Abstract
OBJECTIVE
To examine whether depression symptoms or antidepressant medication (ADM) use predicts the probability of cardiovascular events in overweight/obese individuals with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Preplanned analyses of depression and incident cardiovascular disease (CVD) were performed in the Look AHEAD (Action for Health in Diabetes) weight loss trial after a median follow-up of 9.6 years. Depression symptoms, assessed with the Beck Depression Inventory (BDI), were analyzed both as a continuous and dichotomized variable (BDI score <10 or ≥10). ADM use was coded from participants’ prescription medications. Four composite CVD outcomes were defined in the study protocol. Sex-stratified Cox proportional hazards models were adjusted for a range of baseline covariates.
RESULTS
Depression symptoms were only significantly associated with a composite secondary outcome comprising CVD death, nonfatal myocardial infarction, nonfatal stroke, hospitalized angina, congestive heart failure, peripheral vascular disease, coronary artery bypass graft, and carotid endarterectomy. Significant sex interactions were observed for BDI score and BDI score ≥10. BDI score was significantly associated with higher probability of this composite outcome in men but was not associated with the outcome in women. BDI score ≥10 was positively associated with this composite outcome in men but was negatively associated in women. Exploratory analysis identified a significant BDI ≥10 × ADM use interaction for this composite outcome that differed in men versus women. Men with both BDI score ≥10 and ADM use compared with those with neither had 60% higher probability of the outcome, whereas women with both compared with those with neither had 50% lower probability.
CONCLUSIONS
Sex differences in the association of depression symptoms and ADM use with incident CVD warrant further investigation.
Introduction
Substantial evidence indicates that depression is an independent predictor of cardiovascular disease (CVD) morbidity and mortality in both noncardiac and cardiac populations (1–3). Depression has been estimated to confer a relative risk between 1.5 and 2.5 for cardiac morbidity and mortality in patients with existing coronary artery disease (CAD) and a relative risk of 1.5–2.0 for CAD onset in noncardiac populations (2). Diabetes, which affected 30.2 million people ≥18 years of age, or 12.2% of the U.S. adult population, in 2015 (4) not only is a major risk factor for CVD (5) but also doubles the odds of depression (6). The estimated prevalence of comorbid depression in persons with diabetes is 20% in community samples and 32% in clinical samples (6), and there is evidence suggesting that diabetes and depression may have synergistic effects on some CVD outcomes (3).
The effect of antidepressant medication (ADM) use on CVD risk has not been clearly established and may vary depending on the type of ADM used. Cardiotoxic side effects have been reported for tricyclic antidepressants (TCAs) (7,8). Newer medications such as selective serotonin reuptake inhibitors (SSRIs) have anticoagulation properties and may improve cardiovascular (CV) morbidity and mortality in patients with established CAD (2,7,8). This potential benefit, however, has not been observed in all studies (9), and, among postmenopausal women free of CAD at baseline, SSRI use was associated with increased risk of hemorrhagic and fatal stroke (10).
The Look AHEAD (Action for Health in Diabetes) trial was a multicenter randomized controlled trial in overweight/obese adults with type 2 diabetes, 45–76 years of age, that was designed to compare the efficacy of an intensive lifestyle intervention to promote 5–10% weight loss through reduced caloric intake and increased physical activity with a diabetes support and education control group for preventing incident CVD morbidity and mortality (11,12). Depression symptoms and ADM use were assessed at multiple time points during the trial, and two articles have been published examining the association of depression symptoms and ADM use with CV risk factors both at study entry (13) and over the subsequent 4 years (14). These articles provided evidence that depression symptoms and ADM use were independently and positively associated both cross-sectionally and prospectively with a range of CV risk factors in this large cohort of individuals with type 2 diabetes. The purpose of the present article is to report the results of a preplanned analysis to determine whether depression symptom scores treated as either a continuous measure or dichotomous measure indicating the presence or absence of mild or greater depression or ADM use predict the probability of CVD events over a median of 9.6 years of follow-up.
Research Design and Methods
Study Design
Details of the design and methods of this multicenter randomized controlled clinical trial have been described elsewhere (15). As previously reported (12), the trial was stopped early (median duration of follow-up 9.6 years) based on a futility analysis that found no significant difference between treatment arms on the primary CVD outcome. The Look AHEAD trial is now continuing as a prospective observational cohort study.
Participants
Participants in the Look AHEAD trial (N = 5,145) were recruited from 16 clinical centers in the U.S. (11). Eligibility requirements included the following: age 45–76 years; self-reported type 2 diabetes verified by tested glucose levels, use of glucose-lowering medication, or physician’s report; BMI ≥25 kg/m2 (≥27 kg/m2 if taking insulin); glycosylated hemoglobin (HbA1c) ≤11% (97 mmol/mol); systolic blood pressure (SBP) <160 mmHg; diastolic blood pressure (DBP) <100 mmHg; triglycerides (TGs) <600 mg/dL; the ability to complete a valid maximal exercise test, suggesting it was safe to exercise; and an established relationship with a primary provider. Individuals with a current diagnosis of bipolar disorder or psychosis or who had been hospitalized for depression in the past 6 months were excluded.
The study was approved by the Institutional Review Board at each center, and all participants provided informed consent prior to study participation.
