Abstract
OBJECTIVE
To determine whether self-monitoring of blood glucose (SMBG) is associated with lower HbA1c in youth with type 2 diabetes taking oral medications only or after starting insulin for persistently elevated HbA1c.
RESEARCH DESIGN AND METHODS
Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study participants (n = 699) taking oral medications were asked to perform SMBG twice daily. After reaching primary outcome (PO) (HbA1c ≥8% [64 mmol/mol]) over 6 months or an inability to wean from temporary insulin because of metabolic decompensation), insulin glargine was started. HbA1c and percent of SMBG (SMBG%) (percent days when the meter was used one or more times) before and after PO were analyzed.
RESULTS
SMBG declined over time and was inversely related to HbA1c (P < 0.0001). Of 298 youth who reached PO and started insulin, 282 had SMBG data. At PO, mean ± SD age was 15.8 ± 2.3 years, BMI 35.5 ± 7.9 kg/m2, and HbA1c 9.6 ± 2.0% (81 ± 21.9 mmol/mol); 65.3% were female. Median SMBG% was 40% at PO, which increased to 49% after 6 months and fell to 41% after 1 year on insulin. At PO, 22% of youth checked ≥80% of days, which increased to 25% and fell to 19% after 6 and 12 months using insulin, respectively. At PO, compared with those who checked <80%, youth who checked ≥80% were younger and with a lower BMI, HbA1c, and blood pressure. SMBG ≥80% was associated with ≥1% reduction in HbA1c at 6 and 12 months after insulin initiation.
CONCLUSIONS
Low SMBG adherence was common and associated with higher HbA1c. Optimal SMBG frequency in youth using or not using insulin, and whether less frequent SMBG is a marker for overall worse self-care, require further study.
Introduction
There is general agreement that individuals with type 1 and type 2 diabetes treated with insulin should perform self-monitoring of blood glucose (SMBG) (1). For adults with type 2 diabetes receiving noninsulin therapy, the need for daily SMBG is controversial, with some studies suggesting no improvement in HbA1c, self-care, or quality of life (2–8). This is particularly true for patients on stable regimens and treated with medications such as metformin and rosiglitazone, which are not associated with risk for hypoglycemia. When veterans with stable type 2 diabetes controlled on oral agents or diet therapy were asked to reduce frequency of performing SMBG to twice weekly, there was substantial cost savings without affecting glucose control (2). In the Monitor Trial, adults age >30 years (mean age 61 years) with type 2 diabetes who were not treated with insulin were randomized to not use SMBG or to use once-daily SMBG with or without enhanced messaging. These tailored messages were based on SMBG readings and time of day, were delivered on their meter, and were aimed to educate and motivate the individual with type 2 diabetes. There were no differences between the groups in glycemic control (HbA1c) or health-related quality of life (3). The utility of SMBG in youth with type 2 diabetes not receiving insulin therapy has not been studied. Given the costs and burden of SMBG, understanding whether SMBG is of benefit in this unique population is important.
In the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study, youth with recent-onset type 2 diabetes were randomized to treatment with maximum tolerated doses of metformin plus placebo, metformin plus rosiglitazone, or metformin plus an intensive lifestyle intervention as previously described (9–13). All participants were instructed to perform SMBG twice daily (supplies and incentives for adherence provided). There was a general reduction in medication adherence over time in all treatment groups, but low medication adherence did not predict loss of glycemic control (10). SMBG adherence has not been previously reported.
In TODAY, because participants were initially treated with maximum tolerated doses of metformin plus placebo, rosiglitazone, or lifestyle changes, medication doses were not adjusted on the basis of SMBG results. When insulin therapy was initiated, the dose of insulin was titrated per the blood glucose level obtained by SMBG. SMBG is more important with insulin treatment not only for dose adjustments but also because of the increased risk for hypoglycemia.
The primary objective of the current study was to examine SMBG and its association with HbA1c during the TODAY study before and after insulin therapy was initiated. Our hypothesis was that SMBG would increase after the initiation of insulin therapy and would be associated with better glycemic control. Demographic factors, depression, quality of life, comorbidities, and their relationships to adherence to SMBG are also described with the hope that these results will help to inform the direction of future studies in youth with type 2 diabetes. These analyses are exploratory because the TODAY study was not designed to investigate benefits of SMBG in youth-onset type 2 diabetes.
Research Design and Methods
The TODAY study design and characteristics of participants have been described in detail (9–13) (Supplementary Fig. 1). The collaborative study group included 15 clinical centers, a data coordinating center, and various central laboratories (9). Between July 2004 and February 2009, the trial enrolled 699 youth ages 10–17 years with type 2 diabetes that was diagnosed within 2 years of enrollment, BMI ≥85th percentile, fasting C-peptide >0.6 ng/mL, and absence of pancreatic autoimmunity. Before randomization, participants successfully completed a 2- to 6-month run-in period (13) that included attaining glycemic control (HbA1c <8% [64 mmol/mol] measured monthly for at least 2 months), taking 1,000–2,000 mg of metformin, mastering standard diabetes education, demonstrating ≥80% adherence to study metformin for at least 8 of 12 consecutive weeks, and attending study visits.
