Abstract
Patient: Male, 58
Final Diagnosis: Inferior pancreaticoduodenal artery aneurysm
Symptoms: Bleding
Medication: —
Clinical Procedure: Pancreaticoduodenectomy
Specialty: Surgery
Objective:
Rare disease
Background:
Aneurysm of the inferior pancreaticoduodenal artery (IPDA) is rare among visceral artery aneurysms. Aneurysm and/or pancreatitis may have a causal relation with hemosuccus pancreaticus (HP). HP causes an obscure bleeding in the digestive tract, and this rare disease may lead to life-threatening condition. Although interventional radiology is generally employed as the initial treatment for visceral aneurysms, aneurysmic recanalization is a critical problem.
Case Reports:
A 58-year-old male was incidentally diagnosed as groove pancreatitis, and his pancreatitis was successfully treated by conservative management. One year later, an IPDA aneurysm was detected in image studies. Gastrointestinal bleeding was objectively observed, and a diagnosis of asymptomatic HP was made. Arteriopancreatic duct fistula was suspected, but was not identified. Coil embolization was successfully completed. Six months later, he suffered a relapse of HP, and visited our emergency unit. Pseudocystic lesion around metallic coils were confirmed. Subtotal stomach-preserving pancreaticoduodenectomy without any extended resections was performed. Intentional dissections of nerve plexuses and lymph nodes were all waived. Even a pancreatography of the resected specimen did not clarify his arterio-pancreatic duct fistula. He was discharged at postoperative day 10, and smoothly returned to his work.
Conclusions:
Pancreatic juice-related complications after advanced pancreaticoduodenectomy for malignancies are often intractable. However, simple pancreaticoduodenectomy which omits extended resections and intentional dissections is safe and feasible for benign diseases. After the initial interventional radiology for pancreatic aneurysms, an elective pancreatic surgery should be considered to avoid unwanted recanalization and refractory HP.
MeSH Keywords: Aneurysm; General Surgery; Hemorrhage; Pancreaticoduodenectomy; Pancreatitis; Radiology, Interventional
Background
Visceral artery aneurysm is the third most common intra-abdominal aneurysm after aneurysms of the aortic and iliac arteries [1]. However, visceral artery aneurysm is rare, and the incidence is 0.1% to 0.2% of the population [2,3]. The splenic artery is the most frequent site of aneurysm, and splenic artery aneurysm accounts for approximately 60% of the frequency in visceral artery aneurysms [4]. The second major site of visceral artery aneurysms is the hepatic artery (approximately 20%) [4], and only a certain percentage (approximately <5%) of visceral artery aneurysms occur at the superior mesenteric, celiac, pancreaticoduodenal or gastroduodenal artery [4]. The inferior pancreaticoduodenal artery (IPDA) is considered a rare site for visceral artery aneurysms.
Hemosuccus pancreaticus (HP) is an extremely rare disease, and the frequency has been documented as 0.07% of gastrointestinal bleeding [5]. Sandblom first proposed the term “HP” in 1970 [6], and Longmire and Rose used the term “hemoductal pancreatitis” in 1973 [7]. The HP is defined as bleeding from the ampulla of Vater via the main pancreatic duct [5]. However, making a diagnosis of HP and a definitive identification of an arterio-pancreatic duct fistula (APDF) is difficult [5]. Determining the cause of obscure bleeding in the digestive tract is crucial for successful treatment of HP [5] because HP will lead to massive gastrointestinal bleeding and subsequently a life-threatening condition [5].
As the initial treatment for visceral artery aneurysm, interventional radiology including coil embolization has an acceptable successful rate [8]. However, aneurysmic recanalization during long-term after coil embolization is a critical problem. Aneurysm which repeated HP even after coil embolization had been previously documented [9,10]. Here, in this case report, a thought-provoking case of an IPDA aneurysm which appeared after groove pancreatitis and repeated HP even after coil embolization is reported in detail. We also discuss an indication of surgical approach for arterial aneurysm of the pancreatic artery.