Depression Indicators
Depression symptoms were assessed at baseline, annually in years 1–4, and again at year 8, using the Beck Depression Inventory-1A (BDI-1A), a self-report symptom scale with well-documented psychometric properties (i.e., validity, reliability, and sensitivity to change) across a broad spectrum of clinical and nonclinical populations (16,17). The BDI-1A assesses 21 symptoms, scored 0–3 in order of increasing symptom severity, generating a total scale score ranging from 0 to 63. The presence of elevated depression symptoms, indicating likely mild or greater depression, was defined by a BDI-1A score ≥10. The BDI-1A may also be scored using two subscales by separating the 14 items that assess primarily cognitive and affective components of depression (e.g., guilt and negative mood) from the 7 items that evaluate predominantly somatic components (e.g., sleep and appetite).
Participants brought all prescription medications to their annual clinic assessment visits and these medications (but not the dosages) were recorded by study staff. ADMs were identified using the U.S. Food and Drug Administration classification system. Distinct types of ADM were also identified in the following five categories: serotonin-norepinephrine-dopamine reuptake inhibitor, serotonin-norepinephrine reuptake inhibitor, SSRI, serotonin modulator, and TCA.
Outcome Measures
The four composite CVD outcomes (one primary and three secondary) specified in the Look AHEAD protocol were examined. The primary CVD outcome was the first occurrence of a composite of death from CV causes, nonfatal myocardial infarction (MI), nonfatal stroke, and angina requiring hospitalization. There were three secondary CVD outcomes. Secondary outcome 1 differed from the primary outcome in that it excluded hospitalized angina. Secondary outcome 2 differed from the primary outcome in that it included any death rather than CVD death only. Secondary outcome 3 expanded the scope of secondary outcome 2 to include two additional CV conditions (i.e., congestive heart failure [CHF] and peripheral vascular disease) and two CV procedures (i.e., coronary artery bypass graft [CABG] and carotid endarterectomy). CV events were classified by an Events Adjudication Committee, blinded to treatment arm, that reviewed all pertinent medical records and death certificates to confirm CVD events.
Statistical Analyses
Analyses included all randomized participants (N = 5,145) and were performed using SAS version 9.3 (SAS Institute, Cary, NC). All data were censored at 14 September 2012, the date that the intervention was stopped by the National Institutes of Health (NIH) on recommendation of the Data and Safety Monitoring Board. Median follow-up at that time was 9.6 years. Because patterns in the data suggested that there may be sex differences in the association of depression indicators with CVD outcomes, the preplanned analyses were performed in sex-specific strata. Sex differences in relevant baseline characteristics were examined using χ2 tests for categorical variables and t tests for continuous variables. The association of depression indicators with CVD outcomes was examined using three sequential Cox proportional hazard models. Model 1 included clinic as a stratification variable and intervention assignment as a covariate. Model 2 added baseline age, race/ethnicity, history of CVD, BMI, and waist circumference (WC). Model 3 added additional baseline CV risk factors (i.e., HbA1c, insulin use, SBP, DBP, antihypertensive medication use, LDL cholesterol, HDL cholesterol, total cholesterol, TG, lipid-lowering medication use, ankle-brachial index outside the normal range (<0.9, >1.3) except in secondary outcome 3, smoking, estimated maximal equivalents of task (METs) achieved on stress test, and hormone replacement therapy for women only. Time to event was defined as the time from randomization to first occurrence of the outcome of interest. An individual’s contribution to the number of person-years of exposure was censored at first occurrence of an outcome event, loss to follow-up, or the closing date of 14 September 2012. Tests of the proportional hazard assumptions were performed and met the criteria. Hazard ratios (HRs) and 95% CIs for each depression indicator and CVD outcome were calculated using PROC PHREG. For these preplanned analyses, Bonferroni correction was used to adjust for multiple comparisons within sex groups and for sex × depression indicator interaction effects; thus, a two-sided P value of <0.004 (0.05 ÷ 12) was considered to indicate statistical significance. Separate forest plots were constructed for each depression indicator to display the fully adjusted sex-specific results and the sex × depression interaction effect for each CVD outcome. Depression indicators were analyzed both as baseline predictors of incident CVD events and as time-varying covariates. Because results obtained using the two approaches were very similar, only the results using baseline values of the depression indicators are presented here.
Results
Table 1 shows baseline characteristics of the sample by sex. There was no difference in the distribution of men and women in the study arms. The mean age of women, however, was 2 years less than that of men, and there were significant sex differences in the distribution of race/ethnicity, prior CVD, BMI, and WC. Among women, there was a greater proportion of race/ethnic minorities and higher mean BMI compared with men, while prior CVD was almost 2.5 times higher in men than that in women, and mean WC was higher in men as well. Men and women did not differ significantly on the proportion using insulin, HbA1c level, or mean SBP. Men had a higher mean level of maximal METs achieved; however, sex differences in all remaining covariates were suggestive of a higher level of CVD risk among men. On the other hand, women had values on the three depression indicators suggestive of a worse depression profile. Mean BDI and subscale scores were higher, prevalence of BDI score ≥10 was 1.5 times higher, and ADM use was 1.6 times higher in women compared with men.
Table 1.
