Abstract
Expression of TGF-β1 and miR-99a in patients with early spontaneous abortion and correlation with hormone levels during pregnancy were investigated. A total of 70 pregnant women with early spontaneous abortion diagnosed in Jining No. 1 People's Hospital from February 1, 2015 to May 1, 2018 were selected as the study group, and 83 normal pregnant women who chose abortion for non-medical reasons in the same period as the control group. Enzyme-linked immunosorbent assay (ELISA) was used to detect TGF-β1 and the levels of serum β-HCG, progesterone and estrogen during pregnancy in the two groups, and RT-qPCR to detect the expression of miR-99a, and partial correlation analysis to analyze the correlation of TGF-β1 and miR-99a with the levels of serum β-HCG, progesterone and estrogen in the study group of patients. Expression of β-HCG was significantly lower in the study group than that in the control group, with a statistically significant difference (P<0.001), and that of progesterone was significantly lower in the study group than that in the control group, with a statistically significant difference (P<0.001). Expression of estrogen was significantly lower in the study group than that in the control group, with a statistically significant difference (P<0.001). The partial correlation analysis indicated that the levels of serum β-HCG, progesterone and estrogen were positively correlated with TGF-β1 (r=0.944, 0.868, 0.869, P<0.001), negatively correlated with the expression level of miR-99a (r=−0.944, −0.892, −0.891, all P<0.001). miR-99a was highly expressed in the serum of patients with early spontaneous abortion, but TGF-β1 expression was low. The expression levels of the two factors are related to hormone levels during pregnancy, which are expected to be new candidate molecular diagnostic markers in the diagnosis of early spontaneous abortion.
Keywords: early spontaneous abortion, TGF-β1, miR-99a, β-HCG, progesterone, estrogen
Introduction
Early spontaneous abortion is a common complication in early pregnancy. Abortion before 12 weeks of pregnancy is called early abortion (1). Relevant statistics show that the incidence of abortion accounts for 11–16% of all pregnancies, with that of early spontaneous abortion accounting for more than 79% of the total number of abortion (2). Reasons for abortion are very complicated, such as virus, infection of protozoa, pregnant women with cervical incompetence, ischemia and hypoxia, abnormal rise in blood sugar, abnormal thyroid function, and unhealthy living habits such as smoking, drug use and drinking (3–5). Studies have shown that genetic factors, immune factors, embryonic chromosomal abnormalities and endocrine hormone imbalance of pregnant women during pregnancy can affect pregnancy. Immune factors and endocrine hormone imbalance during pregnancy are important causes of early spontaneous abortion, accounting for 48–59% of them (6). Hormones during pregnancy such as β-human chorionic gonadotropin (β-HCG), progesterone and estrogen all play important roles in maintaining pregnancy (7,8).
A growing number of studies have found that certain factors can cause changes in the expression of microRNA (miRNA), which affects their regulated target genes, leading to changes in hormone levels (9). Studies have found that miR-99a is highly expressed in endometrial cancer tissues, presumably related to abnormal hormone levels, but its specific role in early pregnancy is still unclear (10). With the continuous development of cytokine research, scholars believe that TGF-β1, a cytokine with strong immunosuppressive effect that is closely related to pregnancy, can protect the embryo (11). In the present study, the expression of TGF-β1 and miR-99a in early spontaneous abortion and the correlation with the levels of serum β-HCG, progesterone and estrogen during pregnancy were investigated.
Patients and methods
Specimen collection
A total of 70 pregnant women with early spontaneous abortion diagnosed in Jining No. 1 People's Hospital (Jining, China) from February 1, 2015 to May 1, 2018 were selected as the study group, with an age range of 22–40 years and an average age of 25.79±7.38 years. Another 83 normal pregnant women who chose abortion in the same period were selected as the control group, with an age range of 22–38 years and an average age of 26.04±5.24 years.
The inclusion and exclusion criteria were: i) Only pregnant women who were admitted to Jining No. 1 People's Hospital were included as subjects, without abortion caused by chromosomes, anatomy, endocrine abnormalities, reproductive system infections and autoimmune diseases; ii) In this study, patients with hypertension, hepatitis B virus, gallstone, AIDS and various blood diseases were excluded. The control group excluded pregnant women with abnormal pregnancy history. Subjects signed an informed consent form. This study was approved by the Ethics Committee of Jining No. 1 People's Hospital.