Case Report
A 58-year-old male presented with past history of Mallory-Weiss syndrome, early gastric cancer, and lung adenocarcinoma. Follow-up computed tomography incidentally detected a hypovascular soft tissue in the groove area (Figure 1A). A diagnosis of groove pancreatitis was made, and thereafter, his pancreatitis was successfully treated. However, 1 year later, an artery aneurysm (15 mm in size) was detected in the pancreas head, and the IPDA mainly supplied arterial flow into this aneurysm (Figure 1B, 1C). Iron-deficient anemia and black stool were observed, and a diagnosis of HP was made, though endoscopic examination did not clarify active bleeding. Angiographic finding revealed that the patient’s IPDA aneurysm partially communicated with the gastroduodenal artery (Figures 1D, 2A). Obscure APDF was suspected, but was not identified. Arterial embolization by metallic coils was successfully completed (Figure 2B, 2C), and no blood supply into his aneurysm was confirmed by angiographies not only from the superior mesenteric artery (SMA) but also from the celiac artery (CA).
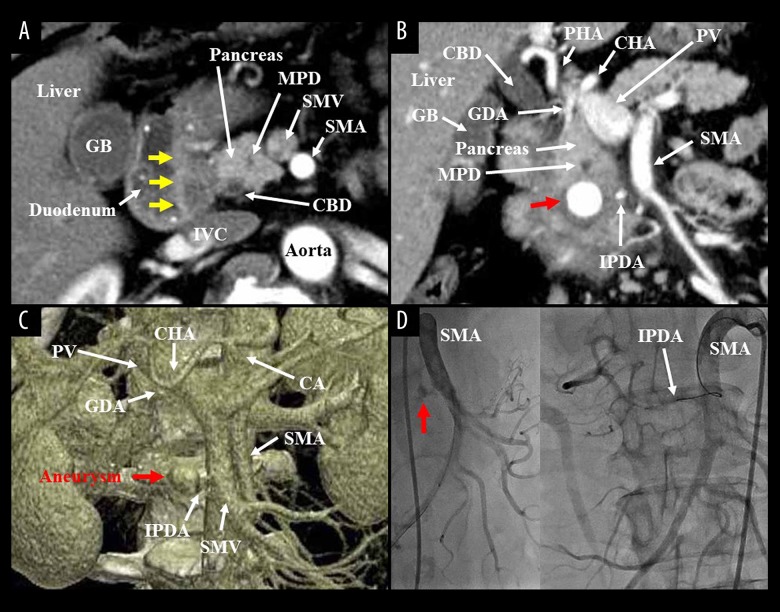
Figure 1.
(A) Computed tomography incidentally detected a hypovascular soft tissue in the groove area (yellow arrows), and a diagnosis of groove pancreatitis was made. Axial image was shown. (B, C) One year after pancreatitis treatment, an artery aneurysm (15 mm in size) was detected in the pancreas head (red arrow). Coronal and 3-dimensional images were shown. (D) Angiographic finding revealed that his IPDA aneurysm (red arrow) partially communicated with the GDA. Obscure APDF was not identified. APDF – arterio-pancreatic duct fistula; CA – celiac artery; CBD – common hepatic duct; CHA – common hepatic artery; GB – gallbladder; GDA – gastroduodenal artery; IPDA – inferior pancreaticoduodenal artery; IVC – inferior vena cava; MPD – main pancreatic duct; PHA – proper hepatic artery; PV – portal vein; SMA – superior mesenteric artery; SMV – superior mesenteric vein.
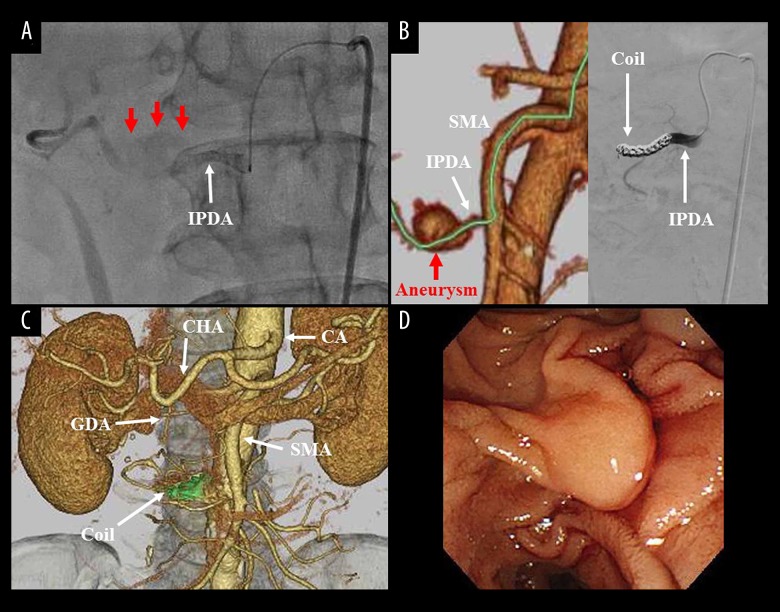
Figure 2.
