Abstract
Purpose
To report the clinical features and epidemiology of uveal melanoma in Ireland.
Methods
This was an observational study of 253 patients with a new diagnosis of uveal melanoma between June 2010 and December 2015. Main outcome measures included demographics, clinical features, age-adjusted incidence, relative survival, overall survival, and distant metastases-free survival.
Results
The mean patient age was 61.7 years. Tumour location was choroidal in 82%, ciliochoroidal in 9%, iridociliary in 2%, and iris in 7%. Treatment modalities included brachytherapy (ruthenium-106 and iodine-125 [64%]), enucleation (27%), and proton beam radiation (8%). The mean age-adjusted incidence of uveal melanoma in Ireland from 2010 to 2015 was 9.5 per million of the population (95% confidence interval [CI]: 8.4–10.7). Four-year relative survival was 81.3% (95% CI: 72.8–87.3). Four-year overall survival was 84% (95% CI: 78–90) and 4-year distant metastases-free survival was 79% (95% CI: 73–86).
Conclusion
Based on this data, the incidence of uveal melanoma in Ireland is high when compared with other reported incidence rates in Europe and worldwide. Relative and observed survival were in keeping with other reported European survival rates.
Keywords: Choroid, Melanoma, Brachytherapy, Epidemiology, Survival, Incidence
Introduction
Uveal melanoma is the most common primary intraocular malignancy in adults, albeit still a rare form of cancer [1]. Treatment of uveal melanoma has evolved over the past 3 decades with eye-conserving treatment options such as proton beam radiotherapy and brachytherapy for treatment of smaller tumours [1]. Despite this therapeutic shift, enucleation is still undertaken for larger tumours not amenable to radiation treatment [1].
The incidence of uveal melanoma across Europe has been shown to range from 2 per million in Spain and southern Italy [2] to over 8 per million in Scotland and the Nordic countries [2]. This geographic variability relating to a decrease in incidence going from a north-to-south gradient suggests a protective role of ocular pigmentation [2]. The incidence in the United States has been reported as 5.1 per million [3]. It is lower in Asia with a reported incidence of 0.60 per million in South Korea [4]. The reported overall relative 5-year survival for uveal melanoma ranges from 68.9 to 81.6% [3, 5].
A dedicated Ocular Oncology Service was established in the Republic of Ireland in 2010. Prior to this, all patients were referred to the United Kingdom for management of uveal melanoma with the exclusion of those treated by primary enucleation. There are no previously published papers reporting the clinical demographics and epidemiology of uveal melanoma in Ireland since the establishment of this dedicated service.
Materials and Methods
All data was retrieved by retrospective analysis of case notes of 253 patients included in this study. Patients were included in the study if they were diagnosed with uveal melanoma between June 2010 and December 2015. All patients in the study were Irish residents at the time of diagnosis. Population estimates were calculated using data from the national Central Statistics Office. This study was approved by the ethics committee of the Royal Victoria Eye and Ear Hospital, Dublin.
Ocular Oncology Service
New cases of uveal melanoma are referred to this service from anywhere in the Republic of Ireland. Diagnosis, initial treatment plan and follow-up are completed in a single department, therefore capture of all cases is robust. Patients are referred to our affiliated oncology service for medical oncology treatment and follow-up. Notification of death is given either directly to our service or via the medical oncology team.
Clinical Examination and Diagnostic Methods
The diagnosis of uveal melanoma was based on clinical and ultrasonographic findings. Clinical evaluation and ultrasound examination were carried out by one specialist in ocular oncology (N.H.). At the time of diagnosis, age, gender, ethnicity, Snellen visual acuity, tumour thickness, largest basal diameter of the tumour, and location of the tumour margins were documented. Tumour biopsy was carried out if there was any uncertainty regarding the diagnosis or at the patient's request. Staging was performed using the American Joint Committee on Cancer (AJCC) staging criteria [6, 7, 8]. All patients were referred for medical oncology follow-up.
Treatment Methods
Treatment modalities available in Dublin include brachytherapy, enucleation and resection in selected iridociliary tumours. There is no proton beam facility on the island of Ireland, so patients requiring proton beam therapy were referred to the Royal Liverpool University Hospital and Clatterbridge Cancer Centre in the United Kingdom. Transpupillary thermotherapy was used as an adjunctive treatment in selected cases. Treatment recommendations were based on tumour size, tumour location, visual potential as well as the patient's needs and preferences.
Proton Beam Radiation
Proton beam radiation was utilised, in general, for uveal melanomas located in the peripapillary or juxtapapillary region. “Juxtapapillary” was defined as any lesion with a posterior margin within 1 disc diameter of the optic nerve [9]. Tumours were categorised as “peripapillary” when the lesion edge was contiguous with the optic disc margin.
Brachytherapy
Brachytherapy was utilised for uveal melanomas measuring up to 10 mm in thickness. In general ruthenium-106 plaques were used to treat tumours up to 5 mm in thickness and iodine-125 was used to treat tumours between 5 and 10 mm in thickness. In some cases, brachytherapy was used to treat tumours greater than 10 mm thickness; e.g., in the case of an “only” eye, or where the patient refused enucleation and accepted the additional risks associated with treating a larger tumour with radiation.
Enucleation
In general, enucleation was reserved for uveal melanomas measuring greater than 10 mm in thickness.
Histology
Histological analysis was performed on all enucleated eyes and cytology was carried out in those cases that underwent fine needle biopsy. Tumours were categorised as spindle, epitheloid or mixed cell type. All histology specimens were analysed for molecular genetic abnormalities. Fluorescence in situ hybridisation studies were carried out on touch prints using the Vysis (Abbott Laboratories Ltd., Dublin, Ireland) CEP 3 probes (specific for the centromeric region of chromosome 3), the Vysis CEP 8 probe (specific for the centromeric region of chromosome 8) and the Vysis MYC (8q24) probe. Extrascleral extension was confirmed on pathological examination.
Incidence
The National Cancer Registry (NCR) in Ireland was used to calculate age-adjusted incidence. All patient data was cross-checked with the Ocular Oncology Service to ensure it was a consistent patient cohort; however, NCR data included all cases diagnosed between 2010 and 2015 nationally (total 269 cases, including those diagnosed between January and May 2010, those who declined referral, and those who were retrospectively diagnosed on death). Age-standardised incidence rates (cases per million per year) for a population aged 0+ years were calculated using age-specific incidence rates weighted by the 1976 European population standard and presented as an annual average for the given time period. International Classification of Disease for Oncology codes (ICD-0–3) for both morphology (8720–8790) and site (C69.2 [retina], C69.3 [choroid], C69.4 [ciliary body] or C69.9 [eye, not further specified]) were used to identify cases. Ocular melanoma cases coded as “retina” (1 case) were included in the analysis if they were confirmed as a miscoding of uveal melanoma by cross-checking clinical details. Those coded as “eye, not further specified” (8 cases) were excluded from the analysis.
