Abstract
Most blood vessels are surrounded by a variable amount of adventitial adipose tissue, perivascular adipose tissue (PVAT), which was originally thought to provide mechanical support for the vessel. It is now known that PVAT secretes a number of bioactive substances including vascular endothelial growth factor, tumor necrosis factor-alpha (TNF-α), leptin, adiponectin, insulin-like growth factor, interleukin-6, plasminogen activator substance, resistin and angiotensinogen. Several studies have shown that PVAT significantly modulated vascular smooth muscle contractions induced by a variety of agonists and electrical stimulation by releasing adipocyte-derived relaxing (ADRF) and contracting factors. The identity of ADRF is not yet known. However, several vasodilators have been suggested including adiponectin, angiotensin 1-7, hydrogen sulfide and methyl palmitate. The anticontractile effect of PVAT is mediated through the activation of potassium channels since it is abrogated by inhibiting potassium channels. Hypertension is characterized by a reduction in the size and amount of PVAT and this is associated with the attenuated anticontractile effect of PVAT in hypertension. However, since a reduction in size and amount of PVAT and the attenuated anticontractile effect of PVAT were already evident in prehypertensive rats with no evidence of impaired release of ADRF, there is the possibility that the anticontractile effect of PVAT was not directly related to an altered function of the adipocytes per se. Hypertension is characterized by low-grade inflammation and infiltration of macrophages. One of the adipokines secreted by macrophages is TNF-α. It has been shown that exogenously administered TNF-α enhanced agonist-induced contraction of a variety of vascular smooth muscle preparations and reduced endothelium-dependent relaxation. Other procontractile factors released by the PVAT include angiotensin II and superoxide. It is therefore possible that the loss could be due to an increased amount of these proinflammatory and procontractile factors. More studies are definitely required to confirm this.
Key Words: Perivascular adipose tissue, Vascular smooth muscle, Anticontractile effect, Adipocyte-derived relaxing factor, Macrophages, Hypertension
Introduction
It is well established that reactivity of the vascular smooth muscle to vasoconstrictors is affected by a variety of factors including age, gender, affinity, temperature, receptor reserve and pathological states [1,2,3,4,5,6]. Even though earlier studies have shown that the vascular endothelium generates a substance, prostacyclin, with vasodilator properties [7,8], the fact that endothelium played a significant role in modulating basal vascular smooth muscle tone and agonist-induced responses through the release of nitric oxide and endothelium-derived contracting and hyperpolarizing factors was not discovered until the 1980s, led by the pioneering work of Furchgott and Zawadski [9]. It is now known that the endothelium modulates vascular smooth muscle tone via the release of a number of vasoactive factors including nitric oxide, prostacyclin, yet-to-be-identified endothelium-derived hyperpolarizing and contracting factors, thromboxane and endothelins.
Another source of factors regulating vascular smooth muscle tone has emerged within the past 20 years and is still evolving. With the exception of cerebral vessels, vascular smooth muscles are surrounded by a variable amount of adipose tissue called perivascular adipose tissue (PVAT) because of its location around blood vessels. The PVAT is made up of brown adipose tissue (BAT) and white adipose tissue (WAT). Both BAT and WAT are innervated by the sympathetic nervous system [10,11,12,13,14,15]. BAT is highly vascularized, metabolically active and associated with energy production via nonshivering thermogenesis. In contrast, WAT is less vascularized and is generally believed to be a storage organ for lipids [16]. The relative distribution of WAT and BAT varies between vessels. PVAT in the abdominal aorta and mesenteric and femoral artery is made up of WAT while PVAT surrounding the aorta is mixed, comprising of both BAT and WAT [17]. PVAT was originally thought to provide a mechanical support for the blood vessels. However it is now known that it functions as a paracrine and endocrine organ secreting a number of bioactive substances including vascular endothelial growth factor, tumor necrosis factor-alpha (TNF-α), leptin, adiponectin, insulin-like growth factor, interleukin-6, plasminogen activator substance, resistin and angiotensinogen [16]. These substances regulate adipocyte metabolism and other cellular processes including vascular smooth muscle tone.
Anticontractile Effect of PVAT
Based on the traditional belief that the primary function of PVAT was to provide mechanical support for the blood vessels, vascular reactivity studies were usually carried out using vessel segments with no attached adipose tissue. The rationale was that the PVAT could affect reactivity either by metabolizing the agonist or preventing access of the agonist to the adventitia layer. Interestingly, the demonstration that the mRNA for angiotensinogen (possibly suggesting local generation of angiotensin II) is expressed [18,19,20] in the PVAT coupled with the fact that there is no barrier between PVAT and the adventitial layer did not change this traditional belief about PVAT or the way vascular reactivity was studied. The first evidence suggesting modulation of vascular smooth muscle tone by PVAT was provided by Soltis and Cassis [21], who reported that PVAT significantly attenuated noradrenaline-induced contraction of the rat aorta. Since PVAT had no effect on contractions induced by phenylephrine and KCl, it has been suggested that the anticontractile effect of PVAT may be due to the uptake of noradrenaline into adrenergic nerves in the fat tissue [21]. However, while confirming the anticontractile effect of PVAT against phenylephrine-induced contractions, Lohn et al. [22] also observed an anticontractile effect of PVAT against 5-HT and angiotensin II as agonists. This observation would suggest that uptake into noradrenaline-containing nerves was not a factor in the anticontractile effect of PVAT since angiotensin II is not a substrate for the uptake process. These early observations of the anticontractile effects of PVAT have been confirmed by several researchers in a variety of vascular smooth muscle preparations [[23,24,25,26,27,28,29,30,31]; Oriowo and Oommen, unpubl. data]. PVAT is also not limited to arterial smooth muscles, as its anticontractile effect has been demonstrated in ring segments of the vena cava [32]. The anticontractile effect of PVAT is not a nonspecific event, since not all agonists are uniformly affected even in the same arterial preparation. Thus, noradrenaline-induced but not phenylephrine- or KCl-induced contractions were attenuated by PVAT in the rat aorta [21] while in the rat mesenteric artery, PVAT attenuated ET-1- and 5-HT-induced but not U 46619-induced contractions [25]. Inhibition of ureteral motility by periureteral adipose tissue has recently been reported by Killian and Bund [33].