Baseline characteristics of sample by sex
| Variable | Men | Women | P value |
|---|---|---|---|
| Treatment assignment, N (%) | 0.8197 | ||
| DSE | 1,038 (49.9) | 1,537 (50.2) | |
| Lifestyle intervention | 1,044 (50.1) | 1,526 (49.8) | |
| Age (years) | 59.93 (0.15) | 57.91 (0.12) | <0.0001 |
| Race/ethnicity, N (%) | <0.0001 | ||
| African American/Black (not Hispanic) | 189 (9.08) | 615 (20.1) | |
| American Indian/Native American/Alaskan Native | 55 (2.64) | 203 (6.63) | |
| Asian/Pacific Islander | 16 (0.77) | 34 (1.11) | |
| White | 1,584 (76.1) | 1,668 (54.5) | |
| Hispanic | 197 (9.46) | 483 (15.8) | |
| Other/mixed | 41 (1.97) | 59 (1.93) | |
| Prior CVD, N (%) | 439 (21.1) | 275 (8.98) | <0.0001 |
| BMI (kg/m2) | 35.18 (0.12) | 36.46 (0.11) | <0.0001 |
| WC (cm) | 118.5 (0.29) | 110.8 (0.24) | <0.0001 |
| Insulin use, N (%) | 389 (18.7) | 5 90 (19.3) | 0.6040 |
| HbA1c (% [mmol/mol]) | 7.26 [56] (0.03) | 7.29 [56] (0.02) | 0.4383 |
| Antihypertensive use, N (%) | 1,588 (76.3) | 2,253 (73.6) | 0.0292 |
| SBP (mmHg) | 128.5 (0.36) | 129.0 (0.32) | 0.2573 |
| DBP (mmHg) | 73.21 (0.20) | 68.05 (0.17) | <0.0001 |
| Lipid-lowering medication use, N (%) | 1,238 (59.5) | 1,384 (45.2) | <0.0001 |
| LDL (mg/dL) | 107.0 (0.68) | 115.9 (0.60) | <0.0001 |
| HDL (mg/dL) | 38.04 (0.20) | 47.20 (0.22) | <0.0001 |
| Total cholesterol (mg/dL) | 182.1 (0.80) | 197.1 (0.69) | <0.0001 |
| TGs (mg/dL) | 191.5 (2.75) | 175.0 (2.00) | <0.0001 |
| ABI outside normal range (<0.9 or >1.3), N (%) | 391 (18.8) | 272 (8.88) | <0.0001 |
| Smoking, N (%) | <0.0001 | ||
| Never | 782 (37.7) | 1,795 (58.7) | |
| Former | 1,198 (57.7) | 1,132 (37.0) | |
| Current | 95 (4.58) | 132 (4.32) | |
| Maximal METs achieved | 7.95 (0.05) | 6.67 (0.03) | <0.0001 |
| HRT use, N (%) | NA | 501 (16.4) | <0.0001 |
| BDI | 4.87 (0.10) | 6.21 (0.09) | <0.0001 |
| Cognitive-affective subscale | 1.98 (2.79 | 2.53 (3.08) | <0.0001 |
| Somatic subscale | 2.89 (2.37) | 3.67 (2.74) | <0.0001 |
| BDI ≥10, N (%) | 283 (13.6) | 649 (21.3) | <0.0001 |
| ADM use, N (%) | 253 (12.6) | 595 (20.1) | <0.0001 |
| Type of ADM used, N (%) | 0.4214 | ||
| SSRI | 149 (55.6) | 378 (57.8) | |
| TCA | 52 (19.4) | 124 (19.0) | |
| SNDRI | 34 (12.7) | 57 (8.7) | |
| SNRI | 17 (6.3) | 49 (7.5) | |
| SerMod | 16 (6.0) | 46 (7.0) |
Data are mean (SE) unless otherwise indicated. ABI, ankle-brachial index; DSE, diabetes support and education; HRT, hormone replacement therapy; NA, not applicable; SerMod, serotonin modulator; SNDRI, serotonin-norepinephrine-dopamine reuptake inhibitor; SNRI, serotonin-norepinephrine reuptake inhibitor. Among AMD users, 1.34% of men and 4.07% of women were taking two types of ADM; 0.44% of men and 0.49% of women were taking three types of ADM.
The total number of events included in the composite CVD outcomes and the frequency of component events are shown in Supplementary Table 1. Some participants experienced multiple events during the same admission; all are included in Supplementary Table 1, but only one contributed to the composite outcome (see footnote in Supplementary Table 1). Supplementary Tables 2–4 provide results for the sequential models stepping in covariates. Among women, none of the depression indicators were significantly associated with CVD outcomes, even in model 1. Among men, BDI score in model 1 was significantly associated with all CVD outcomes except secondary outcome 1. After model 2 covariate adjustments, BDI score remained significantly associated with secondary outcomes 2 and 3, but not with the primary outcome. After model 3 covariate adjustment, BDI score continued to be significantly associated with secondary outcome 3, but the association with secondary outcome 2 became nonsignificant when either lipids/lipid-lowering medication or maximal METs achieved were added to model 2. The results of multivariable models adjusted for all baseline covariates and including a sex × depression interaction term are shown in Figs. 1–3. The only CVD outcome significantly associated with any depression indicator was secondary outcome 3, which included events (CHF, peripheral vascular disease, CABG, carotid endarterectomy) not in other CVD outcomes.
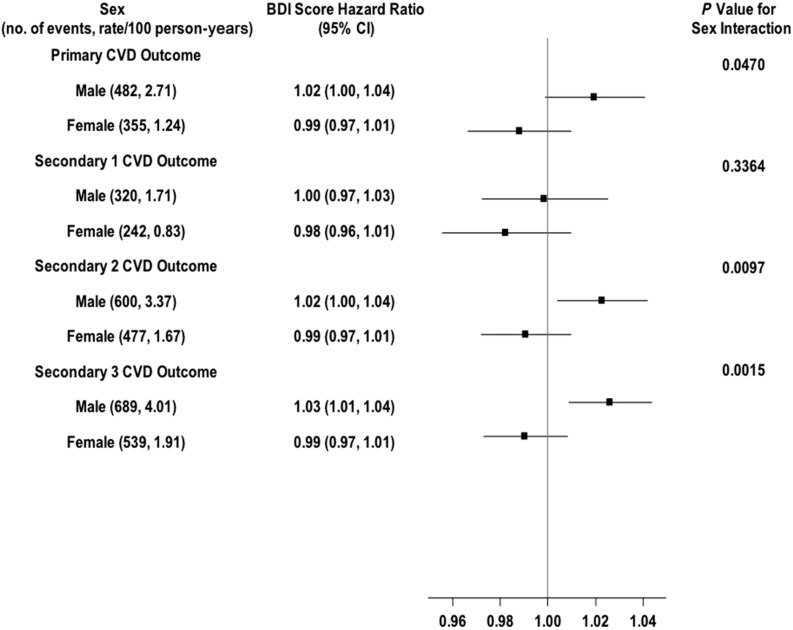
Figure 1.
