Abstract
OBJECTIVE
To study the association of gluten intake with development of islet autoimmunity and progression to type 1 diabetes.
RESEARCH DESIGN AND METHODS
The Diabetes Autoimmunity Study in the Young (DAISY) follows children with an increased risk of type 1 diabetes. Blood samples were collected at 9, 15, and 24 months of age, and annually thereafter. Islet autoimmunity was defined by the appearance of at least one autoantibody against insulin, IA2, GAD, or ZnT8 (zinc transporter 8) in at least two consecutive blood samples. Using food frequency questionnaires, we estimated the gluten intake (in grams per day) annually from 1 year of age. Cox regression modeling early gluten intake, and joint modeling of the cumulative gluten intake during follow-up, were used to estimate hazard ratios adjusted for confounders (aHR).
RESULTS
By August 2017, 1,916 subjects were included (median age at end of follow-up 13.5 years), islet autoimmunity had developed in 178 participants, and 56 of these progressed to type 1 diabetes. We found no association between islet autoimmunity and gluten intake at 1–2 years of age or during follow-up (aHR per 4 g/day increase in gluten intake 1.00, 95% CI 0.85–1.17 and 1.01, 0.99–1.02, respectively). We found similar null results for progression from islet autoimmunity to type 1 diabetes. Introduction of gluten at <4 months of age was associated with an increased risk of progressing from islet autoimmunity to type 1 diabetes compared with introduction at 4–5.9 months (aHR 8.69, 95% CI 1.69–44.8).
CONCLUSIONS
Our findings indicate no strong rationale to reduce the amount of gluten in high-risk children to prevent development of type 1 diabetes.
Introduction
Type 1 diabetes is a common chronic disease in childhood that results from an immune-mediated destruction of pancreatic β-cells, leading to complete and lifelong dependence on exogenous insulin (1). Genetic susceptibility in the development of type 1 diabetes is well established, but increased incidence rates over the past decades strongly suggest an important role of environmental factors in disease development (2–4). Environmental risk factors have not yet been established, and it is currently not possible to prevent the disease. Nutritional factors in childhood may contribute to the development of islet autoimmunity and type 1 diabetes.
Gluten, a storage protein found in wheat, rye, and barley, has been hypothesized to be one of the environmental factors involved in the development of type 1 diabetes (5). Although the mechanism of how gluten could influence the development of type 1 diabetes is unclear, it has been suggested that gluten could change the immune cell populations, giving a more proinflammatory cytokine profile or lead to dysbiosis of the gut microbiota (6,7).
Few prospective studies have examined aspects of gluten intake as a risk factor for islet autoimmunity and type 1 diabetes, and most of these focus on age at the introduction of cereals or gluten-containing foods in infancy. Early introduction of gluten-containing foods has been shown to increase the risk of islet autoimmunity and type 1 diabetes in German high-risk cohorts (8,9). Other prospective studies (10–13) have not found a consistent association between age at the introduction of cereals or gluten-containing foods and the development of islet autoimmunity or type 1 diabetes. Contrary to these studies, The Environmental Determinants of Diabetes in the Young (TEDDY) study (14) recently reported an increased risk of islet autoimmunity with delayed introduction of gluten. An early finding from the Diabetes Autoimmunity Study in the Young (DAISY) (15) was that the timing of the introduction of cereals, but not specifically gluten, was associated with the risk of islet autoimmunity in high-risk children. And a more recent study from DAISY (16) found no association between age at introduction of foods containing wheat and barley and type 1 diabetes. None of the studies looked at the amount of gluten consumed (in grams per day) after infancy.
Given the limited time periods of exposure measurement in previous studies, it is unknown whether gluten intake in other stages of life influences the risk of the development of islet autoimmunity and type 1 diabetes. Our aim was to study the association of age at introduction of gluten and gluten intake in early childhood and throughout childhood and adolescence with the development of islet autoimmunity and progression to type 1 diabetes. We also examined whether these relationships differed by HLA genotype.
Research Design and Methods
DAISY follows children who are at risk for islet autoimmunity and type 1 diabetes. The study is conducted in Denver, CO. Details on the formation of the DAISY cohort and its follow-up have previously been reported (17).
Design and Study Sample
DAISY is a cohort study with longitudinal follow-up from birth, with clinic visits at age 9, 12, 15, and 24 months, and annually thereafter. DAISY participants were recruited from the following two populations:
Children from the general population born in 1993–2006 at the St. Joseph’s Hospital in Denver, CO, without major neonatal morbidities who, through screening by umbilical cord blood for diabetes susceptibility alleles in the HLA region, were identified to have an increased risk for the disease. Of 31,766 screened newborns (87% of eligible children), 1,424 were enrolled in the cohort.
Unaffected children with a first-degree relative with type 1 diabetes in the Denver metropolitan area. A total of 1,123 children were recruited irrespective of their HLA genotype.