Mediators of the Anticontractile Effect of PVAT
Lohn et al. [22] were the first to suggest that the anticontractile effect of PVAT involved the release of a vasodilator (ADRF) from the PVAT, which then diffuses into the adventitia to evoke a relaxation (fig. 1). Using a bioassay system, these researchers demonstrated that the transfer of a small volume of solution from an artery segment with intact PVAT into a bath containing a precontracted artery segment without PVAT resulted in relaxation, indicating the release of a vasorelaxing factor from the artery segment with intact PVAT. This has subsequently been confirmed by others [25,34,35,36]. The identity of ADRF is not yet known. However, it is known that:
Fig. 1.
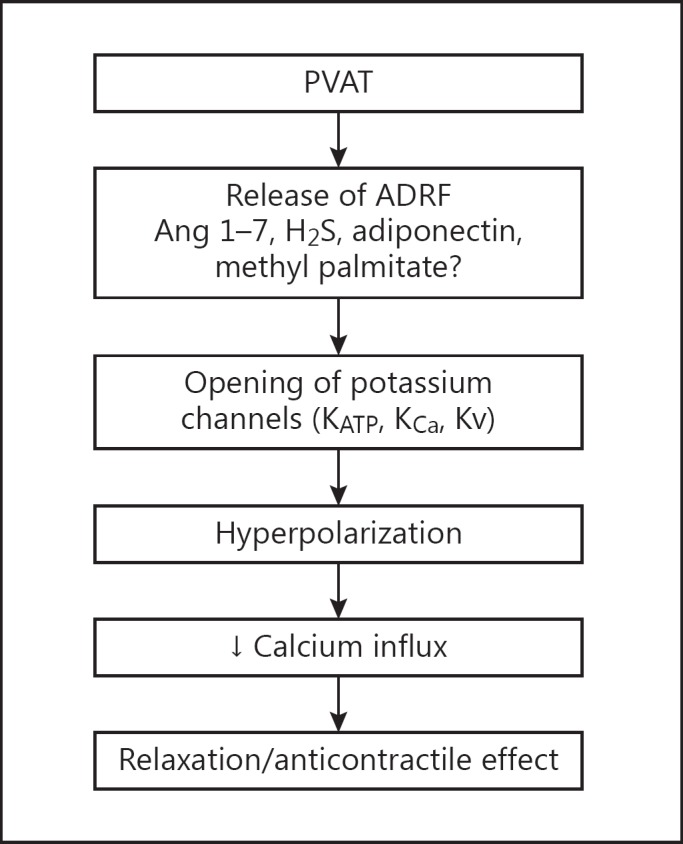
Schematic representation of the effect of PVAT on the vascular smooth muscle. PVAT releases a relaxing factor which activates potassium channels on the vascular smooth muscle membrane leading to hyperpolarization, reduced influx of calcium and relaxation. Ang = Angiotensin; H2S = hydrogen sulfide.
Release of ADRF is calcium dependent.
ADRF produces relaxation by endothelium-dependent (via nitric oxide) and endothelium-independent mechanisms involving activation of potassium channels [25].
The effect of ADRF can be attenuated by genistein, a tyrosine kinase inhibitor, suggesting mediation via tyrosine kinase [22].
Adiponectin is one of the vasodilators released by PVAT [36,37]. Others include angiotensin 1-7 [26,31,38], methyl palmitate [39] and hydrogen sulfide [40,41]. Available evidence suggests that ADRF may vary between tissues [26,31,36,37,38,39,40,41].
PVAT and the Regulation of Resting Vascular Smooth Muscle Tone
Many studies have shown that activation of voltage-dependent potassium channels (Kv channels) modulates basal vascular smooth muscle tone. Since the anticontractile effect of PVAT is mediated via activation of potassium channels, there is the possibility that PVAT, through activation of these channels, could play a role in regulating resting vascular smooth muscle tone. In the mesenteric artery, Verlohren et al. [25] have shown that the resting membrane potential was hyperpolarized and that the hyperpolarization was more in artery segments with intact PVAT than in those without PVAT, suggesting a role for PVAT in regulating resting vascular smooth muscle tone. Abolition of the difference in membrane potential between artery segments with and without PVAT by 4-aminopyridine (4-AP) would confirm a role for Kv channels in PVAT-induced regulation of membrane potential in this artery. Using the increase in perfusion pressure in the presence of 4-AP as an index of regulation of vascular smooth muscle tone, Galvez et al. [28] concluded that PVAT was involved in regulating basal artery tone since 4-AP induced a greater increase in perfusion pressure in a mesenteric vascular bed with intact PVAT. This has been confirmed by Galvez-Prieto et al. [42] using ring segments of the superior mesenteric artery. Zavaritskaya et al. [43] have recently reported more hyperpolarized resting membrane potential in the rat gracilis artery with intact PVAT that was abolished in the presence of XE 991, a selective inhibitor of Kv7 potassium channels. XE 991 produced a depolarization that was accompanied by an increase in basal smooth muscle tone. 4-AP increased resting tension (contraction) of the rat aorta in artery segments with and without intact PVAT. The contraction was significantly greater in artery segments with intact PVAT (fig. 2), confirming a role for PVAT in regulating Kv potassium channel activity and resting tone of the rat aorta [Oriowo and Oommen, unpubl. data]. It would therefore seem that PVAT, through the release of ADRF, is involved in regulating resting membrane potential and vascular smooth muscle tone by a mechanism involving the activation of Kv7 channels.
Fig. 2.