Shown are the number of CVD events and the rate per 100 person-years, the HRs (95% CIs) for a 1-point increment in BDI continuous score for the primary and secondary CVD outcomes in men and women, and the P value for the sex × BDI score interaction effect. The solid vertical line indicates an HR of 1.00 (i.e., no effect).
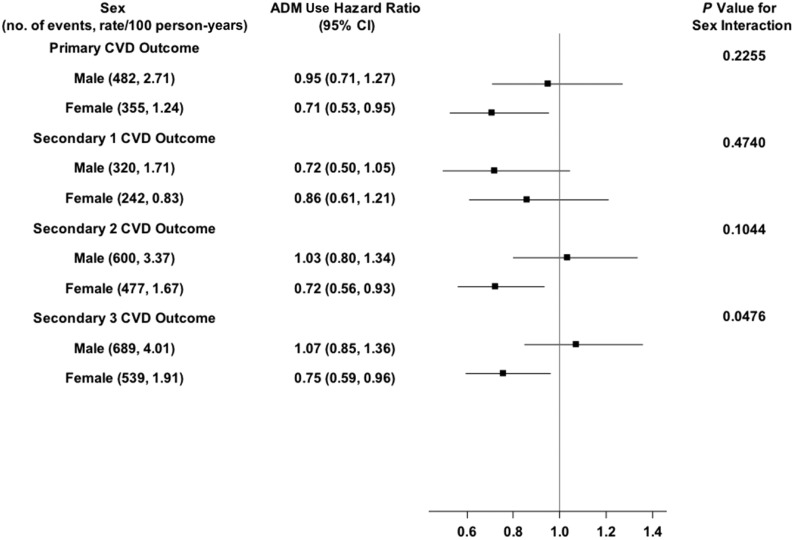
Figure 3.
Shown are the number of CVD events and the rate per 100 person-years, the HRs (95% CIs) for ADM use (ADM) for the primary and secondary CVD outcomes in men and women, and the P value for the sex × ADM use interaction effect. The solid vertical line indicates an HR of 1.00 (i.e., no effect).
For BDI score (Fig. 1), there was a statistically significant sex × BDI interaction effect (P = 0.0015). BDI score was not significantly associated with secondary outcome 3 in women but was significantly associated with increased likelihood of this outcome in men. Each 1-point increment in baseline BDI score was associated with a 3% greater likelihood of the outcome occurring (HR 1.03; 95% CI 1.01–1.04). Analyses examining the association of the separate BDI cognitive-affective and somatic subscales with the composite CVD outcomes showed a similar pattern of sex differences as that observed with the full BDI scale (data not shown).
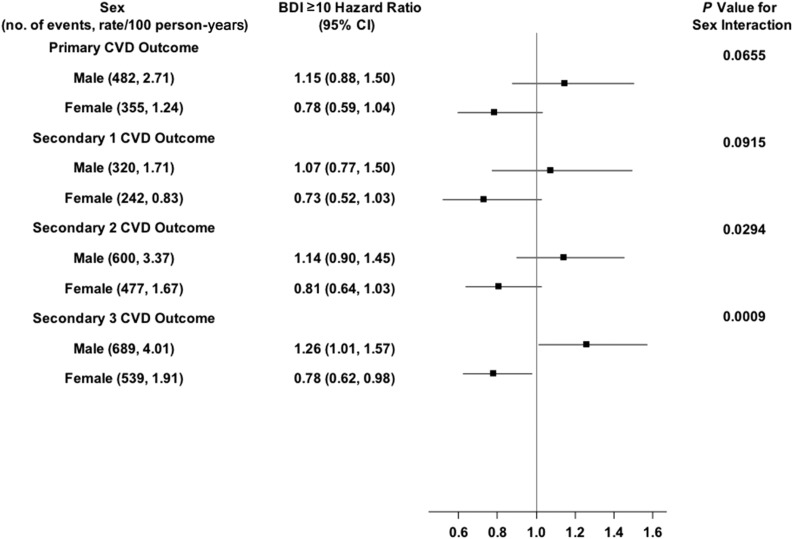
For a BDI score ≥10 (Fig. 2), there was also a statistically significant BDI score ≥10 × sex interaction effect for secondary outcome 3 (P = 0.0009), with the direction of the association opposite in the two sex groups. Men had a 26% greater likelihood of the outcome occurring (HR 1.26; 95% CI 1.01–1.57), whereas women had a 22% lower likelihood of the outcome occurring (HR 0.78; 95% CI 0.62–0.98). However, neither of these associations was statistically significant after Bonferroni correction.
Figure 2.
Shown are the number of CVD events and the rate per 100 person-years, the HRs (95% CIs) for BDI ≥10 for the primary and secondary CVD outcomes in men and women, and the P value for the sex × BDI ≥10 interaction effect. The solid vertical line indicates an HR of 1.00 (i.e., no effect).
