Abstract
Intratumoral heterogeneity, particularly the potential cancer stemness of single cancer cells, has not yet been fully elucidated in human esophageal cancer. Single-cell transcriptome sequencing of two types of esophageal adenocarcinoma (EAC) and two types of esophageal squamous cell carcinoma (ESCC) tissues was performed, and the intratumoral cancer stemness of the types of esophageal cancer were characterized at the single-cell level in the present study. By comparing the transcriptomic profiles of single cancer cells with high and low stemness in individual patients, it was revealed that the overexpression of cell cycle-associated genes in EAC cells was highly correlated with stemness, whereas overexpression of genes involved in the signaling pathways of DNA replication and DNA damage repair was significantly correlated with stemness in ESCC. High expression of these stemness-associated genes was correlated with poor prognosis of patients. Additionally, poly [ADP-ribose] polymerase(PARP)4 was identified as a novel cancer stemness-associated gene in ESCC and its association with survival was validated in a cohort of 121 patients with ESCC. These findings have profound potential implications for the use of cell cycle inhibitors in EAC and PARP inhibitors in ESCC, which may provide novel mechanistic insights into the plasticity of esophageal cancer.
Keywords: esophageal squamous cell carcinoma, esophageal adenocarcinoma, single-cell RNA-Seq, cancer stemness
Introduction
Cancer stemness, which describes the stem-cell functions of cancer cells (1), may have profound implications for tumor aggressiveness and clinical outcome (2,3). The majority of current cancer therapies have been reported to induce cancer stemness, thereby failing due to metastasis and relapse (4–7). In the clinic, strategies that target cancer stemness may thus serve as a novel approach to develop the next generation of cancer therapeutics to suppress cancer relapse and metastasis (8,9).
Numerous signaling pathways have been reported to be functionally associated with the induction of cancer stemness in various cancer types, including WNT signaling (10,11), NOTCH signaling (12), Hippo signaling (13), hypoxia signaling (14), DNA damage repair (15) and RAS signaling (16). However, previous conventional molecular profiling studies have largely relied on bulk-tissue measurements. Considering that clinical cancer tissues are extremely heterogeneous, even within an individual patient (17), characterizing the features of cancer stemness in single cancer cells (i.e., intratumoral cancer stemness) will likely provide more accurate and vital information for identifying the potential pre-existing treatment-resistant cancer cells in the cancer tissue. Furthermore, the inhibition of these cancer stemness-associated signaling pathways will ultimately improve the detection, diagnosis and treatment of cancer.
To characterize the features of intratumoral cancer stemness in two types of esophageal cancer, namely esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC) at a single-cell level, single-cell RNA-sequencing (RNA-Seq) data of ESCC and EAC from the Sequence Read Archive (SRA) database was utilized in the present study. Once the single cancer cells were successfully separated from non-cancer cells in the cancer tissues of patients with EAC and ESCC, cancer stemness analysis was applied to identify high-and low-stemness cancer cells in each patient (18). By comparing these cancer cells within individual patients, the present study intended to identify the signaling pathways and signature genes that were responsible for the cancer stemness of EAC and ESCC. Furthermore, the expression level of these significant genes was validated and their association with prognosis was assessed using The Cancer Genome Atlas (TCGA) cohort and our ESCC cohort.
Materials and methods
Single-cell transcriptome data for bioinformatics analyses
All single-cell transcriptome data were obtained from the SRA (https://www.ncbi.nlm.nih.gov/sra) under the accession no. SRP119465. In these datasets, 5–10 M reads per single cell were generated with Illumina Hiseq4000 (PE150).
RNA-Seq data preprocessing, alignment, normalization and quantification
Fastqc and the R/Bioconductor package ‘ShortRead 1.40.0’ were used to perform quality control of all sequenced data (19). Data were trimmed using Trimmomatic 0.35 to remove and filter low quality and adapter contaminated reads (20). The human genome NCBI GRCh38 and its corresponding transcriptome gene annotation, which can be downloaded from iGenome, was used for read alignment. The Tophat2.1.1 (http://ccb.jhu.edu/software/tophat/) alignment tool was used for alignment with default parameter settings. The fragments per kilobase of transcript per million mapped reads (FPKM) data from Cufflinks output were used for ‘Monocle 2.4.0’ gene differential expression methods (21). A quantile normalization method was applied to the log2 transformed FPKM dataset. In the bulk-cell quantification step, Cufflinks 2.2.1 was used to generate FPKM data with default parameter settings (22).
Cancer stemness analysis
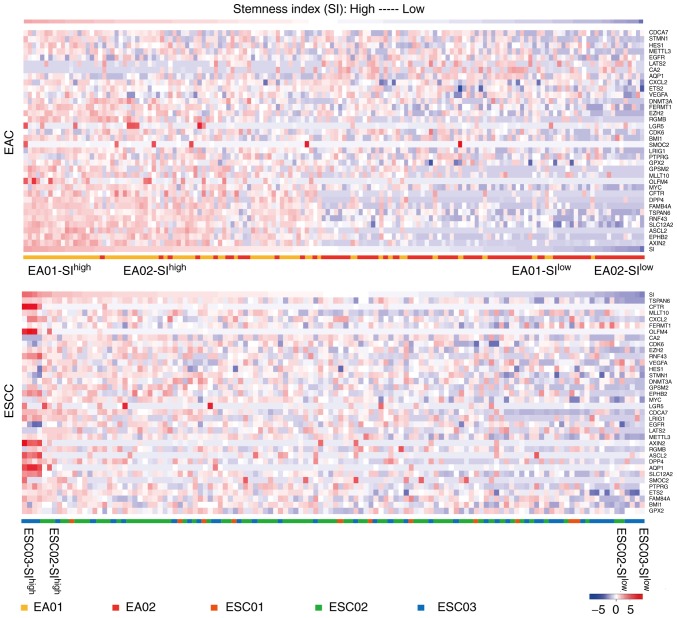
Cancer stemness can be measured according to the expression profiles of stem cell genes, which is a property shared by embryonic and adult stem cells (23). The 35 known stem cell markers were identified according to previous publications, which are listed in Table SI. To quantify the heterogeneity of cancer stemness, a cancer stemness index (SI) was defined by averaging the expression values of these 35 known stem cell markers. Heatmaps for EAC and ESCC were generated and 274 single cancer cells were clustered using the SI level.
