Abstract
We tested the contribution of nucleoside triphosphate (NTP) hydrolysis, ethanol, and organic acid syntheses, and H+-pump ATPases activity in the acidosis of anoxic sycamore (Acer pseudoplatanus) plant cells. Culture cells were chosen to alter NTP pools and fermentation with specific nutrient media (phosphate [Pi]-deprived and adenine- or glycerol-supplied). In vivo 31P- and 13C-nuclear magnetic resonance (NMR) spectroscopy was utilized to noninvasively measure intracellular pHs, Pi, phosphomonoesters, nucleotides, lactate, and ethanol. Following the onset of anoxia, cytoplasmic (cyt) pH (7.5) decreased to 6.8 within 4 to 5 min, whereas vacuolar pH (5.7) and external pH (6.5) remained stable. The NTP pool simultaneously decreased from 210 to <20 nmol g−1 cell wet weight, whereas nuceloside diphosphate, nucleoside monophosphate, and cyt pH increased correspondingly. The initial cytoplasmic acidification was at a minimum in Pi-deprived cells containing little NTP, and at a maximum in adenine-incubated cells showing the highest NTP concentration. Our data show that the release of H+ ions accompanying the Pi-liberating hydrolysis of NTP was the principal cause of the initial cyt pH drop and that this cytoplasmic acidosis was not overcome by H+ extrusion. After 15 min of anoxia, a partial cyt-pH recovery observed in cells supplied with Glc, but not with glycerol, was attributed to the H+-consuming ATP synthesis accompanying ethanolic fermentation. Following re-oxygenation, the cyt pH recovered its initial value (7.5) within 2 to 3 min, whereas external pH decreased abruptly. We suggest that the H+-pumping ATPase located in the plasma membrane was blocked in anoxia and quickly reactivated after re-oxygenation.
The fall in cytoplasmic (cyt) pH following the onset of anoxia is a common phenomenon observed in most organisms, including plants (Roberts, 1984; Raven, 1986; Kennedy et al., 1992; Ratcliffe, 1997). However, the origin of the pH changes occurring in plant cells during anoxia is not yet clearly established as outlined by Ratcliffe (1995). Several parameters are involved in the regulation of intracellular pHs in anoxic plant cells, as recently reviewed by Saglio et al. (1999). Several parameters may contribute to increase the cyt H+ concentration: the passive influx of H+ ions from the external (ext) medium or from the vacuole (vac), the hydrolysis of the pools of Mg nucleoside triphosphate (NTP) and sugar phosphates (Gevers, 1977; Busa and Nuccitelli, 1984), the accumulation of non-processed acidic intermediates like glycolytic compounds (Felle, 1996), the synthesis of lactate (Davies et al., 1974), and a poor CO2 removal. Other parameters have the opposite effect: the synthesis of Ala and γ-aminobutyrate (Menegus et al., 1989), the decarboxylation of organic acids (Roberts et al., 1992), the activation of H+ extrusion at acidic cyt pH (Guern et al., 1991; Xia and Roberts, 1996), and the functioning of the H+-pumping ATPases located at the plasma membrane and tonoplast (Gout et al., 1992). The apparent coincidence between the fall in pH and the synthesis of lactic acid in anoxic tissues suggested that the accumulation of lactate participates in the acidification of the cytoplasm (Roberts et al, 1984; Rivoal and Hanson, 1993). However, anoxia also triggers a cyt acidosis in rice shoots, although the lactate synthesis is very low in this material (Menegus et al., 1991). Furthermore, the fall of cyt pH following the onset of anoxia in maize root tips precedes the accumulation of lactate (Saint-Gès et al., 1991). It was also reported that there is a correlation between the cyt pH and the size of the NTP pool during anoxia. The hydrolysis of NTP liberates H+ ions, contributing to the decrease of the cyt pH, and conversely, the synthesis of NTP should alkalize the cyt pH. On the other hand, the decrease of NTP can also limit the activity of the ATP-dependent H+ pumps of the plasma membrane and tonoplast that have apparent Km for ATP of 0.1 to 0.2 and 0.3 to 0.5 mm, respectively (Sze, 1984), thereby preventing the recovery of the normoxic cyt pH after the initial acidification. Until now the evaluation of the contribution of these different parameters to the initial cyt acidosis was impeded by the absence of data obtained with a unique material in which NTP level could be easily varied and fermentation could be prevented.
In this work experiments were set up to address the following questions: To what extent does the H+-liberating hydrolysis of NTP participate in the initial cyt pH fall? Do the H+ pump ATPases extrude H+ from the cytoplasm in anoxia? What is the contribution of the synthesis of ethanol and organic acids to the regulation of intracellular pHs during anoxia?
To answer these questions heterotrophic cell suspensions from higher plants were utilized in preference to dense tissues. This facilitated the diffusion of gas and substrates during anoxia and improved the homogeneity of the incubation conditions and allowed specific modifications of the physiological state of plant material (Bligny and Leguay, 1987). For example, the importance of NTP hydrolysis in cyt acidosis during anoxia was assessed by diminishing or increasing the cell NTP pool by incubating cells either in a phosphate- (Pi) free (Rébeillé et al., 1982) or in an adenine-supplied nutrient medium (Dorée et al., 1970). The contribution of the synthesis of lactate to the cyt acidosis was assessed by incubating cells on glycerol as a unique carbon source (Aubert et al., 1994), thereby inhibiting fermentation processes.
RESULTS
Changes in pH, Nucleotides, and Hexose Phosphates during Anoxic and Normoxic Transitions
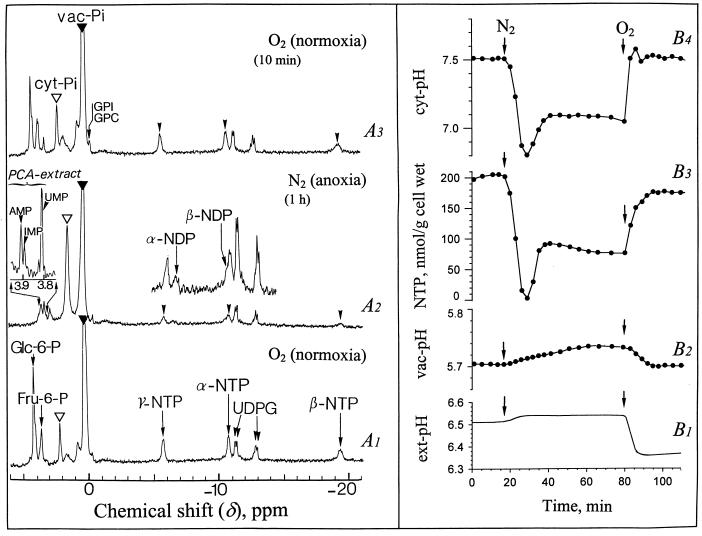
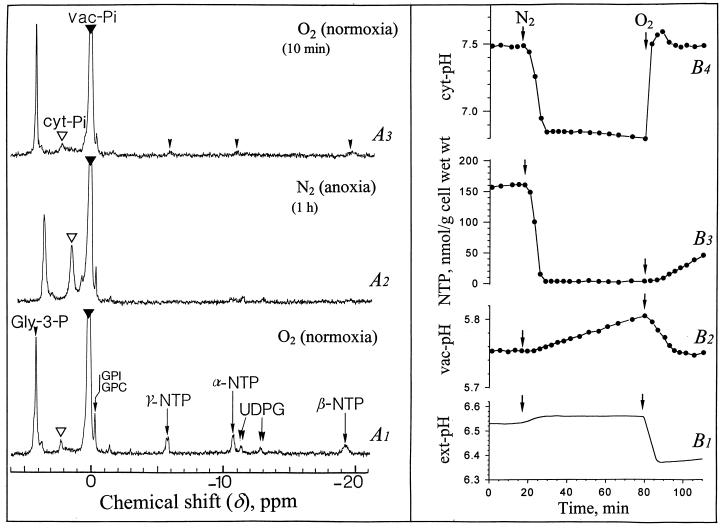
In vivo 31P nuclear magnetic resonance (NMR) spectra obtained from sycamore (Acer pseudoplatanus) cells perfused at pH 6.5 with a Mn2+-free nutrient medium containing 100 μm Pi and supplied with O2 (normoxia) or N2 (anoxia) are shown in Figure 1. The reference spectrum (A1) shows major peaks corresponding to the following compounds, from downfield to upfield: Glc-6-P at 4.30 ppm; Man-6-P as a shoulder at 4.29 ppm; Fru-6-P at 3.78 ppm; inorganic phosphate at 2.28 and 0.40 ppm corresponding to the two separated pools of cyt-Pi (open arrow) and vac-Pi (closed arrow) at pH 7.5 and 5.7, respectively; NTP with three peaks corresponding to γ-, α-, and β-phosphorus at −5.5, −10.6, and −19.2 ppm, respectively; and UDPG with two doublets at −11.0, −11.1, and −12.7, −12.8 ppm, corresponding to β- and α-phosphorus.
Figure 1.