Eligible participants were randomized to one of three treatment arms: 1) metformin plus placebo, 2) metformin plus rosiglitazone, and 3) metformin plus an intensive lifestyle behavior change program. The primary objective of TODAY was to compare the three arms on time to treatment failure (i.e., loss of glycemic control defined as either HbA1c ≥8% [64 mmol/mol] over a 6-month period or inability to wean from temporary insulin therapy within 3 months after acute metabolic decompensation). After an average follow-up of 3.9 years, 319 (45.6%) participants reached the primary outcome (PO), with a median time to treatment failure of 11 months (9). At PO, metformin was continued, rosiglitazone was discontinued, and insulin therapy was initiated with once-daily insulin glargine. Insulin dosing was intensified as needed.
As part of standard diabetes education, all participants were instructed to monitor blood glucose levels twice daily, generally fasting and 2 h postprandial. Glucose meters and monitoring supplies were provided free of charge. Participants were seen every 2 months in year 1 and quarterly thereafter for purposes of medical monitoring and management and distribution of study drug; physical measurements were made, and blood and urine samples were sent to a central study laboratory (9).
Hypertension was defined as blood pressure ≥130/80 mmHg or ≥95th percentile for age, sex, and height; dyslipidemia as LDL ≥130 mg/dL or triglycerides ≥150 mg/dL; and microalbuminuria as urine albumin:creatinine ratio ≥30 μg/mg (9). Health-related quality of life and depressive symptoms were measured by self-report using the Pediatric Quality of Life Inventory and Children’s Depression Inventory as previously described (14–16). Study medication adherence was calculated at each visit as percent of study drug taken on the basis of pill counts; study medication adherence was not normally distributed and was analyzed as above or below 80%, the cutoff used during the study to monitor adequate study medication adherence. The 80% cutoff was chosen on the basis of data in previous publications (17–19).
Participants were instructed to bring their glucose meter to each visit (adherence range 85–92%) for download and review by study staff. Downloaded data were transmitted electronically to the TODAY coordinating center. The SMBG analysis used data from meter downloads. When analyzing data for the first 2 years of intervention when participants were taking oral study drugs only, data collected at visits before the initiation of insulin were included. Percent of SMBG (SMBG%) was computed as the percent of days between study visits on which the meter was used at least once (e.g., for a participant who used the meter on 63 days between visits 90 days apart, SMBG% = 70%).
Participants received incentives at each study visit using a system of points awarded for positive adherence behaviors. For study medication, points were given for bringing pill containers and log books to visits, and more points were earned as adherence levels rose to >80%. For SMBG, at each visit, 1 point was given for bringing the meter to the visit, 1 point for checking once on ≥80% of days, and 2 points for checking at least twice on ≥80% of days. Participants could accumulate points across visits and exchange for gift cards in amounts proportional to the number of points exchanged up to a maximum of incentives worth $150 annually. Incentives were provided throughout the TODAY study for all participants regardless of whether they had reached PO (and whether they were taking insulin).
The protocol was approved by the institutional review boards of the participating institutions. Parents/guardians signed informed consent for children, and youth signed informed assent according to local practice.
Statistical Analysis
Descriptive statistics of behaviors associated with SMBG are based on 548 participants who brought meters to study visits at least 85% of the time during follow-up visits from months 2 to 24. Participants were excluded from the analysis sample if they 1) experienced PO or left the study before 6 months or 2) were administered temporary insulin use during the period under study and followed a different SMBG protocol during that time. Additional analyses examined behaviors and outcomes in a subset of 282 participants who reached PO and started insulin therapy.
Demographic and clinical characteristics were compared between SMBG% groups (≥80% vs. <80%) at the time of PO using the Student t test or Wilcoxon rank sum test for quantitative variables and the χ2 test for categorical variables. Generalized linear mixed models were used for longitudinal analyses to adjust for the repeated measures per participant. Significance was defined as P < 0.05 with no adjustment for multiple comparisons. All analyses were considered exploratory, and statistical significance was defined as P < 0.05.
Results
SMBG During the First 2 Years of TODAY With Use of Oral Glycemic Control Medications Only
In the TODAY study, during the first 2 years of treatment with metformin plus placebo, metformin plus rosiglitazone, or metformin plus an intensive lifestyle intervention, 548 participants (78% of TODAY cohort) brought meters to study visits at least 85% of the time. There were no significant baseline differences in sex, race/ethnicity, highest household education, household annual income, age, diabetes duration, impaired quality of life, presence of depressive symptoms, BMI, percent overweight, and treatment group between participants who brought study meters ≥85% of the time (n = 548) versus those who did not (n = 151). The only significant difference was lower baseline HbA1c in the analysis sample (5.9% [41 mmol/mol] vs. 6.5% [48 mmol/mol]; P < 0.0001). The percent who failed to maintain glycemic control on randomized treatment at 24 months was 39.2% versus 45.6% in the entire cohort at end of study (average follow-up 3.9 years).