Survival
Relative survival of uveal melanoma patients in Ireland was calculated using NCR data covering the follow-up period from 2010 to 2014. This was estimated by comparing observed survival of patients with survival expected in the general population of the same age and sex (based on life tables published by the Central Statistics Office). The Strs algorithm with “Pohar Perme” option (generating what is sometimes termed “net survival”) was used, in Stata 13. Follow-up was until the end of 2014, by matching against death certificates.
All other survival parameters were calculated using patient information taken from the Ocular Oncology Service database.
Overall survival (OS) times were calculated from the date of primary treatment until the date of death (from any cause) or the date of the last follow-up. Cancer-specific survival times were calculated from the date of primary treatment until the date of death from uveal melanoma or the date of the last follow-up. Recurrence-free survival times were calculated from the date of primary treatment until the date of local or distant metastases or the date of death or the date of the last follow-up. Distant metastases-free survival (DMFS) times were calculated from the date of primary treatment until the date of metastases or the date of death or the date of the last follow-up. Enucleation-free survival times were calculated from the date of primary treatment until the date of enucleation or the date of death or the date of the last follow-up. For cancer-specific survival, local recurrence, distant metastases and secondary cancers were not treated as events. Death from uveal melanoma was treated as an event but death from other cancers and non-cancer-related deaths were censored. For recurrence-free survival, local recurrence and distant metastases were treated as events while secondary cancers were not treated as events. All deaths were treated as events.
Statistical Analysis
Categorical variables were analysed using χ2 tests and continuous variables were analysed using the Kruskal-Wallis and Mann-Whitney tests. The Kaplan-Meier method was used to estimate survival times and the log-rank test was used to compare differences in survival. All statistical tests were two-sided and assessed for significance at the 0.05 level. Statistical analyses were carried out using IBM® SPSS® statistical software version 21.
Results
Baseline clinical demographics and treatment are outlined in Table 1. Histology, chromosome 3 and chromosome 8 abnormalities are outlined in Table 2.
Table 1.
Baseline clinical demographics and primary treatment of uveal melanoma in Ireland from 2010 to 2015 (n = 253)
| Sex,n (%) | Stage,n (%) | ||
| Female | 106 (42) | I | 72 (28) |
| Male | 147 (58) | IIA | 61 (24) |
| Age (mean ± SD), years | 61.7±13.9 | IIB | 73 (29) |
| Female,n | IIIA | 30 (12) | |
| 0–15 years | 1 | IIIB | 8 (3) |
| 15–44 years | 15 | IIIC | 1 (0) |
| 45–54 years | 25 | IV | 2 (1) |
| 55–64 years | 27 | Unable to stage | 6 (2) |
| 65–74 years | 26 | Extrascleral extension,n (%) | |
| >75 years | 25 | No | 244 (96) |
| Male,n | Yes | 9 (4) | |
| 0–15 years | 0 | Basal diameter (mean ± SD), mm | 12.6 (3.6) |
| 15–44 years | 16 | Median (range) | 12.6 (1.6 – 21) |
| 45–54 years | 26 | Thickness, mm | |
| 55–64 years | 41 | Mean ± SD | 6.3±3.8 |
| 65–74 years | 41 | Median (range) | 5.0 (1.2–22) |
| >75 years | 24 | Primary treatment,n (%) | |
| Country of birth,n (%) | Brachytherapy | 163 (64) | |
| Republic of Ireland | 242 (96) | Enucleation | 69 (27) |
| Lithuania | 2 | Proton beam radiation | 21 (8) |
| United Kingdom | 3 | Plaque type,n (%) | |
| Poland | 3 | I–125 | 57 (35) |
| France | 2 | Ru–106 | 106 (65) |
| Georgia | 1 | Plaque diameter size,n | |
| Eye,n (%) | Ruthenium | ||
| Left | 115 (45) | 15 mm CCA | 16 |
| Right | 138 (55) | 18 mm CCD | 19 |
| Location,n (%) | 20 mm CCB | 24 | |
| Choroidal | 207 (82) | 20 mm notched COB | 33 |
| Ciliochoroidal | 23 (9) | 25 mm notched COC | 13 |
| Iridociliary | 5 (2) | Iodine | |
| Iris | 18 (7) | 14 mm | 6 |
| Presentation,n (%) | 16 mm | 1 | |
| Ophthalmologist | 187 (74) | 18 mm | 14 |
| Optician | 32 (13) | 20 mm | 37 |
| Emergency Department | 22 (9) | ||
| Other | 12 (5) |
Table 2.
Histology of uveal melanoma patients in Ireland from 2010 to 2015
| Patients (n = 79) | n (%) |
|---|---|
| Histology | |
| Epitheloid | 12 (15) |
| Spindle | 33 (42) |
| Mixed | 34 (43) |
| Monosomy 3/chromosome 8 | |
| + Monosomy 3 + chromosome 8 abnormality | 25 (32) |
| + Monosomy 3 – chromosome 8 abnormality | 13 (l6) |
| – Monosomy 3 + chromosome 8 abnormality | 19 (24) |
| – Monosomy 3 – chromosome 8 abnormality | 15 (19) |
| Data not available | 7 (9) |
+, presence of monosomy 3/chromosome 8 abnormality; –, absence of monosomy 3/chromosome 8 abnormality. Note: it was not routine practice to perform cytology on patients undergoing brachytherapy treatment, hence, the vast majority of histology samples were from patients who underwent enucleation.
Median ophthalmologic follow-up for patients treated with brachytherapy was 28.5 months (28.9 months for iodine-125 [range 0.2–58.3] and 26.6 months for ruthenium-106 [range 0.1–63.2]), 23.3 months (range 1.4–64.2) for those treated with proton beam radiotherapy, and 24.8 months (range 0.2–66.0) for enucleation (p = 0.19).
The mean tumour thickness in males (n = 143) was 6.5 ± 3.8 mm, compared with 6.1 ± 3.7 mm in females (n = 105, p = 0.95). The mean tumour basal diameter was 12.8 ± 3.6 mm in males (n = 142), compared with 12.5 ± 3.6 mm in females (n = 103, p = 0.55).
Almost two thirds of the patients were treated with brachytherapy. The majority were treated with ruthenium-106 plaques (65%; tumour basal diameter 3.0–18.2 mm) while 35% had iodine-125 plaques (tumour basal diameter 6.5–20 mm). Plaque diameters are outlined in Table 1. Thirty-one (19%) of the brachytherapy patients had transpupillary thermotherapy laser as an adjunctive treatment.
The incidence of uveal melanoma is outlined in Table 3.
Table 3.