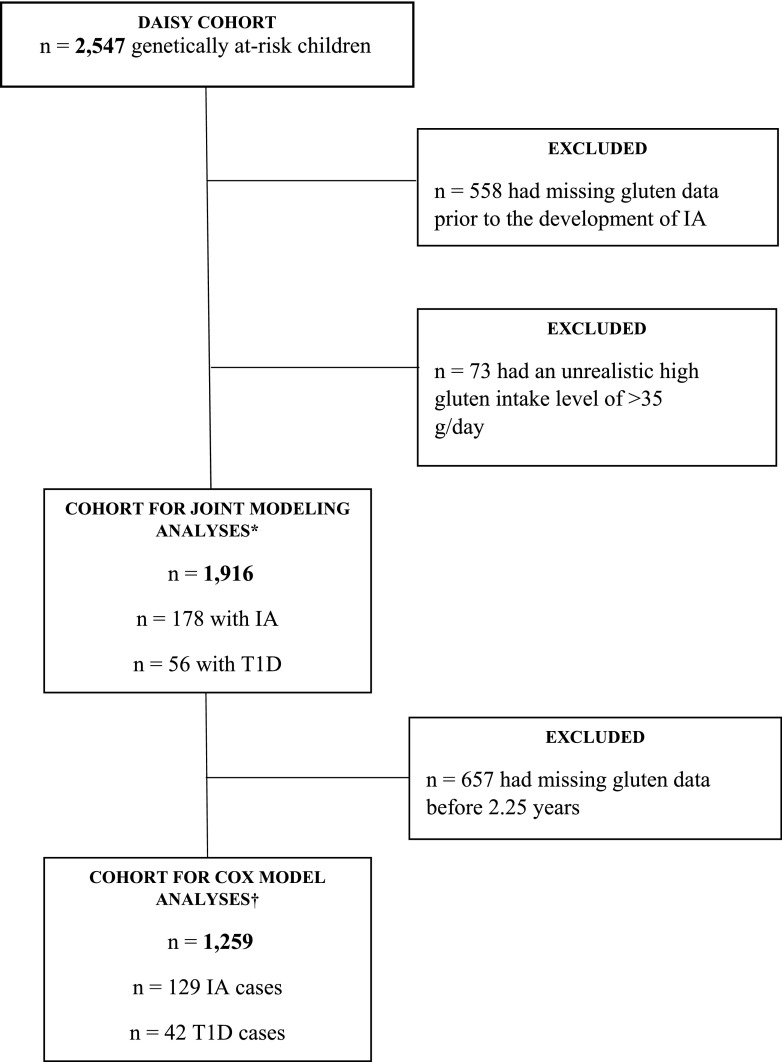
Of 2,547 children included in the DAISY cohort, data for the current study were available for 1,916 participants (Fig. 1).
Figure 1.
Flow chart of the DAISY cohort. IA, islet autoimmunity; T1D, type 1 diabetes. *Cohort for joint modeling analyses was used in analyses of cumulative amount of gluten intake during follow-up and the risk of IA and progression from IA to T1D. †Cohort for Cox model analyses was used in analyses of age at the introduction of gluten, the amount of gluten intake at age 1–2 years, and the risk of IA and progression from IA to T1D.
Parental consent was obtained for all participants, and assent was obtained from children ≥7 years of age. This study has been approved by the Colorado Multiple Institutional Review Board.
Exposure
A priori, we decided to separately examine the effect of three different time periods of gluten exposure: 1) age at first introduction of gluten; 2) amount of gluten intake at age 1–2 years; and 3) cumulative amount of gluten intake during follow-up as a time-varying exposure.
Age at Gluten Introduction
Data for the exposure variable age at gluten introduction was obtained from telephone or face-to-face interviews completed at every third month from 3 to 15 months of age. Age at gluten introduction was grouped into <4, 4–5.9, and ≥6 months, with 4–5.9 months as the reference group, which is in line with previous studies and because of the low frequency of gluten introduction <4 months (16).
Amount of Gluten Intake
At 2 years of age, a semiquantitative food frequency questionnaire (FFQ) measuring the child’s usual dietary intake during the previous year was administered to the parents. The FFQ has been validated in DAISY using biomarkers and 24-h dietary recalls (18,19). The FFQ was administered annually until 10 years of age, after which the participants received a Youth Adolescent Questionnaire (YAQ) annually. The YAQ also covered the usual dietary intake over the previous year and has been validated against 24-h dietary recalls and shown to be reproducible in this age-group (20,21). We have previously shown in DAISY that data from the FFQ and the YAQ can be used in the same analysis when the analysis includes an indicator variable to define the survey method (22). Both the FFQ and the YAQ included common food items with portion sizes and covered the average consumption frequency with alternatives ranging from “never or less than once a month” to “six or more times per day.”
We combined the reported frequency intake of wheat-, rye-, and barley-containing food variables with the reported portion sizes and protein content from online U.S. recipe tools, U.S. Department of Agriculture standardized food recipes, and the U.S. Department of Agriculture food composition database (23). We converted the protein content from flour or grains from each of the food variables into gluten content by using a conversion factor of 0.75, in accordance with a previous study from the U.S. using the same type of FFQ (24). We classified 73 FFQ records (0.4% of total FFQs) as missing because of an unrealistically high gluten intake level of >35 g/day.
Outcomes
We studied two different outcomes, islet autoimmunity and progression from islet autoimmunity to type 1 diabetes.