Proton-decoupled in vivo 31P-NMR spectra of normoxic and anoxic sycamore cells, and evolution of NTP, intracellular pHs (cyt and vac pH), and ext pH. The cells (20 g wet weight) were positioned in a 25-mm NMR tube as described earlier (Roby et al., 1987; Aubert et al., 1994), continuously perfused with a culture medium adjusted to pH 6.5 at time zero, and vigorously bubbled with O2 or N2. In the window of NMR analysis the cells comprised about 50% (w/v) of the total (cell + perfusion medium) volume. The spectra recorded at 20°C with a 0.6-s repetition time were the result of 1,500 transients (15 min). They are referenced to methylene diphosphonic acid peak at 16.38 ppm (not shown on the spectra). The ext pH recording is given on curve B1. The points on the curves B2, B3, and B4 were calculated from 3-min spectra corresponding to a representative experiment chosen in a series of five, as indicated in “Material and Methods.” The inset centered at 3.85 ppm shows a portion of a 31P-NMR spectrum (1,024 transients) obtained from a PCA extract of anoxic cells (1 h N2 bubbling). Peak assignments: GPI and GPC, glycerylphosphoryl-inositol and -choline (note that the chemical shift of these compounds remains constant whatever the pH variations because they are phosphodiesters).
A typical response of Glc-fed sycamore cells to anoxia (1 h) is shown on spectrum A2. The most spectacular effects were a strong decrease of NTP and hexose phosphates, the corresponding accumulation of released Pi in the cytoplasm, and the upfield shift of cyt Pi. Within the first 4 to 5 min following the onset of anoxia, the cyt Pi peak shifted from 2.28 to 1.80 ppm, indicating that the cyt pH decreased from 7.5 to 6.8 (Fig. 1B4). The NTP decreased in parallel from approximately 200 to less than 20 nmol/g cell wet weight (Fig. 1B3). Note that after 15 min of anoxia, cyt pH increased and stabilized at the intermediate value of 7.1 (Fig. 1B4). NTP increased in parallel from <20 to 80 to 100 nmol/g cell wet weight (Fig. 1B3). Nucleoside diphosphate (NDP) identified on spectrum A2 (with α- and β-phosphorus at −5.5 and −10.3 ppm, respectively) averaged 40 nmol/g cell wet weight after 1 h of anoxia. In addition, the inset on the left of spectrum A2 shows the presence, at that time, of a pool of nucleotide monophosphates, AMP, IMP, and UMP at 3.91, 3.89, and 3.81 ppm, respectively. The sum of nucleoside monophosphate (NMP) and NDP roughly corresponded to the disappeared NTP. Phosphomonoesters including Glc-6-P, Fru-6-P, and Man-6-P, which started decreasing after 10 min of anoxia (not shown), were no longer detectable 40 min later (spectrum A2). The phosphate released during the catabolism of nucleotides and sugar phosphates accumulated in the cytoplasm (Fig. 1A2). Its concentration calculated as indicated in “Material and Methods” was 8 to 10 mm. Although cyt Pi was much higher than in normoxia, no sign of Pi outflow toward vacuole or circulating medium was observed. In contrast with cyt pH, vac pH was remarkably stable: constant during the first minutes of anoxia, it increased then by less than 0.02 pH unit (Fig. 1B2). The ext pH, initially adjusted to 6.50, increased first slightly and then remained stable (Fig. 1B1).
After re-oxygenation the cyt pH recovered the normoxic value of 7.5 within 2 to 3 min and the NTP recovered the initial value of 210 nmol g−1 cell wet weight within 5 to 7 min (Fig. 1, B4 and B3). It is interesting that the pH of the perfusion (nutrient) medium decreased abruptly by 0.2 pH units as soon as cyt pH increased (Fig. 1B1), suggesting that the H+-pumping ATPase located in the plasma membrane was reactivated and extruded actively protons outside. It is interesting to note that the spectrum A3 recorded 10 min after re-oxygenation displays a profile quite similar to that of the spectrum A1 recorded prior to anoxia. The pool of accumulated cyt Pi was reused for the synthesis of the soluble P-compounds.
The parallelism in the variations of cyt pH and NTP during anoxia, already reported in maize root tips by Saint-Gès et al. (1991), suggested the existence of a relationship between amplitude of cyt acidosis and size of NTP pool, and prompted us to evaluate the ratio of cyt pH drop versus initial NTP pool decrease.
Relationship between Cyt Acidosis and NTP Pool Size in Anoxic Cells
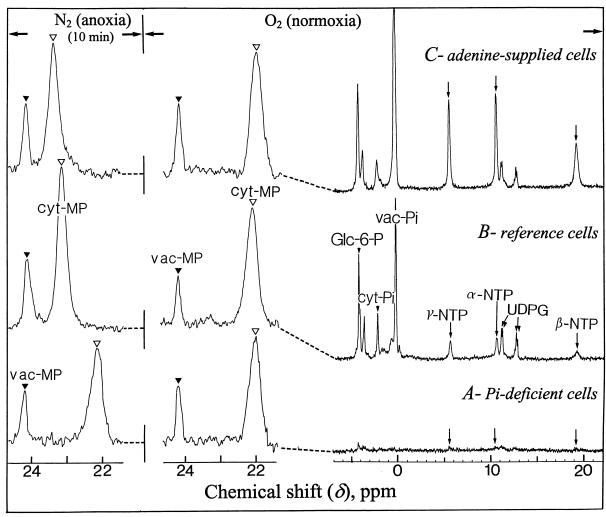
To modify the size of the intracellular NTP pool, cells were incubated in the absence of Pi or in the presence of Pi and adenine. After a 5-d incubation in a Pi-deprived nutrient medium the pools of inorganic phosphate and soluble phosphorylated compounds become very low and the cell growth stops (Rébeillé et al., 1982). However, unlike algae (Theodorou et al., 1991), the Pi deprivation exerts almost no effect on sycamore cell respiration (Rébeillé et al., 1984), indicating that glycolysis (for review, see Plaxton, 1996) delivers respiratory substrates at a normal rate in this material. In addition, as indicated below, fermentation was not affected by phosphate deprivation. The NTP pool of Pi-deprived cells was almost undetectable using in vivo 31P-NMR (Fig. 2, spectrum A), indicating that its concentration was below 20 nmol/g cell wet weight. On the contrary, the NTP pool concentration of Pi-supplied cells (210 nmol/g cell wet weight) was multiplied by 5 to 6 (reaching 1.3 μmol/g cell wet weight) after 12 h incubation in the presence of 1 mm adenine (Fig. 2, spectrum C versus B).
Figure 2.
Proton-decoupled in vivo 31P-NMR spectra of normoxic and anoxic sycamore cells incubated in Pi-deficient (A), standard (B), and adenine-supplied (C) media. The conditions of cell preparation and NMR acquisition are those described in the legend of Figure 1. Adenine-supplied cells were incubated for 12 h in the presence of 1 mm adenine, and Pi-deprived cells were incubated for 5 d in a Pi-free nutrient medium as described in “Material and Methods.” MP was incorporated to cells and used as a cyt pH probe. Prior to NMR analysis all cells were incubated for 6 h in a nutrient medium containing 1 mm MP, but devoid of Pi because MP competes with Pi for the entry into the cell. Note that on the contrary to cyt Pi peak (see Fig. 1), cyt MP peak shifts from upfield to downfield when cyt pH decreases.
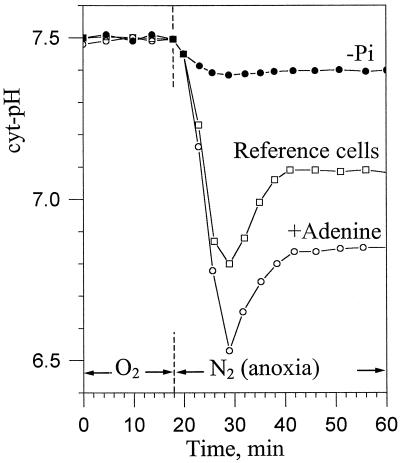
The intracellular pHs of Pi-deprived cells were measured using methylphosphonate (MP) as a non-metabolizable pH probe, as indicated in “Material and Methods.” MP was resolved in two peaks centered at 24.2 and 22.0 ppm corresponding to vac MP (closed arrow) and cyt MP (open arrow), respectively (Fig. 2). The chemical shift of cyt MP in Pi-deficient and Pi-supplied normoxic cells was identical, indicating that the cyt pH of Pi-deprived cells was 7.5, like that of Suc-supplied cells. Under these conditions the comparison of the cyt pH drop following anoxia in Pi-deficient, reference, and adenine-supplied cells (Fig. 2, N2 anoxia) reveals that the acidosis amplitude was related to the size of the intracellular NTP pools (Fig. 3): approximately 0.1 pH unit in cells containing low amounts of NTP (Pi deficient), 0.7 in reference cells, and 1 in NTP-enriched cells (adenine supplied). Intermediate cyt pH decreases were observed in cells partially depleted or enriched in NTP (not shown). These observations suggested that the initial drop of cyt pH following the onset of anoxia originated chiefly from the release of H+ ions, which accompanies the Pi-liberating metabolization of NTP. To further support this conclusion we hydrolyzed 2.5 mm ATP using an alkaline phosphatase purchased from Sigma (St. Louis; 500 μkatals for 3 mL) in a 15 mm phosphate solution (initial pH of 7.5). Under these conditions, which mimic the hydrolysis of cytosolic NTP in the cytoplasm of sycamore cells, with the buffering capacity proposed by Reid et al. (1985) and Mathieu et al. (1986), the pH of the incubation solution decreased by 0.6 pH units. This acidification is comparable to the acidosis observed in anoxic reference cells.
