Abstract
Background:
With social attitudes about marijuana changing and patients sometimes seeking nonmainstream treatment options, the main goal of this study was to investigate the prevalence of, and factors associated with, marijuana use by patients with multiple sclerosis (MS).
Methods:
Adult patients with MS (n = 521) and controls (n = 279) from a study of clinical, neuroimaging, genetic, and environmental factors in MS progression were included. Patients with MS stated whether they had ever used marijuana before MS diagnosis, after MS diagnosis, and in the preceding 3 months as part of an in-person questionnaire. The control group stated whether they had ever used marijuana and in the preceding 3 months.
Results:
The percentage of patients with MS reporting ever use of marijuana was 39.9%, compared with 32.7% of controls. Marijuana use in the preceding 3 months was significantly more prevalent among patients with MS (9.4%) compared with controls (0.4%) (P < .001). Marijuana use was most prevalent in male patients with MS (P = .004) and in patients with MS who used complementary and alternative medicine (P = .045). Cigarette smoking was associated with marijuana use in patients with MS (P < .001) and controls (P = .001). Increasing age was associated with decreasing prevalence of marijuana use in the patients with MS (P < 0.001).
Conclusions:
Patients with MS are more likely to report recent marijuana use than are people without MS. Owing to potential adverse effects, marijuana use by patients with MS may warrant vigilance by MS caregivers, given shifting social attitudes and the trend towards legalization of marijuana in the United States.
Keywords: Cannabis, Marijuana, Multiple sclerosis (MS), Tetrahydrocannabinol (THC)
Multiple sclerosis (MS) is a neurologic disease of the central nervous system that causes physical and cognitive disability. Multiple sclerosis is also associated with a diverse range of symptoms, including pain, spasticity, fatigue, balance problems, heat intolerance, bladder problems, and tremors, all of which adversely affect quality of life.1 Patients with MS often seek nonmainstream treatment options to alleviate their symptoms, such as complementary and alternative medicine (CAM), herbal supplements, and marijuana.2,3
Social perceptions and attitudes regarding marijuana use in the United States have shifted in the past decade. Jones et al4 in 2015 reported that 58% of Americans favored legalization of marijuana, compared with 30% in 2000. The political and legal environment for marijuana use has also changed considerably, particularly in the United States, in part because the legal sale of marijuana is seen as a potential source of tax revenue and in part because of shifting social attitudes among the voting public.4 Marijuana use for medical and recreational purposes is now legal in Colorado, Washington, California, Oregon, Alaska, Massachusetts, Maine, and Nevada, all of which have legalized marijuana use via referendum or legislation.5 Medical marijuana has also been legalized in 29 states.5 However, marijuana is still considered an illicit substance under US federal law.5
The US Food and Drug Administration (FDA) has approved two drugs, dronabinol and nabilone, the active ingredients of which are synthetic derivatives of delta-9-tetrahydrocannabinol (THC), the main psychoactive agent in marijuana.6 Dronabinol is FDA-approved for treating AIDS-associated anorexia and chemotherapy-induced nausea and vomiting.6 Nabilone is FDA-approved for the treatment of nausea and vomiting caused by cancer chemotherapy in patients who do not respond adequately to conventional antiemetic medications. Nabiximols is an oral mucosal spray containing THC and cannabidiol that has been approved in Canada and the United Kingdom for neuropathic pain, spasticity, and overactive bladder7; however, nabiximols is not approved in the United States. In two phase 3 trials, nabiximols did not improve average self-reported numerical rating scale pain scores in patients with cancer.7
The American Academy of Neurology lists oral cannabis extracts as a level A effective treatment for symptoms of spasticity and pain (excluding central neuropathic pain).3,8 There is level B evidence that oral cannabis extracts are probably ineffective for signs of spasticity and tremor.3,8 Synthetic THC is listed as a level B effective treatment for symptoms of spasticity and pain (excluding central neuropathic pain).3,8 Nabiximols is listed as a level B effective treatment for symptoms of spasticity, pain, and urinary frequency.3,8 There is level B evidence that nabiximols is ineffective for signs of spasticity and urinary incontinence, and level C evidence that nabiximols is ineffective for tremor.3,8
Factors associated with marijuana use to treat symptoms of MS have not been extensively researched in the United States. Chong et al9 investigated marijuana use in patients with MS seen at two outpatient neurology clinics in the United Kingdom (n = 254) and found that 43% of patients reported ever use of marijuana. Of the ever users, 68% reported using marijuana to alleviate symptoms of MS.9 In another study by Page et al,10 43% of survey respondents with MS (n = 420) in Canada reported ever use of marijuana and 16% had tried marijuana for medical purposes. Banwell et al11 reported a 54.3% acceptance rate for the legalization of medical marijuana among Canadian patients and found that sleep, pain, anxiety, and spasticity were the most common reasons for marijuana use.
