Abstract
A review is presented of the mechanical damage suffered by tooth crowns. This has been the subject of much recent research, resulting in a need to revise some of the thinking about the mechanisms involved. Damage is classified here by scale into macro-, meso- and microfracture. The focus is on the outer enamel coat because this is the contact tissue and where most fractures start. Enamel properties appear to be tailored to maximize hardness, but also to prevent fracture. The latter is achieved by the deployment of developmental flaws called enamel tufts. Macrofractures usually appear to initiate as extensions of tufts on the undersurface of the enamel adjacent to the enamel-dentine junction and extend from there into the enamel. Cracks that pass from the tooth surface tend to be deflected by an enamel region of high toughness; if they find the surface again, a chip (mesofracture) is produced. The real protection of the enamel-dentine junction here is the layer of decussating inner enamel. Finally, a novel analysis of mechanical wear (microfracture) suggests that the local toughness of the enamel is very important to its ability to resist tissue loss. Enamel and dentine have contrasting behaviours. Seen on a large scale, dentine is isotropic (behaving similarly in all directions) while enamel is anisotropic, but vice versa on a very small scale. These patterns have implications for anyone studying the fracture behaviour of teeth.
Key Words: Tooth enamel, Tooth fracture, Mechanical damage
Introduction
Tooth crowns are exposed lumps of mineralized tissue that represent the only break in the integument of the body. Their function is to break down food particles. However, in the process of doing this, they themselves can easily be damaged. The outermost dental crown tissue is called enamel. The cause of its loss can be either chemical or mechanical. Since enamel is >90% hydroxyapatite by volume, it is vulnerable to chemical attack from acids, dissolving readily when the oral pH falls below 5.5. The erosion of enamel has become a serious problem in modern human populations [1,2,3,4,5,6,7], being attributed mainly to the consumption of acidic carbonated soda drinks [8]. However, gastric regurgitation [9] is also of increasing importance as a cause, particularly as the incidence of gastro-oesophageal reflux in the population increases with an increasing trend towards obesity [10]. However, the focus of this review is on mechanical damage to the enamel. This can be extensive and takes several forms (fig. 1), which are classified below in terms of the extent of the damage to enamel thickness.
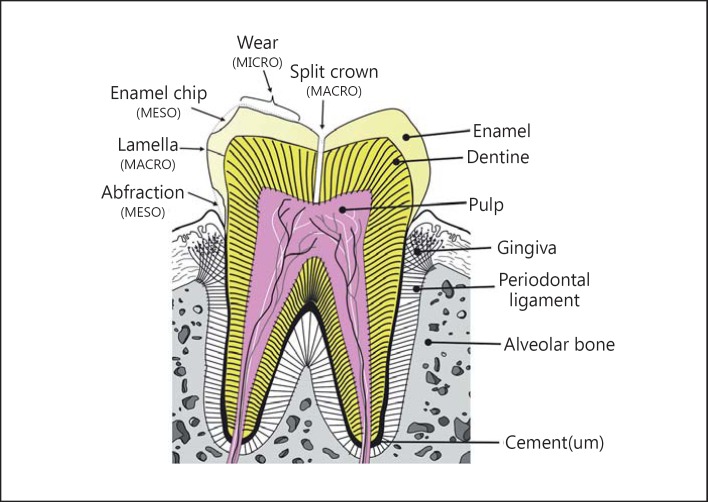
Fig. 1.
Normal tooth structure (right-hand labels) exhibits a thin highly mineralized enamel layer that surfaces the tooth crown. The backbone of the tooth is formed by a tubular bone-like tissue called dentine. A central soft-tissue pulp provides nutritive support for cells that deposit additional dentine internally, thus reducing pulp dimensions. The tooth root is covered by cement (often called cementum). This is also bone-like and bonds the collagen fibres of the periodontal ligament to the tooth. Types of mechanical damage to the enamel (left-hand labels) include split crowns, abfractions, through-enamel faults called lamellae, chipping and wear. ‘Macro', ‘meso' and ‘micro' refer to the fracture scale, i.e. the extent of enamel thickness involvement.
Macrofractures start within the enamel, initiating close to the enamel-dentine junction. These fractures then pass outwards towards the tooth surface. At a minimum, a lamella (a through-enamel crack) results (fig. 1), but extensions of these fractures can result in the loss of large slabs of enamel either underneath the contact point around cusps or near the cervical margin of a crown (fig. 1).
Mesofractures start at the tooth surface from an indentation close to an enamel edge. A crack starts from the undersurface of the indented enamel growing downwards into the tissue. However, the crack is then diverted outwards towards a nearby surface. In the process, a chip of enamel, much smaller than a slab, is removed (fig. 1).
Microfractures result from indentations from very small particles on the enamel surface, leading to the loss of small fragments, i.e. to wear (fig. 1). Probably the best-known type of wear in humans is caused by grinding the teeth during sleep, an activity called ‘bruxism' [7]. Some clinicians believe that this causes loss of more enamel than chemical erosion, but this opinion depends on geography: European clinicians are apparently less inclined to believe this than those based in North America [11].
Does the Loss of Tooth Function Matter?
For virtually all mammals tooth loss does matter because they depend on a functioning dentition in order to eat efficiently. Tooth fractures jeopardize this activity and indications are that accumulating damage is critical to survival. The lifespan of one of the smallest short-tailed shrews, weighing 15-20 g, is limited by wear in the wild. Wild-caught animals generally only live for one breeding season, after which their teeth are heavily worn down to the gums. In contrast, young animals will live for three breeding seasons in captivity with much less wear [12]. At the other end of the scale is the African elephant, weighing 2,700-5,500 kg. Elephants bring their cheek molars into the mouth serially, only having one active molar tooth in each jaw quadrant at a time. Old individuals with very worn last molars either die or seek out vegetation that does not require chewing to swallow [13]. Data for mammals of intermediate size as diverse as primates [14] and marsupials [15,16,17] also support the importance of intact teeth. As an example of the effect of wear on mammalian populations, some of Pahl's [16] data on ringtail possums are shown in figure 2, where it is clear that individuals with very worn dentitions, of whatever age, are disproportionately lost from the population.
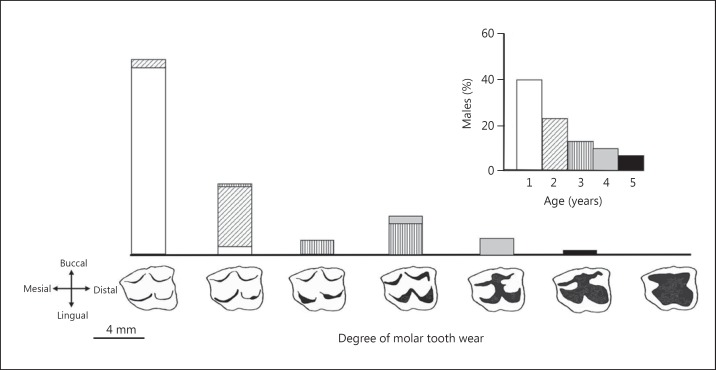
Fig. 2.