Main reagents and instruments
TGF-β1 ELISA detection kit (Shanghai Guyan Biotechnology Co., Ltd., Shanghai, China), progesterone ELISA detection kit (Qingdao Jieshikang Biotechnology Co., Ltd., Qingdao, China), estrogen ELISA detection kit (Qingdao Jieshikang Biotechnology Co., Ltd.), serum β-HCG ELISA detection kit (Qingdao Jieshikang Biotechnology Co., Ltd.), and enzyme microplate reader (Shanghai Haozhuang Instrument Co., Ltd., Shanghai, China) were used in the study.
RNA extraction reagent TRIzol (Nanjing Kebai Biotechnology Co., Ltd., Nanjing, China), reverse transcription kit (Nanjing Kebai Biotechnology Co., Ltd.), miR-99a PCR kit (Thermo Fisher Scientific Co., Ltd., Shanghai, China), and a fluorescence quantitative PCR instrument (Xian Tianlong Technology Co., Ltd., Xian, China) were used in the study. The primers for miR-99a and its internal reference U6 in the kit are shown in Table I.
Table I.
Primers for miR-99a and its internal reference U6.
| Group | Upstream primer sequence | Downstream primer sequence |
|---|---|---|
| miR-99a | 5′-ACACTCCAGCTGGGAACCCGTAGATCCG-3′ | 5′-GGTGTCGTGCAGTCG-3′ |
| U6 | 5′-CTCGCTTCGGCAGCACATATACT-3′ | 5′-ACGCTTCACGAATTTGCGTGTC-3′ |
Detection methods
ELISA detection of TGF-β1 and levels of serum β-HCG, progesterone and estrogen during pregnancy
Elbow venous blood was extracted from all the subjects in a fasting state with a vacuum blood tube before 9:00 in the morning, centrifuged at 3,010 × g for 5 min at 4°C, and then placed in a cryogenic refrigerator at 4°C for use. The experimental procedure was based on ELISA detection kit instructions related to TGF-β1, serum β-HCG, progesterone and estrogen during pregnancy. A sample well to be tested, a standard well and a blank well were set. A total of 100 µl of sample diluent was added to the blank well, 100 µl of sample to be tested or standard sample added to remaining wells, mixed well and sealed with a microplate sealer, prior to incubatoin at 37°C for 30 min. The microplate sealer was carefully removed, with the liquid discarded, and dried. Each well was filled with washing solution, discarded after standing for 30 sec, repeating 5 times and pat dried. A total of 50 µl of the enzyme-labeled reagent was added to each well except for the blank well, washed again after incubation at 37°C for 30 min. The developer was added to each well, mixed well, coloring at 37°C for 15 min in the dark. A total of 50 µl of stop solution was added to each well. The OD value of each well was measured immediately at a wavelength of 450 nm using an enzyme microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The concentrations of TGF-β1, serum β-HCG, progesterone and estrogen during pregnancy were calculated.
RT-qPCR detection of miR-99a
The TRIzol method was used to extract total RNA from blood serum which was then stored in a refrigerator at −80°C until use. The total RNA was reverse-transcribed using Reverse Transcription kit. The reaction system was: 1 µl of M-MLV, 1 µl of Olig (dT), 0.5 µl of RNase inhibitor, 1 µl of dNTPs, RNAse-free water added to 15 µl. Incubation was performed at 38°C for 60 min, and 1 µl of cDNA was taken at 85°C for 5 sec, stored at 4°C. RT-qPCR was used to detect the level of miR-99a in the serum, with its operations referring to the miR-99a SYBR-Green PCR kit protocol (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The reaction procedure was: at 95°C for 1 min, at 95°C for 15 sec, and at 60°C for 20 sec, for a total of 39 cycles. Three replicate wells were set up for each sample. This experiment was repeated 3 times, with U6 as an internal control. All reactions were run 3 times, with U6 as a control. The 2−ΔCq method was used to calculate the expression of mir-99a (12).
Statistical analysis
SPSS 17.0 (Asia Analytics Formerly SPSS China) statistical analysis software was used to analyze the experimental data. The t-test was used for the comparison of mean between two groups, and partial correlation analysis for the correlation. P<0.05 indicates the data difference is statistically significant.