Figure 3.
Anaerobic evolution of the cyt-pH of sycamore cells incubated in Pi-deficient, Pi supplied (reference cells), and adenine-supplied nutrient media. The different points on the curves were calculated from 3-min spectra corresponding to representative experiments chosen in series of five. Experiments were performed under the conditions indicated in the legend of Figures 1 and 2. The perfusing medium was first bubbled with O2, then with N2. The preparation of adenine-supplied and Pi-deficient cells is described in “Material and Methods.”
With time the cyt pH of Pi-deficient cells decreased slowly (not shown), but no partial cyt pH recovery was observed. Different authors relate the partial cyt pH recovery occurring after approximately 15 min anoxia to fermentation processes like ethanol synthesis (Davies et al., 1974; Ratcliffe, 1997). However, the prime cause of the partial re-alkalization of the cytoplasm was unclear. The data in the present study suggest that the resynthesis of NTP, a proton-consuming process, could increase the cyt pH. To substantiate this hypothesis we further analyzed the relationships between fermentation, NTP pool size, and cyt pH.
Relationships between Fermentation, NTP Pool, and Cyt pH in Anoxic Cells
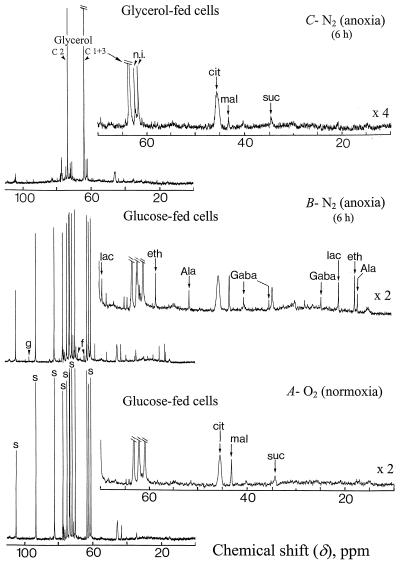
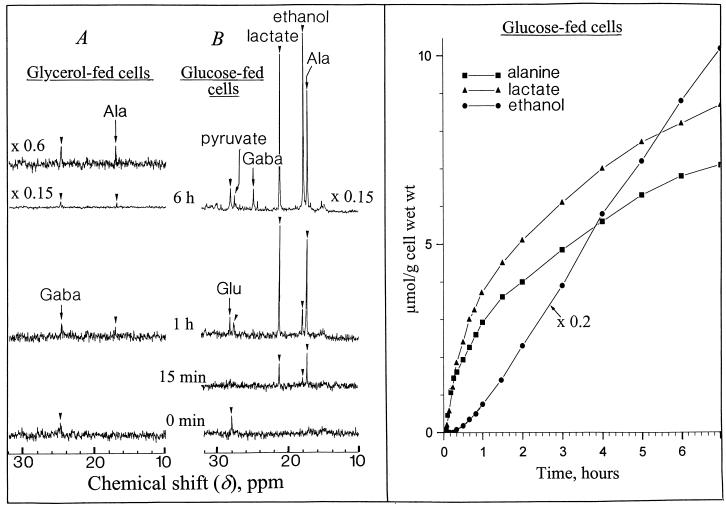
In the following experiments we utilized reference cells incubated in a standard medium containing Glc (Glc-fed cells) or glycerol (glycerol-fed cells) as sole carbon source. Glycerol enters by diffusion to the cyt compartment where it is phosphorylated to sn-glycerol-3-P. This P monoester, which accumulates up to 20 mm in the cytoplasm, is oxidized to dihydroxyacetone-P and sustains the cell respiration via the down steps of glycolysis (Aubert et al., 1994). After 4 to 5 d of incubation in the presence of glycerol, the Glc-6-P, Suc, and starch contents of glycerol-fed cells become negligible since no recycling of triose-P back to hexose-P occurs due to the competitive inhibition of the Glc-6-P isomerase (EC 5.3.1.9) activity by glycerol-3-P (Aubert et al., 1994). As a consequence, the cell growth stops. In anoxic Glc-fed cells the key enzymes involved in the fermentation process (lactate dehydrogenase and alcohol dehydrogenase) allow the continuous oxidation of NADH to NAD+, which is required to sustain the glyceraldehyde-3-P dehydrogenase (EC 1.2.1.12) activity. On the other hand, in anoxic glycerol-fed cells one additional molecule of NAD+ is required for the oxidation of glycerol-3-P to dihydroxyacetone-P catalyzed by sn-glycerol-3-P: NAD+ oxidoreductase (EC 1.1.1.8; Gee et al., 1988). As a consequence, two molecules of NAD+ are required for the metabolization of one molecule of glycerol whereas only one molecule of NAD+ is recycled by fermentation reactions. Therefore, glycerol should not be metabolized in anoxia, and fermentation should not function. This was confirmed by the 13C-NMR spectra (Fig. 4) of normoxic (A) and anoxic (B and C) cells. During a 6-h anoxia Glc-fed cells consumed 18% to 20% of the Suc pool (70–80 μmol g−1 cell wet weight) stored in their vacuole. They simultaneously accumulated lactate, Ala from pyruvate transamination (Vanlerberghe et al., 1991), and γ-aminobutyrate (Gaba) from Glu decarboxylation (Roberts et al., 1992; Ratcliffe, 1995). They also produced appreciable amounts of ethanol (spectrum A versus B) that diffused freely across cell membranes and is present at the same concentration in cells and in perfusing medium (not shown). In contrast, no lactate, Ala, Gaba, or ethanol was distinguishable in natural abundance 13C-NMR spectra of anoxic glycerol-fed cells (spectrum C).
Figure 4.
Proton-decoupled in vivo 13C-NMR spectra (expanded scale from 0–110 ppm) of normoxic and anoxic (A and B) Glc-fed sycamore cells, and anoxic (C) glycerol-fed cells. Normoxic and anoxic spectra of glycerol-fed cells were not significantly different and only the anoxic spectrum was shown. Cells were prepared for NMR analysis as indicated in the legend of Figure 1. The spectra recorded at 20°C with a 1-s repetition time are the result of 3,600 transients (1 h). Glycerol-fed cells were incubated for 1 week in a nutrient medium devoid of Suc and containing 100 mm glycerol. During NMR acquisitions they were perfused with the same nutrient medium. Peak assignments: s, Suc; f, Fru; g, Glc; lac, lactate; eth, ethanol; cit, citrate; mal, malate; suc, succinate; and n.i., not identified.
To improve the signal-to-noise ratio for a precise measurement of the fermentation activity of Glc- and glycerol-fed cells during the first minutes of anoxia, we utilized 100% 13C-enriched substrates d-[1-13C]Glc and d-[3-13C]glycerol, respectively, as indicated in “Material and Methods.” The peaks of the corresponding 13C-enriched carbon of pyruvate, Gaba, lactate, ethanol, and Ala were positioned at 27.2, 24.6, 21.0, 17.6, and 17.0 ppm, respectively (Fig. 5). Lactate and Ala in d-[1-13C]Glc-fed cells were detectable 3 to 5 min after the onset of anoxia. Their accumulation rate was 6 to 7 μmol h−1 g−1 cell wet weight during the 20 first min and then it decreased to approximately 10% of this value. In contrast, the production of ethanol, which started after a delay of approximately 15 min, increased steadily up to a rate of 8 to 9 μmol h−1 g−1 cell wet weight, confirming that the production of ethanol starts after a phase of induction (Davies et al., 1974, Roberts et al., 1984). The production of ethanol by anoxic Glc-fed cells remained stable over several hours. This was also observed in hypoxically pre-treated maize root tips (Xia and Saglio, 1992). However, in contrast with maize root tips, lactate was not excreted in the external medium by sycamore cells, perhaps owing to their cambial origin. It is interesting that the phosphate deficiency did not modify the fermentation activity of anoxic cells with production of lactate and ethanol as long as Glc was the carbon substrate (not shown). In parallel to the production of fermentation compounds during anoxia, the intracellular Suc concentration of Glc-fed cells (80 μmol g−1 cell wet weight) decreased at a rate of 2.1 to 2.5 μmol h−1 g−1 cell wet weight (Fig. 4, A and B), whereas starch (11–12 mg g−1 cell wet weight) remained constant (not shown). This later result is in agreement with the observations of Hill and ap Rees (1995). The absence of starch metabolization, in addition to the arrest of Glc transport through the plasma membrane in anoxic cells (see below) and to the fact that the rate of Suc consumption matched the rate of ethanol, lactate, and Ala synthesis, suggests that the flux of carbon directed toward fermentation reactions originated essentially from endogenous Suc metabolization. In contrast, confirming the observation with natural 13C-abundance glycerol, it was not possible to detect ethanol, lactate, and pyruvate on 13C-NMR spectra of anoxic d-[3-13C]glycerol-fed cells (Fig. 5A). Only very low amounts of Ala and Gaba (approximately 0.2 μmol/g cell wet weight) were detected after 6 h of anoxia. This confirms that no significant fermentation reactions took place in glycerol-fed cells and it indicates that these cells constitute a very suitable material for the analysis of the effects of anoxia on cyt pH when the fermentation activity is negligible.