Islet autoimmunity was defined by the appearance of at least one autoantibody against insulin, the tyrosine phosphatase-like protein IA2, GAD, or zinc transporter 8, twice or more in succession, or being autoantibody positive on one visit and receiving a diagnosis of type 1 diabetes on the next consecutive visit, as described in previous studies (25,26).
Progression from islet autoimmunity to type 1 diabetes was determined by continued follow-up of islet autoimmunity–positive children every 3–6 months. Children were referred to a physician for type 1 diabetes diagnosis if they had a random glucose concentration of >11.1 mmol/L and/or an HbA1c level of >6.2% (44 mmol/mol). The criteria used for type 1 diabetes diagnosis included typical symptoms of polyuria and/or polydipsia and a random glucose concentration of >11.1 mmol/L, or an oral glucose tolerance test with a fasting plasma glucose concentration of ≥7.0 mmol/L or 2-h glucose concentration of >11.1 mmol/L. The details of the intensive monitoring and type 1 diabetes diagnosis protocol have been described previously (27).
Genotyping
We categorized individuals in one of the following three groups based on HLA genotype: DR3/4; DR3/3, DR3/X; DR4/4, DR4/X, and X/X, where X is neither DR3 nor DR4. Details on the genotyping procedure in DAISY have previously been published (17).
Other Variables
Variables considered as potential confounders because they may be associated with the intake of gluten-containing foods and type 1 diabetes development were retrieved from questionnaires or interviews. The variables were child’s sex, family history of type 1 diabetes, parent-reported race-ethnicity of the child, maternal age at the time of delivery, birth weight, cesarean section, parity, maternal smoking status in pregnancy, and maternal education. We also included data on the child’s total energy intake estimated from FFQs, family history of celiac disease (CD), and the appearance of CD autoimmunity (CDA), a preclinical manifestation of CD defined by two consecutive positive tissue transglutaminase autoantibodies tests (28). Variables were categorized as shown in Table 1.
Table 1.
Characteristics of children in the cohort for joint modeling analyses of cumulative amount of gluten intake and risk of islet autoimmunity and progression from islet autoimmunity to type 1 diabetes
| Covariates | Total(N = 1,820) | IA(n = 173) | T1D(n = 49) |
|---|---|---|---|
| Sex, n (%) | |||
| Female | 878 (48.2) | 87 (50.3) | 24 (49.0) |
| Male | 942 (51.8) | 86 (49.7) | 25 (51.0) |
| HLA group, n (%)‡ | |||
| DR3/4 | 405 (22.3) | 33 (19.1) | 12 (24.5) |
| DR3/3, DR3/X | 382 (21.0) | 55 (31.8) | 21 (42.9) |
| DR4/4, DR4/X, DRX/X | 1,033 (56.8) | 85 (49.1) | 16 (32.7) |
| Family history of T1D, n (%) | |||
| No | 910 (50.0) | 63 (36.4) | 16 (32.7) |
| Yes | 910 (50.0) | 110 (63.6) | 33 (67.3) |
| Non-Hispanic white, n (%) | |||
| No | 434 (23.8) | 37 (21.4) | 5 (10.2) |
| Yes | 1,386 (76.2) | 136 (78.6) | 44 (89.8) |
| Maternal age (years) at delivery | |||
| Mean (SD) | 30.3 (5.6) | 30.5 (5.2) | 31.5 (4.7) |
| Age (months) at introduction of gluten, n (%) | |||
| <4 | 145 (8.0) | 12 (6.9) | 5 (10.2) |
| 4.0–5.9 | 614 (33.7) | 58 (33.5) | 19 (38.8) |
| ≥6 | 1,061 (58.3) | 103 (59.5) | 25 (51.0) |
| Family history of CD, n (%) | |||
| No | 1,743 (95.8) | 162 (93.6) | 47 (95.9) |
| Yes | 77 (4.2) | 11 (6.4) | 2 (4.1) |
| Developed CDA, n (%) | |||
| No | 1,656 (91.0) | 155 (89.6) | 44 (89.8) |
| Yes | 164 (9.0) | 18 (10.4) | 5 (10.2) |
IA, islet autoimmunity; T1D, type 1 diabetes. A total of 96 children (of whom IA developed in 5 children) in the joint modeling analysis of the cumulative amount of gluten intake and risk of IA had missing covariates and are not included in the table. A total of seven children who progressed from IA to T1D in the joint modeling analysis of the cumulative amount of gluten intake and risk of progression from IA to T1D had missing covariates and are not included in the table.
‡HLA groups where X is neither DR3 nor DR4.
Statistical Analysis
The statistical software package SAS, version 9.4 of the SAS System for Windows (SAS Institute, Cary, NC), was used for all statistical analyses. Our three gluten exposures were tested for association with two different outcomes, islet autoimmunity and progression from islet autoimmunity to type 1 diabetes, as described above. For islet autoimmunity as the outcome, follow-up time was counted from birth to the first islet autoantibody–positive blood sample or the most recent negative sample as of August 2017. For the progression analysis, follow-up time was counted from the first islet autoantibody–positive sample until type 1 diabetes diagnosis or most recent visit when the child was known not to have type 1 diabetes as of August 2017.