Males in a population of ringtail possums range mostly from 1 to 5 years of age (inset). The main histogram shows wear stages of an upper second molar tooth in terms of the degree of dentinal exposure (black) on its working surface as the covering enamel is lost. Older males tend to have more wear, but this is not inevitable. Virtually no males have extreme wear (last stage to the right).
Evidence for the effect of larger-scale crown fractures, such as wholesale cuspal fractures [18,19,20,21],enamel chipping [22,23,24,25] and the splitting of tooth crowns in half [25], are less well known, but there is no doubt that ingestion and mastication are impaired. The effect is not the same on modern human populations because of ubiquitous preingestive processing and cooking of foods. These processes are of considerable antiquity [26,27,28], making foods easy to eat even when teeth are severely worn. However, before any of these types of fracture can be described and analysed, a short description of both the structure and mechanical properties of the tissue is needed.
Enamel Structure
Enamel forms a highly mineralized thin outer coat on the crown surface (fig. 1, 3). It consists mostly of hydroxyapatite crystals embedded in a matrix of protein and water. Each crystal is between 0.1 and 1 mm in length with cross-sectional dimensions of 30-70 nm [29]. The crystals are thus much bigger than those in other mineralized tissues, such as dentine and bone, and they occupy over 90% of the tissue volume. A few thousand crystals are bundled together into elongated structures called prisms, each 3-5 µm in diameter. Prisms extend almost all the way from the enamel-dentine junction to the tooth surface. The gap between crystals within a prism is only 1-2 nm, but between prisms there are much larger 100-nm crystal-free gaps (fig. 3a), occupied by low molecular weight proteins and water [30]. In the inner enamel, prisms follow a sinuous path (fig. 3b). Adjacent prisms in the longitudinal plane are successively slightly out of phase with each other [31]. The successive phase change leads to the appearance of small parcels of prisms crossing each other, a characteristic called decussation (fig. 3c). In humans, the inner 60-80% of enamel thickness consists of decussating prisms. However, the prisms straighten in the outer enamel, passing in parallel to the tooth surface (fig. 3b). This type of enamel is often called ‘radial enamel' to contrast it with ‘decussating enamel'.
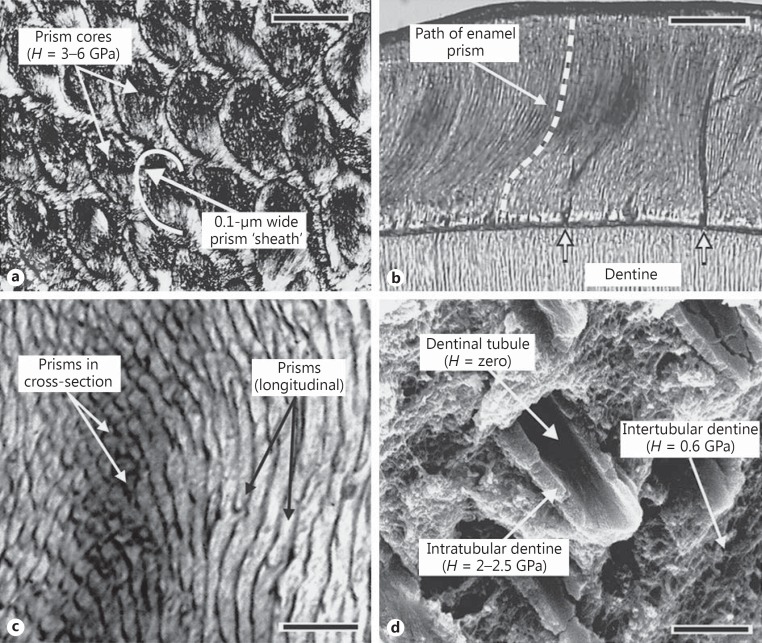
Fig. 3.
Enamel structure contrasted with dentine. The hardness H of structures is indicated where known; data from Cuy et al. [40] and Lucas [90]. a The microstructure of enamel showing prism cross-sections containing crystal bundles surrounded by a crystal-free ‘sheath'. Scale bar 5 μm. b The enamel coat in transverse section showing the wave of prisms in the inner enamel and their straightening near the tooth surface. Arrows show tufts. Scale bar 200 μm. c Longitudinal sections of inner enamel show bands of prisms in cross-section next to bands cut longitudinally due to decussation. Scale bar 5 μm. d A fractured surface of dentine shows a tubule (that in life would contain fluid and a cell process) surrounded by hard intratubular dentine. Thus, dentine is heterogeneous on a scale at which enamel is reasonably homogeneous. Scale bar 3 μm.
In addition to prisms, there are a number of minor structures in enamel that were previously thought to be trivial, but which are now known to be of major importance. Foremost among these are enamel tufts (fig. 3b). These hypocalcified strands, formed during development, resemble widened prism sheaths and extend from the enamel-dentine junction about one third of the way into the enamel [32,33]. Previously thought to be a curiosity, they are now implicated as a key protective mechanism of the tooth crown.
A précis of the development of the tissue is important for understanding some of its features. When the tissue is first formed, the crystallites are thin and large proteins that form the scaffolding of the tissue, and which initiate crystallization, occupy the bulk of the tissue. However, after the enamel thickness is completely formed, the cells that secrete the tissue (ameloblasts) convert into cells that somewhat resemble those lining the small intestine. They start to produce digestive enzymes (matrix metalloproteinases) that break the protein scaffolding down into fragments [34]. The ameloblasts then draw most of these protein pieces, and also much of the water, out from the tissue. This removal allows the crystals to expand in breadth into newly created space. In behaving like this, ameloblasts act at a distance of up to 2 mm and their effectiveness must thus be a function of a diffusion gradient. This appears to set up a parallel gradient in the mature tissue in chemical composition [35,36,37,38,39] that is also mirrored in some mechanical properties, as described below.
Enamel Mechanical Properties
The elastic modulus E of enamel is its resistance to elastic deformation. This is measured in force per unit area (newtons per metre squared: Nm-2). The SI abbreviation for this unit is the pascal (Pa) with 1 Pa = 1 Nm-2. However, this is such a small unit that gigapascals (1 GPa = 109 Nm-2) are required to reduce the number of digits in describing most materials. Mature human enamel is very stiff with an E varying between 70 and 110 GPa [40]. The hardness H of enamel is its resistance to plastic (permanent) deformation. It is again measured in force per unit area (newtons per metre squared: Nm-2). Human enamel is very hard with H = 3-6 GPa (fig. 2a) [40]. Both the elastic modulus and hardness are linearly gradated, with the lowest values near the enamel-dentine junction and the highest at the tooth surface [40,41,42,43]. The properties of the enamel of other mammals vary slightly, but a gradient of hardness and elastic modulus is again usually present [44,45,46,47]. There are unlikely to be big surprises in enamels yet to be examined because both modulus and hardness are subject to a ‘rule of mixtures' wherein the composite tissue is constrained by the stiffest, hardest component of which it is made [48,49]. The upper limits for human enamel fall close to those reported for pure hydroxyapatite [50].