Results
Comparison of levels of β-HCG, progesterone and estrogen between the study and control group
The expression of β-HCG in the study group and the control group was (4802.00±1694.00) mU/l and (10315.00±3999.00) mU/l, respectively, and that of β-HCG was significantly lower in the study group than that in the control group, with a statistically significant difference (P<0.01). Expression of progesterone in the study group and the control group was (30.19±10.57) nmol/l and (108.60±40.60) nmol/l, respectively, and that of progesterone was significantly lower in the study group than that in the control group, with a statistically significant difference (P<0.01). Expression of estrogen in the study group and the control group was (96.33±10.76) nmol/l and (289.14±20.16) nmol/l, respectively, and that of estrogen was significantly lower in the study group than that in the control group, with a statistically significant difference (P<0.01) (Table II).
Table II.
Comparison of levels of β-HCG, progesterone and estrogen and body mass index between the study and control groups.
| Group | Study group (n=70) | Control group (n=83) | t | P-value |
|---|---|---|---|---|
| β-HCG (mU/l) | 4802.00±1694.00 | 10315.00±3999.00 | 10.750 | <0.001 |
| Progesterone (nmol/l) | 30.19±10.57 | 108.60±40.60 | 15.710 | <0.001 |
| Estrogen (ng/l) | 96.33±10.76 | 289.14±20.16 | 71.830 | <0.001 |
Difference in expression of TGF-β1 and miR-99a between the two groups
The expression of TGF-β1 in the study group and the control group was (20.45±10.78) ng/l and (96.01±11.59) ng/l, respectively, and that of TGF-β1 was significantly lower in the study group than that in the control group, with a statistically significant difference (P<0.001). Expression of miR-99a in the study group and the control group was (1.71±0.15) and (0.70±0.03), respectively, and that of miR-99a was significantly higher in the study group than that in the control group, with a statistically significant difference (P<0.001) (Table III).
Table III.
Difference in expression of TGF-β1 and miR-99a in the study and control groups.
| Factor | Study group (n=70) | Control group (n=8) | t | P-value |
|---|---|---|---|---|
| TGF-β1 (ng/l) | 20.45±10.78 | 96.01±11.59 | 41.470 | <0.001 |
| miR-99a | 1.71±0.15 | 0.70±0.03 | 59.970 | <0.001 |