Database for Annotation, Visualization and Integrated Discovery (DAVID) analysis
DAVID (http://david.abcc.ncifcrf.gov) functional annotation bioinformatics microarray analysis was used to identify significantly enriched Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) terms among the given list of genes that were identified to be differentially expressed in response to curcumin. Statistically overrepresented GO and KEGG categories with a P-value of ≤0.05 were considered significant.
Gene expression in online database
Gene expression and survival analysis in various cancer tissues was detected using cBioPortal (http://www.cbioportal.org) and Gene Expression Profiling Interactive Analysis (GEPIA; http://gepia.cancer-pku.cn) according to the protocol (http://gepia.cancer-pku.cn/example.html).
Immunohistochemical (IHC) staining
Human tissues were provided by the Department of Pathology, Hangzhou Cancer Hospital (Hangzhou, China) under a protocol approved by the Institutional Review Board of the Cancer Research Institute, Hangzhou Cancer Hospital. The tissues were collected within 1 h of tumor resection and fixed in formalin for 24 h at room temperature. Dehydration and embedding in paraffin was performed following routine methods. Paraffin sections were cut at 5-µm thickness, then deparaffinized and rehydrated. Once endogenous peroxidase activity was blocked with 3% H2O2 for 10 min at room temperature, the sections were treated with citrate buffer (pH=6.0) in a microwave oven for 10 min for antigen retrieval. Following a pre-incubation with blocking buffer (Perkin Elmer, Waltham, MA, USA) for 10 min, the sections were incubated with primary antibodies in a humidified chamber for 1 h at room temperature. The primary antibody against poly [ADP-ribose] polymerase (PARP)4 (1:20; cat. no. ab24110; Abcam, Cambridge, MA, USA) was diluted with blocking buffer. Once the sections were washed with phosphate buffered saline twice for 5 min, the antigenic binding sites were visualized using the GTVision II Detection System (Gene Tech Co., Ltd., Shanghai, China) according to the manufacturer's instructions. Microscope images were assessed using Image-Pro plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA), and the mean integrated optical density (IOD) of each image was collected. Mean IOD was referred to the average level of positive tissues.
Statistical analysis
Statistical comparisons were made with one-way analysis of variance with Bonferroni's multiple comparison post hoc tests. Data were collected into a spreadsheet program and then imported into statistical software packages (GraphPad Prism version 7.1 for Windows; GraphPad Software, Inc., La Jolla, CA, USA). Survival curves were plotted using the Kaplan-Meier method. Curves were analyzed using the log-rank method and hazard ratios were reported where applicable. P<0.05 was considered to indicate a statistically significant difference.
Results
Intratumoral cancer stemness of EAC and ESCC at single-cell level
Previously, we described the heterogeneity of cancer tissues in 2 patients with EAC and 3 with ESCC through separating and characterizing the expression profiles of different cell types via single-cell transcriptome sequencing (SRP119465) (24). All the single cells were picked arbitrarily. In the present study, it was investigated whether the characterized single cancer cells of EAC and ESCC (150 EAC and 124 ESCC single cells) contain more malignant cancer cells representing cancer stem cells. Thus, all the single cancer cells were evaluated in terms of their expression of the cancer stemness-associated signaling pathways. A small proportion of cancer cells aberrantly overexpressing cancer stemness genes were identified. To quantify the heterogeneity of stemness, cancer stemness analysis was used, which defines a stemness index (SI) by averaging the expression values of 35 known stem cell markers according to previous publications (Table SI) (18). Overall, the distribution of SI values was continuous in EAC and ESCC (Fig. 1). As the cancer tissues consist of single cancer cells, the distribution of SI values may reflect their potential resistance to cancer therapy. Compared with patient EA02, the single cancer cells from patient EA01 demonstrated significantly higher SI values (P<0.01), which was consistent with the poor prognosis outcome of patient EA01 following clinical radiotherapy (data not shown). By contrast, the single cells of these 3 ESCC patients demonstrated uniform distribution with regard to the distribution of cancer stemness, and in bulk level, they exhibited moderate levels of cancer stemness.
Figure 1.
SI values of single cancer cells in EAC and ESCC. The heatmap of 35 stem cell genes whose expression is highly correlated with SI across all single cancer cells is indicated. The high- and low-SI cancer cells of individual patients with EAC or ESCC were selected for further analysis. SI, stemness index; EAC, esophageal adenocarcinoma; ESCC, esophageal squamous cell carcinoma.