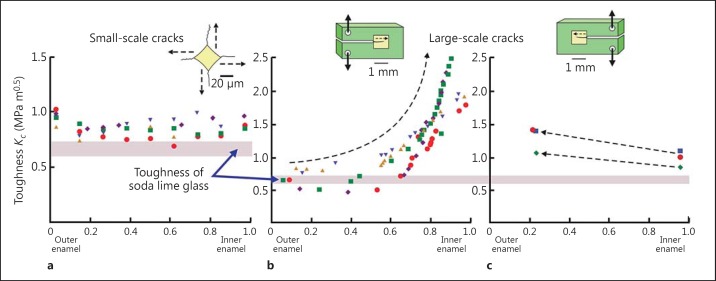
The major mechanical benefit of composite materials constructed from several components, such as enamel, lies not in its stiffness or hardness, but in its fracture toughness, which is the ability of an object to resist the growth of cracks [51]. The toughness of enamel is not graded in the manner of the elastic modulus and hardness because, unlike these properties, it is not bound by limits set by its components. Instead, it benefits from a synergism between these components that produce high toughness in the composite, compared to components that, in isolation, will break very easily. There are two ways to measure toughness, which are easily interconverted. Here we refer to it as ‘fracture toughness', which is the effect that a crack of unit length has on the intensity of stress in a loaded object. This intensity depends on the square root of crack length and thus has units of MNm-2 m0.5 or MPa m0.5. It is symbolized here as Kc. This quantity varies in enamel not in the form of a gentle gradient, but as a function of enamel structure. For very small cracks of the order of size of a few prism diameters (fig. 4a), structure does not matter and the toughness of enamel is uniformly low [52], resembling that of glass [53]. For larger cracks, everything depends on whether they can track along prism sheaths without deviating or whether they encounter decussation [54], in which case the toughness becomes elevated (fig. 4 b, c). Toughness appears to be more elevated when cracks are run from outside in, i.e. the crack starts at the tooth surface and projects towards the dentine (fig. 4b), than when cracks are directed from the inside to outside (fig. 4c) [55]. The ‘inside-out' crack typically becomes unstable in a test because the crack is being opened directly. However, in life, such cracks would be loaded not in direct tension but in compression. Crack opening then depends on tension being developed indirectly at right angles to the load. Under these circumstances, ‘inside-out' cracks in enamel have been shown to be completely stable [56,57,58,59].
Fig. 4.
The varying toughness of enamel reported for small and large scale tests as compared to soda lime glass, a classic brittle material [53]. The horizontal axis denotes the position of a crack with respect to the tooth surface (which is ‘0’ on this axis) and the junction with dentine (innermost enamel is ‘1.0’). a When a pyramidal indenter is pressed onto enamel (producing the diamond-shaped indent shown in surface view), small cracks can extend from its corners. Toughness is always low. Data from Bajaj et al. [52]. b, c To run longer cracks, enamel ‘tablets’ (yellow squares) can be encased within large blocks of material (green boxes) that can be notched into the enamel and then gripped. Cracks can then be opened in the enamel. When cracks are run from the outer enamel to inside (b), toughness increases sharply. Data redrawn from Bajaj and Arola [54] and Yahyazadehfar et al. [55]. Symbols represent data for individual teeth.
A comparison of the properties of enamel and dentine is important in understanding how the whole crown behaves. At the scale of a few prism diameters or less, enamel is effectively isotropic. This makes experimentation on the microfractures that lead to wear entirely tractable. However, on this same scale, dentine is very anisotropic, containing large cylindrical holes surrounded by relatively stiff, hard intratubular (or peritubular) dentine. Between tubules, the intertubular dentine is of much lower elastic modulus and hardness (fig. 3d) [60]. Studies of dentinal wear at the level of the actual mechanical events would thus be extremely difficult. However, at any larger scale, the situation is reversed. Enamel is then very anisotropic, showing the gradient of elastic modulus and hardness, coupled with a strong influence of structure on toughness. In contrast, most of the primary dentine - that formed with the tooth and which abuts the enamel - is relatively homogeneous, with the exception of a thin strip of ‘mantle dentine' directly under the enamel, which is slightly softer and less stiff [61,62,63]. The toughness of dentine varies little for long cracks, hovering between 1.6 and 3 MPa m0.5, but decreasing with dentinal age, presumably because of the gradual accumulation of intratubular dentine [64,65,66,67]. As a consequence of its large-scale uniformity, large cracks in dentine have no real preferred orientation dictated by dentinal structure. In contrast, the structure of enamel has a major directing effect [68].
The Importance of Scale
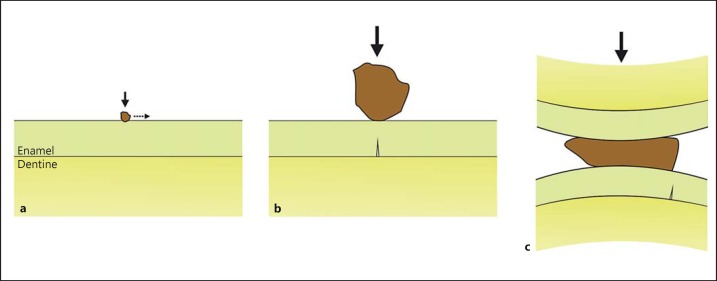
The main ‘scaling factor' that affects enamel damage is nothing intrinsic to the tissue itself, but is a consequence of the effect of the size of particles that contact its external surface [69]. A small particle, of hardness >2.5 times that of enamel [70,71,72], has its effect principally on the tooth surface, indenting/sliding against it at very low forces, causing either plastic deformation or fracture of the enamel (fig. 5a). In contrast, contact with a much larger particle of the same hard material causes the enamel to bend. If the enamel cracks, then it will do so on its deep surface (fig. 5b). Lastly, a contact with a large particle of low hardness, <2.5 times that of enamel, can still damage it. Bending of the enamel under the contact is not likely to produce cracking though, because deformation of the particle will produce a large contact area and a large volume under hydrostatic compression that will suppress fracture. Therefore, when sufficient strain energy is available for fractures to be initiated, they are likely to start well away from the zone of contact where tension can be expressed (fig. 5c). We now discuss the type of fractures seen in teeth with respect to the mechanical behaviour of the tissues.
Fig. 5.
The damage that enamel sustains due to contacts with hard objects (direction and size of force given by the arrows) depends on scale. a A small particle that is much harder than enamel tends to indent its surface, producing microfracture (‘wear‘) if the particle slides. b A large hard particle is more likely to produce a crack on the undersurface of the enamel directly under the contact (a ‘radial' macrofracture) due to enamel flexure. c A softer large particle deforms between teeth. It can still damage the enamel via bending forces, but only away from the contact area, thus forming a ‘margin' macrofracture.