Figure 5.
Proton-decoupled in vivo 13C-NMR spectra (expanded scale from 10–32 ppm) of anoxic glycerol- and Glc-fed sycamore cells (A and B), and evolution of ethanol, lactate, and Ala. The spectra recorded at 20°C with a 1-s repetition time are the result of 900 transients (15 min) at time 0 min, 15 min, and 1 h, and 3,600 transients (1 h) at time 6 h. The nutrient medium of Glc- and glycerol-fed cells contained 250 mg of 100% 13C-enriched d-[1-13C]Glc and d-[3-13C]glycerol, respectively. The different points of the curves were calculated from 7-min spectra corresponding to a representative experiment in a series of five as indicated under “Material and Methods.” Only an upfield part of the spectra (10–32 ppm) showing 13C-enriched methyl and methylene groups of fermentation compounds is given in this figure.
A typical 31P-NMR spectrum of normoxic glycerol-fed cells is shown in Figure 6A1. It differs from the corresponding spectrum of reference cells (Fig. 1A1) by the presence of a dominant phosphomonoester peak of glycerol-3-P centered at 4.1 ppm, and by the absence of Glc-6-P (Aubert et al., 1994). Following the onset of anoxia the position of the cyt Pi peak of glycerol-fed cells shifted from 2.28 to 1.85 ppm (Fig. 6A2), indicating that the cyt pH decreased from 7.50 to 6.85 (Fig. 6B4). This cyt acidification is confirmed by the shift of the glycerol-3-P peak from 4.05 to 3.65 ppm. The pool of NTP decreased in parallel from approximately 150 to less than 20 nmol/g cell wet weight and cyt Pi increased accordingly (Fig. 6A2). Taking into account their smaller cyt volume with respect to the total cell volume, the early response of glycerol-fed cells to anoxia was identical to that of Suc-fed cells (Figs. 1 and 6). In contrast, after 15 min of anoxia there was no partial recovery of the NTP pool in glycerol-fed cells and their cyt pH remained close to 6.85 (Figs. 6B4 versus 1B4). Furthermore, in correlation with the absence of fermentation, the pool of glycerol-3-P did not decrease significantly during anaerobiosis. When anoxia was maintained over 6 h the cyt pH of glycerol-fed cells decreased to 6.6, whereas vac pH increased to 6.3 (not shown). This suggests that protons leaking from the vac to the cytoplasm were not rejected across the tonoplast in the absence of fermentation-linked ATP synthesis. The cell death was observed when cyt pH was decreasing below 6.5.
Figure 6.
Proton-decoupled in vivo 31P-NMR spectra of normoxic and anoxic glycerol-fed sycamore cells, and evolution of NTP, cyt pH, vac pH, and ext pH. The conditions of cell preparation and NMR acquisition are those described in the legend of Figure 1. Prior to NMR analysis cells were incubated for 1 week in a Suc-free nutrient medium containing 100 mm glycerol. The ext pH recording is given on curve B1. The points on the curves B2, B3, and B4 were calculated from 3-min spectra corresponding to a representative experiment chosen in a series of five, as indicated in “Material and Methods.” Gly-3-P, sn-glycerol-3-P.
After re-oxygenation, the cyt pH of glycerol-fed cells rose back to 7.5 within less than 5 min (Fig. 6B4) and the pH of the nutrient medium decreased simultaneously as already observed with Glc-fed cells (Fig. 6B1). It is interesting that the NTP pool remained hardly detectable by in vivo 31P-NMR during the first 15 min of recovery (Fig. 6, A3 and B3). The simplest explanation for this is that oxidative pentose-phosphate pathway is not operating when the cells are fed exclusively with glycerol (Aubert et al., 1994), which would imply that Rib-5-P, indispensable for net synthesis of ATP, is not synthesized. This is in contrast with the situation observed in Glc-fed cells (Fig. 1, A3 and B3). Our results also indicate that a tiny pool of NTP was sufficient to permit the functioning of the plasmalemma H+-pumping ATPase after re-oxygenation.
Arrest of Functioning of the Plasmalemma ATPase in Anoxia
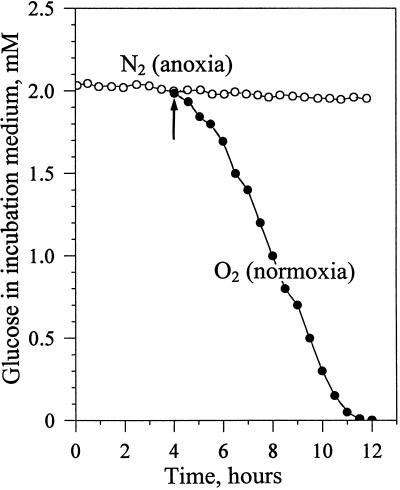
The results presented above suggest that the plasmalemma ATPase does not operate under anoxic conditions. To collect further arguments in favor of this hypothesis we measured, in anoxic cells, nutrient influxes depending on the proton gradient generated by the H+-pump ATPase of the plasmalemma (energy-dependent transports). Figure 7 shows that Glc, which is cotransported with a proton (Bush, 1993), was not significantly taken from the incubation medium maintained at pH 6.5 until oxygenation was restored. In a similar manner, the transport of amino acids and Pi from the incubation medium by proton-coupled symporters was not functioning in anoxia (not shown). In addition, anoxic cells did not incorporate the paramagnetic cation Mn2+, which is incorporated to cells within minutes under normoxia (Roby et al., 1988). This latter observation indicates that the potential gradient (Δψ) maintained across the plasmalemma by the H+-pump ATPase in normoxia was collapsed in anoxia. Taken together, these observations reinforce the hypothesis that the plasmalemma ATPase was not functioning in anoxic cells and raise the question of the cause for this blockage.
Figure 7.
Uptake of Glc by sycamore cells during anoxia and normoxia. Eight grams of reference cells were incubated into flasks containing 200 mL of a fresh Suc-free culture medium supplied with 2 mm Glc and bubbled with N2. In one flask N2 was replaced by O2 after 4 h of anoxia (arrow). The evolution of Glc uptake by cells was determined via the measurement of the Glc present in the culture medium, according to the method of Bergmeyer et al. (1974). The values expressed as concentrations of Glc present in the incubation medium are from a representative experiment chosen in a series of five.
The cause of the blockage of the plasmalemma H+-pump ATPase was not the decrease of the NTP pool, because the cyt pH was normally regulated in normoxic Pi-deficient cells containing much lower amounts of NTP (Fig. 2). One could hypothesize that the significant accumulation of NDP and NMP observed in anoxia (Fig. 1A2) inhibited the functioning of the plasmalemma ATPase. To check this possibility reference cells were incubated in the presence of 200 μm cyanide, which blocks the mitochondrial electron chain at the level of the cytochrome c oxidase. Under these conditions the NTP pool decreased by 60% to 70%, with a corresponding increase of NMP and NDP, thus mimicking the effect of anoxia (not shown). However, after a transient acidification concomitant to the partial hydrolysis of the NTP pool, the cyt pH of cyanide-incubated cells returned to 7.4 to 7.5 in the absence of NTP pool recovery. This indicates that the H+-pump ATPase of the plasmalemma was functioning in cyanide-incubated cells despite the presence of significant NDP and NMP pools. One other hypothesis is that the enzyme activity was blocked by a transducer of anoxia signal released during anoxia. It is interesting that Subbaiah et al. (1994) showed that calcium ions are released from intracellular stores in the cytosol of anoxic cells and Lino et al. (1998) observed that free Ca2+ ions trigger the blockage of the proton pumps. To substantiate the hypothesis of a calcium-mediated blockage of the proton pumps in anoxic sycamore cells we utilized cells cultivated in a calcium-free nutrient medium. After 7 d the calcium content of sycamore cells was reduced by approximately 80%. When submitted to anoxia the cyt pH of these calcium-deprived cells decreased by only 0.2 to 0.3 pH unit (not shown). On the other hand, the sequestration of external Ca2+ with 1 mm EGTA added in the incubation medium did not affect the cyt acidosis consecutive to anoxia, suggesting therefore that Ca2+ should derive from a cell compartment and not from the external medium.