Correlation of TGF-β1 and miR-99a with levels of serum β-HCG, progesterone and estrogen in study group of patients
Correlation of TGF-β1 with levels of serum β-HCG, progesterone and estrogen in the study group of patients
The results of partial correlation analysis showed that the expression of TGF-β1 was positively correlated with that of β-HCG in the serum of pregnant women with early spontaneous abortion (r=0.944, P<0.001). Expression of TGF-β1 was positively correlated with that of progesterone in the serum of pregnant women with early spontaneous abortion (r=0.868, P<0.001). TGF-β1 was positively correlated with that of estrogen in the serum of pregnant women with early spontaneous abortion (r=0.869, P<0.001) (Fig. 1).
Figure 1.
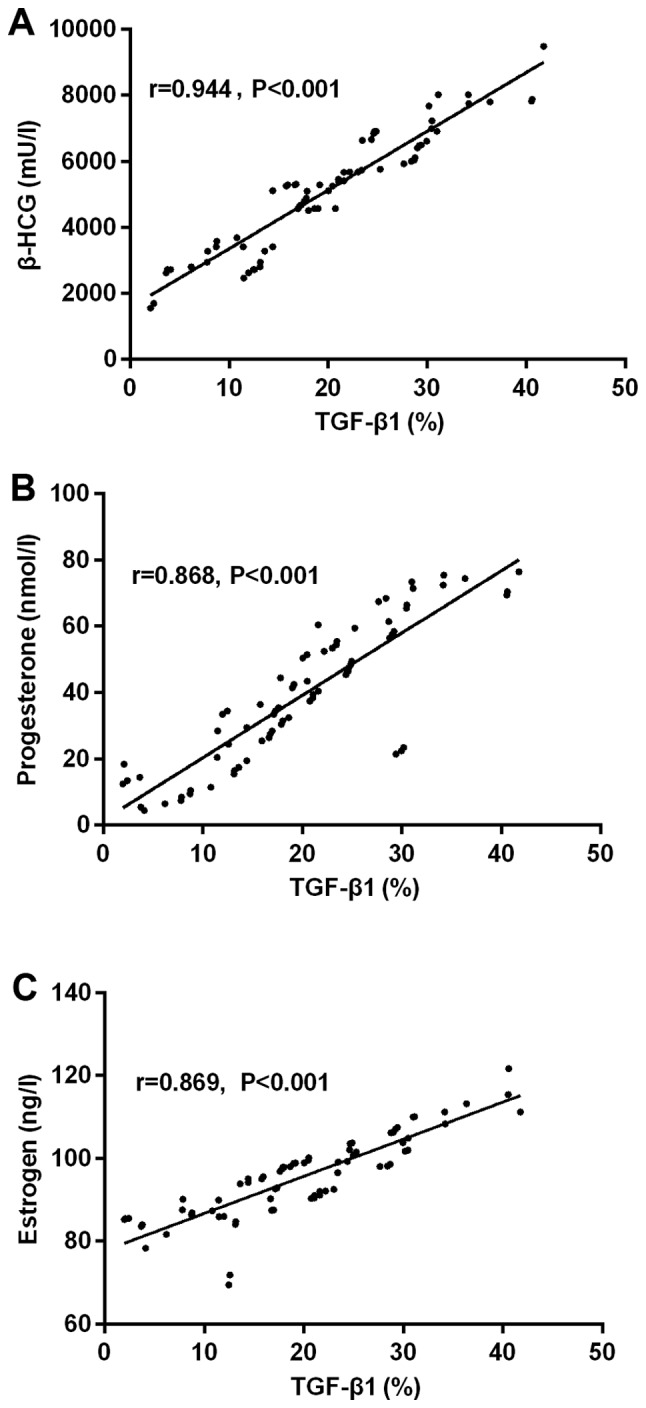
Correlation of TGF-β1 with serum hormone contents in patients with early spontaneous abortion. (A) The results of partial correlation analysis showed the expression of TGF-β1 was positively correlated with that of β-HCG in the serum of pregnant women with early spontaneous abortion (r=0.944, P<0.001). (B) Expression of TGF-β1 was positively correlated with that of progesterone in the serum of pregnant women with early spontaneous abortion (r=0.868, P<0.001). (C) TGF-β1 was positively correlated with that of estrogen in the serum of pregnant women with early spontaneous abortion (r=0.869, P<0.001).
Correlation of miR-99a with levels of serum β-HCG, progesterone and estrogen in the study group of patients
The results of partial correlation analysis showed that the expression of miR-99a was negatively correlated with that of β-HCG in the serum of pregnant women with early spontaneous abortion (r=−0.944, P<0.001). Eexpression of miR-99a was negatively correlated with that of progesterone in the serum of pregnant women with early spontaneous abortion (r=−0.892, P<0.001), and that of miR-99a was negatively correlated with that of estrogen in the serum of pregnant women with early spontaneous abortion (r=−0.891, P<0.001) (Fig. 2).
Figure 2.
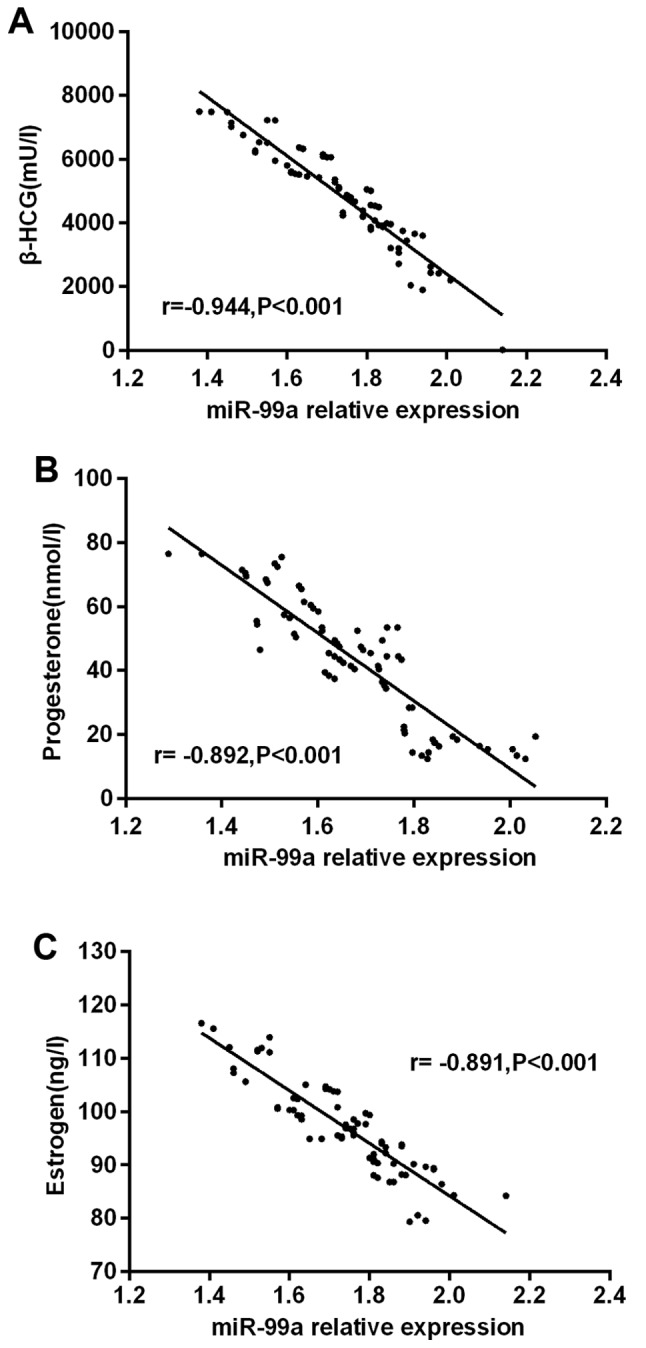