For ADM use (Fig. 3), there was some suggestion of a sex difference in its association with secondary outcome 3. ADM use was associated with a 25% lower likelihood of the outcome in women (HR 0.75; 95% CI 0.59–0.96), but was not associated with the outcome in men (HR 1.07; 95% CI 0.85–1.36). Neither the association in women, however, nor the ADM use × sex interaction was statistically significant after Bonferroni correction.
Two exploratory analyses were performed to gain further insights into the results of these preplanned analyses: 1) an analysis to examine whether there was a BDI ≥10 × ADM use interaction in men and women for secondary outcome 3, and 2) whether specific component events were driving the finding that depression indicators were associated only with composite secondary outcome 3. Bonferroni correction was not applied for these exploratory analyses.
For the first analysis, the following four indicator variables were created: BDI <10 + no ADM use, BDI <10 + ADM use, BDI ≥10 + no ADM use, and BDI ≥10 + ADM use. BDI <10 + no ADM use was used as the reference category. In women, BDI ≥10 + ADM use was significantly associated with a 50% decreased likelihood of secondary outcome 3 occurring (HR 0.50; 95% CI 0.32–0.78; P = 0.0020), whereas in men it was significantly associated with a 60% increased likelihood of this outcome occurring (HR 1.60; 95% CI 1.12–2.28; P = 0.0099). The BDI score ≥10 × ADM use interaction effect was statistically significant in men (P = 0.04) and trended toward significance in women (P = 0.07).
For the second exploratory analysis, there was a sufficient number of events for any death, nonfatal MI, hospitalized angina, CABG, and CHF (Supplementary Table 1) to examine associations with the depression indicators separately in men and women. In men, BDI score was associated with increased likelihood of hospitalized angina (HR 1.03; 95% CI 1.00–1.06; P = 0.0483), BDI ≥10 was associated with none of these events, and ADM use was significantly associated with decreased likelihood of nonfatal MI (HR 0.47; 95% CI 0.27–0.82; P = 0.0077). In women, BDI score and BDI score ≥10 were associated with none of these events, whereas ADM use was significantly associated with decreased likelihood of both hospitalized angina (HR 0.52; 95% CI 0.32–0.86; P = 0.0114) and CABG (HR 0.60; 95% CI 0.39–0.93; P = 0.0211).
Conclusions
In the current study, we performed sex-stratified analyses of the association of three depression indicators with incident CVD outcomes because patterns in the data suggested that this association might differ between men and women. The results of multivariable analyses, adjusting for trial design and a wide range of baseline covariates, identified significant sex differences in the association of depression indicators with secondary outcome 3, which was the only CVD outcome significantly associated with any depression indicator. Depression symptoms treated as a continuous BDI score were significantly associated with higher probability of secondary outcome 3 in men but were not associated with this outcome in women. A similar pattern of sex differences was observed in separate analyses with the BDI subscales. The sex × BDI score interaction effect was statistically significant, supporting the conclusion that depression symptoms were differentially associated with this outcome in men compared with women. A statistically significant sex interaction effect with elevated depression symptoms (BDI score ≥10) was also observed for secondary outcome 3 (i.e., the direction of the association was opposite for men and women), with BDI score ≥10 associated with increased likelihood of the outcome in men and decreased likelihood in women. The ADM use × sex interaction effect was not statistically significant; however, exploratory analyses identified a significant BDI ≥10 × ADM use interaction that differed in the two sex groups. Men with both BDI score ≥10 and ADM use compared with those with neither had a 60% higher probability of this outcome (P = 0.0099), whereas women with both compared with those with neither had 50% lower probability (P = 0.0020). The BDI score ≥10 × ADM use interaction effect was statistically significant in men and trended toward significance in women.
The finding of no significant association between BDI score and CVD composite outcomes in women was unexpected as some evidence suggests that women with diabetes who are depressed have more rapid development of CVD compared with those who are not depressed (18). It should be noted, however, that previous population-based, prospective studies examining sex differences in the depression-CVD association have yielded mixed results. Some studies found increased risk of incident CVD outcomes in women but not men (19–22), some found increased risk in men but not women (23), and others found increased risk in both men and women (1,24). These mixed results may be due, at least in part, to variations in design characteristics across studies, including the baseline age of the population, instrument to assess depression, CV/mortality end points, length of follow-up, and covariates included in multivariable analyses. Although they provide inconsistent evidence about whether men or women are at greater risk of CVD outcomes, these studies as well as our own suggest a need for a more careful and systematic investigation of sex differences in the association of depression with CVD and the mechanisms that account for observed differences. It has been noted (25) that the vast majority of mechanistic studies examining the link between depression and CVD have not reported sex-specific results.
Given the earlier Look AHEAD trial publication that reported higher 4-year incidence of CVD risk factors among participants using ADM (14), we hypothesized that both men and women who were ADM users would have a higher probability of incident CVD outcomes compared with those who were nonusers. Thus, the lack of significant associations between ADM use and any of the CVD composite outcomes in either men or women in the preplanned analyses was unexpected, as was the finding in exploratory analyses of a significant BDI ≥10 × ADM use interaction effect for secondary outcome 3. Several studies in the psychiatry and psychopharmacology literature, however, have reported sex differences in antidepressant response to different types of ADM. For example, a randomized treatment trial comparing a TCA to an SSRI ADM found that men compared with women were more likely to show a favorable response to the TCA, while women were more likely than men to show a favorable response to the SSRI. Among postmenopausal women, however, there was no difference in response to these two types of ADM, leading investigators to hypothesize that female sex hormones may enhance response to SSRIs and inhibit response to TCAs. In this regard, it should be noted that 82.1% of women in the Look AHEAD trial were postmenopausal at baseline. Another study using data from 15 placebo-controlled trials found that the magnitude of women’s antidepressant response with SSRIs was twice as high as that in men and posited that the higher concentration of adipose tissue in women may allow for greater accumulation and slower release of SSRIs, which are lipophilic in nature (26). That study, as well as a large treatment trial of SSRIs in which women demonstrated a 33% greater likelihood of depression remission versus men, also posited sex-specific biological differences in the serotonergic system as a plausible mechanism for observed sex differences (27). A comprehensive discussion of mechanisms underlying sex differences in antidepressant response to different types of ADM is provided by Yonkers and Brawman-Mintzer (28).