(A) The IPDA is a main feeder of his aneurysm (red arrows). (B, C) After simulation analysis of the access path (green line), arterial embolization by metallic coils (green area) was successfully completed. (D) Six months after coil embolization, endoscopic examination clearly showed vinous duodenal juice. CA – celiac artery; CHA – common hepatic artery; GDA – gastroduodenal artery; IPDA – inferior pancreaticoduodenal artery; SMA – superior mesenteric artery.
Six months later, he visited our emergency unit due to massive hematemesis. Hematochezia was observed. Endoscopic examination showed not active bleeding but vinous duodenal juice (Figure 2D). Endoscopic ultrasound (Figure 3A) and magnetic resonance imaging (Figure 3B) showed a pancreatic pseudocyst around metallic coils (22 mm in size). His hidden APDF was not clarified in the endoscopic retrograde pancreatography (Figure 3C), and then, an endoscopic nasopancreatic drainage tube was placed (Figure 3D). Bloody pancreatic juice was observed in the tube discharge. A diagnosis of HP was made.
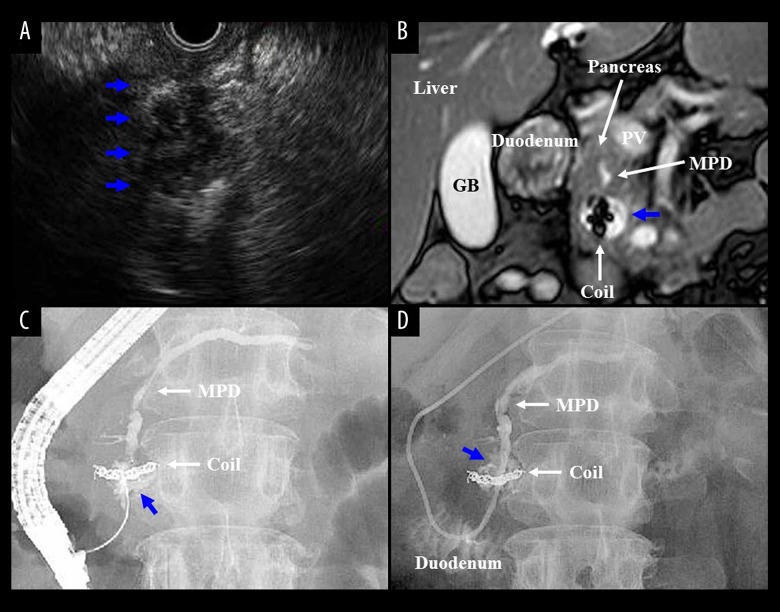
Figure 3.
(A, B) Endoscopic ultrasound (A) and magnetic resonance imaging (B) detected pancreatic pseudocyst (22 mm in size) (blue arrows). (C, D) Endoscopic nasopancreatic drainage tube was placed, and bloody discharge was observed. A diagnosis of HP was made. Endoscopic retrograde pancreatography (C) and pancreatography via an endoscopic nasopancreatic drainage tube (D) detected a pseudocystic lesion around metallic coils (blue arrows), not his hidden APDF. APDF – arterio-pancreatic duct fistula; GB – gallbladder; HP – hemosuccus pancreaticus; MPD – main pancreatic duct.