The main goal of this study was to investigate the prevalence of marijuana use in patients with MS compared with controls. Another goal was to investigate the associations between marijuana use and clinical factors, such as MS disease course, use of disease-modifying therapies (DMTs), and disability level, and demographic characteristics, such as education level, smoking status, and CAM use.
Methods
Study Population
Study Setting
The data were obtained from an ongoing prospective study of clinical, genetic, and environmental risk factors in MS at the Jacobs Neurological Institute/Baird MS Research Center of the State University of New York at Buffalo.12,13 This was a single-center, prospective, observational study. The University at Buffalo Human Subjects Institutional Review Board approved the study protocol. All participants provided written informed consent at enrollment.
Inclusion and Exclusion Criteria
For this substudy, only individuals aged 18 years or older were included. People whose responses regarding marijuana use were “don't know” and those who declined to answer were excluded.
Clinical Assessments
The enrolled patients underwent neurologic examinations. Answers to a comprehensive questionnaire were collected in person by an interviewer. The responses to the questionnaire were transcribed by the interviewer. All participants were enrolled and answered the questionnaire prior to the legalization of medical marijuana in New York State in July 2014. The questionnaire included patients' responses to whether they had used marijuana before MS diagnosis, after MS diagnosis, and in the 3 months preceding the questionnaire. Controls provided responses to whether they had used marijuana in the past and in the preceding 3 months.
Data Analysis
Patients with MS were assigned “yes” to marijuana ever use if they answered “yes” to any of the following marijuana use categories: before MS diagnosis, after MS diagnosis, or in the preceding 3 months. For the control group, marijuana ever use was defined as answering “yes” regarding past use or use in the preceding 3 months.
Data on age (continuous variable), gender (female or transgender female, male or transgender male), education level (college or no college), MS disease course (relapsing remitting, secondary progressive, or primary progressive), DMT treatment status (receiving DMT, not receiving DMT), smoking status (smoked > 100 [tobacco] cigarettes, have not smoked > 100 cigarettes), living status (living alone, or living with a partner and spouse), race (white, African American, Hispanic Latino, Asian, or other), and CAM use (ever used CAM, never used CAM) were obtained from the study database. The following defined CAM: acupuncture, aromatherapy, Ayurveda, Chinese herbal medicine, chiropractic care, electromagnetic therapy, homeopathy, hypnosis, massage, naturopathy, qigong, Reiki, therapeutic touch, bee stings, or other. For all binary variables, the odds ratio (OR) was computed and reported.
The differences between the means of the continuous variable (eg, age between participants who reported marijuana use and those who did not) were assessed using independent samples t tests. The differences in distributions of categorical variables (eg, gender, education level, DMT treatment status) between participants who reported marijuana use and those who did not were assessed using the Fisher exact test for binary variables and the χ2 test for the nonbinary categorical variable.