For the analysis of time-invariant exposures (age at gluten introduction and amount of gluten intake at 1–2 years of age), we used the Cox proportional hazards model. We used a joint modeling approach for the analysis of the time-varying gluten exposure (cumulative amount of gluten intake during follow-up). Briefly, joint longitudinal survival modeling estimates the association between the random subject effects of longitudinal gluten measurements during follow-up and time-to-event data (29). We modeled exposure during follow-up using a quadratic curve that fits changing eating patterns over time. A piecewise exponential distribution with four internal knots was determined to be the best fit for the baseline hazard function for the parametric survival model used in the joint modeling approach. The longitudinal and survival models were linked using a cumulative association structure (30).
In the Cox model for analysis of time-invariant exposures, we included shared frailty to incorporate cluster effects from correlated time-to-event data among siblings. Efron’s approximation was used to handle ties in the event times. Gluten intake was modeled both as a continuous variable (in grams per day) and as tertiles of gluten intake. Testing interaction terms between gluten intake and follow-up time, we found no violation of the proportional hazards assumption.
We tested for interactions between HLA groups and gluten exposure in both models. The primary adjustment variables were the child’s total energy intake, family history of CD, timing of CDA appearance, HLA genotype, family history of type 1 diabetes, parent reported race-ethnicity, sex, and maternal age at delivery. Birth weight, cesarean section, parity, maternal smoking status in pregnancy, and maternal education were not included in the final models after backward elimination. In the analysis using the cumulative amount of gluten intake during follow-up as the exposure, we also adjusted for type of FFQ (FFQ or YAQ) used. In all analyses using the amount of gluten intake as the exposure, we adjusted for age at gluten introduction, and in the analyses using age at introduction of gluten as the exposure, we adjusted for the amount of gluten intake at age 1–2 years (Table 2).
Table 2.
Association between gluten exposures and development of islet autoimmunity and progression from IA to type 1 diabetes
| TotalN (%) | IAn (%) | UnadjustedHR (95% CI) | AdjustedHR (95% CI) | |
|---|---|---|---|---|
| Development of IA | ||||
| Exposure | ||||
| Gluten (per 4 g/day) over time* | 1,820 (100) | 173 (100) | 1.00 (0.99–1.02) | 1.01 (0.99–1.02) |
| Gluten (per 4 g/day) at age 1–2 years† | 1,235 (100) | 128 (100) | 1.02 (0.90–1.15) | 1.00 (0.85–1.18) |
| Tertiles 1–2 years | ||||
| Low (0.0–9.0 g/day) | 415 (33.6) | 38 (29.7) | 1 | 1 |
| Middle (9.0–13.1 g/day) | 404 (32.7) | 44 (34.4) | 1.18 (0.76–1.84) | 1.07 (0.68–1.67) |
| High (13.1–34.9 g/day) | 416 (33.7) | 46 (35.9) | 1.20 (0.77–1.86) | 1.05 (0.62–1.77) |
| Age at gluten introduction (months)‡ | ||||
| <4 | 79 (6.4) | 7 (5.47) | 0.89 (0.39–2.01) | 0.97 (0.45–2.09) |
| 4.0–5.9 | 415 (33.6) | 41 (32.0) | 1 | 1 |
| ≥6 | 741 (60.0) | 80 (62.5) | 1.08 (0.74–1.59) | 1.06 (0.73–1.56) |
| TotalN (%) | T1Dn (%) | UnadjustedHR (95% CI) | AdjustedHR (95% CI) | |
| Progression from IA to T1D | ||||
| Exposure | ||||
| Gluten (per 4 g/day) over time* | 164 (100) | 49 (100) | 1.02 (0.99–1.04) | 1.02 (0.99–1.04) |
| Gluten (per 4 g/day) at age 1–2 years† | 128 (100) | 42 (100) | 1.00 (0.81–1.24) | 0.86 (0.57–1.29) |
| Tertiles 1–2 years | ||||
| Low (1.9–8.7 g/day) | 38 (29.7) | 13 (31.0) | 1 | 1 |
| Middle (9.1–13.1 g/day) | 44 (34.4) | 15 (35.7) | 1.24 (0.59–2.61) | 0.81 (0.28–2.33) |
| High (13.2–26.2 g/day) | 46 (35.9) | 14 (33.3) | 1.03 (0.48–2.20) | 0.63 (0.18–2.18) |
| Age at gluten introduction (months)‡ | ||||
| <4 | 7 (5.5) | 6 (14.3) | 5.09 (1.64–15.8) | 8.69 (1.69–44.8) |
| 4.0–5.9 | 41 (32.0) | 14 (33.3) | 1 | 1 |
| ≥6 | 80 (62.5) | 22 (52.4) | 0.76 (0.37–1.55) | 0.77 (0.31–1.88) |
IA, islet autoimmunity; T1D, type 1 diabetes.
*Adjusted for the child’s total energy intake, age at introduction of gluten, family history of CD, appearance of CDA, HLA genotype, family history of T1D, type of FFQ, parent-reported race-ethnicity, sex, and maternal age at delivery.
†Adjusted for the child’s total energy intake, age at introduction of gluten, family history of CD, HLA genotype, family history of T1D, parent-reported race-ethnicity, sex, and maternal age at the time of delivery.