Cell cycle signaling is associated with high cancer stemness of EAC
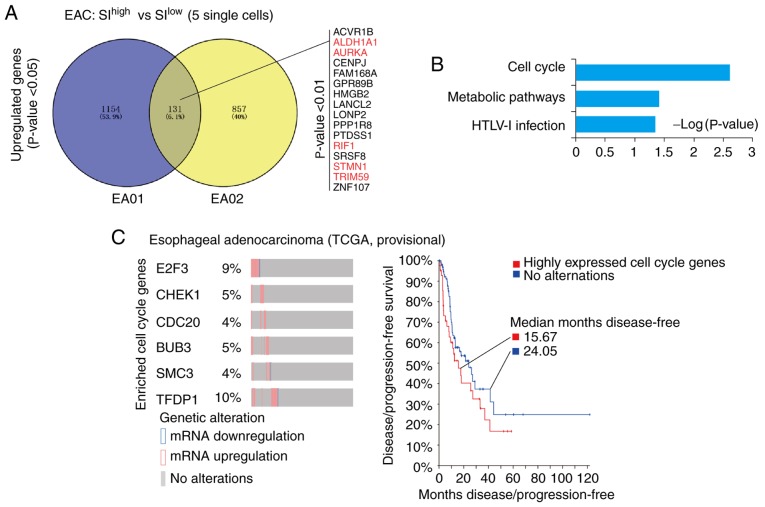
Due to the deep sequencing data of single-cell transcriptome (5–10 M reads per single cell), the detailed features of single cancer cells with high SI values were investigated. The SIhigh single cancer cells (top 5) were compared with SIlow single cancer cells (top 5) in each patient with EAC (EA01 and EA02), and the respective differentially expressed genes (DEGs) were identified. Venny analyses (http://bioinfogp.cnb.csic.es/tools/venny/) of these DEGs in EA01 and EA02 indicated that 131 genes were upregulated, among which 16 genes were significantly upregulated (P<0.01; Fig. 2A), including the previously reported cancer stemness-associated genes aldehyde dehydrogenase 1 (ALDH1A1) (25–29), aurora kinase A (AURKA) (30–33), Replication Timing Regulatory Factor 1 (RIF1) (34–36), stathmin 1 (STMN1) (37,38) and Tripartite Motif Containing 59 (TRIM59) (39,40) (Table SII). Following this, KEGG and GO analyses was applied to these 131 genes, which indicated that genes associated with the ‘cell cycle’ and ‘metabolic pathways’ were significantly enriched (P=0.0025 and P=0.039, respectively; Fig. 2B). Taken together, unlike previous bulk measurements (41), the methods used in the present study allowed the identification of significant genes and signaling pathways that were associated with the cancer stemness of EAC at the single-cell level.
Figure 2.
Upregulated genes in the cell cycle are responsible for the high cancer stemness of EAC. (A) Venny analysis of upregulated genes in the cancer stemness of EA01 and EA02. A total of 131 genes were upregulated in the high-stemness single cancer cells of EA01 and EA02. Among these, 16 genes were identified with P<0.01. Previously reported stemness-associated genes were marked in red; references were listed in Table SI. (B) Database for Annotation, Visualization and Integrated Discovery-Kyoto Encyclopedia of Genes and Genomes analysis of upregulated genes in EAC. The signaling pathway of cell cycle and metabolic pathways were significantly enriched in the cancer stemness of EAC (P<0.05). (C) Dynamic expression of EAC cancer stemness-associated cell cycle genes in TCGA data as assessed with cBioPortal. E2F3, CHEK1, CDC20, BUB3, SMC3 and TFDP1 (EAC cancer stemness-associated cell cycle genes) were significantly upregulated in some patients with EAC (percentage is given), which correlated with poor prognosis in patients (median disease-free: 15.67 vs. 24.05 months). EAC, esophageal adenocarcinoma; SI, stemness index; TCGA, The Cancer Genome Atlas; E2F3, E2F transcription factor 3; CHEK1, checkpoint kinase 1; CDC20, cell division cycle 20; SMC3, structural maintenance of chromosomes protein 3; TRDP1, transcription factor Dp-1; HTLV-1, human T-lymphotropic virus type 1; ACVR1B, activin A receptor type 1B; ALDH1A1, aldehyde dehydrogenase 1 family member A1; AURKA, aurora kinase A; RIF1, replication timing regulatory factor 1; STMN1, stathmin 1; TRIM59, tripartite motif containing 59; CENPJ, centromere protein J; FAM168A, family with sequence similarity 168 member A; GPR89B, G protein-coupled receptor 89B; HMGB2, high mobility group box 2; LANCL2, lanthionine synthetase C-like 2; LONP2, lon peptidase 2, peroxisomal; PPP1R8, protein phosphatase 1 regulatory subunit 8; PTDSS1, phosphatidylserine synthase 1; SRSF8, serine and arginine rich splicing factor 8; ZNF107, zinc finger protein 107.
Cancer stem cells are believed to constitute a principal cellular source for tumor progression and therapeutic drug resistance (42). Therefore, the small proportion of ‘stem cell-like’ carcinoma cells likely represents the true cancer stem cells of esophageal cancer. In cBioPortal of TCGA-EAC (http://www.cbioportal.org), it was indicated that the cell cycle-associated genes identified in the present single-cell data were significantly upregulated in some patients with EAC (TCGA, EAC provisional). In particular, 6 genes [E2F Transcription Factor 3 (E2F3), checkpoint kinase 1 (CHEK1), Cell Division Cycle 20 (CDC20), BUB3, Structural Maintenance of Chromosomes protein 3 (SMC3) and Transcription Factor Dp-1 (TFDP1)] were upregulated in 4–10% of patients with EAC, as indicated in Fig. 2C. Notably, the patients with EAC who expressed a high level of these cell cycle-associated genes exhibited poorer prognosis with regard to disease/progression-free survival compared with patients without altered expression of these genes (median months disease-free: 15.67 vs. 24.06; Fig. 2C).