Correlation of miR-99a with serum hormone contents in patients with early spontaneous abortion. The results of partial correlation analysis showed that (A) the expression of miR-99a was negatively correlated with that of β-HCG in the serum of pregnant women with early spontaneous abortion (r=−0.944, P<0.001). (B) Expression of miR-99a was negatively correlated with that of progesterone in the serum of pregnant women with early spontaneous abortion (r=−0.892, P<0.001). (C) miR-99a was negatively correlated with that of estrogen in the serum of pregnant women with early spontaneous abortion (r=−0.891, P<0.001).
Discussion
The main reasons of abortion in early pregnancy are the lack of nutrition provided by the mother causes the fetal development in the uterus to cease, or the abdomen of the pregnant woman is squeezed and injured, causing the fetal position to be unstable or the development of the fetus itself is unhealthy. In addition to the mothers nutritional supply to the fetus and external physical factors, the chromosome of the mother to the fetus and endocrine imbalance are also main reasons of spontaneous abortion in early pregnancy (13,14). Synthesizing and secreting endocrine hormones and different hormones to enter the blood, endocrine cells have different effects on different tissue cell metabolism of the human body, producing different physiological activities (15). The important hormones during pregnancy are β-HCG, progesterone and estrogen, which have important effects on physiological activities in pregnant women and the fetus (16). β-HCG is a glycoprotein that has a great influence on maintaining the stability of the uterine environment (17). Estrogen prepares the implantation of the embryo in the uterus (18). On the basis of estrogenic effects during pregnancy, progesterone keeps the fertilized egg implanted in the uterus and maintains a smooth pregnancy (19). The detection of β-HCG, progesterone and estrogen is important for the diagnosis of early pregnancy. Studies have shown that TGF-β1 and miR-99a are associated with changes in pathological phenomena of pregnancy and hormone levels during pregnancy. TGF-β1 expression is low in the serum of pregnant womens peripheral blood, and the elevated expression level of miR-99a will inhibit hormone expression levels during pregnancy (16,20). In this study, the expression of TGF-β1 and miR-99a in early spontaneous abortion and the correlation with the levels of serum β-HCG, progesterone and estrogen during pregnancy were investigated.
