Abstract
The question whether sucrose (Suc) is present inside plastids has been long debated. Low Suc levels were reported to be present inside isolated chloroplasts, but these were argued to be artifacts of the isolation procedures used. We have introduced Suc-metabolizing enzymes in plastids and our experiments suggest substantial Suc entry into plastids. The enzyme levansucrase from Bacillus subtilis efficiently synthesizes fructan from Suc. Targeting of this enzyme to the plastids of tobacco (Nicotiana tabacum) and potato (Solanum tuberosum) plants leads to high-level fructan accumulation in chloroplasts and amyloplasts, respectively. Moreover, introduction of this enzyme in amyloplasts leads to an altered starch structure. Expression of the yeast invertase in potato tuber amyloplasts results in an 80% reduction of total Suc content, showing efficient hydrolysis of Suc by the plastidic invertase. These observations suggest that Suc can enter plastids efficiently and they raise questions as to its function and metabolism in this organelle.
Plastids are of tremendous metabolic importance. Next to photosynthesis they are involved in the synthesis of fatty acids, amino acids, starch, and many compounds of secondary metabolism. This diverse metabolic capacity of plastids requires an extensive array of selective transporting systems for interaction with other cellular compartments. Plastids are surrounded by two membranes, the inner and the outer membrane. In the inner membrane of the plastid envelope, many metabolite specific transporters are present, whereas the outer membrane contains non-specific porin-like channels. The envelope outer membrane was proposed to be non-selective and permeable for many small molecules (Heldt and Sauer, 1971). However, recent data suggest that outer membranes can also act as selective and regulated molecular sieves (Flügge, 2000; Neuhaus and Wagner, 2000; Soll et al., 2000).
Several metabolite transporters in plastids have now been identified (Emes and Neuhaus, 1997; Flügge, 1998; Neuhaus and Wagner, 2000). The well-known triose phosphate/phosphate translocator exports the triose phosphates generated by photosynthetic CO2 fixation into the cytosol. The phosphoenolpyruvate/phosphate translocator is responsible for the import of phosphoenolpyruvate into plastids for several plastidic metabolic pathways, like the shikimate pathway or amino acid synthesis (Streatfield et al., 1999). Another phosphate antiporter is the Glc-6-P/phosphate translocator (Naeem et al., 1997; Wischmann et al., 1999). The imported Glc-6-P in amyloplasts can be used for starch biosynthesis or in the oxidative pentose phosphate pathway (Naeem et al., 1997). Next to sugar-phosphates, unphosphorylated carbohydrates like Glc and maltose can be transported (Schleucher et al., 1998) and recently a gene encoding plastidic Glc translocator was identified (Weber et al., 2000). Furthermore, plastids contain transporters involved in ammonia and nitrogen assimilation, transporting Glu, Gln, and oxaloacetate in exchange for malate or Glu. An ADP/ATP translocator is present for the supply of ATP as a driving-force for biosynthetic processes.
Only limited information is available on the subcellular compartmentation of many metabolites in vivo. For plastids such research is mostly focused on the metabolites involved in the pentose phosphate route in chloroplasts or on metabolites involved in starch biosynthesis in amyloplasts like ADP-Glc, Glc-1-P, and Glc-6-P. One metabolite whose presence inside plastids has been debated over the years is Suc. Metabolite localization studies usually show no, or very low, Suc compartmentation to plastids (Heldt and Sauer, 1971; Wang and Nobel, 1971; Heineke et al., 1994). However, significant Suc levels were reported for chloroplasts of frost-hardy cabbage leaves (Santarius and Milde, 1977). Moreover, Suc can be imported in early stages of chloroplast development (Hampp and Schmidt, 1976). Thus, plastid membrane permeability for Suc may depend on environmental and developmental conditions. In these cases it is unknown how Suc enters the plastids, nor whether it plays a role in metabolic processes in these organelles.
The enzyme levansucrase from Bacillus subtilis converts Suc into fructan, a polymer of Fru. This fructosyltransferase catalyzes the synthesis of fructans by transferring the Fru-unit from Suc to a fructosyl-acceptor with concomitant release of Glc (Dedonder, 1966). Expression of this enzyme in different plant species results in the accumulation of fructans, often to high levels (Ebskamp et al., 1994; Caimi et al., 1996; Röber et al., 1996; Turk et al., 1997).
Here we report on high-level fructan accumulation in transgenic tobacco (Nicotiana tabacum) and potato (Solanum tuberosum) harboring a plastid-targeted levansucrase. These fructans accumulate inside chloroplasts and amyloplasts of transgenic tobacco and potato plants, respectively. To further investigate the presence of Suc in plastids we introduced a yeast invertase in the potato tuber amyloplast. The total Suc content of these tubers was reduced up to 80%. These results suggest a substantial Suc entry into plastids.