DISCUSSION
The results presented in this article demonstrate that the proton-releasing metabolization of NTP was at the origin of the cyt acidosis established in sycamore cells immediately after the imposition of anoxia. The metabolization of the pools of hexose phosphates starts after the initial decrease of NTP and did not participate in the initial cyt pH change. The amplitude of the cyt pH decrease was related to the size of the NTP pool present in cells before the onset of anoxia (Fig. 3); negligible in Pi-deficient cells containing very little NTP, but maximum in adenine-supplied cells enriched in NTP. It would be interesting to determine if there was a tight proportionality between the decrease of NTP and the decrease of cyt pH. It is unfortunate that the quantification of the contribution of NTP hydrolysis to cyt pH acidification in anoxia is hampered by the uncertainty as to the buffering capacity of the cytosol where NTPs are hydrolyzed and where the released Pi (the NMR pH-probe) accumulates. However, assuming a buffering capacity of 15 μmol H+/g cell wet weight per pH unit, and admitting that the hydrolysis of one molecule of MgNTP liberates three protons, one can estimate that the initial cyt pH decrease (0.7 pH unit) corresponds roughly to the liberation of protons following the hydrolysis of approximately 2.5 mm NTP, which is the NTP concentration in the cytosol of reference cells. This estimate is consistent with the decrease of pH measured in vitro during the hydrolysis of 2.5 mm ATP in an incubation medium initially adjusted to pH 7.5 and buffered by 15 mm Pi. In cells containing five times more NTP (adenine supplied), the increase of the pH drop consecutive to anoxia remained, however, lower than expected (Fig. 3). This discrepancy may be explained by a low activity of the H+ pumps in anoxia when cyt pH falls markedly below neutrality (Xia and Roberts, 1996). In addition, the buffering capacity of cyt cell compartments varies according to their pH (Oja et al., 1999).
A second observation in favor of the existence of a tight relationship between the variations of cyt pH and the variations of the NTP pool size in anoxic cells is that cyt pH increased in parallel to the partial recovery of the NTP pool 15 min after the onset of anoxia (Fig. 1). The synthesis of ATP from ADP and Pi, which is an alkalizing process, permitted the increase of cyt pH from 6.85 to 7.1. In anoxic Glc-fed cells the partial recovery of the NTP pool was concomitant to the start of a continuous production of ethanol (Fig. 5). On the contrary, in glycerol-fed cells characterized by the absence of fermentation, there was no partial NTP recovery and we observed no cyt pH increase. As a consequence, these results confirm that in anoxic cells the short-term fluctuations of cyt pH are NTP-dependent and indicate that ethanolic fermentation permitted, via the regulation of the NTP pool size, the increase of cyt pH up to a new equilibrium state.
The long-term steady-state regulation of intracellular pHs starting after approximately 20 min anoxia does not seem to be dominated by the NTP pool size in sycamore cells. For example, in Pi-deficient anoxic cells cyt pH remained stable to 7.4 to 7.3 despite the very low NTP pool. This confirms the observations of Xia et al. (1995) in acclimated root tips poisoned with fluoride to lower the NTP pool size, and suggests that long-term cyt pH regulation involved other mechanisms. One can recall the synthesis of lactate (Davies et al., 1974), the metabolism of succinate, malate, and amino acids (Roberts et al., 1992), and the ethanolic fermentation (for review, see Tadege et al., 1999). According to Roberts et al. (1984) and Fox et al. (1995), the production of ethanol by anoxic tissues is induced by the acidification of the cytoplasm. Here ethanol was steadily produced after an induction phase during which the cyt pH was below 7.0 (Fig. 1B4). After the 1st h of anoxia the rate of ethanol synthesis (8–9 μmol h−1 g−1 cell wet weight, Fig. 5) was approximately 5 times higher than the rate of lactate, Ala, and Gaba synthesis, which was declining. Ethanol was the major metabolic end product during long-term anaerobiosis in sycamore cells, confirming the observations of Good and Muench (1993) on barley root tissue. Assuming that the H+ pump of the plasma membrane was not functioning, our data suggest that the cyt pH regulation in the long-term involved an equilibrium between acidifying and alkalizing processes like the syntheses of lactate, Ala, and Gaba. Our results indicate that in anoxic sycamore cells, the synthesis of Ala and Gaba matched that of lactate (Figs. 4 and 5).
In well-oxygenated cells the H+-pumping activity of the plasma membrane plays a major role in counteracting cytoplasmic acidification (Gout et al., 1992). Since it is highly sensitive to cyt pH (Slayman, 1987), it was expected to combat against the acidosis induced by anoxia as long as the pool of ATP was not limiting. Nevertheless, several observations indicate that the proton pump of the plasmalemma was not operating under anaerobiosis: (1) the capacity of cells to incorporate metabolites cotransported with protons like Glc or phosphate was abolished (Fig. 7); (2) H+ ions accumulated in the cytoplasm during the hydrolysis of NTP were not ejected into the external medium during anoxia; and (3) upon re-oxygenation the cyt pH increased immediately, whereas ext-pH decreased, indicating that the functioning of the plasmalemma ATPase was quickly restored. The cyt pH recovered the initial value of 7.5 within 2 to 3 min and remained stable due to the central role played by the plasmalemma H+-pumping ATPase in the homeostasis of the cyt pH in normoxic higher plant cells (Gout et al., 1992). What may be the cause for the arrest of functioning of the plasmalemma ATPase in anoxia? Different hypotheses were proposed such as: it is a consequence of the diminution of the ATP pool; NDP and NMP that accumulated significantly following the onset of anoxia exert an inhibitory effect; and the enzyme activity is blocked by the absence of O2 directly or via a messenger released under anaerobiosis.
The first hypothesis can be rejected for two reasons: An NTP pool much lower than that present in anoxic Glc-fed cells was sufficient to sustain the full recovery of cyt pH in glycerol-fed cells after re-oxygenation (Fig. 6, B3 and B4); and the cyt pH of Pi-deprived cells is regulated at 7.5, like the cyt pH of reference cells, despite the very small size of the NTP pool (Fig. 2). The second hypothesis seems also unlikely since the cyt pH is regulated at the normal pH of 7.4 to 7.5 in cyanide-treated cells in which the NTP pool decreased by 60% to 70% and the NMP + NDP pools increased correspondingly. In support of the third hypothesis, our observation that the cyt acidosis following anoxia was lower in calcium-deficient cells than in normal cells suggests that calcium ions are released from intracellular stores and block the functioning of the proton pumps. This is in good agreement with the observations of Lino et al. (1998) and Subbaiah et al. (1994, 1998). According to these later authors, calcium is released in anoxic maize cells by depolarized mitochondria. Upon return to normoxia the recovery of the normoxic intracellular pHs, with a concomitant acidification of the outer medium, was observed within 2 to 3 min (Fig. 1, B1 and B4). It is interesting that Sedbrook et al. (1996) observed that free Ca2+ ions disappear quickly from anoxic cells following re-oxygenation. We suggest that this permits the immediate activation of the plasmalemma H+-pumping ATPase. Calcium is also implicated in the expression of various genes, including those encoding glycolytic and ethanolic fermentation enzymes (Sedbrook et al., 1996; Ratcliffe, 1997). As a consequence, the release of free Ca2+ in the cytosol of anoxic cells should favor the induction of the ethanolic fermentation activity that was observed after the 15 first min of anoxia. In an indirect manner, it should favor the partial recovery of the NTP pool and the increase of cyt pH.
The anoxia-induced increase in the glycolytic flux (Pasteur effect) tends to restore, at least partially, the NTP pool. Given the accumulation of fermentation compounds (10–11 μmol h−1 g−1 cell wet weight), and the value of cell respiration in normoxia (17–20 μmol O2 h−1 g−1 cell wet weight; Rébeillé et al., 1985), one can estimate that the glycolytic flux is enhanced by 70% to 80% in anoxia. However, it is far from sufficient to sustain a rate of NTP synthesis close to the normoxic value since the oxidation of one molecule of Glc permits the production of only two molecules of ATP in anoxia (corresponding to glycolytic steps of Glc metabolization) versus 38 in normoxia. The initial stock of vacuolar Suc (70–80 μmol g−1 cell wet weight), which was consumed by only 18% to 20% after 6 h of anoxia (Fig. 4), seems sufficient to sustain a stronger glycolytic flux and permits the maintenance of significant pools of phosphorylated carbohydrates in anoxia. Nevertheless, the hexose phosphate pool decreased below the 31P-NMR threshold of detection within less than 1 h of anoxia (Fig. 1A2). It recovered its initial value shortly after re-oxygenation (Fig. 1A3). In anoxic maize root tips, Bouny and Saglio (1996) demonstrated that the major limiting step of glycolysis was the phosphorylation of hexoses by kinases. For these authors the maintenance of the glycolytic flux in hypoxically pretreated tissues is explained by a combination of a rise in kinase activities and decreased inhibition due to higher cyt pH and ATP pools. However, according to Chang et al. (2000), acclimation to anoxia requires more than just enhanced fermentation via increased levels of enzymes. In anoxic sycamore cells there was no Glc transport through the plasmalemma and no starch metabolization. As a consequence, the main pool of available carbohydrates was the Suc present in the vacuole. Our results suggest that in this case, the release of Suc from the vacuole could be the limiting factor of the glycolytic activity. The utilization of Suc as a source of energy depends on its cleavage into hexoses catalyzed by invertase (EC 3.2.1.26), which cleaves Suc into the two monosaccharides or Suc synthase (EC 2.4.1.13), which converts Suc in the presence of UDP into UDP-Glc and Fru. Besides isoforms of vacuolar and extracellular invertases, most plant species contains isoforms of at least two cytosolic invertases (Sturm, 1999) with pH optima between 7.0 and 7.8 (Gallagher and Pollock, 1998). It is surprising that the UDP-Glc pool that is in equilibrium with the hexose phosphate pools in normoxic cells did not decrease in anoxia (see UDPG after 1 h of anoxia, Fig. 1A2) despite the disappearance of hexose phosphates. This can be explained considering that the pyrophosphate-dependent 6-phosphofructo-1-phosphotransferase (EC 2.7.1.90), which generates PPi for UDP-Glc pyrophosphorylase (EC 2.7.7.9) functioning is inhibited by inorganic phosphate (Stitt, 1990) and that Pi accumulates considerably in the cytosol of anoxic cells. It is also possible that the excess of Pi present in the cytosol under anaerobiosis exerts a direct effect on UDP-Glc pyrophosphorylase activity. Because starch and UDP-Glc seem to be not utilized under anaerobiosis we are forced to conclude that in the absence of oxygen Suc is channeled via invertase to sustain the glycolytic pathway. On a physiological point of view, this limitation of carbohydrate consumption could be beneficial to tissues enduring prolonged anoxic situation in so much that it avoids the exhaustion of carbohydrates and consequently favors the recovery process after re-oxygenation. The blockage of the plasmalemma H+-pumping ATPase is also beneficial to anoxic tissues because it limits the consumption of ATP during a period when the recycling of this nucleotide is drastically reduced due to the absence of oxidative phosphorylation.