In addition to their antidepressant effects, both TCAs and SSRIs have effects that may directly impact cardiac risk. TCAs may increase cardiac risk by altering heart rate and rhythm; SSRIs may lower cardiac risk by decreasing platelet aggregation (2,7,29). ADMs may therefore affect CVD outcomes not only via their impact on depression but also on physiological/biological factors that alter cardiac risk. There was no statistically significant difference between sex groups in the type of ADM used, and over half of men and women (55.6% and 57.8%, respectively) who took ADMs were taking SSRIs. Women’s greater antidepressant response to SSRIs compared with that of men may partially explain why women with a BDI score ≥10 and using ADM had a substantially lower probability of secondary outcome 3 relative to those with neither condition. The reason why men with a BDI score ≥10 and ADM use compared with those with neither had a 60% higher probability of secondary outcome 3 is less clear. Although men may have a smaller antidepressant response to SSRIs compared with women, there is no evidence to suggest that SSRIs adversely affect depression or cardiac risk in men. A possible explanation may be derived from an American Psychological Association report (30) indicating that men are less likely than women to seek help for all mental health problems and are particularly resistant to seeking help for depression and experience greater discomfort than women with emotional expression. Consistent with national trends (25), the proportion of ADM users in our study was 70% higher in women than in men, and women were more likely to use ADMs at lower levels of depression symptoms. Thus, men may not only have waited until they experienced higher levels of depression symptoms before seeking treatment, but once they began using ADMs were less adherent and less likely to engage in concomitant cognitive behavioral therapy, resulting in a higher probability of adverse cardiac events.
Given its frequency and specificity to secondary outcome 3, it seems likely that CABG was a major component accounting for the fact that significant main effects were found only for this composite outcome. Exploratory component analyses also called attention to potential differences in the relationship of depression symptoms and ADM use to different types of CVD events and to potential sex differences in these relationships. The observed negative association of ADM use with nonfatal MI in men when the overall association with composite secondary outcome 3 was positive is consistent with the known concern that use of composite outcomes may sometimes mask relationships with individual component events when these differ from that observed for the composite outcome as a whole (31,32).
This study has several limitations. Although analyses were preplanned and based on data collected in a randomized controlled trial, the present analyses comprise an observational study controlling for treatment arms. More severe levels of depression symptoms were not represented in the sample; however, a dose-response relationship between depression and CVD across the full range of depression symptom scores has been found in other studies (33). ADMs are prescribed for indications other than depression (e.g., smoking cessation, social anxiety disorder, peripheral neuropathy), but we did not confirm that all participants taking ADMs were doing so because of depression. In addition, we did not obtain information about the dosage or duration of ADM treatment or about concomitant treatments such as cognitive behavioral therapy. Important areas for future investigation thus include sex differences in ADM prescribing, reaching therapeutic dose, treatment adherence in ADM use, and the use of concomitant treatments for depression also shown to be effective. We examined the moderating effect of sex on the association of depression indicators with CVD outcomes, but we did not explore the data further to identify other potential moderators or perform mediation analyses to identify potential intervening variables that might explain the moderating effects of sex on the association between depression indicators and CVD outcomes (34). Although we discuss factors that may mediate the observed sex × depression interaction effect, we did not have the necessary data to perform a formal mediation analysis. Last, these results may not apply to persons without diabetes or to people with diabetes who would not volunteer for a clinical trial or meet the strict eligibility criteria for this clinical trial. Notably, eligibility included passing a maximal exercise tolerance test (which suggests participants would have lower risk of CVD) and not having severe depression.
The study also has important strengths. It was conducted in a large, multiethnic cohort of individuals with type 2 diabetes and, to our knowledge, is the first study to examine sex differences in the association of depression with CVD in a cohort comprised exclusively of individuals with type 2 diabetes. The cohort had substantial numbers of both men and women with high rates of retention and no differential dropout, and sex × depression interaction effects were formally tested in the analyses. P values were corrected for multiple comparisons with the exception of the two exploratory analyses. CV events were classified by an Events Adjudication Committee, blinded to treatment arm, that reviewed all pertinent medical records and death certificates to confirm CVD events. Participants brought all prescription medications to the annual study visit, and ADM use and type were coded using a standardized classification system. The study examined not only depression symptoms but also ADM use as predictors of CVD outcomes. The latter is particularly important as data from the National Health and Nutrition Examination Surveys, 2011–2014, found that 12.7% of Americans ≥12 years old take ADM; among persons 40–59 years old, 11.6% of men and 21.2% of women take ADM; and among persons ≥60 years old, 12.6% of men and 24.4% of women take ADM (35).
