Abstract
We describe an inexpensive autocidal ovitrap for Aedes aegypti that uses cross-linked polyacrylamide (PAM) gel as the oviposition substrate. Aedes aegypti readily laid eggs on PAM gel that had been hydrated with either hay infusion or water. Aedes aegypti larvae that hatched from their eggs desiccated on the surface of the PAM gel. We tested the effects of gel hydration, texture, and type of attractant on trap performance, and compared the capture rates of standard ovitraps with those of PAM gel ovitraps in the field. The results showed that the number of eggs did not vary over a range of gel hydration levels (40–100%) and that more eggs were recovered from ovitraps containing coarse gel than from those containing homogenized gel. PAM gel hydrated with hay infusion was more attractive to gravid female mosquitoes than gel hydrated with water. In the field, the number of eggs recovered from autocidal ovitraps with PAM gel was similar to that recovered from standard ovitraps with hay infusion.
Keywords: Aedes aegypti, autocidal ovitrap, vector, surveillance, control
Autocidal ovitraps (AOs) are designed to attract gravid female Aedes aegypti, collect their eggs, and prevent the emergence of adult mosquitoes. Ovitraps have been developed to attract ovipositing female Ae. aegypti using the preferences of the mosquitoes for color, texture, shape, and size of the container as well as the odor and taste of the water (Fay and Perry 1965). The most common attractants for ovipositing female mosquitoes include dark-colored containers and either water or hay infusion. In standard AOs, hatching larvae are eliminated by mechanical barriers that block air exchange (Chan et al. 1977) or by biopesticides (Ritchie and Long 2003, Regis et al. 2008). Autocidal ovitraps have been used for mosquito surveillance and control purposes in several countries (Azil et al. 2011). Autocidal sticky traps designed to capture ovipositing mosquitoes use similar principles of attraction to AOs (Ordonez-Gonzales et al. 2001; Chadee and Ritchie 2010; Lee et al. 2013).
This study aimed at developing an inexpensive AO for Ae. aegypti that did not use pesticides. We observed that female Ae. aegypti readily laid their eggs on the surface of a cross-linked polyacrylamide (PAM) gel, also known as plant gel. PAM crystals absorb water and other solutions and increase several hundred times in volume, forming a water-insoluble gel. Because most of the water is trapped in the cross-linked polymer structure, it is not available to the developing larvae, and they desiccate on the gel’s surface. PAM crystals have been used as a substitute for water in cemetery flower vases because they keep flowers fresh and prevent mosquito production (http://www.fightthebite.net/download/ecomanagement/Cemetery%20Flyer.pdf). PAM crystals can be re-hydrated several times.
We used the F1 progeny of Ae. aegypti collected from San Juan City between May 2009 and June 2009. Mosquitoes were reared at 26 ± 1.0 °C, 65–80 % RH, and a 12:12 (L:D) photoperiod in pans containing 150 larvae and 1 L dechlorinated water. They were fed increasing amounts of finely ground rabbit chow during development (0.7–1.6 mg/larva/day). After a minimum of 3 days after adult emergence (range, 3–7 days), the mosquitoes had daily access to porcine blood for 5 consecutive days, and were then maintained for an additional 3 days prior to use.
We tested 4 levels of PAM gel (DNB Designs, Inc., Littleton, CO) hydration (40, 60, 80, and 100%) in small black plastic ovicups (150 mL, 7 × 4 cm) as an oviposition substrate for attracting female Ae. aegypti. These ovicups were compared with those containing undiluted hay infusion and germination paper (but no PAM gel) as the oviposition substrate (Reiter et al. 1991). The PAM crystals absorbed hay infusion to a maximum of 133.3 times their mass. Hydration levels were obtained by mixing 7.5, 9.38, 12.5, and 18.76 grams of PAM crystals per liter of undiluted hay infusion. Polyacrylamide gel was homogenized to a fine slurry using a blender (2–5 min). We used 20 black plastic buckets (4 L; 15.5 × 19 cm) with a screen as test cages, each containing 10 gravid female Ae. aegypti, and an ovicup (100 mL of PAM gel or hay infusion) with a given treatment (5 treatments × 4 replicates). The number of eggs per ovicup was registered after 24 h. Analysis of variance (ANOVA) was used to test the null hypothesis that the mean number of eggs per ovicup did not differ with the level of gel hydration (40, 60, 80, 100%, hay infusion); the difference was not significant (Table 1; P> 0.05). The number of eggs on PAM gel was the highest with the 80% hydration treatment, and this number was not significantly higher than in ovicups with hay infusion (Table 1).
Table 1.