DNA replication and DNA damage repair-associated genes are enriched in high-cancer stemness cells in ESCC
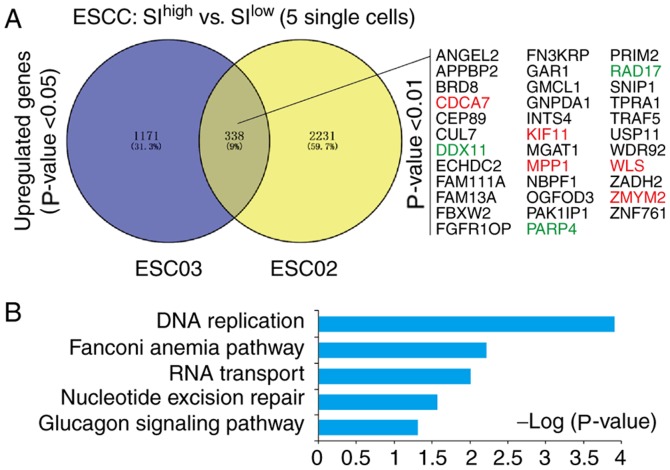
Cancer stemness analysis was performed using SI values on single cancer cells in the single-cell data of ESCC. Due to the few single cancer cells in patient ESC01, this patient was excluded in further analysis. The DEGs of SIhigh single cancer cells (top 5) and SIlow single cancer cells (top 5) in patient ESC02 and ESC03 were identified. Venny analyses of these DEGs indicated that 338 genes were upregulated, among which 35 genes were significantly upregulated (P<0.01; Fig. 3A), including previously reported cancer stemness-associated genes cell division cycle-associated protein 7 (CDCA7) (43), Kinesin Family Member 11 (KIF11) (44–46), Wnt ligand secretion mediator (WLS) (47,48) and zinc finger MYM-type containing 2 (ZMYM2) (49,50) (Table SIII). These 338 genes were further analyzed with KEGG and GO analyses, which indicated that they were significantly enriched in ‘DNA replication’ (P=1.25×10−04), ‘Fanconi anemia pathway’ (P=0.006), ‘RNA transport’ (P=0.0097) and ‘nucleotide excision repair’ (P=0.027; Fig. 3B).
Figure 3.
Signature genes and signaling pathways responsible for the high cancer stemness of ESCC. (A) Venny analysis of upregulated genes in the cancer stemness of ESC02 and ESC03. A total of 338 upregulated genes were identified. Among these, a total of 35 significantly upregulated genes were listed (P<0.01). Previously reported stemness-associated genes were marked in red; the references were given in Table SII. (B) Database for Annotation, Visualization and Integrated Discovery-Kyoto Encyclopedia of Genes and Genomes analysis of upregulated genes in ESCC. The signaling pathways of DNA replication, Fanconi anemia pathway, RNA transport and nucleotide excision repair were significantly enriched in the cancer stemness of ESCC (P<0.05). ESCC, esophageal squamous cell carcinoma; SI, stemness index; ANGEL2, angel homolog 2; APPBP2, amyloid β precursor protein binding protein 2; BRD8, bromodomain containing 8; CDCA7, cell division cycle associated 7; CEP89, centrosomal protein 89; CUL7, cullin 7; DDX11, DEAD/H-Box Helicase 11; ECHDC2, enoyl-CoA hydratase domain containing 2; FAM111A, family with sequence similarity 111 member A; FAM13A, family with sequence similarity 13 member A; FBXW2, F-Box and WD repeat domain containing 2; FGFR10P, FGFR1 oncogene partner; FN3KRP, fructosamine 3 kinase related protein; GAR1, GAR1 ribonucleoprotein; GMCL1, germ cell-less, spermatogenesis associated 1; GNPDA1, glucosamine-6-phosphate deaminase 1; INTS4, integrator complex subunit 4; KIF11, kinesin family member 11; MGAt1, mannosyl (alpha-1,3-)-glycoprotein beta-1,2-N-acetylglucosaminyltransferase; MPP1, membrane palmitoylated protein 1; NBPF1, NBPF member 1; OGFOD3, 2-oxoglutarate and iron dependent oxygenase domain containing 3; PAK1IP1, p21-activated protein kinase-interacting protein 1; PARP4, poly(ADP-ribose) polymerase family member 4; PRIM2, DNA primase subunit 2; RAD17, RAD17 checkpoint clamp loader component; SNIP1, Smad nuclear interacting protein 1; TPRA1, transmembrane protein adipocyte associated 1; TRAF5, TNF receptor associated factor 5; USP11, ubiquitin specific peptidase 11; WDR92, WD repeat domain 92; WLS, Wntless Wnt ligand secretion mediator; ZADH2, zinc binding alcohol dehydrogenase domain containing 2; ZMYM2, zinc finger MYM-type containing 2; ZNF761, zinc finger protein 761.
Identification of PARP4 as a potential marker of high cancer stemness for ESCC
Among the cancer stemness features of ESCC, it was indicated that several DNA damage repair-associated genes (DDX11, RAD17 and PARP4) were significantly upregulated within the more stringently upregulated genes (35 genes) (Fig. 3A; Fig. S1A). Furthermore, in the GEPIA database (http://gepia.cancer-pku.cn), it was identified that PARP4 was upregulated in various human cancers (Fig. S1B), and highly expressed PARP4 was correlated with poor prognosis in cervical squamous cell carcinoma and lung squamous cell carcinoma, but not EAC (Fig. S2).
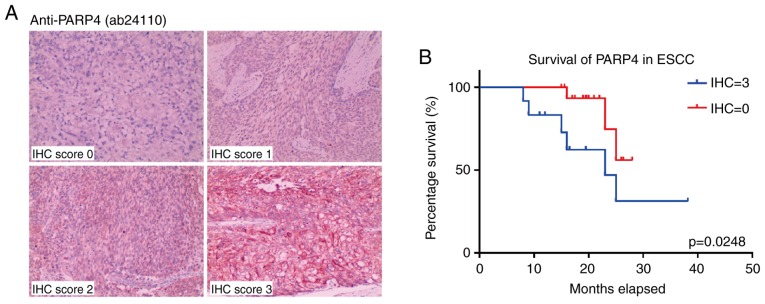
In order to validate the correlation between the expression level of PARP4 and the prognosis of these patients with ESCC, an ESCC cohort with 121 patients was assessed in the present study (Table SIV). Immunohistochemical scoring for the cancer tissues from these patients indicated that high PARP4 expression was associated with poorer survival (Fig. 4A), particularly in patients with higher PARP4 scores (12 patients, IHC score=3; P=0.0248; Fig. 4B). Notably, the majority of patients with ESCC exhibited moderate expression of PARP4 (55 patients, IHC score=1; 36 patients, IHC score=2; Fig. S3A), and their overall survival was longer than that of patients with high PARP4 (IHC score=3) but shorter than that of patients with low PARP4 (IHC score=0; Fig. S3B).