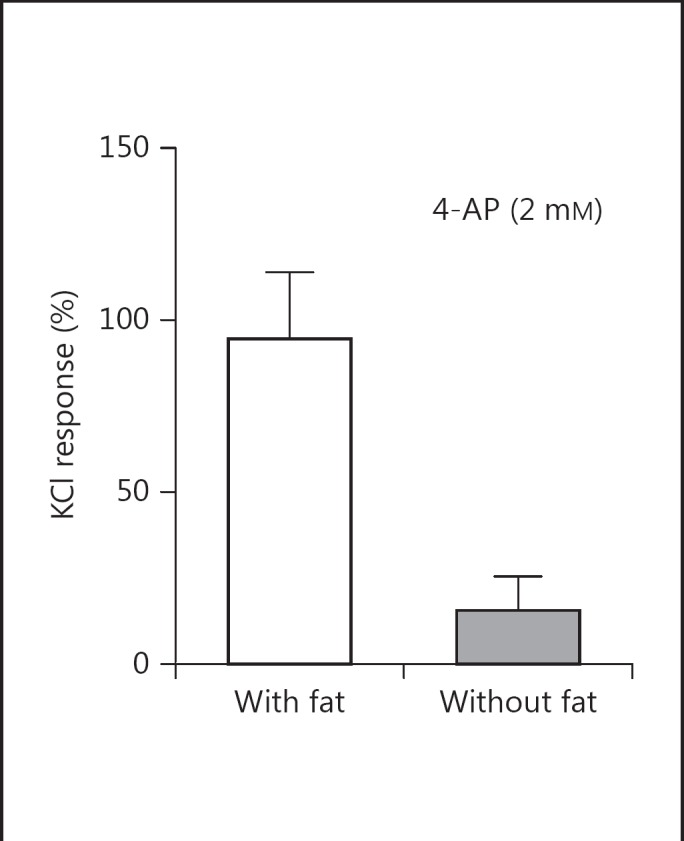
Bar chart showing 4-AP-induced contractions of rat thoracic aorta ring segments with and without attached PVAT.
Role of Potassium Channel Activation in the Anticontractile Effect of PVAT
In addition to regulating resting vascular smooth muscle tone, activation of potassium channels has also been shown to modulate agonist-induced contractions in a variety of vascular smooth muscles. When activated, these channels cause hyperpolarization accompanied by reduced influx of extracellular calcium into the smooth muscle cells, leading to reduced contractions (fig. 1). Earlier studies have shown that the anticontractile effect of PVAT was attenuated by increasing potassium concentration to between 60 and 80 mM. This would suggest a role for potassium channel activation in the anticontractile effect of PVAT [22,25]. However, available evidence (described below) tends to suggest that the type of potassium channels involved in the anticontractile effect varies with the arterial preparation under investigation. In the rat aorta, blockade of ATP-dependent potassium channels (KATP channels) with glibenclamide abolished the anticontractile effect of PVAT [22]. On reexamination, however, it was found that glibenclamide increased the contractile responses to 5-HT similarly in aorta segments with or without PVAT, suggesting that activation of KATP channels may not explain the effect of PVAT on 5-HT-induced contractions of the rat aorta [44]. Oriowo and Oommen [unpubl. data] have observed that blockade of calcium-activated potassium channels with tetraethylammonium enhanced 5-HT-induced contraction of rat aorta segments with or without PVAT and that the enhancement was less in artery segments with intact PVAT, suggesting that tetraethylammonium-sensitive KCa channel activation was not involved in the anticontractile effect of PVAT in the aorta. However, recent studies have shown that an anticontractile effect of PVAT on noradrenaline-induced contractions in small mesenteric mouse arteries was not observed in the presence of BKCa channel inhibitor or in BKCa knockout mice [45]. In addition, it was also observed that PVAT from BKCa knockout mice had no effect on noradrenaline-induced contractions [46]. This would suggest that activation of the BKCa channel was involved in the anticontractile effect of PVAT in small mesenteric mouse arteries, thus indicating that the role of the BKCa channel in the anticontractile effect of PVAT could be vessel and/or species specific.
In the superior mesenteric artery preparation, treatment with 4-AP and 3,4-diaminopyridine (both blockers of Kv channels) abolished the anticontractile effect of PVAT, confirming that the difference in reactivity to 5-HT in artery segments with and without PVAT resulted from activation of Kv channels [25]. A role for these channels in the anticontractile effect of PVAT has also been demonstrated in small mesenteric arteries [45]. Iberiotoxin and tetraethylammonium at concentrations that specifically block KCa channels enhanced 5-HT-induced contractions in artery segments with and without PVAT and did not discriminate between the two types of preparations, indicating that activation of KCa channels modulated 5-HT-induced contractions of the mesenteric artery but was not responsible for the anticontractile effect of PVAT. A recent study [43] in the gracilis muscle artery has confirmed a role for Kv channels in mediating the anticontractile effect of PVAT. These investigators observed that XE 991 and linopirdine (both selective inhibitors of Kv7 channels) abolished the anticontractile effect of PVAT against 5-HT-induced contractions in the gracilis muscle artery, thus confirming the involvement of the Kv7 channel isoform in the anticontractile effect of PVAT [43]. It could therefore be concluded that there is a regional/species variation in the role of potassium channel isoforms in the anticontractile effect of PVAT. This is supported by the observation [26,32] that ADRF release from vessel segments with intact PVAT and which is thought to be angiotensin 1-7 (the proposed transferable factor) relaxed ring segments of the rat aorta and inferior vena cava through activation of the BKCa channel and Kv channels, respectively.
Effect of Hypertension on the Anticontractile Effect of PVAT
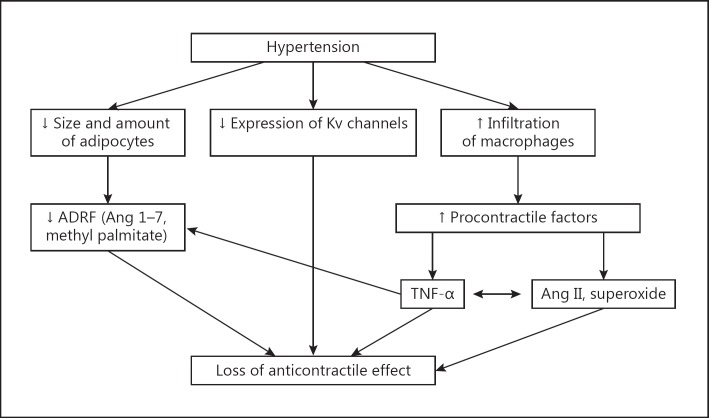
Several studies [23,28,31,38,39,42,45,46,47,48] have examined the effect of hypertension on the anticontractile effect of PVAT. The general agreement in all these studies is that the anticontractile effect of PVAT is attenuated in artery segments from hypertensive rats irrespective of the experimental model used. The mechanism responsible for the loss of anticontractile effect of PVAT is however not yet known. It could be due to impaired release of ADRF resulting from the reduced amount and size of PVAT, reduced expression and function of potassium channels in the adipose tissue or the vascular smooth muscle or increased production of contractile factor(s) (fig. 3).