First, the levels of β-HCG, progesterone and estrogen were detected using ELISA. The results showed that the expression was significantly lower in the study group than in the control group, with a statistically significant difference (P<0.001). It is speculated that hormone levels during pregnancy in patients with early pregnancy abortion are significantly lower than those in normal pregnancy. In addition, a large number of clinical studies show that hormone levels during pregnancy in normal pregnancy are significantly higher than those in patients with early pregnancy abortion, consistent with our findings (21). Next, ELISA and qRT-PCR were used to detect TGF-β1 and miR-99a, respectively, in the serum of included patients with early spontaneous abortion. The results showed that the expression of TGF-β1 was significantly lower in the study group than that in the control group, with a statistically significant difference (P<0.001), that of miR-99a was significantly higher in the study group than that in the control group, with a statistically significant difference (P<0.001). This is similar to the findings of Turcatel et al (22). qRT-PCR was used by them to detect TGF-β1 and miR-99a in the serum of patients with abortion. It is found that the expression of TGF-β1 in the serum of patients with abortion is significantly lower than that of normal pregnant women, while that of miR-99a in the serum of patients with abortion is significantly higher than that of normal pregnant women, with statistically significant difference (all P<0.05). Finally, the correlation of TGF-β1 and miR-99a in the serum with the levels of β-HCG, progesterone and estrogen was analyzed in patients with early spontaneous abortion. The results showed that TGF-β1 was positively correlated with the levels of β-HCG, progesterone and estrogen in the serum of patients (r=0.944, 0.868, 0.869, P<0.001), but that of miR-99a was negatively correlated with them (r=−0.944, −0.892, −0.891, all P<0.001). In the detection of hormone levels during pregnancy in patients with abortion, Dong et al (23) found that TGF-β1 and miR-99a in the serum of patients are closely related to hormone levels during pregnancy. In addition, TGF-β1 in the serum of patients is positively correlated with hormone levels during pregnancy, while that of miR-99a is negatively correlated.
In this study, the number of subjects included was small, which may cause some contingency on the experimental results.
In summary, miR-99a is highly expressed in the serum of patients with early spontaneous abortion, but TGF-β1 expression is low. The expression levels of the two factors are related to hormone levels during pregnancy. By monitoring the expression levels of miR-99a and TGF-β1 in the serum of pregnant women, according to the correlation the hormones during pregnancy, the concentration in the serum of their peripheral blood can be adjusted. Compared with monitoring hormone levels during pregnancy, this adjustment is more convenient and intuitive. miR-99a and TGF-β1 are expected to be new candidate molecular diagnostic markers in the diagnosis of early spontaneous abortion.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors contributions
JX performed the ELISA and wrote the manuscript. JX and YC were responsible for PCR. Both authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Jining No. 1 People's Hospital (Jining, China). Patients who participated in the present study had complete clinical data. Signed informed consents were obtained from the patients or the guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Mekinian A, Cohen J, Kayem G, Carbillon L, Nicaise-Roland P, Gaugler B, Darai E, Bornes M, Fain O. Unexplained recurrent early miscarriages: Role of immunomodulation? Rev Med Interne. 2017;38:264–268. doi: 10.1016/j.revmed.2016.08.001. (In French) [DOI] [PubMed] [Google Scholar]
- 2.Tunç E, Tanrıverdi N, Demirhan O, Süleymanova D, Çetinel N. Chromosomal analyses of 1510 couples who have experienced recurrent spontaneous abortions. Reprod Biomed Online. 2016;32:414–419. doi: 10.1016/j.rbmo.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Granat NE, Frolova OG, Iankova MF, Ilovaĭskaia SF. Causes of miscarriage. Med Sestra. 1978;37:37–40. (In Russian) [PubMed] [Google Scholar]
- 4.Dean DD, Agarwal S, Tripathi P. Connecting links between genetic factors defining ovarian reserve and recurrent miscarriages. J Assist Reprod Genet. 2018;35:2121–2128. doi: 10.1007/s10815-018-1305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Regan L, Rai R. Epidemiology and the medical causes of miscarriage. Best Pract Res Clin Obstet Gynaecol. 2000;14:839–854. doi: 10.1053/beog.2000.0123. [DOI] [PubMed] [Google Scholar]