Incidence of uveal melanoma in Ireland (cases of uveal melanoma per million of the population)
| 2010–2015 | Males | Females | Total |
|---|---|---|---|
| 2010 | 20 | 14 | 34 |
| 2011 | 21 | 15 | 36 |
| 2012 | 34 | 27 | 61 |
| 2013 | 36 | 18 | 54 |
| 2014 | 18 | 21 | 39 |
| 2015 | 20 | 25 | 45 |
| Annual average | 25 | 20 | 45 |
| Age-standardised rate (95% CI) | 11.1 (9.3–12.9) | 8.2 (6.7–9.7) | 9.5 (8.4–10.7) |
| Male:female directly standardised rate ratio | 1.35 (1.06–1.73) |
Disease Control
During the course of the study, 6 brachytherapy patients had subsequent enucleations; 4 due to local recurrence and 2 due to complications of radiation retinopathy. One patient had a subsequent exenteration due to local orbital recurrence 39 months following primary enucleation. Of note, in that case, there was scleral vascular channel invasion but no evidence of macroscopic or microscopic extrascleral extension at the time of primary enucleation. Two of those who had subsequent enucleations for local recurrence later developed metastatic disease.
Table 4 outlines the number of patients with enucleation as a primary treatment who developed metastases, according to histological and chromosomal alteration groups. It is important to note that it was not routine practice during this time to perform biopsy for cytology or cytogenetics on patients undergoing brachytherapy treatment, hence, the vast majority of histology samples were from patients who underwent primary enucleation.
Table 4.
Metastases following enucleation as a primary treatment for uveal melanoma in Ireland from 2011 to 2015 (n = 69) by histology and chromosomal alteration
| Enucleated,n (%), a | Developed metastatic disease,n (%), b | Median time free from metastatic disease, months, c | |
|---|---|---|---|
| Histology | |||
| Epitheloid | 10 (14) | 5 (50) | 8.3 |
| Spindle | 30 (43) | 4 (13) | 27.5 |
| Mixed | 29 (42) | 10 (34) | 13.0 |
| Chromosomal alterations | |||
| + Monosomy 3 + chromosome 8 | 25 (36) | 13 (52) | 15.4 |
| + Monosomy 3 – chromosome 8 | 10 (14) | 3 (30) | 16.2 |
| – Monosomy 3 + chromosome 8 | 17 (25) | 2 (12) | 41.5 |
| – Monosomy 3 – chromosome 8 | 11 (16) | 0 | 26.2 |
| Data not available | 6 (9) | 1 (17) | |
+, presence of monosomy 3/chromosome 8 abnormality; –, absence of monosomy 3/chromosome 8 abnormality. Monosomy 3 data not available for 6 patients. Chromosome 8 data not available for 5 patients.
Percent of enucleated cases.
Percent of those enucleated who developed metastases.
Months from enucleation to either metastatic disease, death, or last contact.