Associations between marijuana use variables and Expanded Disability Status Scale (EDSS) scores were investigated using ordinal regression, with EDSS score as the dependent variable and age, gender, and marijuana use variable of interest as predictor variables. A multivariate logistic regression was used to investigate the associations between marijuana use as a dependent variable and the following predictor variables: age, gender, ever-smoking cigarettes, MS disease course, and CAM use. Subsequently a stepwise regression analysis (entry P = .05, removal P = .1) was done.
All statistical analyses were conducted using IBM SPSS Statistics for Windows version 24.0 (IBM Corp, Armonk, NY).
Results
Demographic and Clinical Characteristics
This substudy included 521 patients with MS and 279 controls. Table 1 summarizes the demographic characteristics of both groups. Both groups were mostly female (MS: 71.0%, controls: 63.8%) and of white race/ethnicity (MS: 93.1%, controls: 86.6%). The mean ± SD age was 47.6 ± 11.1 years for the MS group compared with 45.9 ± 15.1 years for the control group.
Table 1.
Demographic and clinical characteristics of patients with MS and controls
| Characteristic | Patients with MS | Controls |
|---|---|---|
| Gender, female: male (% female) | 370:151 (71.0) | 178:101 (63.8) |
| MS disease course | ||
| Relapsing remitting | 345 (66.2) | |
| Secondary progressive | 139 (26.7) | |
| Primary progressive | 37 (7.1) | |
| Race/ethnicity | ||
| White | 483 (93.1) | 240 (86.6) |
| African American | 22 (4.2) | 22 (7.9) |
| Hispanic Latino | 9 (1.7) | 3 (1.1) |
| Asian | 2 (0.4) | 9 (3.2) |
| Other | 3 (0.6) | 2 (.7) |
| Age, y | 47.6 ± 11.1 | 45.9 ± 15.1 |
| MS disease duration, y | 15.2 ± 10.5 | |
| Treatment duration, y | 4.32 ± 3.88 | |
| EDSS score, median [IQR] | 3.0 [4.0] | |
Note: Unless otherwise indicated, values are given as number (percentage) or mean ± SD.
Abbreviations: EDSS: Expanded Disability Status Scale; IQR: inter-quartile range; MS, multiple sclerosis.
Most of the patients with MS (66.2%) had relapsing-remitting MS. The median (interquartile range) EDSS score for patients with MS was 3.0 (4.0). The mean ± SD disease duration and treatment duration were 15.2 ±10.5 and 4.32 ± 3.9 years, respectively.
Prevalence of Marijuana Use
Ever use of marijuana was not significantly more prevalent in the MS group compared with the control group (39.9% vs 32.7%) (Fisher exact test, P = .70). Marijuana use in the past 3 months was significantly higher in the MS group (9.4%) compared with the control group (0.4%) (Fisher exact test, P < .001). Fewer patients reported having used marijuana after they were diagnosed with MS (16.1%) compared with before their MS diagnosis (37.2%) (Fisher exact test, P < .001).
Demographic Factors and Marijuana Use
Ever use of marijuana decreased with increasing age in the MS group (P < .001) but not in the control group. Gender was significantly associated with marijuana ever use among patients with MS, with more males reporting marijuana ever use (males 49.7% vs females 35.9%; OR, 1.76; P = .004). Gender, however, was not associated with ever use of marijuana in the control group, and education and race were not associated with marijuana ever use in either group. Ever use of marijuana was significantly associated with living status in the MS group only, with patients who lived with a partner or spouse having the highest prevalence of use (5.2%, P = .007). Cigarette smoking was associated with marijuana ever use in both patients with MS (53.2% smokers vs 25.4% nonsmokers; OR, 3.33; P < .001) and controls (54.6% smokers vs 27.0% nonsmokers; OR, 3.26; P < .001). The dependence of the prevalence of ever use of marijuana in the MS and control groups on gender and smoking status is summarized in Figures 1A and 1B, respectively.
Figure 1.