This work shows that the metabolic activity of normoxic cells prior to experiments interferes little with the initial acidosis following the onset of anoxia, shedding a doubt on the claim that acidifying protons arise from non-processed substrates like glycolytic and respiratory intermediates (Felle, 1996). In support of this conclusion we observed that anoxia triggered a comparable cyt acidosis in cells containing very different organic acid pools, but similar NTP pools like Glc- and glycerol-fed cells (Figs. 4 and 6), and that the amplitude of the cyt pH decrease in Pi-deficient cells is small despite a normal fermentation activity. In addition, the synthesis of potentially acidifying organic acids accumulated during fermentation, like lactate, did not contribute to the initial cyt pH drop since it started later, confirming the observations of Saint-Gès et al. (1991) with maize root tips. These latter results are consistent with the observation that rice shoots exhibit a decrease in cyt pH immediately after the beginning of anaerobiosis even though these tissues are almost devoid of lactic acid fermentation (Rivoal et al., 1989; Menegus et al., 1991); and, in maize root tips, a stronger anoxia-induced acidosis was observed when the induction of fermentation pathway was inhibited by cycloheximide pretreatment (Chang et al., 2000).
MATERIAL AND METHODS
Suspension-cultured sycamore (Acer pseudoplatanus) cells were grown at 20°C in a liquid nutrient medium containing Suc (or Glc) as sole carbon source (Bligny, 1977). The culture medium was kept at a volume of 250 mL and stirred continuously at 60 rpm. Under these conditions the cell number doubling time is 40 to 48 h after a lag phase of approximately 1 d. The maximum cell density is attained after 7 to 8 d of growth. Cell suspensions were maintained in exponential growth by weekly subcultures. The age of cells refers to the day of subculturing. The so-called reference cells were 4-d-old cells. In addition, Pi-deprived and glycerol-fed cells were used. Pi-deprived cells were reference cells that were rinsed three times by successive resuspensions in a fresh Pi-free culture medium and incubated in the same medium for 5 d. Glycerol-fed cells were reference cells that were rinsed three times with a culture medium devoid of Suc and placed in a Suc-free culture medium containing 100 mm glycerol as unique carbon source for 5 d. The growth of Glc- and Suc-fed cells was identical. The growth of Pi-starved and glycerol-fed cells stopped after 2 to 3 d of culture, but it could restart readily after the addition of Pi or Suc. The cell wet weight was measured after straining culture aliquots for 20 s onto a glass-fiber filter (pressure of suction, 9 × 104 Pa).
NMR Analyses
In Vivo Measurements
Spectra were recorded on a spectrometer (AMX 400, WB, Bruker, Billerica, MA) equipped with a multinuclear 25-mm probe tuned at 161.9 and 100.6 MHz for 31P- and 13C-NMR, respectively. To optimize the homogeneity of the cell incubation conditions and to maximize the signal-to-noise ratio we utilized an experimental arrangement derived from that of Roby et al. (1987). Cells (20 g wet weight) were introduced in the 25-mm NMR tube on a porous plate placed near the bottom of the tube. The porous plate was crossed by a central output glass tube; two short tubes (inlet and safety output tubes) were positioned 2 cm above the surface of the sedimented cells. A peristaltic pump attached to these tubes circulated the nutrient medium (200 mL) through the cells, and then through the porous plate, and recycled it via a 500-mL reservoir. In this reservoir the medium was bubbled with O2 or N2. The composition of the incubation medium was 1 mm KCl, 5 mm KNO3, 0.5 mm MgSO4, 0.5 mm Ca(NO3)2, with or without 0.1 mm KH2PO4, and 4 mm Glc or 8 mm glycerol. The pH of the external medium was adjusted to 6.50 before experiments and recorded using a pH electrode (Urectron 6, Tacussel, France), which tip was immersed in the reservoir of perfusing medium.
Conditions for 31P-NMR acquisition utilized 70-μs pulses (50°C) at 0.6-s intervals and a sweep width of 9.8 kHz. Broad-band decoupling at 2.5 W during acquisition and 0.5 W during the delay was applied using the Waltz sequence; the signal was digitized at 4,000 data points zero-filled to 8,000 and processed with a 2-Hz line broadening. Spectra were referenced to methylene diphosphonic acid (pH 8.9) contained in a coaxial capillary that was inserted inside the output tube (Roby et al., 1987) at 16.38 ppm.
Conditions for 13C-NMR acquisition utilized 70-μs pulses (90°C) at 5.6-s intervals and a sweep width of 20.73 kHz. Broad-band decoupling at 4 W during acquisition and 0.5 W during the delay was applied using the Waltz sequence; the signal was digitized using 16,000 data points zero-filled to 32,000 and processed with a 2-Hz line broadening. Spectra were referenced to hexamethyldisiloxane at 2.7 ppm. To improve the detection of the fermentation products during anoxia d-[1-13C]Glc and sn-d-[3-13C]glycerol were used as carbon source in nutrient media at the concentration of 1 mg/mL, as specified in text. These compounds were purchased from Leman (St. Quentin, France).
To set up anoxia the bubbling gas was switched from O2 to N2. The CO2 produced by cell respiration, ethanolic fermentation, or Glu decarboxylation to Gaba was evacuated to the atmosphere by the gas bubbling in the medium reservoir (gas flow, 1 L/min). To limit the formation of a CO2 gradient between the top and the bottom of the perfused mass of cells, the nutrient medium was circulated at 120 mL/min.
Intracellular pH were estimated from the chemical shift (δ) of the cyt and vac Pi pools as described by Gout et al. (1992). In the case of Pi-deprived cells MP was utilized as a very suitable probe for cyt pH measurements using 31P-NMR (Pugin et al., 1997). Sycamore cells incorporated an average of 0.8 to 1 μmol MP/g cell wet weight after a 6-h incubation in a Pi-free culture medium containing 1 mm MP. The incorporated MP split into 5 mm cyt MP and 0.2 mm vac MP. Using cells containing Pi and Pi + MP, it was verified that the intracellular pHs measured from the chemical shift of Pi or MP were identical.
In Vitro Measurements
Perchloric acid (PCA) extracts were prepared from 10 g of cells as described by Aubert et al. (1996). Spectra were obtained on a spectrometer (AMX 400) equipped with a 10-mm multinuclear probe tuned at 161.9 and 100.6 MHz for 31P- and 13C-NMR, respectively. The deuterium resonance of 2H2O (100 μL added per milliliter of extract) was used as a lock signal.
Conditions for 31P-NMR acquisition utilized 15-μs pulses (70°C) at 3.6-s intervals and a sweep width of 8.2 kHz. Broad-band decoupling at 1 W during acquisition and 0.5 W during delay was applied using the Waltz sequence; the signal was digitized using 8,000 data points zero-filled to 16,000 and processed with a 0.2-Hz line broadening. Spectra were referenced to methylene diphosphonic acid (pH 8.9) at 16.38 ppm. Divalent paramagnetic cations were chelated by the addition of corresponding amounts of 1,2-cyclohexylenedinitrilotetraacetic acid.
Conditions for 13C-NMR acquisition utilized 19-μs pulses (90°C) at 6-s intervals and a sweep width of 20 kHz. Broad-band decoupling at 2.5 W during acquisition and 0.5 W during the delay was applied using the Waltz sequence; the signal was digitized using 32,000 data points zero-filled to 64,000 and processed with a 0.2-Hz line broadening. 13C-NMR spectra are referenced to hexamethyldisiloxane at 2.7 ppm. Mn2+ ions were chelated by the addition of 1 mm 1,2-cyclohexylenedinitrilotetraacetic acid.
