Abstract
A 68-year-old man presented with rapid progressive visual loss caused by a progressive local invasive sinonasal intestinal-type adenocarcinoma (ITAC) with intracranial invasion. The local relapse of ITAC in the ethmoid sinus was previously treated with palliative radiotherapy and carboplatin-paclitaxel, without response, hence disease progression was seen. Ophthalmological examination revealed irreversible blindness of the left eye and a dramatic progressive visual loss of the right eye. Due to important visual loss caused by optic nerve invasion, a palliative treatment with cisplatin-5-fluorouracyl was started. This therapy resulted in a good clinical response with a regression of the local mass and a partial recovery of the vision.
Keywords: Intestinal-type sinonasal adenocarcinoma, Cisplatin-5-fluorouracyl, Systemic therapy
Introduction
Sinonasal intestinal-type adenocarcinoma (ITAC) is a rare cancer which is closely related to professional exposure to wood dusts or leather [1, 2]. Due to the rarity of sinonasal cancers, there is a paucity of data regarding strategy for their optimal treatment. A multimodal therapeutic approach is mandatory, which is based on complete surgical resection and post-operative radiotherapy [3]. Outcomes have improved with the use of transnasal endoscopic surgery [4, 5]. The implementation of systemic therapy for locally advanced disease could result in a better outcome [6]. There is no standard treatment for irresectable local recurrences or metastasis. The current schedule in the palliative setting is based on the experience in the neoadjuvant setting with cisplatin-5-FU and has shown good clinical responses [3]. Further studies are needed to precisely define the role of systemic therapy and identify the optimal schedule in a palliative setting.
Case Presentation
A 68-year-old man was referred to our hospital in May 2017 with rapid visual loss caused by a local tumor progression with intracranial invasion. The patient was diagnosed with a left sinonasal adenocarcinoma fourteen years before, and was treated with surgery via lateral rhinotomy followed by postoperative radiotherapy. A year before, a local relapse was diagnosed, histopathologically identified as a well differentiated intestinal-type adenocarcinoma. The relapse was considered inoperable at the treating hospitals and chemotherapy with carboplatin-paclitaxel was applied. Ten months later, the disease progressed under this treatment, and a reirradiation (10 × 3 Gy) was performed. In spite of this, further progression was observed after five months with a rapid progressive visual loss. The patient complained of bilateral blurred vision and difficulties to read. Ophthalmological examination revealed irreversible blindness of the left eye based on optic nerve invasion, and fast evolving visual loss of the good right eye. Magnetic resonance imaging (MRI) brain shows a large mass bilateral in the naso-ethmoidal sinuses, with a large intracranial component with important frontal cerebral edema, dimensions were 7.3 × 8.1 × 6.9 (Fig. 1B). The patient was able to count fingers at a maximal distance of 2 m. Due to the rapid progression of the visual loss, the patient was treated with high doses corticosteroids. One week later, ophthalmological examination showed further worsening of visual loss (counting fingers only possible at very short distance) and steroid treatment was stopped. Chemotherapy with cisplatin-5-fluorouracil (cisplatin 100 mg/msq [day 1] and 5-FU 1,000 mg/msq day 1 – day 4) was applied, despite the somewhat impaired kidney function after nephrectomy in the past.
Fig. 1.
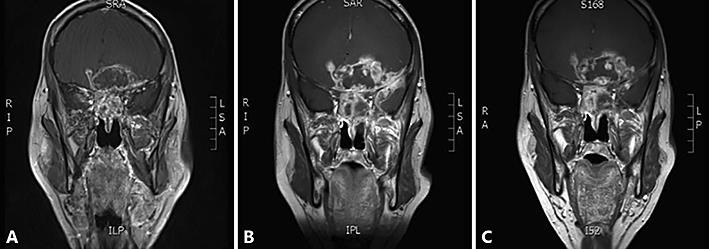
Mass evolution. A Mass after initial treatment with carboplatin-paclitaxel and reirradiation (10 × 3 Gy) in March 2017. B Tumor growth 4 months later, dimensions were 7.3 × 8.1 × 6.9 cm. C At the level of the optic nerve no clear difference is seen after three cycles cisplatin-5-fluorouracyl.