The mean tumour thickness at presentation in patients who did not have metastatic disease at the last follow-up was 5.9 mm as compared to 8.9 mm in those who did have metastatic disease (p < 0.0005). Similarly, the mean basal diameter was 12.3 mm in those who did not have metastatic disease as compared to 14.9 mm in those who did develop metastatic disease (p < 0.001).
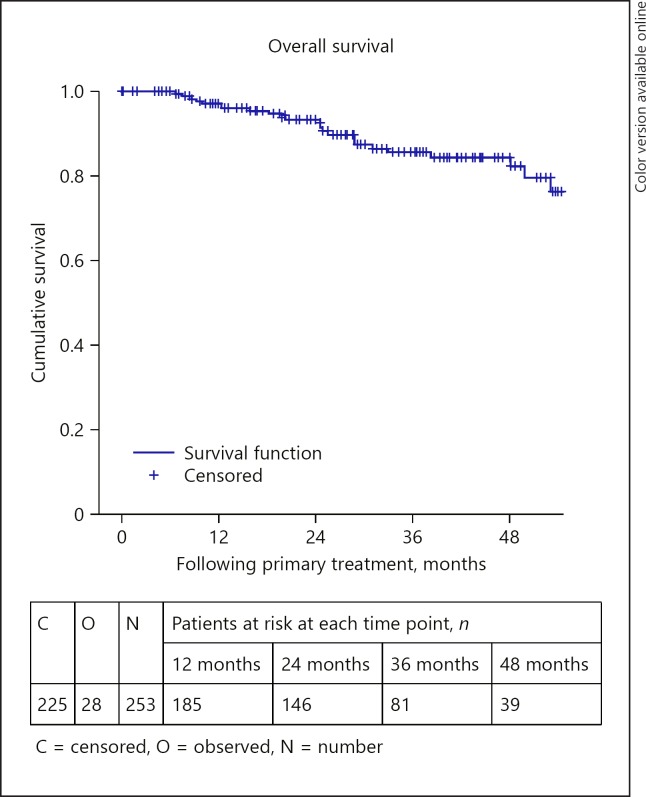
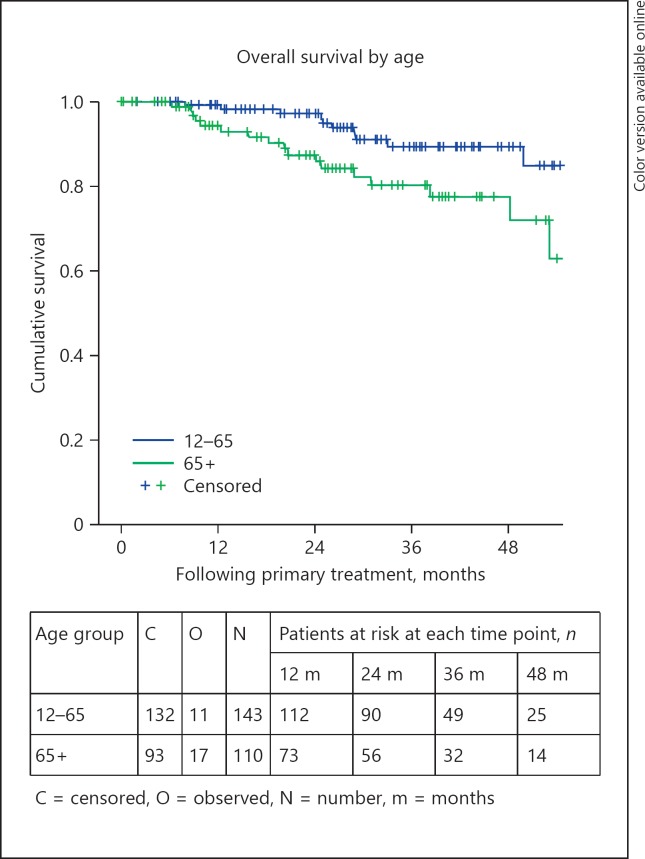
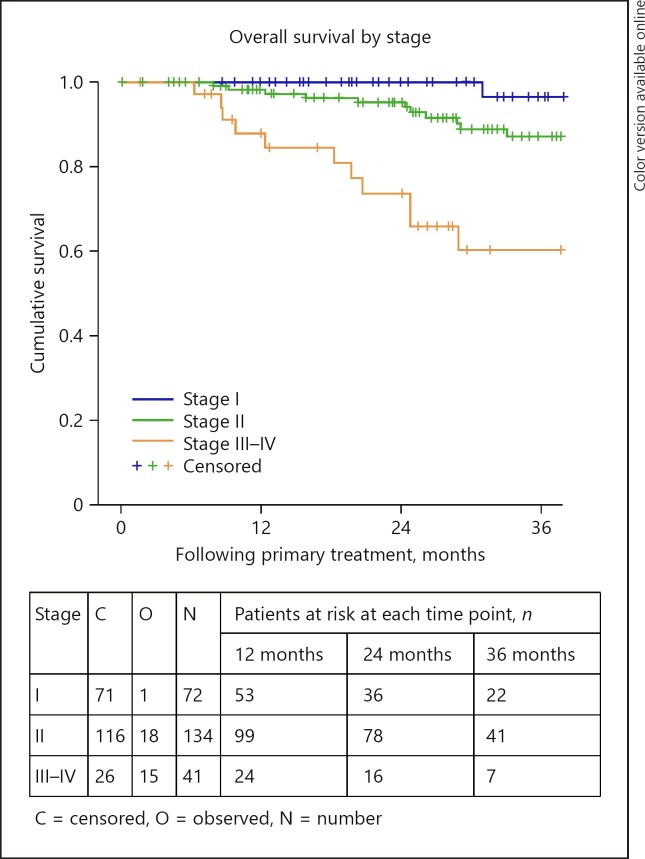
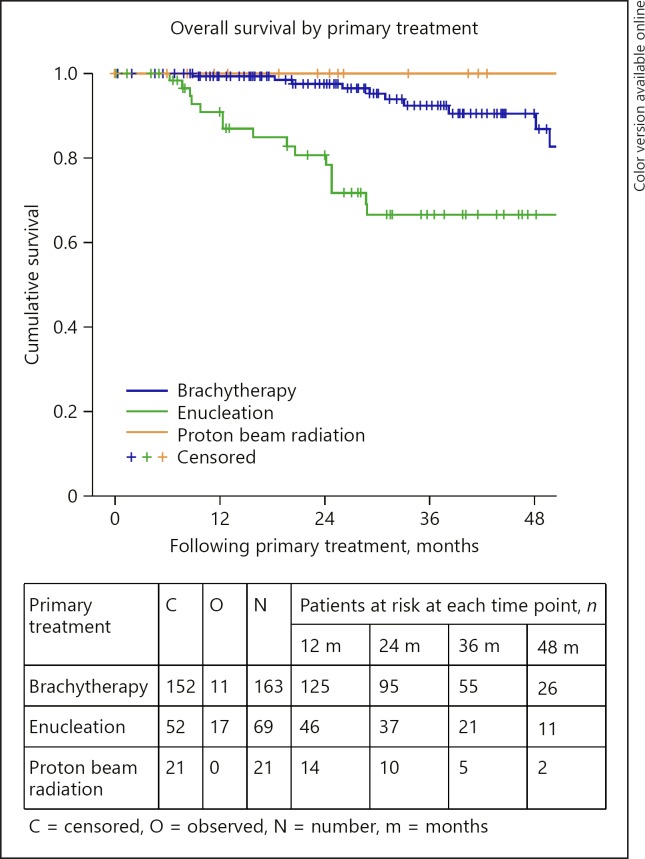
Relative survival is outlined in Table 5. The OS and DMFS curves are shown in Figures 1, 2, 3, 4, 5, 6. The OS curves were curtailed at 52 months when the cumulative probability of survival was 80 and 77%, respectively, and when 29 and 24 cases, respectively, were remaining. The 3- and 4-year OS rates were 86% (95% confidence interval [CI]: 80–91) and 84% (95% CI: 78–90), respectively (Fig. 1). OS was statistically significantly different depending on age group (p = 0.017), AJCC stage (p < 0.0005), tumour thickness (p = 0.013), and the primary treatment received (p < 0.0005). The 3-year OS rates were 85% (95% CI: 78–93%) and 72% (95% CI: 60–83%), for those aged 12–65 and 65+ years, respectively (Fig. 2). The 3-year OS rates were 97% (95% CI: 90–103), 87% (95% CI: 80–94), and 60% (95% CI: 41–79), for those with stage I, stage II, and stage III–IV disease, respectively (Fig. 3). The 3-year OS rates were 89% (95% CI: 83–94) and 70% (95% CI: 53–87) for those with tumours measuring less than or equal to 10 mm and those measuring 10 mm or more, respectively. The 3-year OS rates were 92% (95% CI: 87–98), 66% (95% CI: 53–80), and 100% for those treated with brachytherapy, enucleation, and proton beam radiation, respectively (Fig. 4). OS did not differ significantly by sex (p = 0.803). The 3-year OS rates were 86% (95% CI: 72–101), 57% (95% CI: 25–89), and 58% (95% CI: 37–80) for spindle, epitheloid and mixed histology, respectively. The 3-year OS rates were 66% (95% CI: 50–82) and 79% (95% CI: 60–98) for the presence and absence of chromosomal abnormality, respectively.
Table 5.
Five-year relative survival of patients diagnosed with uveal melanoma from 2010 to 2014, based on National Cancer Registry data, by sex and age-group
| 5-year relative survival (95% CI) | n | |
|---|---|---|
| Total | 77.3% (68.3–83.9) | 192 |
| Sex | ||
| Male | 79.3% (67.0–87.4) | 105 |
| Female | 74.4% (60.5–83.9) | 87 |
| Age group | ||
| 15–44 | 85.7% (61.4–95.2) | 24 |
| 45–54 | 65.9% (46.9–79.4) | 44 |
| 55–64 | 80.2% (64.1–89.5) | 52 |
| 65–74 | 81.8% (62.4–91.8) | 43 |
| 75+ | 62.4% (31.6–82.3) | 29 |
Fig. 1.
Overall survival.
Fig. 2.
Overall survival by age.
Fig. 3.
Overall survival by stage.
Fig. 4.
Overall survival by primary treatment.
Fig. 5.