Identification and Quantification
The assignment of resonance of inorganic phosphate and soluble Pi-containing compounds to specific peaks observed on in vivo 31P-NMR spectra was carried out with the help of the spectra of PCA extracts prepared from the samples frozen immediately after in vivo analyses (Roberts and Jardetzky, 1981; Roby at al., 1987; Aubert et al., 1994). The assignments of resonance of 13C peaks were carried out according to Gout et al. (1993). In all experiments the spectra of standard solutions of known compounds were compared with that of PCA extracts of sycamore cells. The definitive assignments were made after running a series of spectra obtained by addition of the authentic compounds to the PCA extracts at different pHs.
Identified compounds were quantified from the surface of their resonance peaks using fully relaxed conditions for spectra acquisition (pulses at 20-s intervals). Peak intensities were calibrated by comparison with spectra obtained after the addition of known amounts of the corresponding authentic compounds. The average extraction rate of the soluble cell compounds using the technique of PCA extraction was estimated to 75% to 80% according to Aubert et al. (1996). The in vivo quantification of ethanol was done by comparison of the surface of its resonance peaks with those of other quantified compounds, taking into account the free ethanol diffusion across cell membranes and the relative cell volume in the NMR-analyzed area (50%). To calculate intracellular concentrations, the knowledge of the relative volume of the different cell compartments is required. Subcellular volumes were estimated from electron micrographs of cells according to Winter et al. (1994). In 1 g of Glc-fed cells there was typically 0.13 mL cyt and 0.80 mL vac, and in 1 g of glycerol-fed cells there was 0.08 mL cyt and 0.86 mL vac. According to Aubert et al. (1994), this difference originates from the fact that the vac volume of glycerol-fed cells increases before cell growth stops, but not the cyt volume. In both types of cells the cytosol occupied nearly 60% of the cyt volume.
Each NMR experiment was repeated at least five times with cells originating from independent cultures.
Measurement of Glc and Starch: Uptake of Glc by Cells
Glc was measured with hexokinase and Glc-6-P dehydrogenase (EC 1.1.1.49) as described by Bergmeyer et al. (1974). For starch determination, cells (200 mg wet weight) were rinsed, dropped into 3 mL of ice-cold 0.5 m NaOH, and left at 0°C for 15 min. Portions (1 mL) were vigorously stirred in a Potter-Elvehjem homogenizer to disrupt cells and plastids, and were neutralized with 10 m HCl. Aliquots (500 μL) were then incubated for 1 h at pH 4.6 and 35°C with 500 μL of 1 mg/mL amyloglucosidase (EC 3.2.1.3; Sigma) in sodium acetate. Glc released from starch hydrolysis was measured as indicated above.
The uptake of Glc by cells was measured as follows: 8 g of 4-d-old sycamore cells was incubated in 200 mL of a fresh Suc-free culture medium containing 2 mm Glc. Fractions of 1 mL of cell suspension were taken with time and placed in 1.5-mL Eppendorf tubes for 1 min to permit the sedimentation of cells and the separation of the supernatant. Then, 200-μL aliquots of the cell-free supernatant were taken for the measurement of Glc. The uptake of Glc was measured from the disappearance of Glc from the supernatants.
Cell Enrichment with d-[3-13C]Glycerol and d-[1-13C]Glc
Glycerol-fed cells were rinsed three times with a glycerol-free culture medium to eliminate the unlabeled glycerol initially present in cells and replace it by 100% 13C-enriched d-[3-13C]glycerol (1 mg/mL perfusion medium). Glycerol diffuses freely across cell membranes and equilibrates between outside medium and cell compartments within 1 to 2 min (Aubert et al., 1994). We verified that this equilibration was not dependent upon cell oxygenation.
On the contrary, Glc was not incorporated to cells in anoxia (see “Results”). For this reason, intracellular carbohydrates pools were labeled with 13C prior to experiments. After a 24-h incubation in a Suc-free nutrient medium to decrease unlabeled sucrose by 90% (Rébeillé et al., 1985), cells were incubated for 12 h in the presence of 100% 13C-enriched d-[1-13C]Glc (1 mg/mL) to restore Suc with 13C-enrichment on the C-1 and C-6 carbon atoms of the glycosyl and fructosyl moieties of the molecule (Keeling et al., 1988).
LITERATURE CITED
- Aubert S, Gout E, Bligny R, Douce R. Multiple effects of glycerol on plant cell metabolism. J Biol Chem. 1994;269:21420–21427. [PubMed] [Google Scholar]
- Aubert S, Gout E, Bligny R, Marty-Mazars D, Barrieu F, Alabouvette J, Marty F, Douce R. Ultrastructural and biochemical characterization of autophagy in higher plant cells subjected to carbon deprivation: control by the supply of mitochondria with respiratory substrates. J Cell Biol. 1996;133:1251–1263. doi: 10.1083/jcb.133.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmeyer HU, Bernt E, Schmidt F, Stork H. d-Glucose: determination with hexokinase and glucose-6-phosphate dehydrogenase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. Vol. 3. New York: Academic Press; 1974. pp. 1196–1201. [Google Scholar]
- Bligny R. Growth of suspension cultured Acer pseudoplatanus cells in automatic culture units of large volume. Plant Physiol. 1977;59:502–505. doi: 10.1104/pp.59.3.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligny R, Leguay JJ. Techniques of cell suspension culture. Methods Enzymol. 1987;148:3–16. [Google Scholar]
- Bouny JM, Saglio PH. Glycolytic flux and hexokinase activities in anoxic maize root tips acclimated by hypoxic pretreatment. Plant Physiol. 1996;111:187–194. doi: 10.1104/pp.111.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busa WB, Nuccitelli R. Metabolic regulation via intracellular pH. Am J Physiol. 1984;246:409–438. doi: 10.1152/ajpregu.1984.246.4.R409. [DOI] [PubMed] [Google Scholar]
- Bush DR. Proton-coupled sugar and amino acid transporters in plants. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:513–542. [Google Scholar]
- Chang WP, Huang L, Shen M, Webster C, Burlingame AL, Roberts J. Patterns of protein synthesis and tolerance of anoxia in root tips of maize seedlings acclimated to a low-oxygen environment, and identification of proteins by mass spectrometry. Plant Physiol. 2000;122:295–317. doi: 10.1104/pp.122.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DD, Grego S, Kenworthy P. The control of the production of lactate and ethanol by higher plants. Planta. 1974;118:297–310. doi: 10.1007/BF00385580. [DOI] [PubMed] [Google Scholar]
- Dorée M, Leguay JJ, Sadorge P, Terrine C, Guern J. Utilisation d'adénine exogène par les tissus végétaux: II Rôle de l'adénine pyrophosphoribosyltransférase. Physiol Vég. 1970;8:515–528. [Google Scholar]
- Felle HH. Control of cytoplasmic pH under anoxic conditions and its implication for plasma membrane proton transport in Medicago sativa root hairs. J Exp Bot. 1996;47:967–973. [Google Scholar]
- Fox GG, McCallan NR, Ratcliffe RG. Manipulating cytoplasmic pH under anoxia: a critical test of the role of pH in the switch from aerobic to anaerobic metabolism. Planta. 1995;195:324–330. [Google Scholar]
- Gallagher JA, Pollock CJ. Isolation and characterization of a cDNA clone from Lolium temulentum L. encoding for a sucrose hydrolytic enzyme which shows alkaline/neutral invertase activity. J Exp Bot. 1998;49:789–795. [Google Scholar]
- Gee RW, Byerrum RU, Gerber DW, Tolbert NE. Dihydroxyacetone phosphate reductase in plants. Plant Physiol. 1988;86:98–103. doi: 10.1104/pp.86.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevers W. Generation of protons by metabolic processes in heart cells. J Mol Cell Cardiol. 1977;9:867–874. doi: 10.1016/s0022-2828(77)80008-4. [DOI] [PubMed] [Google Scholar]
- Good AG, Muench DG. Long-term anaerobic metabolism in root tissue: metabolic products of pyruvate metabolism. Plant Physiol. 1993;101:1163–1168. doi: 10.1104/pp.101.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gout E, Bligny R, Douce R. Regulation of intracellular pHs in higher plant cell: carbon-13 and phosphorus-31 nuclear magnetic resonance studies. J Biol Chem. 1992;267:111–173. [PubMed] [Google Scholar]
- Gout E, Bligny R, Pascal N, Douce R. 13C nuclear magnetic resonance studies of malate and citrate synthesis and compartmentation in higher plant cells. J Biol Chem. 1993;268:3986–3992. [PubMed] [Google Scholar]
- Guern J, Felle HH, Mathieu Y, Kurkdjian A. Regulation of intracellular pH in plant cell. Int Rev Cytol. 1991;127:111–173. [Google Scholar]
- Hill SA, ap Rees T. The effect of anoxia on the control of carbohydrate metabolism in ripening bananas. Planta. 1995;197:313–323. [Google Scholar]