RESULTS
Fructan Accumulation in Tobacco Chloroplasts
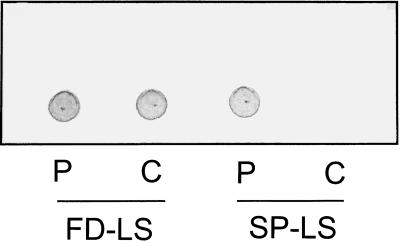
For plastid localization of the B. subtilis levansucrase, the mature sacB gene was fused to the ferredoxin chloroplast targeting sequence. This chimeric construct was placed under the control of the constitutive cauliflower mosaic virus 35S promoter, the alfa alfa mosaic virus translational enhancer, and the nopaline synthase terminator. Tobacco plants were transformed and transformants were identified by selection for kanamycin resistance. Twenty independent 35S-ferredoxin- (FD) LS transformants were grown on soil in the greenhouse. The transgenic tobacco plants showed a different phenotype compared with wild-type (WT) plants. Lower leaves showed early bleaching and necrosis. Fructan levels were determined in the mature leaves and ranged from 0.2% to over 10% of dry weight (data not shown). Levansucrase enzymatic activity was determined in leaf extracts incubated with radioactive 14C-Suc followed by thin-layer chromatography (TLC) separation and autoradiography. Enzymatic activity could be detected in leaves with more than 3% of dry weight fructan, although this enzymatic activity was near the detection limit (results not shown). Four transgenic plants, which accumulated 0.5%, 1.0%, 2.7%, and 10% of dry weight as fructans, respectively, were self-pollinated and the offspring was used for further analysis. Plastidic localization was analyzed by preparing protoplasts from plants containing 2.7% fructan. From these protoplasts, Percoll gradient-purified chloroplasts were isolated and fructan levels were determined. Based on chlorophyll content, equal amounts of fructan were detected in protoplasts and chloroplasts (Fig. 1), indicating a quantitative localization of fructans in plastids. As a control, transgenic 35S-SP-LS tobacco plants were used. Here, levansucrase is fused to the N-terminal 110 amino acids of sweet potato sporamin, which results in fructan accumulation in the endomembrane system (Turk et al., 1997). In these plants fructans were detected in the protoplast, but no signal was observed in the chloroplast extract, showing that fructans as such do not copurify with chloroplasts (Fig. 1). These results show that fructans are present inside the chloroplast and that the transit peptide of ferredoxin targets the levansucrase enzyme to this compartment as was previously observed for a host of other proteins. The accumulation of such high fructan levels (up to 10% of dry weight) in chloroplasts is remarkable since in plastids Suc levels were reported to be absent or very low. Moreover, the Km of levansucrase for Suc is 20 mm (Dedonder, 1966).
Figure 1.
TLC fructan analysis of extracts from protoplasts (P) and Percoll gradient-purified chloroplasts (C) from 35S-FD-LS and 35S-SP-LS tobacco plants. Based on chlorophyll, equal amounts of protoplast and chloroplast extracts were loaded.
Fructan Accumulation in Potato Tuber Amyloplasts
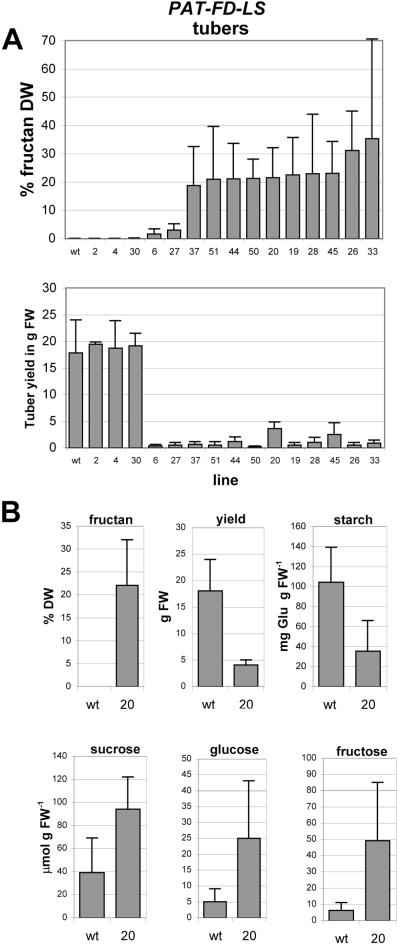
The 35S-FD-LS construct was also introduced in potato plants. The plants were allowed to tuberize and in these tubers the fructan levels varied from 0.1% to 5% dry weight with an average of 2.7%. These results show that also potato plastids contain sufficient substrate for fructan synthesis. In an attempt to restrict expression of amyloplast-targeted levansucrase to potato tubers, the patatin promoter was used (Wenzler et al., 1989). In these PAT-FD-LS plants, up to 35% of dry weight fructan was observed (Fig. 2a), showing that much higher fructan levels can accumulate in potato tubers.
Figure 2.
A, Average fructan content and tuber yield of PAT-FD-LS potato tubers. Presented is the average of a number of plants of each line grown in the greenhouse for 4.5 months. B, Quantitation of neutral sugars, starch, and fructans in tubers of greenhouse-grown potato plants, WT (n = 7) and PAT-FD-LS line 20 (n = 5).
Sugar analysis of line PAT-FD-LS-20 with a tuber yield of 4 ± 1 g and a tuber fructan content of approximately 22% of dry weight revealed that tuber starch content was reduced, whereas Suc, Glc, and Fru levels were elevated (Fig. 2b). Thus, elevated sugar levels were detected in these fructan-accumulating potato plants as previously observed for endomembrane-targeted levansucrase (Turk et al., 1997).