Fig. 3.
Schematic presentation of the possible mechanisms underlying the loss of anticontractile effect of PVAT in hypertension. Ang = Angiotensin.
Prehypertensive Rats
Galvez-Prieto et al. [42] reported that 5-HT-induced contraction was greater in the mesenteric vascular bed from prehypertensive 4-week-old spontaneously hypertensive rats (SHR) compared with Wistar-Kyoto (WKY) rats. These authors also reported a reduction in the amount and function of PVAT in these animals, leading to the suggestion that a reduction in the amount and function of PVAT preceded the development of hypertension. Even though it would appear that loss of anticontractile activity of PVAT correlated with a reduced amount of PVAT [28,42], no cause and effect relationship has been established. Using the pressor response to 4-AP as an index of ADRF release, Galvez-Prieto et al. [42] observed that the increase in perfusion pressure induced by 4-AP was not different between WKY and SHR, suggesting that basal release of ADRF was not different at this prehypertensive stage. However, since Kv channels are also involved in the relaxing effect of ADRF on vascular smooth muscle, similar 4-AP-induced vasoconstriction of the perfused mesenteric vascular bed in WKY and prehypertensive 4-week-old SHR would also rule out a dysfunction of the Kv7 potassium channels located on the vascular smooth muscle. This would imply that the loss of anticontractile effect of PVAT in the mesenteric vascular bed of prehypertensive SHR was not due to impaired ADRF release or impaired vascular smooth muscle Kv channel function.
Hypertensive Rats
In contrast to no change in the 4-AP-induced increase in perfusion pressure recorded in the mesenteric vascular bed of prehypertensive rats, 4-AP-induced increase in perfusion pressure was significantly reduced in the mesenteric vascular bed from hypertensive rats. This could suggest reduced release of ADRF or impaired expression and function of vascular smooth muscle Kv channels or both. An increased release of contracting factors cannot be ruled out. The reduced anticontractile effect of PVAT in established hypertension has been shown to be associated with a reduced release of vasodilators, leptin [28,47] and methyl palmitate [39], from the PVAT. However, the observation that the direct dilator effect of leptin on angiotensin II-induced vasoconstriction was attenuated in artery segments from SHR [49] would indicate that whatever mechanism mediates leptin-induced vascular smooth muscle relaxation is also impaired in vessels from SHR. This would suggest that the loss of vasodilator response to leptin in hypertension occurs at the level of PVAT and vascular smooth muscle. It has been reported that the loss of anticontractile effect of PVAT in hypertension could be due to impaired functioning of the Kv7 channels [46]. This is consistent with the demonstration of a significant reduction in the expression of Kv channels in SHR [50] and the observation that 4-AP potentiated 5-HT-induced contraction only in WKY artery segments with intact PVAT. These observations are, however, in contrast to those reported by Zavaritskaya et al. [43] in the gracilis artery. These authors showed that the anticontractile effect of PVAT was attenuated in gracilis muscle arteries of SHR even though the inhibitory effects of potassium channel openers (measure of channel function) were similar in artery segments from SHR and Wistar rats. This would probably suggest that the reduced anticontractile effect of PVAT in the gracilis artery from SHR is associated with a reduced release of ADRF but not the expression and function of vascular smooth muscle Kv7 potassium channels. Thus, hypertension is associated with reduced release of ADRF, irrespective of the vascular smooth muscle preparation studied, while its effect on the expression and function of Kv7 potassium channels appeared to be vessel specific.
Infiltration of Macrophages as a Possible Reason for the Loss of Anticontractile Effect of PVAT
It is clearly evident that the loss of anticontractile effect of PVAT in prehypertensive and hypertensive rats appeared to be mediated via different mechanisms [43,46,49]. Even though both stages of hypertension are characterized by a similar reduction in the amount and size of the adipose tissue, the release of ADRF and the function of the membrane potassium channels were impaired in artery segments from hypertensive but not prehypertensive SHR [28,39,42,47]. This raises the question as to what common mechanisms mediate the loss of anticontractile effect of PVAT in these animals. It is being postulated here that the loss of anticontractile effect of PVAT could be due to the release of contractile factors from the PVAT. This is based on the fact that in addition to ADRF, PVAT also produces contracting factors including angiotensin II and superoxide ions. An attenuated anticontractile effect of PVAT is commonly observed in artery segments from hypertensive and obese rats even though these pathological states are characterized by a reduction (hypertension) and increase (obesity) in the amount and size of PVAT. These observations would suggest that the loss of anticontractile effect of PVAT is not dependent on changes in the size and amount of adipocytes per se. Infiltration of macrophages into adipose tissues has been observed in a variety of pathological states including obesity, inflammation and hypoxia, and could be responsible for the loss of anticontractile activity (as described below). In a recent study, Withers et al. [51] concluded that activation of macrophages is responsible for the loss of anticontractile effect of PVAT in inflamed perivascular fat. This was based on the observation that noradrenaline-induced contractions of artery segments isolated from wild-type and macrophage-deficient mice was not different, with or without fat, indicating loss of anticontractile effect of PVAT in the aorta. Vascular inflammation is a prominent feature of hypertension [52,53,54,55] and it has been reported that macrophages accumulate in the vascular wall during hypertension [52,55,56,57]. Wenzel et al. [52] have shown that depletion of monocytes, which are precursors of macrophages, prevented experimentally induced hypertension in mice. This has been confirmed using a chemokine antagonist in deoxycorticosterone/salt hypertension in mice [55]. It is therefore quite possible that the loss of anticontractile effect of PVAT in hypertension could be due to an accumulation of proinflammatory factors released by the macrophages. TNF-α is produced by macrophages and the level is elevated in hypertension [58,59,60,61]. The increase in TNF-α was observed even at the prehypertensive stage [62]. Previous studies [63,64,65] have shown that TNF-α increased agonist-induced contractions in a variety of vascular smooth muscle preparations. Therefore, there is the possibility that the loss of the anticontractile effect of PVAT in hypertension could be due to a release of TNF-α, which then exerts a procontractile effect by a mechanism involving generation of reactive oxygen species and angiotensin II [62,66]. The observations that treatment with TNF-α antagonist reduced angiotensin-induced hypertension [67,68] and that chronic blockade of angiotensin AT1 receptor significantly reduced circulating levels of TNF-α [69] are consistent with the existence of cross-talk between angiotensin II and TNF-α (fig. 3). Increased secretion of TNF-α could also lead to a reduction in the secretion of vasodilator cytokines by the PVAT. Hajri et al. [66] have reported that TNF-α reduced the secretion of adiponectin. A similar observation was reported by Fain et al. [70]. TNF-α has also been reported to interfere with nitric oxide-mediated vasodilation [64,71,72,73].