‡Adjusted for the child’s total energy intake, gluten intake at age 1–2 years, family history of CD, HLA genotype, family history of T1D, parent-reported race-ethnicity, sex, and maternal age at the time of delivery.
Sensitivity and Subgroup Analyses
Having two or more autoantibodies increases the risk of progressing from islet autoimmunity to type 1 diabetes (31). We therefore repeated the main analyses where only those with multiple (two or more) autoantibodies at first islet autoimmunity visit or later were followed up until islet autoimmunity or most recent follow-up, or until type 1 diabetes diagnosis or last visit with islet autoimmunity but no known type 1 diabetes as of August 2017.
We repeated all analyses with breastfeeding as a covariate since breastfeeding could have an influence on other aspects of infant feeding and the risk of developing type 1 diabetes (11,12).
Because of the recent finding from TEDDY where the introduction of gluten at 9 months or after predicted an increased risk of islet autoimmunity (14), we recategorized age at the introduction of gluten into <4, 4–5.9, 6–8.9, and ≥9 months to further investigate if late introduction was associated with islet autoimmunity or progression to type 1 diabetes.
Results
By August 2017, 1,916 subjects were included (median age at end of follow-up 13.5 years), 178 participants had islet autoimmunity, and 56 of these progressed to type 1 diabetes.
Gluten Intake at 1–2 Years of Age
Between the ages of 1 and 2 years, the average daily gluten intake was 11.4 g (SD 5.61 g). We found no association between amount of gluten intake at 1–2 years and the development of islet autoimmunity (hazard ratio adjusted for confounders [aHR] 1.00, 95% CI 0.85–1.17) (Table 2) per 4 g increase in gluten intake, equal to a slice of bread, or progression from islet autoimmunity to type 1 diabetes (aHR 0.86, 95% CI 0.57–1.29) (Table 2). The aHR for association between gluten intake at 1–2 years of age and the risk of the development of islet autoimmunity or progression to type 1 diabetes did not vary across different HLA groups (all aHRs close to 1.00).
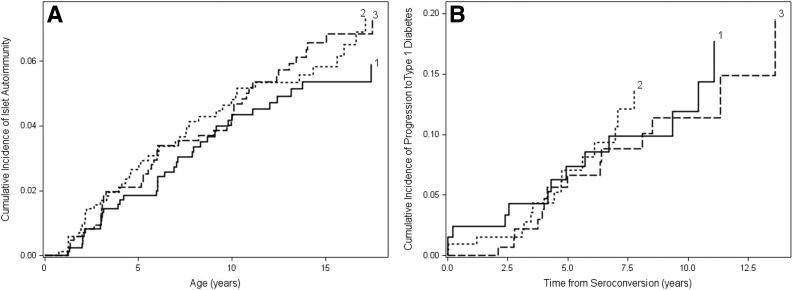
When comparing subjects in the lowest third of gluten intake at age 1–2 years to those in the middle and highest third, we found no association with risk of the development islet autoimmunity (Fig. 2A and Table 2) or progression from islet autoimmunity to type 1 diabetes (Fig. 2B and Table 2). The aHR for association between gluten intake tertile and the risk of islet autoimmunity or progression to type 1 diabetes did not vary appreciably by HLA group (all aHRs close to 1.00).
Figure 2.
Probability of islet autoimmunity (A) and progression to type 1 diabetes (B) according to tertiles for the amount of gluten intake at age 1–2 years. 1 = lowest third; 2 = middle third; 3 = highest third. When comparing subjects in the lowest third of gluten intake at age 1–2 years to those in the middle and highest third, we found no association with the risk of the development of islet autoimmunity or progression from islet autoimmunity to type 1 diabetes (all P values >0.47).
Cumulative Gluten Intake During Follow-up
We found no association between the cumulative amount of gluten intake during follow-up and the development of islet autoimmunity (aHR per 4 g increase in gluten intake 1.01, 95% CI 0.99–1.02) (Table 2). At age 6 years, this corresponded to an aHR of 1.03 (95% CI 0.95–1.12) per SD of the cumulative amount of gluten intake. The cumulative amount of gluten intake was not associated with progression from islet autoimmunity to type 1 diabetes (aHR 1.02, 95% CI 0.99–1.04) (Table 2). The aHR for association between the total amount of gluten intake during follow-up and the risk of the development of islet autoimmunity or progressing to type 1 diabetes did not vary appreciably across different HLA groups (all HRs close to 1.00).
Age at Introduction of Gluten
We found no associations between age at introduction of gluten and the development of islet autoimmunity for introduction before 4 months of age (aHR 0.97, 95% CI 0.45–2.09), or for 6 months of age or later (aHR 1.06, 95% CI 0.73–1.56) compared with introduction at 4–5.9 months of age (Table 2). However, the introduction of gluten before 4 months of age was associated with an increased risk of progressing from islet autoimmunity to type 1 diabetes when compared with introduction at 4–5.9 months of age (aHR 8.69, 95% CI 1.69–44.8) (Table 2). No association was found for introduction at 6 months or later compared with introduction at 4–5.9 months of age (aHR 0.77, 95% CI 0.31–1.88) (Table 2).