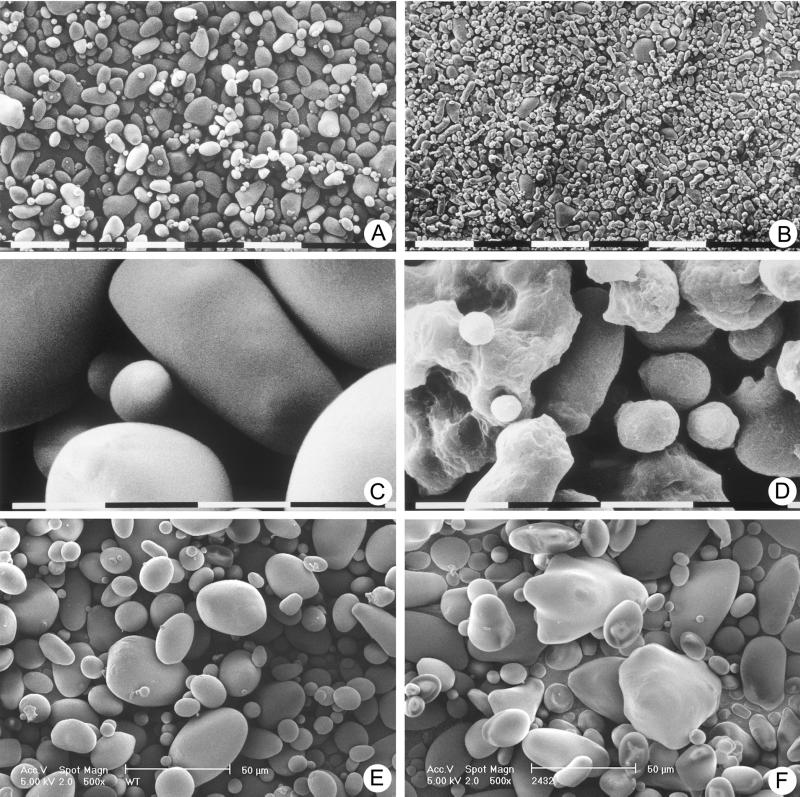
In the 35S-FD-LS and the PAT-FD-LS potato plants, starch granule morphology was affected. In Figure 3, a through d, starch granules derived from 35S-FD-LS tubers are shown compared with those of WT using scanning electron microscopy (SEM). Potato plants accumulating fructan in the endomembrane system (Turk et al., 1997) did not show an effect on granule morphology. Thus, the altered granule morphology is specific for plants harboring a plastid-targeted levansucrase. It is most likely due to the presence of fructan in the starch granules (N. Gerrits, unpublished data).
Figure 3.
SEM analysis of starch granules from potato tubers at magnifications of 150× (A and B), 2,400× (C and D), and 500× (E and F). Granules were isolated from WT tubers (A, C, and E) and 35S-FD-LS tubers containing 4.8% of dry weight fructan (B and D) and FD-INV tubers line 24–32 (F). The bar segments represent lengths of 100 μm (A and B), 10 μm (C and D), or 50 μm (E and F).
Invertase Activity in Tuber Amyloplasts
Levansucrase and the fructan produced could in theory affect membrane transport properties. Therefore, another Suc-hydrolyzing activity was introduced in plastids. For this, the invertase of Saccharomyces cerevisiae was used. This invertase was introduced into potato plastids using the FD plastid-targeting signal. The FD-invertase (FD-INV) construct was fused to the tuber-specific patatin promoter and introduced into potato (var. Kardal). To test for plastid localization of the yeast invertase protein, chloroplasts were isolated from greenhouse-grown potato leaves of transgenic line 24–31. The leakiness of the patatin promoter in leaves was already observed in fructan accumulating lines and also in FD-INV lines, elevated invertase activity was observed in leaves. In two independent experiments invertase activity was determined by measuring Glc release from Suc (Cairns, 1987). The total invertase activity present in leaves of the transgenic plants was 3.6 ± 0.7 nmol Glu min−1 mg−1 chlorophyll versus 1.7 ± 0.3 nmol Glu min−1 mg−1 chlorophyll in WT. In purified intact chloroplasts the invertase activity was 2.9 ± 0.4 nmol Glu min−1 mg−1 chlorophyll, representing 80% of the total invertase activity in leaves and suggesting a quantitative localization of the FD-INV protein to plastids.
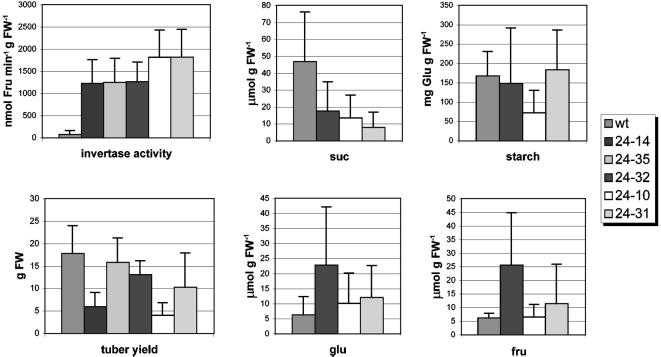
Tubers were harvested from greenhouse-grown plants after four and one-half months and were analyzed. The invertase activity detected in the transgenic tubers varied between 1,263 ± 444 nmol min−1 g−1 fresh weight for line 24–32 and 1,818 ± 616 nmol min−1 g−1 fresh weight for line 24–31, representing an invertase activity of 27 times the WT level (approximately 67 nmol min−1 g−1 fresh weight). In some invertase lines the tuber yield was reduced (Fig. 4), but no correlation was found between invertase activity and tuber yield.
Figure 4.
Analysis of potato tubers of PAT-FD-INV lines with an elevated average invertase activity in the amyloplast (line 24–14 [n = 8], line 24–35 [n = 8], line 24–32 [n = 8], line 24–10 [n = 6], and line 24–31 [n = 7]) compared with WT (n = 7) tubers. Tubers were harvested after growth for 4.5 months in the greenhouse.