Summary of Experimental Findings
PVAT, in addition to providing mechanical support for the blood vessels, regulates vascular smooth muscle tone through the release of ADRF and adipocyte-derived contracting factors. The identity of ADRF is unknown, but its release is calcium dependent and involves activation of potassium channels. The anticontractile effect of PVAT has been demonstrated in arteries (conduit and resistance) and veins. This anticontractile effect is abolished by blocking potassium channels (mostly voltage-gated channels). Hypertension is associated with an impaired anticontractile effect of PVAT, suggesting a role for PVAT in the pathogenesis of hypertension. Studies in SHR have shown that the loss of anticontractile effect of PVAT is associated with a reduction in the size and amount of PVAT. However, the fact that the loss of anticontractile effect of PVAT was already present in prehypertensive SHR at a time when no reduction in the release of ADRF could be demonstrated would suggest that altered size and amount of the adipocytes per se was not responsible for the impaired anticontractile effect of PVAT in hypertension. More studies are needed to examine the role of adipocytokines released by macrophages that have been shown to accumulate in the PVAT in hypertension.
Clinical Perspectives
PVAT is now well recognized as a modulator of vascular smooth muscle tone and its anticontractile effect has been demonstrated in both arteries and veins. This anticontractile effect of PVAT is attenuated in hypertension, a condition that is associated with remodeling of the smooth muscle and increased peripheral vascular resistance. The exact mechanism underlying the loss of anticontractile effect is not known. Recent studies [51,52,53,54,55,56,57] have shown that hypertension is characterized by a low-grade inflammation associated with infiltration of the adipose tissue by macrophages and an increase in the release of TNF-α, angiotensin II and reactive oxygen species. It has been reported that cross-talk exists between the renin/angiotensin system and TNF-α release. Thus, treatment with TNF-α antagonist reduced angiotensin-induced hypertension while chronic blockade of angiotensin AT1 receptor significantly reduced circulating levels of TNF-α. Treatment with ACE inhibitors and AT1 receptor antagonists is associated with increased circulating levels of angiotensin 1-7 [74]. Recent studies [75,76] have shown that angiotensin 1-7 decreased the production of proinflammatory cytokines by the adipose tissue. It is therefore possible that the increase in adipose TNF-α levels in hypertension could be due to reduced release of angiotensin 1-7, which has been reported to occur in hypertension. Increased secretion of TNF-α could also lead to a reduction in the secretion of vasodilators adiponectin, nitric oxide and angiotensin 1-7 by PVAT. Thus PVAT would play a significant role in the pathogenesis of hypertension by increasing the release of procontractile factors while at the same time reducing the generation of vasodilators. In addition, these studies would suggest that treatment with ACE inhibitors and AT1 receptor antagonists would prevent the inflammation and restore the balance between vasoconstrictor and vasodilator factors produced by PVAT and hence its anticontractile effect.
Disclosure Statement
The author declares no conflict of interest.
Acknowledgments
This study was supported by a grant (MR 01/12) from the Research Sector, Kuwait University, Kuwait. The author is grateful to Mrs. Elsie Oommen for her excellent technical assistance and to Professor Sam Kombiam and Professor Charles Ezeamuzie for their helpful comments.
References
- 1.Dyer DC. Evidence for differences in alpha-adrenergic receptor affinity in stress susceptible swine. Experientia. 1982;38:1343–1344. [Google Scholar]
- 2.Flavahan NA, Vanhoutte PM. Effect of cooling on alpha-1 and alpha-2 adrenergic responses in canine saphenous and femoral veins. J Pharmacol Exp Ther. 1986;238:139–147. [PubMed] [Google Scholar]
- 3.Stevens MJ, Moulds RF. Factors determining α-adrenoceptor-mediated responses of human digital arteries and metacarpal veins. Clin Sci. 1985;68:315–335. doi: 10.1042/cs068s031. [DOI] [PubMed] [Google Scholar]
- 4.Chu ZM, Beilin LJ. Mechanisms of vasodilatation in pregnancy: studies of the role of prostaglandins and nitric oxide in changes of vascular reactivity in the in situ blood perfused mesentery of pregnant rats. Br J Pharmacol. 1993;109:322–329. doi: 10.1111/j.1476-5381.1993.tb13573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oriowo MA, Bevan JA, Bevan RD. Variation in sensitivity of six cat and rat arteries to norepinephrine can be related to differences in agonist affinity and receptor reserve. J Pharm Exp Ther. 1989;251:16–20. [PubMed] [Google Scholar]
- 6.Oriowo MA, Chandrasekhar B, Kadavil EA. α1-Adrenoceptor subtypes mediating noradrenaline-induced contraction of pulmonary artery from pulmonary hypertensive rats. Eur J Pharmacol. 2003;482:255–263. doi: 10.1016/j.ejphar.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Herman AG, Moncada S, Vane JR. Formation of prostacyclin (PGl2) by different layers of the arterial wall. Arch Int Pharmacodyn Ther. 1977;227:162–163. [PubMed] [Google Scholar]
- 8.Moncada S, Herman AG, Higgs EA, et al. Differential formation of prostacyclin (PGX or PGI2) by layers of the arterial wall. An explanation for the anti-thrombotic properties of vascular endothelium. Thromb Res. 1977;11:323–344. doi: 10.1016/0049-3848(77)90185-2. [DOI] [PubMed] [Google Scholar]