Figure 4.
Upregulated PARP4 correlates with poor prognosis and overall survival in the ESCC cohort. (A) Representative images of PARP4-stained ESCC samples resected from patients in each IHC score category: 0, 1, 2 and 3 (scale bar, 200 µm). (B) Survival analysis based on IHC score of PARP4. IHC=3: scores 3; IHC=0: scores 0. Significance was determined using the Gehan-Breslow-Wilcoxon test. IHC, immunohistochemical; PARP4, poly(ADP-ribose) polymerase family member 4; ESCC, esophageal squamous cell carcinoma.
Discussion
Current cancer treatments ultimately fail owing to metastasis and relapse, which is primarily due to the heterogeneity of cancer tissues (51). Novel methodologies to evaluate and design strategies to reduce the therapeutic resistance in cancer tissues are urgently required for advancing cancer treatment in the clinic (3). Although our increased understanding of the genomic, transcriptomic and proteomic complexity of tumor heterogeneity further highlights the extreme heterogeneity of cancer cells, it has been indicated that the heterogeneity of intratumoral cancer cells was considerably more complicated than originally conjectured. Previous results have indicated that targeting cancer stemness can be used as a strategy to develop the next generation of cancer therapeutics to suppress cancer relapse and metastasis (8). To this end, in the present study, single-cell transcriptome sequencing was utilized to describe the detailed expression profiles of intratumoral cancer stemness in EAC and ESCC at single-cell level.
With the combined expression of potential cancer stemness genes, the SI was generated for each of the single cells, and the heterogeneity of cancer cells was characterized to separate the potential cancer relapse-associated cancer stem cells (18). With the deep sequencing of single-cell transcriptome, detailed features of these potential cancer stem cells could be investigated. This approach can identify a large number of potential cancer stem cell-associated genes and signaling pathways, which may be useful therapeutic targets for future clinical trials.
In EAC, a number of upregulated genes in high-stemness cancer cells were indicated, including previously reported cancer stemness-associated genes ALDH1A1 (25–29), AURKA (30–33), RIF1 (34–36), STMN1 (37,38) and TRIM59 (39,40). These genes were significantly enriched in the ‘cell cycle signaling pathway’. In particular, AURKA and CDC20 genes were significantly enriched in the ‘cell cycle signaling pathway’. This finding holds considerable promise, as in the clinic numerous compounds are already utilized that can target components of cell cycle signaling (52), including cyclin-dependent kinase (CDK)4/CDK6 (palbociclib, ribociclib and abemaciclib), aurora kinases (AT9283 and MLN8237), Wee1 kinase (MK-1775), KSP (ispinesib) and tubulin (taxanes and vinca alkaloids). Based on the characterization of intratumoral cancer stemness in EAC provided in the present study, it was considered that combining such cell cycle inhibitors with current standard treatments might be worthwhile to reduce the potential cancer stemness-associated therapeutic resistance in EAC.
In ESCC, with the exception of the previously reported cancer stemness-associated genes CDCA7 (43), KIF11 (44–46), WLS (47,48) and ZMYM2 (49,50), the present study identified the cancer stemness-associated signaling pathways of DNA replication and DNA damage repair; therefore, these pathways may represent the features of cancer stemness in single ESCC cancer cells. Furthermore, the DNA damage repair-associated factor PARP4 was identified as a novel potential cancer stemness marker in ESCC, the expression of which correlated with patient prognosis. PARP4 may thus serve as a novel therapeutic target for the inhibition of cancer stemness in ESCC. Notably, several compounds are also currently utilized in the clinic that can target PARP-1 (PARP-1 inhibitors) (53). Considering that these have been used to treat various types of cancer (54–56), with excellent outcomes reported in non-small cell lung cancer (57), such PARP-1 inhibitors may serve as effective supplementary therapy to conventional anticancer agents for ESCC (58,59).
In conclusion, through transcriptomic analyses of single cancer cells, the present research demonstrated the features of intratumoral cancer stemness in EAC and ESCC. The diversity of cancer stemness within and between different types of cancer implied that the treatment of EAC and ESCC should be approached differently in future clinical therapy. Furthermore, the detailed profiles of intratumoral cancer stemness in EAC and ESCC provided several indications for applying treatment strategies against specific cancer stemness features in future cancer therapy clinical trials.
Supplementary Material
Acknowledgements
The authors thank the Novogene Company for providing the sequencing support. The sequenced data were analyzed by Xingbao Health Co. Ltd.
Glossary
Abbreviations
- DEG
differentially expressed gene
- EAC
esophageal adenocarcinoma
- ESCC
esophageal squamous cell carcinoma
- FPKM
fragments per kilobase of transcript per million mapped reads
- GO
Gene Ontology
- IHC
immunohistochemistry
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- ScRNA-Seq
single-cell RNA sequencing
- SI
stemness index
- SRA
Sequence Read Archive
- TCGA
The Cancer Genome Atlas
Funding
The present work was supported by grants from the Hangzhou Health Science and Technology Project (grant no. 2017A28), Zhejiang Health Science and Technology Project (grant no. 20180533B64), National Natural Science Foundation of China (grant nos. 81672994 and 31860306), Zhejiang Provincial Foundation for Natural Sciences (grant nos. LY14H160005 and LZ15H220001) and the Zhejiang Provincial Medical Scientific Research Foundation of China (grant nos. 2015KYB325 and 2015PYA009).
Availability of data and materials
All data generated or analyzed during this study are included in this published article. The sequencing RAW data analyzed during the current study are available in the NCBI SRA database (https://www.ncbi.nlm.nih.gov/sra), with accession no. SRP119465.
