Abstract
Background
A wide variety of grafts have been introduced with the aim of improving the outcomes of traditional native tissue repair (colporrhaphy) for vaginal prolapse.
Objectives
To determine the safety and effectiveness of transvaginal mesh or biological grafts compared to native tissue repair for vaginal prolapse.
Search methods
We searched the Cochrane Incontinence Group Specialised Register, which contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, ongoing trials registers, and handsearching of journals and conference proceedings (6 July 2015). We also contacted researchers in the field.
Selection criteria
Randomised controlled trials (RCTs) comparing different types of vaginal repair (mesh, biological graft, or native tissue).
Data collection and analysis
Two review authors independently selected trials, assessed risk of bias, and extracted data. The primary outcomes were awareness of prolapse, repeat surgery, and recurrent prolapse on examination.
Main results
We included 37 RCTs (4023 women). The quality of the evidence ranged from very low to moderate. The main limitations were poor reporting of study methods, inconsistency, and imprecision.
Permanent mesh versus native tissue repair
Awareness of prolapse at one to three years was less likely after mesh repair (risk ratio (RR) 0.66, 95% confidence interval (CI) 0.54 to 0.81, 12 RCTs, n = 1614, I2 = 3%, moderate‐quality evidence). This suggests that if 19% of women are aware of prolapse after native tissue repair, between 10% and 15% will be aware of prolapse after permanent mesh repair.
Rates of repeat surgery for prolapse were lower in the mesh group (RR 0.53, 95% CI 0.31 to 0.88, 12 RCTs, n = 1675, I2 = 0%, moderate‐quality evidence). There was no evidence of a difference between the groups in rates of repeat surgery for continence (RR 1.07, 95% CI 0.62 to 1.83, 9 RCTs, n = 1284, I2 = 21%, low‐quality evidence). More women in the mesh group required repeat surgery for the combined outcome of prolapse, stress incontinence, or mesh exposure (RR 2.40, 95% CI 1.51 to 3.81, 7 RCTs, n = 867, I2 = 0%, moderate‐quality evidence). This suggests that if 5% of women require repeat surgery after native tissue repair, between 7% and 18% in the permanent mesh group will do so. Eight per cent of women in the mesh group required repeat surgery for mesh exposure.
Recurrent prolapse on examination was less likely after mesh repair (RR 0.40, 95% CI 0.30 to 0.53, 21 RCTs, n = 2494, I2 = 73%, low‐quality evidence). This suggests that if 38% of women have recurrent prolapse after native tissue repair, between 11% and 20% will do so after mesh repair.
Permanent mesh was associated with higher rates of de novo stress incontinence (RR 1.39, 95% CI 1.06 to 1.82, 12 RCTs, 1512 women, I2 = 0%, low‐quality evidence) and bladder injury (RR 3.92, 95% CI 1.62 to 9.50, 11 RCTs, n = 1514, I2 = 0%, moderate‐quality evidence). There was no evidence of a difference between the groups in rates of de novo dyspareunia (RR 0.92, 95% CI 0.58 to 1.47, 11 RCTs, n = 764, I2 = 21%, low‐quality evidence). Effects on quality of life were uncertain due to the very low‐quality evidence.
Absorbable mesh versus native tissue repair
There was very low‐quality evidence for the effectiveness of either form of repair at two years on the rate of awareness of prolapse (RR 1.05, 95% CI 0.77 to 1.44, 1 RCT, n = 54).
There was very low‐quality evidence for the effectiveness of either form of repair on the rate of repeat surgery for prolapse (RR 0.47, 95% CI 0.09 to 2.40, 1 RCT, n = 66).
Recurrent prolapse on examination was less likely in the mesh group (RR 0.71, 95% CI 0.52 to 0.96, 3 RCTs, n = 292, I2 = 21%, low‐quality evidence)
The effect of either form of repair was uncertain for urinary outcomes, dyspareunia, and quality of life.
Biological graft versus native tissue repair
There was no evidence of a difference between the groups at one to three years for the outcome awareness of prolapse (RR 0.97, 95% CI 0.65 to 1.43, 7 RCTs, n = 777, low‐quality evidence).
There was no evidence of a difference between the groups for the outcome repeat surgery for prolapse (RR 1.22, 95% CI 0.61 to 2.44, 5 RCTs, n = 306, I2 = 8%, low‐quality evidence).
The effect of either approach was very uncertain for recurrent prolapse (RR 0.94, 95% CI 0.60 to 1.47, 7 RCTs, n = 587, I2 = 59%, very low‐quality evidence).
There was no evidence of a difference between the groups for dyspareunia or quality of life outcomes (very low‐quality evidence).
Authors' conclusions
While transvaginal permanent mesh is associated with lower rates of awareness of prolapse, repeat surgery for prolapse, and prolapse on examination than native tissue repair, it is also associated with higher rates of repeat surgery for prolapse or stress urinary incontinence or mesh exposure (as a composite outcome), and with higher rates of bladder injury at surgery and de novo stress urinary incontinence. The risk‐benefit profile means that transvaginal mesh has limited utility in primary surgery. While it is possible that in women with higher risk of recurrence the benefits may outweigh the risks, there is currently no evidence to support this position.
Limited evidence suggests that absorbable mesh may reduce rates of recurrent prolapse on examination compared to native tissue repair, but there was insufficient evidence on absorbable mesh for us to draw any conclusions for other outcomes. There was also insufficient evidence for us to draw any conclusions regarding biological grafts compared to native tissue repair.
In 2011, many transvaginal permanent meshes were voluntarily withdrawn from the market, and the newer, lightweight transvaginal permanent meshes still available have not been evaluated within a RCT. In the meantime, these newer transvaginal meshes should be utilised under the discretion of the ethics committee.
Keywords: Female; Humans; Surgical Mesh; Absorbable Implants; Awareness; Randomized Controlled Trials as Topic; Recurrence; Reoperation; Reoperation/statistics & numerical data; Secondary Prevention; Secondary Prevention/statistics & numerical data; Urinary Incontinence, Stress; Urinary Incontinence, Stress/surgery; Uterine Prolapse; Uterine Prolapse/prevention & control; Uterine Prolapse/psychology; Uterine Prolapse/surgery; Vagina; Vagina/surgery
Plain language summary
Transvaginal mesh or grafts compared with native tissue repair for vaginal prolapse
Review question
Should transvaginal mesh or biological grafts or native tissue be utilised to repair vaginal prolapse?
Background
Pelvic organ prolapse is common, affecting as many as 50% of women who have had children. The traditional method of repairing vaginal prolapse using native tissue is associated with high rates of recurrence. It is thought that transvaginal grafts made of absorbable or permanent mesh or biological material may improve the outcomes of prolapse surgery.
Study characteristics
We evaluated 37 randomised controlled trials (4023 women) comparing transvaginal grafts versus traditional native tissue repair for repairing vaginal prolapse. The evidence is current to July 2015.
Key results
Low to moderate quality evidence suggests that there are advantages to using transvaginal permanent mesh compared to native tissue repair, including lower rates of awareness of prolapse, repeat surgery for prolapse, and recurrent prolapse on examination. The evidence suggests that if 19% of women are aware of prolapse after native tissue repair, between 10% and 15% will be aware of prolapse after permanent mesh repair. If the rate of recurrent prolapse on examination after a native tissue repair is assumed to be 38%, the risk would be between 11% and 20% after a repair with transvaginal permanent mesh. However, there are also problems associated with permanent transvaginal mesh. If we assume that 5% of women require repeat surgery for prolapse or urinary incontinence or mesh exposure (any of the three) after native tissue repair, the risk would be between 7% and 18% after permanent mesh repair. Eight per cent of women in the mesh groups required repeat surgery for mesh exposure.
Low quality evidence suggests that absorbable mesh may reduce the risk of recurrent prolapse on examination compared to native tissue repair, but there is insufficient evidence on absorbable mesh for us to draw any conclusions for other outcomes.
Low quality evidence suggests there is no difference between biological grafts and native tissue repair on rates of awareness of prolapse or reoperation for prolapse. Due to the very low quality of evidence, the impact of the interventions on prolapse on examination was uncertain.
While permanent mesh has some advantages over native tissue, there are also disadvantages in its routine use. Many transvaginal permanent meshes were withdrawn from use in 2011, and the newer, lightweight transvaginal permanent meshes still available have not been evaluated within a randomised study.
Quality of the evidence
Overall, the quality of the evidence ranged from very low to moderate. The main limitations were poor reporting of study methods, inconsistency, and imprecision.
Summary of findings
Summary of findings for the main comparison. Any transvaginal permanent mesh versus native tissue repair for vaginal prolapse.
| Any transvaginal permanent mesh versus native tissue repair for vaginal prolapse | ||||||
| Population: women with vaginal prolapse Settings: surgical Intervention: any transvaginal permanent mesh versus native tissue repair | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Native tissue repair | Any transvaginal permanent mesh | |||||
|
Awareness of prolapse review 1 to 3 years |
188 per 1000 | 124 per 1000 (101 to 152) |
RR 0.66 (0.54 to 0.81) |
1614 (12 RCTs) |
⊕⊕⊕⊝ moderate1 | |
|
Repeat surgery ‐ prolapse review 1 to 3 years |
32 per 1000 | 17 per 1000 (10 to 28) | RR 0.53 (0.31 to 0.88) | 1675 (12 RCTs) | ⊕⊕⊕⊝ moderate1 | |
| Repeat surgery ‐ continence surgery | 26 per 1000 |
28 per 1000 (16 to 48) |
RR 1.07 (0.62 to 1.83) |
1284 (9 RCTs) |
⊕⊕⊝⊝ low1,2 | |
|
Repeat surgery ‐ surgery for prolapse, SUI, or mesh exposure review 1 to 3 years |
48 per 1000 | 114 per 1000 (72 to 181) | RR 2.40 (1.51 to 3.81) | 867 (7 studies) | ⊕⊕⊕⊝ moderate1 | |
|
Recurrent prolapse review 1 to 3 years |
381 per 1000 | 152 per 1000 (114 to 202) | RR 0.40 (0.30 to 0.53) | 2494 (21 studies) | ⊕⊕⊝⊝ low1,4 | I2 = 73% |
| Bladder injury | 5 per 1000 | 21 per 1000 (9 to 51) | RR 3.92 (1.62 to 9.5) | 1514 (11 studies) | ⊕⊕⊕⊝ moderate1 | |
|
De novo dyspareunia (pain during sexual intercourse) review 1 to 3 years |
95 per 1000 | 88 per 1000 (55 to 140) | RR 0.92 (0.58 to 1.47) | 764 (11 studies) | ⊕⊕⊝⊝ low1,2 | |
|
De novo stress urinary incontinence review 1 to 3 years |
96 per 1000 | 133 per 1000 (101 to 174) | RR 1.39 (1.06 to 1.82) | 1512 (12 studies) | ⊕⊕⊝⊝ low1,3 | |
|
Quality of life review 1 to 2 years |
The mean quality of life in the mesh groups was 0.05 standard deviations higher (0.20 lower to 0.30 higher). This is an imprecise finding that is consistent with a small benefit in either group, or else no difference between the groups | 665 (7 studies) |
⊕⊝⊝⊝ very low1, 2,4 |
I2 = 60% | ||
| *The basis for the assumed risk is the median control group risk across studies The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; SUI: stress urinary incontinence | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded one level due to serious risk of bias: most of the studies were at unclear or high risk of bias associated with poor reporting of methods, including failure by many to describe satisfactory methods of allocation concealment or blinding. A minority of studies did not report use of blinding at all.
2Downgraded one level due to serious imprecision: findings compatible with benefit in either group or with no clinically meaningful difference between the groups.
3Downgraded one level due to serious imprecision: findings compatible with benefit in native tissue group or with no clinically meaningful difference between the groups.
4Downgraded one level due to serious inconsistency: substantial statistical heterogeneity.
Summary of findings 2. Absorbable mesh versus native tissue repair for vaginal prolapse.
| Absorbable mesh versus native tissue repair for vaginal prolapse | ||||||
|
Population: women with vaginal prolapse
Settings: surgical
Intervention: absorbable mesh Control: native tissue repair | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Native tissue repair | Absorbable mesh | |||||
|
Awareness of prolapse at 2 years |
724 per 1000 | 760 per 1000 (558 to 1000) | RR 1.05 (0.77 to 1.44) | 54 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
|
Repeat surgery for prolapse (stage 2 or more) at 2 years |
125 per 1000 | 59 per 1000 (11 to 300) | RR 0.47 (0.09 to 2.40) | 66 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
|
Recurrent prolapse at 3 months to 2 years |
429 per 1000 | 304 per 1000 (223 to 411) | RR 0.71 (0.52 to 0.96) | 292 (3 studies) | ⊕⊕⊝⊝ low3,4 | |
| Bladder injury | Not reported in the included studies | |||||
|
De novo dyspareunia (pain during sexual intercourse) review 1 to 3 years |
Not reported in the included studies | |||||
|
Stress urinary incontinence at 2 years |
593 per 1000 | 818 per 1000 (563 to 1000) | RR 1.38 (0.95 to 2) | 49 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
|
Quality of life at 2 years |
The mean quality of life score was the same in both groups, when measured using a severity score of 1 to 10 (mean difference 0, 95% CI ‐2.82 to 2.82) | 54 (1 study) | ⊕⊝⊝⊝ very low1,2 | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded one level due to serious risk of attrition bias: at two years 18% not included in analysis. 2Downgraded two levels due to very serious imprecision: single small trial with confidence interval compatible with benefit in either arm or no effect. Low event rate. 3Downgraded one level due to serious risk of attrition bias in 2/3 studies. 4Downgraded one level due to serious imprecision: low overall event rate (n = 101). 5Downgraded one level due to serious risk of bias: unclear whether outcome assessment was blinded.
Summary of findings 3. Biological repair versus native tissue repair for vaginal prolapse.
| Biological repair versus native tissue repair for vaginal prolapse | ||||||
|
Population: women with vaginal prolapse
Settings: surgical
Intervention: biological repair Control: native tissue repair | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Native tissue repair | Biological repair | |||||
|
Awareness of prolapse at 1 to 3 years |
105 per 1000 | 102 per 1000 (68 to 151) | RR 0.97 (0.65 to 1.43) | 777 (7 studies) | ⊕⊕⊝⊝ low1,2 | |
|
Repeat prolapse surgery 1 to 2 years |
43 per 1000 | 52 per 1000 (26 to 105) | RR 1.22 (0.61 to 2.44) | 306 (5 studies) | ⊕⊕⊝⊝ low3,4 | |
|
Recurrent prolapse at 1 year |
295 per 1000 | 277 per 1000 (177 to 434) | RR 0.94 (0.60 to 1.47) | 587 (7 studies) | ⊕⊝⊝⊝ very low3,5,6 | |
| Bladder injury | Not estimable as only 1 event occurred (in the native tissue group) | 137 (1 study) |
||||
| Bowel injury | Not estimable as only 1 event occurred (in the biological repair group) | 137 (1 study) |
||||
|
De novo dyspareunia (pain during sexual intercourse) review 1 to 3 years |
177 per 1000 | 150 per 1000 (35 to 648) | RR 0.85 (0.20 to 3.67) | 37 (1 study) | ⊕⊝⊝⊝ very low3,8 | |
|
De novo urinary stress incontinence at 1 year |
Not estimable ‐ no events occurred | 56 (1 study) | ||||
|
Quality of life at 1 year |
The mean quality of life in the biological repair group was 0.05 standard deviations lower (0.48 lower to 0.38 higher). This is an imprecise finding that is consistent with a small benefit in either group, or else no difference between the groups | 84 (2 studies) | ⊕⊝⊝⊝ very low9 | |||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded one level due to serious risk of bias: four of the studies at high or unclear risk of bias associated with blinding status. 2Downgraded one level due to serious imprecision: confidence intervals compatible with benefit in either group or with no difference between the groups. 3Downgraded one level due to imprecision: confidence interval compatible with benefit in either group or with no difference between groups. 4Downgraded one level due to serious risk of bias in 3/5 studies: two studies at high risk of attrition bias, and one study not blinded. 5Downgraded one level due to serious risk of bias: three studies rated at high risk of attrition bias, detection bias, and other bias (conflict of interest), respectively. 6Downgraded one level due to serious inconsistency: I2 = 59% indicating substantial statistical heterogeneity.
7Downgraded one level due to serious risk of bias: blinding status unclear.
8Downgraded two levels due to very serious imprecision: single small study, only six events.
9Downgraded one level due to serious risk of attrition bias, and a further two levels due to very serious imprecision: only 84 participants.
Background
Description of the condition
Pelvic organ prolapse is common and is seen on examination in 40% to 60% of parous women (Handa 2004; Hendrix 2002). The annual aggregated rate of associated surgery in the USA is in the range of 10 to 30 per 10,000 women (Brubaker 2002). Pelvic organ prolapse is the descent of one or more of the pelvic organs (uterus, vagina, bladder, or bowel). The different types of prolapse include:
upper vaginal prolapse (apical prolapse), i.e. uterus, vaginal vault (after hysterectomy when the top of the vagina drops down);
anterior vaginal wall prolapse, i.e. cystocele (bladder descends), urethrocele (urethra descends), paravaginal defect (pelvic fascia defect);
posterior vaginal wall prolapse, i.e. enterocele (small bowel descends), rectocele (rectum descends), perineal deficiency.
A woman can present with prolapse of one or more of these sites.
The aetiology of pelvic organ prolapse is complex and multi‐factorial. Possible risk factors include pregnancy, childbirth, congenital or acquired connective tissue abnormalities, denervation or weakness of the pelvic floor, ageing, hysterectomy, menopause, and factors associated with chronically raised intra‐abdominal pressure (Bump 1998; Gill 1998; MacLennan 2000).
Women with prolapse commonly have a variety of pelvic floor symptoms, only some of which are directly related to the prolapse. Generalised symptoms of prolapse include pelvic heaviness; bulge, lump or protrusion coming down from the vagina; a dragging sensation in the vagina; or backache. Symptoms of bladder, bowel, or sexual dysfunction are frequently present. For example, women may need to reduce the prolapse digitally to aid urinary voiding or defecation. These symptoms may be directly related to the prolapsed organ, for example poor urinary stream when a cystocele is present or obstructed defecation in case of a rectocele. They may also be independent of the prolapse, for example symptoms of overactive bladder when a cystocele is present, or irritable bowel when a rectocele is present.
Description of the intervention
Treatment of prolapse depends on the severity of the prolapse, its symptoms, the woman's general health, and surgeon preference and capabilities. Options available for treatment are conservative, mechanical, or surgical interventions.
Generally, conservative or mechanical treatments are considered for women with a mild degree of prolapse, those who wish to have more children, the frail, or those women unwilling to undergo surgery. Separate Cochrane reviews have considered conservative and mechanical interventions (Adams 2004; Hagen 2011). There was no good evidence to guide management in either of these reviews.
A wide variety of abdominal and vaginal surgical techniques are available for the treatment of prolapse (see Appendix 1). The most common procedures are anterior repair (colporrhaphy) for anterior vaginal wall prolapse and posterior repair (colporrhaphy) for posterior vaginal wall prolapse. Together, anterior and posterior compartment surgery account for the majority of all prolapse operations Haya 2015 . Two main approaches can be used.
Vaginal approaches include vaginal hysterectomy, anterior or posterior vaginal wall repair (colporrhaphy), McCall culdoplasty, Manchester repair (amputation of the cervix with uterus suspension to the cardinal ligaments), prespinous and sacrospinous colpopexy, enterocele ligation, paravaginal repair, Le Fort's procedure, and perineal reconstruction.
Abdominal approaches include hysterectomy, sacral colpopexy, paravaginal repair, vault suspending and uterosacral ligament plication, enterocele ligation, and posterior vaginal wall repair. Abdominal surgery can be performed through an open incision or keyhole incisions via the laparoscope or robot.
A combination of these procedures may be employed in the surgical correction of prolapse, as frequently more than one type of prolapse may occur.
In addition to the variety of prolapse operations, the surgeon must choose whether to use absorbable sutures such as polyglycolic acid‐based materials (for example polyglactin), delayed‐absorption sutures such as polydioxanone, or non‐absorbable sutures such as polypropylene. Furthermore, over the last decade in an effort to reduce the recurrence rate of prolapse and given the success of mesh used in continence surgery, at sacral colpopexy, and at abdominal hernias, surgeons have utilised grafts at transvaginal repairs.
Graft material can be synthetic (for example permanent polypropylene or absorbable polyglactin mesh) or biological. Biological grafts can be further divided into autologous (using a person's own tissue, such as fascial sheath), alloplastic (from animals, for example porcine dermis), or homologous (for example cadaveric fascia lata).
The choice of operation depends on a number of factors, which include the nature, site, and severity of the prolapse; whether there are additional symptoms affecting urinary, bowel, or sexual function; the general health of the woman; and surgeon preference and capability. Concomitant procedures to treat or prevent urinary incontinence are often performed.
To aid the assessment of the success of surgery, clear pre‐ and postoperative site‐specific vaginal grading and details of the operative intervention should be recorded in the reports.
How the intervention might work
The aims of surgery include:
the restoration of normal vaginal anatomy;
the restoration or maintenance of normal bladder function;
the restoration or maintenance of normal bowel function;
the restoration or maintenance of normal sexual function.
The restoration of normal anatomy is achieved by utilising grafts as an alternative to the native tissue repair. The graft is utilised to prevent the descent of the bladder into the vagina, the bowel moving forward into the vagina, or the uterus or upper vagina descending towards or beyond the vaginal opening.
Why it is important to do this review
The wide variety of surgical treatments available for prolapse indicates the lack of consensus as to the optimal treatment. No clinical guidelines exist to identify the preferred surgical intervention. The most reliable evidence is likely to come from the consideration of randomised controlled trials, and this is the basis for our review. The aim is to help identify optimal practice and to highlight where there is a need for further research.
This review should be read as part of a series of six Cochrane reviews relating to the surgical management of prolapse including:
Surgery for women with anterior compartment prolapse.
Surgery for women with posterior compartment prolapse.
Surgery for women with apical compartment prolapse.
Continence outcomes in pelvic organ prolapse surgery.
Transvaginal grafts or mesh compared with native tissue repair for vaginal prolapse (current review).
Peri‐operative interventions at prolapse surgery.
This review evaluating any transvaginal grafts as compared to native tissue repairs was not reported separately in the Cochrane surgery for pelvic organ prolapse 2013 review, and thus represents a new evaluation. We have included 13 new trials, Dahlgren 2011, da Silveira 2014, Delroy 2013, De Tayrac 2013, Gupta 2014, Lamblin 2014, Qatawneh 2013, Robert 2014, Rudnicki 2014, Sung 2012, Svabik 2014, Tamanini 2014, and Turgal 2013, and a three‐year update of Iglesia 2010 since the last review.
Objectives
To determine the safety and effectiveness of transvaginal mesh or biological grafts compared to native tissue repair for vaginal prolapse.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
We required that studies include at least 20 participants in each arm.
Types of participants
Adult women seeking treatment for symptomatic pelvic organ prolapse (either primary or recurrent).
Pelvic organ prolapse includes:
anterior vaginal wall prolapse (cystocele, urethrocele, paravaginal defect);
upper vaginal prolapse (apical prolapse), i.e. prolapse of the uterine or vaginal vault in those who have undergone a hysterectomy;
posterior vaginal wall prolapse (enterocele, rectocele, perineal deficiency).
Types of interventions
Trials including any type of transvaginal graft compared with transvaginal native tissue repair. Grafts included absorbable or permanent mesh materials or biological implants. We also evaluated concomitant operations to treat or prevent urinary incontinence.
Types of outcome measures
Primary outcomes
1. Awareness of prolapse
Defined as affirmative response to questions relating to awareness of prolapse or vaginal bulge, or affirmative response to question three of pelvic floor distress inventory (PFDI‐20), “Do you usually have a bulge or something falling out that you can see or feel in the vaginal area?”.
2. Repeat surgery
2.1 Surgery for prolapse
2.2 Surgery for stress urinary incontinence
2.3 Surgery for prolapse, stress urinary incontinence, or mesh exposure (composite outcome)
3. Recurrent prolapse
Defined as any stage 2 or greater vaginal prolapse (Pelvic Organ Prolapse Quantification (POPQ): prolapse ‐ 1 cm above the hymen or below).
Secondary outcomes
4. Adverse events
4.1 Death (related to surgery)
4.2 Mesh exposure
4.3 Injury to the bladder or bowel
4.4 Surgery for mesh exposure
5. Prolapse outcomes
5.1 Objective failure
5.1.1 Stage 2 or greater anterior compartment prolapse (point Ba at or beyond 1 cm inside the introitus)
5.1.2 Stage 2 or greater apical compartment prolapse (point C at or beyond 1 cm inside the introitus)
5.1.3 Stage 2 or greater posterior vaginal compartment prolapse (point Bp at or beyond 1cm inside the introitus)
-
5.1.4 POPQ scores describe nine measurements of the vagina to quantify and describe vaginal prolapse. For simplicity, we have reported four of these basic measurements:
Point Ba on POPQ measurement (range ‐3 to +10 cm). Point Ba is approximately midpoint of the anterior vaginal wall
Point Bp on POPQ measurements (range ‐3 to +10 cm). Point Bp is approximately midpoint of posterior vaginal wall
Point C on POPQ measurements range from ‐10 cm to non‐determined limit). Point C describes the vaginal apex (upper vagina)
Total vaginal length (TVL) in cm range (0 to 14 cm): TVL is length from the vaginal entrance to apex (cervix or vaginal cuff)
6. Bladder function
For example:
6.1 Stress urinary incontinence
6.2 De novo stress urinary incontinence
6.3 Bladder overactivity or urge incontinence
6.4 De novo bladder overactivity or urge incontinence
7. Bowel function
For example:
7.1 De novo faecal incontinence
7.2 De novo obstructed defecation
8. Sexual function
8.1 De novo dyspareunia
8.2 Prolapse and Incontinence Sexual Questionnaire (PISQ‐12): range 0 to 48, the higher the score the better the sexual function
9. Quality of life and satisfaction measured by questionnaire
9.1 Patient Global Impression of Improvement (PG1‐1): data presented as 7‐point Likert scale and responses of "much" or "very much" better considered affirmative and presented as dichotomous outcome
9.2 Prolapse Quality of Life questionnaire (PQOL): range 0 to 100, the higher the score the greater the dysfunction
9.3 Pelvic Floor Distress Inventory (PFDI‐20): range 0 to 300, the higher the score the greater the dysfunction
9.4 Pelvic Floor Impact Questionnaire (PFIQ‐7): range 0 to 300, the higher the score the greater the dysfunction
10. Measures associated with surgery
10.1 Operating time
10.2 Blood transfusion
10.3 Length of hospital stay
Search methods for identification of studies
We did not impose any language limits, however we did not include trials with fewer than 20 participants in each treatment group.
Electronic searches
This review drew on the search strategy developed for the Cochrane Incontinence Group. We identified relevant trials from the Group's Specialised Register of controlled trials which is described, along with the Review Group search strategy, under the Group's module in the Cochrane Library. The Register contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, MEDLINE in process, ClinicalTrials.gov, WHO ICTRP, and handsearching of journals and conference proceedings. We searched the Incontinence Group Specialised Register on 6 July 2015 using the Group's own keyword system; we have provided the search terms used in Appendix 2.
Searching other resources
We handsearched conference proceedings for the International Urogynecology Society (IUGA) and International Continence Society (ICS) for podium presentations from 2012 to 2014. We searched the reference lists of relevant articles and contacted researchers in the field.
Data collection and analysis
Selection of studies
Two review authors assessed titles and, if available, abstracts of all possibly eligible studies for compliance with the review inclusion criteria. Two review authors then independently assessed full reports of each study likely to be eligible. We have listed excluded studies with the reasons for their exclusion in the Characteristics of excluded studies table.
Data extraction and management
At least two review authors independently undertook data extraction, and comparisons were made to ensure accuracy. Discrepancies were resolved by discussion or by referral to a third party. Where trial data were not reported adequately, we attempted to acquire the necessary information from the trialist.
Assessment of risk of bias in included studies
Two review authors independently assessed the included studies for risk of bias using the Cochrane 'Risk of bias' assessment tool (www.cochrane‐handbook.org) in order to assess: selection (random sequence generation and allocation concealment); performance (blinding of participants and personnel); detection (blinding of outcome assessors); attrition (incomplete outcome data); reporting (selective reporting); and other bias. Disagreements were resolved by discussion or by a third review author. We described all judgements fully and presented the conclusions in the 'Risk of bias' table, which was incorporated into the interpretation of review findings by means of sensitivity analyses (see below).
Measures of treatment effect
For dichotomous data, we used the numbers of events in the control and intervention groups of each study to calculate Mantel‐Haenszel risk ratios. For continuous data, if all studies reported exactly the same outcomes, we calculated mean difference between treatment groups. If similar outcomes were reported on different scales, we planned to calculate the standardised mean difference. We presented 95% confidence intervals for all outcomes. We compared the magnitude and direction of effect reported by studies with how they are presented in the review, taking account of legitimate differences. We interpreted the standardised mean difference as follows: an effect size of 0.2 is a small effect, an effect size of 0.5 is a medium effect, and an effect size of 0.8 is a large effect (Cohen 1988).
Unit of analysis issues
All analyses were per woman randomised.
Dealing with missing data
We analysed the data on an intention‐to‐treat basis as far as possible and made attempts to obtain missing data from the original trialist. Where these were unobtainable, we analysed only the available data.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity by the measure of the I2. We took an I2 measurement greater than 50% to indicate substantial heterogeneity (Higgins 2003).
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, we aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data. If there were 10 or more studies in an analysis, we planned to use a funnel plot to explore the possibility of small‐study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
If the studies were sufficiently similar, we combined the data using a fixed‐effect model in the following three comparisons:
-
Transvaginal permanent mesh versus native tissue repair, stratified by type of repair:
Anterior compartment permanent mesh versus native tissue
Multi‐compartment (apical, anterior, and/or posterior) permanent mesh repair versus native tissue
Absorbable mesh versus native tissue
Biological graft versus native tissue
An increase in the odds of a particular outcome, which may be beneficial (for example patient's global impression of improvement ) or detrimental (for example reoperation for prolapse), is displayed graphically in the meta‐analyses to the right of the centre‐line, and a decrease in the odds of an outcome to the left of the centre‐line.
Subgroup analysis and investigation of heterogeneity
Where data were available, we considered the separate evidence for the primary outcomes within the following subgroups:
Anterior compartment repair only
Multi‐compartment repair (apical and/or anterior and/or posterior)
We investigated differences between subgroups by means of a formal test for significance (Chi2 test). We interpreted a low P value (< 0.05) as evidence of differences between the subgroups (variation in effect estimates beyond chance). We also computed an I2 statistic to describe the percentage of the variability in effect estimates from the different subgroups that is due to genuine subgroup differences rather than sampling error (chance) (Higgins 2011).
If we detected substantial heterogeneity, we explored possible explanations in sensitivity analyses. We took any statistical heterogeneity into account when interpreting the results, especially if there was any variation in the direction of effect as described above.
Where there was substantial heterogeneity, we used a random‐effects model.
Sensitivity analysis
We conducted sensitivity analyses for the primary outcomes to determine whether the conclusions were robust to arbitrary decisions made regarding the eligibility and analysis. These analyses included consideration of whether the review conclusions would have differed if:
eligibility had been restricted to studies without high risk of bias (defined as studies with low risk of bias for sequence generation and allocation concealment, and not at high risk of bias in any domain);
a random‐effects model had been adopted;
the summary effect measure had been odds ratio rather than risk ratio.
Overall quality of the body of evidence: 'Summary of findings' table
We prepared a 'Summary of findings' table using GRADEpro software (GRADEPRO 2014). This table evaluated the overall quality of the body of evidence for the main review outcomes, using GRADE criteria (study limitations (that is risk of bias), consistency of effect, imprecision, indirectness, and publication bias). Judgements about evidence quality (high, moderate, low, or very low) were justified, documented, and incorporated into reporting of results for each outcome.
If we judged there to be serious risk of bias, inconsistency, imprecision, indirectness, or suspicion of publication bias, we downgraded the evidence by one level (for each domain affected). We downgraded the evidence by two levels if the risk was considered very serious.
Results
Description of studies
Results of the search
Thirty‐seven trials evaluated transvaginal graft repair compared with a native tissue repair (Ali 2006; Allahdin 2008; Al‐Nazer 2007; Altman 2011; Carey 2009; Dahlgren 2011; da Silveira 2014; Delroy 2013; De Tayrac 2008; De Tayrac 2013; Feldner 2010; Gandhi 2005; Guerette 2009; Gupta 2014; Halaska 2012; Hviid 2010; Iglesia 2010; Lamblin 2014; Menefee 2011; Meschia 2004a; Meschia 2007; Nguyen 2008; Nieminen 2008; Paraiso 2006; Qatawneh 2013; Robert 2014; Rudnicki 2014; Sand 2001; Sivaslioglu 2008; Sung 2012; Svabik 2014; Tamanini 2014; Thijs 2010; Turgal 2013; Vollebregt 2011; Weber 2001; Withagen 2011).
We also evaluated Gutman 2013, which is a three‐year update of Iglesia 2010, and two studies, Ek 2010 and Ek 2011, which are ancillary reports to Altman 2011. The flow of literature through the assessment process is shown in the PRISMA flowchart (Figure 1).
1.