- Keeling PL, Wood JR, Tyson RH, Bridges IG. Starch biosynthesis in developing wheat grain. Plant Physiol. 1988;87:311–319. doi: 10.1104/pp.87.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy RA, Rumpho ME, Fox TC. Anaerobic metabolism in plants. Plant Physiol. 1992;100:1–6. doi: 10.1104/pp.100.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lino B, Baizabal-Aguirre VM, Gonzáles de la Vara LE. The plasma-membrane H+-ATPase from beet root is inhibited by a calcium-dependent phosphorylation. Planta. 1998;204:352–359. doi: 10.1007/s004250050266. [DOI] [PubMed] [Google Scholar]
- Mathieu Y, Guern J, Péan M, Pasquier C, Beloeil JC, Lallemand JY. Cytoplasmic pH regulation in Acer pseudoplatanus cells: II. Possible mechanisms involved in pH regulation during acid load. Plant Physiol. 1986;82:846–852. doi: 10.1104/pp.82.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegus F, Cattaruzza L, Chersi A, Fronza G. Differences in the anaerobic lactate-succinate production and the changes of cell sap pH for plants with high and low resistance to anoxia. Plant Physiol. 1989;90:29–32. doi: 10.1104/pp.90.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegus F, Cattaruzza L, Mattana M, Beffagna N, Ragg E. Response to anoxia in rice and wheat seedlings: changes in the pH of intracellular compartments, glucose-6-phosphate level, and metabolic rate. Plant Physiol. 1991;95:760–767. doi: 10.1104/pp.95.3.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oja V, Savchenko G, Jakob B, Heber U. pH and buffer capacities of apoplastic and cytoplasmic cell compartments in leaves. Planta. 1999;209:239–249. doi: 10.1007/s004250050628. [DOI] [PubMed] [Google Scholar]
- Plaxton WC. The organization and regulation of plant glycolysis. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:185–214. doi: 10.1146/annurev.arplant.47.1.185. [DOI] [PubMed] [Google Scholar]
- Pugin A, Frachisse JM, Tavernier E, Bligny R, Gout E, Douce R, Guern J. Early events induced by the elicitor cryptogein in tobacco cells: involvement of a plasma membrane NADPH oxidase and activation of glycolysis and pentose phosphate pathway. Plant Cell. 1997;9:2077–2091. doi: 10.1105/tpc.9.11.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe RG. Metabolic aspect of the anoxic response in plant tissues. In: Smirnoff N, editor. Environment and Plant Metabolism, Flexibility and Acclimation. Oxford: BIOS Scientific Publishers; 1995. pp. 111–127. [Google Scholar]
- Ratcliffe RG. In vivo NMR studies of the metabolic response of plant tissues to anoxia. Ann Bot Suppl A. 1997;79:39–48. [Google Scholar]
- Raven JA. Biochemical disposal of excess H+ in growing plants? New Phytol. 1986;104:175–206. [Google Scholar]
- Rébeillé F, Bligny R, Douce R. Regulation of Pi uptake by Acer Pseudoplatanus cells. Arch Biochem Biophys. 1982;219:371–378. doi: 10.1016/0003-9861(82)90168-0. [DOI] [PubMed] [Google Scholar]
- Rébeillé F, Bligny R, Douce R. Is the cytosolic Pi concentration a limiting factor for plant cell respiration? Plant Physiol. 1984;74:355–359. doi: 10.1104/pp.74.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rébeillé F, Bligny R, Martin JB, Douce R. Effect of sucrose starvation on sycamore (Acer pseudoplatanus) cell carbohydrate and Pi status. Biochem J. 1985;226:679–684. doi: 10.1042/bj2260679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid RJ, Laughman BC, Ratcliffe RG. 31P NMR measurements of cytoplasmic pH changes in maize root tips. J Exp Bot. 1985;36:889–897. [Google Scholar]
- Rivoal J, Hanson AD. Evidence for a large and sustained glycolytic flux to lactate in anoxic roots of some members of halophytic genus Limonium. Plant Physiol. 1993;101:553–560. doi: 10.1104/pp.101.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivoal J, Ricard B, Pradet A. Glycolytic and fermentative enzyme induction during anaerobiosis in rice seedlings. Plant Physiol Biochem. 1989;27:43–51. [Google Scholar]
- Roberts JKM. Study of plant metabolism in vivo using NMR spectroscopy. Annu Rev Plant Physiol. 1984;35:375–386. [Google Scholar]
- Roberts JKM, Callis J, Wemmer D, Walbot V, Jardetzky O. Mechanism of cytoplasmic pH regulation in hypoxic maize root tips and its role in survival under hypoxia. Proc Natl Acad Sci USA. 1984;81:3379–3383. doi: 10.1073/pnas.81.11.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JKM, Hooks MA, Miaullis AP, Edwards S, Webster C. Contribution of malate and amino acid metabolism to cytoplasmic pH regulation in hypoxic maize root tips studied using nuclear magnetic resonance spectroscopy. Plant Physiol. 1992;98:480–487. doi: 10.1104/pp.98.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JKM, Jardetzky O. Monitoring of cellular metabolism by NMR. Biochim Biophys Acta. 1981;639:53–76. doi: 10.1016/0304-4173(81)90005-7. [DOI] [PubMed] [Google Scholar]
- Roby C, Bligny R, Douce R, Tu SI, Pfeffer P. Facilitated transport of Mn2+ in sycamore (Acer pseudoplatanus) cells and excised maize root tips: a comparative in vivo 31P NMR study. Biochem J. 1988;252:401–408. doi: 10.1042/bj2520401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roby C, Martin JB, Bligny R, Douce R. Biochemical changes during sucrose deprivation in higher plant cells: phosphorus-31 nuclear magnetic resonance studies. J Biol Chem. 1987;262:5000–5007. [PubMed] [Google Scholar]
- Saglio P, Germain V, Ricard B. The response of plants to oxygen deprivation: role of enzyme induction in the improvement of tolerance to anoxia. In: Lerner HR, editor. Plant Responses to Environmental Stresses. New York: Marcel Dekker; 1999. pp. 373–393. [Google Scholar]
- Saint-Gès V, Roby C, Bligny R, Pradet A, Douce R. Kinetic studies of the variation of cytoplasmic pH, nucleotide triphosphate (31P-NMR) and lactate during normoxic and anoxic transitions in maize root tips. Eur J Biochem. 1991;200:477–482. doi: 10.1111/j.1432-1033.1991.tb16207.x. [DOI] [PubMed] [Google Scholar]
- Sedbrook JC, Kronebusch PJ, Borisy GG, Trewavas AJ, Masson PH. Transgenic AEQUORIN reveals organ-specific cytosolic Ca2+ responses to anoxia in Arabidopsis thaliana seedlings. Plant Physiol. 1996;111:243–257. doi: 10.1104/pp.111.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slayman CL. The plasma membrane ATPase of Neurospora crassa: a proton-pumping electroenzyme. J Bioenerg Biomembr. 1987;19:1–20. doi: 10.1007/BF00769728. [DOI] [PubMed] [Google Scholar]
- Stitt M. Fructose-2,6-bisphosphate as a regulatory molecule in plants. Annu Rev Plant Physiol Plant Mol Biol. 1990;41:153–185. [Google Scholar]
- Sturm A. Invertases: primary structures, functions, and roles in plant development, and sucrose partitioning. Plant Physiol. 1999;121:1–7. doi: 10.1104/pp.121.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaiah CC, Bush DS, Sachs MM. Elevation of cytosolic calcium precedes anoxic gene expression in maize suspension cultured cells. Plant Cell. 1994;6:1747–1762. doi: 10.1105/tpc.6.12.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaiah CC, Bush DS, Sachs MM. Contribution to the anoxic Ca2+ signal in maize suspension-cultured cells. Plant Physiol. 1998;118:759–771. doi: 10.1104/pp.118.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H. H+-translocating ATPases of the plasma membrane and tonoplast of plant cells. Physiol Plant. 1984;61:683–691. [Google Scholar]
- Tadege M, Dupuis I, Kuhlemeier C. Ethanolic fermentation: new functions for an old pathway. Trends Plant Sci. 1999;4:320–325. doi: 10.1016/s1360-1385(99)01450-8. [DOI] [PubMed] [Google Scholar]
- Theodorou M, Elrifi IR, Turpin DH, Plaxton WC. Effects of phosphorus limitation on respiratory metabolism in green alga Selenastrum minutum. Plant Physiol. 1991;95:1089–1095. doi: 10.1104/pp.95.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, Joy KW, Turpin DH. Anaerobic metabolism in the N-limited green alga Selenastrum minutum: III. Alanine is the product of anaerobic ammonium assimilation. Plant Physiol. 1991;95:655–658. doi: 10.1104/pp.95.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter H, Robinson DG, Heldt HW. Subcellular volumes and metabolite concentrations in spinach leaves. Planta. 1994;193:530–535. [Google Scholar]
- Xia JH, Roberts JKM. Regulation of H+ extrusion and cytoplasmic pH in maize root tips acclimated to a low-oxygen environment. Plant Physiol. 1996;111:227–233. doi: 10.1104/pp.111.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia JH, Saglio PH. Lactic acid efflux as a mechanism of hypoxic acclimation of maize root tips to anoxia. Plant Physiol. 1992;100:40–46. doi: 10.1104/pp.100.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia JH, Saglio PH, Roberts JKM. Nucleotide levels do not critically determine survival of maize root tips acclimated to a low-oxygen environment. Plant Physiol. 1995;108:589–595. doi: 10.1104/pp.108.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]