Prevalence of depression is about twice as high in women as in men (24,36), and substantial evidence indicates that women differ from men in multiple aspects of CAD, including age of onset, presenting symptoms, traditional and psychosocial risk factors, and outcomes following MI and CABG (36,37). This is one of the few studies, however, that has examined sex differences in the association of depression symptoms and ADM use with CVD. The results provide evidence that, among individuals with type 2 diabetes, there are differences between men and women in the effects of both depression symptoms and ADM use on the probability of incident CVD events. Careful consideration should be given to the implications of these findings for the design of future studies and the management of depression and ADM use in the presence of type 2 diabetes.
Supplementary Material
Article Information
Acknowledgments. Dr. Richard Rubin (Johns Hopkins School of Medicine, now deceased) wrote the initial proposal for this article. This publication is dedicated to his memory. Some of the information contained herein was derived from data provided by the Bureau of Vital Statistics, New York City Department of Health and Mental Hygiene.
Funding. This research was funded by the NIH through cooperative agreements with the National Institute of Diabetes and Digestive and Kidney Diseases: DK-57136, DK-57149, DK-56990, DK-57177, DK-57171, DK-57151, DK-57182, DK-57131, DK-57002, DK-57078, DK-57154, DK-57178, DK-57219, DK-57008, DK-57135, and DK-56992. Additional funding was provided by the National Heart, Lung, and Blood Institute, National Institute of Nursing Research, National Center on Minority Health and Health Disparities, NIH Office of Research on Women’s Health, and the Centers for Disease Control and Prevention. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The Indian Health Service provided personnel, medical oversight, and use of facilities. Additional support was received from The Johns Hopkins Medical Institutions Bayview General Clinical Research Center (M01-RR-02719), the Massachusetts General Hospital Mallinckrodt General Clinical Research Center and the Massachusetts Institute of Technology General Clinical Research Center (M01-RR-01066), the Harvard Clinical and Translational Science Center (RR-025758-04), the University of Colorado Health Sciences Center General Clinical Research Center (M01-RR-00051) and Clinical Nutrition Research Unit (P30-DK-48520), the University of Tennessee at Memphis General Clinical Research Center (M01-RR-0021140), the University of Pittsburgh General Clinical Research Center (GCRC) (M01-RR-000056), the Clinical Translational Research Center (CTRC) funded by the Clinical and Translational Science Award (UL1-RR-024153) and NIH grant (DK-046204), the Department of Veterans Affairs VA Puget Sound Health Care System Medical Research Service, and the Frederic C. Bartter General Clinical Research Center (M01-RR-01346).
The opinions expressed in this article are those of the authors and do not necessarily reflect the views of the Indian Health Service or other funding sources.
Duality of Interest. The following organizations have committed to make major contributions to Look AHEAD trial: FedEx Corporation; Health Management Resources; LifeScan, Inc., a Johnson & Johnson Company; OPTIFAST of Nestlé HealthCare Nutrition, Inc.; Hoffmann-La Roche Inc.; Abbott Nutrition; and Slim-Fast Brand of Unilever North America. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. H.P.H. contributed to the design of the study, interpreted the data, and drafted/revised the manuscript. S.A.G. contributed to the design of the study, performed the statistical analyses, interpreted the data, and reviewed/edited the manuscript. R.R.W., S.Z.Y., K.C.J., M.C., T.A.W., E.S.H., B.V.D., and W.C.K. contributed to the design of the study, interpreted the data, and reviewed/edited the manuscript. S.A.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT00017953, clinicaltrials.gov
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-0575/-/DC1.
A complete list of The Look AHEAD Research Group can be found in the Supplementary Data online.
References
- 1.Ariyo AA, Haan M, Tangen CM, et al.; Cardiovascular Health Study Collaborative Research Group . Depressive symptoms and risks of coronary heart disease and mortality in elderly Americans. Circulation 2000;102:1773–1779 [DOI] [PubMed] [Google Scholar]
- 2.Lett HS, Blumenthal JA, Babyak MA, et al. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med 2004;66:305–315 [DOI] [PubMed] [Google Scholar]
- 3.Egede LE, Nietert PJ, Zheng D. Depression and all-cause and coronary heart disease mortality among adults with and without diabetes. Diabetes Care 2005;28:1339–1345 [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2017. Atlanta, GA, Centers for Disease Control and Prevention, U.S. Department of Health and Human Services, 2017 [Google Scholar]
- 5.Benjamin EJ, Virani SS, Callaway CW, et al.; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics–2018 update: a report from the American Heart Association [published correction appears in Circulation 2018;137:e493]. Circulation 2018;137:e67–e492 [DOI] [PubMed] [Google Scholar]
- 6.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care 2001;24:1069–1078 [DOI] [PubMed] [Google Scholar]
- 7.Cohen HW, Gibson G, Alderman MH. Excess risk of myocardial infarction in patients treated with antidepressant medications: association with use of tricyclic agents. Am J Med 2000;108:2–8 [DOI] [PubMed] [Google Scholar]
- 8.Whooley MA, Wong JM. Depression and cardiovascular disorders. Annu Rev Clin Psychol 2013;9:327–354 [DOI] [PubMed] [Google Scholar]
- 9.Taylor CB, Youngblood ME, Catellier D, et al.; ENRICHD Investigators . Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry 2005;62:792–798 [DOI] [PubMed] [Google Scholar]