PRISMA study flow diagram.
Included studies
Study design and setting
All of the 37 included studies were parallel‐group randomised controlled trials (RCTs). They were conducted in 15 countries (Italy, USA, Australia, UK, the Netherlands, Finland, Belgium, Canada, Chile, Czech Republic, Denmark, France, India, Sweden, and Turkey). Fifteen trials were multi‐centre randomised trials (Altman 2011; Dahlgren 2011; da Silveira 2014; Delroy 2013; De Tayrac 2013; Guerette 2009; Halaska 2012; Iglesia 2010; Menefee 2011; Meschia 2007; Nieminen 2008; Rudnicki 2014; Sung 2012; Vollebregt 2011; Withagen 2011).
Participants
The studies evaluated 4023 women, with 1986 undergoing transvaginal graft repairs and 2037 undergoing traditional native tissue repair (colporrhaphy).
Interventions
-
Polypropylene permanent mesh versus native tissue: 25 RCTs made this comparison (Ali 2006; Al‐Nazer 2007; Altman 2011; Carey 2009; da Silveira 2014; Delroy 2013; De Tayrac 2008; De Tayrac 2013; Gupta 2014; Halaska 2012; Iglesia 2010; Lamblin 2014; Menefee 2011; Meschia 2004a; Nguyen 2008; Nieminen 2008; Qatawneh 2013; Rudnicki 2014; Sivaslioglu 2008; Svabik 2014; Tamanini 2014; Thijs 2010; Turgal 2013; Vollebregt 2011; Withagen 2011).
Anterior compartment repair: 17 RCTs compared permanent mesh versus native tissue for anterior compartment repair (Ali 2006; Al‐Nazer 2007; Altman 2011; Delroy 2013; De Tayrac 2013; Gupta 2014; Lamblin 2014; Menefee 2011; Nguyen 2008; Nieminen 2008; Qatawneh 2013; Rudnicki 2014; Sivaslioglu 2008; Tamanini 2014; Thijs 2010; Turgal 2013; Vollebregt 2011).
Multi‐compartment repair: Eight RCTs compared permanent mesh versus native tissue for apical, anterior, and/or posterior repair (Carey 2009; da Silveira 2014; De Tayrac 2008; Halaska 2012; Iglesia 2010; Meschia 2004a; Svabik 2014; Withagen 2011).
Absorbable mesh versus native tissue: three RCTs made this comparison (Allahdin 2008; Sand 2001; Weber 2001).
Biological graft repair versus native tissue: 10 RCTs made this comparison (Dahlgren 2011; Feldner 2010; Gandhi 2005; Guerette 2009; Hviid 2010; Menefee 2011; Meschia 2007; Paraiso 2006; Robert 2014; Sung 2012).
Outcomes
Most studies reported at least one of our primary outcomes and reported data in a form suitable for analysis:
Eighteen reported awareness of prolapse (Allahdin 2008; Al‐Nazer 2007; Altman 2011; Carey 2009; Dahlgren 2011; De Tayrac 2013; Gandhi 2005; Gupta 2014; Hviid 2010; Iglesia 2010; Lamblin 2014; Meschia 2004a; Nieminen 2008; Paraiso 2006; Qatawneh 2013; Vollebregt 2011).
Nineteen reported repeat surgery for prolapse, incontinence, or for the composite outcome (prolapse, incontinence, or mesh surgery) (Allahdin 2008; Altman 2011; da Silveira 2014; De Tayrac 2013; Feldner 2010; Guerette 2009; Halaska 2012; Hviid 2010; Iglesia 2010; Lamblin 2014; Menefee 2011; Nguyen 2008; Nieminen 2008; Paraiso 2006; Qatawneh 2013; Robert 2014; Tamanini 2014; Thijs 2010; Turgal 2013; Vollebregt 2011; Withagen 2011).
Twenty‐five reported recurrent prolapse on objective examination (Allahdin 2008; Al‐Nazer 2007; Carey 2009; De Tayrac 2008; De Tayrac 2013; Feldner 2010; Gandhi 2005; Halaska 2012; Hviid 2010; Iglesia 2010; Menefee 2011; Nguyen 2008; Nieminen 2008; Paraiso 2006; Qatawneh 2013; Robert 2014; Rudnicki 2014; Sand 2001; Sivaslioglu 2008; Svabik 2014; Tamanini 2014; Turgal 2013; Vollebregt 2011; Weber 2001; Withagen 2011).
Two studies did not report any of our primary outcomes, but did report at least one of our secondary outcomes (Ali 2006; Delroy 2013).
All trials reported outcomes with at least one year's follow‐up, apart from one, Ali 2006, which had only six months' follow‐up. Eight trials reported two‐year outcomes (Allahdin 2008; Delroy 2013; Guerette 2009; Lamblin 2014;Menefee 2011; Meschia 2007; Tamanini 2014;Weber 2001), and three trials reported three‐year outcomes (Dahlgren 2011; Iglesia 2010; Nieminen 2008).
Where studies reported "mesh erosion" and did not differentiate this from "mesh exposure", we have included the data in analyses of mesh exposure.
We have provided full details of the included trials in the Characteristics of included studies table.
Excluded studies
We excluded five studies from the review (Altman 2013; Balci 2011; Chao 2012; Juneja 2010; Tincello 2009). We have provided full details in the Characteristics of excluded studies table.
Risk of bias in included studies
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Thirty of the included studies (30/37) adequately described sequence generation, and 17 described an adequate method of allocation concealment (for example remote allocation or use of consecutively numbered, sealed, opaque envelopes).
We rated seven studies that did not clearly describe an adequate method of sequence generation as at unclear risk of bias in this domain.
We rated 18 studies that did not describe an adequate method of allocation concealment as at unclear risk in this domain, and we rated two studies as at high risk of bias, as they either did not use allocation concealment, in Tamanini 2014, or we suspected a high potential for bias (Withagen 2011).
See Figure 2 for details.
Blinding
Eight trials performed blinding of women and the postoperative reviewer (Allahdin 2008; Altman 2011; Iglesia 2010; Menefee 2011; Nguyen 2008; Paraiso 2006; Robert 2014; Sung 2012). Non‐surgeons conducted outcome assessments in 10 trials (Al‐Nazer 2007; da Silveira 2014; Delroy 2013; Feldner 2010; Iglesia 2010; Meschia 2007; Paraiso 2006; Sung 2012; Svabik 2014; Weber 2001).
We rated eight studies as at low risk of performance bias, 19 as at unclear risk, and ten as at high risk of bias in this domain.
We rated 12 RCTs as at low risk of detection bias, 17 as at unclear risk, and eight as at high risk of bias in this domain.
Incomplete outcome data
Loss to follow‐up varied, ranging from zero, in Allahdin 2008 and Meschia 2004a, to 53%, in Guerette 2009 (49/93). Weber also reported a significantly higher loss to follow‐up in one arm of the trial (ultra‐lateral anterior vaginal wall repair) (Weber 2001).
We rated 22 RCTs as at low risk of attrition bias, five as at unclear risk, and 10 as at high risk of bias in this domain.
Selective reporting
Thirty‐two studies clearly reported at least one of our primary outcomes and were deemed to be at low risk of selective reporting. We rated three studies as at unclear risk of selective reporting because they did not report any of our primary outcomes (Ali 2006; Delroy 2013), or else did not report data separately for the two groups (Weber 2001). We rated one study as at high risk of selective reporting because the choice of primary outcome appeared to be inconsistent (Withagen 2011).
Other potential sources of bias
All trials reported baseline descriptive characteristics, and there was no evidence of a difference between the groups, except in three trials: in Sand 2001, previous hysterectomy was more common in the mesh overlay group; in Withagen 2011, women in the native tissue group had greater degree prolapse at point A posterior (Ap), point B posterior (Bp), and genital hiatus (GH) compared to the mesh group, and prior sacral colpopexy was three times more frequent in the mesh group; and in Lamblin 2014, the rate of concomitant hysterectomy was twice as common in the vaginal colposuspension group (77%) as in the mesh group (33%, P < 0.001).
All trials reported preoperative prolapse status, but two trials did not specifically report equal distribution and severity of prolapse between groups (Ali 2006; Sand 2001), and Weber 2001 included 7% of women with stage 1 anterior vaginal wall prolapse preoperatively (at time of inclusion), which would also have been classified as a postoperative success.
We rated 12 RCTs as at low risk of other bias, 16 as at unclear risk, and six as at high risk of bias in this domain.
Effects of interventions
See: Table 1; Table 2; Table 3
1. Permanent mesh versus native tissue repair
Twenty‐five RCTs made this comparison. They compared permanent mesh versus native tissue repair in women having either anterior or multi‐compartment repair (Ali 2006; Al‐Nazer 2007; Altman 2011; Carey 2009; da Silveira 2014; Delroy 2013; De Tayrac 2008; De Tayrac 2013; Gupta 2014; Halaska 2012; Iglesia 2010; Lamblin 2014; Menefee 2011; Meschia 2004a; Nguyen 2008; Nieminen 2008; Qatawneh 2013; Rudnicki 2014; Sivaslioglu 2008; Svabik 2014; Tamanini 2014; Thijs 2010; Turgal 2013; Vollebregt 2011; Withagen 2011).
Primary outcomes
1.1 Awareness of prolapse (one‐ to three‐year review)
Women who had permanent transvaginal mesh repair were less likely to report awareness of prolapse than women who had native tissue repair (risk ratio (RR) 0.66, 95% confidence interval (CI) 0.54 to 0.81, 12 RCTs, n = 1614, I2 = 3%, moderate‐quality evidence). This suggests that if 19% of women are aware of prolapse after native tissue repair, between 10% and 15% will be aware of prolapse after permanent mesh repair. (Analysis 1.1; Figure 4)
1.1. Analysis.

Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 1 Awareness of prolapse (1‐3 years).
4.

Forest plot of comparison: 1 Any transvaginal permanent mesh versus native tissue repair, outcome: 1.1 Awareness of prolapse (1 to 3 years).
1.1.1 Subgroup analysis by extent of repair
When we subgrouped the analysis by extent of repair, there was no evidence of a significant difference between the subgroups: test for subgroup differences: Chi2 = 0.01, df = 1 (P = 0.94), I2 = 0%.
1.2 Repeat surgery (one‐ to three‐year review)
1.2.1 Surgery for prolapse
The rate of repeat surgery for prolapse was lower in the mesh group (RR 0.53, 95% CI 0.31 to 0.88, 12 RCTs, n = 1675; I2= 0%, moderate‐quality evidence). This suggests that if 3% of women undergo repeat prolapse surgery after traditional repair, between 1% and 3% will require repeat prolapse surgery after transvaginal mesh repair. (Analysis 1.2)
1.2. Analysis.

Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 2 Repeat surgery (1‐3 years).
1.2.2 Surgery for stress urinary incontinence
There was no evidence of a difference between the groups in the rate of repeat surgery for stress urinary incontinence (RR 1.07, 95% CI 0.62 to 1.83, 9 RCTs, n = 1284, I2 = 21%, low‐quality evidence). (Analysis 1.2)
1.2.3 Surgery for prolapse, stress urinary incontinence, or mesh exposure
Women who had a transvaginal mesh repair were more likely to undergo repeat surgery for prolapse, stress urinary incontinence, or mesh exposure than those undergoing native tissue repair (RR 2.40, 95% CI 1.51 to 3.81, 7 RCTs, n = 867, I2 = 0%, moderate‐quality evidence). This suggests that if 5% of women who undergo native tissue repair require subsequent surgery to manage prolapse, stress urinary incontinence, or mesh exposure, between 7% and 18% would require repeat surgery after transvaginal permanent mesh repair. (Analysis 1.2)
1.3 Recurrent prolapse (stage 2 or greater prolapse on examination at any vaginal site) (one‐ to three‐year review)
Women who had transvaginal mesh repair were less likely to have stage 2 or greater prolapse on examination at any vaginal site than after a native tissue repair (random‐effects model; RR 0.40, 95% CI 0.30 to 0.53, 21 RCTs, n = 2494; I2 = 73%, low‐quality evidence). This suggests that if 38% of women have prolapse on examination after native tissue repair, between 11% and 20% will have prolapse on examination after transvaginal mesh repair. Heterogeneity was high for this analysis, mainly due to differences in the effect size in studies of multi‐compartment repair. However, the direction of effect was consistent. (Analysis 1.3)
1.3. Analysis.

Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 3 Recurrent prolapse (any) at 1‐3 years.
1.3.1 Subgroup analysis by extent of repair
The test for subgroup differences indicated a statistically significant difference between the two subgroups: test for subgroup differences: Chi2 = 6.97, df = 1 (P = 0.008), I2 = 85.7%.
• 1.3.1.1 Anterior repair only
When the analysis was limited to anterior compartment repair, the benefit in the mesh group was more pronounced, and statistical heterogeneity was much reduced (RR 0.33, 95% CI 0.26 to 0.40, 15 RCTs, n = 1748, I2 = 10%). (Analysis 1.3)
• 1.3.1.2 Multi‐compartment repair
When the analysis was limited to studies of multi‐compartment repair, the benefit in the mesh group persisted but to a lesser magnitude (random‐effects model; RR 0.59, 95% CI 0.40 to 0.87, 6 RCTs, n = 746, I2 = 76%). (Analysis 1.3)
Secondary outcomes
1.4 Adverse events
1.4.1 Death
None of the included studies reported this outcome.
1.4.2 Mesh exposure (19 RCTs, one‐ to three‐year review)
While a woman undergoing a native tissue repair has no risk of mesh exposure, overall 134/1097 (12%) women in the transvaginal permanent mesh groups had mesh exposure (Table 4).
1. Mesh exposure following transvaginal permanent mesh.
| Study ID | Repair events | Repair total | Exposure events | Exposure total |
| Ali 2006 abstract | 0 | 43 | 3 | 46 |
| Al‐Nazer 2007 | 0 | 23 | 1 | 21 |
| Altman 2011 | 0 | 182 | 21 | 183 |
| Carey 2009 | 0 | 60 | 5 | 62 |
| da Silveira 2014 | 0 | 81 | 18 | 88 |
| Delroy 2013 | 0 | 39 | 2 | 40 |
| Gupta 2014 | 0 | 54 | 4 | 44 |
| Halaska 2012 | 0 | 72 | 16 | 79 |
| Iglesia 2010 | 0 | 33 | 5 | 32 |
| Lamblin 2014 | 0 | 35 | 2 | 33 |
| Menefee 2011 | 0 | 24 | 2 | 28 |
| Nguyen 2008 | 0 | 38 | 2 | 37 |
| Nieminen 2008 | 0 | 96 | 18 | 104 |
| Qatawneh 2013 | 0 | 63 | 4 | 53 |
| Sivaslioglu 2008 | 0 | 42 | 3 | 43 |
| Thijs 2010 abstract | 0 | 48 | 9 | 48 |
| Turgal 2013 | 0 | 20 | 3 | 20 |
| Vollebregt 2011 | 0 | 51 | 2 | 53 |
| Withagen 2011 | 0 | 84 | 14 | 83 |
| Total | 134 | 1097 |
• 1.4.2.1 Subgroup analysis by extent of repair
Anterior repair only: Mesh exposure was reported in 10% (76/753) women after anterior permanent mesh repairs (Table 5).
2. Mesh exposure versus anterior compartment repairs.
| Study ID | Repair events | Repair total | Exposure events | Exposure total |
| Ali 2006 abstract | 0 | 43 | 3 | 46 |
| Al‐Nazer 2007 | 0 | 23 | 1 | 21 |
| Altman 2011 | 0 | 182 | 21 | 183 |
| Delroy 2013 | 0 | 39 | 2 | 40 |
| Gupta 2014 | 0 | 54 | 4 | 44 |
| Lamblin 2014 | 0 | 35 | 2 | 33 |
| Menefee 2011 | 0 | 24 | 2 | 28 |
| Nguyen 2008 | 0 | 38 | 2 | 37 |
| Nieminen 2008 | 0 | 96 | 18 | 104 |
| Qatawneh 2013 | 0 | 63 | 4 | 53 |
| Sivaslioglu 2008 | 0 | 42 | 3 | 43 |
| Thijs 2010 abstract | 0 | 48 | 9 | 48 |
| Turgal 2013 | 0 | 20 | 3 | 20 |
| Vollebregt 2011 | 0 | 51 | 2 | 53 |
| Total | 76 | 753 |
Multi‐compartment repair: Mesh exposure was reported in 17% (58/344) women after multi‐compartment mesh repair (Table 6).
3. Mesh exposure versus multi‐compartment repairs.
| Study ID | Repair events | Repair total | Exposure events | Exposure total |
| Carey 2009 | 0 | 60 | 5 | 62 |
| da Silveira 2014 | 0 | 81 | 18 | 88 |
| Halaska 2012 | 0 | 72 | 16 | 79 |
| Iglesia 2010 | 0 | 33 | 5 | 32 |
| Withagen 2011 | 0 | 84 | 14 | 83 |
| Total | 58 | 344 |
1.4.3 Injuries to the bladder or bowel
Women undergoing a transvaginal permanent mesh repair were more likely to have a bladder injury than those undergoing a native tissue repair (RR 3.92, 95% CI 1.62 to 9.50, 11 RCTs, n = 1514, I2 = 0%, moderate‐quality evidence). This suggests that if the bladder injury rate at a native tissue repair was 0.5%, then between 1% and 6% of women would have a bladder injury at a transvaginal mesh repair. (Analysis 1.4)
1.4. Analysis.

Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 4 Injuries bladder or bowel.
Only a single trial reported bowel injury as an outcome, and there was no evidence of a difference between the two groups (RR 3.26, 95% CI 0.13 to 78.81, 1 RCT, n = 169). (Analysis 1.4)
1.4.4 Surgery for mesh exposure (one‐ to three‐year review)
Surgery for mesh exposure was required in 8% of women (100/1227) (Table 7).
4. Surgery for mesh exposure following any transvaginal permanent mesh.
| Study ID | Surgery for mesh exposure | Total number of women in mesh group |
| Altman 2011 | 6 | 186 |
| Carey 2009 | 3 | 62 |
| da Silveira 2014 | 7 | 88 |
| De Tayrac 2013 | 4 | 66 |
| Delroy 2013 | 2 | 40 |
| Gupta 2014 | 2 | 44 |
| Halaska 2012 | 10 | 79 |
| Iglesia 2010 | 3 | 32 |
| Lamblin 2014 | 2 | 33 |
| Nguyen 2008 | 2 | 37 |
| Nieminen 2008 | 14 | 104 |
| Qatawneh 2013 | 4 | 53 |
| Rudnicki 2014 | 5 | 78 |
| Sivaslioglu 2008 | 3 | 43 |
| Svabik 2014 | 2 | 36 |
| Tamanini 2014 | 7 | 42 |
| Thijs 2010 abstract | 4 | 48 |
| Turgal 2013 | 3 | 20 |
| Vollebregt 2011 | 2 | 53 |
| Withagen 2011 | 5 | 83 |
| Total | 100 | 1227 |
1.5 Prolapse outcomes
1.5.1 Objective failure (one‐ to three‐year review)
• 1.5.1.1 Objective failure of anterior compartment
Women who had a transvaginal mesh repair were less likely to have a stage 2 or greater anterior compartment prolapse on examination than those undergoing a native tissue repair (RR 0.45, 95% CI 0.36 to 0.55, 13 RCTs, n = 1406, I2 = 35%). (Analysis 1.5)
1.5. Analysis.

Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 5 Objective failure of anterior compartment (cystocoele).
Subgroup analysis by extent of repair
The test for subgroup differences indicated a statistically significant difference between the two subgroups: test for subgroup differences: Chi2 = 9.76, df = 1 (P = 0.002), I2 = 89.8%.
Anterior repair only: When the analysis was limited to studies of anterior compartment repair, the benefit in the mesh group was more pronounced (RR 0.36, 95% CI 0.28 to 0.47, 9 RCTs, n = 1004, I2 = 0%).
Multi‐compartment repair: When the analysis was limited to studies of multi‐compartment repair, there was no conclusive evidence of a difference between the groups (RR 0.73, 95% CI 0.51 to 1.06, 4 RCTs, n = 402, I2 = 0%). (Analysis 1.5)
• 1.5.1.2 Objective failure of apical compartment
None of the included studies reported this outcome.
• 1.5.1.3 Objective failure of posterior vaginal compartment
There was no evidence of a difference between the groups in rates of grade 2 or greater posterior compartment prolapse (RR 0.64, 95% CI 0.29 to 1.42, 3 RCTs, n = 226, I2 = 0%). (Analysis 1.6)
1.6. Analysis.

Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 6 Objective failure of posterior compartment (rectocoele).
• 1.5.1.4 Pelvic Organ Prolapse Quantification (POPQ) scores
Point Ba (mid‐anterior vaginal wall)
Evidence suggested that Point Ba on the mid‐anterior vaginal wall had better support after transvaginal permanent mesh repair than after native tissue repair (random‐effects model; MD ‐0.93, 95% CI ‐1.27 to ‐0.59, 10 RCTs, n = 1125, I2 = 86%). This result should be interpreted very cautiously as there was substantial heterogeneity between studies. However, the direction of effect was consistent. (Analysis 1.7)
1.7. Analysis.

Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 7 POPQ assessment (any mesh).
Point C (vaginal apex)
There was no evidence of a difference between the groups at Point C (random‐effects model; mean difference (MD) ‐0.45, 95% CI ‐1.13 to 0.23, 8 RCTs, n = 925, I2 = 82%). (Analysis 1.7). This result should be interpreted very cautiously as there was substantial heterogeneity between studies, and the directions of effect were not consistent.
Point Bp (mid‐posterior vaginal wall)
There was no evidence of a difference between the groups at Point Bp (random‐effects model; MD 0.05, 95% CI ‐0.34 to 0.44, 7 RCTs, n = 832, I2 = 86%). This result should be interpreted very cautiously as there was substantial heterogeneity between studies, and the directions of effect were not consistent. (Analysis 1.7)
• 1.5.1.5 Total vaginal length (cm)
There was no evidence of a difference between the groups in total vaginal length (random‐effects model; MD 0.07, 95% CI ‐0.25 to 0.40; 5 RCTs, n = 611; I2 = 43%). This result should be interpreted very cautiously as there was substantial heterogeneity between studies, and the directions of effect were not consistent. (Analysis 1.7)
1.6 Bladder function
1.6.1 Stress urinary incontinence
None of the included studies reported this outcome.
1.6.2 De novo stress urinary incontinence ( one‐ to three‐year review)
Women undergoing a transvaginal permanent mesh repair were more likely to develop de novo stress urinary incontinence than those undergoing native tissue repair (RR 1.39, 95% CI 1.06 to 1.82, 12 RCTs, n = 1512, I2 = 0%, low‐quality evidence). This suggests that if 10% of women developed urinary stress incontinence after native tissue repair, 10% to 17% would develop urinary stress incontinence after a transvaginal permanent mesh repair. (Analysis 1.8)
1.8. Analysis.

Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 8 Bladder function: de novo stress urinary incontinence (1‐3 years).
• 1.6.2.1 Subgroup analysis by site of repair
When we subgrouped the analysis by extent of repair, there was no evidence of a significant difference between the subgroups: test for subgroup differences: Chi2 = 0.14, df = 1 (P = 0.71), I2 = 0%.
1.6.3 De novo bladder voiding difficulties or urgency
There was no evidence of a difference between the groups in the rate of de novo voiding disorder, urgency, detrusor overactivity, or overactive bladder (RR 0.75, 95% CI 0.35 to 1.63, 3 RCTs, n = 236, I2 = 0%). (Analysis 1.9)
1.9. Analysis.

Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 9 De novo voiding disorder, urgency, detrusor overactivity or overactive bladder.
1.7 Bowel function
1.7.1 De novo faecal incontinence or obstructed defecation
None of the included studies reported this outcome in a format suitable for analysis.
1.8 Sexual function
1.8.1 De novo dyspareunia (one‐ to three‐year review)
There was no evidence of a difference between the groups in the rate of de novo dyspareunia (RR 0.92, 95% CI 0.58 to 1.47, 11 RCTs, n = 764; I2 = 21%). (Analysis 1.10)
1.10. Analysis.

Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 10 De novo dyspareunia (1‐3 years).
• 1.8.1.1 Subgroup analysis by extent of repair
When we subgrouped the analysis by extent of repair, there was no evidence of a significant difference between the subgroups: test for subgroup differences: Chi2 = 1.05, df = 1 (P = 0.31), I2 = 4.7%.
1.8.2 Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire
There was no evidence of a difference between the groups in prolapse‐specific sexual function questionnaire scores (MD ‐0.13, 95 CI ‐0.40 to 0.13, 7 RCTs, n = 857, I2 = 0%). (Analysis 1.11)
1.11. Analysis.

Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 11 Sexual function (1‐3 years).
1.9 Quality of life and satisfaction measures (one‐ to two‐year review)
Quality of life was measured by post‐treatment scores (end scores) on the Prolapse Quality of Life Questionnaire (3 RCTs) or the Pelvic Floor Impact Questionnaire (4 RCTs).
When we combined data to calculate standardised mean differences, we found no evidence of a difference between the groups (standardised mean difference (SMD) 0.05, 95% CI ‐0.20 to 0.30, 7 RCTs, 665 women, I2 = 60%; Analysis 1.12). These findings should be interpreted with caution as there was substantial heterogeneity between studies, and the directions of effect were not consistent.
1.12. Analysis.

Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 12 Quality of life: continuous data (1‐2 years):.
One study reported this outcome using a dichotomous measure for Patient Global Impression of Improvement. There was no evidence of a difference between the groups in the number of women who reported feeling "much or very much better" (RR 1.00, 95% CI 0.80 to 1.25, 1 RCT, n = 168). (Analysis 1.13)
1.13. Analysis.

Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 13 Quality of life: dichotomous data "much or very much better".
1.10 Measures associated with surgery
1.10.1 Operating time (mins)
Twelve studies reported this outcome. While the evidence strongly suggests shorter operating time in non‐mesh group due to significant heterogeneity (I2 = 97%) and inconsistency in the direction of effect the data were not pooled. Mean operating time ranged across studies from 53 minutes longer in the mesh group to 11 minutes shorter in the mesh group. (Analysis 1.14)
1.14. Analysis.

Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 14 Operating time (minutes).
• 1.10.1.1 Subgroup analysis by extent of repair
Anterior compartment repair: We did not pool studies due to extreme heterogeneity (I2 = 97%) and inconsistency in the direction of effect. Five of the ten studies reported that mean operating time was at least 15 minutes longer in the mesh group. Four studies found no difference between the groups, and one reported that the mean operating time was five minutes shorter in the mesh group.
Multi‐compartment repair: When multi‐compartment repairs were considered in isolation, the mean operating time was shorter in the mesh group (MD ‐7.48 minutes, 95% CI ‐10.87 to ‐4.08, 3 RCTs, n = 295, I2 = 0%). (Analysis 1.14) (data shown unpooled)
1.10.2 Blood transfusion
There was no evidence of a difference between the groups in the rate of blood transfusion (RR 1.55, 95% CI 0.88 to 2.72, 6 RCTs, n = 723, I2 = 0%). (Analysis 1.15)
1.15. Analysis.

Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 15 Blood transfusion.
1.10.3 Length of hospital stay (days) (7 RCTs)
There was no evidence of a difference between the groups in duration of admission (random‐effects model; MD ‐0.06 days, 95% CI ‐0.03 to 0.18, 7 RCTs, n = 953, I2 = 68%). (Analysis 1.16)
1.16. Analysis.

Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 16 Length of stay in hospital (days).
See Table 1
2.0 Absorbable mesh versus native tissue repair
Three trials evaluated the effects of using absorbable polyglactin (Vicryl) mesh inlay to augment prolapse repairs (Allahdin 2008; Sand 2001; Weber 2001). We pooled limited data from these trials. In Weber 2001, data from non‐mesh native tissue arms were combined.
Primary outcomes
2.1 Awareness of prolapse (two‐year review)
A single trial reported no evidence of a difference in awareness of prolapse between women undergoing absorbable mesh repair and those undergoing native tissue vaginal repair (colporrhaphy) (RR 1.05, 95% CI 0.77 to 1.44, 1 RCT, n = 54, very low‐quality evidence). This suggests that if 72% of women are aware of prolapse after native tissue repair, then between 55% and 100% would be aware of prolapse after an absorbable mesh repair. (Analysis 2.1)
2.1. Analysis.

Comparison 2 Absorbable mesh versus native tissue repair, Outcome 1 Awareness of prolapse (2 year review).
2.2 Repeat surgery (two‐year review)
A single trial reported no evidence of a difference between the two groups in the rate of repeat surgery for prolapse (RR 0.47, 95% CI 0.09 to 2.40, 1 RCT, n = 66, very low‐quality evidence). This suggests that if 13% of women required repeat surgery for prolapse after a native tissue repair, then between 1% to 30% would require repeat surgery for prolapse after an absorbable mesh repair. (Analysis 2.2)
2.2. Analysis.

Comparison 2 Absorbable mesh versus native tissue repair, Outcome 2 Repeat surgery for prolapse (2 years).
2.3 Recurrent prolapse (three‐months to two‐year review)
Three RCTs reported this outcome. Two RCTs had follow‐up of one year, in Sand 2001, or nearly two years, in Weber 2001. The third, Allahdin 2008, had only three months' follow‐up for this outcome.
Rates of any recurrent prolapse on examination were lower in the absorbable mesh group (RR 0.71, 95% CI 0.52 to 0.96, 3 RCTs, n = 292, I2 = 21%, low‐quality evidence). However, this finding was sensitive to choice of statistical model, and was not statistically significant when we used a random‐effects model (RR 0.74, 95% CI 0.51 to 1.06). This suggests that if 43% of women had recurrent prolapse on examination after native tissue repair, then between 22% and 41% would have recurrent prolapse after an absorbable mesh repair. (Analysis 2.3)
2.3. Analysis.

Comparison 2 Absorbable mesh versus native tissue repair, Outcome 3 Recurrent prolapse (3 months ‐2 years).
2.3.1 Subgroup analysis by extent of repair
There was no evidence of a difference between the two subgroups: test for subgroup differences: Chi2 = 0.14, df = 1 (P = 0.71), I2 = 0%.
Secondary outcomes
2.4 Adverse events
2.4.1 Death (2 RCTs)
No deaths related to surgery were reported. (Analysis 2.4)
2.4. Analysis.

Comparison 2 Absorbable mesh versus native tissue repair, Outcome 4 Death.
Other adverse events were unreported in the included studies.
2.5 Prolapse outcomes
2.5.1 Objective failure (one‐ to two‐year review)
• 2.5.1.1 Objective failure of anterior compartment (2 RCTs)
There was no evidence of a difference between the groups in grade 2 or greater anterior compartment prolapse on examination (RR 0.72, 95% CI 0.53 to 0.98, 2 RCTs, n = 226; I2 = 57%, very low‐quality evidence). (Analysis 2.5)
2.5. Analysis.
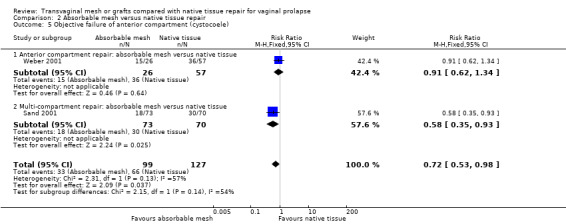
Comparison 2 Absorbable mesh versus native tissue repair, Outcome 5 Objective failure of anterior compartment (cystocoele).
• 2.5.1.2 Objective failure of apical compartment
None of the included studies reported this outcome.
• 2.5.1.3 Objective failure of posterior compartment (1 RCT)
There was no evidence of a difference between the groups in grade 2 or greater posterior compartment prolapse on examination (RR 1.13, 95% CI 0.40 to 3.19, 1 RCT, n = 132, very low‐quality evidence). (Analysis 2.6)
2.6. Analysis.

Comparison 2 Absorbable mesh versus native tissue repair, Outcome 6 Objective failure of posterior compartment (rectocoele).
• 2.5.1.4 POPQ scores
None of the included studies reported this outcome.
2.6 Bladder function
2.6.1 Postoperative stress urinary incontinence (two‐year review)
There was no evidence of a difference between the groups in the rate of postoperative stress incontinence (RR 1.38, 95% CI 0.95 to 2.00, 1 RCT, n = 49, very low‐quality evidence). (Analysis 2.7)
2.7. Analysis.

Comparison 2 Absorbable mesh versus native tissue repair, Outcome 7 Stress urinary incontinence.
Other outcomes were not reported in the included studies.
2.7 Bowel function
2.7.1 De novo faecal incontinence or obstructed defecation
None of the included studies reported this outcome in a format suitable for analysis.
2.8 Sexual function
None of the included studies reported this outcome.
2.9 Quality of life
2.9.1 Prolapse Quality of Life Questionnaire (1 RCT, 2‐year review)
A single trial reported no evidence of a difference between the groups in quality of life scores, measured using end scores on a 0 to 10 visual analogue scale ("How much do prolapse symptoms interfere with everyday life?" 0 = not at all, 10 = a great deal) (MD 0.00, 95% CI ‐2.82 to 2.82, 1 RCT, n = 54). (Analysis 2.8)
2.8. Analysis.

Comparison 2 Absorbable mesh versus native tissue repair, Outcome 8 Quality of life (2 years).
2.10 Measures associated with surgery
None of the included studies reported these outcomes.
See Table 2
3. Biological graft versus native tissue repair
Ten studies compared biological grafts versus native tissue; eight were porcine grafts (Dahlgren 2011; Feldner 2010; Hviid 2010; Menefee 2011; Meschia 2007; Paraiso 2006; Robert 2014; Sung 2012), one cadaveric (Gandhi 2005), and one was bovine (Guerette 2009).
Primary outcomes
3.1 Awareness of prolapse (one to three year review)
There was no evidence of a difference between the groups (RR 0.97, 95% CI 0.65 to 1.43, 7 RCTs, n = 777, I2 = 27%, low‐quality evidence). This suggests that if 10% of women were aware of prolapse after a native tissue repair, between 7% and 15% would be aware of prolapse after biological graft repair. (Analysis 3.1; Figure 5)
3.1. Analysis.

Comparison 3 Biological repair versus native tissue repair, Outcome 1 Awareness of prolapse (1‐3 year).
5.

Forest plot of comparison: 3 Biological repair versus native tissue repair, outcome: 3.1 Awareness of prolapse (1 to 3 years).
3.2 Repeat surgery (one‐ to two‐year review)
3.2.1 Surgery for prolapse
There was no evidence of a difference between the groups (RR 1.22, 95% CI 0.61 to 2.44, 5 RCTs, n = 306, I2 = 8%, low‐quality evidence). This suggests that if 4% of women required repeat prolapse surgery after native tissue repair, then between 3% to 10% would require repeat prolapse surgery after biological graft repair. (Analysis 3.2)
3.2. Analysis.

Comparison 3 Biological repair versus native tissue repair, Outcome 2 Repeat prolapse surgery (1‐2 years).
3.2.2 Surgery for stress urinary incontinence
None of the included studies reported this outcome.
3.3 Recurrent prolapse (one‐year review)
There was no evidence of a difference between the groups (RR 0.94, 95% CI 0.60 to 1.47, 7 RCTs, n = 587, I2 = 59%, very low‐quality evidence). This suggests that if 30% of women had recurrent prolapse after a native tissue repair, then between 18% and 33% would have recurrent prolapse on examination after a biological graft repair. (Analysis 3.3)
3.3. Analysis.
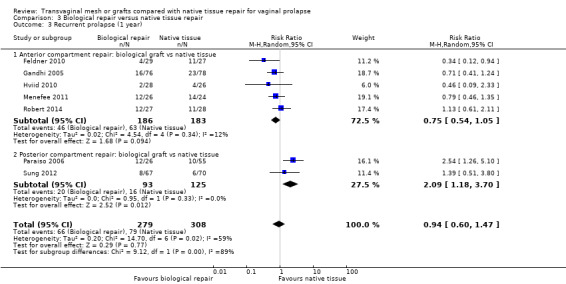
Comparison 3 Biological repair versus native tissue repair, Outcome 3 Recurrent prolapse (1 year).
3.3.1 Subgroup analysis by extent of repair
The test for subgroup differences indicated a statistically significant difference between the two subgroups: test for subgroup differences: Chi² = 9.12, df = 1 (P = 0.003), I² = 89.0%
Anterior repair only: When the analysis was limited to studies of anterior compartment repair, there was no conclusive evidence of a difference between the groups (RR 0.75, 95% CI 0.54 to 1.05, 5 RCTs, n=369, I2=12%)
Posterior compartment repair: When the analysis was limited to studies of posterior compartment repair, there was a higher risk of prolapse in the native tissue group (RR 2.09, 95% CI 1.18 to 3.70, 2 RCTs, n=218, I2=0%)
Secondary outcomes
3.4 Adverse events
3.4.3 Injury to the bladder or bowel
There was no evidence of a difference between the groups for this outcome, and only one event occurred in each comparison (bladder injury: RR 0.35, 95% CI 0.01 to 8.40, 1 RCT, n = 137; bowel injury: RR 3.13, 95% CI 0.13 to 75.57, 1 RCT, n = 137, very low‐quality evidence). (Analysis 3.4)
3.4. Analysis.

Comparison 3 Biological repair versus native tissue repair, Outcome 4 Injuries to bladder or bowel.
3.5 Prolapse outcomes
3.5.1 Objective failure (one‐year review)
• 3.5.1.1 Objective failure of anterior compartment
Women who had biological graft repair were less likely to have an objective failure of the anterior compartment than women having native tissue repair (RR 0.66, 95% CI 0.46 to 0.96, 6 RCTs, n = 570, I2 = 33%). (Analysis 3.5)
3.5. Analysis.

Comparison 3 Biological repair versus native tissue repair, Outcome 5 Objective failure of anterior compartment (cystocele).
• 3.5.1.2 Objective failure of apical compartment
None of the included studies reported this outcome.
• 3.5.1.3 Objective failure of posterior vaginal compartment (3 RCTs)
There was no evidence of a difference between the groups (random‐effects model; RR 1.16, 95% CI 0.39 to 3.51, 3 RCTs, n = 283, I2 = 80%). (Analysis 3.6). This result should be interpreted cautiously as there was substantial heterogeneity between studies, and the directions of effect were not consistent.
3.6. Analysis.

Comparison 3 Biological repair versus native tissue repair, Outcome 6 Objective failure of posterior compartment (rectocele).
• 3.5.1.4 POPQ scores
Point Ba (mid‐anterior vaginal wall)
Evidence suggested that there was greater support at point Ba after the biological graft repair compared to native tissue repair (MD ‐0.50, 95% CI ‐0.98 to ‐0.02, 1 RCT, n = 56). (Analysis 3.7)
3.7. Analysis.
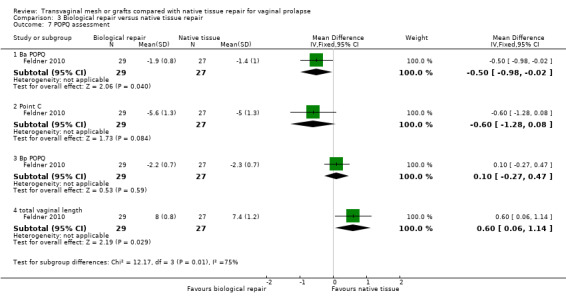
Comparison 3 Biological repair versus native tissue repair, Outcome 7 POPQ assessment.
Point C (vaginal apex)
There was no evidence of a difference between the groups at point C (MD ‐0.60, 95% CI ‐1.28 to 0.08, 1 RCT, n = 56). (Analysis 3.7)
Point Bp (mid‐posterior vaginal wall)
There was no evidence of a difference between the groups at point Bp (MD 0.10, 95% CI ‐0.27 to 0.47, 1 RCT, n = 56). (Analysis 3.7)
Total vaginal length
Total vaginal length was longer after the biological repair compared to native tissue repair (MD 0.60, 95% CI 0.06 to 1.14, 1 RCT, n = 56). (Analysis 3.7)
3.6 Bladder function
3.6.1 De novo stress urinary incontinence (1 RCT)
One study (n = 93) reported de novo stress urinary incontinence, but there were no events. (Analysis 3.8)
3.8. Analysis.