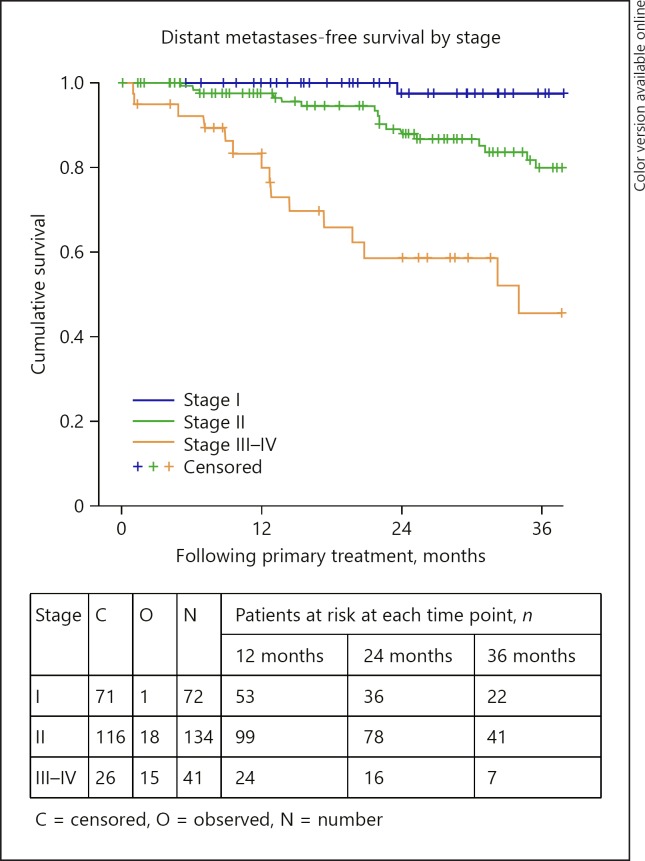
Distant metastases-free survival by stage.
Fig. 6.
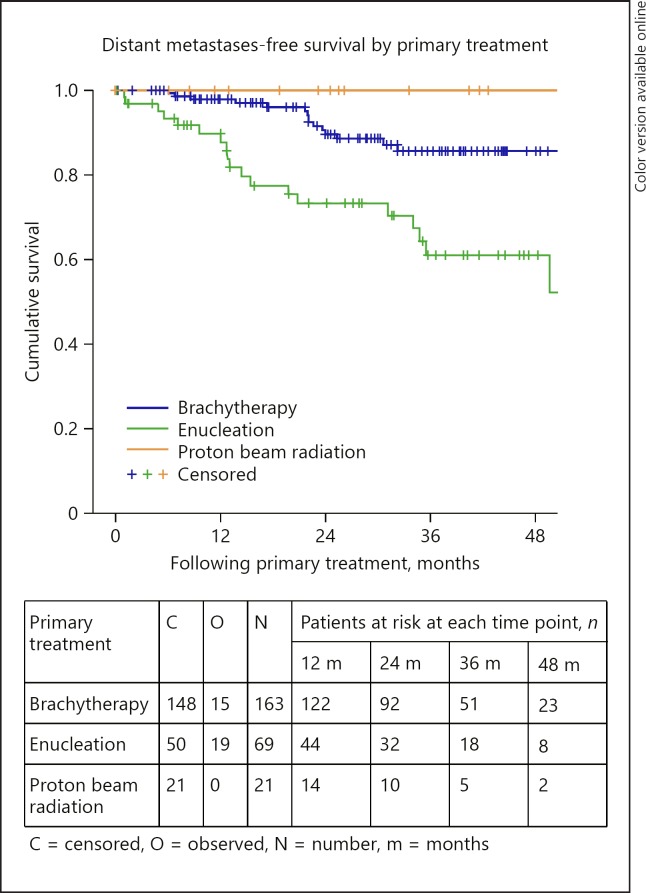
Distant metastases-free survival by primary treatment.
The 4-year cancer-specific survival was 88% (95% CI: 82–93) at 4 years. The median recurrence-free survival was 15.4 months (95% CI: 7.4–23.4); 7.7 months (95% CI: 0–15.6) for those with local recurrence and 15.4 months (95% CI: 6.8–24) for those with distant metastatic disease. The recurrence-free survival rates at 3 and 4 years were 76% (95% CI: 69–82) and 74% (95% CI: 67–82), respectively. The median enucleation-free survival was 62 months. The enucleation-free survival at 3 and 4 years following eye-conserving treatment was 97% (95% CI: 94–100) and 96% (95% CI: 91–100), respectively.
The 3- and 4-year DMFS rates were 79% (95% CI: 73–86) in each case. DMFS was statistically significantly different depending on AJCC stage (p < 0.0005), extrascleral extension (p = 0.018), tumour thickness (p = 0.002), and the primary treatment received (p < 0.0005). The 3-year DMFS was 97% (95% CI: 90–100), 87% (95% CI: 80–94) and 60% (95% CI: 41–79) for those with stage I, stage II, and stage III–IV disease, respectively (Fig. 5). The 3-year DMFS was 83% (95% CI: 76–90) and 60% (95% CI: 41–79) for those with tumours measuring less than or equal to 10 mm and for those with tumours measuring greater than 10 mm, respectively. The 3-year DMFS was 86% (95% CI: 79–93), 61% (95% CI: 46–76), and 100% for those treated with brachytherapy, enucleation, and proton beam radiation, respectively (Fig. 6). DMFS did not differ significantly by age group (p = 0.067) or sex (p = 0.435).
Discussion
The age-adjusted incidence of uveal melanoma in our study was 11.1 per million in males, 8.2 per million in females, and 9.5 per million overall. The largest published series looking at incidence rates of uveal melanoma in Europe from 1983 to 1994 found an incidence of uveal melanoma of between 2 and 8 per million for a population aged ≥0 years [2]. The study by Virgili et al. [2] also described a geographic variability that was related to a north-to-south gradient. They did, however, comment that there was no gradient noted within the Nordic, eastern European or United Kingdom when considered alone. They described how the geographic variability related to latitude may be attributable to increased light exposure functioning as a positive risk factor or darker skin pigmentation and increased ocular pigmentation functioning as a protective factor [2, 10, 11, 12, 13, 14, 15, 16]. Singh and Topham [17] tabulated a summary of 22 published reports on incidence of uveal melanoma worldwide from 1961 to 2001. The incidence rates ranged from 0.3 per million in Japan to between 9 and 10.4 per million in Norway, Connecticut (USA), Sweden, East Germany, and Ohio (USA) [17, 18, 19, 20, 21, 22, 23, 24]. A more recent large series looking at the incidence of uveal melanoma in the United States over a 36-year period from 1973 to 2008, predominantly occurring in Caucasian adults, demonstrated a figure of 5.1 per million [3]. The incidence in Ireland, when compared with these studies, appears to be remarkably high. It is important to note that many of these studies examined data from more than 10 years. Despite these considerations, the higher incidence rate of uveal melanoma in Ireland appears significant and would be in keeping with the recognised predisposing factors to uveal melanoma of pale skin colour, light eye colour and inability to tan, which are stereotypical traits in the native Irish population [13]. It is also worth considering the theory of solar ultraviolet light, via its role in vitamin D photosynthesis, having a protective effect against the development of uveal melanoma [25].