Reduced Suc Content in PAT-FD-INVTubers
Three lines were selected for further analysis. Two lines with a tuber yield comparable with WT: line 24–32 with the lowest invertase activity (1,263 ± 444 nmol min−1 g−1 fresh weight) and line 24–31 with the highest invertase activity (1,818 ± 616 nmol min−1 g−1 fresh weight). The third line selected, line 24–10, showed a reduced tuber yield and an invertase activity comparable with line 24–31 (1,813 ± 635 nmol min−1 g−1 fresh weight).
The sugar content of the tubers was analyzed using HPLC. In all transgenic tubers total Suc content was reduced compared with WT (47 ± 29 μmol g−1 fresh weight). Line 24–31, showing the highest invertase activity, has the lowest Suc content of approximately 8 μmol g−1 fresh weight. The Suc content of lines 24–10 and 24–32 are approximately 14 and 18 μmol g−1 fresh weight, respectively (Fig. 4). The Suc extraction and quantification procedures were tested by adding a known amount of Suc to WT and invertase tuber slices before extraction. In these tests no hydrolysis of added Suc during extraction was detected (data not shown), confirming that the invertase enzymatic activity is properly inactivated during the extraction procedure and does not affect the Suc concentration measured. Thus, a reduction of total Suc content in the transgenic tubers up to 80% correlates with the elevated invertase activity detected in transgenic tubers.
In line 24–32 variability was observed in Glc and Fru content (Fig. 4). However, the level of monosaccharides in the tubers of 24–10 and 24–31 are comparable with WT. This implies that the monosaccharides released during the hydrolysis of Suc by yeast invertase are efficiently shuttled back into intermediary metabolism. The starch levels in line 24–31 (183 ± 102 mg Glu g−1 fresh weight) and 24–32 (148 ± 144 mg Glu g−1 fresh weight) are comparable with WT (167 ± 63 mg Glu g−1 fresh weight). Only in line 24–10 was the starch content reduced to 73 ± 58 mg Glu g−1 fresh weight (Fig. 4). From these results we conclude that reduced tuber Suc levels does not significantly affect tuber starch content.
SEM studies were performed to study the effect of invertase activity in the amyloplast on starch granule morphology. Starch was isolated from different invertase lines, but only results of line 24–32 are presented (Fig. 3, e and f). The granules appeared to be more angular compared with WT granules, but the surface of the granules is smooth like WT.
DISCUSSION
Introduction of the FD-LS construct in tobacco results in high-level fructan accumulation reaching 10% of dry weight in leaves. The fructan was localized to the plastids, confirming correct targeting of levansucrase. This high fructan accumulation is remarkable since it implies that sufficient Suc must be available in plastids. Plastidic Suc concentrations were reported to be very low and the observation that fructans accumulate to high levels suggests a continuous Suc influx into these organelles. Suc is probably the major fructosyl donor in tobacco chloroplasts, but we cannot exclude the possibility that other compounds also play such a role. For example, sugars like raffinose can also act as fructosyl donor for levansucrase in vitro, but to our knowledge there are no reports to suggest the presence of such sugars in plastids. No in vitro fructan synthesis by levansucrase was detected with Fru-6-P as a possible fructosyl donor, even in the presence of low Suc concentrations for priming (data not shown).
The yeast invertase was targeted to the plastid using the same FD plastid-targeting sequence as has been used for levansucrase. It is remarkable that this amyloplast-targeted yeast invertase led up to an 80% reduction of total tuber Suc content. From literature it is known that cytosolic or apoplastic expression of yeast invertase in potato led to an over 90% reduction in Suc. In these cytosolic and apoplastic lines invertase activity was elevated to 19 and 66 times WT level, respectively (Sonnewald et al., 1997), compared with up to 27 times WT level for amyloplast-targeted invertase. Such a large reduction of total Suc content by an amyloplast-targeted invertase implies that the entry of Suc into the amyloplast is an efficient process. This entry rate is sufficient to lower the cytosolic Suc content, suggesting that Suc is readily taken up by plastids. If Suc is taken up by WT plastids to the same extent it must be metabolized or exported somehow, since no Suc accumulates in plastids of WT plants. Until now, no Suc transporters have been localized to the plastid envelopes, so it is unclear how Suc enters plastids.
It is unlikely that there is significant yeast invertase activity in the cytosol during transport to the amyloplast since in potato tubers with cytosolically expressed invertase, starch content and tuber yield are reduced and the tuber number is increased (Sonnewald et al., 1997). The amyloplast-targeted invertase does not result in a reduction of starch content of tubers and total yield and tuber number are unaltered; thus, the contribution of a possible cytosolic activity is not likely to be significant. Suc was proposed to signal starch accumulation in tubers (Geiger et al., 1998) and seeds (Weber et al., 1998). It is interesting that in our study low Suc and normal starch levels are detected, suggesting that induction of starch biosynthetic enzymes does not involve cytosolic Suc.
It is unclear why tuber yield in the fructan-accumulating potato lines is reduced. The signal for tuber initiation and tuber filling is presently unknown. Suc levels are elevated in fructan accumulating lines, indicating that there is sufficient substrate for starch accumulation even in lines accumulating high levels of fructan. Moreover, normal starch levels and tuber yield can be observed in the invertase lines with reduced Suc levels. Thus, Suc availability seems not to reduce tuber yield. A reduced tuber yield is also observed when fructans accumulate in other cellular compartments (Pilon-Smits et al., 1996).