- 9.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 10.Slavin BG, Ballard KW. Morphological studies on the adrenergic innervation of white adipose tissue. Anat Rec. 1978;191:377–389. doi: 10.1002/ar.1091910310. [DOI] [PubMed] [Google Scholar]
- 11.Ballard K, Malmfors T, Rosell S. Adrenergic innervation and vascular patterns in canine adipose tissue. Microvasc Res. 1974;8:164–171. doi: 10.1016/0026-2862(74)90091-0. [DOI] [PubMed] [Google Scholar]
- 12.Bartness TJ, Song CK. Brain-adipose tissue neural crosstalk. Physiol Behav. 2007;91:343–351. doi: 10.1016/j.physbeh.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowers RR, Festuccia WT, Song CK, Shi H, et al. Sympathetic innervation of white adipose tissue and its regulation of fat cell number. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1167–R1175. doi: 10.1152/ajpregu.00558.2003. [DOI] [PubMed] [Google Scholar]
- 14.Romijn JA, Fliers E. Sympathetic and parasympathetic innervation of adipose tissue: metabolic implications. Curr Opin Clin Nutr Metab Care. 2005;8:440–444. doi: 10.1097/01.mco.0000172586.09762.55. [DOI] [PubMed] [Google Scholar]
- 15.Giordano A, Song CK, Bowers RR, et al. White adipose tissue lacks significant vagal innervation and immunohistochemical evidence of parasympathetic innervation. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1243–R1255. doi: 10.1152/ajpregu.00679.2005. [DOI] [PubMed] [Google Scholar]
- 16.Szasz T, Webb RC. Perivascular adipose tissue: more than just structural support. Clin Sci. 2012;122:1–12. doi: 10.1042/CS20110151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao YJ. Dual modulation of vascular function by perivascular adipose tissue and its potential correlation with adiposity/lipoatrophy-related vascular dysfunction. Curr Pharm Des. 2007;13:2185–2192. doi: 10.2174/138161207781039634. [DOI] [PubMed] [Google Scholar]
- 18.Campbell DJ, Habener JF. Cellular localization of angiotensinogen gene expression in brown adipose tissue and mesentery: quantification of messenger ribonucleic acid abundance using hybridization in situ. Endocrinology. 1987;121:1616–1626. doi: 10.1210/endo-121-5-1616. [DOI] [PubMed] [Google Scholar]
- 19.Cassis LA, Lynch KR, Peach MJ. Localization of angiotensinogen messenger RNA in rat aorta. Circ Res. 1988;62:1259–1262. doi: 10.1161/01.res.62.6.1259. [DOI] [PubMed] [Google Scholar]
- 20.Cassis LA, Saye J, Peach MJ. Location and regulation of rat angiotensinogen messenger RNA. Hypertension. 1988;11:591–596. doi: 10.1161/01.hyp.11.6.591. [DOI] [PubMed] [Google Scholar]
- 21.Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp Hypertens A. 1991;13:277–296. doi: 10.3109/10641969109042063. [DOI] [PubMed] [Google Scholar]
- 22.Lohn M, Dubrovska G, Lauterbach B, et al. Periadventitial fat releases a vascular relaxing factor. FASEB J. 2002;16:1057–1063. doi: 10.1096/fj.02-0024com. [DOI] [PubMed] [Google Scholar]
- 23.Zeng ZH, Zhang ZH, Luo BH, et al. The functional changes of the perivascular adipose tissue in spontaneously hypertensive rats and the effects of atorvastatin therapy. Clin Exp Hypertens. 2009;31:355–363. doi: 10.1080/10641960902977916. [DOI] [PubMed] [Google Scholar]
- 24.Rebolledo A, Rebolledo OR, Marra CA, et al. Early alterations in vascular contractility associated to changes in fatty acid composition and oxidative stress markers in perivascular adipose tissue. Cardiovasc Diabetol. 2010;9:65. doi: 10.1186/1475-2840-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verlohren S, Dubrovska G, Tsang SY, et al. Visceral periadventitial adipose tissue regulates arterial tone of mesenteric arteries. Hypertension. 2004;44:271–276. doi: 10.1161/01.HYP.0000140058.28994.ec. [DOI] [PubMed] [Google Scholar]
- 26.Lee RM, Lu C, Su LY, et al. Endothelium-dependent relaxation factor released by perivascular adipose tissue. J Hypertens. 2009;27:782–790. doi: 10.1097/HJH.0b013e328324ed86. [DOI] [PubMed] [Google Scholar]
- 27.Huang F, Lezama MA, Ontiveros JA, et al. Effect of losartan on vascular function in fructose-fed ears: the role of perivascular adipose tissue. Clin Exp Hypertens. 2010;32:98–104. doi: 10.3109/10641960902993129. [DOI] [PubMed] [Google Scholar]
- 28.Galvez B, de Castro J, Herold D, et al. Perivascular adipose tissue and mesenteric vascular function in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol. 2006;26:1297–1302. doi: 10.1161/01.ATV.0000220381.40739.dd. [DOI] [PubMed] [Google Scholar]
- 29.Gao YJ, Takemori K, Su LY, et al. Perivascular adipose tissue promotes vasoconstriction: the role of superoxide anion. Cardiovasc Res. 2006;71:363–373. doi: 10.1016/j.cardiores.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Gao YJ, Lu C, Su LY, et al. Modulation of vascular function by perivascular adipose tissue: the role of endothelium and hydrogen peroxide. Br J Pharmacol. 2007;151:323–331. doi: 10.1038/sj.bjp.0707228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu C, Su LY, Lee RM, et al. Alterations in perivascular adipose tissue structure and function in hypertension. Eur J Pharmacol. 2011;656:68–73. doi: 10.1016/j.ejphar.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 32.Lu C, Zhao AX, Gao YJ, et al. Modulation of vein function by perivascular adipose tissue. Eur J Pharmacol. 2011;657:111–116. doi: 10.1016/j.ejphar.