The mean age at diagnosis in this study was 61 years, which is in keeping with other studies that recognise uveal melanoma as increasing in incidence with age [17, 26]. In relation to other published series, there is increasing evidence to support a higher incidence of uveal melanoma in men [17, 26]. Damato and Coupland [27] described gender differences in relation to tumour location with a trend towards thicker, posterior tumours in men and involvement of the ciliary body and iris occurring more frequently in females. Zloto et al. [28] found similarly that men had more posterior tumours and had an increased rate of metastasis and an increased melanoma-related mortality. In this study, 58% of subjects were male. However, we found no statistical difference in the site of the tumour, basal diameter or tumour thickness between men and women.
Despite the availability of multiple treatment options, OS for uveal melanoma patients has not changed in the past 3 decades. Approximately, 50% of uveal melanoma patients will develop metastatic disease within 3 decades of diagnosis [29], with median survival following diagnosis of metastases ranging from 6–12 months [3, 5, 29, 30, 31, 32, 33] to 12–24 months [34, 35, 36, 37] in studies that reported retrospective analyses of specific treatments. Many of the patients in the latter studies were under surveillance for early detection, hence, lead time bias may be somewhat contributing to their longer survival times. Historically, uveal melanoma size at diagnosis was believed to be the most important clinical prognostic factor related to prognosis and survival [30, 31]. However, recent studies have clearly underscored the importance of cytogenetics (aberrations in chromosome 1, 3, 6, and 8 and mutations in BRCA1-associated protein 1, BAP1, or the splicing factor SF3B1dd) [38, 39, 40, 41, 42] and gene expression profile (class 2) [43, 44, 45, 46] in determining prognosis in uveal melanoma. Additionally, other recognised clinical and histopathological negative predictive factors include ciliary body location, diffuse tumour configuration, extraocular extension, epitheloid cell type, and advanced staging [47, 48, 49, 50, 51, 52, 53, 54, 55, 56]. In keeping with some of these recognised negative predictive factors, patients in our study with large tumours treated with enucleation, epitheloid histology, and chromosome 3 abnormalities had a worse prognosis (Table 4). Additionally, those with advanced staging and extrascleral extension also had a worse outcome. It is also worth noting that the survival difference noted due to primary treatment received was not as a result of superior efficacy of radiation over enucleation but rather due to patient selection according to tumour size at presentation.
This is the first study to describe the epidemiology of uveal melanoma in Ireland since the establishment of a dedicated ocular oncology service in 2010. It is important to note that due to the limited follow-up in our 5-year group, we did not report on 5-year survival, hence this is a limitation of this paper. Despite this, our data offers considerable insight into the incidence and survival in this cohort of patients.
Despite the numerous advances in treatment of uveal melanoma, survival will ultimately not be improved without significant advances in systemic treatments. Fortunately, systemic treatments continue to evolve and hopefully in time will effectively improve OS for uveal melanoma patients in Ireland and worldwide.
Statement of Ethics
Ethical approval was granted for this study from the Ethics and Medical Research Committee of the Royal Victoria Eye and Ear Hospital, Dublin.
Disclosure Statement
None of the authors have any proprietary interests or conflicts of interest related to this submission. This submission has not been published anywhere previously and is not simultaneously being considered for any other publication.
Funding Sources
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Acknowledgements
The support by Prof. Heinrich Heimann, Royal Liverpool University Hospital, UK and Dr. Ernie Marshall, Clatterbridge Cancer Centre, UK is gratefully acknowledged.
References
- 1.Shields CL, Kels JG, Shields JA. Melanoma of the eye: revealing hidden secrets, one at a time. Clin Dermatol. 2015 Mar-Apr;33((2)):183–96. doi: 10.1016/j.clindermatol.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Virgili G, Gatta G, Ciccolallo L, Capocaccia R, Biggeri A, Crocetti E, et al. EUROCARE Working Group Incidence of uveal melanoma in Europe. Ophthalmology. 2007 Dec;114((12)):2309–15. doi: 10.1016/j.ophtha.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 3.Singh AD, Turell ME, Topham AK. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 2011 Sep;118((9)):1881–5. doi: 10.1016/j.ophtha.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 4.Park SJ, Oh CM, Kim BW, Woo SJ, Cho H, Park KH. Nationwide Incidence of Ocular Melanoma in South Korea by Using the National Cancer Registry Database (1999-2011) Invest Ophthalmol Vis Sci. 2015 Jul;56((8)):4719–24. doi: 10.1167/iovs.15-16532. [DOI] [PubMed] [Google Scholar]
- 5.Virgili G, Gatta G, Ciccolallo L, Capocaccia R, Biggeri A, Crocetti E, et al. EUROCARE Working Group Survival in patients with uveal melanoma in Europe. Arch Ophthalmol. 2008 Oct;126((10)):1413–8. doi: 10.1001/archopht.126.10.1413. [DOI] [PubMed] [Google Scholar]
- 6.Cancer Staging Manual AJ, editor. New York: Springer; 2010. 7th. [Google Scholar]
- 7.International Validation of the American Joint Committee on Cancer's 7th Edition Classification of Uveal Melanoma JAMA ophthalmology. 2015;133:376–383. doi: 10.1001/jamaophthalmol.2014.5395. [DOI] [PubMed] [Google Scholar]
- 8.Kujala E, Damato B, Coupland SE, Desjardins L, Bechrakis NE, Grange JD, et al. Staging of ciliary body and choroidal melanomas based on anatomic extent. J Clin Oncol. 2013 Aug;31((22)):2825–31. doi: 10.1200/JCO.2012.45.2771. [DOI] [PubMed] [Google Scholar]