Sensitivity and Subgroup Analyses
Analyses including only those positive for two or more autoantibodies gave the same conclusions. The increased risk of progressing from having two or more autoantibodies to type 1 diabetes when gluten was introduced at <4 months of age, compared with introduction at 4–5.9 months of age, remained significant (P = 0.04) with an aHR of 22.1, whereas all other results remained nonsignificant (P > 0.05). Including breastfeeding as a covariate did not have an impact on our findings compared with our primary analysis presented above (data not shown). Introduction of gluten at age 6–8.9 and ≥9 months of age was not significantly associated with islet autoimmunity or progression from islet autoimmunity to type 1 diabetes compared with introduction at 4–5.9 months (data not shown).
Conclusions
In this cohort of children at high risk for type 1 diabetes, the amount of gluten intake at age 1–2 years, and during follow-up, did not predict the development of islet autoimmunity or progression to type 1 diabetes, whereas the early introduction of gluten-containing foods was associated with an increased risk of progression from islet autoimmunity to type 1 diabetes.
The main strengths of the study are the frequent assessment of islet autoantibodies and the prospective design, with validated FFQs to assess diet throughout childhood and adolescence. The prospective design, where dietary data are assessed before the outcome, minimizes the risk of recall bias that can influence the results in retrospective studies. Given that a change in diet could be a result of islet autoimmunity, a prospective design reduces the possibility of reverse causality. The detailed data on potential confounders and predictors for type 1 diabetes is another strength. Because it could be hypothesized that environmental exposures may have different effects by HLA genotype, it is a major strength to have detailed data at this level, giving us the opportunity to explore potential interactions. Another strength of this study is the long follow-up period, with frequent collection of dietary data, which made it possible to investigate the association of gluten intake throughout childhood and adolescence with islet autoimmunity and type 1 diabetes development.
Although results from this high-risk group may not be generalizable to the general population, the study represents the majority of the population in whom type 1 diabetes will develop. Since national and international food composition databases do not contain information on gluten amount, we estimated the amount of gluten intake from prospectively assessed self-reported data, and, as for other studies, large imprecisions in our estimations are likely. Our estimated amount of gluten intake was similar to but slightly higher than those in other cohorts that have estimated the amount of gluten intake (32–34). The cohorts use varying methods to estimate the amount of gluten intake in different populations and settings and are not directly comparable, but the estimate can be used within the respective study without leading to biased results.
The study is observational, which means that we cannot exclude unmeasured confounding as an explanation for our findings. We cannot rule out the possibility that the statistically significant association we identified was a chance finding or that lack of power explains the lack of other associations we investigated. Adjusting for variables that previously have been thought to influence islet autoimmunity or type 1 diabetes did not affect our results.
To our knowledge, this is the first report from an investigation of the amount of gluten intake beyond infancy and the risk of the development of islet autoimmunity and progression from islet autoimmunity to type 1 diabetes. In addition to studying the amount of gluten intake at age 1–2 years, the unique structure of the DAISY study gave us the opportunity to study the amount of gluten intake not just at a very early age but also throughout childhood and adolescence.
In contrast to the recent TEDDY study (14), we did not find any association between age at the introduction of gluten and the risk of islet autoimmunity. A small randomized controlled trial, BABYDIET (10), found no significant effect of delaying the introduction of gluten from 6 to 12 months of age. It could be speculated that the amount of gluten introduced in combination with the timing is important for the risk of islet autoimmunity and type 1 diabetes, but we are not aware of any such published data. We found, however, that the early introduction of gluten was associated with an increased risk of progression from islet autoimmunity to type 1 diabetes. It must be stressed that these results should be interpreted cautiously because of the low numbers of children introduced to gluten before 4 months of age. This finding is in line with a previous finding from a high-risk cohort with type 1 diabetes as an outcome (9), whereas others did not find any association (11–13), including a previous report from DAISY (16). However, it is important to mention that these studies investigated risk from birth to type 1 diabetes in all children, not just in those who had islet autoimmunity. The one study that investigated progression from islet autoimmunity to type 1 diabetes had a different exposure using cereals including oats (13).
A gluten-free diet in a period of 6–12 months after seroconversion but before type 1 diabetes diagnosis had a limited impact on the level of autoantibodies and progression to type 1 diabetes in two very small intervention studies, including children at high risk (35,36). However, a study of NOD mice showed that a gluten-free diet prevented the development of diabetes (37). We are not able to exclude any potential effect of a gluten-free diet on the risk of islet autoimmunity and type 1 diabetes as this would require a large randomized controlled trial.
Our finding of the early introduction of gluten before 4 months of age as a potential risk factor for progression to type 1 diabetes supports general infant feeding recommendations from the American Academy of Pediatrics. Given our finding of no association between the amount of gluten intake at age 1–2 years or throughout childhood and adolescence and the risk of islet autoimmunity and progression to type 1 diabetes, we conclude that there is no rationale to reduce the amount of gluten during childhood and adolescence in the high-risk population to prevent the development of type 1 diabetes.
Article Information
Acknowledgments. The authors thank all of the children and their families who participated in DAISY, who generously volunteered their time and knowledge.