Comparison 3 Biological repair versus native tissue repair, Outcome 8 De novo urinary stress incontinence.
3.6.2 De novo urinary dysfunction (bladder overactivity and voiding dysfunction) (2 RCTs)
There was no evidence of a difference between the groups (RR 0.81, 95% CI 0.29 to 2.26, 2 RCTs, n = 93, I2 = 0%). (Analysis 3.9)
3.9. Analysis.

Comparison 3 Biological repair versus native tissue repair, Outcome 9 De novo voiding disorders, urgency, detrusor overactivity or overactive bladder.
3.7 Bowel function
3.7.1 De novo faecal incontinence or obstructed defecation
None of the included studies reported this outcome in a form suitable for analysis.
3.8 Sexual function
3.8.1 De novo dyspareunia (one‐year review)
There was no evidence of a difference between the groups, but only six events were reported (RR 0.85, 95% CI 0.20 to 3.67, 1 RCT, n = 37, very low‐quality evidence). (Analysis 3.10)
3.10. Analysis.

Comparison 3 Biological repair versus native tissue repair, Outcome 10 De novo dyspareunia (1 year).
3.8.2 Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire
There was no evidence of a difference between groups (MD 1, 95% CI ‐2.33 to 4.33, 1 RCT, n = 35, very low‐quality evidence).
3.9 Quality of life (1‐year review)
Two studies used validated prolapse‐specific quality of life questionnaires in which higher scores indicated greater dysfunction, and there was no evidence of a difference between the groups (SMD ‐0.05, 95% CI ‐0.48 to 0.38, 2 RCTs, n = 84, I2 = 0%). (Analysis 3.12)
3.12. Analysis.
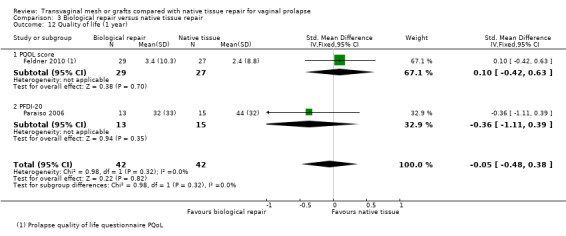
Comparison 3 Biological repair versus native tissue repair, Outcome 12 Quality of life (1 year).
3.10 Measures associated with surgery
3.10.1 Operating time
Duration of surgery was longer after native tissue repair than after biological graft repair (MD 10.34 minutes, 95% CI 6.31 to 14.36, 4 RCTs, n = 232, I2 = 0%). (Analysis 3.13)
3.13. Analysis.

Comparison 3 Biological repair versus native tissue repair, Outcome 13 Operating time (minutes).
3.10.2 Blood transfusion
Only one study reported this outcome in a format suitable for analysis. There was no evidence of a difference between the groups (RR 2.13, 95% CI 0.14 to 32.90, 1 RCT, n = 100).
3.10.3 Length of hospital stay
None of the included studies reported this outcome.
See Table 3
Other analyses
We conducted the prespecified sensitivity analyses by study quality, choice of statistical model, and choice of effect estimate. None of these substantially influenced the main findings, except for Analysis 2.3, which was sensitive to the choice of statistical model. As noted above, when we used a random‐effects model there was no significant difference between biological graft repair and native tissue repair in the rate of recurrent prolapse on examination.
We constructed a funnel plot for Analysis 1.3, which included 13 studies comparing permanent mesh versus native tissue repair, for the outcome of recurrent prolapse. The funnel plot was not strongly suggestive of publication bias. (Figure 6)
6.

Funnel plot of comparison: 1 Any transvaginal permanent mesh versus native tissue repair, outcome: 1.3 Recurrent prolapse (any) at 1 to 3 years.
Discussion
Summary of main results
When any transvaginal permanent mesh was compared to any native tissue vaginal repair, the advantages included decreased awareness of prolapse, prolapse on examination, and reoperation for prolapse. However, the rate of bladder injury, de novo stress urinary incontinence, and reoperation for prolapse, stress urinary incontinence or mesh exposure (composite outcome) was lower after a native tissue repair. This risk profile suggests, at best, that the utilisation of transvaginal permanent mesh needs to be individualised to those who accept the benefits and risk of these interventions. The quality of the evidence supporting these findings ranges from low to moderate for most comparisons.
Overall completeness and applicability of evidence
The 37 trials we assessed in this review allow an extensive review of the transvaginal grafts compared to native tissue repairs. The primary outcomes of awareness of prolapse, repeat surgery, and recurrent prolapse were generally well reported. Significant variation exists in the definitions of primary and secondary outcomes, which reduced our ability to include these outcomes in a meta‐analysis. Reporting of standardised anatomical, functional, and adverse events related to pelvic organ prolapse interventions in RCTs will reduce these problems in the future. Data relating to the impact of interventions on bowel function and cost evaluation of the interventions were poorly reported. Since the publication of the first version of this review, many of the transvaginal permanent meshes have been voluntarily withdrawn from the market. To date, the newer, lightweight transvaginal permanent meshes that remain on the market have not been evaluated under the auspices of a RCT.
While the rate of mesh exposure reported is consistent with previous reports, one of the main concerns in the FDA 2011 transvaginal mesh alert was the rate of vaginal pain or dyspareunia, or both, which accounted for over one‐third of the reported adverse events. Contrary to this, in the nearly 2500 women undergoing a transvaginal mesh repair in this systematic review, surgery for vaginal pain or dyspareunia related to the transvaginal mesh was barely mentioned. One possible explanation for this disparity is that while the incidence of vaginal pain requiring surgery following transvaginal mesh surgery may be low, the individual morbidity may be greater than with native tissue repairs, resulting in a higher tendency to report this adverse event. In this review, validated quality of life and pelvic floor function questionnaires were unable to detect a difference between the groups. Finally, the generally short follow‐up time after transvaginal polypropylene mesh intervention may not identify all the potential adverse events.
Quality of the evidence
The risk of bias in primary studies has generally decreased since our previous review, with the randomisation process being well reported. Reporting of allocation concealment, Consolidated Standards of Reporting Trials (CONSORT) flow diagrams, and methods of blinding of participants and reviewers is improving.
The quality of evidence when comparing transvaginal permanent mesh to native tissue was low to moderate for most outcomes, the most common limitations being poor reporting of methods, imprecision and inconsistency (Table 1).
The quality of evidence comparing absorbable mesh to native tissue repairs was generally very low to low, reflecting smaller, older studies with poor reporting of methods, high rates of attrition, and lack of blinding, and imprecision (Table 2).
The quality of evidence comparing biological grafts to native tissue repairs was very low to low, reflecting poor reporting of study methods, lack of clarity with regard to blinding of assessors, and imprecision (Table 3).
Overall, the main limitations were poor reporting of study methods, inconsistency, and imprecision.
Potential biases in the review process
The authors of this review did not conduct any of the trials being evaluated. The review authors have no conflicts of interest to report.
Agreements and disagreements with other studies or reviews
Recent reviews have evaluated the safely of transvaginal mesh in the treatment of female pelvic floor. The 2015 European Commission report on the safety of transvaginal meshes utilised in urogynaecology surgery concluded that the implantation of any mesh for the treatment of POP via the vaginal route should be only considered in complex cases, in particular after failed primary repair surgery (SCENHIR 2015). The New Zealand Accident Compensation Corporation also reported in 2015 on complications related to all surgical meshes for hernia, urinary incontinence and prolapse surgeries. They found that the rate of complications related to transvaginal polypropylene mesh was five time higher when utilised in prolapse repairs compared to both urinary incontinence and hernia repairs (ACC 2015). Both of these findings are relatively consistent with our findings.
The UK Medicines and Healthcare Products Regulatory Agency (MHRA) reported in late 2014 after reviewing literature until 2012, taking submissions from support groups, reviewing adverse events reports made to MHRA, and engaging with professional organisations and regulatory bodies worldwide (MHRA 2014). The full report was extensive and concluded that based on the available data:10.1002/14651858.CD006375.pub3
for the majority of women, the use of vaginal mesh implants is safe and effective;
when these products are used correctly they can help alleviate the very distressing symptoms of stress urinary incontinence and pelvic organ prolapse, and as such the benefits still outweigh the risks.
Our review suggests that while permanent transvaginal mesh is associated with a greater reduction in prolapse on examination, awareness of prolapse and reoperation for prolapse than native tissue repairs, it is associated with increased morbidity, including a higher rate of bladder injury, de novo stress urinary incontinence, and reoperation rates for prolapse, stress urinary incontinence, and/or mesh exposure. The rate of mesh exposure was 12%, and surgery for mesh exposure was required in 8%, accounting for most of the reoperations for mesh complications. We conclude, in contrast to the MHRA 2014 report, that while there may be individual cases of anterior compartment prolapse where mesh utilisation may be warranted, it cannot be considered a first‐line treatment option for pelvic organ prolapse, due to the associated morbidity.
Furthermore, and in contrast to the MHRA 2014 report, we have highlighted that most of data informing our report was derived from transvaginal mesh products that were voluntarily removed from the market in 2012, and that transvaginal mesh products currently available for use have not been evaluated by RCTs. We believe it is prudent that until such data become available, the currently available transvaginal mesh products should be utilised in a clinical setting under the discretion of the local ethics committee.
A recent Cochrane systematic review (Ford 2015) assessed mid‐urethral sling operations for the treatment of women with stress urinary incontinence. It included comparisons of different surgical routes, different types of synthetic tape and types of tape insertion. The review authors concluded that the surgery has a good safety profile and is highly effective in the short and medium term. This review has limited applicability to the current review, as it included women with or without pelvic prolapse; most trials did not report whether prolapse was present. Moreover none of the trials directly compared traditional anterior repair (with native tissue) to mid‐urethral synthetic sling.
Authors' conclusions
Implications for practice.
While transvaginal permanent mesh is associated with lower rates of awareness of prolapse, repeat surgery for prolapse, and prolapse on examination than native tissue repair, it is also associated with higher rates of repeat surgery for prolapse or stress urinary incontinence or mesh exposure (as a composite outcome), and with higher rates of bladder injury at surgery and de novo stress urinary incontinence. The risk‐benefit profile means that transvaginal mesh has limited utility in primary surgery. While it is possible that in women with higher risk of recurrence the benefits may outweigh the risks, there is currently no evidence to support this position.
Limited evidence suggests that absorbable mesh may reduce the risk of recurrent prolapse on examination compared to native tissue repair, but there was insufficient evidence on absorbable mesh for us to draw any conclusions for other outcomes.
In 2011, many of the transvaginal permanent meshes evaluated in this review were voluntarily withdrawn from the market. To date, the newer, lightweight transvaginal permanent meshes that remain of the market have not been evaluated within a RCT. Until such data become available, these newer transvaginal meshes should be utilised under the discretion of the ethics committee.
Implications for research.
In the short term, urgent evaluation of newer, lighter transvaginal mesh products that remain on the market is required. Unfortunately, at least two trials have received ethical committee approval comparing the new lightweight mesh with either sacral colpopexy or transanal repair (NCT01097200; NCT01497171), but have been terminated due to difficulty in recruiting or lack of funding. These products should also be compared to native tissue repairs and sacral colpopexy. In the medium to long term, the development of newer, self rejuvenating products through tissue engineering and bio‐design should be funded, and the efficacy, safety, and cost of the interventions assessed. A cost‐benefit analysis of transvaginal mesh is needed, and the long‐term outcomes of meshes already evaluated should also be undertaken.
What's new
| Date | Event | Description |
|---|---|---|
| 8 November 2017 | Amended | Acknowledgements section edited to recognise the contribution of the Cochrane Incontinence Group's Information Specialist Sheila Wallace; detail added to External sources of support by NIHR, UK |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 2, 2016
| Date | Event | Description |
|---|---|---|
| 27 May 2016 | Amended | Clarified wording re composite outcome "repeat surgery for prolapse or stress urinary incontinence or mesh exposure" |
| 6 July 2015 | New search has been performed | A comparison of transvaginal grafts versus native tissue repairs was formerly part of the 2013 Cochrane review "Surgical management of pelvic organ prolapse in women". We now present this as a separate review. Twelve new trials are included that were not in the previous review: Dahlgren 2011,da Silviera 2014,Delroy 2013,De Tayrac 2013,Gupta 2014, Qatawneh 2013,Robert 2014,Rudnicki 2014,Sung 2012,Svabik 2014,Tamanini 2014,Turgal 2013, and we also include a three‐year follow‐up of Iglesia 2010. |
| 14 April 2010 | Amended | Changed citation, added conflicts |
| 17 November 2009 | New citation required but conclusions have not changed | Full reports of 59 potentially eligible studies were assessed; for this update, 23 new
eligible studies were assessed (Ali 2006a; Allahdin 2008; Al‐Nazer 2007a; Barber 2006; Biller 2008; Borstad 2008; Braun 2007a; Carramao
2008a; Constantini 2008; De Tayrac 2008; Dietz
2008a; Glavind 2007; Guerette 2006a; Lim 2007a; Meschia
2007a; Natale 2007; Natale 2009; Nguyen
2008; Nieminen 2008; Pantazis 2008a;
Schierlitz 2007a; Segal 2007; Sivaslioglu 2008).
Overall, 17 studies were excluded from the review, six during this update (Barber 2006;
Biller 2008; Carramao 2008a; Glavind 2007; Meschia
2007a; Segal 2007); full details are given in the Characteristics of excluded
studies table. In this the second update, 18 new trials were added (Ali 2006; Allahdin 2008; Al‐Nazer 2007; Borstad 2008; Braun 2007a; Constantini 2007; Constantini 2008; De Tayrac 2008; Dietz 2008a; Guerette 2006; Lim 2007; Natale 2007; Natale 2009; Nguyen 2008; Nieminen 2008; Pantazis 2008; Schierlitz 2007; Sivaslioglu 2008), and three previously included studies were updated (Brubaker 2008; Meschia 2007; Roovers 2004). |
| 9 February 2009 | New search has been performed | New search Feb 2009 |
| 10 October 2008 | Amended | Converted to new review format |
| 17 April 2007 | New citation required and conclusions have changed | Substantive Update Issue 3 2007. 22 RCTs (8 new included trials). The findings are still insufficient to provide robust evidence to support current and new practice (such as whether to perform a concurrent continence operation, or to use mesh or grafts) |
Acknowledgements
We acknowledge the work of Elisabeth J Adams and Suzanne Hagen as coauthors on the original review, and Charis Glazener as coauthor on the original review and update. The authors of the 2016 update would like to thank Sheila Wallace, Information Specialist of the Cochrane Incontinence Review Group, for designing the search strategy and running the searches for this review. We are also grateful for the assistance of Helen Nagels and Cindy Farquhar of the Cochrane Gynaecology and Fertility Group in the preparation of this new review.
Appendices
Appendix 1. Types of operations
Sacral colpopexy
Aim to correct upper genital tract prolapse
Indication Usually reserved for recurrent prolapse of the upper vagina (recurrent cystocele, vault or enterocele) or massive vaginal eversion
Surgical technique
Usually performed under general anaesthesia
Performed through an incision on the lower abdomen or keyhole
The bladder and rectum are freed from the vagina and permanent mesh supports the front and back wall of the vagina
This mesh is secured to the sacrum (upper tailbone)
Peritoneum (lining of the abdominal cavity) is closed over the mesh
Other repairs are performed as required at the same time including paravaginal repair, perineoplasty, colposuspension or rectopexy
Bowel preparation is required prior to the surgery
McCall culdoplasty
Indications
Vault prolapse or an enterocele
Often performed at the time of vaginal hysterectomy to prevent future prolapse
Surgical technique
After the uterus is removed at the time of hysterectomy the uterosacral ligaments are identified and incorporated into the closure of the peritoneum and upper vagina using one to two sutures
An anterior or posterior vaginal repair is often performed at the same time
Sacrospinous fixation
Aim This surgery offers support to the upper vagina, minimising risk of recurrent prolapse at this site. The advantage of this surgery is that vaginal length is maintained.
Indication Upper vaginal prolapse (uterine or vault prolapse, enteroceles)
This procedure can be used in reconstructive vaginal surgery where increased vaginal length is required.
Procedure
The procedure can be performed under regional or general anaesthesia
A routine posterior vaginal incision is made and extended to the top of the vagina
Using sharp dissection, the vagina is freed from the underlying rectovaginal fascia and rectum until the pelvic floor (puborectalis) muscle is seen
Using sharp and blunt dissection, the sacrospinous ligament running from the ischial spine to the sacral bone is palpated and identified
Two sutures are placed through the strong ligament and secured to the top of the vagina. This results in increased support to the upper vagina. There is no shortening of the vagina
Other fascial defects in the vagina are repaired, and the vaginal skin is closed
Anterior vaginal repair (colporrhaphy)
Indication
Prolapse of the bladder or urethra
Sometimes used to treat urinary stress incontinence
Surgical technique
The procedure can be performed under regional or general anaesthesia
The vagina overlying the bladder and urethra is incised in the midline
Dissection in a plane directly below the vagina allows the damaged fascia supporting the bladder and urethra to be exposed
The fascia is plicated in the midline using delayed absorbable or permanent sutures
Sometimes excessive vaginal skin is removed
The vaginal skin is then closed
Other sites of prolapse are then repaired as required
Posterior vaginal repair and perineoplasty
Indications Treatment of rectocele (rectum bulges or herniates forward into the vagina) and defects of the perineum (area separating entrance of the vagina and anus)
Aim correct defects in the rectovaginal fascia separating rectum and vagina while allowing bowel function to be maintained or corrected without interfering with sexual function
Surgical technique
An incision is made on the posterior wall of the vagina starting at the entrance and finishing at the top of the vagina
Dissecting the vagina and rectovaginal fascia from the vagina until the pelvic floor muscles (puborectalis) are located
Defects in the fascia are corrected by centrally plicating the fascia using delayed absorption sutures
The perineal defects are repaired by placing deep sutures into the perineal muscles to build up the perineal body
The overlying vaginal and vulval skin is then closed
A pack is usually placed into the vagina and a catheter into the bladder at the end of surgery
Anterior or posterior vaginal repair, or both (colporrhaphy)
Indications:
Anterior repair: treatment for prolapse of bladder (bladder bulges forward into the vagina; cystocele) or urethra.
Posterior repair: correction of bowel prolapse (rectum bulges forward into the vagina; rectocele).
Vault repair: treat prolapse of upper vagina.
Depending on the side of the defect, the repair can either be anterior, posterior, vault, or total. The repair is achieved by the placement of permanent mesh, which may result in a stronger repair.
Surgical technique
The procedure can be performed under regional or general anaesthesia. Anterior vaginal repair
Midline incision to the vagina overlying the bladder and urethra
Dissection in a plane directly below the vagina and lateral of the bladder allows the damaged fascia supporting the bladder to be exposed
The fascia is plicated in the midline using sutures
Mesh can be used to reinforce the repair and can be used as an inlay or anchored through the obturator foramen and exiting through small incisions at both sides of the upper inner thigh
The vaginal skin is closed
Posterior and vault repair
An incision is made to the posterior wall of the vagina
Dissection below the vagina identifies the rectovaginal fascia and opens the space between the rectum and the pelvic floor muscle to the sacrospinous ligaments
Defects in the fascia are corrected by centrally plicating the fascia using sutures
Mesh can be used to reinforce the repair and can be used as an inlay or anchored bilaterally to the pelvic side wall and exiting through a small incision approximately 3 cm lateral and down from the anus
The vaginal skin is then closed
Vaginal paravaginal repair
Aim The objective of this surgery is to reattach detached lateral vaginal fascia to its normal point of insertion on the lateral side wall. This firm area of attachment is termed the white line or arcus tendineus fascia pelvis.
Indication The repair of anterior wall prolapse due to defects of the lateral supporting tissues
Procedure The procedure can be performed under regional or general anaesthesia.
Routine anterior repair The sharp dissection of the vagina from the bladder fascia continues laterally until the pelvic side wall can be identified.
Permanent or delayed absorbable sutures are placed from the lateral vagina to the firm pelvic side wall tissue (white line or arcus tendineus fascia pelvis). Three to four sutures are placed on each side.
A routine anterior repair with midline plication of the fascia, trimming of excess vaginal skin as required, and closure of the vaginal skin.
Appendix 2. Searches
Search strategy:
The Incontinence Group Specialised Register was searched using the Group's own keyword system (all searches were of the keyword field of Reference Manager 2012). The search terms used were:
({design.cct*} OR {design.rct*}) AND ({topic.prolapse*}) AND ({intvent.surg*})
Date of the most recent search of the register for this review: 6 July 2015.
Search registered trials: clinicaltrials.gov: date 1/6/2015 Terms: "Vaginal prolapse" "Surgery prolapse" with 175 trials identified
Data and analyses
Comparison 1. Any transvaginal permanent mesh versus native tissue repair.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Awareness of prolapse (1‐3 years) | 12 | 1614 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.54, 0.81] |
| 1.1 Anterior compartment:mesh vs native tissue | 9 | 1172 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.51, 0.84] |
| 1.2 Multicompartment: mesh vs native tissue | 4 | 442 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.46, 0.97] |
| 2 Repeat surgery (1‐3 years) | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Prolapse | 12 | 1675 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.31, 0.88] |
| 2.2 Continence surgery | 9 | 1284 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.62, 1.83] |
| 2.3 Surgery for prolapse, SUI or mesh exposure | 7 | 867 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.40 [1.51, 3.81] |
| 3 Recurrent prolapse (any) at 1‐3 years | 21 | 2494 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.30, 0.53] |
| 3.1 Anterior compartment repair: mesh versus native tissue | 15 | 1748 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.26, 0.40] |
| 3.2 Multi‐compartment repair: mesh versus native tissue | 6 | 746 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.40, 0.87] |
| 4 Injuries bladder or bowel | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Bladder injury | 11 | 1514 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.92 [1.62, 9.50] |
| 4.2 Bowel injury | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.26 [0.13, 78.81] |
| 5 Objective failure of anterior compartment (cystocoele) | 13 | 1406 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.36, 0.55] |
| 5.1 Anterior compartment repair: mesh versus native tissue | 9 | 1004 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.28, 0.47] |
| 5.2 Multi‐compartment repair: mesh versus native tissue | 4 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.51, 1.06] |
| 6 Objective failure of posterior compartment (rectocoele) | 3 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.29, 1.42] |
| 6.1 Mesh vs native tissue | 3 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.29, 1.42] |
| 7 POPQ assessment (any mesh) | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Point Ba POPQ | 10 | 1125 | Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐1.27, ‐0.59] |
| 7.2 Point C POPQ | 8 | 925 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.13, 0.23] |
| 7.3 Point Bp | 7 | 832 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.34, 0.44] |
| 7.4 total vaginal length | 5 | 611 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.25, 0.40] |
| 8 Bladder function: de novo stress urinary incontinence (1‐3 years) | 12 | 1512 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.06, 1.82] |
| 8.1 Anterior compartment: mesh vs native tissue | 8 | 1205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.00, 2.11] |
| 8.2 Multi compartment : mesh vs native tissue | 4 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.90, 1.92] |
| 9 De novo voiding disorder, urgency, detrusor overactivity or overactive bladder | 3 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.35, 1.63] |
| 10 De novo dyspareunia (1‐3 years) | 11 | 764 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.58, 1.47] |
| 10.1 Anterior compartment: mesh vs native tissue | 8 | 643 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.60, 1.93] |
| 10.2 Multicompartment: mesh vs native tissue | 3 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.29, 1.42] |
| 11 Sexual function (1‐3 years) | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 PISQ score | 7 | 857 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.40, 0.13] |
| 12 Quality of life: continuous data (1‐2 years): | 7 | 665 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.20, 0.30] |
| 12.1 PQOL end score | 3 | 331 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.31, 0.49] |
| 12.2 Pelvic floor impact questionnaire end score | 4 | 334 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.34, 0.37] |
| 13 Quality of life: dichotomous data "much or very much better" | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.80, 1.25] |
| 13.1 PGI‐I | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.80, 1.25] |
| 14 Operating time (minutes) | 13 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 14.1 Anterior compartment: mesh vs native tissue | 10 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 Multicompartment: mesh vs native tissue | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Blood transfusion | 6 | 723 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.88, 2.72] |
| 16 Length of stay in hospital (days) | 7 | 953 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.30, 0.18] |
Comparison 2. Absorbable mesh versus native tissue repair.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Awareness of prolapse (2 year review) | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.77, 1.44] |
| 2 Repeat surgery for prolapse (2 years) | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.09, 2.40] |
| 3 Recurrent prolapse (3 months ‐2 years) | 3 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.52, 0.96] |
| 3.1 Any site stage 2 or more | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.10, 2.70] |
| 3.2 Anterior compartment | 2 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.53, 0.98] |
| 4 Death | 2 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 absorbable mesh versus native tissue repair | 2 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Objective failure of anterior compartment (cystocoele) | 2 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.53, 0.98] |
| 5.1 Anterior compartment repair: absorbable mesh versus native tissue | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.62, 1.34] |
| 5.2 Multi‐compartment repair: absorbable mesh versus native tissue | 1 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.35, 0.93] |
| 6 Objective failure of posterior compartment (rectocoele) | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.40, 3.19] |
| 6.1 Multi‐compartment repair: absorbable mesh versus native tissue | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.40, 3.19] |
| 7 Stress urinary incontinence | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Postoperative SUI | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.95, 2.00] |
| 8 Quality of life (2 years) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.82, 2.82] |
| 8.1 VAS QoL | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.82, 2.82] |
Comparison 3. Biological repair versus native tissue repair.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Awareness of prolapse (1‐3 year) | 7 | 777 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.65, 1.43] |
| 1.1 Anterior compartment repair: biological graft vs native tissue | 4 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.45, 1.23] |
| 1.2 Multicompartment repair: biological graft vs native tissue | 1 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.55 [1.04, 19.92] |
| 1.3 Posterior compartment repair: biological graft vs native tissue | 2 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.41, 1.94] |
| 2 Repeat prolapse surgery (1‐2 years) | 5 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.61, 2.44] |
| 3 Recurrent prolapse (1 year) | 7 | 587 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.60, 1.47] |
| 3.1 Anterior compartment repair: biological graft vs native tissue | 5 | 369 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.54, 1.05] |
| 3.2 Posterior compartment repair: biological graft vs native tissue | 2 | 218 | Risk Ratio (M‐H, Random, 95% CI) | 2.09 [1.18, 3.70] |
| 4 Injuries to bladder or bowel | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 bladder injury | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.40] |
| 4.2 bowel injury | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.13, 75.57] |
| 5 Objective failure of anterior compartment (cystocele) | 6 | 570 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.46, 0.96] |
| 6 Objective failure of posterior compartment (rectocele) | 3 | 283 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.39, 3.51] |
| 7 POPQ assessment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Ba POPQ | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐0.98, ‐0.02] |
| 7.2 Point C | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.28, 0.08] |
| 7.3 Bp POPQ | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.27, 0.47] |
| 7.4 total vaginal length | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [0.06, 1.14] |
| 8 De novo urinary stress incontinence | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 De novo voiding disorders, urgency, detrusor overactivity or overactive bladder | 2 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.29, 2.26] |
| 10 De novo dyspareunia (1 year) | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.20, 3.67] |
| 11 Sexual function (1 year) | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐2.33, 4.33] |
| 11.1 PISQ | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐2.33, 4.33] |
| 12 Quality of life (1 year) | 2 | 84 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.48, 0.38] |
| 12.1 PQOL score | 1 | 56 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.42, 0.63] |
| 12.2 PFDI‐20 | 1 | 28 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.36 [‐1.11, 0.39] |
| 13 Operating time (minutes) | 4 | 232 | Mean Difference (IV, Fixed, 95% CI) | 10.34 [6.31, 14.36] |
| 14 Blood transfusion | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [0.14, 32.90] |
3.11. Analysis.