Effects of PAM gel hydration (%) and texture on Aedes aegypti oviposition in experimental ovitraps.
| Treatments | Test | |||||
|---|---|---|---|---|---|---|
| Eggs collected per ovitrap at varying levels of PAM gel hydration (%; average ± 95% CI) | 40 | 60 | 80 | 100 | Hay infusion | One-way ANOVA |
| 375 ± 118 | 374 ± 110 | 509 ± 64 | 268 ± 65 | 398 ± 156 | F4,15 = 2.4; P> 0.05 | |
| Percentages (± 95% CI) of eggs collected per ovitrap with varying texture and attractants | Coarse, hydrated with hay infusion | Coarse, hydrated with water | Fine, hydrated with hay infusion | Fine, hydrated with water | Two-way ANOVA |
|
| 0.37 ± 0.06 | 0.21 ± 0.06 | 0.32 ± 0.05 | 0.10 ± 0.04 | Texture F1,12= 7.9; P< 0.05 Attractant F1,12= 46.5; P< 0.01 Texture x Attractant F1, 12 = 1.2; P > 0.05 |
||
The effects of PAM gel texture (coarse vs. homogenized gel) and attractant (gel hydrated with hay infusion vs. water) were examined. Crystals were hydrated to maximum capacity overnight using either water or undiluted hay infusion. Half the material was left intact (coarse gel), whereas the other half was homogenized using a blender (2 min). A total of 4 treatments combining gel texture (coarse vs. homogenized gel) and gel attractant (water vs. hay infusion) were simultaneously compared. Each PAM gel type was added to an ovicup that was placed on top of a screened bucket (4 L) containing 2 L of 15% hay infusion, which in turn was placed inside a black 20-L pail with a lid bearing a bottomless cup and an opening of 8.9 cm, so that the ovicups and buckets could not be directly seen by the female mosquitoes. Between 60 and 100 gravid female mosquitoes were released in a room (29.7 m3) with the 4 pails, 1 in each corner, and the eggs were recovered from the ovicups after 22 h. The experiment was repeated 4 times, and the treatments were rotated among the pails each time. A 2-way ANOVA comparing the mean proportions of eggs laid in the ovicups showed significant effects of texture (coarse vs. homogenized gel; P < 0.05) and attractant (water vs. hay infusion; P < 0.01), but no interaction (P > 0.05; Table 1). The mean proportion of eggs on PAM gel hydrated with hay infusion (0.34 ± 0.04) was higher than that on PAM gel hydrated with water (0.16 ± 0.05). The proportion of eggs recovered on coarse PAM gel (0.29 ± 0.07) was higher than that recovered on homogenized PAM gel (0.21 ± 0.08).
We compared Ae. aegypti oviposition between standard ovitraps (hay infusion) and PAM gel ovitraps (crystals hydrated with hay infusion) under field conditions. Pairs of black standard ovicups containing either 150 mL of undiluted hay infusion or 150 mL of PAM gel hydrated with undiluted hay infusion (both ovicups lined with germination paper) were placed at least 6 m apart at each of 18 residences in Reparto Metropolitano, San Juan, Puerto Rico, and recovered after 48 h. We drilled 2 drainage holes (~6 mm in diameter) near the base of the ovicup containing PAM gel. We added 50 mL of undiluted hay infusion to the PAM gel ovicups just before placing them in the field. A total of 656 eggs (18.2 ± 15.2 eggs / trap /day) were recovered from 8 of the 18 hay-infusion ovitraps, whereas only 88 eggs (0.9 ± 1.5) were recovered from 3 of the 18 PAM gel ovicups. The odor of the PAM gel hydrated with undiluted hay infusion was not found to be as strong as that of the hay infusion itself.