Plastidic levansucrase and invertase affect starch granule morphology. Granules isolated from FD-LS lines are small and irregular compared with smooth and oval-shaped WT granules. The starch granules from FD-INV plants show a triangular morphology compared with WT. These results show that levansucrase and invertase affect granule morphology differently when targeted to plastids. Further analysis showed that starch isolated from FD-LS plants possesses altered physical properties (N. Gerrits and J. Vincken, unpublished data).
Suc hydrolysis by invertase produces Glc and Fru, but elevated levels of these monosaccharides were not observed. It is apparent that there is efficient shuttling of the released monosaccharides in intermediary metabolism. Glc is known to stimulate respiration and this results in decreased starch accumulation (Geiger et al., 1998; Trethewey et al., 1998). We did not observe such a reduction in starch and most likely, the monosaccharides are used somehow in the starch biosynthetic pathway. Hexokinases and fructokinases were reported to be associated with plastids, but it is unclear whether these enzymes are present inside these organelles (Stitt et al., 1978; Schnarrenberger, 1990). An outer membrane associated hexokinase was recently reported (Wiese et al., 1999).
Our findings suggest that there must be a substantial Suc flux into chloroplasts and amyloplasts. More research is necessary to determine the rate and mechanism of Suc entry into plastids and the way in which Suc is metabolized in this organelle. Moreover, it raises the question as to a possible physiological and metabolic role of Suc in this organelle.
MATERIAL AND METHODS
Generation of 35S-FD-LS and 35S-SP-L Plants
Plasmid pSTU94 was constructed by cloning the 0.5-kb NcoI-BamHI fragment encoding the FD peptide from Silene pratensis from pETFD100 (Pilon et al., 1995) into NcoI-BglII-digested vector pMTL22 (Chambers et al., 1988). PSTU42 (Turk et al., 1997), containing the sacB gene from Bacillus subtilis that was digested with NcoI, blunted with mung bean nuclease, digested with XhoI, and the 1.9-kb fragment was cloned in the Eco47III-XhoI-digested vector pSTU94 to yield pSTU113. A 1.6-kb NcoI-BamHI fragment encoding the FD-levansucrase hybrid protein was cloned in the NcoI-BamHI-digested vector pPA2 (Turk et al., 1997) to yield pSTU176. A 3.0-kb SmaI-XhoI fragment was subsequently cloned in a pBin19-derived binary vector (Bevan, 1984; Frisch et al., 1995) to yield pSTU192. The construction of pSTU134 encoding the sporamin-levansucrase hybrid protein was described previously (Turk et al., 1997).
Plasmids pSTU134 and pSTU192 were transformed into Agrobacterium tumefaciens strain LBA4404 using electroporation (Mattanovich et al., 1989) and introduced into tobacco (Nicotiana tabacum var. Samsun NN) and potato (Solanum tuberosum var. Kardal) using the leaf disc (Horsch et al., 1985) and the shoot transformation method (Visser, 1991), respectively. Regenerated plants named 35S-SP-LS (pSTU134) and 35S-FD-LS plants (pSTU192) were selected for kanamycin resistance and were grown on Murashige and Skoog medium (Murashige and Skoog, 1962).
Generation of PAT-FD-LS and PAT-FD-INV Plants
Plasmid pPFL19 was constructed by cloning the NcoI-SalI fragment from pSTU 113 containing the FD plastid-targeting signal fused to the gene encoding for levansucrase and the nopaline synthase terminator into the NcoI-SalI-digested vector pMOG1139 (MOGEN International, Leiden, The Netherlands) containing the patatin promoter. The XhoI-SalI fragment from pPFL19 was cloned in a pBIN19-derived binary vector (Bevan, 1984; Frisch et al., 1995) to yield pPFL21.
Plasmid pPFGI-23 was constructed by cloning the BstEII-SalI fragment from pJK-6 into BstEII-SalI-digested vector pPFL-19 to place the construct under control of the patatin promoter. The BstEII-SalI fragment encodes part of the FD plastid-targeting signal (S. pratensis) derived from pETFD100 (Pilon et al., 1995) fused with the Eco47III site to the second amino acid (Thr at position 848 of the sequence) of yeast invertase (SUC2 gene) and the terminator Tnos at the BamHI site introduced at position 2,456 in the invertase sequence. The XhoI-SalI fragment from pPFGI-23 encoding for the plastid targeted yeast invertase under control of the patatin promoter and the nopaline synthase terminator was cloned into a pBIN19-derived binary vector (Bevan, 1984; Frisch et al., 1995) to yield pPFGI-24.
The plasmids pPFL-21 and pPFGI-24 were transformed into A. tumefaciens strain LBA4404 using electroporation (Mattanovich et al., 1989) and introduced into potato (var. Kardal) using the shoot transformation method (Visser, 1991). Regenerated plants named PAT-FD-LS (pPFL-21) and PAT-FD-INV (pPFGI-24) were selected for kanamycin resistance and were grown on Murashige and Skoog medium (Murashige and Skoog, 1962).
Isolation of Tobacco Protoplasts and Chloroplasts
Isolation of tobacco protoplasts was done as described previously (Turk et al., 1997). Protoplasts (5 × 106) were resuspended in 3 mL of chloroplast isolation buffer (0.33 m sorbitol, 2 mm EDTA, 1 mm MnCl2, 1 mm MgCl2, 0.1% [w/v] fatty acid-free bovine serum albumin, 1% [v/v] Percoll, and 50 mm HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid]/KOH, pH 7.3) and the protoplasts were lysed by forcing the suspension through a 14-μm filter. Intact chloroplasts were isolated by separating the organelles on a continuous Percoll gradient as described (Cline et al., 1985).