Macrofracture
Large-scale damage to a tooth crown is completely incompatible with its continued use. The most common form of such fracture is a through-enamel crack called a lamella (fig. 1). Lamellae are absent from newly erupted teeth, but accumulate during life. Although such lamellae were long thought to start at the enamel surface and progress as ‘outside-in' cracks [73], experimental evidence now suggests that instead they grow from inside to outside as extensions of enamel tufts [57,58]. There is a forest of tufts all around the enamel-dentine junction ready to pick this strain energy up [74,75]. These cracks grow slowly, requiring a sharply increasing force to extend [57,58]. The relationship between crack extension and force is known, with a dependence on enamel toughness and thickness, but additionally on the square root of tooth size. The presence of lamellae can be used to establish a history of force use on a tooth [76].
In themselves, lamellae are relatively innocuous. However, the major issue with these types of crack is a potentially catastrophic fracture, which results in the loss of large slabs of enamel, either part of a cusp (against a hard particle; fig. 5b) or part of the margin of a tooth (against a softer particle; fig. 5c). Margin fractures are often called ‘abfractions' in the human dental literature [77]. They depend on tooth dimensions in a different manner to that which affects cuspal (radial) cracks (fig. 6). This is because they stem from tensile stresses known as ‘hoop' stresses [59]. These stresses gain their name from the design of barrels, where metal hoops around the circumference stop internal cracks in the barrels from propagating outwards. Human teeth are very vulnerable to such margin cracks because they lack any protection in the region where the tooth crown meets the root (fig. 1). This marginal region is buttressed in many mammals, but not in humans [69,78].
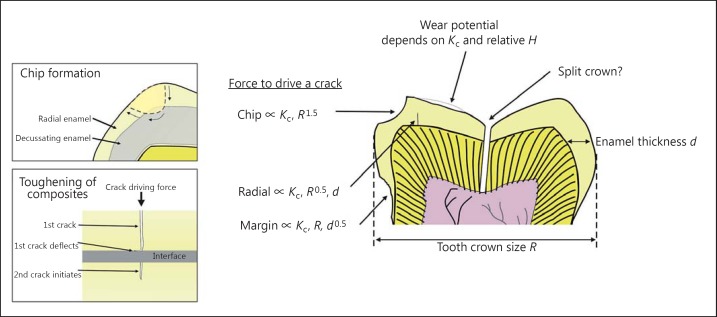
Fig. 6.
The dependence of different scales of fracture events on enamel properties and tooth crown dimensions. Relationships have been firmly established for radial, margin and chipping fractures. Note that the degree of dependence on crown dimensions varies. These relationships were established by Lee et al. [76] and Lucas et al. [86]. The upper box shows the usual path of a chip (small arrows show its growth path), following a prism sheath in the outer radial enamel, being deflected by decussation and then seeking the shortest path to a surface again along a sheath. The lower box shows the typical toughening mechanism of composites. A force drives a primary crack. That crack meets an interface of low stiffness and deflects. The force cannot easily push the primary crack further, but if the force builds, a secondary crack can initiate on the other side of the interface, leaving a ‘bridge' between the two that will eventually fail, causing fragmentation.
Mesofracture
The chipping of enamel (fig. 1) is very common in mammals in general [22,23,24,25], being particularly frequent in certain archaic and modern human populations [79,80,81]. It is also often seen by dentists in a clinical setting, where it is mostly viewed as an aesthetic issue [82]. Yet, a general theory of chipping resistance in materials has only recently started to be developed [83]. Provided the complexities of resistance to fracture seen in human enamel are ignored, and the tissue is treated as responding elastically with little toughness, then a remarkably simple treatment of chipping resistance in ceramic materials can be applied to enamel [84]. Evidence suggests that a crack starts at the surface at a contact close to an edge as what are termed ‘median' vents [84]. As it extends downwards, energetic considerations favour the crack turning towards the edge, so releasing a chip of tissue. The analysis of chipping resembles those for macrofractures that start from deep in the enamel, but with a differing dependence on tooth dimensions (fig. 6). Again, chipping gives evidence of the force that produced it [24].
A crack that starts on the tooth surface and grows from outside inwards would encounter increasing resistance as it does so because inner decussating enamel would obstruct its progress (fig. 4b). Thus, part of the explanation for the number of chips on human teeth, and the relative rarity of cracks that have grown from outside inwards to reach the enamel-dentine junction, is that the inner decussating enamel is there to protect the integrity of the tooth (fig. 6). A mesofracture is preferable to a split crown that could result if the crack continued its original path and passed into the dentine.
Microfracture
Microfracture refers to the mechanical wear of the tooth surface whereby small amounts of material are lost from the crown surface. These events accumulate over time to be seen by the naked eye as macroscopic wear (fig. 1). Dentists distinguish tissue lost from tooth-tooth contacts, which they call attrition, from abrasion, which is damage produced by small foreign objects that enter the mouth [85]. Here, we use terms that refer to the processes involved. ‘Abrasion' involves the removal of tissue from a surface either as a ribbon that curls away from the surface (as in the wear of metals) or as a chip. It leaves marks that are sharp V-shaped grooves (fig. 7). Only small particles that are >2.5 times as hard as enamel can do this. Particles that are less hard can only produce plastic deformation of the enamel surface. This is called ‘rubbing'. Material can be moved on the surface, forming a groove surrounded by a mound of tissue, but no material is immediately lost. Such mounds are not seen with abrasion (fig. 7).
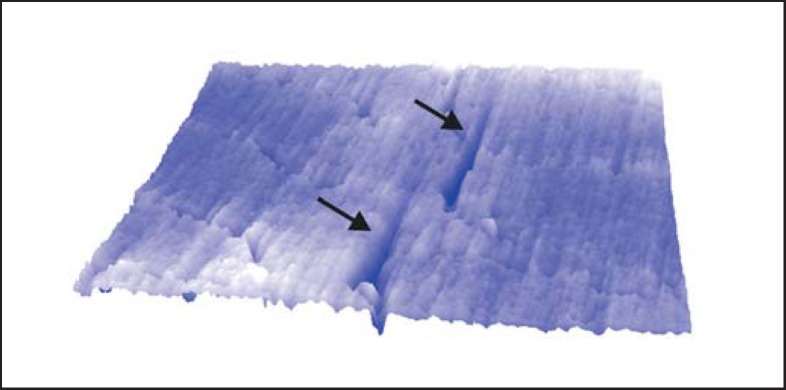
Fig. 7.
A three-dimensional AFM (atomic force microscopic) image of an enamel surface, each side 16.5 µm in length, showing two large V-shaped marks (arrows) produced experimentally by contact with a single piece of quartz. This particle has made two scratches because of its irregular shape. Note that there are no raised mounds around the scratches. This is typical of abrasion.