Comparison 3 Biological repair versus native tissue repair, Outcome 11 Sexual function (1 year).
3.14. Analysis.

Comparison 3 Biological repair versus native tissue repair, Outcome 14 Blood transfusion.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Al‐Nazer 2007.
| Methods | Single‐centre RCT for stage 2 POPQ prolapse PC‐generated randomisation 2‐year follow‐up No CONSORT statement Blinding not stated Authors state power of 85% need sample size of 20 in each arm |
|
| Participants | 40 randomised in abstract, however 44 were randomised, 4 of whom failed to return
postoperatively and were excluded Inclusion criteria: stage 2 POPQ cystocele with no plans of pregnancy in 12 months Exclusion criteria: contemplating pregnancy, women with paravaginal defects, needing continence surgery, prior colposuspension or vaginal surgery, immunocompromised, or diabetics |
|
| Interventions | A (n = 23): anterior colporrhaphy AC 0 polyglactin (Vicryl) suture B (n = 21): self styled armless soft polypropylene (Gynemesh) mesh without AC |
|
| Outcomes | Assessed at 6 weeks, 3 months, then every 6 months to 2 years postop Reports the following review outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated number tables |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes to ensure allocation concealment; as not consecutive sealed, opaque envelopes unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Reviewers blinded except when mesh exposure occurred |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 1‐year, group A 20/23, group B 20/21 |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Unclear risk | Funding not stated; authors no COI |
Ali 2006.
| Methods | Single‐centre RCT Inclusion grade 3 or 4 cysto‐urethrocele (BW halfway system) No exclusion No power Randomisation and concealment, blinding not stated 6/12 follow‐up |
|
| Participants | No CONSORT N = 108 Inclusion: women with grade 3 or 4 cysto‐urethrocele (BW halfway system) There were no significant differences between the groups regarding preoperative storage symptoms, urodynamics, and degree of prolapse |
|
| Interventions | A (54): anterior colporrhaphy alone B (54): anterior colporrhaphy with tension‐free polypropylene (Gynemesh PS) overlay |
|
| Outcomes | Assessed at 6 months' postop Reports the following review outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | At 6 months, group A 43/54, group B 46/54; greater than 15% loss to follow‐up at 6 months |
| Selective reporting (reporting bias) | Unclear risk | Did not report any of the primary outcomes of this review; only has 6 months' follow‐up |
| Other bias | Unclear risk | No statement about funding |
Allahdin 2008.
| Methods | Single‐centre RCT comparing vaginal fascial repair with or without polyglactin mesh
and with polydioxanone or polyglactin sutures, 2 x 2 factorial design PC randomisation, "secure" remote concealment Blinded women, ward staff, and follow‐up assessor Follow‐up 3 months with exam, 6 months with non‐validated questionnaire, 2 years with validated questionnaire |
|
| Participants | 73 randomised, 7 ineligible after randomisation, 66 in trial Lost to follow‐up: 8 at 3 months; 4 at 6 months; 12 at 2 years Inclusion: grade 2 or more prolapse (unclear examination technique), anterior or posterior prolapse, or both Concomitant procedures: vaginal hysterectomy 14; cervical amputation (Manchester) 18; tension‐free vaginal tape 13 |
|
| Interventions | Comparing vaginal fascial repair with or without polyglactin mesh and with
polydioxanone or polyglactin sutures, 2 x 2 factorial design A (32): fascial repair plus polyglactin mesh overlay B (34): fascial repair without mesh C (33): repair of fascia with polydioxanone sutures D (33): repair of fascia with polyglactin sutures |
|
| Outcomes | Assessed at 3 months', 6 months', and 2 years' postop Reports the following review outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Secure method of concealment of randomisation (remote computer allocation) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant‐completed questionnaires, data entry blinded to randomisation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Equal non‐response between the groups at 2 years, medical records seen for all non‐responders |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Low risk | Unfunded study |
Altman 2011.
| Methods | Multi‐centre RCT: 53 centres, 58 surgeons 90% powered to detect 20% difference between groups with 1% type 1 error, central randomisation PC Participant blinded Reviews conducted 2 and 12 months by surgeon 1/3, non‐surgeon 2/3 Completed pre‐ and 1‐year UDI and PISQ‐12 |
|
| Participants | 1685 screened; 389 randomised Underwent surgery: A 182, B 191 Lost to follow‐up A 7, B 14 (1 year: A 182, B 186) Inclusion: > 18 yrs, ≥ stage 2 symptomatic cystocele POPQ Exclusion: previous cancer of any pelvic organ, systemic glucocorticoid treatment, insulin‐treated diabetes, an inability to participate or to provide consent, or need concomitant surgery |
|
| Interventions | A (182): anterior colporrhaphy slow absorption monofilament thread, sham skin
markings, excessive trimming vagina discouraged B (191): Gynecare transvaginal anterior mesh (Prolift), absorbable sutures, excessive vaginal trimming discouraged, catheter care discretion surgeon |
|
| Outcomes | Assessed at 1‐year postop Reports the following review outcomes at 1 year:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Secure concealment with remote computer |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Women blinded (sham skin markings) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Reviewers surgeon 1/3, non‐surgeon 2/3 Woman‐completed questionnaires |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1‐year AC 174/182; mesh 186/191 |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | High risk | Funded by Karolinska Institute and Ethicon; conflict of interest statements of members of Nordic transvaginal mesh group who were reviewers of surgery were not reported |
Carey 2009.
| Methods | Single‐centre RCT CONSORT: no Randomisation: computer generated Allocation concealment: N/S Women, surgeons, and reviewers not blinded 12 months' follow‐up |
|
| Participants | Inclusion criteria: women who were recommended vaginal surgery for anterior and
posterior compartment with ≥ grade 2 prolapse Exclusion criteria: only requiring anterior or posterior compartment surgery, apical prolapse beyond the hymen, or those requiring abdominal mesh surgery Randomised: 139 (A 70, B 69); 10 women breached study protocol, and 11 more recruited. All were analysed Lost to follow‐up: A 6, B 9 Analysed 12 months: A 63, B 61 |
|
| Interventions | A (70): traditional anterior and posterior fascial plication using polydioxanone
sutures B (69): anterior and posterior repair with Gynemesh PS augmentation |
|
| Outcomes | Assessed at 6 months and 1 year postop Reports the following review outcomes at 1 year:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Native tissue 63/70; mesh 63/69 1 year |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | High risk | Funding not stated: authors' conflict of interest financial agreement with Ethicon manufacturer of product evaluated in study |
da Silveira 2014.
| Methods | Multi‐centre (4) RCT for stage 3 to 4 POPQ (any compartment) Brazil Computerised randomisation Sample size n = 90 in each group, 90% power and allowing 20% loss of follow‐up No ITT analysis Women unblinded Reviewers blinded |
|
| Participants | Inclusion criteria: grade 3 to 4 POP (any POPQ measurement > +1) No exclusion criteria 199 screened, 184 randomised Native tissue n = 90 randomised, n = 81 completed 1 year Mesh n = 94 randomised, n = 88 1 year |
|
| Interventions | Gp A: site‐specific native tissue: site‐specific anterior and/or posterior 1.0
non‐absorbable suture (polypropylene), apical 1.0 non‐absorbable sacrospinous right; uterine prolapse hysterectomy in both groups Gp B: mesh group: polypropylene macroporous monofilament Prolift mesh Concomitant surgery allowed Prior to study each centre performed at least 3 surgeries Hb 24 hours postop Assessed 1 week 1, 6, 12 months Pain assessed variable rating scale Gp A: 74/90 anterior compartment prolapse ∓ other surgery, posterior alone n = 7, apical alone n = 9 Gp B: mesh group similar breakdown, mid‐urethral slings: 5/90 native tissue, 9/94 mesh; vaginal hysterectomy: 32/90, 29/94 |
|
| Outcomes | Assessed at 1‐year postop Reports the following review outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not able to be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Native tissue: randomised 90, 1 year 81 completed Mesh: randomised 94, 1 year 88 |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Low risk | J&J donated product; no financial input study |
Dahlgren 2011.
| Methods | Multi‐centre (8) Swedish open RCT Computer‐generated block randomisation stratified for each centre Allocation concealment in opaque, sealed envelopes Sample size was based on the assumption that a 15% difference in objective cure rate after 3 years between the implant‐augmented repair and the traditional colporrhaphy with 90% power should be significant at a 5% level. It was estimated that 160 women, 80 in each arm of the study, including a drop‐out of 10%, were needed 3‐year review ITT and CONSORT guidelines reporting not stated |
|
| Participants | Inclusion: recurrent (prior surgery on the prolapsing site) POP in anterior or
posterior compartment, or both No exclusion criteria 135 randomised Gp A native tissue repair 66, and 3 years 60/66 Gp B porcine dermis repair 65, and 3 years 65/68 |
|
| Interventions | Standardised surgery with 2 meeting workshops prior to study Native tissue repair: midline fascial plication interrupted polydioxanone suture, vagina closed polyglactin absorbable suture Porcine: porcine dermal implant (Pelvicol, Bard Sweden) as inlay with no fascial plication: inlay anchored to vaginal wall and fascia 6‐8 polydioxanone sutures, vagina closed polyglactin suture Concomitant mid‐urethral sling, apical support, and levator plication performed as required |
|
| Outcomes | Assessed at 3 months and 3 years Reports the following review outcomes:
|
|
| Notes | Did not reach sample size as slow to recruit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated blocked randomisation list stratified for each centre |
| Allocation concealment (selection bias) | Unclear risk | Sealed, opaque envelopes (not stated if consecutive or not) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Nil |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Gp A 60/68 and Gp B 65/68 completed 3‐year review |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Low risk | No COI; funded by local research institutes |
De Tayrac 2008.
| Methods | Multi‐centre RCT comparing infracoccygeal sacropexy and sacrospinous suspension for
uterine or vaginal vault prolapse No CONSORT statement Power calculation: yes, 77 required in each arm. Recruitment stopped after change in mesh material (multi‐filament mesh replaced by monofilament) No ITT analysis No data on type of randomisation, blinding strategy, or allocation concealment No definition of cure or failure Mean follow‐up 16.8 months (range 1.5 to 32) both arms Prolapse assessment: POPQ Validated questionnaires: PFDI, PFIQ, PISQ‐12, French version |
|
| Participants | Inclusion: symptomatic uterine or vaginal vault prolapse (stage 2 or higher) Exclusion: isolated cystocele, stage 1 prolapse, rectal prolapse, and intestinal inflammatory disease 49 randomised 4 lost to follow‐up 45 analysed |
|
| Interventions | A (21): infracoccygeal sacropexy (multi‐filament polypropylene tape, posterior
IVS) B (24): sacrospinous suspension Concomitant surgery: cystocele repair, posterior repair, hysterectomy, suburethral tape Types of repair and indications for repair were not described |
|
| Outcomes | Assessed at "medium term" follow‐up (mean 16.8 months postop, range 1.5 to 32) Reports the following review outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Participant‐completed questionnaires |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 1 year 45/49 completed review |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Unclear risk | COI or funding unstated |
De Tayrac 2013.
| Methods | Multi‐centre (12 French hospitals) RCT 12‐month review Randomisation by drawing lots, stratified by centre, allocation concealment not discussed Intention to treat stated yes, but women already randomised were removed if cystotomy occurred during surgery CONSORT guidelines Sample size of 194 provided 80% power to detect 20% difference with an alpha error 5% and drop‐out rate 10% Assessors not clear |
|
| Participants | Inclusion criteria: symptomatic stage 2 anterior wall prolapse, aged 60 years or
older Exclusion criteria: steroids, poorly controlled diabetes, prior pelvic radiation, untreated vaginal or urinary infection, ascites, bladder injury during the procedure All used preoperative estrogen therapy 163 included, 162 randomised Gp A 82, 1 year 67/82 Gp B 80, 1 year 66/60 Preop demographics and potential confounders similar in both groups, except colorectal impact was greater group A |
|
| Interventions | Gp A: anterior colporrhaphy no mesh (plication of fascia with 2.0 polyglactin
absorbable suture), uterosacral colpopexy and hysterectomy as required Gp B: anterior polypropylene macroporous mesh (Ugtex, Sofradim, Covidien) 4‐armed transobturator mesh fixed with 2 x 2.0 permanent polypropylene sutures to uterine isthmus or uterosacral ligaments and 2 x 2.0 polyglactin sutures to inferior edge of pubic rami; vaginal trimming minimised Concomitant surgery mid‐urethral sling, hysterectomy, and any native tissue repair, however no other transvaginal mesh intervention included |
|
| Outcomes | Assessed at 1‐year follow‐up Reports the following review outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation by drawing lots? |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Gp A 82, 1 year 67/82 Gp B 80, 1 year 66/80 (20% attrition). 2 women who had bladder injury were excluded from analysis |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | High risk | Author COI with Sofradim, who provided partial funding and whose product was being evaluated. 2 women who had bladder injury were excluded from analysis; this outcome not reported clearly in both groups |
Delroy 2013.
| Methods | Single‐centre non‐inferiority RCT Computer‐generated random number list Allocation at inclusion with surgeon aware only in operating theatre Envelopes allocation Sample size: 35 in each group, 80% power to detect 5% significant change with 10% drop‐out ITT analysis Assessors blinded Women unblinded |
|
| Participants | Any anterior POP point Ba ≥ +1 on POPQ Excluded malignant urogenital disease, prior radiation, acute genitourinary infection, connective tissue disorders, steroid treatments, insulin‐dependent diabetes |
|
| Interventions | All procedures under spinal by 3 experienced surgeons 1. AC: plicate fascia purse string 0 polyglactin (Vicryl), vaginal trimming, transvaginal trocar‐guided polypropylene mesh (kits donated by Promedon) Nazca TC (Promedon, Córdoba, Argentina) I prepubic and 2 transobturator macroporous monofilament; vagina closed overlapping fashion 355 accessed, 79 randomised AC 39 completed, 1‐year review n = 39 2. Anterior mesh 40 randomised, 40 completed 1‐year review Concomitant surgery as required |
|
| Outcomes | Assessed at 1 year Reports the following review outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation tables |
| Allocation concealment (selection bias) | Unclear risk | Envelopes (opaque?, sealed?) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 79 randomised, and all completed 1‐year review |
| Selective reporting (reporting bias) | Unclear risk | Did not clearly report any of the primary outcomes of this review |
| Other bias | Low risk | Funded by Federal University of Sao Paulo, Brazil; Promedon contributed product free
of charge No author COI |
Feldner 2010.
| Methods | Single‐centre RCT Randomisation and allocation concealment described Evaluated 1 year after AC as compared to small intestine submucosa graft Blinded reviewers Sample size of 60 women was required to achieve a significance level of 0.05 and a power of 80%. This was based on the assumptions of a 25% difference in cure rates between the groups with a 10% loss to follow‐up rate |
|
| Participants | Inclusion criteria: women with point Ba ≥ ‐1 Exclusion criteria: hypertension, prior radiation, pelvic sepsis, diabetes, and chronic illness Concomitant surgery allowed including vaginal hysterectomy if greater than stage 2 uterine prolapse |
|
| Interventions | Gp A (27) AC with interrupted 0 polyglactin (Vicryl) sutures GP B (29) non‐cross‐linked xenograft porcine small intestine submucosa 7 x 10 cm with dissection to suprapubic arch fixed with 0 prolene x3 each side |
|
| Outcomes | Assessed at 1 year Reports the following review outcomes at 1 year:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Centrally controlled allocation concealment appropriate |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded reviewers and participant‐completed validated questionnaires |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1 year: Gp A 20/27(74%); Gp B 22/29 (76%) |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Low risk | No COI and no external funding |
Gandhi 2005.
| Methods | Single‐centre RCT (computer‐generated, opaque envelopes, adequate concealment) AC with and without fascia lata for primary or recurrent anterior vaginal wall prolapse | |
| Participants | 162 signed consent form 154 randomised A 76, B 78 Loss to follow‐up 2 in B, but in results 78 and 77 analysed respectively Inclusion: anterior vaginal wall prolapse to hymen or beyond on straining; > 18 years of age; willing to comply with return visits Concomitant surgery: vaginal hysterectomy in 49%/47%; sacrospinous fixation in 43%/42% (all cases with vaginal vault prolapse to mid‐vagina or beyond); posterior repair in 99%/94%; Coopers' ligament sling in 67%/55%; mid‐urethral sling 13%/10% Enterocele: A 75%, B 73% Baseline voiding dysfunction (slow stream): A 48/68, B 42/65 | |
| Interventions | A (76): "ultra‐lateral" midline plication of anterior endopelvic connective tissue using polyglactin (Vicryl) buttress sutures (as described by Weber 2001), plus additional cadaveric fascia lata patch (Tutoplast) anchored at the lateral limits of the colporrhaphy B (78): as above without allograft | |
| Outcomes | Assessed at 1 year Reports the following review outcomes:
|
|
| Notes | Unclear participant numbers (disparity with loss to follow‐up) Questionnaires not used in all participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque, consecutive envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data largely complete; 2/155 lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Unclear risk | No COI or funding statement |
Guerette 2009.
| Methods | Multi‐centre RCT 24‐month follow‐up Randomisation computer generated Allocation concealment without blinding of women or surgeon Not according CONSORT Sample size was calculated by estimating a recurrence rate of 35% with AC and 10% with graft reinforcement. Assuming a 2‐tailed hypothesis test with 5% type 1 error and 80% power, 80 women would be required. We enrolled 94 women assuming a drop‐out rate of 15% |
|
| Participants | Randomised: Gp A 47, Gp B 47 2 years: Gp A 33, Gp B 26 Examination: Gp A 27, Gp B 17 Inclusion criteria: point Ba ≥ ‐1 Exclusion criteria: total vaginal length < 6 cm, severe atrophy, isolated paravaginal defect, allergic to bovine material, prior vaginal implant surgery, or ulceration |
|
| Interventions | A (n = 46): AC B (n = 44): AC with bovine pericardium collagen matrix graft reinforcement |
|
| Outcomes | Assessed at 6 months, 1 year, and 2 years Reports the following review outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes opened in theatre (not consecutive) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated if assessors blinded, participant‐completed questionnaire |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Equal losses in both groups; only 50% completed 2‐year review |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | High risk | Extensive COI reported; study partly funded Synovis Life Technologies, whose bovine pericardium product was being evaluated |
Gupta 2014.
| Methods | Single‐centre RCT India Computer‐generated randomisation Allocation concealment: not stated Blinding of participants and reviewers: not stated Sample size 106 with 80% power to detect 21% difference between the groups with 5% type 1 error |
|
| Participants | Inclusion criteria: stage 2 or greater anterior compartment prolapse Exclusion criteria: SUI, dominant post‐vaginal prolapse, suspected malignancy, vaginal infections |
|
| Interventions | Group A: AC 2.0 polyglactin (Vicryl); n = 54, 1 year n = 41 Group B: self‐styled 4‐arms monofilament polypropylene mesh (Vypro mesh, J&J); n = 52, 1 year n = 44 |
|
| Outcomes | Assessed at 6 months, 1 year Reports the following review outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No statement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No statement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Gp A 41/54, Gp B 44/52 at 1 year (20% attrition) |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Unclear risk | No COI statement |
Halaska 2012.
| Methods | Multi‐centre randomised trial Computer‐generated randomisation table Allocation concealment not defined 70% power to detect 20% difference in groups |
|
| Participants | Inclusion criteria: central posthysterectomy vault prolapse: POPQ greater or equal
stage 2 Exclusion criteria: pelvic malignancy, < 18 years, prior radiotherapy, requiring hysterectomy Allocated: Gp A 83, Gp B (Mesh) 85 1 year: Gp A 72, Gp B 79 Recurrence defined as stage 2 or greater POPQ Not clear who performed assessments |
|
| Interventions | Gp A (83) anterior repair. Sacrospinous colpopexy (2x non‐absorbable sutures Nurolon)
± posterior repair (approximation of levator muscles) and moderate excision of redundant
vagina GP B (85) total Prolift mesh secured with 2.0 PDS Intervention performed by surgeons with greater than 20 cases experience of each type of surgery |
|
| Outcomes | Assessed at 1 year Reports the following review outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 year Gp A 72/83; Gp B 79/85 (89%) |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Low risk | Funded by grant from Czech Ministry of Health, authors no COI |
Hviid 2010.
| Methods | Single‐centre RCT Computer‐generated randomisation and allocation concealment were appropriate with sealed envelopes opened in operating room Reviews by non‐blinded surgeon No concomitant surgery 80% power to detect 20% difference between the groups with 5% type 1 error: 60 randomised |
|
| Participants | Inclusion criteria: symptomatic prolapse point Ba ≥ ‐1 Exclusion criteria: defects posterior or apical compartment, prior pelvic surgery, history of collagen or endocrine disorders Allocated: Gp A 31, Gp B 30 1 year: Gp A 26, Gp B 28 |
|
| Interventions | A (31): 2.0 interrupted polyglactin (Vicryl) plication B (30): no plication, Pelvicol porcine dermis 4 x 7 cm anchored with 2.0 polyglactin (Vicryl) sutures No concomitant surgery |
|
| Outcomes | Assessed at 1 year Reports the following review outcomes:
|
|
| Notes | Irregularities exist: methods failure defined as e Ba ≥ ‐1 results > ‐1; in table 2 Gp A range Ba 2 to 8, and states in table 3 that 4 had stage 2 prolapse |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Sealed, non‐transparent, consecutive envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Reviewers not blinded, participant‐completed questionnaires |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 year: Gp A 26/31, Gp B 28/30 (88%) |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Unclear risk | No COI declared; no statement funding |