Authors' contributions
HW conceived the study. YL, HW and QH performed the experiments. RZ and ZL performed the IHC score analysis. JY and HW analyzed the data. HW, JY and ZL prepared the manuscript. MJ and SW reviewed the results, participated in the discussion of the manuscript and were also involved in the conception of the study. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Human tissues were provided by the Department of Pathology, Hangzhou Cancer Hospital following approval by the Institutional Review Board of the Cancer Research Institute, Hangzhou Cancer Hospital. Written, informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wong DJ, Segal E, Chang HY. Stemness, cancer and cancer stem cells. Cell Cycle. 2008;7:3622–3624. doi: 10.4161/cc.7.23.7104. [DOI] [PubMed] [Google Scholar]
- 2.Monteiro J, Fodde R. Cancer stemness and metastasis: Therapeutic consequences and perspectives. Eur J Cancer. 2010;46:1198–1203. doi: 10.1016/j.ejca.2010.02.030. [DOI] [PubMed] [Google Scholar]
- 3.Khosrotehrani K, Roy E. Tell me about your stemness. I'll give your cancer risk! Cell Death Differ. 2017;24:6–7. doi: 10.1038/cdd.2016.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghisolfi L, Keates AC, Hu X, Lee DK, Li CJ. Ionizing radiation induces stemness in cancer cells. PLoS One. 2012;7:e43628. doi: 10.1371/journal.pone.0043628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu X, Ghisolfi L, Keates AC, Zhang J, Xiang S, Lee DK. Induction of cancer cell stemness by chemotherapy. Cell Cycle. 2012;11:2691–2698. doi: 10.4161/cc.21021. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Z, Duan Q, Zhao H, Liu T, Wu H, Shen Q, Wang C, Yin T. Gemcitabine treatment promotes pancreatic cancer stemness through the Nox/ROS/NF-kappaB/STAT3 signaling cascade. Cancer Lett. 2016;382:53–63. doi: 10.1016/j.canlet.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Liu L, Yang L, Yan W, Zhai J, Pizzo DP, Chu P, Chin AR, Shen M, Dong C, Ruan X, et al. Chemotherapy induces breast cancer stemness in association with dysregulated monocytosis. Clin Cancer Res. 2018;24:2370–2382. doi: 10.1158/1078-0432.CCR-17-2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Rogoff HA, Keates S, Gao Y, Murikipudi S, Mikule K, Leggett D, Li W, Pardee AB, Li CJ. Suppression of cancer relapse and metastasis by inhibiting cancer stemness. Proc Natl Acad Sci USA. 2015;112:1839–1844. doi: 10.1073/pnas.1424171112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang ZM, Chen Y, Luo ML. Targeting stemness: Implications for precision medicine in breast cancer. Adv Exp Med Biol. 2017;1026:147–169. doi: 10.1007/978-981-10-6020-5_7. [DOI] [PubMed] [Google Scholar]
- 10.Fodde R, Brabletz T. Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol. 2007;19:150–158. doi: 10.1016/j.ceb.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Lou Y, Wang H, Zheng X, Dong Q, Sun J, Han B. Wnt signaling regulates the stemness of lung cancer stem cells and its inhibitors exert anticancer effect on lung cancer SPC-A1 cells. Med Oncol. 2015;32:95. doi: 10.1007/s12032-014-0462-1. [DOI] [PubMed] [Google Scholar]
- 12.Jin L, Vu T, Yuan G, Datta PK. STRAP promotes stemness of human colorectal cancer via epigenetic regulation of the NOTCH pathway. Cancer Res. 2017;77:5464–5478. doi: 10.1158/0008-5472.CAN-17-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu T, Li Z, Yang Y, Ji W, Yu Y, Niu X, Zeng Q, Xia W, Lu S. The Hippo/YAP1 pathway interacts with FGFR1 signaling to maintain stemness in lung cancer. Cancer Lett. 2018;423:36–46. doi: 10.1016/j.canlet.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Yun Z, Lin Q. Hypoxia and regulation of cancer cell stemness. Adv Exp Med Biol. 2014;772:41–53. doi: 10.1007/978-1-4614-5915-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Li F, Huang Q, Zhang Z, Zhou L, Deng Y, Zhou M, Fleenor DE, Wang H, Kastan MB, Li CY. Self-inflicted DNA double-strand breaks sustain tumorigenicity and stemness of cancer cells. Cell Res. 2017;27:764–783. doi: 10.1038/cr.2017.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carné Trécesson S, Souazé F, Basseville A, Bernard AC, Pécot J, Lopez J, Bessou M, Sarosiek KA, Letai A, Barillé-Nion S, et al. BCL-XL directly modulates RAS signalling to favour cancer cell stemness. Nat Commun. 2017;8:1123. doi: 10.1038/s41467-017-01079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sottoriva A, Spiteri I, Piccirillo SG, Touloumis A, Collins VP, Marioni JC, Curtis C, Watts C, Tavaré S. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci USA. 2013;110:4009–4014. doi: 10.1073/pnas.1219747110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, Courtois ET, Sengupta D, Tan Y, Chen KH, Goh JJL, Kong SL, Chua C, Hon LK, Tan WS, et al. Reference component analysis of single-cell transcriptomes elucidates cellular heterogeneity in human colorectal tumors. Nat Genet. 2017;49:708–718. doi: 10.1038/ng.3818. [DOI] [PubMed] [Google Scholar]