The performance of AOs was further explored under field conditions. The AO consisted of a black 20 L pail with a white lid (~30 cm in diameter) and a funnel cup with an 8.8-cm diameter trap entrance, containing a 4 L bucket with 2 L of undiluted hay infusion and a cover screen with a recessed ovicup containing the attraction medium (Fig. 1). The ovicups were inserted into and supported by a polyvinyl chloride (PVC) plastic tube (8.7 × 19 cm). Drainage holes (1 mm) were drilled near the top of both the ovicups and the 4 L buckets. We compared the number of eggs deposited in standard ovicups containing 300 mL of undiluted hay infusion and germination paper with that deposited in ovicups containing 300 mL of PAM gel hydrated with undiluted hay infusion (Fig. 1). The ovicups containing the PAM gel were designed to hold coarse gel in a lower chamber and a thin layer of homogenized PAM gel in a removable upper chamber to facilitate egg counting. The upper chamber was made by cutting the lower portion of a plastic cup and replacing the bottom with a glued fine screen mesh (60 µm) to allow the passage of water and to keep contact with the hydrated gel below. Although not used in this experiment, the PAM gel cup should also have minute holes drilled at the bottom to allow the hay infusion contained in the bucket to keep the gel hydrated through the inundated PVC tube. A total of 30 houses was selected in Reparto Metropolitano for the field experiment (at least 50 m apart). Each house was randomly assigned 1 of the 2 treatments, and the ovitraps were placed in sheltered locations. After 3 days, egg papers from the standard ovicups and upper chambers of the PAM gel ovicups were removed and counted. The infusion, germination paper, and PAM gel in the ovicups were replaced and the treatments were rotated at each house. After 3 days, the eggs were collected from the ovitraps as before. A total of 6098 (68 ± 20 eggs / trap / day; mean ± 95% CI) and 5474 (61 ± 19) Ae. aegypti eggs were collected by the hay infusion ovitraps and PAM gel AOs, respectively. A paired t-test showed lack of statistical differences in the number of eggs (square-root transformed) collected by each device (t = 0.32; d.f. = 29; P > 0.05). These numbers of eggs per ovicup per day in AOs were higher than those observed in previous studies (Smith et al. 2009). Average and 95% CI eggs per ovitrap per day in two neighborhoods of San Juan city were 29.8 ± 1.0 and 31.9 ± 1.1 from May to December of 2008 (Barrera et al. 2011). Reiter et al. (1991) reported an average of 38 eggs per ovitrap with hay infusion per day from the same neighborhood that we studied.
Fig. 1.
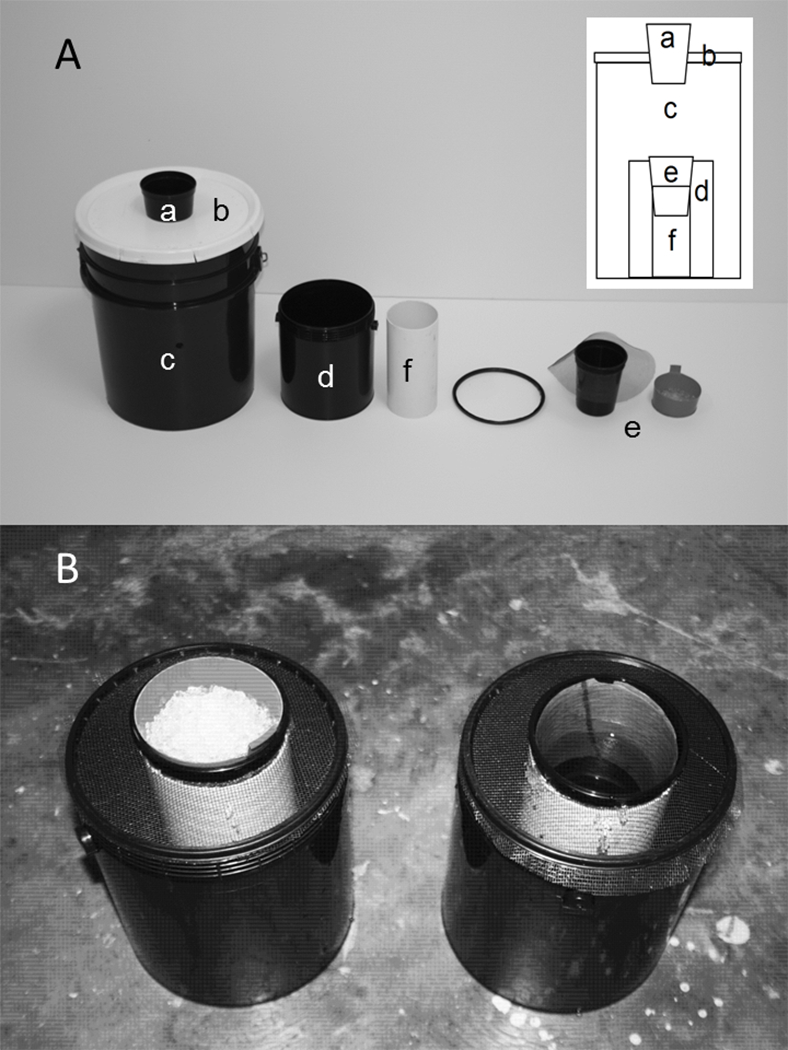
Elements of the autocidal ovitrap (AO) (A) consisting of a funnel cup with an 8.8 cm diameter entrance (a) transecting a white pail lid (b), a black 20 L pail (c), a 4 L bucket (d) containing hay infusion, and an ovicup with an upper, inner black or red (as shown) chamber holding PAM gel (e) supported by a 19-cm long PVC pipe (f). Assembled AO containing PAM gel (left) or hay infusion plus germination paper (right) (B).