Iglesia 2010.
| Methods | Multi‐centre RCT Double blinded Power calculation included Randomisation computer generated stratified for presence uterine prolapse, allocation concealment, CONSORT guidelines met, no ITT analysis |
|
| Participants | 173 excluded variety reasons Gp A 33, Gp B 32 Lost to follow‐up: Gp A 0, Gp B 0 Prior to surgery all demographic details similar between the 2 groups, except Gp B had lower POPDI‐6 score than Gp A Inclusion criteria: ≥ 21 yrs, grade 2 to 4 (POPQ) uterovaginal or vaginal prolapse who agreed to undergo vaginal surgery, available for 12 months' review, and can complete questionnaires Exclusion criteria: multiple medical contraindications, short vagina, uterus > 12 weeks size, desire future fertility, and postpartum |
|
| Interventions | Gp A: uterosacral colpopexy with polytetrafluoroethylene sutures or sacrospinous
colpopexy (Gortex sutures) and hysterectomy performed if uterus present Gp B: if point C or D on POPQ was ≥ ‐3 apical suspension with total vaginal mesh (Prolift), and if C or D was < ‐3 anterior Prolift was utilised. No T incisions were performed, and hysterectomy performed if uterus present |
|
| Outcomes | Assessed at 1, 2, and 3 years Reports the following review outcomes (at 3 years unless otherwise stated):
|
|
| Notes | The ethics committee stopped the study prior to completion due to predetermined stopping criteria of mesh erosion rate of > 15% being reached, with 65 of the desired sample size of 90 having undergone interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Consecutive, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 years: Gp A 26/32, Gp B 25/33 |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Low risk | Funded American Urogynecologic Society Foundation and MedStar research; authors reported no COI |
Lamblin 2014.
| Methods | Single‐centre RCT France Computer‐generated, 6‐block randomisation Allocation concealment: not stated Blinding: no women or reviewers Intention to treat: not stated |
|
| Participants | Inclusion criteria: stage 3 or greater anterior compartment prolapse Exclusion criteria: pregnancy, family not completed, prior cancer or radiation, poorly controlled diabetes mellitus, polypropylene sensitivity, immunocompromised. Concomitant surgery performed |
|
| Interventions | Gp A: AC with bilateral vaginal colposuspension (Ethibond suture) n = 35, at 2 years n
= 32 Gp B: polypropylene transobturator mesh (Perigee AMS) n = 33, at 2 years n = 31 More women underwent hysterectomy (77%) in the colposuspension group compared with 33% in the mesh group. P < 0.001 |
|
| Outcomes | Assessed at 3 months, 1 year, and 2 years Reports the following review outcomes at 2 years:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 2 years Gp A 32/35, Gp B 31/33 |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Unclear risk | Funding by the Claude Bernard University. Authors no COI. Measures of variance very low for some outcomes; attempt to check data with primary authors unsuccessful |
Menefee 2011.
| Methods | Double‐blind, triple‐arm RCT Randomisation, allocation concealment, N/S power 33 in each group 80% power to detect 35% difference with 5% type 2 error 2‐year review |
|
| Participants | Inclusion criteria: women ≥ 18 years of age with a POPQ point Ba of ≥ 0 Exclusion criteria: N/S Concomitant surgery: hysterectomy, colpopexy, posterior repair, continence at surgeons discretion |
|
| Interventions | 99 randomised Gp A: 32 standard AC using midline plication with delayed absorbable suture Gp B: 31 vaginal paravaginal repair using free‐hand formed porcine dermis graft (PelvicolTM) Gp C: 36 vaginal paravaginal repair using free‐formed polypropylene mesh. All graft material was secured to the arcus tendineus fascia pelvis using a CapioTM device with permanent monofilament suture |
|
| Outcomes | Assessed at 2 years Reports the following review outcomes at 2 years:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 years: Gp A 24/32; Gp B 26/31; Gp C 28/36 |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | High risk | Authors report COI with companies producing product evaluated and funded by Boston Scientific, whose product Capio was being evaluated |
Meschia 2004a.
| Methods | RCT (computer‐generated number table, opaque envelopes) on posterior IVS and sacrospinous fixation for vault prolapse Median follow‐up: Gp A 19, Gp B 17 months | |
| Participants | 66 randomised, no stratification A 33, B 33 No withdrawals or losses to follow‐up Inclusion: vault (vaginal cuff) prolapse ICS stage 2 or more Baseline stress urinary incontinence: A 11/33, B 7/33 Baseline overactive bladder: A 14/33, B 11/33 Baseline voiding dysfunction: A 19/33, B 18/33 Women in group A were significantly younger than those in group B (63 yrs vs 68 yrs, P < 0.05) | |
| Interventions | Gp A (33): infracoccygeal sacropexy (posterior IVS) using multifilament polypropylene tape Gp B (33): sacrospinous ligament fixation (vaginal sacrospinous colpopexy) Concomitant surgery: anterior (A 64%, B 66%) and posterior (70%, 88%) repair, high closure of pouch of Douglas if indicated (36%, 42%) | |
| Outcomes | Reports the following review outcomes at median 17‐ to 19‐month follow‐up:
|
|
| Notes | Abstract and further data from authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | PC‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% reviewed |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Unclear risk | No statement about funding |
Meschia 2007.
| Methods | Multi‐centre RCT (computer generated) on primary surgery anterior vaginal wall
prolapse
Allocation concealed Power calculation: 90 in each arm required Follow‐up: 2 years ITT analysis: yes, including those women with missing data at 2 years but with 1 year follow‐up completed |
|
| Participants | 206 randomised
Lost to follow‐up 5: Gp A 2, Gp B 3
Inclusion: primary
anterior prolapse POPQ point Ba ‐1 (≥ stage 2)
Exclusion: none
Baseline
SUI: A 22/100, B 18/106
Baseline overactive bladder: A 44/100, B 35/106
Baseline sexually active: A 65/100, B 74/106; with dyspareunia: A 12/65, B 11/74 No differences between the 2 groups with respect to demographic and clinical characteristics At 2 years number available for analysis: 176 (A 91, B 85) ITT analysis: 201 analysed (A 103, B 98) |
|
| Interventions | A (100): interrupted fascial plication polyglactin (Vicryl) 00 with porcine dermis graft (Pelvicol overlay) fixed with PDS suburethrally and uterosacral cardinal ligament distally B (106): surgery as above without Pelvicol overlay Concomitant surgery standardised Vaginal hysterectomy McCall culdoplasty, posterior compartment defect fascial plication | |
| Outcomes | Assessed at 1 year Reports the following review outcomes at 1 year:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No participant‐completed questionnaires |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 2 years: 91/100 native tissue versus biological 85/106 |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Unclear risk | No statement about funding |
Nguyen 2008.
| Methods | Single‐centre RCT on anterior vaginal prolapse CONSORT statement: yes Power calculation: 38 in each arm Type of randomisation: computer generated Blinding strategy: primary surgeon ‐ until the surgery day; women, research nurse, and medical assistant remained blinded Allocation concealment: sealed, opaque envelopes Definition of cure: Ant wall POPQ stage < 2, "Optimal support" = Aa and Ba at stage 0, "Satisfactory" = Aa and Ba at stage 1 and improved from preop staging Follow‐up: 12 months (full publication) and 24 months (abstract only) Prolapse assessment: POPQ |
|
| Participants | Inclusion: 21 years and older with POPQ stage 2 or greater anterior prolapse requiring
surgical correction Exclusion: pregnancy (present or contemplated), prior repair with graft, systemic infection, compromised immune system, uncontrolled diabetes mellitus, previous pelvic irradiation/cancer, polypropylene allergy, scheduled for concomitant Burch or pubovaginal sling Randomised: 76 Withdrawals: 1 Lost to follow‐up: 1 Analysed: 76 |
|
| Interventions | Gp A (38): AC with delayed absorbable (PDS) sutures Gp B (38): AC + polypropylene 4‐armed mesh kit repair (Perigee, American Medical Systems) Concomitant surgery: vaginal hysterectomy, bilateral salpingo‐oophorectomy, uterosacral suspension, mid‐urethral tape, site‐specific rectocele repair, perineoplasty, Apogee mesh kit repair Concomitant prolapse and suburethral tape surgeries were performed in both groups |
|
| Outcomes | Assessed at 1 year Reports the following review outcomes at 1 year:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Women blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded; participant‐completed questionnaires |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 year: Gp A 37/38, Gp B 37/38 |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Unclear risk | No statement about funding |
Nieminen 2008.
| Methods | Multi‐centre RCT on anterior vaginal prolapse CONSORT statement: yes Power calculation: 101 in each arm Type of randomisation: computer generated Allocation concealment: opaque envelopes Blinding strategy: not specified, but lack of a non‐surgical blinded outcome reviewer Definition of cure: less than stage 2 prolapse at Aa or Ba Follow up: 24 months Prolapse assessment: POPQ |
|
| Participants | Inclusion: postmenopausal women with symptomatic anterior vaginal wall prolapse to the
hymen or beyond Exclusion: apical defect indicating vaginal fixation or SUI necessitating surgery or the main symptomatic prolapse component was in the posterior vaginal wall. Also women with gynaecological tumour or malignancy calling for laparotomy or laparoscopy, and those with untreated vaginal infection Randomised: 202 Withdrawals: 1 Lost to follow‐up: 1 Analysed: 200 No significant differences in baseline demographics, prior hysterectomy, or prolapse surgeries between the 2 groups |
|
| Interventions | Gp A (96): AC using a 0 or 2/0 multifilament suture Gp B (104): AC + self tailored (from a 6 x 11 cm mesh patch) 4‐armed low‐weight polypropylene mesh Type of mesh: non‐absorbable monofilament polypropylene (Parietene light, Sofradim, France) Sutures for AC: absorbable 0 or 2/0 multifilament suture Concomitant surgery: vaginal hysterectomy, posterior repair, culdoplasty as required, no concomitant continence surgeries were performed |
|
| Outcomes | Assessed at 2 months, 1, 2, and 3 years Reports the following review outcomes at 3 years:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 years: 95/104 (92%) vs 85/96 (89%) |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Unclear risk | Some inconsistencies in data across publications at different follow‐up times |
Paraiso 2006.
| Methods | Single‐centre RCT (computer‐generated randomisation by sealed envelopes with blinded research nurse) 106 randomised to posterior colporrhaphy (37), site‐specific repair (37), site‐specific repair augmented with porcine small intestine submucosa (32: Fortagen, Organogenesis). Study funded with unrestricted research grant from Organogenesis | |
| Participants | 106 women Inclusion: grade 2 or greater posterior vaginal wall prolapse with or without other prolapse or incontinence or gynaecological procedures Exclusion: concomitant colorectal procedures, allergy to pork | |
| Interventions | Gp A (37): posterior colporrhaphy as per Maher 2‐0 Ethibond Gp B (37): site‐specific repair Cundiff 2‐0 Ethibond Gp C (32): as in B with 4 x 8 cm porcine small intestine submucosa graft inlay (Fortagen) | |
| Outcomes | Assessed at 1 year and 2 years (few 2‐year data reported) Reports the following review outcomes at 1 year:
|
|
| Notes | Ongoing study: initial full‐text review after 1 year ITT basis CONSORT statement Independent nurse review Limited sample size | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded non‐surgeon reviewer |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 17 months 99/106 completed; gps unclear |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Unclear risk | Unrestricted research grant from Organogenesis, whose product was being evaluated |
Qatawneh 2013.
| Methods | Single‐centre RCT Jordan 57 in each group had 80% power to detect 25% difference between the groups with a 5% type 1 error with a 10% drop‐out rate No ITT analysis |
|
| Participants | Inclusion criteria: symptomatic stage 3 or greater utero‐vaginal prolapse in all
compartments: primary and recurrent Exclusion criteria: less than grade 3 prolapse in any compartment, any prior surgery with implants for pelvic floor defects, prior radiation, those wishing uterine preservation |
|
| Interventions | AC group (n = 65): 2.0 PDS plication Mesh group (n = 64): self shaped polypropylene (Gynemesh) 15 x 3 cm with 2 arms retropubic space without suturing Concomitant continence surgery if needed and vaginal hysterectomy in those with uterine prolapse All underwent sacrospinous colpopexy and posterior colporrhaphy |
|
| Outcomes | Assessed at 6 weeks, then every 6 months. Median follow‐up 28/29 months, range 6 to
10 Reports the following review outcomes at 1 year:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated: POPQ assessment by independent investigator |
| Incomplete outcome data (attrition bias) All outcomes | High risk | AC group: 63/65; mesh group 53/64 at median 28‐month review; follow‐up times variable |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Unclear risk | Funded by Cook Medical |
Robert 2014.
| Methods | Parallel‐group RCT | |
| Participants | Included: women with a cystocele requiring surgical management Excluded: allergy to graft material, immunocompromised, non‐English speaking, unavailable for follow‐up Concomitant surgery or previous non‐anterior prolapse surgery were not exclusion criteria. |
|
| Interventions | Small intestine mesh‐augmented procedure vs same anterior repair without mesh | |
| Outcomes | Assessed at 1 year Reports the following review outcomes at 1 year:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised randomisation through university obstetrics & gynaecology department data manager |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Follow‐up assessment by examining physician blinded to allocation with no involvement in clinical care |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 55/57 women randomised (96%) were included in analysis for objective outcomes and 57/57 (100%) for subjective outcomes |
| Selective reporting (reporting bias) | Low risk | Reports expected review outcomes |
| Other bias | Low risk | Supplier of product (Cook) partially funded study, however the blinded nature of participants and reviewers overcomes potential biases |
Rudnicki 2014.
| Methods | Multi‐centre (6) international RCT Nordic countries: Norway, Sweden, Denmark, and
Finland: Block computer‐generated randomisation list Allocation concealment: opaque, sealed envelopes ITT analysis Sample size: 130 women allowed 80% power to detect 20% difference with an alpha error of 5% and a drop‐out rate of 15% Assessors: surgeons Women unblinded Surgeons trained to ensure uniform surgery performed |
|
| Participants | Inclusion criteria: ≳ 55 years, anterior wall prolapse stage 2 POPQ Aa or Ba ≳ ‐1 Exclusion criteria: previous major pelvic surgery with the exception of a hysterectomy for reasons other than genital prolapse, previous vaginal surgery, or hysterectomy for POP; concomitant prolapse of the uterus or an enterocele of stage 1 or higher; previous incontinence sling surgery performed through the obturator membrane; current treatment with corticosteroids; or a history of genital or abdominal cancer All surgery covered intra‐operative antibiotics and pre‐ and post‐local oestrogens Concomitant surgery allowed posterior repair |
|
| Interventions | AC group: interrupted absorbable suture fascial plication, vaginal trimming and
closure with running unlocked absorbable suture Mesh group: biosynthetic system monofilament polypropylene mesh with central portion coated in absorbable hydrophylic porcine collagen film Bard Avaulta Plus anterior 169 available randomisation with 161 randomised AC: 79 randomised, 1 year 76 Mesh: 82 randomised, 1 year 78 |
|
| Outcomes | Assessed at 3 months, 1 year, and 3 years Reports the following review outcomes at 1 year:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked computer‐generated randomisation list for each of 4 countries |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded (unable to blind) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Surgeons evaluated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1‐year evaluation/randomised AC 76/79, mesh 78/82 |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Unclear risk | No COI |
Sand 2001.
| Methods | Single‐centre RCT (computer‐generated number table) Vaginal repair with or without polyglactin (Vicryl) mesh overlay for cystocele and rectocele Follow‐up: Gp A 12 months, Gp B 12 months | |
| Participants | 143 women Inclusion: cystocele to or beyond hymenal ring on standing Exclusion: less than 18 years of age, pregnancy, contemplating pregnancy within 1 year, paravaginal defect only, anterior enterocele 161 randomised 1 excluded (anterior enterocele) 17 lost to follow‐up | |
| Interventions | Gp A (70): no mesh: Vicryl plication of anterior endopelvic fascia
Gp B (73):
mesh: as above with Vicryl mesh folded underneath trigone and cuff and secured Vicryl to
fascia; also added to posterior wall if posterior repair performed
Posterior
repair performed: A: 67/70, B: 65/73 Standardised concomitant surgery Review by surgeon |
|
| Outcomes | Assessed at 2, 6, 12 weeks and 1 year Reports the following review outcomes at 1 year:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 143/170 (84%) completed 1‐year review |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Unclear risk | No COI statement |
Sivaslioglu 2008.
| Methods | Single‐centre RCT comparing polypropylene mesh surgery with site‐specific surgery in
the treatment of cystocele CONSORT statement: yes Power calculation: 45 in each arm Type of randomisation: computer generated Blinding strategy: no (assessment was performed by non‐blinded reviewers) Allocation concealment: not specified Definition of cure/failure: "Acceptable cure" defined as cystocele less than ‐1 cm (stage 1 POPQ) Follow‐up: mean 12 months (range 8 to 16) Prolapse assessment: POPQ |
|
| Participants | Inclusion: primary cystocele Exclusion: SUI, concomitant rectocele or enterocele or recurrent cystocele Randomised: 90 (45 to each arm) Analysed: 85 Lost to follow‐up: 5 |
|
| Interventions | A (42): site‐specific polyglactin 910 anterior repair B (43): self styled 4‐armed polypropylene (Parietene, Sofradim, France) mesh, no anterior repair Concomitant surgery not standardised, management of concomitant apical prolapse was not specified in either group |
|
| Outcomes | Assessed at 6 weeks, 6 months, and annually Reports the following review outcomes at mean follow‐up of 1 year (range 8 to 16 months):
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Non‐blinded reviewers; objective assessment was participant‐completed questionnaires |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram: 1 year Gp A 42/45, Gp B 43/45 |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Low risk | No funding and no COI |
Sung 2012.
| Methods | 2‐centre, double‐blinded randomised control trial Allocation concealment: sealed envelopes Randomisation block and stratified site Women and assessors blinded (women unblinded 12 months) Based on a study by Kohli et al (Kohli 2003) assuming that graft use is associated with a 93% anatomic success rate, 63 women per group would be needed to detect a 20% difference at .05 and .20. We aimed to recruit 160 women (80 women per group) to account for drop‐out |
|
| Participants | Inclusion criteria: women with stage 2 or greater symptomatic rectocele (defined as vaginal bulge, defecatory symptoms, or both) electing surgical repair were eligible Exclusion criteria: < 18 years, women undergoing concomitant sacrocolpopexy or colorectal procedures, history of porcine allergy, connective tissue disease, pelvic malignancy, pelvic radiation, inability to understand English, or unable or unwilling to consent or comply with follow‐up. All other vaginal prolapse repairs and anti‐incontinence procedures were included | |
| Interventions | Gp A: 70 controls midline plication or site‐specific repair Gp B: 67 midline plication or site‐specific repair with 4 x 7 cm subintestinal submucosal graft over the repair and secured to levator ani fascia using interrupted No. 2‐0 polyglycolic acid and inferiorly to the perineal body using No. 2‐0 polyglycolic acid sutures. Excess vaginal tissue was trimmed in all women, and the posterior vaginal incision was closed using 2‐0 polyglycolic acid sutures. The deep and superficial transverse perineal muscles and bulbocavernosus muscles were re‐approximated using No. 0 polyglycolic acid sutures, and concomitant perineorrhaphy was performed in all women |
|
| Outcomes | Assessed at 6 months and 1 year Reports the following outcomes at median 12.2 to 12.5 months (range 10 to 43 months):
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded reviewers |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 year Gp A 70/80, Gp B 67/79 |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Low risk | No financial COI; grant funding National Institute of Child and Human Health |
Svabik 2014.
| Methods | Single‐centre RCT Computer randomisation on patient hospitalisation numbers Allocation concealment: not stated Women unblinded Postop unblinded due to surgeries Sample size 30 in each group allowed 80% power to detect a 45% difference with an alpha error of 5% ITT analysis: not stated |
|
| Participants | Inclusion criteria: symptomatic posthysterectomy patients with at least 2‐compartment
prolapse (with affected apical/vault compartment, stage 2 or higher (POPQ)), requesting
pelvic floor reconstructive surgery, and diagnosed with a complete unilateral or
bilateral avulsion injury Exclusion criteria: nil further stated Assessment pre‐ and postoperative POPQ examination, 4D ultrasonography with acquisition of volume data sets at rest, during pelvic floor muscle contraction, and on maximum Valsalva manoeuvre, PISQ‐12, POPDI, UDI, CRADI 142 reviewed and 72 excluded (70 no avulsion, 2 refused) Sacrospinous fixation: 34, 1 year 31 Mesh: 36, 1 year 36 |
|
| Interventions | Native tissue sacrospinous fixation: all cases: anterior repair with 2.0 polyglactin
(Vicryl Plus) (Ethicon), posterior high levatorplasty Vicryl Plus 1: 2x Nurolon 1.0
(Ethicon) permanent R sacrospinous ligament Mesh: Prolift total (Ethicon): 3 arms each side with mesh secured to apex with Vicryl Plus 2.0 and to introitus posteriorly Primary outcome: failure defined: Ba, C, or Bp at hymen or below Uterosacral suspension definition ≳ 10 mm descent of the bladder below the lower margin of the symphysis pubis on maximum Valsalva |
|
| Outcomes | Assessed at 3 months and at 1 year Reports the following review outcomes at 1 year:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Computer randomisation based on hospital number? |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No, cannot be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No, cannot blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 year 31/34 sacrospinous fixation, mesh 36/36 |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Low risk | Funded by Czech Ministry of health and Charles University in Prague; 1 author financial COI |
Tamanini 2014.