Funding. N.A.L.-B. was supported by Helse Sør-Øst RHF. F.D., J.S., A.E.B., K.C.W., R.K.J., M.J.R., and J.M.N. were supported by the National Institutes of Health (grants R01-DK-104351 and R01-DK-32493). K.M., K.S., G.T., and L.C.S. were supported by the Norwegian Research Council (grant 221909/F20).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. N.A.L.-B. was involved in study concept and design and the acquisition of data, analyzed and interpreted the data, did the statistical analyses, drafted the manuscript, critically revised the manuscript for important intellectual content, and approved the final manuscript submitted and the authorship list. F.D. was involved in study concept and design, analyzed and interpreted the data, did the statistical analyses, critically revised the manuscript for important intellectual content, and approved the final manuscript submitted and the authorship list. K.M., J.S., K.C.W., M.J.R., and J.M.N. were involved in study concept and design and the acquisition of data; analyzed and interpreted the data; critically revised the manuscript for important intellectual content; and approved the final manuscript submitted and the authorship list. A.E.B., G.J., K.S., G.T., L.C.S., and R.K.J. were involved in study concept and design; analyzed and interpreted the data; critically revised the manuscript for important intellectual content; and approved the final manuscript submitted and the authorship list. J.M.N. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018.
Footnotes
This article is part of a special article collection available at http://care.diabetesjournals.org/evolution-nutritional-therapy.
References
- 1.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet 2014;383:69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patterson CC, Gyürüs E, Rosenbauer J, et al. . Trends in childhood type 1 diabetes incidence in Europe during 1989-2008: evidence of non-uniformity over time in rates of increase. Diabetologia 2012;55:2142–2147 [DOI] [PubMed] [Google Scholar]
- 3.Harjutsalo V, Sund R, Knip M, Groop PH. Incidence of type 1 diabetes in Finland. JAMA 2013;310:427–428 [DOI] [PubMed] [Google Scholar]
- 4.Skrivarhaug T, Stene LC, Drivvoll AK, Strøm H, Joner G; Norwegian Childhood Diabetes Study Group . Incidence of type 1 diabetes in Norway among children aged 0-14 years between 1989 and 2012: has the incidence stopped rising? Results from the Norwegian Childhood Diabetes Registry. Diabetologia 2014;57:57–62 [DOI] [PubMed] [Google Scholar]
- 5.Barbeau WE, Bassaganya-Riera J, Hontecillas R. Putting the pieces of the puzzle together - a series of hypotheses on the etiology and pathogenesis of type 1 diabetes. Med Hypotheses 2007;68:607–619 [DOI] [PubMed] [Google Scholar]
- 6.Antvorskov JC, Josefsen K, Engkilde K, Funda DP, Buschard K. Dietary gluten and the development of type 1 diabetes. Diabetologia 2014;57:1770–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serena G, Camhi S, Sturgeon C, Yan S, Fasano A. The role of gluten in celiac disease and type 1 diabetes. Nutrients 2015;7:7143–7162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ziegler AG, Schmid S, Huber D, Hummel M, Bonifacio E. Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. JAMA 2003;290:1721–1728 [DOI] [PubMed] [Google Scholar]
- 9.Chmiel R, Beyerlein A, Knopff A, Hummel S, Ziegler AG, Winkler C. Early infant feeding and risk of developing islet autoimmunity and type 1 diabetes. Acta Diabetol 2015;52:621–624 [DOI] [PubMed] [Google Scholar]
- 10.Beyerlein A, Chmiel R, Hummel S, Winkler C, Bonifacio E, Ziegler AG. Timing of gluten introduction and islet autoimmunity in young children: updated results from the BABYDIET study. Diabetes Care 2014;37:e194–e195 [DOI] [PubMed] [Google Scholar]
- 11.Lund-Blix NA, Stene LC, Rasmussen T, Torjesen PA, Andersen LF, Rønningen KS. Infant feeding in relation to islet autoimmunity and type 1 diabetes in genetically susceptible children: the MIDIA study. Diabetes Care 2015;38:257–263 [DOI] [PubMed] [Google Scholar]
- 12.Lund-Blix NA, Dydensborg Sander S, Størdal K, et al. . Infant feeding and risk of type 1 diabetes in two large Scandinavian birth cohorts. Diabetes Care 2017;40:920–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hakola L, Takkinen HM, Niinistö S, et al. . Infant feeding in relation to the risk of advanced islet autoimmunity and type 1 diabetes in children with increased genetic susceptibility: a cohort study. Am J Epidemiol 2018;187:34–44 [DOI] [PubMed] [Google Scholar]
- 14.Uusitalo U, Lee HS, Andrén Aronsson C, et al.; TEDDY Study Group . Early infant diet and islet autoimmunity in the TEDDY study. Diabetes Care 2018;41:522–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norris JM, Barriga K, Klingensmith G, et al. . Timing of initial cereal exposure in infancy and risk of islet autoimmunity. JAMA 2003;290:1713–1720 [DOI] [PubMed] [Google Scholar]