Recent evidence suggests that abrasion can take place at forces of 1 mN or less [86], which is negligible considering that maximum bite forces in modern humans can reach 1 kN, six orders of magnitude higher [24]. Clearly, if conditions are right, large amounts of wear can take place by abrasion. Several factors help to prevent this. Firstly, abrasion depends on the presence of ingested particles that are much harder than enamel. It also depends on sliding contacts because this is much more important in producing wear than static contacts. No food tissue eaten by any mammal is as hard as enamel, but grit and dust particles that enter the mouth ingested with food can be much harder [86]. Luckily, unless these particles are sufficiently sharp, this will not result in abrasive wear [83,87,88]. The criterion for ‘sufficient sharpness' is actually set by enamel toughness. Most airborne particles are actually quite smooth-surfaced, and the only way to generate sufficient sharpness is if the particles are broken in the mouth. This produces an audible noise and, luckily, mechanoreceptors in the periodontal ligament of the tooth (fig. 1) are present to pick up the sub-newtonian loads at which dust and grit particles break, so alerting an individual to damage [89]. Very soft particles of whatever size do little mechanical damage to an enamel surface, but there exists an in-between category of particle that is roughly as hard as enamel. Such particles can rub the tooth surface, indenting it and doing damage very similar to that which teeth do to each other [86]. Such damage, however, is a plastic indentation without any immediate resulting tissue loss, so strictly it is not wear when considered as a single event.
Overview of Mechanical Damage to Enamel
For the first time, several of the patterns of damage to a tooth crown can be expressed quantitatively in terms of the force that produces them (fig. 6). The extent to which properties and crown dimensions affect this force depends on the type of fracture. Margin and radial cracks, despite their similar origin near the enamel-dentine junction (fig. 5b, c), depend on the local thickness of the enamel and on crown dimensions, but with differing exponents (fig. 6). While it is difficult to express resistance to mechanical wear in the same detail, it is clear that enamel hardness (compared to that being contacted) and toughness are the critical properties that resist damage.
The discovery that a standard pattern of cracking in enamel runs ‘inside-out' has many implications for researchers investigating the protection of a tooth, unknown even a few years ago [90]. Currently, much research is focused on how the enamel-dentine junction is protected from fractures that cross from the enamel into the dentine [63,91,92,93,94,95,96]. It might be ironic if most of the protection came from the fact that the general direction of cracks is from close to the enamel-dentine junction, travelling in the opposite direction to that which would fracture into dentine. The role of the mantle dentine may be to encourage the enamel to flex, and thus to strain the tufts (fig. 5b, c). The tufts extend only when forces are high enough [97], thus protecting the dentinal core by directing cracks in the opposite direction. Such cracks that do pass from the exterior inwards may well be deflected by decussating enamel; of course, this may sacrifice a chip of enamel, but it saves the tooth (fig. 6).
The factors that control the splitting of tooth crowns in half are less well known. One theory proposes that the relation for chipping (equation in fig. 6 and top inset) applies also to splitting [25], with the difference that the latter is a consequence of a more central position for crack initiation on the crown [24]. Recent developments have extended some of this theory to variation in crown height [98] and to include the presence of multiple cusps [99].
Judging from experiments [52,54,55], the cracking of enamel in macrofractures is likely to follow the typical toughening pattern of composites shown in figure 6 (bottom inset). A primary crack traverses a homogeneous region of the material, e.g. a prism core. It then reaches an interface, which we could identify in enamel as the prism sheath. Here, the crack deflects because the stiffness of the small-protein gel in the sheath is so low that strain energy cannot be built up to advance it; thus, it opens for a short distance along the interface [100]. If the force continues to build, then a secondary crack may initiate on the other side of the sheath in the next prism core. The toughening comes from the fact that the force to start this secondary crack is far higher than that which started the primary one, with the ratio depending on the width of the interface [101]. The gap between the two cracks then remains as a bridging ligament that restricts the opening of the whole fracture as it advances. This pattern is repeated across successive interfaces until, in the end, the ligaments fail with eventual union of the whole crack and the loss of material. This mechanism can amplify toughness in an extraordinary manner, sometimes to be several thousand times higher than the toughness of isolated components. The organization of biological composite tissues is designed to promote the effectiveness of these mechanisms. Teeth do not ‘want' to be fractured, and virtually no biological tissue ‘wants' to end up being broken down by the teeth as food. Thus, a composite structure is common to most biological tissues.
Conclusion
There is still a lot to learn about tooth crown fractures, and little has yet been done at the same level on root fractures, which are clinically important. Yet the groundwork has been laid for a new era of understanding, particularly of enamel. Some say that the tissue behaves like metal [102], others that it is glassy [58]. We conclude here that, dependent on the influence of structure and the scale at which it acts, both may be correct.
Disclosure Statement
The authors have no conflicts of interest to disclose.
Acknowledgements
We acknowledge support from Kuwait University General Facilities Projects GD02/11 and GE01/07. We thank Lidia Arockia Thai for the image in figure 6.