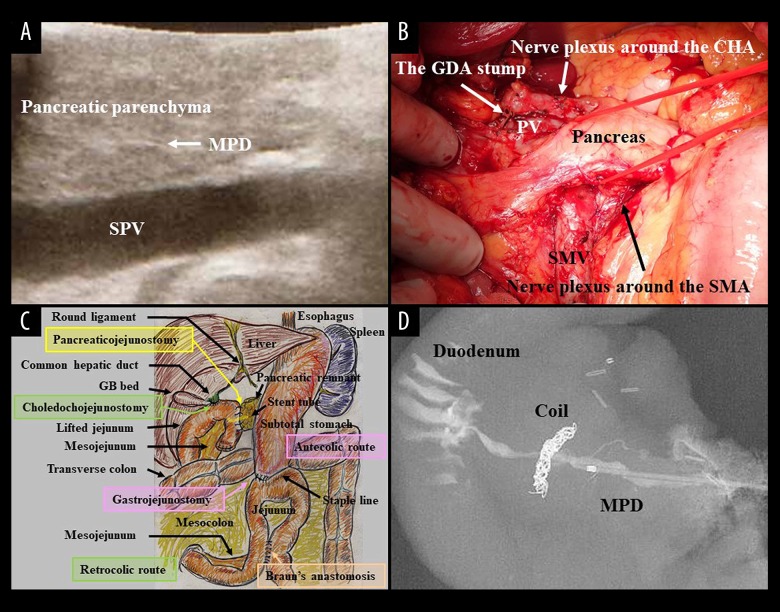
At approximately 60 years of age, subtotal stomach-preserving pancreaticoduodenectomy (SSpPD) without any extended resections was simply performed. Also, intentional dissections of nerve plexuses and lymph nodes were all waived. Pseudocystic lesion and metallic coils were observed in the pancreas head by intraoperative ultrasound (Figure 4A). Dense tissues were observed at the groove area (Figure 4B). Operative time was 483 minutes. Blood loss was 955 mL, and blood transfusion of red blood cell (280 mL) was required. Nerve plexuses around the SMA, CA, and other arteries (e.g., the common hepatic artery) were all preserved. The arterial root of the IPDA which branched from the SMA was intact. The IPDA and gastroduodenal artery were ligated and then cut at each root, respectively (Figure 4C, 4D). Normal pancreatic parenchyma and duct were confirmed at the pancreatic body by intraoperative ultrasound (Figure 5A), and the pancreas was cut at the normal portion after a tunneling of the pancreas head (Figure 5B).
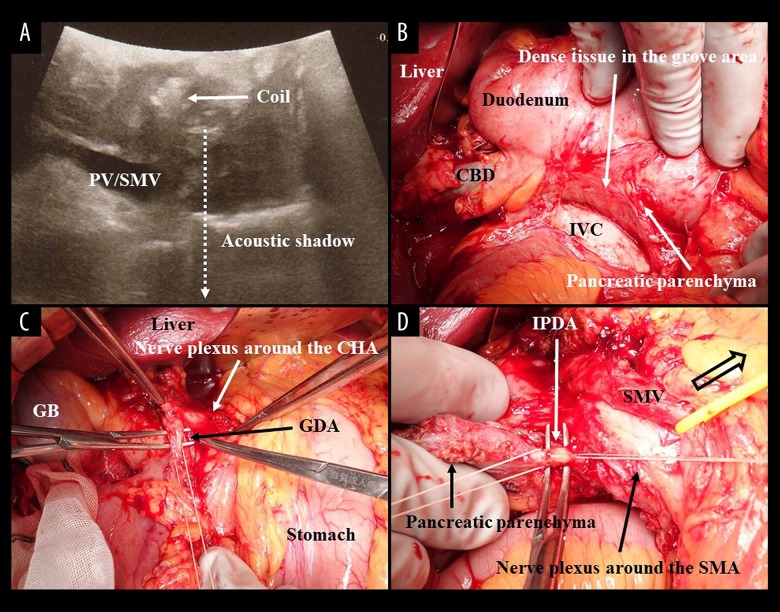
Figure 4.