- 9.Sagoo MS, Shields CL, Mashayekhi A, Freire J, Emrich J, Reiff J, Komarnicky L, Shields JA. Plaque radiotherapy for juxtapapillary choroidal melanoma overhanging the optic disc in 141 consecutive patients. Archives of ophthalmology (Chicago, Ill : 1960) 2008;126:1515–1522. doi: 10.1001/archopht.126.11.1515. [DOI] [PubMed] [Google Scholar]
- 10.Guénel P, Laforest L, Cyr D, Févotte J, Sabroe S, Dufour C, et al. Occupational risk factors, ultraviolet radiation, and ocular melanoma: a case-control study in France. Cancer Causes Control. 2001 Jun;12((5)):451–9. doi: 10.1023/a:1011271420974. [DOI] [PubMed] [Google Scholar]
- 11.Lutz JM, Cree I, Sabroe S, Kvist TK, Clausen LB, Afonso N, et al. Occupational risks for uveal melanoma results from a case-control study in nine European countries. Cancer Causes Control. 2005 May;16((4)):437–47. doi: 10.1007/s10552-004-5029-6. [DOI] [PubMed] [Google Scholar]
- 12.Relethford JH. Hemispheric difference in human skin color. Am J Phys Anthropol. 1997 Dec;104((4)):449–57. doi: 10.1002/(SICI)1096-8644(199712)104:4<449::AID-AJPA2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 13.Shields CL, Shields JA. Ocular melanoma: relatively rare but requiring respect. Clin Dermatol. 2009 Jan-Feb;27((1)):122–33. doi: 10.1016/j.clindermatol.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Tucker MA, Shields JA, Hartge P, Augsburger J, Hoover RN, Fraumeni JF., Jr Sunlight exposure as risk factor for intraocular malignant melanoma. N Engl J Med. 1985 Sep;313((13)):789–92. doi: 10.1056/NEJM198509263131305. [DOI] [PubMed] [Google Scholar]
- 15.Vajdic CM, Kricker A, Giblin M, McKenzie J, Aitken J, Giles GG, et al. Eye color and cutaneous nevi predict risk of ocular melanoma in Australia. Int J Cancer. 2001 Jun;92((6)):906–12. doi: 10.1002/ijc.1281. [DOI] [PubMed] [Google Scholar]
- 16.Vajdic CM, Kricker A, Giblin M, McKenzie J, Aitken J, Giles GG, et al. Sun exposure predicts risk of ocular melanoma in Australia. Int J Cancer. 2002 Sep;101((2)):175–82. doi: 10.1002/ijc.10579. [DOI] [PubMed] [Google Scholar]
- 17.Singh AD, Topham A. Incidence of uveal melanoma in the United States: 1973-1997. Ophthalmology. 2003 May;110((5)):956–61. doi: 10.1016/S0161-6420(03)00078-2. [DOI] [PubMed] [Google Scholar]
- 18.Abrahamsson M. Malignant melanoma of the choroid and the ciliary body 1956-1975 in Halland and Gothenburg. Incidence, histopathology and prognosis. Acta Ophthalmol (Copenh) 1983 Aug;61((4)):600–10. doi: 10.1111/j.1755-3768.1983.tb04350.x. [DOI] [PubMed] [Google Scholar]
- 19.Bergman L, Seregard S, Nilsson B, Ringborg U, Lundell G, Ragnarsson-Olding B. Incidence of uveal melanoma in Sweden from 1960 to 1998. Invest Ophthalmol Vis Sci. 2002 Aug;43((8)):2579–83. [PubMed] [Google Scholar]
- 20.Davidorf FH, Knupp JA. Epidemiology of ocular melanoma. Incidence and geographic relationship in Ohio (1967-1977) Ohio State Med J. 1979 Sep;75((9)):561–4. [PubMed] [Google Scholar]
- 21.Kaneko A. [Malignant ophthalmic tumors] Nihon Rinsho. 1993 Jan;51(Suppl):1013–20. [PubMed] [Google Scholar]
- 22.Lommatzsch PK, Staneczek W, Bernt H. [Epidemiologic study of new cases of intraocular tumors in East Germany 1961-1980] Klin Monatsbl Augenheilkd. 1985 Dec;187((6)):487–92. doi: 10.1055/s-2008-1054382. [DOI] [PubMed] [Google Scholar]
- 23.Mork T. Malignant neoplasms of the eye in Norway. Incidence, treatment and prognosis. Acta Ophthalmol (Copenh) 1961;39((5)):824–31. doi: 10.1111/j.1755-3768.1961.tb07747.x. [DOI] [PubMed] [Google Scholar]
- 24.Strickland D, Lee JA. Melanomas of eye: stability of rates. Am J Epidemiol. 1981 Jun;113((6)):700–2. doi: 10.1093/oxfordjournals.aje.a113150. [DOI] [PubMed] [Google Scholar]
- 25.Moan J, Cicarma E, Setlow R, Porojnicu AC, Grant WB, Juzeniene A. Time trends and latitude dependence of uveal and cutaneous malignant melanoma induced by solar radiation. Dermatoendocrinol. 2010 Jan;2((1)):3–8. doi: 10.4161/derm.2.1.11745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nichols EE, Richmond A, Daniels AB. Disparities in Uveal Melanoma: patient Characteristics. Semin Ophthalmol. 2016;31((4)):296–303. doi: 10.3109/08820538.2016.1154176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Damato BE, Coupland SE. Differences in uveal melanomas between men and women from the British Isles. Eye (Lond) 2012 Feb;26((2)):292–9. doi: 10.1038/eye.2011.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zloto O, Pe'er J, Frenkel S. Gender differences in clinical presentation and prognosis of uveal melanoma. Invest Ophthalmol Vis Sci. 2013 Jan;54((1)):652–6. doi: 10.1167/iovs.12-10365. [DOI] [PubMed] [Google Scholar]
- 29.Kujala E, Mäkitie T, Kivelä T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2003 Nov;44((11)):4651–9. doi: 10.1167/iovs.03-0538. [DOI] [PubMed] [Google Scholar]
- 30.The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V Twelve-year mortality rates and prognostic factors: COMS report No. 28. Archives of ophthalmology (Chicago, Ill : 1960) 2006;124:1684–1693. doi: 10.1001/archopht.124.12.1684. [DOI] [PubMed] [Google Scholar]
- 31.Diener-West M, Hawkins BS, Markowitz JA, Schachat AP. A review of mortality from choroidal melanoma. II. A meta-analysis of 5-year mortality rates following enucleation, 1966 through 1988. Archives of ophthalmology (Chicago, Ill : 1960) 1992;110:245–250. doi: 10.1001/archopht.1992.01080140101036. [DOI] [PubMed] [Google Scholar]
- 32.Diener-West M, Reynolds SM, Agugliaro DJ, Caldwell R, Cumming K, Earle JD, et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Archives of ophthalmology (Chicago, Ill : 1960) 2005;123:1639–1643. doi: 10.1001/archopht.123.12.1639. [DOI] [PubMed] [Google Scholar]
- 33.Gamel JW, McLean IW, McCurdy JB. Biologic distinctions between cure and time to death in 2892 patients with intraocular melanoma. Cancer. 1993 Apr;71((7)):2299–305. doi: 10.1002/1097-0142(19930401)71:7<2299::aid-cncr2820710721>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 34.Edelhauser G, Schicher N, Berzaczy D, Beitzke D, Höeller C, Lammer J, et al. Fotemustine chemoembolization of hepatic metastases from uveal melanoma: a retrospective single-center analysis. AJR Am J Roentgenol. 2012 Dec;199((6)):1387–92. doi: 10.2214/AJR.11.7748. [DOI] [PubMed] [Google Scholar]