After three cycles, MRI showed no clear difference of the optic nerve invasion (Fig. 1C), but, more importantly, the patient experienced a progressive visual improvement, enabling him to watch TV again and read subtitles. Ophthalmologic reevaluation confirmed visual recovery of the right eye from counting fingers to a visual acuity of 0.7 after four cycles of cis-5-FU. With time, there was also an improvement in visual field. However, there was no recuperation of the visual acuity in the left eye. The initiated palliative therapy thus resulted in a good objective and subjective response. The patient remained progression-free one year after start of the treatment. The chemotherapy was well tolerated with only modest nausea, itching and polyneuropathy grade 1 in the lower limbs. In addition, the second cycle was postponed because of grade 3 neutropenia with spontaneous recuperation. After three cycles the dose was reduced to 75% considering the limited kidney function, which eventually remained stable with an estimated glomerular filtration rate (eGFR) of 69 mL/min/1.73 m2.
Discussion
Sinonasal intestinal-type adenocarcinomas (ITACs) are epithelial tumors of the nasal cavity and the paranasal sinuses. ITACs represents 3 % of all neoplasms occurring in the head and neck region, and 8 to 25% of all malignant sinonasal cancers [1]. It is a rare cancer which is closely related to professional exposure to wood dusts or leather. In contrast to most other typically squamous cell carcinomas of the head and neck, tobacco, alcohol and human papillomavirus do not seem to be risk factors for the development of ITAC [2].
ITAC's develop especially in men, demonstrated by a male: female ratio of 21: 1, and the mean onset is between 60 to 65 years of age [2]. The usual clinical presentation of sinonasal cancers is unilateral or bilateral epistaxis, rhinorrhea and nasal obstruction which are indistinguishable from chronic sinus inflammation. Patients often present with extensive tumoral invasion of neighboring organs and tissues such as the eyes, optic nerves and chiasm, frontal and temporal lobes of the brain and brainstem [3]. The nonspecific symptoms explain the delay in diagnosis, with frequently an interval of 6 months from the first symptoms to diagnosis [3]. The disease has a 5-year overall survival rate of 68% [4]. Depending on the extension of the tumor in surrounding structures, patients may develop additional symptoms such as headache and nausea or ocular symptoms with proptosis, diplopia and visual loss as in the presented case due to intracranial growth.
Due to the rarity and the variety of histological types of sinonasal cancers, there is a paucity of data regarding strategy for their optimal treatment. A multimodality therapeutic approach is mandatory. In general, the treatment is based on complete surgical resection followed by postoperative radiotherapy [3]. Outcomes have improved with the use of transnasal endoscopic surgery [4, 5]. 5-years overall survival, disease-specific survival and recurrence-free survival were 68, 82 and 62% respectively [4]. The mean local recurrence rate in the literature is 30% [7]. Local recurrences are common, because wide margin resection of these tumors is not always possible. Cantu et al. showed that most relapses were local (88%), followed by distant metastasis (9%) [2]. Adjuvant radiotherapy is administered to decrease the risk of local relapse. Using modern radiation techniques, doses can be delivered more conformal to the target in comparison with the conventional radiation delivery. This can result in a reduction of severe late toxicity without compromising disease control [8].
The inclusion of systemic therapy (chemotherapy, targeted agents or immunotherapy) may offer improvement of local control rates and reduction of the frequency of distant metastasis, as well as better survival for patients with irresectable disease. Induction chemotherapy has shown encouraging results and could play a role in the multimodal approach of patients with advanced sinonasal tumors [9, 10]. To date, there are no phase III or other prospective trials in recurrent or metastatic ITACs. Therefore, the treatment choice is mainly based on case reports [1]. The most frequently employed chemotherapeutic agents are cisplatin (cis), associated with 5-fluorouracil (5-FU), taxane, ifosfamide or vincristine [3]. However, there is no standard treatment for irresectable local recurrences or metastasis in ITAC's. The current schedule in the palliative setting is based on the experience in the neoadjuvant setting with cis-5-FU derived from the activity of this chemotherapy schedule in squamous cell cancer of the head and neck and adenocarcinoma of the gastro-intestinal tract. In the search of new systemic treatment options, the molecular profile of ITAC cells has partially been elucidated. Licitra et al. suggested that ITAC with a nonfunctional p53 protein have a significantly worse prognosis in terms of both overall survival and disease-free survival [6]. This indicated the existence of two different ITAC subgroups defined by differences in TP 53 mutational status, reflecting differences in prognosis. However, no druggable targets for personalized medicine have been found yet and further studies are needed.
We present a case of a man with an irresectable local recurrence of an ITAC in the ethmoid sinus that was heavily pretreated, but never with cisplatin. Because important loss of vision caused by invasion of the optical nerve, we started a palliative treatment with cisplatin-5-FU. The therapy demonstrated a good clinical response with a nice partial and durable recovery of vision and some regression of the tumoral mass. The patient remains progression-free one year after start of the treatment. Importantly, the patient had been treated previously with a carboplatin-based regimen without success, suggesting that cisplatin-based chemotherapy is more effective in this setting.