SMBG was performed as instructed (at least twice a day) 59% of the time initially, but this was not sustained and fell to <50% by the end of the first year of follow-up. As shown in Supplementary Fig. 2A, the percent of days during which SMBG was performed only once daily, twice daily, or three or more times daily was 22%, 46%, and 15% at month 2 and 23%, 28%, and 10% at year 2, respectively. The percent of days during which SMBG was not performed at all doubled from 23% between baseline and the 2-month visit to 46% at the 2-year visit. More than 90% of participants performed SMBG at least twice a week (Supplementary Fig. 2B). The percent of weeks during which SMBG was performed once, twice, or three or more times per week was 32%, 23%, and 91% at month 2 and 31%, 24%, and 81% at year 2, respectively.
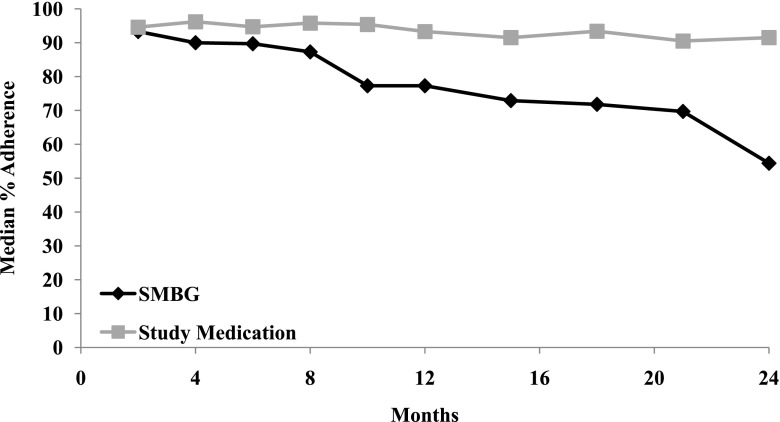
As shown in Fig. 1, over the first 2 years of the study, oral study medication adherence (defined as ≥80%) remained relatively stable, whereas SMBG adherence declined (interaction P < 0.0001). Median SMBG% decreased over time from 93.3% at month 2 to 54.4% at 2 years (P < 0.0001) (Fig. 1). Overall, the median percent of days that SMBG occurred at least once a day was ∼82%. Adherence to recommended SMBG was not associated with sex, race/ethnicity, highest household education, household annual income, diabetes duration, or treatment group (Supplementary Table 1). Younger participants showed greater adherence to SMBG procedures; median SMBG% across all 24 months of follow-up was 90.0% among 10- to 12-year-olds, 84.4% among 13- to 15-year-olds, and 73.2% among 16- to 18-year-olds.
Figure 1.
Adherence (≥80%) to SMBG and study medications (metformin ± rosiglitazone) over the first 2 years of TODAY.
In TODAY, the presence at baseline of depressive symptoms was related to worse medication adherence (10). It was therefore of interest to examine the possible association of depression with SMBG adherence. In addition, studies examining SMBG in adults with non–insulin-treated type 2 diabetes reported either no effect or a negative impact of SMBG on quality of life, but this was not examined in youth (5,7). In the current study, there was no significant relationship between SMBG% and presence of clinically depressive symptoms (P = 0.1150) or impaired quality of life (P = 0.4426).
Four comorbidities were examined: hypertension, LDL dyslipidemia, triglyceride dyslipidemia, and microalbuminuria. These comorbidities require additional therapy, including treatment with oral medications. The burdens (additional medications as well as emotional burden) of comorbidities could affect adherence to SMBG. To assess the effect of burden of comorbidities on SMBG%, the number of comorbidities present was categorized at each visit (0, 1, 2, or 3–4). There was a statistically significant association between the number of comorbidities and SMBG% (P = 0.0061) (Supplementary Fig. 3). SMBG% remained at 80–85% across 0, 1, or 2 comorbidities and fell to 43% in participants with 3–4 comorbidities.