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 33.Killian LM, Bund SJ. The inhibition of ureteral motility by periureteral adipose tissue. ISRN Urol. 2012;2012:312487. doi: 10.5402/2012/312487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao YJ, Zeng ZH, Teoh K, et al. Perivascular adipose tissue modulates vascular function in the human internal thoracic artery. J Thorac Cardiovasc Surg. 2005;130:1130–1136. doi: 10.1016/j.jtcvs.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 35.Malinowski M, Deja MA, Gołba KS, et al. Perivascular tissue of internal thoracic artery releases potent nitric oxide and prostacyclin-independent anticontractile factor. Eur J Cardiothorac Surg. 2008;33:225–231. doi: 10.1016/j.ejcts.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Greenstein AS, Khavandi K, Withers SB, et al. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation. 2009;119:1661–1670. doi: 10.1161/CIRCULATIONAHA.108.821181. [DOI] [PubMed] [Google Scholar]
- 37.Fesus G, Dubrovska G, Gorzelniak K, et al. Adiponectin is a novel humoral vasodilator. Cardiovasc Res. 2007;75:719–727. doi: 10.1016/j.cardiores.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 38.Lee RM, Bader M, Alenina N, et al. Mas receptors in modulating relaxation induced by perivascular adipose tissue. Life Sci. 2011;89:467–472. doi: 10.1016/j.lfs.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 39.Lee YC, Chang HH, Chiang CL, et al. Role of perivascular adipose tissue-derived methyl palmitate in vascular tone regulation and pathogenesis of hypertension. Circulation. 2011;124:1160–1171. doi: 10.1161/CIRCULATIONAHA.111.027375. [DOI] [PubMed] [Google Scholar]
- 40.Bełtowski J, Jamroz-Wiśniewska A. Modulation of H2S metabolism by statins: a new aspect of cardiovascular pharmacology. Antioxid Redox Signal. 2012;17:81–94. doi: 10.1089/ars.2011.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wojcicka G, Jamroz-Wisniewska A, Atanasova P, et al. Differential effects of statins on endogenous H2S formation in perivascular adipose tissue. Pharmacol Res. 2011;63:68–76. doi: 10.1016/j.phrs.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 42.Galvez-Prieto B, Dubrovska G, Cano MV, et al. A reduction in the amount and anticontractile effect of periadventitial mesenteric adipose tissue precedes hypertension development in spontaneously hypertensive rats. Hypertens Res. 2008;31:1415–1423. doi: 10.1291/hypres.31.1415. [DOI] [PubMed] [Google Scholar]
- 43.Zavaritskaya O, Zhuravleva N, Schleifenbaum J, et al. Role of KCNQ channels in skeletal muscle arteries and periadventitial vascular dysfunction. Hypertension. 2013;61:151–159. doi: 10.1161/HYPERTENSIONAHA.112.197566. [DOI] [PubMed] [Google Scholar]
- 44.Gollasch M. Vasodilator signals from perivascular adipose tissue. Br J Pharmacol. 2012;165:633–642. doi: 10.1111/j.1476-5381.2011.01430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lynch FM, Withers SB, Yao Z, et al. Perivascular adipose tissue-derived adiponectin activates BKCa channels to induce anticontractile responses. Am J Physiol Heart Circ Physiol. 2013;304:H786–H795. doi: 10.1152/ajpheart.00697.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li R, Andersen I, Aleke J, et al. Reduced anti-contractile effect of perivascular adipose tissue on mesenteric small arteries from spontaneously hypertensive rats: role of Kv7 channels. Eur J Pharmacol. 2013;698:310–315. doi: 10.1016/j.ejphar.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 47.Gálvez-Prieto B, Somoza B, Gil-Ortega M, et al. Anticontractile effect of perivascular adipose tissue and leptin are reduced in hypertension. Front Pharmacol. 2012;3:103. doi: 10.3389/fphar.2012.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee RM, Ding L, Lu C, et al. Alteration of perivascular adipose tissue function in angiotensin II-induced hypertension. Can J Physiol Pharmacol. 2009;87:944–953. doi: 10.1139/y09-088. [DOI] [PubMed] [Google Scholar]
- 49.Rodríguez A, Frühbeck G, Gómez-Ambrosi J, et al. The inhibitory effect of leptin on angiotensin II-induced vasoconstriction is blunted in spontaneously hypertensive rats. J Hypertens. 2006;24:1589–1597. doi: 10.1097/01.hjh.0000239295.17636.6e. [DOI] [PubMed] [Google Scholar]
- 50.Jepps TA, Chadha PS, Davis AJ, et al. Downregulation of Kv7.4 channel activity in primary and secondary hypertension. Circulation. 2011;124:602–611. doi: 10.1161/CIRCULATIONAHA.111.032136. [DOI] [PubMed] [Google Scholar]
- 51.Withers SB, Agabiti-Rosci C, Livingstone DM, et al. Macrophage activation is responsible for loss of anticontractile function in inflamed perivascular fat. Arterioscler Thromb Vasc Biol. 2011;31:908–913. doi: 10.1161/ATVBAHA.110.221705. [DOI] [PubMed] [Google Scholar]
- 52.Wenzel P, Knorr M, Kossmann S, et al. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation. 2011;124:1370–1381. doi: 10.1161/CIRCULATIONAHA.111.034470. [DOI] [PubMed] [Google Scholar]
- 53.Guzik TJ, Marvar PJ, Czesnikiewicz-Guzik M, et al. Perivascular adipose tissue as a messenger of the brain-vessel axis: role in vascular inflammation and dysfunction. J Physiol Pharmacol. 2007;58:591–610. [PubMed] [Google Scholar]
- 54.Ebrahimian T, Li MW, Lemarié CA, et al. Mitogen-activated protein kinase-activated protein kinase 2 in angiotensin II-induced inflammation and hypertension: regulation of oxidative stress. Hypertension. 2011;57:245–254. doi: 10.1161/HYPERTENSIONAHA.110.159889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chan CT, Moore JP, Budzyn K, et al. Reversal of vascular macrophage accumulation and hypertension by a CCR2 antagonist in deoxycorticosterone/salt-treated mice. Hypertension. 2012;60:1207–1212. doi: 10.1161/HYPERTENSIONAHA.112.201251. [DOI] [PubMed] [Google Scholar]