Fructans were isolated by lysing the chloroplasts in 100 μL of sterile water at room temperature followed by centrifugation at 12,000 g for 5 min. This extraction procedure was performed three times and the supernatants were combined.
Isolation of Potato Chloroplasts
Leaf material was harvested and incubated in the dark on water overnight at room temperature to reduce starch content. The veins were removed and the leaves were cut into small slices in grinding buffer containing 50 mm HEPES/KOH, pH 7.3, 0.33 m sorbitol, 1 mm MgCl2, 1 mm MnCl2, 2 mm EDTA, and 0.1% (w/v) fatty acid-free bovine serum albumin. After homogenizing using a polytron, the suspension was filtered through Miracloth (Calbiochem, La Jolla, CA) and was centrifuged for 3 min at 1,500g. The pellet was suspended in 1 to 2 mL of grinding buffer and put on a pre-formed Percoll gradient (40% [v/v] Percoll, 60 min at 27,000g, 4°C, brake off) and centrifuged for 10 min at 5,000 rpm in a HB4 rotor (Swing out) with rotorbrake off. The intact chloroplasts were isolated and 1 volume of grinding buffer was added before pelleting the chloroplast at 12,000g for 3 min. The chloroplasts were washed with 1 mL of grinding buffer at 1,000 rpm for 1 min in the Eppendorf centrifuge. Invertase activity was determined by adding 180 μL of 0.1 m Suc in 0.02 m NaAc, pH 4.7, to a 20-μL sample and incubating at 30°C for 30 min followed by inactivating at 95°C for 5 min. Glc was measured using a colorimetric assay (Cairns, 1987). Invertase activity was calculated on a chlorophyll base. Chlorophyll was determined by using 5 to 10 μL of leaf extract or purified chloroplasts suspended in 200 μL of water. After adding 800 μL of 100% (w/v) acetone the suspension was mixed and centrifuged for 5 min at 12,000g. Chlorophyll amount was determined at 652 nm (Bruinsma, 1961).
Potato Tuber Invertase Assay
Fifty to 100 mg of plant material was homogenized in 25 μL of invertase buffer (50 mm HEPES-KOH, pH 7.4, 5 mm MgCl2, 0, 1% [v/v] Triton X-100, and 10% [v/v] glycerol) and was centrifuged at 12,000g for 5 min at 4°C. Ten microliters of supernatant was incubated with 90 μL of a fresh solution of 100 mm Suc in 20 mm NaAc, pH 4.7, at 30°C for 30 min. The solution was inactivated at 95°C for 3 min and was spotted on silica gel TLC foils (Schleicher & Schuell, Dassel, Germany). The TLC was developed three times in 90:10 acetone:water and was stained with a Fru-specific urea-phosphoric spray as described by Wise et al. (1955). The Fru spot was quantified by scanning the urea spray-stained TLC foil using the Pharmacia Biotech Imagemaster VDS (San Francisco).
Isolation of Starch, Fructans, and Other Nonstructural Carbohydrates
Fructan and other soluble carbohydrates were isolated and quantified as described (Ebskamp et al., 1994; Turk et al., 1997). For starch isolation potato tubers were ground in extraction buffer (50 mm Tris-HCl, pH 7.4, 10 mm EDTA, 1 mm NaS2O5, and 1 mm dithiothreitol) and the homogenate was filtered through two layers of Miracloth (Calbiochem-Novabiochem). Starch granules were allowed to sediment at 4°C by gravity flow for 48 h. The supernatant was removed and the granules were washed twice with two volumes of extraction buffer, twice with water, and once with acetone. In between the washing steps the granules were allowed to sediment for 24 h. This material was used for SEM.
For the determination of soluble sugar in transgenic invertase plants, ±100 mg of plant material was extracted with 80% (v/v) ethanol at 80°C for 30 min in a total volume of 1 mL. After spinning for 5 min at 12,000g the supernatant was evaporated in a speedvac and resolved in 1 mL of water. For HPLC detection the samples were treated with ion-exchange resin (AG 501-X8, Bio-Rad, Hercules, CA)/PVP (Merck, Darmstadt, Germany) 2:1 (w/w), and was filtered through a 0.22-μm filter. The samples were analyzed on an Aminex HPX-87C column using water as an eluent.
Starch Determination
Plant material (± 200 mg of leaf or tuber) was extracted three times with 300 μL of water. The pellet was dissolved in 2 mL of dimethyl sulfoxide and 0.5 mL of 9.25% (v/v) HCl at 60°C for 1 h. After incubation the mixture was neutralized with 10 n NaOH and diluted in 0.1 m citrate buffer (pH 4.6) to a final volume of 10 mL. Five microliters of the hydrolyzed starch sample was incubated with 5 units of amyloglucosidase (Boehringer Mannheim, Basel) in a final volume of 250 μL in 0.1 m citrate buffer (pH 4.6) overnight at 37°C. Glc was determined as described in Cairns (1987).
ACKNOWLEDGMENTS
The authors would like to thank W.A.M. van Maurik for SEM on starch granules and Dr. Ted Slaghek of the Agrotechnological Research Institute for helpful discussions.