- 19.Morgan M, Anders S, Lawrence M, Aboyoun P, Pages H, Gentleman R. ShortRead: A bioconductor package for input, quality assessment and exploration of high-throughput sequence data. Bioinformatics. 2009;25:2607–2608. doi: 10.1093/bioinformatics/btp450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolger AM, Lohse M, Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trapnell C, Cacchiarelli D, Grimsby J, Pokharel P, Li S, Morse M, Lennon NJ, Livak KJ, Mikkelsen TS, Rinn JL. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat Biotechnol. 2014;32:381–386. doi: 10.1038/nbt.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with tophat and cufflinks. Nat Protoc. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: Models and concepts. Annu Rev Med. 2007;58:267–284. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 24.Wu H, Yu J, Li Y, Hou Q, Zhou R, Zhang N, Jing Z, Jiang M, Li Z, Hua Y, et al. Single-cell RNA sequencing reveals diverse intratumoral heterogeneities and gene signatures of two types of esophageal cancers. Cancer Lett. 2018;438:133–143. doi: 10.1016/j.canlet.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 25.Kulsum S, Sudheendra HV, Pandian R, Ravindra DR, Siddappa G, R N, Chevour P, Ramachandran B, Sagar M, Jayaprakash A, et al. Cancer stem cell mediated acquired chemoresistance in head and neck cancer can be abrogated by aldehyde dehydrogenase 1 A1 inhibition. Mol Carcinog. 2017;56:694–711. doi: 10.1002/mc.22526. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Wang L, Cui W, Yuan X, Lin L, Cao Q, Wang N, Li Y, Guo W, Zhang X, et al. Targeting ALDH1A1 by disulfiram/copper complex inhibits non-small cell lung cancer recurrence driven by ALDH-positive cancer stem cells. Oncotarget. 2016;7:58516–58530. doi: 10.18632/oncotarget.11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meng E, Mitra A, Tripathi K, Finan MA, Scalici J, McClellan S, Madeira da Silva L, Reed E, Shevde LA, Palle K, et al. ALDH1A1 maintains ovarian cancer stem cell-like properties by altered regulation of cell cycle checkpoint and DNA repair network signaling. PLoS One. 2014;9:e107142. doi: 10.1371/journal.pone.0107142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas ML, de Antueno R, Coyle KM, Sultan M, Cruickshank BM, Giacomantonio MA, Giacomantonio CA, Duncan R, Marcato P. Citral reduces breast tumor growth by inhibiting the cancer stem cell marker ALDH1A3. Mol Oncol. 2016;10:1485–1496. doi: 10.1016/j.molonc.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang L, Ren Y, Yu X, Qian F, Bian BS, Xiao HL, Wang WG, Xu SL, Yang J, Cui W, et al. ALDH1A1 defines invasive cancer stem-like cells and predicts poor prognosis in patients with esophageal squamous cell carcinoma. Mod Pathol. 2014;27:775–783. doi: 10.1038/modpathol.2013.189. [DOI] [PubMed] [Google Scholar]
- 30.Chen C, Song G, Xiang J, Zhang H, Zhao S, Zhan Y. AURKA promotes cancer metastasis by regulating epithelial-mesenchymal transition and cancer stem cell properties in hepatocellular carcinoma. Biochem Biophys Res Commun. 2017;486:514–520. doi: 10.1016/j.bbrc.2017.03.075. [DOI] [PubMed] [Google Scholar]
- 31.Eterno V, Zambelli A, Villani L, Tuscano A, Manera S, Spitaleri A, Pavesi L, Amato A. AurkA controls self-renewal of breast cancer-initiating cells promoting wnt3a stabilization through suppression of miR-128. Sci Rep. 2016;6:28436. doi: 10.1038/srep28436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang N, Wang C, Wang Z, Zona S, Lin SX, Wang X, Yan M, Zheng FM, Li SS, Xu B, et al. FOXM1 recruits nuclear aurora kinase A to participate in a positive feedback loop essential for the self-renewal of breast cancer stem cells. Oncogene. 2017;36:3428–3440. doi: 10.1038/onc.2016.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng F, Yue C, Li G, He B, Cheng W, Wang X, Yan M, Long Z, Qiu W, Yuan Z, et al. Nuclear AURKA acquires kinase-independent transactivating function to enhance breast cancer stem cell phenotype. Nat Commun. 2016;7:10180. doi: 10.1038/ncomms10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.GursesCila HE, Acar M, Barut FB, Gunduz M, Grenman R, Gunduz E. Investigation of the expression of RIF1 gene on head and neck, pancreatic and brain cancer and cancer stem cells. Clin Invest Med. 2016;39:27500. doi: 10.25011/cim.v39i6.27500. [DOI] [PubMed] [Google Scholar]
- 35.Li P, Ma X, Adams IR, Yuan P. A tight control of Rif1 by Oct4 and Smad3 is critical for mouse embryonic stem cell stability. Cell Death Dis. 2015;6:e1588. doi: 10.1038/cddis.2014.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mei Y, Peng C, Liu YB, Wang J, Zhou HH. Silencing RIF1 decreases cell growth, migration and increases cisplatin sensitivity of human cervical cancer cells. Oncotarget. 2017;8:107044–107051. doi: 10.18632/oncotarget.22315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li M, Yang J, Zhou W, Ren Y, Wang X, Chen H, Zhang J, Chen J, Sun Y, Cui L, et al. Activation of an AKT/FOXM1/STMN1 pathway drives resistance to tyrosine kinase inhibitors in lung cancer. Br J Cancer. 2017;117:974–983. doi: 10.1038/bjc.2017.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obayashi S, Horiguchi J, Higuchi T, Katayama A, Handa T, Altan B, Bai T, Bao P, Bao H, Yokobori T, et al. Stathmin1 expression is associated with aggressive phenotypes and cancer stem cell marker expression in breast cancer patients. Int J Oncol. 2017;51:781–790. doi: 10.3892/ijo.2017.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sang Y, Li Y, Song L, Alvarez AA, Zhang W, Lv D, Tang J, Liu F, Chang Z, Hatakeyama S, et al. TRIM59 promotes gliomagenesis by inhibiting TC45 dephosphorylation of STAT3. Cancer Res. 2018;78:1792–1804. doi: 10.1158/0008-5472.CAN-17-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Z, Ji Z, Wang Y, Li J, Cao H, Zhu HH, Gao WQ. TRIM59 is up-regulated in gastric tumors, promoting ubiquitination and degradation of p53. Gastroenterology. 2014;147:1043–1054. doi: 10.1053/j.gastro.