- 35.Farolfi A, Ridolfi L, Guidoboni M, Milandri C, Calzolari F, Scarpi E, et al. Liver metastases from melanoma: hepatic intra-arterial chemotherapy. A retrospective study. J Chemother. 2011 Oct;23((5)):300–5. doi: 10.1179/joc.2011.23.5.300. [DOI] [PubMed] [Google Scholar]
- 36.Mariani P, Piperno-Neumann S, Servois V, Berry MG, Dorval T, Plancher C, Couturier J, Levy-Gabriel C, Lumbroso-Le Rouic L, Desjardins L, Salmon RJ. Surgical management of liver metastases from uveal melanoma: 16 years' experience at the Institut Curie. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2009;35:1192–1197. doi: 10.1016/j.ejso.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto A, Chervoneva I, Sullivan KL, Eschelman DJ, Gonsalves CF, Mastrangelo MJ, et al. High-dose immunoembolization: survival benefit in patients with hepatic metastases from uveal melanoma. Radiology. 2009 Jul;252((1)):290–8. doi: 10.1148/radiol.2521081252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amaro A, Gangemi R, Piaggio F, Angelini G, Barisione G, Ferrini S, et al. The biology of uveal melanoma. Cancer Metastasis Rev. 2017 Mar;36((1)):109–40. doi: 10.1007/s10555-017-9663-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koopmans AE, Verdijk RM, Brouwer RW, van den Bosch TP, van den Berg MM, Vaarwater J, et al. van IWF, Kilic E, de Klein A: Clinical significance of immunohistochemistry for detection of BAP1 mutations in uveal melanoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology. Inc. 2014;27:1321–30. doi: 10.1038/modpathol.2014.43. [DOI] [PubMed] [Google Scholar]
- 40.Kilic E, Naus NC, van Gils W, Klaver CC, van Til ME, Verbiest MM, et al. Concurrent loss of chromosome arm 1p and chromosome 3 predicts a decreased disease-free survival in uveal melanoma patients. Invest Ophthalmol Vis Sci. 2005 Jul;46((7)):2253–7. doi: 10.1167/iovs.04-1460. [DOI] [PubMed] [Google Scholar]
- 41.Kilic E, van Gils W, Lodder E, Beverloo HB, van Til ME, Mooy CM, et al. Clinical and cytogenetic analyses in uveal melanoma. Invest Ophthalmol Vis Sci. 2006 Sep;47((9)):3703–7. doi: 10.1167/iovs.06-0101. [DOI] [PubMed] [Google Scholar]
- 42.White VA, Chambers JD, Courtright PD, Chang WY, Horsman DE. Correlation of cytogenetic abnormalities with the outcome of patients with uveal melanoma. Cancer. 1998 Jul;83((2)):354–9. [PubMed] [Google Scholar]
- 43.Onken MD, Worley LA, Ehlers JP, Harbour JW. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res. 2004 Oct;64((20)):7205–9. doi: 10.1158/0008-5472.CAN-04-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Onken MD, Worley LA, Tuscan MD, Harbour JW. An accurate, clinically feasible multi-gene expression assay for predicting metastasis in uveal melanoma. J Mol Diagn. 2010 Jul;12((4)):461–8. doi: 10.2353/jmoldx.2010.090220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tschentscher F, Hüsing J, Hölter T, Kruse E, Dresen IG, Jöckel KH, et al. Tumor classification based on gene expression profiling shows that uveal melanomas with and without monosomy 3 represent two distinct entities. Cancer Res. 2003 May;63((10)):2578–84. [PubMed] [Google Scholar]
- 46.van Gils W, Lodder EM, Mensink HW, Kiliç E, Naus NC, Brüggenwirth HT, et al. Gene expression profiling in uveal melanoma: two regions on 3p related to prognosis. Invest Ophthalmol Vis Sci. 2008 Oct;49((10)):4254–62. doi: 10.1167/iovs.08-2033. [DOI] [PubMed] [Google Scholar]
- 47.Affeldt JC, Minckler DS, Azen SP, Yeh L. Prognosis in uveal melanoma with extrascleral extension. Archives of ophthalmology (Chicago, Ill : 1960) 1980;98:1975–1979. doi: 10.1001/archopht.1980.01020040827006. [DOI] [PubMed] [Google Scholar]
- 48.Shields CL, Furuta M, Thangappan A, Nagori S, Mashayekhi A, Lally DR, Kelly CC, Rudich DS, Nagori AV, Wakade OA, Mehta S, Forte L, Long A, Dellacava EF, Kaplan B, Shields JA. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Archives of ophthalmology (Chicago, Ill : 1960) 2009;127:989–998. doi: 10.1001/archophthalmol.2009.208. [DOI] [PubMed] [Google Scholar]
- 49.Shields CL, Kaliki S, Furuta M, Fulco E, Alarcon C, Shields JA. American Joint Committee on Cancer classification of posterior uveal melanoma (tumor size category) predicts prognosis in 7731 patients. Ophthalmology. 2013 Oct;120((10)):2066–71. doi: 10.1016/j.ophtha.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 50.Coupland SE, Campbell I, Damato B. Routes of extraocular extension of uveal melanoma: risk factors and influence on survival probability. Ophthalmology. 2008 Oct;115((10)):1778–85. doi: 10.1016/j.ophtha.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 51.Damato B, Duke C, Coupland SE, Hiscott P, Smith PA, Campbell I, et al. Cytogenetics of uveal melanoma: a 7-year clinical experience. Ophthalmology. 2007 Oct;114((10)):1925–31. doi: 10.1016/j.ophtha.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 52.de la Cruz PO, Jr, Specht CS, McLean IW. Lymphocytic infiltration in uveal malignant melanoma. Cancer. 1990 Jan;65((1)):112–5. doi: 10.1002/1097-0142(19900101)65:1<112::aid-cncr2820650123>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 53.Folberg R, Pe'er J, Gruman LM, Woolson RF, Jeng G, Montague PR, et al. The morphologic characteristics of tumor blood vessels as a marker of tumor progression in primary human uveal melanoma: a matched case-control study. Hum Pathol. 1992 Nov;23((11)):1298–305. doi: 10.1016/0046-8177(92)90299-i. [DOI] [PubMed] [Google Scholar]
- 54.Foss AJ, Alexander RA, Jefferies LW, Hungerford JL, Harris AL, Lightman S. Microvessel count predicts survival in uveal melanoma. Cancer Res. 1996 Jul;56((13)):2900–3. [PubMed] [Google Scholar]
- 55.McLean MJ, Foster WD, Zimmerman LE. Prognostic factors in small malignant melanomas of choroid and ciliary body. Archives of ophthalmology (Chicago, Ill : 1960) 1977;95:48–58. doi: 10.1001/archopht.1977.04450010050004. [DOI] [PubMed] [Google Scholar]
- 56.Seregard S, Kock E. Prognostic indicators following enucleation for posterior uveal melanoma. A multivariate analysis of long-term survival with minimized loss to follow-up. Acta Ophthalmol Scand. 1995 Aug;73((4)):340–4. doi: 10.1111/j.1600-0420.1995.tb00039.x. [DOI] [PubMed] [Google Scholar]