SMBG Compared 2 Years Before and After Insulin Initiation
There were 319 TODAY participants who reached PO during the study. Among these, 298 were started on insulin therapy, and 282 of the 298 had SMBG data at the time of PO. TODAY participant characteristics at the time of insulin initiation (n = 282) are shown in Table 1. At PO, mean ± SD age was 15.8 ± 2.3 years, and 65.3% of participants were female, 38.3% non-Hispanic black, 16.3% Hispanic, and 7.1% non-Hispanic white. BMI was 35.5 ± 7.9 kg/m2, and HbA1c was 9.6 ± 2.0% (81 ± 21.9 mmol/mol). Compared with those who checked <80% at PO, youth who checked ≥80% were younger (14.7 vs. 16.0 years) and had a lower BMI (33.2 vs. 36.2 kg/m2), lower HbA1c (9.1% [76 mmol/mol] vs. 9.7% [83 mmol/mol]), and lower blood pressure (114/68 vs. 118/71 mmHg; all P < 0.05).
Table 1.
Characteristics of the TODAY study participants who reach PO at time of insulin initiation overall and by SMBG%
| Characteristic | At time of insulin initiation | SMBG% ≥80% | SMBG% <80% | P value | BMI-adjusted P value |
|---|---|---|---|---|---|
| n | 282 | 61 | 221 | ||
| Age (years) | 15.8 ± 2.3 | 14.7 ± 2.1 | 16.0 ± 2.3 | <0.0001 | — |
| Female | 65.3 | 67.2 | 64.7 | 0.7158 | — |
| Race/ethnicity | |||||
| Black non-Hispanic | 38.3 | 34.4 | 39.4 | 0.0500 | — |
| Hispanic | 16.3 | 27.9 | 13.1 | ||
| White non-Hispanic | 7.1 | 4.9 | 7.7 | ||
| Other | 38.3 | 32.8 | 39.8 | ||
| Highest household education | |||||
| High school or less | 55.9 | 54.1 | 56.4 | 0.5856 | — |
| College/associates degree | 31.5 | 29.5 | 32.1 | ||
| Graduate degree | 12.5 | 16.4 | 11.5 | ||
| Household annual income | |||||
| <$25,000 | 45.5 | 48.2 | 44.7 | 0.8998 | — |
| $25,000–$49,000 | 35.8 | 33.9 | 36.3 | ||
| ≥$50,000 | 18.7 | 17.9 | 19.0 | ||
| Treatment group | |||||
| Metformin only | 39.0 | 41.0 | 38.5 | 0.6453 | — |
| Metformin and rosiglitazone | 27.7 | 43.0 | 29.0 | ||
| Metformin and lifestyle | 33.3 | 36.1 | 32.6 | ||
| Physical examination | |||||
| BMI (kg/m2) | 35.5 ± 7.9 | 33.2 ± 7.3 | 36.2 ± 7.9 | 0.0088 | — |
| Systolic BP (mmHg) | 117.3 ± 11.5 | 114.3 ± 11.2 | 118.1 ± 11.5 | 0.0248 | — |
| Diastolic BP (mmHg) | 70.8 ± 9.3 | 68.4 ± 9.6 | 71.4 ± 9.1 | 0.0238 | — |
| Metabolic | |||||
| HbA1c (%) | 9.6 ± 2.0 | 9.1 ± 1.9 | 9.7 ± 2.0 | 0.0223 | 0.0086 |
| HbA1c (mmol/mol) | 81.5 ± 22.1 | 75.7 ± 21.2 | 83.1 ± 22.2 | ||
| Comorbidities | |||||
| Hypertension | 41.1 | 29.5 | 44.3 | 0.0371 | 0.2788 |
| LDL dyslipidemia | 9.9 | 4.9 | 11.3 | 0.1393 | 0.0788 |
| Triglyceride dyslipidemia | 34.0 | 24.6 | 36.7 | 0.0784 | 0.0477 |
| Microalbuminuria | 19.9 | 19.7 | 19.9 | 0.9672 | 0.9182 |
Data are mean ± SD or percent unless otherwise indicated. The P values are calculated from the t test or Wilcoxon rank-sum test for continuous variables and from the χ2 test for categorical variables. BP, blood pressure.