Footnotes
This work was financially supported by the Ministry of Economic Affairs, by the Ministry of Education, Culture, and Science, and by the Ministry of Agriculture, Nature Management, and Fishery in the framework of an industrial relevant research program of the Netherlands Association of Biotechnology Centers in the Netherlands. S.T. was financially supported by the European Union-Food and Agro-Industrial Research (program no. PL–96–1896).
LITERATURE CITED
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acid Res. 1984;12:8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruinsma J. A comment on the spectrophotometric determination of chlorophyll. Biochim Biophys Acta. 1961;52:576–578. doi: 10.1016/0006-3002(61)90418-8. [DOI] [PubMed] [Google Scholar]
- Caimi PG, McCole LM, Klein TM, Kerr PS. Fructan accumulation and sucrose metabolism in transgenic maize endosperm expressing a Bacillus amyloliquefaciens SacB gene. Plant Physiol. 1996;110:355–363. doi: 10.1104/pp.110.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns AJ. Colorimetric microtiter plate assay of glucose and fructose by enzyme-linked formazan production: applicability to the measurement of fructosyl transferase activity. Anal Biochem. 1987;167:270–278. doi: 10.1016/0003-2697(87)90163-1. [DOI] [PubMed] [Google Scholar]
- Chambers SP, Prior SE, Barstow DA, Minton NP. The pMTL nic-cloning vectors: I. Improved pUC polylinker regions to facilitate the use of sonicated DNA for nucleotide sequencing. Gene. 1988;68:139–149. doi: 10.1016/0378-1119(88)90606-3. [DOI] [PubMed] [Google Scholar]
- Cline K, Werner-Washburne M, Lubben T, Keegstra K. Precursors to two nuclear-encoded chloroplast proteins bind to the outer envelope membrane before being imported into chloroplasts. J Biol Chem. 1985;260:3691–369. [PubMed] [Google Scholar]
- Dedonder R. Levansucrase from Bacillus subtilis. In: Neufeld EF, Ginsburg V, editors. Methods in Enzymology. New York: Academic Press; 1966. pp. 500–505. [Google Scholar]
- Ebskamp MJM, van der Meer IM, Spronk BA, Weisbeek PJ, Smeekens SCM. Accumulation of fructose polymers in transgenic tobacco. BioTechnology. 1994;12:272–274. doi: 10.1038/nbt0394-272. [DOI] [PubMed] [Google Scholar]
- Emes MJ, Neuhaus HE. Metabolism and transport in non-photosynthetic plastids. J Exp Bot. 1997;48:1995–2005. [Google Scholar]
- Flügge U. Metabolite transporters in plastids. Curr Opin Plant Biol. 1998;1:201–206. doi: 10.1016/s1369-5266(98)80105-2. [DOI] [PubMed] [Google Scholar]
- Flügge U. Transport in and out of plastids: does the outer envelope membrane control the flow? Trends Plant Sci. 2000;5:135–137. doi: 10.1016/s1360-1385(00)01578-8. [DOI] [PubMed] [Google Scholar]
- Frisch DA, Harris-Haller LW, Yokubaitis NT, Thomas TL, Hardin SH, Hall TC. Complete sequence of the binary vector Bin19. Plant Mol Biol. 1995;27:405–409. doi: 10.1007/BF00020193. [DOI] [PubMed] [Google Scholar]
- Geiger M, Stitt M, Geigenberger P. Metabolism in slices from growing potato tubers responds differently to addition of sucrose and glucose. Planta. 1998;206:234–244. [Google Scholar]
- Hampp R, Schmidt HW. Changes in envelope permeability during chloroplast development. Planta. 1976;129:69–73. doi: 10.1007/BF00390916. [DOI] [PubMed] [Google Scholar]
- Heineke D, Wildenberger K, Sonnewald U, Willmitzer L, Heldt HW. Accumulation of hexoses in leaf vacuoles: studies with transgenic tobacco plants expressing yeast-derived invertase in the cytosol, vacuole or apoplasm. Planta. 1994;194:29–33. [Google Scholar]
- Heldt HW, Sauer F. The inner membrane of the chloroplast envelope as site of specific metabolite transport. Biochim Biophys Acta. 1971;234:83–91. doi: 10.1016/0005-2728(71)90133-2. [DOI] [PubMed] [Google Scholar]
- Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT. A simple and general method for transferring genes into plants. Science. 1985;227:1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Mattanovich D, Rüker F, da Camara Machado A, Laimer M, Reguer F, Steinkellner H, Himmler G, Katinger H. Efficient transformation of Agrobacterium spp. by electroporation. Nucleic Acids Res. 1989;17:6747. doi: 10.1093/nar/17.16.6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Naeem M, Tetlow IJ, Emes MJ. Starch synthesis in amyloplasts purified from developing potato tubers. Plant J. 1997;11:1095–1103. [Google Scholar]
- Neuhaus HE, Wagner R. Solute pores, ion channels, and metabolite transporters in the outer and inner envelope membranes of higher plant plastids. Biochim Biophys Acta. 2000;1465:307–323. doi: 10.1016/s0005-2736(00)00146-2. [DOI] [PubMed] [Google Scholar]
- Pilon M, Wienk H, Sips W, de Swaaf M, Talboom I, van't Hof R, de Korte-Kool G, Demel R, Weisbeek P, de Kruijff B. Functional domains of the ferredoxin transit sequence involved in chloroplast import. J Biol Chem. 1995;270:3882–3893. doi: 10.1074/jbc.270.8.3882. [DOI] [PubMed] [Google Scholar]