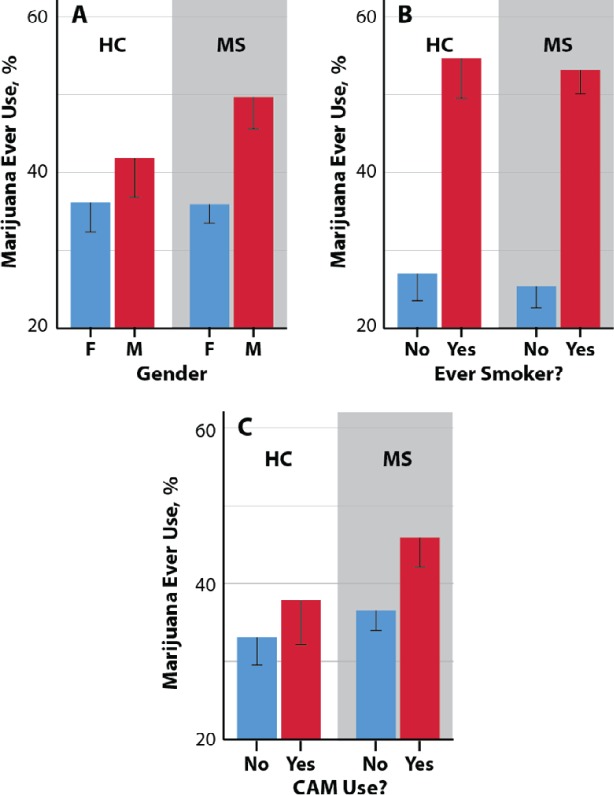
Dependence of participants reporting marijuana ever use on gender (A), ever smoker status (B), and complementary and alternative medicine (CAM) use status (C) in patients with multiple sclerosis (MS) and healthy control (HC) group
Clinical Factors and Marijuana Use
The associations between marijuana use and MS disease course, disability level, current DMT status, and CAM use were also investigated. Marijuana use in the past 3 months was associated with disease course (P < .001). The percentages of patients with relapsing-remitting, secondary progressive, and primary progressive MS reporting marijuana use in the previous 3 months were 18.3%, 12.3%, and 13.9%, respectively. The use of CAM was associated with marijuana use in patients with MS (P = .045) but not in controls. The associations between marijuana ever use and CAM use in both groups are summarized in Figure 1C. No association was found between marijuana ever use in patients with MS and EDSS scores.
Multivariate Analyses
To address the contribution of the covariates acting together, multivariate logistic regression analyses were conducted, followed by stepwise regression. Ever use of marijuana was the dependent variable, and age, gender, smoking status, MS disease course, and CAM use were treated as predictor variables. The multivariate logistic regression identified gender (P = .004), age (P = .0039), cigarette smoking (P < .001), and any CAM use (P = .006) as significant factors associated with marijuana ever use. A follow-up stepwise regression analysis showed that gender (P = .002), age (P = .016), smoking status (P < .001), and CAM use (P = .003) remained significant predictors of marijuana ever use.
Discussion
The purpose of the present study was to investigate the factors associated with reported marijuana use in patients with MS and to compare the prevalence of reported marijuana use in patients with MS to that of controls. Our investigation of marijuana use provides useful information regarding the demographic and clinical characteristics of patients with MS and controls who report marijuana use in the United States. Patients with MS were more likely to report recent marijuana use (within the past 3 months) compared with controls. A greater prevalence of marijuana ever use was observed in younger patients, and in those who reported smoking more than 100 cigarettes in their lifetime in both the MS and control groups. We also found a greater prevalence of marijuana ever use in males versus females in the patients with MS. Marijuana ever use was not associated with race or education level in the MS group. There was a positive association between marijuana ever use and CAM use in the MS group.