- 56.Bush E, Maeda N, Kuziel WA, et al. CC chemokine receptor 2 is required for macrophage infiltration and vascular hypertrophy in angiotensin II-induced hypertension. Hypertension. 2000;36:360–363. doi: 10.1161/01.hyp.36.3.360. [DOI] [PubMed] [Google Scholar]
- 57.Ishibashi M, Hiasa K, Zhao Q, et al. Critical role of monocyte chemoattractant protein-1 receptor CCR2 on monocytes in hypertension-induced vascular inflammation and remodeling. Circ Res. 2004;94:1203–1210. doi: 10.1161/01.RES.0000126924.23467.A3. [DOI] [PubMed] [Google Scholar]
- 58.Ferreri NR, Zhao Y, Takizawa H, et al. Tumor necrosis factor-α-angiotensin interactions and regulation of blood pressure. J Hypertens. 1997;15:1481–1484. doi: 10.1097/00004872-199715120-00016. [DOI] [PubMed] [Google Scholar]
- 59.Bautista LE, Vera LM, Arenas IA, et al. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-α) and essential hypertension. J Hum Hypertens. 2005;19:149–154. doi: 10.1038/sj.jhh.1001785. [DOI] [PubMed] [Google Scholar]
- 60.Sriramula S, Haque M, Majid DS, et al. Involvement of tumor necrosis factor-α in angiotensin II-mediated effects on salt appetite, hypertension, and cardiac hypertrophy. Hypertension. 2008;51:1345–1351. doi: 10.1161/HYPERTENSIONAHA.107.102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Al-Daghri NM, Bindahman LS, Al-Attas OS, et al. Increased circulating ANG II and TNF-α represents important risk factors in obese Saudi adults with hypertension irrespective of diabetic status and BMI. PLoS One. 2012;7:e51255. doi: 10.1371/journal.pone.0051255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mazor R, Itzhaki O, Sela S, et al. Tumor necrosis factor-α: a possible priming agent for the polymorphonuclear leukocyte-reduced nicotinamide-adenine dinucleotide phosphate oxidase in hypertension. Hypertension. 2010;55:353–362. doi: 10.1161/HYPERTENSIONAHA.109.144154. [DOI] [PubMed] [Google Scholar]
- 63.Matsuki T, Duling BR. TNF-α modulates arteriolar reactivity secondary to a change in intimal permeability. Microcirculation. 2000;7:411–418. [PubMed] [Google Scholar]
- 64.Giardina JB, Green GM, Cockrell KL, et al. TNF-α enhances contraction and inhibits endothelial NO-cGMP relaxation in systemic vessels of pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2002;293:R130–R143. doi: 10.1152/ajpregu.00704.2001. [DOI] [PubMed] [Google Scholar]
- 65.Arenas IA, Armstrong SJ, Xu Y, et al. Tumor necrosis factor-α and vascular angiotensin II in estrogen-deficient rats. Hypertension. 2006;48:497–503. doi: 10.1161/01.HYP.0000235865.03528.f1. [DOI] [PubMed] [Google Scholar]
- 66.Hajri T, Tao H, Wattacheril J, et al. Regulation of adiponectin production by insulin: interactions with tumor necrosis factor-α and interleukin-6. Am J Physiol Endocrinol Metab. 2011;300:E350–E360. doi: 10.1152/ajpendo.00307.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guzik TJ, Hoch NE, Brown KA, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sriramula S, Cardinale JP, Francis J. Inhibition of TNF in the brain reverses alterations in RAS components and attenuates angiotensin II-induced hypertension. PLoS One. 2013;8:e63847. doi: 10.1371/journal.pone.0063847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsutamoto T, Wada A, Maeda K, et al. Angiotensin II type 1 receptor antagonist decreases plasma levels of tumor necrosis factor-α, interleukin-6 and soluble adhesion molecules in patients with chronic heart failure. J Am Coll Cardiol. 2000;35:714–721. doi: 10.1016/s0735-1097(99)00594-x. [DOI] [PubMed] [Google Scholar]
- 70.Fain JN, Buehrer B, Tichansky DS, et al. Regulation of adiponectin release and demonstration of adiponectin mRNA as well as release by the non-fat cells of human omental adipose tissue. Int J Obes (Lond) 2008;32:429–435. doi: 10.1038/sj.ijo.0803745. [DOI] [PubMed] [Google Scholar]
- 71.Greenberg S, Xie J, Wang Y, et al. Tumor necrosis factor-alpha inhibits endothelium-dependent relaxation. J Appl Physiol. 1993;74:2394–2403. doi: 10.1152/jappl.1993.74.5.2394. [DOI] [PubMed] [Google Scholar]
- 72.Arenas IA, Armstrong SJ, Xu Y, et al. Chronic tumor necrosis factor-α inhibition enhances NO modulation of vascular function in estrogen-deficient rats. Hypertension. 2005;46:76–81. doi: 10.1161/01.HYP.0000168925.98963.ef. [DOI] [PubMed] [Google Scholar]
- 73.Wimalasundera R, Fexby S, Regan L, et al. Effect of tumour necrosis factor-α and interleukin-1β on endothelium-dependent relaxation in rat mesenteric resistance arteries in vitro. Br J Pharmacol. 2003;138:1285–1294. doi: 10.1038/sj.bjp.0705168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ferrario CM, Jessup J, Gallagher PE, et al. Effects of renin-angiotensin system blockade on renal angiotensin-(1-7) forming enzymes and receptors. Kidney Int. 2005;68:2189–2196. doi: 10.1111/j.1523-1755.2005.00675.x. [DOI] [PubMed] [Google Scholar]
- 75.Giani JF, Muñoz MC, Pons RA, et al. Angiotensin-(1-7) reduces proteinuria and diminishes structural damage in renal tissue of stroke-prone spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2011;300:F272–F282. doi: 10.1152/ajprenal.00278.2010. [DOI] [PubMed] [Google Scholar]
- 76.Santos SH, Fernandes LR, Pereira CS, et al. Increased circulating angiotensin-(1-7) protects white adipose tissue against development of a proinflammatory state stimulated by a high-fat diet. Regul Pept. 2012;178:64–70. doi: 10.1016/j.regpep.2012.06.009. [DOI] [PubMed] [Google Scholar]