- Pilon-Smits EAH, Ebskamp MJM, Jeuken MJW, van der Meer IM, Visser RGF, Weisbeek PJ, Smeekens JCM. Microbial fructan production in transgenic potato plants and tubers. Industrial Crops Prod. 1996;5:35–46. [Google Scholar]
- Röber M, Geider K, Müller-Röber B, Willmitzer L. Synthesis of fructans in tubers of transgenic starch-deficient potato plants does not result in an increased allocation of carbohydrates. Planta. 1996;199:528–536. doi: 10.1007/BF00195183. [DOI] [PubMed] [Google Scholar]
- Santarius KA, Milde H. Sugar compartmentation in frost-hardy and partially deharded cabbage leaf cells. Planta. 1977;136:163–166. doi: 10.1007/BF00396193. [DOI] [PubMed] [Google Scholar]
- Schleucher J, Vanderveer PJ, Sharkey TD. Export of carbon from chloroplasts at night. Plant Physiol. 1998;118:1439–1445. doi: 10.1104/pp.118.4.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnarrenberger C. Characterization and compartmentation in green leaves, of hexokinases of different specificities of glucose, fructose, and mannose and for nucleoside triphosphates. Planta. 1990;181:249–255. doi: 10.1007/BF02411547. [DOI] [PubMed] [Google Scholar]
- Soll J, Böltner B, Wagner R, Hinnah SC. The chloroplast outer envelope: a molecular sieve? Trends Plant Sci. 2000;5:137–138. doi: 10.1016/s1360-1385(00)01579-x. [DOI] [PubMed] [Google Scholar]
- Sonnewald U, Hajirezaei M, Kossmann J, Heyer A, Tretheway RN, Willmitzer L. Increased potato tuber size resulting from apoplastic expression of a yeast invertase. Nat Biotechnol. 1997;15:794–797. doi: 10.1038/nbt0897-794. [DOI] [PubMed] [Google Scholar]
- Stitt M, Bulpin PV, ap Rees T. Pathway of starch breakdown in photosynthetic tissues of Pisum sativum. Biochim Biophys Acta. 1978;344:200–214. doi: 10.1016/0304-4165(78)90223-4. [DOI] [PubMed] [Google Scholar]
- Streatfield SJ, Weber A, Kinsman EA, Häusler RE, Li J, Post-Beittenmiller D, Kaiser WM, Pyke KA, Flügge U, Chory J. The phosphoenolpyruvate/phosphate translocator is required for phenolic metabolism, palisade cell development, and plastid-dependent nuclear gene expression. Plant Cell. 1999;11:1609–1621. doi: 10.1105/tpc.11.9.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trethewey RN, Geigenberger P, Riedel K, Hajirezaei M, Sonnewald U, Stitt M, Riesmeier JW, Willmitzer L. Combined expression of glucokinase and invertase in potato tubers leads to a dramatic reduction in starch accumulation and a stimulation of glycolysis. Plant J. 1998;15:109–118. doi: 10.1046/j.1365-313x.1998.00190.x. [DOI] [PubMed] [Google Scholar]
- Turk SCHJ, de Roos K, Scotti P, Weisbeek P, Smeekens SCM. The vacuolar sorting domain of sporamin transports GUS, but not levansucrase, to the plant vacuole. New Phytol. 1997;136:29–38. [Google Scholar]
- Visser RGF. Regeneration and transformation of potato by Agrobacterium tumefaciens. In: Lindsey K, editor. Plant Culture Manual B5. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1991. pp. 1–9. [Google Scholar]
- Wang CT, Nobel PS. Permeability of pea chloroplasts to alcohols and aldoses as measured by reflection coefficients. Biochim Biophys Acta. 1971;241:200–212. doi: 10.1016/0005-2736(71)90317-8. [DOI] [PubMed] [Google Scholar]
- Weber A, Servaites JC, Geiger DR, Kofler H, Hille D, Gröner F, Hebbeker U, Flügge U. Identification, purification and molecular cloning of a putative plastidic glucose translocator. Plant Cell. 2000;12:787–801. doi: 10.1105/tpc.12.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H, Heim U, Golombek S, Borisjuk L, Manteuffel R, Wobus U. Expression of a yeast-derived invertase in developing cotyledons of Vicia narbonensis alters the carbohydrate state and affects storage functions. Plant J. 1998;16:163–172. doi: 10.1046/j.1365-313x.1998.00282.x. [DOI] [PubMed] [Google Scholar]
- Wenzler HC, Mignery A, Fisher LM, Park WD. Analysis of a chimeric class-I patatin-GUS gene in transgenic potato plants: high-level expression in tubers and sucrose-inducible expression in cultured leaf and stem explants. Plant Mol Biol. 1989;12:41–50. doi: 10.1007/BF00017446. [DOI] [PubMed] [Google Scholar]
- Wiese A, Gröner F, Sonnewald U, Deppner H, Lerchl J, Hebbeker U, Flügge U, Weber A. Spinach hexokinase I is located in the outer envelope membrane of plastids. FEBS Lett. 1999;461:13–18. doi: 10.1016/s0014-5793(99)01417-9. [DOI] [PubMed] [Google Scholar]
- Wischmann B, Nielsen TH, Møller BL. In vitro biosynthesis of phosphorylated starch in intact potato amyloplasts. Plant Physiol. 1999;119:455–462. doi: 10.1104/pp.119.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise C, Dimler RJ, Davis HA, Rist CE. Determination of easily hydrolyzable fructose units in dextran preparations. Anal Biochem. 1955;27:33–36. [Google Scholar]