- 6.Makino T. Recurrent reproductive wastage and immunologic factors. Am J Reprod Immunol. 2002;48:266–268. doi: 10.1034/j.1600-0897.2002.01114.x. [DOI] [PubMed] [Google Scholar]
- 7.Luo Y, He GP. Correlative analysis of postpartum depression. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2007;32:460–465. (In Chinese) [PubMed] [Google Scholar]
- 8.Yahi D, Ojo NA, Mshelia GD. Effects of dexamethasone on progesterone and estrogen profiles and uterine progesterone receptor localization during pregnancy in Sahel goat in Semi-Arid region. J Anim Sci Technol. 2017;59:12. doi: 10.1186/s40781-017-0137-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferraro L, Ravo M, Nassa G, Tarallo R, De Filippo MR, Giurato G, Cirillo F, Stellato C, Silvestro S, Cantarella C, et al. Effects of oestrogen on microRNA expression in hormone-responsive breast cancer cells. Horm Cancer. 2012;3:65–78. doi: 10.1007/s12672-012-0102-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Zhang Z, Zhang X, Lin Y, Luo T, Xiao Z, Zhou Q. A dual PI3K/AKT/mTOR signaling inhibitor miR-99a suppresses endometrial carcinoma. Am J Transl Res. 2016;8:719–731. [PMC free article] [PubMed] [Google Scholar]
- 11.Lygnos MC, Pappa KI, Papadaki HA, Relakis C, Koumantakis E, Anagnou NP, Eliopoulos GD. Changes in maternal plasma levels of VEGF, bFGF, TGF-beta1, ET-1 and sKL during uncomplicated pregnancy, hypertensive pregnancy and gestational diabetes. In Vivo. 2006;20:157–163. [PubMed] [Google Scholar]
- 12.Livak KJ, Scmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 13.Zhang HK, Luo FW, Geng Q, Li J, Liu QZ, Chen WB, Li F, Xie JS. Analysis of fetal chromosomal karyotype and etiology in 252 cases of early spontaneous abortion. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2011;28:575–578. doi: 10.3760/cma.j.issn.1003-9406.2011.05.024. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 14.Tara F, Lotfalizadeh M, Moeindarbari S. The effect of diagnostic amniocentesis and its complications on early spontaneous abortion. Electron Physician. 2016;8:2787–2792. doi: 10.19082/2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alter RC. Abortion outcome as a function of sex-role identification. Psychol Women Q. 1984;8:211–233. doi: 10.1111/j.1471-6402.1984.tb00633.x. [DOI] [PubMed] [Google Scholar]
- 16.Gol M, Altunyurt S, Cimrin D, Guclu S, Bagci M, Demir N. Different maternal serum hCG levels in pregnant women with female and male fetuses: Does fetal hypophyseal--adrenal--gonadal axis play a role? J Perinat Med. 2004;32:342–345. doi: 10.1515/JPM.2004.064. [DOI] [PubMed] [Google Scholar]
- 17.Di Lorenzo G, Ceccarello M, Cecotti V, Ronfani L, Monasta L, Vecchi Brumatti L, Montico M, DOttavio G. First trimester maternal serum PIGF, free β-hCG, PAPP-A, PP-13, uterine artery Doppler and maternal history for the prediction of preeclampsia. Placenta. 2012;33:495–501. doi: 10.1016/j.placenta.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Ravindranath N, Moudgal RN. Effect of a specific estrogen antibody on pregnancy establishment in the bonnet monkey (Macaca radiata) Fertil Steril. 1990;54:1162–1167. doi: 10.1016/S0015-0282(16)54022-9. [DOI] [PubMed] [Google Scholar]
- 19.Dobrzyn K, Smolinska N, Szeszko K, Kiezun M, Maleszka A, Rytelewska E, Kaminski T. Effect of progesterone on adiponectin system in the porcine uterus during early pregnancy. J Anim Sci. 2017;95:338–352. doi: 10.2527/jas.2016.0732. [DOI] [PubMed] [Google Scholar]
- 20.Yoshihara A, Noh JY, Mukasa K, Suzuki M, Ohye H, Matsumoto M, Kunii Y, Watanabe N, Suzuki N, Kameda T, et al. Serum human chorionic gonadotropin levels and thyroid hormone levels in gestational transient thyrotoxicosis: Is the serum hCG level useful for differentiating between active Graves disease and GTT? Endocr J. 2015;62:557–560. doi: 10.1507/endocrj.EJ14-0596. [DOI] [PubMed] [Google Scholar]
- 21.Brown JB, Evans JH, Beischer NA, Campbell DG, Fortune DW. Hormone levels in threatened abortion. J Obstet Gynaecol Br Commonw. 1970;77:690–700. doi: 10.1111/j.1471-0528.1970.tb03594.x. [DOI] [PubMed] [Google Scholar]
- 22.Turcatel G, Rubin N, El-Hashash A, Warburton D. MIR-99a and MIR-99b modulate TGF-β induced epithelial to mesenchymal plasticity in normal murine mammary gland cells. PLoS One. 2012;7:e31032. doi: 10.1371/journal.pone.0031032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong F, Zhang Y, Xia F, Yang Y, Xiong S, Jin L, Zhang J. Genome-wide miRNA profiling of villus and decidua of recurrent spontaneous abortion patients. Reproduction. 2014;148:33–41. doi: 10.1530/REP-14-0095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