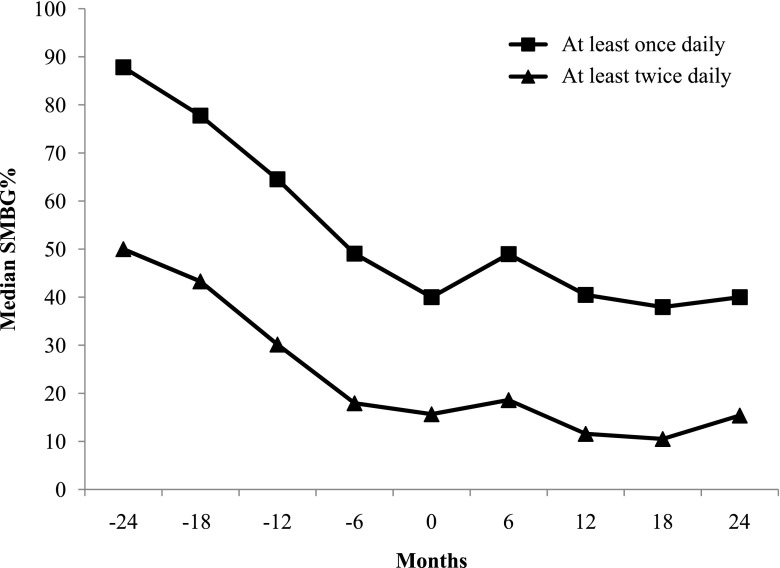
Median SMBG%, defined as SMBG at least once daily on ≥80% of days, was 40.0% at PO (n = 282), which increased to 49.0% 6 months after starting insulin therapy (n = 181) and returned to 40.5% and 40.0% after 1 year (n = 145) and 2 years (n = 94) of insulin therapy, respectively (Fig. 2). Median SMBG%, defined as SMBG at least twice daily on ≥80% of days, was 16% at PO, which increased slightly to 19% and then decreased to 15% by 2 years. At PO, 22% of youth (n = 61) checked ≥80% of days, and 42% (n = 119) checked ≥50%. Those checking ≥80% of days increased to only 25% (n = 46) after 6 months of insulin therapy and returned to 19% (n = 28) after 1 year of insulin treatment.
Figure 2.
Median SMBG% 24 months before and after insulin initiation for participants performing SMBG at least once daily and at least twice daily. Month 0 is the time the participant reached PO and initiated insulin therapy.
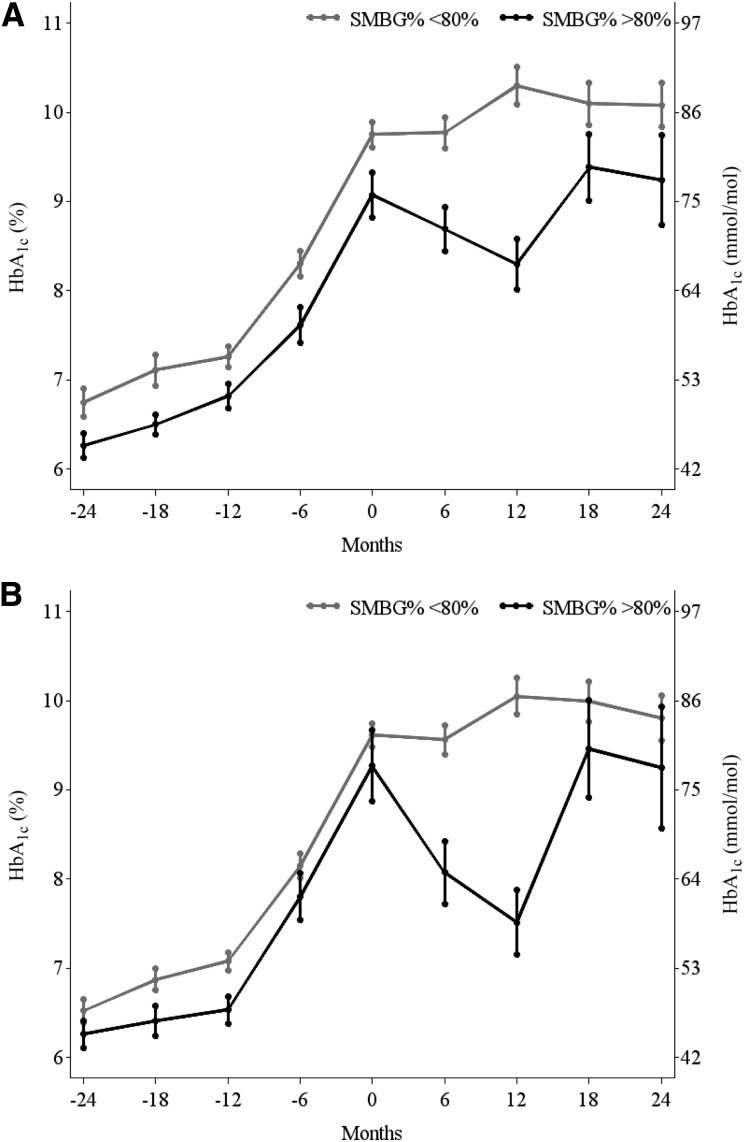
As shown in Fig. 3, performing SMBG, defined as SMBG at least once daily (Fig. 3A) or at least twice daily (Fig. 3B) on ≥80% of days, was associated with a lower HbA1c. Six months and 1 year after insulin initiation, participants with SMBG ≥80% had a ≥1% reduction in HbA1c. HbA1c reduction for those checking at least twice daily was 1.5% and 2.5%, respectively, at 6 months and 1 year after starting insulin therapy; however, these improvements were not sustained.
Figure 3.
HbA1c (mean ± SE) 24 months before and after insulin initiation among SMBG% ≥80% vs. <80% for participants performing SMBG at least once daily (A) and at least twice daily (B). Month 0 is the time the participant reached PO and initiated insulin therapy.