We determined whether Ae. aegypti hatching larvae survived on PAM gel. The upper chambers of ovicups containing PAM gel and eggs were collected weekly for 3 weeks from 20 PAM gel AOs placed at houses in the field. Egg collection containers were maintained in the laboratory for 15 days to assess whether immature Ae. aegypti could complete development on saturated PAM gel. The number of live larvae was recorded every 3 days, and water was added to the PAM gel to replace any loss due to evaporation. The average number of live larvae observed in the egg collection chambers brought from the field was 8.1 ± 6.8; however, none of the larvae were able to survive beyond the second instar on the PAM gel. These results show that cross-linked PAM gel can be used as an autocidal oviposition substrate for Ae. aegypti. This AO is a component of a sticky gravid ovitrap that we have developed to capture ovipositing female Ae. aegypti and the eggs of those female mosquitoes that might manage to escape from the trap (CDC, unpublished; patent pending PCT/US12/25462). This AO can also be used as a stand-alone device to monitor oviposition activity and to control Ae. aegypti populations. Future investigations are needed to determine the effectiveness of these AOs without the 20 L pail.
Acknowledgments
We thank the residents of San Juan municipality for their cooperation and hospitality. We also acknowledge the exceptional field support provided by Belkis Caban, Veronica Acevedo, Gilberto Felix, Juan Medina, Angel Berrios, Jesus Flores, Orlando Gonzalez, Jose Gonzalez, and Luis Rivera.
REFERENCES CITED
- Azil AH, Li M, Williams CR. 2011. Dengue vector surveillance programs: a review of methodological diversity in some endemic and epidemic countries. Asia Pac J Public Health 23:827–842. [DOI] [PubMed] [Google Scholar]
- Barrera R, Amador M, Mackay AJ. 2011. Population dynamics of Aedes aegypti and dengue as influenced by weather and human behavior in San Juan, Puerto Rico. PLoS Negl Trop Dis 5:e1378. doi: 10.1371/journal.pntd.0001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadee DD, Ritchie SA. 2010. Efficacy of sticky and standard ovitraps for Aedes aegypti in Trinidad, West Indies. J Vector Ecol 35: 395–400. [DOI] [PubMed] [Google Scholar]
- Chan KL, Kiat NS, Koh TK. 1977. An autocidal ovitrap for the control and possible eradication of Aedes aegypti. Southeast Asian J Trop Med Public Health 8:56–62. [PubMed] [Google Scholar]
- Fay RW, Perry AS. 1965. Laboratory studies of ovipositional preferences of Aedes aegypti. Mosq News 25:276–281. [Google Scholar]
- Lee C, Vythilingam I, Chong CS, Razak MAA, Tan CH, Pok KY, Lee-Ching Ng LC. 2013. Gravitraps for management of dengue clusters in Singapore. Am. J. Trop. Med. Hyg. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordoñez-Gonzalez JG, Mercado-Hernandez R, Flores-Suarez AE, Fernandez-Salas I. 2001. The use of sticky ovitraps to estimate dispersal of Aedes aegypti in northeastern Mexico. J Am Mosq Control Assoc 17: 93–97. [PubMed] [Google Scholar]
- Regis L, Monteiro AM, Melo-Santos MA, Silveira JC, Furtado AF, Acioli RV, Santos GM, Nakazawa M, Carvalho MS, Ribeiro PJ, de Souza WV. 2008. Developing new approaches for detecting and preventing Aedes aegypti population outbreaks: basis for surveillance, alert and control system. Mem Inst Oswaldo Cruz 103:50–59. [DOI] [PubMed] [Google Scholar]
- Reiter P, Amador M, Colon N. 1991. Enhancement of the CDC ovitrap with hay infusions for daily monitoring of Aedes aegypti populations. J Am Mosq Control Assoc 7:52–55. [PubMed] [Google Scholar]
- Ritchie SA, Long S. 2003. Does S-methoprene affect oviposition by Aedes aegypti in an ovitrap? J Am Mosq Control Assoc 19:170–171. [PubMed] [Google Scholar]
- Smith J, Amador M, Barrera R. 2009. Seasonal and habitat effects on dengue and West Nile virus vectors in San Juan, Puerto Rico. J Am Mosq Control Assoc 25: 38–46. [DOI] [PubMed] [Google Scholar]