(A) Pseudocystic lesion and metallic coils were observed by intraoperative ultrasound. (B) Dense tissues were observed at the groove area. (C) Nerve plexuses around the SMA, CA and other arteries (e.g., the CHA) were all preserved. The SMV was retracted by a vessel tape (clear arrow), and the arterial root of the IPDA from the SMA was confirmed as intact. The GDA (C) and IPDA (D) were ligated and then cut at each root, respectively. CBD – common hepatic duct; CHA – common hepatic artery; GB – gallbladder; GDA – gastroduodenal artery; IPDA – inferior pancreaticoduodenal artery; IVC – inferior vena cava; PV – portal vein; SMA – superior mesenteric artery; SMV – superior mesenteric vein.
Figure 5.
(A) Normal pancreatic parenchyma and duct were confirmed at the pancreatic body by intraoperative ultrasound. (B) The pancreas was cut at the normal portion after a tunneling of the pancreas head. Simple SSpPD without extended resections and intentional dissections was performed. Nerve plexuses and lymph nodes were all preserved. (C) Inherent reconstructions during SSpPD were done by modified Child’s method with Braun’s anastomosis. (D) Even a pancreatography of the resected specimen with a higher pressure of contrast dye injection did not clarify his peculiar APDF. APDF – arteriopancreatic duct fistula; CHA – common hepatic artery; GB – gallbladder; GDA – gastroduodenal artery; MPD – main pancreatic duct; SMA – superior mesenteric artery; SMV – superior mesenteric vein; SSpPD – subtotal stomach-preserving pancreaticoduodenectomy; SPV – splenic vein.
Inherent reconstructions during SSpPD were done by modified Child’s method with Braun’s anastomosis (Figure 5C). During pancreaticojejunostomy, intraductal lost stent (Pancreatic duct tube, 5Fr, burled, MD41515; Sumitomo Bakelite Co., Ltd., Tokyo, Japan) was placed, and a duct-to-jejunal anastomosis was made by 4 interrupted sutures (polydioxanone [4-0 PDS II, violet, RB-1, Z712D; Ethicon, Inc., Cincinnati, OH, USA]). Intentional approximation of the pancreatic stump and jejunal wall was made by 4 interrupted sutures (polyvinylidene fluoride [4-0 ASSP504-0IIN, ASFLEX, 75 cm; Konoseisakusho Co., Ltd., Ichikawa, Chiba, Japan]). Choledochojejunostomy was made by interrupted sutures (polydioxanone). Linear stapler (GST system [blue cartridge] and Echelon Flex; Ethicon, Inc.) with an antecedent compression was employed for gastrojejunostomy [11], and the entry hole was closed by hand suture in the layer-to-layer fashion [11]. Braun’s anastomosis was also made by hand suture in the layer-to-layer fashion. Mesenteric gaps were all closed to avoid postoperative hernia.
Drains were placed nearly at the anastomoses of pancreaticojejunostomy, choledochojejunostomy and gastrojejunostomy. Even a pancreatography of the resected specimen with a higher pressure of contrast dye injection did not clarify his peculiar APDF (Figure 5D).
Postoperative course was uneventful without any complications. He was discharged at postoperative day 10, and then, he smoothly returned to work.
Discussion
The HP is mostly caused by acute or chronic pancreatitis, and an aneurysm is also often associated with HP [5]. However, a causal relationship between HP and pancreatitis/aneurysm is still not established [5]. In our patient’s case, his IPDA aneurysm developed after groove pancreatitis, and his HP occurred after pancreatitis and aneurysm. Our experience will support an opinion that pancreatitis and/or aneurysm trigger to occur HP.
Unexpected HP may result in a life-threatening condition [5]. Interventional radiology is initially applied for visceral artery aneurysm with a higher successful rate [3,8]. However, aneurysms which repeated HP even after coil embolization had been previously documented. Peri- and intra-pancreatic arteries build a unique pancreatic arcade, and this characteristic flow results in an aneurysmic recanalization [9,10]. Although surgical strategies of pancreatic pseudocyst have been established [12], a pseudocyst itself did not affect a surgical indication in our case. Contrastingly, a refractory HP even after coil embolization forced us to choose a surgical approach for his IPDA aneurysm.