Conclusion
The treatment of sinonasal intestinal-type adenocarcinomas (ITACs) requires a multimodal approach. Employment of systemic therapy for locally advanced disease could result in better outcome, and optimize the therapeutic armamentarium for this rare cancer. This case report illustrates a good clinical response with cisplatin-5-FU that allowed preservation of vision in an irresectable local recurrence of an ITAC. In this patient cisplatin-5FU was far superior to carboplatin-paclitaxel. We suggest using cisplatin-based regimens rather than carboplatin-based regimens in the treatment of recurrent/metastatic ITAC, particularly in case of symptomatic disease.
Statement of Ethics
The ethics committee research UZ / KU Leuven has no objection and takes note of the fact that it concerns a retrospective description of a case that is completely anonymous.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.Hoeben A, van de Winkel L, Hoebers F, Kross K, Driessen C, Slootweg P, et al. Intestinal-type sinonasal adenocarcinomas: the road to molecular diagnosis and personalized treatment. Head Neck. 2016 Oct;38((10)):1564–70. doi: 10.1002/hed.24416. [DOI] [PubMed] [Google Scholar]
- 2.Cantu G, Solero CL, Mariani L, Lo Vullo S, Riccio S, Colombo S, et al. Intestinal type adenocarcinoma of the ethmoid sinus in wood and leather workers: a retrospective study of 153 cases. Head Neck. 2011 Apr;33((4)):535–42. doi: 10.1002/hed.21485. [DOI] [PubMed] [Google Scholar]
- 3.Bossi P, Saba NF, Vermorken JB, Strojan P, Pala L, de Bree R, et al. The role of systemic therapy in the management of sinonasal cancer: A critical review. Cancer Treat Rev. 2015 Dec;41((10)):836–43. doi: 10.1016/j.ctrv.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Camp S, Van Gerven L, Poorten VV, Nuyts S, Hermans R, Hauben E, et al. Long-term follow-up of 123 patients with adenocarcinoma of the sinonasal tract treated with endoscopic resection and postoperative radiation therapy. Head Neck. 2016 Feb;38((2)):294–300. doi: 10.1002/hed.23900. [DOI] [PubMed] [Google Scholar]
- 5.Van Gerven L, Jorissen M, Nuyts S, Hermans R, Vander Poorten V. Long-term follow-up of 44 patients with adenocarcinoma of the nasal cavity and sinuses primarily treated with endoscopic resection followed by radiotherapy. Head Neck. 2011 Jun;33((6)):898–904. doi: 10.1002/hed.21556. [DOI] [PubMed] [Google Scholar]
- 6.Licitra L, Suardi S, Bossi P, Locati LD, Mariani L, Quattrone P, et al. Prediction of TP53 status for primary cisplatin, fluorouracil, and leucovorin chemotherapy in ethmoid sinus intestinal-type adenocarcinoma. J Clin Oncol. 2004 Dec;22((24)):4901–6. doi: 10.1200/JCO.2004.05.071. [DOI] [PubMed] [Google Scholar]
- 7.de Gabory L, Maunoury A, Maurice-Tison S, Merza Abdulkhaleq H, Darrouzet V, Bébéar JP, et al. Long-term single-center results of management of ethmoid adenocarcinoma: 95 patients over 28 years. Ann Surg Oncol. 2010 Apr;17((4)):1127–34. doi: 10.1245/s10434-010-0933-3. [DOI] [PubMed] [Google Scholar]
- 8.Dirix P, Vanstraelen B, Jorissen M, Vander Poorten V, Nuyts S. Intensity-modulated radiotherapy for sinonasal cancer: improved outcome compared to conventional radiotherapy. Int J Radiat Oncol Biol Phys. 2010 Nov;78((4)):998–1004. doi: 10.1016/j.ijrobp.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 9.Choussy O, Ferron C, Védrine PO, Toussaint B, Liétin B, Marandas P, et al. GETTEC Study Group Adenocarcinoma of Ethmoid: a GETTEC retrospective multicenter study of 418 cases. Laryngoscope. 2008 Mar;118((3)):437–43. doi: 10.1097/MLG.0b013e31815b48e3. [DOI] [PubMed] [Google Scholar]
- 10.Knegt PP, Ah-See KW, vd Velden LA, Kerrebijn J. Adenocarcinoma of the ethmoidal sinus complex: surgical debulking and topical fluorouracil may be the optimal treatment. Arch Otolaryngol Head Neck Surg. 2001 Feb;127((2)):141–6. doi: 10.1001/archotol.127.2.141. [DOI] [PubMed] [Google Scholar]