References
- 1.Smith BGN. Dental erosion, attrition and abrasion. Dent Pract. 1975;214:347–355. [PubMed] [Google Scholar]
- 2.Smith BGN, Bartlett DW, Robb ND. The prevalence, etiology and management of tooth wear in the United Kingdom. J Prosthet Dent. 1997;78:367–372. doi: 10.1016/s0022-3913(97)70043-x. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett DW, Evans DF, Smith BGN. The relationship between gastro-oesophageal reflux disease and dental erosion. J Oral Rehabil. 1996;23:289–297. doi: 10.1111/j.1365-2842.1996.tb00855.x. [DOI] [PubMed] [Google Scholar]
- 4.Kelleher M, Bishop K. Tooth surface loss: an overview. Br Dent J. 1999;186:61–66. doi: 10.1038/sj.bdj.4800020a2. [DOI] [PubMed] [Google Scholar]
- 5.Smith BGN, Robb ND. The prevalence of toothwear in 1,007 dental patients. J Oral Rehabil. 1996;23:232–239. doi: 10.1111/j.1365-2842.1996.tb00846.x. [DOI] [PubMed] [Google Scholar]
- 6.Azzopardi A, Bartlett DW, Watson TF, et al. A literature review of the techniques to measure tooth wear and erosion. Eur J Prosthodont Restor Dent. 2000;8:93–97. [PubMed] [Google Scholar]
- 7.van Rijkom HM, Truin GJ, Frencken JEFM, et al. Prevalence, distribution and background variables of smooth-bordered tooth wear in teenagers in the Hague, the Netherlands. Caries Res. 2002;36:147–154. doi: 10.1159/000057874. [DOI] [PubMed] [Google Scholar]
- 8.Moazzez R, Smith BGN, Bartlett DW. Oral pH and drinking habit during ingestion of a carbonated drink in a group of adolescents with dental erosion. J Dent. 2000;28:395–397. doi: 10.1016/s0300-5712(00)00020-8. [DOI] [PubMed] [Google Scholar]
- 9.Bartlett DW, Evans DF, Anggiansah A, et al. A study of the association between gastro- oesophageal reflux and palatal dental erosion. Br Dent J. 1996;181:125–131. doi: 10.1038/sj.bdj.4809187. [DOI] [PubMed] [Google Scholar]
- 10.Jacobson BC, Somers SC, Fuchs CS, et al. Body-mass index and symptoms of gastroesophageal reflux in women. N Engl J Med. 2006;354:2340–2348. doi: 10.1056/NEJMoa054391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartlett DW, Phillips K, Smith B. A difference in perspective - the North American and European interpretations of tooth wear. Int J Prosthodont. 1999;12:401–408. [PubMed] [Google Scholar]
- 12.Pearson OP. Longevity of the short-tailed shrew. Am Midl Nat. 1945;34:531–546. [Google Scholar]
- 13.Sikes SK. The African elephant, Loxodonta africana: a field method for the estimation of age. J Zool. 1968;154:235–248. [Google Scholar]
- 14.King SJ, Arrigo-Nelson SJ, Pochron ST, et al. Dental senescence in a long-lived primate links infant survival to rainfall. Proc Natl Acad Sci USA. 2005;102:16579–16583. doi: 10.1073/pnas.0508377102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanyon JM, Sanson GD. Koala (Phascolarctos cinereus) dentition and nutrition. II. Implications of tooth wear in nutrition. J Zool. 1986;209:169–181. [Google Scholar]
- 16.Pahl LI. Survival, age determination and population age structure of the common ringtail possum, Pseudocheirus peregrinus, in a Eucalyptus woodland and a Leptospermum thicket in southern Victoria. Aust J Zool. 1987;35:625–639. [Google Scholar]
- 17.Logan M, Sanson GD. The effect of tooth wear on the feeding behaviour of free-ranging koalas (Phascolarctos cinereus, Goldfuss) J Zool. 2002;256:63–69. [Google Scholar]
- 18.van Valkenburgh., B Incidence of tooth breakage among large, predatory mammals. Am Nat. 1988;131:291–302. [Google Scholar]
- 19.van Valkenburgh B, Hertel F. Tough times at La Brea: tooth breakage in large carnivores of the late Pleistocene. Science. 1993;261:456–459. doi: 10.1126/science.261.5120.456. [DOI] [PubMed] [Google Scholar]
- 20.van Valkenburgh., B Feeding behavior in free-ranging, large African carnivores. J Mammal. 1996;77:240–254. [Google Scholar]
- 21.van Valkenburgh., B Costs of carnivory: tooth fracture in Pleistocene and Recent carnivorans. Biol J Linn Soc. 2009;96:68–81. [Google Scholar]
- 22.Wallace JA. Tooth chipping in the australopithecines. Nature. 1973;244:117–118. doi: 10.1038/244117a0. [DOI] [PubMed] [Google Scholar]
- 23.Wallace JA. Dietary adaptations of Australopithecus and early Homo. In: Tuttle R, editor. Paleoanthropology, Morphology and Paleoecology. Mouton: The Hague; 1975. pp. 203–223. [Google Scholar]
- 24.Constantino P, Lee JJ-W, Chai H, et al. Tooth chipping can reveal the diet and bite forces of fossil hominins. Biol Lett. 2010;6:719–722. doi: 10.1098/rsbl.2010.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chai H, Lee JJ-W, Lawn BR. On the chipping and splitting of teeth. J Mech Behav Biomed Mater. 2011;4:315–321. doi: 10.1016/j.jmbbm.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Wrangham RW, Jones JH, Laden G, et al. The raw and the stolen: cooking and the ecology of human origins. Curr Anthropol. 1999;40:567–594. [PubMed] [Google Scholar]
- 27.Carmody RN, Weintraub GS, Wrangham RW. Energetic consequences of thermal and nonthermal food processing. Proc Natl Acad Sci USA. 2011;108:19199–19203. doi: 10.1073/pnas.1112128108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lucas PW. Cooking clue to human dietary diversity. Proc Natl Acad Sci USA. 2011;108:19101–19102. doi: 10.1073/pnas.1116813108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daculsi G, Menanteau J, Kerebel LM, et al. Length and shape of enamel crystals. Calc Tissue Int. 1984;36:550–555. doi: 10.1007/BF02405364. [DOI] [PubMed] [Google Scholar]
- 30.Frank RM, Nalbandian S. Ultrastructure of amelogenesis. In: Miles AEW, editor. Structural and Chemical Organisation of Teeth. London: Academic Press; 1968. pp. 399–462. [Google Scholar]
- 31.Osborn JW. Variation in structure and development of enamel. In: Melcher AH, Zarb GA, editors. Dental Enamel: Oral Science Reviews. vol 3. Copenhagen: Munksgaard; 1973. pp. 3–83. [PubMed] [Google Scholar]
- 32.Sognnaes RF. The organic elements of enamel. II. The organic framework of the internal part of the enamel, with special regard to the organic basis for theb so-called tufts and Schreger's bands. J Dent Res. 1949;28:549–557. doi: 10.1177/00220345490280060401. [DOI] [PubMed] [Google Scholar]
- 33.Osborn JW. The 3-dimensional morphology of the tufts in human enamel. Acta Anat. 1969;73:481–495. doi: 10.1159/000143313. [DOI] [PubMed] [Google Scholar]