Pancreaticoduodenectomy (PD) was first attempted by Codivilla in 1898 [13], and was thereafter successfully performed by Kausch in 1912 [14]. In 1935, Whipple demonstrated that classical PD was technically feasible and compatible with a reasonable function after recovery [15]. Pylorus-preserving PD was first introduced by Watson in 1944 [16], and Traverso and Longmire subsequently documented the usefulness of this surgery [17]. In order to reduce delayed gastric emptying, SSpPD was initially described during the 1990s in Japan [18]. Hayashibe et al. were the first to report clinical outcomes of pylorus-preserving PD compared to SSpPD in 2007 [19]. The term “artery-first approach” was first coined by Weitz in 2010 [20]. All PDs inherently require 3 reconstructions: digestive tract, biliary tree, and pancreatic duct. Reconstructive techniques (e.g., the Whipple, Child, and Imanaga procedures) have been introduced [15,21–24], and have already undergone some modifications [25,26]. Hence, surgical procedures during all PDs have been currently well-established in detail.
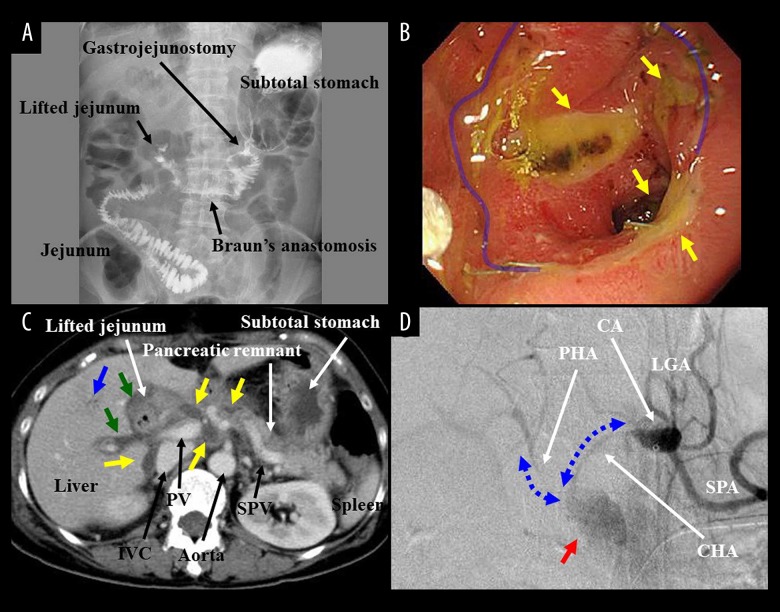
A simple question has arisen: Is a PD for pancreatic aneurysm and HP risky business? The risk of PD has fallen to 5% to 10% [25,27], though PD easily causes postoperative complications (e.g., delayed gastric emptying, gastrointestinal ulcer, postoperative cholangitis, anastomotic leakage, intraperitoneal abscess, intractable pancreatic fistula, pseudoaneurysm, and massive bleeding). Actual findings of typical complications after advanced SSpPDs with extended resections of vessels and surrounding organs and/or intentional dissections of nerve plexuses and lymph nodes were summarized in Figure 6. Especially, pancreatic juice-related complications (i.e., pancreatic leakage and subsequent fistula) after advanced PDs have refractory symptoms, and these intractable complications may result in fatal outcomes (e.g., septic state and aneurysm rupture).
Figure 6.