- 16.Frederiksen B, Kroehl M, Lamb MM, et al. . Infant exposures and development of type 1 diabetes mellitus: the Diabetes Autoimmunity Study in the Young (DAISY). JAMA Pediatr 2013;167:808–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rewers M, Bugawan TL, Norris JM, et al. . Newborn screening for HLA markers associated with IDDM: Diabetes Autoimmunity Study in the Young (DAISY). Diabetologia 1996;39:807–812 [DOI] [PubMed] [Google Scholar]
- 18.Parrish LA, Marshall JA, Krebs NF, Rewers M, Norris JM. Validation of a food frequency questionnaire in preschool children. Epidemiology 2003;14:213–217 [DOI] [PubMed] [Google Scholar]
- 19.Brady H, Lamb MM, Sokol RJ, et al. . Plasma micronutrients are associated with dietary intake and environmental tobacco smoke exposure in a paediatric population. Public Health Nutr 2007;10:712–718 [DOI] [PubMed] [Google Scholar]
- 20.Rockett HR, Wolf AM, Colditz GA. Development and reproducibility of a food frequency questionnaire to assess diets of older children and adolescents. J Am Diet Assoc 1995;95:336–340 [DOI] [PubMed] [Google Scholar]
- 21.Rockett HR, Breitenbach M, Frazier AL, et al. . Validation of a youth/adolescent food frequency questionnaire. Prev Med 1997;26:808–816 [DOI] [PubMed] [Google Scholar]
- 22.Lamb MM, Ross CA, Brady HL, Norris JM. Comparison of children’s diets as reported by the child via the Youth/Adolescent Questionnaire and the parent via the Willett food-frequency questionnaire. Public Health Nutr 2007;10:663–670 [DOI] [PubMed] [Google Scholar]
- 23.United States Department of Agriculture. USDA food composition databases [Internet], 2017. Available from https://ndb.nal.usda.gov/ndb/. Accessed 5 June 2017
- 24.Lebwohl B, Cao Y, Zong G, et al. . Long term gluten consumption in adults without celiac disease and risk of coronary heart disease: prospective cohort study. BMJ 2017;357:j1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu L, Rewers M, Gianani R, et al. . Antiislet autoantibodies usually develop sequentially rather than simultaneously. J Clin Endocrinol Metab 1996;81:4264–4267 [DOI] [PubMed] [Google Scholar]
- 26.Barker JM, Barriga KJ, Yu L, et al.; Diabetes Autoimmunity Study in the Young . Prediction of autoantibody positivity and progression to type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY). J Clin Endocrinol Metab 2004;89:3896–3902 [DOI] [PubMed] [Google Scholar]
- 27.Stene LC, Barriga K, Hoffman M, et al. . Normal but increasing hemoglobin A1c levels predict progression from islet autoimmunity to overt type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY). Pediatr Diabetes 2006;7:247–253 [DOI] [PubMed] [Google Scholar]
- 28.Bao F, Yu L, Babu S, et al. . One third of HLA DQ2 homozygous patients with type 1 diabetes express celiac disease-associated transglutaminase autoantibodies. J Autoimmun 1999;13:143–148 [DOI] [PubMed] [Google Scholar]
- 29.Asar Ö, Ritchie J, Kalra PA, Diggle PJ. Joint modelling of repeated measurement and time-to-event data: an introductory tutorial. Int J Epidemiol 2015;44:334–344 [DOI] [PubMed] [Google Scholar]
- 30.Mauff K, Steyerberg EW, Nijpels G, van der Heijden AAWA, Rizopoulos D. Extension of the association structure in joint models to include weighted cumulative effects. Stat Med 2017;36:3746–3759 [DOI] [PubMed] [Google Scholar]
- 31.Ziegler AG, Rewers M, Simell O, et al. . Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013;309:2473–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crespo-Escobar P, Mearin ML, Hervás D, et al. . The role of gluten consumption at an early age in celiac disease development: a further analysis of the prospective PreventCD cohort study. Am J Clin Nutr 2017;105:890–896 [DOI] [PubMed] [Google Scholar]
- 33.Hoppe C, Trolle E, Gondolf UH, Husby S. Gluten intake in 6-36-month-old Danish infants and children based on a national survey. J Nutr Sci 2013;2:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoppe C, Gøbel R, Kristensen M, et al. . Intake and sources of gluten in 20- to 75-year-old Danish adults: a national dietary survey. Eur J Nutr 2017;56:107–117 [DOI] [PubMed] [Google Scholar]
- 35.Hummel M, Bonifacio E, Naserke HE, Ziegler AG. Elimination of dietary gluten does not reduce titers of type 1 diabetes-associated autoantibodies in high-risk subjects. Diabetes Care 2002;25:1111–1116 [DOI] [PubMed] [Google Scholar]
- 36.Pastore MR, Bazzigaluppi E, Belloni C, Arcovio C, Bonifacio E, Bosi E. Six months of gluten-free diet do not influence autoantibody titers, but improve insulin secretion in subjects at high risk for type 1 diabetes. J Clin Endocrinol Metab 2003;88:162–165 [DOI] [PubMed] [Google Scholar]
- 37.Funda DP, Kaas A, Bock T, Tlaskalová-Hogenová H, Buschard K. Gluten-free diet prevents diabetes in NOD mice. Diabetes Metab Res Rev 1999;15:323–327 [DOI] [PubMed] [Google Scholar]