- 34.Lu Y, Papagerakis P, Yamakoshi Y, et al. Functions of KLK4 and MMP-20 in dental enamel formation. Biol Chem. 2008;389:695–700. doi: 10.1515/BC.2008.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weidmann SM, Weatherall JA, Hamm SM. Variations of enamel density in sections of human teeth. Arch Oral Biol. 1967;12:85–97. doi: 10.1016/0003-9969(67)90145-8. [DOI] [PubMed] [Google Scholar]
- 36.Weatherall JA, Robinson C, Hiller CR. Distribution of carbonate in thin sections of dental enamel. Caries Res. 1968;2:1–9. doi: 10.1159/000259538. [DOI] [PubMed] [Google Scholar]
- 37.Robinson C, Weatherall JA, Hallsworth AS. Variation in composition of dental enamel within thin ground tooth sections. Caries Res. 1971;5:44–57. doi: 10.1159/000259731. [DOI] [PubMed] [Google Scholar]
- 38.Theuns HM, Driessens FCM, van Dijk JWE, et al. Experimental evidence for a gradient in the solubility and in the rate of dissolution of human enamel. Caries Res. 1986;20:24–31. doi: 10.1159/000260916. [DOI] [PubMed] [Google Scholar]
- 39.Macena MSA, Leite MLAS, Gouveia CL, et al. A comparative study on component volumes from outer to inner dental enamel in relation to enamel tufts. Arch Oral Biol. 2014;59:568–577. doi: 10.1016/j.archoralbio.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Cuy JL, Mann AB, Livi KJ, et al. Nanoindentation mapping of the mechanical properties of human molar tooth enamel. Arch Oral Biol. 2002;47:281–291. doi: 10.1016/s0003-9969(02)00006-7. [DOI] [PubMed] [Google Scholar]
- 41.He L-H, Swain MV. Enamel - a functionally graded natural coating. J Dent. 2009;37:596–603. doi: 10.1016/j.jdent.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 42.Jeng Y-R, Lin T-T, Hsu H-M, et al. Human enamel rod presents anisotropic nanotribological properties. J Mech Behav Biomed Mater. 2011;4:515–522. doi: 10.1016/j.jmbbm.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 43.An B, Wang R, Arola D, et al. The role of property gradients on the mechanical behavior of human enamel. J Mech Behav Biomed Mater. 2012;9:63–72. doi: 10.1016/j.jmbbm.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 44.Darnell L, Teaford M, Livi K, et al. Variations in the mechanical properties of Alouatta palliata molar enamel. Am J Phys Anthropol. 2010;141:7–15. doi: 10.1002/ajpa.21126. [DOI] [PubMed] [Google Scholar]
- 45.Lee JJ-W, Morris D, Constantino P, et al. Properties of tooth enamel in great apes. Acta Biomater. 2010;12:4560–4565. doi: 10.1016/j.actbio.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 46.Constantino PJ, Lee JJW, Morris D, et al. Adaptation to hard object eating in sea otters and hominins. J Hum Evol. 2011;61:89–96. doi: 10.1016/j.jhevol.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 47.Campbell SE, Cuozzo FP, Sauther ML, et al. Nanoindentation of lemur enamel: an ecological investigation of mechanical property variations within and between sympatric species. Am J Phys Anthropol. 2012;148:178–190. doi: 10.1002/ajpa.21582. [DOI] [PubMed] [Google Scholar]
- 48.Harris B. The mechanical behaviour of composite materials. In: Vincent JFV, Currey JD, editors. The Mechanical Properties of Biological Materials. Cambridge: Cambridge University Press; 1980. pp. 37–74. [Google Scholar]
- 49.Ashby MF. Criteria for selecting the components of composites. Acta Met Mater. 1993;41:1313–1335. [Google Scholar]
- 50.Kumar RR, Wang M. Modulus and hardness evaluations of sintered bioceramic powders and functionally graded bioactive composites by nano-indentation technique. Mater Sci Eng. 2002;A338:230–236. [Google Scholar]
- 51.Lucas PW, Turner IM, Dominy NJ, et al. Mechanical defences to herbivory. Ann Bot. 2000;86:913–920. [Google Scholar]
- 52.Bajaj D, Park S, Quinn GD, et al. Fracture processes and mechanisms of crack growth resistance in human enamel. J Mater. 2010;62:76–82. [Google Scholar]
- 53.Lucas PW, Constantino PJ, Chalk J, et al. Indentation as a technique to assess the mechanical properties of fallback foods. Am J Phys Anthrop. 2009;140:643–652. doi: 10.1002/ajpa.21026. [DOI] [PubMed] [Google Scholar]
- 54.Bajaj D, Arola DD. On the R-curve behavior of human tooth enamel. Biomaterials. 2009;30:4037–4046. doi: 10.1016/j.biomaterials.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yahyazadehfar M, Bajaj D, Arola DD. Hidden contributions of the enamel rods on the fracture resistance of human teeth. Acta Biomat. 2013;9:4806–4814. doi: 10.1016/j.actbio.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lawn BR, Lee JJ, Constantino PJ, et al. Predicting failure in mammalian enamel. J Mech Behav Biomed Mat. 2009;2:33–42. doi: 10.1016/j.jmbbm.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 57.Lee JJ-W, Kwon J-Y, Chai H, et al. Fracture modes in human teeth. J Dent Res. 2009;88:224–228. doi: 10.1177/0022034508330055. [DOI] [PubMed] [Google Scholar]
- 58.Chai H, Lee JJ-W, Constantino P, et al. Remarkable resilience of teeth. Proc Natl Acad Sci USA. 2009;106:7289–7293. doi: 10.1073/pnas.0902466106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chai H, Lee JJ-W, Kwon J-Y, et al. A simple model for enamel fracture from margin cracks. Acta Biomat. 2009;5:1663–1667. doi: 10.1016/j.actbio.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 60.Kinney JH, Balooch M, Marshall SJ, et al. Hardness and Young's modulus of human peritubular and intertubular dentine. Arch Oral Biol. 1996;41:9–13. doi: 10.1016/0003-9969(95)00109-3. [DOI] [PubMed] [Google Scholar]
- 61.Osborn JW. Dentine hardness and incisor wear in the beaver (Castor fiber) Acta Anat. 1969;72:123–132. doi: 10.1159/000143242. [DOI] [PubMed] [Google Scholar]
- 62.Renson CE, Braden M. The experimental deformation of human dentine by indenters. Arch Oral Biol. 1971;16:563–572. doi: 10.1016/0003-9969(71)90059-8. [DOI] [PubMed] [Google Scholar]
- 63.Zaslansky P, Friesem AA, Weiner S. Structure and mechanical properties of the soft zone separating bulk dentin and enamel in crowns of human teeth: insight into tooth function. J Struct Biol. 2006;153:188–199. doi: 10.1016/j.jsb.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 64.El Mowlafy OM, Watts DC. Fracture toughness of human dentin. J Dent Res. 1986;65:677–681. doi: 10.1177/00220345860650050901. [DOI] [PubMed] [Google Scholar]
- 65.Iwamoto N, Ruse ND. Fracture toughness of human dentin. J Biomed Mater Res A. 2003;66:507–512. doi: 10.1002/jbm.a.10005. [DOI] [PubMed] [Google Scholar]
- 66.Imbeni V, Nalla RK, Bosi C, et al. In vitro fracture toughness of human dentin. J Biomed Mater Res A. 2003;66:1–9. doi: 10.1002/jbm.a.10548. [DOI] [PubMed] [Google Scholar]