| Methods | Single‐unit raffle randomisation prior to surgery No allocation concealment described Surgeons and women unblinded Unclear who performed assessments (blinded?) 2 surgeons performed 2 surgeries with mesh kit prior to surgery Sample size: 100 women to 80% power to detect 26% difference between the groups with alpha error of 5% with 20% loss to follow‐up at 2 years |
|
| Participants | 122 reviewed, 100 randomised AC 55, 1 year 54, 2 years 50 Mesh 45, 1 year 43, 2 years 42 Inclusion criteria: 45 years old or older, with AVWP ≥ 2 (POPQ stage) without previous surgical correction or with previous surgical treatment of AVWP without the use of polypropylene mesh were selected Exclusion criteria: women who were previously treated (due to AVWP or SUI) using polypropylene mesh, who were receiving oncological treatment, with altered Papanicolaou smear exam or with uterine bleeding, with genital or acute urinary infection, women who didn't commit to ambulatory follow‐up or who refused the written informed consent All preop Urodynamics |
|
| Interventions | Spinal anaesthesia with antibiotics Nazca TC kit (Promedon, Córdoba, Argentina) monofilament macroporous 4 arms (1 prepubic and 1 transobturator each side) concomitant surgery as required: hysterectomy, apical or posterior repair AC group 2.0 polyglactin (Vicryl) fascial plication mid‐urethral sling if SUI on preop Urodynamics (14/55) |
|
| Outcomes | Assessed at 1 year and 2 years Reports the following review outcomes at 2 years:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Raffle randomisation 55 in AC and 45 in mesh |
| Allocation concealment (selection bias) | High risk | No allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | AC group 55 and 42 completed 2 years (42/55) Mesh group 45 and 42 completed 2 years (42/45) |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Low risk | No COI reported |
Thijs 2010.
| Methods | Multi‐centre and multi‐national RCT Randomisation and allocation concealment: N/S 90% power to detect 20% difference UDI prolapse domain at 1 year with 5% type 1 error with 38 in each group |
|
| Participants | Gp A (48): AC Gp B (48): Perigee transobturator polypropylene mesh Gp A: 35 AC only, 5 SSF, 5 hysterectomy, 6 mid‐urethral sling Gp B: 34 Perigee only, 4 SSF, 8 hysterectomy, 1 mid‐urethral sling |
|
| Interventions | Inclusion criteria: stage 2 or more cystocele Excluded if anterior was not the leading prolapse Concomitant surgery allowed Stage 2 or more uterine prolapse hysterectomy or SSF SUI mid‐urethral sling |
|
| Outcomes | Assessed at 6 months and 1 year Reports the following review outcomes at 1 year:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No clear numbers supplied in abstract |
| Selective reporting (reporting bias) | Low risk | Reports 1 of our primary review outcomes |
| Other bias | Unclear risk | No statement about funding |
Turgal 2013.
| Methods | Parallel‐group RCT | |
| Participants | Inclusion: grade 2 or 3 cystocele Exclusion: urinary incontinence, previous gynaecological operation, concomitant rectocele or enterocele, recurrent cystocele |
|
| Interventions | Polypropylene mesh surgery (20 women) vs AC (20 women) | |
| Outcomes | Assessed at 6 weeks, 6 months, 1 year Reports the following review outcomes at 1 year:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Allocated by computer programme" |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All 40/40 randomised women were included in analysis |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Low risk | Reports "no conflict of interest". No other potential bias identified |
Vollebregt 2011.
| Methods | Multi‐centre RCT Randomisation was computerised, and stratification was performed for the presence of uterine descent ≥ 2. No blinding of group assignment was performed Allocation concealment: N/S Power 80 to detect 25% difference in groups with 5% type 1 error from sample size of 50 in each group |
|
| Participants | Inclusion criteria: ≥ stage 2 cystocele Exclusion criteria: history of urogynaecological surgery for pelvic organ prolapse or incontinence, cancer or COPD, concomitant urinary stress incontinence with an indication for surgical correction, recurrent lower urinary tract infections (> 3 culture proven infections/year), maximum bladder capacity < 300 ml, an indication for hysterectomy, and women with childbearing potential and inadequate birth control measures Randomised: A 64, B 61 Withdrawals prior to surgery: A 2, B 2 12 months: A 51, B 53 |
|
| Interventions | Gp A: AC Gp B: trocar‐guided transobturator synthetic mesh (Avaulta) |
|
| Outcomes | Assessed at 6 months and 1 year Reports the following review outcomes at 1 year:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Research nurse from online list |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Reviewers blinded by strapping thighs prior to review |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 year AC 55/56, mesh 55/58 |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Low risk | No funding and no COI |
Weber 2001.
| Methods | RCT (computer‐generated random number tables. Sealed envelopes concealed assignment) comparing 3 surgical techniques 3 arms, 1 centre Length of follow‐up: A + B + C, 23.3 months | |
| Participants | 83 women Inclusion: all women undergoing cystocele repair Exclusion: continence surgery, i.e. colposuspension or sling 114 randomised 5 withdrawals 26 lost to follow‐up (A 2: B 15: C 9), leaving 83 in trial | |
| Interventions | Gp A (33): anterior repair: midline plication without tension 0 PDS
Gp B (24):
ultra‐lateral: dissection to pubic rami laterally, plication paravaginal with tension 0
PDS interrupted
Gp C (26): anterior repair plus mesh: standard plication midline
polyglactin (Vicryl) mesh overlay, Vicryl sutures Number and level of surgeons unknown |
|
| Outcomes | Assessed at 6 months, 1 year, and 2 years Reports the following review outcomes at median follow‐up 23 months (range 4.5 to 44.4 months)
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 83/114 randomised women included in analysis (73%) |
| Selective reporting (reporting bias) | Unclear risk | Main review outcomes reported, but no comparative data for most outcomes |
| Other bias | Unclear risk | No statement about funding. Significant disparity between total numbers in Table 4 and actual numbers with prolapse reported |
Withagen 2011.
| Methods | Multi‐centre RCT 13 centres; 22 surgeons Randomisation list computer generated for each centre. Allocation concealment not discussed and woman, surgeon, and assessor (surgeons) not blinded Surgeons underwent specific Prolift mesh training Full power calculation completed |
|
| Participants | Randomised: Gp A 99, Gp B 95 1‐year examination: A 84, B 83 Inclusion criteria: recurrent stage 2 or higher anterior or posterior wall prolapse, or both Exclusion criteria: pregnancy, future pregnancy, prior vaginal mesh repair, a compromised immune system or any other condition that would compromise healing, previous pelvic irradiation or cancer, blood coagulation disorders, renal failure, upper urinary tract obstruction, renal failure and upper urinary tract obstruction, or presence of large ovarian cysts or myomas |
|
| Interventions | Gp A: conventional surgery was performed at the discretion of the surgeon, although
absorbable sutures were specified and hysterectomies permitted Gp B: standardised and structured in the tension‐free vaginal mesh: performed as described by Fatton (Fatton 2007), and no hysterectomies were performed or T incisions allowed |
|
| Outcomes | Assessed at 6 months and 1 year Reports the following review outcomes at 1 year:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | High risk | Allocation concealment not described. Unfortunately, preoperatively group A is significantly different than group B, as demonstrated by having greater degree prolapse at Ap, Bp, and GH in Table 7; having significantly higher number with ≥ stage 2 apical compartment prolapse in those in Table I undergoing prior apical surgery, (36% (16/45) in group A versus 18% (10/56) in group B (P = 0.04, odds ratio 2.54)); and finally prior sacral colpopexy was 3 times as frequent in group B. Only the final anomaly is acknowledged |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Non‐blinded reviewers; participant‐completed questionnaires |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Gp A 84/99, Gp B 83/98 |
| Selective reporting (reporting bias) | High risk | Primary outcome definition inconsistent |
| Other bias | High risk | Funded by university research fund; all authors reported financial support from Ethicon, which manufactures product being evaluated by non‐blinded reviewers |
AC = anterior colporrhaphy AVWP = anterior vaginal wall prolapse BW = Baden‐Walker CI = confidence interval COI = conflict of interest CONSORT = Consolidated Standards of Reporting Trials CRADI = Colorectal‐Anal Distress Inventory Hb = haemoglobin ICS = International Continence Society ITT = intention to treat IVS = intravaginal slingplasty N/S = not specified PDS = absorbable polydioxanone surgical suture PFDI = Pelvic Floor Distress Inventory PFIQ = Pelvic Floor Impact Questionnaire PGI‐I = Patient Global Impression of Improvement PISQ = Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire POP = pelvic organ prolapse POPDI = Pelvic Organ Prolapse Distress Inventory POPIQ = Pelvic Organ Prolapse Impact Questionnaire POPQ = Pelvic Organ Prolapse Quantification (according to ICS) PQOL= Prolapse Quality of Life Questionnaire QOL = quality of life RCT = randomised controlled trial SD = standard deviation SSF = sacrospinous fixation SUI = stress urinary incontinence (symptom diagnosis) UDI = Urogenital Distress Inventory
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Altman 2013 | Not a RCT |
| Balci 2011 | Not a RCT |
| Chao 2012 | Assessment of impact of traction on uterine prolapse without any surgical intervention |
| Juneja 2010 | Juneja and colleagues compared hysterectomy (n = 9) versus no hysterectomy (n = 7) for uterine prolapse in conjunction with posterior infracoccygeal colpopexy in a pilot randomised study. Due to a predefined decision that papers with fewer than 20 women in each treatment group would not be included in the review, the manuscript was excluded |
| Tincello 2009 | Tincello et al report a pilot randomised patient preference study comparing colposuspension or tension‐free vaginal tape for urinary incontinence at time of anterior repair for prolapse. 31 women were recruited, however only 4 (2 in each arm) were randomised. Due to a predefined decision that papers with fewer than 20 women in each treatment group would not be included in the review, the manuscript was excluded |
RCT = randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
ACTRN12612000236897.
| Trial name or title | Puborectalis sling RCT ‐ a study on reducing pelvic organ prolapse recurrences following prolapse surgery |
| Methods | Multi‐centre RCT |
| Participants | Pelvic organ prolapse |
| Interventions | Vaginal repair and hysterectomy with and without mesh |
| Outcomes | Prolapse on uterosacral suspension |
| Starting date | 2012 |
| Contact information | https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12612000236897 |
| Notes | Ongoing? |
ISRCTN60695184.
| Trial name or title | PROSPECT (PROlapse Surgery: Pragmatic Evaluation and randomised Controlled Trials) |
| Methods | RCT |
| Participants | Women having prolapse surgery |
| Interventions | Anterior and posterior repair (colporrhaphy) with or without non‐absorbable or biological mesh inlay, or mesh kit |
| Outcomes | Prolapse symptoms (POP‐SS), prolapse stage (POPQ), economic outcomes |
| Starting date | 01 09 2009 |
| Contact information | c.glazener@abdn.ac.uk |
| Notes | Health Technology Assessment‐funded study in UK ongoing |
NCT00743535.
| Trial name or title | Anterior defect correction with mesh plus treatment of stress incontinence with transobturator or transvaginal approach |
| Methods | RCT |
| Participants | Prolapse and SUI |
| Interventions | Anterior mesh repair tension‐free vaginal tape compared to anterior mesh repair with transobturator tape |
| Outcomes | |
| Starting date | 2008 |
| Contact information | |
| Notes | Slow recruitment; study terminated |
NCT00955448.
| Trial name or title | Trial of small intestine submucosa (SIS) mesh for anterior repair |
| Methods | RCT |
| Participants | Anterior prolapse |
| Interventions | Anterior repair versus SIS biograft (Cook) |
| Outcomes | |
| Starting date | 2009 |
| Contact information | https://clinicaltrials.gov/show/NCT00955448 |
| Notes | Study completed; unable to identify publication as yet |
NCT01095692.
| Trial name or title | ATHENA |
| Methods | RCT |
| Participants | Women with occult urinary incontinence |
| Interventions | POP + SUI surgery vs POP surgery alone |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
NCT01097200.
| Trial name or title | Sacrocolpopexy versus vaginal mesh procedure for pelvic prolapse (Elevate) |
| Methods | RCT |
| Participants | Vaginal prolapse |
| Interventions | Laparoscopic sacral colpopexy versus Elevate transvaginal mesh |
| Outcomes | |
| Starting date | 2010 |
| Contact information | http://ClinicalTrials.gov/show/NCT01097200 |
| Notes | No longer recruiting |
NCT01497171.
| Trial name or title | The ELEGANT Trial: Elevate Transvaginal Mesh vs Anterior Colporrhaphy |
| Methods | RCT |
| Participants | Anterior prolapse |
| Interventions | Anterior repair versus Elevate (AMS) anterior mesh |
| Outcomes | |
| Starting date | 2011 |
| Contact information | http://ClinicalTrials.gov/show/NCT01497171 |
| Notes | Study ended due to funding termination |
NCT01594372.
| Trial name or title | Laparoscopic to vaginal surgery for uterine prolapse |
| Methods | RCT |
| Participants | Uterine prolapse |
| Interventions | Laparoscopic supracervical hysterectomy and sacral colpopexy versus vaginal hysterectomy and uterosacral colpopexy |
| Outcomes | |
| Starting date | 2012 |
| Contact information | |
| Notes | Terminated as unable to offer laparoscopy |
NCT01637441.
| Trial name or title | Prosthetic Pelvic Organ Prolapse Repair (PROSPERE) |
| Methods | RCT |
| Participants | Cystocele |
| Interventions | Lap sacral colpopexy versus vaginal mesh procedure (unspecified) |
| Outcomes | |
| Starting date | 2012 |
| Contact information | https://clinicaltrials.gov/show/NCT01637441 |
| Notes | Study active but not recruiting? |
NCT01762384.
| Trial name or title | Laparoscopic sacral colpopexy versus modified total pelvic floor reconstructive surgery for apical prolapse stage III‐IV |
| Methods | RCT |
| Participants | Uterine and vault prolapse |
| Interventions | Lap sacrocolpopexy versus vaginal mesh repair with Gynemesh |
| Outcomes | |
| Starting date | 2012 |
| Contact information | https://clinicaltrials.gov/show/NCT01762384 |
| Notes | Ongoing recruiting |
NCT01802281.
| Trial name or title | Study of Uterine Prolapse Procedures ‐ Randomised Trial (SUPeR) |
| Methods | RCT |
| Participants | Uterine prolapse |
| Interventions | Mesh hysteropexy (Uphold LITE) versus vaginal hysterectomy uterosacral suspension |
| Outcomes | |
| Starting date | 2013 |
| Contact information | https://clinicaltrials.gov/show/NCT01802281 |
| Notes | Ongoing |
NTR1197.
| Trial name or title | CUPIDO 1 and CUPIDO 2 |
| Methods | RCT |
| Participants | Women with SUI (CUPIDO 1) and women with occult SUI (CUPIDO 2) |
| Interventions | POP + SUI surgery vs POP surgery alone |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Ongoing |
IIQ = Incontinence Impact Questionnaire POP = pelvic organ prolapse POPQ = Pelvic Organ Prolapse Quantification POP‐SS = Prolapse Symptom Score RCT = randomised controlled trial SUI = stress urinary incontinence (symptom diagnosis)
Differences between protocol and review
This review is the result of updating the review 'Surgical management of pelvic organ prolapse in women'. As a result of the update, we decided to split the review into six reviews.
This review should be read as part of a series of six Cochrane reviews relating to the surgical management of prolapse including:
Surgery for women with anterior compartment prolapse.
Surgery for women with posterior compartment prolapse.
Surgery for women with apical compartment prolapse.
Continence outcomes in pelvic organ prolapse surgery.
Transvaginal grafts or mesh compared with native tissue repair for vaginal prolapse.
Perioperative interventions at prolapse surgery.
Differences from the published review methods were a reduction in the number of outcomes and limiting this review to studies that compared native tissue with mesh (absorbable and permanent) and biological grafts.
Contributions of authors
All review authors contributed to writing the protocol. Four review authors (C Maher, C Schmid, B Feiner, K Baessler) assessed the relevance and eligibility of studies for inclusion in the review and then assessed the quality of included studies. Four review authors (C Maher, C Schmid, K Baessler, B Feiner) independently extracted data from trial reports, interpreted the results, and contributed to the writing of the draft version of the review.
Sources of support
Internal sources
-
Cochrane, UK.
Cochrane Review Support Programme: Pelvic organ prolapse reviews
External sources
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to the Cochrane Incontinence Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, the NIHR, the NHS or the Department of Health.
Declarations of interest
The lead review author, Christopher Maher, is an author of two trials of pelvic prolapse (Maher 2004a; Maher 2011)
The other review authors declare that they have no conflicts of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Ali 2006 {published data only}
- Ali S, Han HC, Lee LC. A prospective randomized trial using Gynemesh PS (trademark) for the repair of anterior vaginal wall prolapse (Abstract number 292). International Urogynecology Journal and Pelvic Floor Dysfunction 2006;17 Suppl 2:221. [Google Scholar]
Allahdin 2008 {published data only}
- Allahdin S, Glazener C, Bain C. A randomised controlled trial evaluating the use of polyglactin mesh, polydioxanone and polyglactin sutures for pelvic organ prolapse surgery. Journal of Obstetrics and Gynaecology 2008;28(4):427‐31. [DOI] [PubMed] [Google Scholar]
- Madhuvrata P, Glazener C, Boachie C, Allahdin S, Bain C. A randomised controlled trial evaluating the use of polyglactin (Vicryl) mesh, polydioxanone (PDS) or polyglactin (Vicryl) sutures for pelvic organ prolapse surgery: outcomes at 2 years. Journal of Obstetrics and Gynaecology 2011;31(5):429‐35. [DOI] [PubMed] [Google Scholar]
Al‐Nazer 2007 {published data only}
- Al‐Nazer MA, Ismail WA, Gomaa IA. Comparative study between anterior colporrhaphy versus vaginal wall repair with mesh for management of anterior vaginal wall prolapse (Abstract number 84). International Urogynecology Journal and Pelvic Floor Dysfunction 2007;18 Suppl 1:49‐50. [Google Scholar]
- El‐Nazer M, Gomaa I, Ismail Madkour W, Swidan K, El‐Etriby M. Anterior colporrhaphy versus repair with mesh for anterior vaginal wall prolapse: a comparative clinical study. Archives of Gynecology and Obstetrics 2012;286:965‐72. [DOI] [PubMed] [Google Scholar]
Altman 2011 {published data only}
- Altman D, Väyrynen T, Engh ME, Axelsen S, Falconer C, for the Nordic Transvaginal Mesh Group. Anterior colporrhaphy versus transvaginal mesh for pelvic‐organ prolapse. The New England Journal of Medicine 2011;364(19):1826‐36. [41463] [DOI] [PubMed] [Google Scholar]
- Ek M, Altman D, Elmér C, Gunnarsson J, Falconer C, Tegerstedt G. Clinical efficacy of a trocar guided mesh kit for the repair of anterior lateral defects (Abstract number 556). Proceedings of the 41st Annual Meeting of the International Continence Society (ICS), 2011 Aug 29 to Sept 2, Glasgow, Scotland. 2011.
- Ek M, Tegerstedt G, Falconer C, Kjaeldgaard A, Rezapour M, Rudnicki M, et al. Urodynamic assessment of anterior vaginal wall surgery: A randomized comparison between colporrhaphy and transvaginal mesh. Neurourology and Urodynamics 2010;29:527‐31. [39589] [DOI] [PubMed] [Google Scholar]
Carey 2009 {published data only}
- Carey M, Higgs P, Goh J, Lim J, Leong A, Krause H, Cornish A. Vaginal repair with mesh versus colporrhaphy for prolapse: a randomised controlled trial. BJOG 2009;116(10):1380‐6. [32066] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dahlgren 2011 {published data only}
- Dahlgren E, Kjolhede P on behalf of the RPOP‐PELVICOL Study Group. Long‐term outcome of porcine skin graft in surgical treatment of recurrent pelvic organ prolapse. An open randomized controlled multicenter study. Acta Obstetricia Et Gynecologica Scandinavica 2011;90:1393‐401. [DOI] [PubMed] [Google Scholar]
da Silveira 2014 {published data only}
- dos Reis Brandão da Silveira S, Haddad JM, Jármy‐Di Bella Z, Nastri F, Kawabata M, Silva Carramão S, et al. Multicenter, randomized trial comparing native vaginal tissue repair and synthetic mesh repair for genital prolapse surgical treatment. International Urogynecology Journal 2015;3:335‐42. [DOI] [PubMed] [Google Scholar]
Delroy 2013 {published data only}
- Delroy CA, Castro Rde A, Dias MM, Feldner PC, Bortolini MA, Girao MS. The use of transvaginal synthetic mesh for anterior vaginal wall prolapse repair: a randomized controlled trial. International Urogynecology Journal 2013;24(11):1899‐907. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Tayrac 2008 {published data only}
- Tayrac R, Mathe ML, Bader G, Deffieux X, Fazel A, Fernandez H. Infracoccygeal sacropexy or sacrospinous suspension for uterine or vaginal vault prolapse. International Journal of Gynaecology and Obstetrics 2008;100(2):154‐9. [DOI] [PubMed] [Google Scholar]
De Tayrac 2013 {published data only}
- Tayrac R, Cornille A, Eglin G, Guilbaud O, Mansoor A, Alonso S, et al. Comparison between trans‐obturator trans‐vaginal mesh and traditional anterior colporrhaphy in the treatment of anterior vaginal wall prolapse: results of a French RCT. International Urogynecology Journal 2013;24:1651‐61. [DOI] [PubMed] [Google Scholar]
Feldner 2010 {published data only}
- Feldner PC Jr, Castro RA, Cipolotti LA, Delroy CA, Sartori MG, Girao MJ. Anterior vaginal wall prolapse: a randomized controlled trial of SIS graft versus traditional colporrhaphy. International Urogynecology Journal and Pelvic Floor Dysfunction 2010;21(9):1057‐63. [40053] [DOI] [PubMed] [Google Scholar]
- Feldner PC Jr, Castro RA, Delroy CA, Dias MM, Sartori MG, Girao MJ. Surgical treatment of anterior vaginal wall prolapse: comparison of small intestine submucosa (SIS) graft and traditional repair (Abstract number 160). International Urogynecology Journal 2009;20 Suppl 2:S208‐9. [39890] [Google Scholar]
Gandhi 2005 {published data only}
- Gandhi S, Goldberg RP, Kwon C, Koduri S, Beaumont JL, Abramov Y, et al. A prospective randomized trial using solvent dehydrated fascia lata for the prevention of recurrent anterior vaginal wall prolapse. American Journal of Obstetrics and Gynecology 2005;192:1649‐54. [DOI] [PubMed] [Google Scholar]
- Gandhi S, Kwon C, Goldberg RP, Abramov Y, Beaumont JL, Koduri S, et al. A randomized controlled trial of fascia lata for the prevention of recurrent anterior vaginal wall prolapse. Neurourology and Urodynamics 2004;23(5/6):558. [Google Scholar]
- Kwon C, Goldberg R, Evaston IL, Koduri S, Franklin WI, Gandhi S, et al. Preliminary results of a prospective randomized trial of tutoplast processed fascia lata to prevent recurrent cystoceles and rectoceles. The Journal of Urology 2002;167:203. [Google Scholar]
Guerette 2009 {published data only}
- Guerette NL, Aguirre O, VanDrie DM, Biller DH, Davila GW. Multi‐center, randomized, prospective trial comparing anterior colporrhaphy alone to bovine pericardium collagen matrix graft reinforced anterior colporrhaphy: 12‐month analysis (Abstract number 11). International Urogynecology Journal and Pelvic Floor Dysfunction 2006;17 Suppl 2:63‐4. [Google Scholar]