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 41.Malta TM, Sokolov A, Gentles AJ, Burzykowski T, Poisson L, Weinstein JN, Kamińska B, Huelsken J, Omberg L, Gevaert O, et al. Machine learning identifies stemness features associated with oncogenic dedifferentiation. Cell. 2018;173:338–354.e15. doi: 10.1016/j.cell.2018.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14:611–629. doi: 10.1038/nrclinonc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guiu J, Bergen DJ, De Pater E, Islam AB, Ayllon V, Gama-Norton L, Ruiz-Herguido C, González J, López-Bigas N, Menendez P, et al. Identification of Cdca7 as a novel Notch transcriptional target involved in hematopoietic stem cell emergence. J Exp Med. 2014;211:2411–2423. doi: 10.1084/jem.20131857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imai T, Oue N, Sentani K, Sakamoto N, Uraoka N, Egi H, Hinoi T, Ohdan H, Yoshida K, Yasui W, et al. KIF11 is required for spheroid formation by oesophageal and colorectal cancer cells. Anticancer Res. 2017;37:47–55. doi: 10.21873/anticanres.11287. [DOI] [PubMed] [Google Scholar]
- 45.Jiang M, Zhuang H, Xia R, Gan L, Wu Y, Ma J, Sun Y, Zhuang Z. KIF11 is required for proliferation and self-renewal of docetaxel resistant triple negative breast cancer cells. Oncotarget. 2017;8:92106–92118. doi: 10.18632/oncotarget.20785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Venere M, Horbinski C, Crish JF, Jin X, Vasanji A, Major J, Burrows AC, Chang C, Prokop J, Wu Q, et al. The mitotic kinesin KIF11 is a driver of invasion, proliferation, and self- renewal in glioblastoma. Sci Transl Med. 2015;7:304ra143. doi: 10.1126/scitranslmed.aac6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Augustin I, Dewi DL, Hundshammer J, Erdmann G, Kerr G, Boutros M. Autocrine wnt regulates the survival and genomic stability of embryonic stem cells. Sci Signal. 2017;10:eaah6829. doi: 10.1126/scisignal.aah6829. [DOI] [PubMed] [Google Scholar]
- 48.Augustin I, Dewi DL, Hundshammer J, Rempel E, Brunk F, Boutros M. Immune cell recruitment in teratomas is impaired by increased wnt secretion. Stem Cell Res. 2016;17:607–615. doi: 10.1016/j.scr.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 49.Ren M, Cowell JK. Constitutive notch pathway activation in murine ZMYM2-FGFR1-induced T-cell lymphomas associated with atypical myeloproliferative disease. Blood. 2011;117:6837–6847. doi: 10.1182/blood-2010-07-295725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ren M, Qin H, Wu Q, Savage NM, George TI, Cowell JK. Development of ZMYM2-FGFR1 driven AML in human CD34+ cells in immunocompromised mice. Int J Cancer. 2016;139:836–840. doi: 10.1002/ijc.30100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rubben A, Araujo A. Cancer heterogeneity: Converting a limitation into a source of biologic information. J Transl Med. 2017;15:190. doi: 10.1186/s12967-017-1290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mills CC, Kolb EA, Sampson VB. Recent advances of cell-cycle inhibitor therapies for pediatric cancer. Cancer Res. 2017;77:6489–6498. doi: 10.1158/0008-5472.CAN-17-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lord CJ, Ashworth A. PARP inhibitors: Synthetic lethality in the clinic. Science. 2017;355:1152–1158. doi: 10.1126/science.aam7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weaver AN, Cooper TS, Rodriguez M, Trummell HQ, Bonner JA, Rosenthal EL, Yang ES. DNA double strand break repair defect and sensitivity to poly ADP-ribose polymerase (PARP) inhibition in human papillomavirus 16-positive head and neck squamous cell carcinoma. Oncotarget. 2015;6:26995–277007. doi: 10.18632/oncotarget.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nasuno T, Mimaki S, Okamoto M, Esumi H, Tsuchihara K. Effect of a poly(ADP-ribose) polymerase-1 inhibitor against esophageal squamous cell carcinoma cell lines. Cancer Sci. 2014;105:202–210. doi: 10.1111/cas.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wurster S, Hennes F, Parplys AC, Seelbach JI, Mansour WY, Zielinski A, Petersen C, Clauditz TS, Münscher A, Friedl AA, Borgmann K. PARP1 inhibition radiosensitizes HNSCC cells deficient in homologous recombination by disabling the DNA replication fork elongation response. Oncotarget. 2016;7:9732–9741. doi: 10.18632/oncotarget.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramalingam SS, Blais N, Mazieres J, Reck M, Jones CM, Juhasz E, Urban L, Orlov S, Barlesi F, Kio E, et al. Randomized, placebo-controlled, phase II study of veliparib in combination with carboplatin and paclitaxel for advanced/metastatic non-small cell lung cancer. Clin Cancer Res. 2017;23:1937–1944. doi: 10.1158/1078-0432.CCR-15-3069. [DOI] [PubMed] [Google Scholar]
- 58.Zhan L, Qin Q, Lu J, Liu J, Zhu H, Yang X, Zhang C, Xu L, Liu Z, Cai J, et al. Novel poly (ADP-ribose) polymerase inhibitor, AZD2281, enhances radiosensitivity of both normoxic and hypoxic esophageal squamous cancer cells. Dis Esophagus. 2016;29:215–223. doi: 10.1111/dote.12299. [DOI] [PubMed] [Google Scholar]
- 59.Yasukawa M, Fujihara H, Fujimori H, Kawaguchi K, Yamada H, Nakayama R, Yamamoto N, Kishi Y, Hamada Y, Masutani M. Synergetic effects of PARP inhibitor AZD2281 and cisplatin in oral squamous cell carcinoma in vitro and in vivo. Int J Mol Sci. 2016;17:272. doi: 10.3390/ijms17030272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article. The sequencing RAW data analyzed during the current study are available in the NCBI SRA database (https://www.ncbi.nlm.nih.gov/sra), with accession no. SRP119465.