- 67.Nazari A, Bajaj D, Zhang D, et al. Aging and the reduction in fracture toughness of human dentin. J Mech Behav Biomed Mat. 2009;2:550–559. doi: 10.1016/j.jmbbm.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Waters NE. Some mechanical and physical properties of teeth. In: Vincent JFV, Currey JD, editors. The Mechanical Properties of Biological Materials. Cambridge: Cambridge University Press; 1980. pp. 99–135. [Google Scholar]
- 69.Lucas PW, Constantino P, Wood BA, et al. Dental enamel as a dietary indicator in mammals. Bioessays. 2008;30:374–285. doi: 10.1002/bies.20729. [DOI] [PubMed] [Google Scholar]
- 70.Tabor D. Oxford: Clarendon; 1951. Hardness of Metals; pp. 1–175. [Google Scholar]
- 71.Atkins AG, Felbeck DK. Applying mutual indentation hardness phenomena to service failures. Met Eng Q. 1974;22:55–61. [Google Scholar]
- 72.Atkins AG. Topics in indentation hardness. Met Sci. 1982;16:127–137. [Google Scholar]
- 73.Sognnaes RF. The organic elements of enamel. IV. The gross morphology and the histological relationship of the lamellae to the organic framework of the enamel. J Dent Res. 1950;29:260–269. doi: 10.1177/00220345500290030201. [DOI] [PubMed] [Google Scholar]
- 74.Amizuka N, Uchida T, Fukae M, et al. Ultrastructural and immunocytochemical studies of enamel tufts in human permanent teeth. Arch Histol Cytol. 1992;55:179–190. doi: 10.1679/aohc.55.179. [DOI] [PubMed] [Google Scholar]
- 75.Amizuka N, Uchida T, Nozawa-Inoue K, et al. Ultrastructural images of enamel tufts in human permanent teeth. J Oral Biosci. 2005;47:33–41. [Google Scholar]
- 76.Lee JJ-W, Constantino P, Lucas PW, et al. Fracture in teeth - a diagnostic for inferring tooth function and diet. Biol Rev. 2011;86:959–974. doi: 10.1111/j.1469-185X.2011.00181.x. [DOI] [PubMed] [Google Scholar]
- 77.Grippo JO. Abfraction: a new classification of hard tissue lesions of teeth. J Esthet Dent. 1991;3:14–18. doi: 10.1111/j.1708-8240.1991.tb00799.x. [DOI] [PubMed] [Google Scholar]
- 78.Anderson PSL, Gill PG, Rayfield EJ. Modeling the effects of cingula structure on strain patterns and potential fracture in tooth enamel. J Morphol. 2011;272:50–65. doi: 10.1002/jmor.10896. [DOI] [PubMed] [Google Scholar]
- 79.Turner CG, Cadien JD. Dental chipping in Aleuts, Eskimoes and Indians. Am J Phys Anthopol. 1969;31:303–310. doi: 10.1002/ajpa.1330310305. [DOI] [PubMed] [Google Scholar]
- 80.Hylander WL. The adaptive significance of Eskimo craniofacial morphology. In: Dahlberg A, Garber TM, editors. Orofacial Growth and Development. Paris: Mouton; 1977. pp. 129–169. [Google Scholar]
- 81.Scott GR, Winn JR. Dental chipping: contrasting patterns of microtrauma in Inuit and European populations. Int J Osteoarchaeol. 2011;21:723–731. [Google Scholar]
- 82.Andreasen JO, Andreason FM, Andersson L. ed 4. Copenhagen: Blackwell Munksgaard; 2007. Textbook and Color Atlas of Traumatic Injuries to the Teeth. [Google Scholar]
- 83.Atkins AG. Oxford: Elsevier Press; 2009. The Science and Engineering of Cutting. [Google Scholar]
- 84.Chai H, Lawn BR. A universal relation for edge chipping from sharp contacts in brittle materials and its use as a simple means of toughness evaluation. Acta Mater. 2007;55:2555–2561. [Google Scholar]
- 85.Lucas PW, Omar R. New perspectives on tooth wear. Int J Dent. 2012;2012:287573. doi: 10.1155/2012/287573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lucas PW, Omar R, Al-Fadhalah K, et al. Mechanisms and causes of wear in tooth enamel: implications for hominin diets. J R Soc Interface. 2013;10:20120923. doi: 10.1098/rsif.2012.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Atkins AG, Liu JH. Toughness and the transition between cutting and rubbing in abrasive contacts. Wear. 2007;262:146–159. [Google Scholar]
- 88.Atkins T. Toughness and processes of material removal. Wear. 2009;267:1764–1771. [Google Scholar]
- 89.Lucas PW, van Casteren A, Al-Fadhalah K, et al. The role of dust, grit and phytoliths in tooth wear. Ann Zool Fenn. 2014;51:143–152. [Google Scholar]
- 90.Lucas PW. Cambridge: Cambridge University Press; 2004. Dental Functional Morphology: How Teeth Work. [Google Scholar]
- 91.Lin CP, Douglas WH. Structure-property relations and crack resistance at the bovine dentin-enamel junction. J Dent Res. 1994;73:1072–1078. doi: 10.1177/00220345940730050901. [DOI] [PubMed] [Google Scholar]
- 92.White SN, Paine ML, Luo W, et al. The dentino-enamel junction is a broad transitional zone uniting dissimilar bioceramic composites. J Am Ceram Soc. 2000;83:238–240. [Google Scholar]
- 93.Marshall GW, Jr, Bolooch M, Gallagher RR, et al. Mechanical properties of the dentinoenamel junction: AFM studies of nanohardness, elastic modulus and fracture. J Biomed Mater Res. 2001;54:87–95. doi: 10.1002/1097-4636(200101)54:1<87::aid-jbm10>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 94.Marshall SJ, Balooch M, Habelitz S, et al. The dentin-enamel junction - a natural multilevel interface. J Eur Ceram Soc. 2003;23:2897–2904. [Google Scholar]
- 95.Imbeni V, Kruzic JJ, Marshall GW, et al. The dentin-enamel junction and the fracture of human teeth. Nat Mater. 2005;4:229–232. doi: 10.1038/nmat1323. [DOI] [PubMed] [Google Scholar]
- 96.White SN, Miklus VG, Chang PP, et al. Controlled failure mechanisms toughen the dentino-enamel junction zone. J Prosthet Dent. 2005;94:330–335. doi: 10.1016/j.prosdent.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 97.Chai H, Lee JJ-W, Lawn BR. Fracture of tooth enamel from incipient microstructural defects. J Mech Behav Biomed Mater. 2010;3:116–120. doi: 10.1016/j.jmbbm.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 98.Lawn BR, Bush MB, Barani A, et al. Inferring biological evolution from fracture patterns in teeth. J Theoret Biol. 2013;338:59–65. doi: 10.1016/j.jtbi.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 99.Barani A, Bush MB, Lawn BR. Role of multiple cusps in tooth fracture. J Mech Behav Biomed Mater. 2014;35:85–92. doi: 10.1016/j.jmbbm.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 100.He M-Y, Hutchinson JW. Crack deflection at an interface between dissimilar elastic materials. Int J Solids Structures. 1989;25:1053–1067. [Google Scholar]
- 101.Lee JJ-W, Lloyd IK, Chai H, et al. Arrest, deflection, penetration and reinitiation of cracks in brittle layers across adhesive interlayers. Acta Mater. 2007;55:5859–5866. [Google Scholar]
- 102.He LH, Swain MV. Enamel: a ‘metallic-like' deformable biocomposite. J Dent. 2007;35:431–437. doi: 10.1016/j.jdent.2006.12.002. [DOI] [PubMed] [Google Scholar]