- Guerette NL, Peterson TV, Aguirre OA, VanDrie DM, Biller DH, Davila GW. Anterior repair with or without collagen. Obstetrics and Gynecology 2009;114:59‐65. [DOI] [PubMed] [Google Scholar]
Gupta 2014 {published data only}
- Gupta B, Vaid NB, Suneja A, Guleria K, Jain S. Anterior vaginal prolapse repair: A randomised trial of traditional anterior colporrhaphy and self‐tailored mesh repair. South African Journal of Obstetrics and Gynaecology 2014;20(3):47‐50. [Google Scholar]
Halaska 2012 {published data only}
- Halaska M, Maxova K, Sottner O, Svabik K, Mlcoch M, Kolarik D, et al. A multicentre randomized prospective controlled study comparing sacrospinous fixation and transvaginal mesh in the treatment of post‐hysterectomy vaginal vault prolapse. American Journal of Obstetrics and Gynecology 2012;207(301):e1‐7. [DOI] [PubMed] [Google Scholar]
Hviid 2010 {published data only}
- Hviid U, Hviid TV, Rudnicki M. Porcine skin collagen implants for anterior vaginal wall prolapse: a randomised prospective controlled study. International Urogynecology Journal 2010;21(5):529‐34. [39449] [DOI] [PubMed] [Google Scholar]
Iglesia 2010 {published data only}
- Gutman R, Nosti P, Sokol A, Sokol E, Peterson J, Wang H, Iglesia C. Three‐year outcome of vaginal mesh for prolapse, a randomized controlled trial. Obstetrics & Gynecology 2013;122(4):770‐7. [Clinicaltrials.gov: NCT00475540] [DOI] [PubMed] [Google Scholar]
- Iglesia CB, Sokol AI, Sokol ER, Kudish BI. Vaginal mesh for prolapse: a randomized controlled trial. Obstetrics and Gynecology 2010;116(2 Pt 1):293‐303. [39891] [DOI] [PubMed] [Google Scholar]
- Sokol AI, Iglesia CB, Kudish BI, Gutman RE, Shveiky D, Bercik R, et al. One‐year objective and functional outcomes of a randomized clinical trial of vaginal mesh for prolapse. American Journal of Obstetrics and Gynecology 2012;206(1):86.e1‐9. [DOI: 10.1016/j.ajog.2011.08.003; 42158] [DOI] [PubMed] [Google Scholar]
Lamblin 2014 {published data only}
- Lamblin G, Van‐Nieuwenhuyse A, Chabert P, Lebail‐Carval K, Moret S, Mellier G. A randomized controlled trial comparing anatomical and functional outcome between vaginal colposuspension and transvaginal mesh. International Urogynecology Journal 2014;25:961–70. [DOI] [PubMed] [Google Scholar]
Menefee 2011 {published data only}
- Dyer K, Nguyen J, Lukacz E, Simsiman A, Luber K, Menefee S. The Optimal Anterior Repair Study (OARS): a triple arm randomized double blinded clinical trial of standard colporrhaphy, porcine dermis or polypropylene mesh augmented anterior vaginal wall repair (Abstract number 252). Neurourology and Urodynamics 2009;28(7):894‐5. [39346] [Google Scholar]
- Dyer K, Nguyen J, Simsiman A, Lukacz E, Luber K, Menefee S. The Optimal Anterior Repair Study (OARS): a triple arm randomized double blinded clinical trial of standard colporrhaphy versus vaginal paravaginal repair with porcine dermis graft or polypropylene mesh (Abstract number 281). Neurourology and Urodynamics 2010;29(6):1207‐8. [40164] [Google Scholar]
- Menefee SA, Dyer KY, Lukacz ES, Simsiman AJ, Luber KM, Nguyen JN. Colporrhaphy compared with mesh or graft‐reinforced vaginal paravaginal repair for anterior vaginal wall prolapse: a randomized controlled trial. Obstetrics and Gynecology 2011;118(6):1337‐44. [42866] [DOI] [PubMed] [Google Scholar]
Meschia 2004a {published and unpublished data}
- Meschia M, Gattei U, Pifarotti P, Spennacchio M, Longatti D, Barbacini P. Randomized comparison between infracoccygeal sacropexy (posterior IVS) and sacrospinous fixation in the management of vault prolapse (Abstract number 614). Proceedings of the Joint Meeting of the International Continence Society (34th Annual Meeting) and the International Urogynecological Association, 2004 Aug 23‐27, Paris. 2004.
Meschia 2007 {published and unpublished data}
- Kocjancic E, Crivellaro S, Bernasconi F, Magatti F, Frea B, Meschia M. A two years follow‐up, prospective randomized study on cystocele repair with or without Pelvicol (trademark) implant (Abstract number 1374). Proceedings of the Annual Meeting of the American Urological Association, 19‐24 May 2007, Anaheim (CA). 2007.
- Meschia M, Pifarotti P, Bernasconi F, Magatti F, Riva D, Kojancic E. Porcine skin collagen implants to prevent anterior vaginal wall prolapse recurrence: A multicentre, randomized study. The Journal of Urology 2007;177:192‐5. [DOI] [PubMed] [Google Scholar]
- Meschia M, Pifarotti P, Magatti F, Bernasconi F, Riva D, Kojancic E. Porcine skin collagen implants (Pelvicol) (trademark) to prevent anterior vaginal wall prolapse recurrence: a randomized study (Abstract). Neurourology and Urodynamics 2005;24(5/6):587‐8. [Google Scholar]
Nguyen 2008 {published and unpublished data}
- Nguyen JN, Burchette RJ. Anatomy and visceral function after anterior vaginal prolapse repair: a randomized controlled trial (Abstract number 42). Proceedings of the 29th Annual Meeting of the American Urogynecologic Society (AUGS), Sept 4‐6, Chicago. 2008.
- Nguyen JN, Burchette RJ. Outcome after anterior vaginal prolapse repair. Randomized controlled trial. Obstetrics and Gynecology 2008;111(4):891‐8. [DOI] [PubMed] [Google Scholar]
Nieminen 2008 {published data only}
- Hiltunen R, Nieminen K, Takala T, Heiskanen E, Merikari M, Niemi K, et al. Low‐weight polypropylene mesh for anterior vaginal wall prolapse: a randomized controlled trial. Obstetrics and Gynecology 2007;110(2 pt 2):455‐62. [DOI] [PubMed] [Google Scholar]
- Nieminen K, Hiltunen R, Heiskanen E, Takala T, Niemi K, Merikari M, et al. Symptom resolution and sexual function after anterior vaginal wall repair with or without polypropylene mesh. International Urogynecology Journal. 2008;19(12):1611‐6. [DOI] [PubMed] [Google Scholar]
- Nieminen K, Hiltunen R, Takala T, Heiskanen E, Merikari M, Niemi K, et al. Outcomes after anterior vaginal wall repair with mesh: a randomized, controlled trial with a 3 year follow‐up. American Journal of Obstetrics and Gynecology 2010;203(3):235.e1‐8. [40020] [DOI] [PubMed] [Google Scholar]
Paraiso 2006 {published data only}
- Paraiso M, Barber M, Muir T, Walters M. Rectocele repair: A randomized trial of three surgical techniques including graft augmentation. American Journal of Obstetrics and Gynecology 2006;195:1762‐71. [DOI] [PubMed] [Google Scholar]
Qatawneh 2013 {published data only}
- Qatawneh A, Al‐Kazaleh F, Saleh S, Thekrallah F, Bata M, Sumreen I, et al. Transvaginal cystocele repair using tension‐free polypropylene mesh at the time of sacrospinous colpopexy for advanced uterovaginal prolapse: a prospective randomised study. Gynecological Surgery 2013;10:79‐85. [Google Scholar]
Robert 2014 {published data only}
- Robert M, Girard I, Brennand E, Tang S, Birch C, Murphy M, et al. Absorbable mesh augmentation compared with no mesh for anterior prolapse: a randomized controlled trial. Obstetrics and Gynecology 2014;123(2 Part 1):288‐94. [DOI] [PubMed] [Google Scholar]
Rudnicki 2014 {published data only}
- Rudnicki M, Laurikainen E, Pogosean R, Kinne I, Jakobsson U, Teleman P. A 3–year follow‐up after anterior colporrhaphy compared with collagen‐coated transvaginal mesh for anterior vaginal wall prolapse: a randomised controlled trial. BJOG 2015;published online:1‐7. [DOI] [PubMed] [Google Scholar]
- Rudnicki M, Laurikainen E, Pogosean R, Kinne I, Jakobsson U, Teleman P. Anterior colporrhaphy compared with collagen‐coated transvaginal mesh for anterior vaginal wall prolapse: a randomised controlled trial. BJOG 2014;121:102‐11. [DOI] [PubMed] [Google Scholar]
Sand 2001 {published data only}
- Goldberg RP, Koduri S, Lobel RW, Culligan PJ, Tomezsko JE, Winkler HA, et al. Long‐term effects of three different anti‐incontinence procedures on the posterior compartment (Abstract). Proceedings of the International Continence Society (ICS) 31st Annual Meeting; 2001 Sept 18‐21; Seoul, Korea. 2001.
- Sand PK, Koduri S, Lobel RW, Winkler HA, Tomezsko J, Culligan PJ, et al. Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystoceles and rectoceles. American Journal of Obstetrics and Gynecology 2001;184(7):1357‐64. [DOI] [PubMed] [Google Scholar]
Sivaslioglu 2008 {published data only}
- Sivaslioglu AA, Unlubilgin E, Dolen I. A randomized comparison of polypropylene mesh surgery with site‐specific surgery in the treatment of cystocoele. International Urogynecology Journal. 2008;19(4):467‐71. [DOI] [PubMed] [Google Scholar]
Sung 2012 {published data only}
- Sung VW, Rardin CR, Raker CA, Lasala CA, Myers DL. Porcine subintestinal submucosal graft augmentation for rectocele repair: a randomized controlled trial. Obstetrics and Gynecology 2012;119(1):125‐33. [42876] [DOI] [PMC free article] [PubMed] [Google Scholar]
Svabik 2014 {published data only}
- Svabik K, Martan A, Masata J, El‐Haddad R, Hubka P. Comparison of vaginal mesh repair with sacrospinous vaginal colpopexy in the management of vaginal vault prolapse after hysterectomy in patients with levator ani avulsion: a randomized controlled trial. Ultrasound in Obstetrics and Gynecology 2014;43(4):365‐71. [DOI] [PubMed] [Google Scholar]
Tamanini 2014 {published data only}
- Tamanini JTN, Oliveira Souza Castro RC, Tamanini JM, Castro RA, Sartori MGF, Girão MJBC. A prospective, randomized and controlled trial for the treatment of anterior vaginal wall prolapse: medium‐term follow‐up. The Journal of Urology 2015;193(4):1298‐304. [DOI] [PubMed] [Google Scholar]
- Tamanini JTN, Oliveira Souza Castro RC, Tamanini JM, Castro RA, Sartori MGF, Girão MJBC. Treatment of anterior vaginal wall prolapse with and without polypropylene mesh: a prospective, randomized and controlled trial ‐ Part I. Int Braz J Urol 2013;39(4):519‐30. [DOI] [PubMed] [Google Scholar]
Thijs 2010 {published data only}
- Thijs S, Deprest J, Ridder D, Claerhout F, Roovers J. A randomized controlled trial of anterior colporraphy and Perigee™ as a primary surgical correction of symptomatic cystocele (Abstract number 96). International Urogynecology Journal and Pelvic Floor Dysfunction 2010;21 Suppl 1:S142‐3. [40133] [Google Scholar]
Turgal 2013 {published data only}
- Turgal M, Sivaslioglu A, Yildiz A, Dolen I. Anatomical and functional assessment of anterior colporrhaphy versus polypropylene mesh surgery in cystocele treatment. European Journal of Obstetrics and Gynecology and Reproductive Biology 2013;170(2):555‐8. [DOI] [PubMed] [Google Scholar]
Vollebregt 2011 {published data only}
- Vollebregt A, Fischer K, Gietelink D, Vaart CH. Primary surgical repair of anterior vaginal prolapse: a randomised trial comparing anatomical and functional outcome between anterior colporrhaphy and trocar‐guided transobturator anterior mesh. British Journal of Obstetrics and Gynaecology 2011;118(12):1518‐27. [42606] [DOI] [PubMed] [Google Scholar]
- Vollebregt A, Gietelink D, Fischer K, Vaart H. One year results of colporrhaphy anterior versus a trocar guided transobturator synthetic mesh in primary cystocele repair: a randomized controlled trial (Abstract number 51). Neurourology and Urodynamics 2010;29(6):880‐2. [40124] [Google Scholar]
Weber 2001 {published data only}
- Weber AM, Walters MD, Piedmonte MR, Ballard LA. Anterior colporrhaphy: a randomized trial of three surgical techniques. American Journal of Obstetrics and Gynecology 2001;185(6 Pt 1):1299‐306. [DOI] [PubMed] [Google Scholar]
Withagen 2011 {published and unpublished data}
- Milani AL, Withagen MI, The HS, Nedelcu‐Van der Wijk I, Vierhout ME. Sexual function following trocar‐guided mesh or vaginal native tissue repair in recurrent prolapse: A randomized controlled trial. The Journal of Sexual Medicine 2011;8(10):2944‐53. [42064] [DOI] [PubMed] [Google Scholar]
- Withagen MI, Milani AL, Boon Den J, Vervest HA, Vierhout ME. Tension free vaginal mesh compared to conventional vaginal prolapse surgery in recurrent prolapse; a randomized controlled trial (Abstract number 090). International Urogynaecology Journal 2009;20 Suppl 2:S153‐4. [39885] [Google Scholar]
- Withagen MI, Milani AL, Boon J, Vervest HA, Vierhout ME. Trocar‐guided mesh compared with conventional vaginal repair in recurrent prolapse: a randomized controlled trial. Obstetrics and Gynecology 2011;117(2 Pt 1):242‐50. [40881] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Altman 2013 {published data only}
- Altman D, Mooller Bek K, Mikkola T, Gunnarsson J, Ellstrom Engh M, Falconer C. Intra‐and perioperative morbidity following pelvic organ prolapse repair using a transvaginal suture capturing mesh device compared to trocar guided transvaginal mesh and traditional colporraphy. Neurourology and Urodynamics 2013;32(6):873‐4. [Google Scholar]
Balci 2011 {published data only}
- Balci O, Capar M, Acar A, Colakoglu MC. Balci technique for suspending vaginal vault at vaginal hysterectomy with reduced risk of vaginal vault prolapse. Journal of Obstetrics and Gynaecology Research 2011;37(7):762‐9. [DOI] [PubMed] [Google Scholar]
Chao 2012 {published data only}
- Chao FL, Rosamilia A, Dwyer PL, Polyakov A, Schierlitz L, Agnew G. Does pre‐operative traction on the cervix approximate intra‐operative uterine prolapse? A randomised controlled trial. International Urogynecology Journal 2012;23(4):417‐22. [DOI] [PubMed] [Google Scholar]
Juneja 2010 {published data only}
- Juneja M, Munday D, Kopetz V, Barry C. Hysterectomy vs no hysterectomy for uterine prolapse in conjunction with posterior infracococcygeal colpopexy ‐ a randomised pilot study 12 months review (Abstract number 692). Proceedings of the Joint Meeting of the International Continence Society (ICS) and the International Urogynecological Association, 2010 Aug 23‐27, Toronto, Canada. 2010.
Tincello 2009 {published data only}
- Tincello DG, Kenyon S, Slack M, Toozs‐Hobson P, Mayne C, Jones D, et al. Colposuspension or TVT with anterior repair for urinary incontinence and prolapse: results of and lessons from a pilot randomised patient‐preference study (CARPET 1). British Journal of Obstetrics and Gynaecology 2009;116(13):1809‐14. [DOI] [PubMed] [Google Scholar]
- Tincello DG, Mayne CJ, Toozs‐Hobson P, Slack M. Randomised controlled trial of colposuspension versus anterior repair plus TVT for urodynamic stress incontinence with anterior vaginal prolapse: proposal (Abstract). Proceedings of the International Continence Society, 11th Annual Scientific Meeting; 2004 Mar 18‐19; Bournemouth, United Kingdom. 2004:46. [17170]
References to ongoing studies
ACTRN12612000236897 {unpublished data only}
- ACTRN12612000236897. Puborectalis sling RCT ‐ a study on reducing pelvic organ prolapse recurrences following prolapse surgery. http://www.anzctr.org.au/ACTRN12612000236897.aspx (accessed 24/2/2012).
ISRCTN60695184 {published data only}
- ISRCTN60695184. Clinical and cost‐effectiveness of surgical options for the management of anterior and/or posterior vaginal wall prolapse: two randomised controlled trials within a comprehensive cohort study (PROSPECT). www.controlled‐trials.com/ISRCTN60695184 (accessed 13 April 2010). [DOI] [PMC free article] [PubMed]
NCT00743535 {published data only}
- NCT00743535. Anterior defect correction with mesh plus treatment of stress incontinence with transobturator or transvaginal approach. http://ClinicalTrials.gov/show/NCT00743535 (accessed April 2013).
NCT00955448 {published data only}
- NCT00955448. Trial of small intestine submucosa (SIS) mesh for anterior repair. https://clinicaltrials.gov/show/NCT00955448 (accessed August 2013).
NCT01095692 {published data only}
- NCT01095692. Evaluating the necessity of TOT implantation in women with pelvic organ prolapse and occult stress urinary incontinence (ATHENA). http://clinicaltrials.gov/ct2/show/NCT01095692 (accessed 19 April 2011). [41350]
NCT01097200 {published data only}
- NCT01097200. Sacrocolpopexy versus vaginal mesh procedure for pelvic prolapse (Elevate). https://clinicaltrials.gov/show/NCT01097200 (accessed May 2015).
NCT01497171 {unpublished data only}
- NCT01497171. The ELEGANT Trial: Elevate transvaginal mesh vs. anterior colporrhaphy. http://ClinicalTrials.gov/show/NCT01497171 (accessed 2011).
NCT01594372 {unpublished data only}
- NCT01594372. Comparison laparoscopic to vaginal surgery for uterine prolapse. http://ClinicalTrials.gov/show/NCT01594372 (accessed 2012).
NCT01637441 {unpublished data only}
- NCT01637441. Prosthetic Pelvic Organ Prolapse Repair (PROSPERE). https://clinicaltrials.gov/show/NCT01637441 (accessed 2012).
NCT01762384 {published data only}
- NCT01762384. Laparoscopic sacral colpopexy versus modified total pelvic floor reconstructive surgery for apical prolapse stage III‐IV. https://clinicaltrials.gov/show/NCT01762384 (accessed 2012).
NCT01802281 {unpublished data only}
- NCT01802281. Study of uterine prolapse procedures ‐ randomized trial (SUPeR). https://clinicaltrials.gov/show/NCT01802281 (last accessed July 2015).
NTR1197 {published data only}
- NTR1197. Concomitant surgery and urodynamic investigation in genital prolapse and stress incontinence. A diagnostic study including outcome evaluation. CUPIDO 1: Vaginal prolapse repair and mid urethral sling procedure in women with genital prolapse and predominant stress urinary incontinence. Netherlands Trial Register. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=1197 (accessed June 2012). [34193]
- Steen A, Ploeg M, Dijkgraaf MG, V, Roovers JP. Protocol for the CUPIDO trials; multicenter randomized controlled trials to assess the value of combining prolapse surgery and incontinence surgery in patients with genital prolapse and evident stress incontinence (CUPIDO I) and in patients with genital prolapse and occult stress incontinence (CUPIDO II). BMC Women's Health 2010;10:16. [39877] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
ACC 2015
- Accident Compenstaion Corporation (ACC). Surgical Mesh Review: Analysis of Treatment Injury Claims 1 July 2005 to 30 June 2014. http://www.acc.co.nz/for‐providers/clinical‐best‐practice/WPC138052 2015.
Adams 2004
- Adams E, Thomson A, Maher C, Hagen S. Mechanical devices for pelvic organ prolapse in women. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD004010.pub2] [DOI] [PubMed] [Google Scholar]
Brubaker 2002
- Brubaker L, Bump R, Jacquetin B, Schuessler B, Weidner A, Zimmern P, et al. Pelvic organ prolapse. Incontinence: 2nd International Consultation on Incontinence. 2nd Edition. Plymouth: Health Publication Ltd, 2002:243‐65. [Google Scholar]
Bump 1998
- Bump R, Norton P. Epidemiology and natural history of pelvic floor dysfunction. Obstetrics and Gynecology Clinics of North America 1998;25(4):723‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd Edition. Lawrence Erlbaum Associates, 1988. [Google Scholar]
Ek 2010
- Ek M, Tegerstedt G, Falconer C, Kjaeldgaard A, Rezapour M, Rudnicki M, et al. Urodynamic assessment of anterior vaginal wall surgery: A randomized comparison between colporraphy and transvaginal mesh. Neurourology and Urodynamics 2010;29:527‐31. [39589] [DOI] [PubMed] [Google Scholar]
Ek 2011
- Ek M, Altman D, Elmér C, Gunnarsson J, Falconer C, Tegerstedt G. Clinical efficacy of a trocar guided mesh kit for the repair of anterior lateral defects (Abstract number 556). Proceedings of the 41st Annual Meeting of the International Continence Society (ICS), 2011 Aug 29 to Sept 2, Glasgow, Scotland. 2011.
Fatton 2007
- Fatton B, Amblard J, Debodinance P, Cosson M, Jacqutin B. Transvaginal repair of genital prolapse: preliminary results of a new tension‐free vaginal mesh (Prolift technique) ‐ a case series multicentric study. International Urogynecology Journal and Pelvic Floor Dysfunction 2007;18:743–52. [DOI] [PubMed] [Google Scholar]
FDA 2011
- Food, Drug Administration (FDA). Surgical mesh for POP and SUI repair: FDA executive summary. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/ObstetricsandGynecologyDevices/UCM270402.pdf (accessed 8‐9 September 2011).
Ford 2015
- Ford AA, Rogerson L, Cody JD, Ogah J. Mid‐urethral sling operations for stress urinary incontinence in women. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD006375.pub3] [DOI] [PubMed] [Google Scholar]
Gill 1998
- Gill EJ, Hurt WG. Pathophysiology of pelvic organ prolapse. Obstetrics and Gynecology Clinics of North America 1998;25(4):759‐69. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADEPRO 2014 [Computer program]
- GRADEpro 2014. [Computer program on www.gradepro.org]. McMaster University, Version December 2015.
Gutman 2013
- Gutman R, Nosti P, Sokol A, Sokol E, Peterson J, Wang H, et al. Three‐year outcome of vaginal mesh for prolapse, a randomized controlled trial. Obstetrics & Gynecology 2013;122(4):770‐7. [Clinicaltrials.gov: NCT00475540] [DOI] [PubMed] [Google Scholar]
Hagen 2011
- Hagen S, Stark D. Conservative prevention and management of pelvic organ prolapse in women. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD003882.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Handa 2004
- Handa VL, Garrett E, Hendrix S, Gold E, Robbins J. Progression and remission of pelvic organ prolapse: a longitudinal study of menopausal women. American Journal of Obstetrics and Gynecology 2004;190(1):27‐32. [DOI] [PubMed] [Google Scholar]
Haya 2015
- Haya N, Baessler K, Christmann‐Schmid C, Tayrac R, Dietz V, Guldberg R, et al. Prolapse and continence surgery in countries of the Organization for Economic Co‐operation and Development in 2012. American Journal of Obstetrics and Gynecology 2015;212(6):755‐7. [DOI] [PubMed] [Google Scholar]
Hendrix 2002
- Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Women's Health Initiative: gravity and gravidity. American Journal of Obstetrics and Gynecology 2002;186(6):1160‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kohli 2003
- Kohli N, Miklos JR. Dermal graft‐augmented rectocele repair. Int Urogynecol J Pelvic Floor Dysfunct 2003;14:146‐9. [DOI] [PubMed] [Google Scholar]
MacLennan 2000
- MacLennan AH, Taylor AW, Wilson DH, Wilson D. The prevalence of pelvic floor disorders and their relationship to gender, age, parity and mode of delivery. British Journal of Obstetrics and Gynaecology 2000;107(12):1460‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Maher 2004a
- Maher CF, Qatawneh AM, Dwyer PL, Carey MP, Cornish A, Schluter PJ. Abdominal sacral colpopexy or vaginal sacrospinous colpopexy for vaginal vault prolapse: A prospective randomized study. American Journal of Obstetrics and Gynecology 2004;190(1):20‐6. [DOI] [PubMed] [Google Scholar]
Maher 2011
- Maher CF, Feiner B, Decuyper EM, Nichlos CJ, Hickey KV, O'Rourke P. Laparoscopic sacral colpopexy versus total vaginal mesh for vaginal vault prolapse: a randomized trial. American Journal of Obstetrics and Gynecology 2011;204(4):e361‐7. [DOI] [PubMed] [Google Scholar]
MHRA 2014
- Medicines and Healthcare Products Regulatory Agency (MHRA). A summary of the evidence on the benefits and risks of vaginal mesh implants. https://www.gov.uk/government/publications/vaginal‐mesh‐implants‐summary‐of‐benefits‐and‐risks (Accessed Oct 2014).
Milani 2011
- Milani AL, Withagen MI, The HS, Nedelcu‐Van der Wijk I, Vierhout ME. Sexual function following trocar‐guided mesh or vaginal native tissue repair in recurrent prolapse: A randomized controlled trial. The Journal of Sexual Medicine 2011;8(10):2944‐53. [42064] [DOI] [PubMed] [Google Scholar]
SCENHIR 2015
- Epstein M, Emri I, Hartemann P, Hoet P, Leitgeb N, Martínez Martínez L, et al. The safety of surgical meshes used in urogynecological surgery. Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) 3 December 2015, issue http://ec.europa.eu/health/scientific_committees/consultations/public_consultations/scenihr_consultation_27_en.htm.
References to other published versions of this review
Maher 2004
- Maher C, Baessler K, Glazener CMA, Adams EJ, Hagen S. Surgical management of pelvic organ prolapse in women. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004014.pub2] [DOI] [PubMed] [Google Scholar]


