IL-6 polymorphisms and HBV-related liver diseases: A meta-analysis. Int J Clin Exp Med. 2015;8:17036–17045. [PMC free article] [PubMed] [Google Scholar]
- 22.Lan T, Chang L, Wu L, Yuan YF. IL-6 plays a crucial role in HBV infection. J Clin Transl Hepatol. 2015;3:271–276. doi: 10.14218/JCTH.2015.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chinese Society of Hepatology, Chinese Medical Association: Chinese Society of Infectious Diseases, Chinese Medical Association, corp-author. The guidelines of prevention and treatment for chronic Hepatitis B. Zhonghua Gan Zang Bing Za Zhi. 2005;13:881–891. (In Chinese) [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Badding MA, Schwegler-Berry D, Park JH, Fix NR, Cummings KJ, Leonard SS. Sintered indium-tin oxide particles induce pro-inflammatory responses in vitro, in part through inflammasome activation. PLoS One. 2015;10:e0124368. doi: 10.1371/journal.pone.0124368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6:a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 29.Tone M, Powell MJ, Tone Y, Thompson SA, Waldmann H. IL-10 gene expression is controlled by the transcription factors Sp1 and Sp3. J Immunol. 2000;165:286–291. doi: 10.4049/jimmunol.165.1.286. [DOI] [PubMed] [Google Scholar]
- 30.Yao X, Huang J, Zhong H, Shen N, Faggioni R, Fung M, Yao Y. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther. 2014;141:125–139. doi: 10.1016/j.pharmthera.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Luo Q, Ma X, Wahl SM, Bieker JJ, Crossley M, Montaner LJ. Activation and repression of interleukin-12 p40 transcription by erythroid Kruppel-like factor in macrophages. J Biol Chem. 2004;279:18451–18456. doi: 10.1074/jbc.M400320200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aarstad HH, Vintermyr OK, Ulvestad E, Kross K, Heimdal JH, Aarstad HJ. In vitro-stimulated IL-6 monocyte secretion and in vivo peripheral blood T lymphocyte activation uniquely predicted 15-year survival in patients with head and neck squamous cell carcinoma. PLoS One. 2015;10:e0129724. doi: 10.1371/journal.pone.0129724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varshney J, Subramanian S. MicroRNAs as potential target in human bone and soft tissue sarcoma therapeutics. Front Mol Biosci. 2015;2:31. doi: 10.3389/fmolb.2015.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liz J, Esteller M. lncRNAs and microRNAs with a role in cancer development. Biochim Biophys Acta. 2016;1859:169–176. doi: 10.1016/j.bbagrm.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 35.Pauley KM, Satoh M, Chan AL, Bubb MR, Reeves WH, Chan EK. Upregulated miR-146a expression in peripheral blood mononuclear cells from rheumatoid arthritis patients. Arthritis Res Ther. 2008;10:R101. doi: 10.1186/ar2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abou-Zeid A, Saad M, Soliman E. MicroRNA 146a expression in rheumatoid arthritis: Association with tumor necrosis factor-alpha and disease activity. Genet Test Mol Biomarkers. 2011;15:807–812. doi: 10.1089/gtmb.2011.0026. [DOI] [PubMed] [Google Scholar]
- 37.Yamasaki K, Nakasa T, Miyaki S, Yamasaki T, Yasunaga Y, Ochi M. Angiogenic microRNA-210 is present in cells surrounding osteonecrosis. J Orthop Res. 2012;30:1263–1270. doi: 10.1002/jor.22079. [DOI] [PubMed] [Google Scholar]
- 38.Li X, Gibson G, Kim JS, Kroin J, Xu S, van Wijnen AJ, Im HJ. MicroRNA-146a is linked to pain-related pathophysiology of osteoarthritis. Gene. 2011;480:34–41. doi: 10.1016/j.gene.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X, Kroin JS, Kc R, Gibson G, Chen D, Corbett GT, Pahan K, Fayyaz S, Kim JS, van Wijnen AJ, et al. Altered spinal microRNA-146a and the microRNA-183 cluster contribute to osteoarthritic pain in knee joints. J Bone Miner Res. 2013;28:2512–2522. doi: 10.1002/jbmr.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Curtale G, Citarella F, Carissimi C, Goldoni M, Carucci N, Fulci V, Franceschini D, Meloni F, Barnaba V, Macino G. An emerging player in the adaptive immune response: MicroRNA-146a is a modulator of IL-2 expression and activation-induced cell death in T lymphocytes. Blood. 2010;115:265–273. doi: 10.1182/blood-2009-06-225987. [DOI] [PubMed] [Google Scholar]
- 41.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larner-Svensson HM, Williams AE, Tsitsiou E, Perry MM, Jiang X, Chung KF, Lindsay MA. Pharmacological studies of the mechanism and function of interleukin-1beta-induced miRNA-146a expression in primary human airway smooth muscle. Respir Res. 2010;11:68. doi: 10.1186/1465-9921-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.