- 10.Smoller JW, Allison M, Cochrane BB, et al. Antidepressant use and risk of incident cardiovascular morbidity and mortality among postmenopausal women in the Women’s Health Initiative study. Arch Intern Med 2009;169:2128–2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pi-Sunyer X, Blackburn G, Brancati FL, et al.; Look AHEAD Research Group . Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care 2007;30:1374–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wing RR, Bolin P, Brancati FL, et al.; Look AHEAD Research Group . Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes [published correction appears in N Engl J Med 2014;370:1866]. N Engl J Med 2013;369:145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubin RR, Gaussoin SA, Peyrot M, et al.; Look AHEAD Research Group . Cardiovascular disease risk factors, depression symptoms and antidepressant medicine use in the Look AHEAD (Action for Health in Diabetes) clinical trial of weight loss in diabetes. Diabetologia 2010;53:1581–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubin RR, Peyrot M, Gaussoin SA, et al.; Look AHEAD Research Group . Four-year analysis of cardiovascular disease risk factors, depression symptoms, and antidepressant medicine use in the Look AHEAD (Action for Health in Diabetes) clinical trial of weight loss in diabetes. Diabetes Care 2013;36:1088–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryan DH, Espeland MA, Foster GD, et al.; Look AHEAD Research Group . Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials 2003;24:610–628 [DOI] [PubMed] [Google Scholar]
- 16.Beck AT, Steer RA. Beck Depression Inventory: Manual. San Antonio, TX, The Psychological Corporation, 1987 [Google Scholar]
- 17.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev 1988;8:77–100 [Google Scholar]
- 18.Clouse RE, Lustman PJ, Freedland KE, Griffith LS, McGill JB, Carney RM. Depression and coronary heart disease in women with diabetes. Psychosom Med 2003;65:376–383 [DOI] [PubMed] [Google Scholar]
- 19.Haukkala A, Konttinen H, Uutela A, Kawachi I, Laatikainen T. Gender differences in the associations between depressive symptoms, cardiovascular diseases, and all-cause mortality. Ann Epidemiol 2009;19:623–629 [DOI] [PubMed] [Google Scholar]
- 20.Williams SA, Kasl SV, Heiat A, Abramson JL, Krumholz HM, Vaccarino V. Depression and risk of heart failure among the elderly: a prospective community-based study. Psychosom Med 2002;64:6–12 [DOI] [PubMed] [Google Scholar]
- 21.Mendes de Leon CF, Krumholz HM, Seeman TS, et al. Depression and risk of coronary heart disease in elderly men and women: New Haven EPESE, 1982-1991. Established Populations for the Epidemiologic Studies of the Elderly. Arch Intern Med 1998;158:2341–2348 [DOI] [PubMed] [Google Scholar]
- 22.Gilmour H. Depression and risk of heart disease. Health Rep 2008;19:7–17 [PubMed] [Google Scholar]
- 23.Penninx BW, Guralnik JM, Mendes de Leon CF, et al. Cardiovascular events and mortality in newly and chronically depressed persons > 70 years of age. Am J Cardiol 1998;81:988–994 [DOI] [PubMed] [Google Scholar]
- 24.Ferketich AK, Schwartzbaum JA, Frid DJ, Moeschberger ML. Depression as an antecedent to heart disease among women and men in the NHANES I study. National Health and Nutrition Examination Survey. Arch Intern Med 2000;160:1261–1268 [DOI] [PubMed] [Google Scholar]
- 25.Hayes SN. Broken-hearted women: the complex relationship between depression and cardiovascular disease. Womens Health (Lond) 2009;5:709–725 [DOI] [PubMed] [Google Scholar]
- 26.Khan A, Brodhead AE, Schwartz KA, Kolts RL, Brown WA. Sex differences in antidepressant response in recent antidepressant clinical trials. J Clin Psychopharmacol 2005;25:318–324 [DOI] [PubMed] [Google Scholar]
- 27.Young EA, Kornstein SG, Marcus SM, et al. Sex differences in response to citalopram: a STAR*D report. J Psychiatr Res 2009;43:503–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yonkers KA, Brawman-Mintzer O. The pharmacologic treatment of depression: is gender a critical factor? J Clin Psychiatry 2002;63:610–615 [DOI] [PubMed] [Google Scholar]
- 29.Goldston K, Baillie AJ. Depression and coronary heart disease: a review of the epidemiological evidence, explanatory mechanisms and management approaches. Clin Psychol Rev 2008;28:288–306 [DOI] [PubMed] [Google Scholar]
- 30.American Psychological Association. Men: a different depression [Internet], 2005. Available from http://www.apa.org/research/action/men.aspx. Accessed 8 November 2017
- 31.Freemantle N, Calvert M, Wood J, Eastaugh J, Griffin C. Composite outcomes in randomized trials: greater precision but with greater uncertainty? JAMA 2003;289:2554–2559 [DOI] [PubMed] [Google Scholar]
- 32.Goldberg R, Gore JM, Barton B, Gurwitz J. Individual and composite study endpoints: separating the wheat from the chaff. Am J Med 2014;127:379–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rowan PJ, Haas D, Campbell JA, Maclean DR, Davidson KW. Depressive symptoms have an independent, gradient risk for coronary heart disease incidence in a random, population-based sample. Ann Epidemiol 2005;15:316–320 [DOI] [PubMed] [Google Scholar]
- 34.Fairchild AJ, MacKinnon DP. A general model for testing mediation and moderation effects. Prev Sci 2009;10:87–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pratt LA, Brody DJ, Gu Q. Antidepressant Use Among Persons Aged 12 and Over: United States, 2011–2014. Hyattsville, MD, National Center for Health Statistics, 2017. (NCHS Data Brief No. 283) [PubMed] [Google Scholar]
- 36.Möller-Leimkühler AM. Gender differences in cardiovascular disease and comorbid depression. Dialogues Clin Neurosci 2007;9:71–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lawton JS. Sex and gender differences in coronary artery disease. Semin Thorac Cardiovasc Surg 2011;23:126–130 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.