SMBG ≥80% (SMBG at least once daily) was also associated with less hypertension at PO (29.5 vs. 44.3%; P = 0.0371), but this difference was no longer present 1 year after insulin initiation (Table 1). There were no significant differences between groups (SMBG ≥80% vs. <80%) in the percent of participants with microalbuminuria or elevated LDL cholesterol or triglycerides over time.
Conclusions
To our knowledge, these are the first prospective analyses of SMBG use in youth with type 2 diabetes treated initially with oral agents and later with insulin as a result of deterioration in glycemic control. We observed a decline in SMBG over time. Adherence remained generally low, even for those who eventually required insulin therapy, and was related to higher HbA1c. Because all TODAY study participants received glucose monitoring devices, monitoring supplies, and medications, the cost of these items was not a barrier. Although greater use of SMBG was associated with lower HbA1c, it is unclear whether the better glycemic control when taking oral study drug was directly related to adherence to SMBG or whether SMBG use generally reflected better adherence to diabetes self-care (i.e., proper medication use, diet, physical activity). It is possible that SMBG use motivated a subset of this cohort to engage in positive behavior change, but this was not specifically studied in the TODAY study.
SMBG% fell as age increased, similar to reports of reduced glucose monitoring in mid-older adolescents (20,21). Given the disappointing results of the intensive lifestyle intervention in TODAY (9), it is unlikely that SMBG alone would significantly influence diet and physical activity in these youth. Although adherence to daily SMBG was low, >80% of participants tested on average three or more times per week.
TODAY youth were asked to check their glucose levels twice daily, but this frequency may not be necessary for youth-onset non–insulin-treated type 2 diabetes. Similarly, the 80% cutoff for SMBG adherence, which was based on previously published work on mediation adherence, is considered arbitrary. In adults who are stable and taking oral medications alone, less frequent SMBG has been shown to be sufficient and to result in lower cost and burden (2–8). The optimal frequency of SMBG in youth with type 2 diabetes on oral agents only was not investigated in TODAY.
The role of providing incentives to improve adherence is unclear. Little is known about the effectiveness of incentives in improving adherence to glucose monitoring in youth with type 2 diabetes. In a randomized trial in which daily financial incentives were used to improve adherence to glucose monitoring in adolescents and young adults with type 1 diabetes, increased monitoring at the end of 3 months was observed but quickly declined when incentives were no longer provided (22). In TODAY, incentives for SMBG adherence were given for several years, but despite these incentives, SMBG adherence declined over time. The use of incentives in improving monitoring and glycemic control in youth with type 2 diabetes will require further study.
When barriers and strategies for oral medication adherence were examined in TODAY, forgetting was the most common barrier reported, and better family support was the most common strategy provided (23). Older teens have less parental/family involvement and competing demands that can affect performing self-management tasks as well as a tendency to make decisions contrary to authority figures. In the current analyses of TODAY participants with youth-onset type 2 diabetes, we demonstrate that adherence to SMBG was worse than adherence to study medication during the first 2 years of intervention and, unlike medication adherence, not related to the presence of depressive symptoms. Possible reasons may include the discomfort and inconvenience of performing SMBG, the belief that SMBG is not as important as medication adherence, study burden, and/or families choosing to focus on only one task (medication adherence) given resistance of adolescents to follow suggestions for change from adults. There could have been a sense of futility during the first 2 years of TODAY because oral medications were not altered on the basis of SMBG results, so there may have been no noticeable benefits. Futility, however, cannot explain low SMBG adherence after insulin initiation because insulin dosing was based on SMBG results. In addition, SMBG data were derived from meter downloads, which are more difficult to manipulate than our assessment of adherence with medication using pill counts and not pill consumption. We have no other verifiable measure of medication adherence, and therefore, pill dumping before visits may have been ongoing long before SMBG began to deteriorate. Finally, the fall in SMBG also was related to having more (3,4) comorbid conditions.
Once insulin treatment is needed, it is generally accepted that glucose monitoring should be used to guide therapy. Studies in adults with type 2 diabetes have suggested that structured SMBG and programs that provide feedback on the basis of SMBG results, with recommendations for treatment changes, are important, but it is unknown whether such programs would be effective in adolescents (24–28). After insulin initiation, we found a transient small improvement in participants with SMBG ≥80%, which was related to better glycemic control, but this was not sustained. Overall, participants with SMBG ≥80% had a greater reduction in HbA1c. Unfortunately, the majority of participants taking insulin had SMBG <80%. This result was disappointing but not surprising given the patient population (adolescents); it is not known whether the participants were similarly nonadherent to taking insulin as well. In the TODAY study, participants did not use continuous glucose monitoring (CGM) devices. Future studies should investigate whether the use of CGM, which provides more information with less discomfort and burden, can improve glycemic outcomes in this challenging population. Novel approaches are needed to help adolescents with type 2 diabetes to improve self-care behaviors important for their long-term health.