(A) During advanced SSpPD, reconstructions were made by modified Child’s method with Braun’s anastomosis. Upper gastrointestinal examination at postoperative day 6 was shown. Atonic stomach and intestinal hypoperistalsis were observed. Oral intake was severely disturbed for 10 days because of delayed gastric emptying. (B) Endoscopic findings after advanced SSpPD revealed multiple ulcers (yellow arrows) at the anastomosis (blue line) and jejunum. Gastrojejunostomy was remade at postoperative day 99 because of refractory ulcers and jejunal stenosis. (C) Pancreatic leakage was observed from postoperative day 1 after advanced SSpPD. Abscess formations (yellow arrows) were detected around the pancreaticojejunostomy and IVC by enhanced computed tomography at postoperative day 9. Postoperative cholangitis also occurred. Parabiliary plexuses were swelled (green arrows), and cholangiolitic air (blue arrow) was observed. Fasting and antibiotics were required for 1 week. (D) Advanced SSpPD for pancreatic cancer was performed as a conversion surgery after chemotherapy. According to the tumor extent, the IVC was partially resected, and paraaortic lymph nodes were fully dissected. Intraperitoneal abscess due to pancreatic leakage developed at postoperative day 15. Although local drainage and antibiotics administration were continued, the stump of gastroduodenal artery was suddenly ruptured at postoperative 28. Massive bleeding (red arrow) was observed in an emergent angiography. Owing to intentional dissections of nerve plexuses and lymph nodes, angiographic forms of the CHA and PHA notably became thin and frizzy (blue arrows). Covered stents were placed into the CHA and PHA, and both hepatic arterial flow and complete hemostasis were obtained simultaneously. CA – celiac artery; CHA – common hepatic artery; GB – gallbladder; GDA – gastroduodenal artery; IVC – inferior vena cava; LGA – left gastric artery; MPD – main pancreatic duct; PHA – proper hepatic artery; PV – portal vein; SMA – superior mesenteric artery; SMV – superior mesenteric vein; SPA – splenic artery; SPV – splenic vein; SSpPD – subtotal stomach-preserving pancreaticoduodenectomy.
Even after a coil embolization of the pancreatic artery, peri- and intra-pancreatic arteries will build a unique pancreatic arcade which causes an aneurysmic recanalization [9,10]. Moreover, a refractory HP may be fatal. After the initial interventional radiology for pancreatic aneurysms, an elective pancreatic surgery should be considered to avoid unwanted recanalization and refractory HP.
Conclusions
Our IPDA aneurysm repeated HPs even after the initial coil embolization, and a SSpPD might have a therapeutic potential for IPDA aneurysm and HP. The authors hope this thought-provoking case will be informative for physicians in the field of the pancreas.
Acknowledgments
T. Hori originally drew the schema.
Footnotes
Conflict of interest
None.
References:
- 1.Al-Habbal Y, Christophi C, Muralidharan V. Aneurysms of the splenic artery – a review. Surgeon. 2010;8:223–31. doi: 10.1016/j.surge.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Lee SA, Jae HJ, Ahn S, et al. Endovascular treatment of a saccular aneurysm in the celiomesenteric trunk: A case report and review of literature. Vasc Specialist Int. 2018;34:44–47. doi: 10.5758/vsi.2018.34.2.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Rijn MJ, Ten Raa S, Hendriks JM, Verhagen HJ. Visceral aneurysms: Old paradigms, new insights? Best Pract Res Clin Gastroenterol. 2017;31:97–104. doi: 10.1016/j.bpg.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Stanley JC, Thompson NW, Fry WJ. Splanchnic artery aneurysms. Arch Surg. 1970;101:689–97. doi: 10.1001/archsurg.1970.01340300045009. [DOI] [PubMed] [Google Scholar]
- 5.Yu P, Gong J. Hemosuccus pancreaticus: A mini-review. Ann Med Surg. 2018;28:45–48. doi: 10.1016/j.amsu.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandblom P. Gastrointestinal hemorrhage through the pancreatic duct. Ann Surg. 1970;171:61–66. doi: 10.1097/00000658-197001000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Longmire WP, Jr, Rose AS., 3rd Hemoductal pancreatitis. Surg Gynecol Obstet. 1973;136:246–50. [PubMed] [Google Scholar]