There are limitations to these analyses. The TODAY study was not designed to test the effectiveness of SMBG in improving glycemic control in non–insulin-treated youth with type 2 diabetes. All participants were instructed to use SMBG (participants were not randomized to SMBG). Health literacy was not measured, and we do not know how these families prioritized type 2 diabetes management tasks. Given the design of this clinical trial, we report associations and are not able to address causation. Strengths of our report include the long-term follow-up of the TODAY cohort of youth-onset type 2 diabetes and the ability to assess SMBG both before and after insulin therapy was needed. Given the lack of data available on SMBG in youth with type 2 diabetes, our findings help to fill an important void in the pediatric type 2 diabetes literature. The burden and cost of SMBG are great. Future studies are needed to inform providers and patients about the best modes and frequency of glucose monitoring in youth with type 2 diabetes receiving non–insulin-based and insulin-based therapies.
In conclusion, the majority of youth with type 2 diabetes of >1 year duration were not adherent to SMBG, even after glycemic control deteriorated on oral agents and insulin therapy was required, but those who used SMBG had lower HbA1c. More studies are needed in youth with type 2 diabetes to better understand patient beliefs regarding benefits and barriers to SMBG as well as other self-care tasks and to inform best practices for recommending and responding to SMBG and CGM to improve glycemic outcomes and prevent future diabetes-related complications.
Supplementary Material
Article Information
Acknowledgments. The authors gratefully acknowledge the participation and guidance of the American Indian partners associated with the clinical center located at the University of Oklahoma Health Sciences Center, including members of the Absentee Shawnee Tribe, Cherokee Nation, Chickasaw Nation, Choctaw Nation of Oklahoma, and Oklahoma City Area Indian Health Service.
The opinions expressed in this article are those of the authors and do not necessarily reflect the views of the respective tribes and the Indian Health Service.
Funding. This work was completed with funding from the National Institute of Diabetes and Digestive and Kidney Diseases and the National Institutes of Health Office of the Director through grants U01-DK-61212, U01-DK-61230, U01-DK-61239, U01-DK-61242, and U01-DK-61254.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The National Institute of Diabetes and Digestive and Kidney Diseases project office was involved in all aspects of the study, including design and conduct, collection, management, analysis, and interpretation of the data, review and approval of the manuscript, and decision to submit the manuscript for publication.
Duality of Interest. R.S.W. is participating in clinical trials sponsored by Medtronic Minimed, Diasome Pharmaceuticals, Oramed Ltd., and Mylan GmbH. The TODAY Study Group thanks the following companies for donations in support of the study’s efforts: Becton, Dickinson and Company; Bristol-Myers Squibb; Eli Lilly and Company; GlaxoSmithKline; LifeScan, Inc.; Pfizer; and Sanofi. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. R.S.W. wrote the manuscript. B.H.B. conducted the statistical analyses and wrote sections of the manuscript. P.M., M.E.L., N.B.G., N.W.-A., L.M.L., C.L.C., N.C., B.E.S., R.A.B., N.C.-J., and M.W.H. wrote sections of the manuscript and reviewed and edited the manuscript. B.H.B. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018.
Footnotes
Clinical trial reg. no. NCT00081328, clinicaltrials.gov
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-1854/-/DC1.
A complete list of individuals in the TODAY Study Group can be found in the Supplementary Data online.
References
- 1.American Diabetes Association. 6 Glycemic targets: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018;41(Suppl. 1):S55–S64 [DOI] [PubMed] [Google Scholar]
- 2.Meier JL, Swislocki AL, Lopez JR, Noth RH, Bartlebaugh P, Siegel D. Reduction in self-monitoring of blood glucose in persons with type 2 diabetes results in cost savings and no change in glycemic control. Am J Manag Care 2002;8:557–565 [PubMed] [Google Scholar]
- 3.Young LA, Buse JB, Weaver MA, et al.; Monitor Trial Group . Glucose self-monitoring in non-insulin-treated patients with type 2 diabetes in primary care settings: a randomized trial. JAMA Intern Med 2017;177:920–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davidson MB. Counterpoint: self-monitoring of blood glucose in type 2 diabetic patients not receiving insulin: a waste of money. Diabetes Care 2005;28:1531–1533 [DOI] [PubMed] [Google Scholar]
- 5.Malanda UL, Welschen LM, Riphagen II, Dekker JM, Nijpels G, Bot SD. Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev 2012;1:CD005060. [DOI] [PubMed] [Google Scholar]
- 6.Zhu H, Zhu Y, Leung SW. Is self-monitoring of blood glucose effective in improving glycaemic control in type 2 diabetes without insulin treatment: a meta-analysis of randomised controlled trials. BMJ Open 2016;6:e010524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simon J, Gray A, Clarke P, Wade A, Neil A, Farmer A; Diabetes Glycaemic Education and Monitoring Trial Group . Cost effectiveness of self monitoring of blood glucose in patients with non-insulin treated type 2 diabetes: economic evaluation of data from the DiGEM trial. BMJ 2008;336:1177–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farmer AJ, Wade AN, French DP; DiGEM Trial Group. Blood glucose self-monitoring in type 2 diabetes: a randomised controlled trial. Health Technol Assess 2009;13:iii–iv, ix–xi, 1–50. [DOI] [PubMed]
- 9.Zeitler P, Hirst K, Pyle L, et al.; TODAY Study Group . A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012;366:2247–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katz LL, Anderson BJ, McKay SV, et al.; TODAY Study Group . Correlates of medication adherence in the TODAY cohort of youth with type 2 diabetes. Diabetes Care 2016;39:1956–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeitler P, Epstein L, Grey M, et al.; TODAY Study Group . Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes 2007;8:74–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Copeland KC, Zeitler P, Geffner M, et al.; TODAY Study Group . Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab 2011;96:159–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laffel L, Chang N, Grey M, et al.; TODAY Study Group . Metformin monotherapy in youth with recent onset type 2 diabetes: experience from the prerandomization run-in phase of the TODAY study. Pediatr Diabetes 2012;13:369–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varni JW, Limbers CA, Burwinkle TM. Impaired health-related quality of life in children and adolescents with chronic conditions: a comparative analysis of 10 disease clusters and 33 disease categories/severities utilizing the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes 2007;5:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovacs M. Children’s Depression Inventory Manual. North Tonawanda, NY, Multi-Health Systems Inc, 1992 [Google Scholar]
- 16.Beck AT, Steer RA. Beck Depression Inventory II. San Antonio, TX, Psychological Corp, 1996 [Google Scholar]
- 17.Walker EA, Molitch M, Kramer MK, et al.; DPP Research Group . Adherence to preventive medications: predictors and outcomes in the Diabetes Prevention Program. Diabetes Care 2006;29:1997–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zinman B, Harris SB, Neuman J, et al. . Low-dose combination therapy with rosiglitazone and metformin to prevent type 2 diabetes mellitus (CANOE trial): a double-blind randomised controlled study. Lancet 2010;376:103–111 [DOI] [PubMed] [Google Scholar]
- 19.Haynes RB. A critical review of the “determinants” of patient compliance with therapeutic regimens. In Compliance With Therapeutic Regimens. Sackett DL, Haynes RB, Eds. Baltimore, MD, Johns Hopkins University Press, 1976, p. 26–39 [Google Scholar]
- 20.Hanghøj S, Boisen KA. Self-reported barriers to medication adherence among chronically ill adolescents: a systematic review. J Adolesc Health 2014;54:121–138 [DOI] [PubMed] [Google Scholar]
- 21.Butz AM. Evidence-based practice: what is the evidence for medication adherence in children? J Pediatr Health Care 2006;20:338–341 [DOI] [PubMed] [Google Scholar]
- 22.Wong CA, Miller VA, Murphy K, et al. . Effect of financial incentives on glucose monitoring adherence and glycemic control among adolescents and young adults with type 1 diabetes: a randomized clinical trial. JAMA Pediatr 2017;171:1176–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venditti EM, Tan K, Chang N, et al.; TODAY Study Group . Barriers and strategies for oral medication adherence among children and adolescents with Type 2 diabetes. Diabetes Res Clin Pract 2018;139:24–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenwood DA, Blozis SA, Young HM, Nesbitt TS, Quinn CC. Overcoming clinical inertia: a randomized clinical trial of a telehealth remote monitoring intervention using paired glucose testing in adults with type 2 diabetes. J Med Internet Res 2015;17:e178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kempf K, Tankova T, Martin S. ROSSO-in-praxi-international: long-term effects of self-monitoring of blood glucose on glucometabolic control in patients with type 2 diabetes mellitus not treated with insulin. Diabetes Technol Ther 2013;15:89–96 [DOI] [PubMed] [Google Scholar]
- 26.Mannucci E, Antenore A, Giorgino F, Scavini M. Effects of structured versus unstructured self-monitoring of blood glucose on glucose control in patients with non-insulin-treated type 2 diabetes: a meta-analysis of randomized controlled trials. J Diabetes Sci Technol 2018;12:183–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bosi E, Scavini M, Ceriello A, et al.; PRISMA Study Group . Intensive structured self-monitoring of blood glucose and glycemic control in noninsulin-treated type 2 diabetes: the PRISMA randomized trial. Diabetes Care 2013;36:2887–2894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kan K, Zhu W, Lu F, et al. . Contribution of structured self-monitoring of blood glucose to the glycemic control and the quality of life in both insulin- and noninsulin-treated patients with poorly controlled diabetes. Diabetes Technol Ther 2017;19:707–714 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.