- 8.Tulsyan N, Kashyap VS, Greenberg RK, et al. The endovascular management of visceral artery aneurysms and pseudoaneurysms. J Vasc Surg. 2007;45:276–83. doi: 10.1016/j.jvs.2006.10.049. [DOI] [PubMed] [Google Scholar]
- 9.Miyake T, Sakai M, Ueda S, et al. Arteriopancreatic duct fistula in juvenile pancreatitis. A cause of massive gastrointestinal hemorrhage. Dig Dis Sci. 1981;26:760–64. doi: 10.1007/BF01316869. [DOI] [PubMed] [Google Scholar]
- 10.Kuzuya A, Mizuno K, Miyake H, et al. Hemosuccus pancreaticus caused by rupture of a true splenic artery aneurysm following a failure of coil embolization. Ann Vasc Surg. 2006;20:130–33. doi: 10.1007/s10016-005-9100-x. [DOI] [PubMed] [Google Scholar]
- 11.Kitano T, Yasukawa D, Aisu Y, Hori T. Overlap anastomosis for digestive reconstruction during laparoscopic distal gastrectomy with intensive regional lymph node dissection: Physiological impact of preserving the mesenteric autonomic nerves in the lifted jejunal limb. Surg Res Pract. 2018;2018:4938341. doi: 10.1155/2018/4938341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frey CF. Pancreatic pseudocyst – operative strategy. Ann Surg. 1978;188:652–62. doi: 10.1097/00000658-197811000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Are C, Dhir M, Ravipati L. History of pancreaticoduodenectomy: Early misconceptions, initial milestones and the pioneers. HPB. 2011;13:377–84. doi: 10.1111/j.1477-2574.2011.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kausch W. Das carnimom der papilla duodeni und seine radikale entfernung. Beitr Klin Chir. 1912;78:439–86. [in German] [Google Scholar]
- 15.Whipple AO, Parsons WB, Mullins CR. Treatment of carcinoma of the ampulla of Vater. Ann Surg. 1935;102:763–79. doi: 10.1097/00000658-193510000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watson K. Carcinoma of ampulla of Vater. Successful radical resection. Br J Surg. 1944;31:368–73. [Google Scholar]
- 17.Traverso LW, Longmire WP., Jr Preservation of the pylorus in pancreaticoduodenectomy. Surg Gynecol Obstet. 1978;146:959–62. [PubMed] [Google Scholar]
- 18.Huang W, Xiong JJ, Wan MH, et al. Meta-analysis of subtotal stomach-preserving pancreaticoduodenectomy vs pylorus preserving pancreaticoduodenectomy. World J Gastroenterol. 2015;21:6361–73. doi: 10.3748/wjg.v21.i20.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashibe A, Kameyama M, Shinbo M, Makimoto S. The surgical procedure and clinical results of subtotal stomach preserving pancreaticoduodenectomy (SSPPD) in comparison with pylorus preserving pancreaticoduodenectomy (PPPD) J Surg Oncol. 2007;95:106–9. doi: 10.1002/jso.20608. [DOI] [PubMed] [Google Scholar]
- 20.Weitz J, Rahbari N, Koch M, Buchler MW. The ‘artery first’ approach for resection of pancreatic head cancer. J Am Coll Surg. 2010;210:e1–4. doi: 10.1016/j.jamcollsurg.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 21.Imanaga H. A new method of pancreaticoduodenectomy designed to preserve liver and pancreatic function. Surgery. 1960;47:577–86. [PubMed] [Google Scholar]
- 22.Whipple AO. Observations on radical surgery for lesions of the pancreas. Surg Gynecol Obstet. 1946;82:623–31. [PubMed] [Google Scholar]
- 23.Child CG. Carcinoma of the duodenum. Ann Surg. 1943;118:838–42. doi: 10.1097/00000658-194311000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Child CG. Pancreaticojejunostomy and other problems associated with the surgical management of carcinoma involving the head of the pancreas: Report of five additional cases of radical pancreaticoduodenectomy. Ann Surg. 1944;119:845–55. doi: 10.1097/00000658-194406000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peters JH, Carey LC. Historical review of pancreaticoduodenectomy. Am J Surg. 1991;161:219–25. doi: 10.1016/0002-9610(91)91134-5. [DOI] [PubMed] [Google Scholar]
- 26.Sparkman RS. Two physicians named Whipple. Am J Surg. 1995;170:306–7. doi: 10.1016/s0002-9610(05)80024-8. [DOI] [PubMed] [Google Scholar]
- 27.Kimura Y, Yasukawa D, Aisu Y, Hori T. Imanaga’s first method for reconstruction with preservation of mesojejunal autonomic nerves during pylorus-preserving pancreatoduodenectomy. Am J Case Rep. 2018;19:608–13. doi: 10.12659/AJCR.908817. [DOI] [PMC free article] [PubMed] [Google Scholar]