The overall prevalence of marijuana ever use among patients with MS in our study was 39.9%, which is comparable to the 43% reported in previous studies of patients with MS in the United Kingdom9 and Canada.10 The National Institute of Drug Abuse14 estimates that 35.9% of 12th graders in the United States and 12.2% of US citizens aged 26 years or older report marijuana use in the past year.
The outcomes of future clinical trials of pharmaceutical-grade, cannabis-based medicine for the treatment of symptoms related to MS have the potential to influence patients' perceptions of smoked marijuana, particularly in states where marijuana is legal. Patients eager to adopt marijuana as part of their treatment regimen may overlook the risks associated with the lack of control over the dose ingested, exposure to marijuana cigarette smoke, and lack of standardization of dose form with smoked marijuana, none of which have been extensively investigated in a clinical setting. Interestingly, in a placebo-crossover study of 37 participants by Corey-Bloom et al,15 spasticity decreased by 2.74 points on the Ashworth scale and pain reduced by an average of 5.28 points on the visual analogue scale after patients had smoked cannabis for 3 days. However, a significant decrease in cognition was reported among patients just after they had smoked marijuana, as measured with the Paced Auditory Serial Addition Test.15 These findings suggest that smoked marijuana may alleviate some of the symptoms associated with MS, but that its apparent effect on cognition remains a concern.15 In addition, as marijuana use becomes more mainstream, reports of adverse events associated with it could modify public and patient perceptions.
Recently, Cofield et al16 examined survey responses from 5481 participants in the North American Research Committee on Multiple Sclerosis (NARCOMS) Registry, which assessed interest in and use of marijuana before and after MS diagnosis. The researchers found that 64% of the participants reported marijuana use prior to diagnosis, and 47% would consider marijuana as a possible supplement to treat MS symptoms.16 Additionally, 91% of respondents believed that marijuana use should be legalized in some form.16 The results from this study highlight the interest in legalization of marijuana use among patients with MS.
The present study was cross-sectional and has all the limitations of such a design, including the risks of recall bias. During this study, marijuana was illicit for medicinal purposes in New York State. Thus, it is possible that marijuana use was underreported among the study participants. Despite this limitation, the present study provides a useful profile of the factors associated with marijuana use in patients with MS. A questionnaire study of patients with MS (n = 220) conducted in Nova Scotia by Clark et al17 found that men were significantly more likely to use medical cannabis than women, which corroborates our findings in this study population. However, Chong et al9 found no differences in prevalence of marijuana use between male and female patients with MS in the United Kingdom.
In the present study, we found no evidence for associations between marijuana use in patients with MS and educational level or race. These results are concordant with the findings from Chong et al9, who investigated marijuana use in 254 patients with MS in the United Kingdom. Our findings, which indicate a negative association between age and marijuana use for treatment in MS, were not observed in the studies conducted in the United Kingdom or Canada.9,17 However, the age dependence in our MS sample is consistent with the age-dependent pattern of marijuana use in the US population at large.5
Interestingly, use of CAM was also associated with marijuana ever use in patients with MS. To our knowledge, our study is the first to investigate the associations of marijuana use with CAM use in patients with MS. Our findings suggest that patients with MS who use CAM may be more likely to use marijuana as an alternative to standardized DMT for treatment of MS-related symptoms.
In conclusion, we have identified several potential demographic and clinical characteristics associated with self-treatment with marijuana in patients with MS. With the increasing interest in use of marijuana to treat the debilitating symptoms associated with chronic diseases such as MS, there is a need for well-controlled clinical studies to evaluate the safety, feasibility, and effectiveness of such treatment.
PRACTICE POINTS
The prevalence of patients with MS reporting ever use of marijuana was similar to that of controls. In both the MS and control groups marijuana use was significantly associated with cigarette smoking.
Patients with MS reported recent use of marijuana (within preceding 3 months) more than controls.
Greater prevalence of marijuana use was associated with male gender, younger age, relapsing-remitting MS disease course, cigarette smoking, use of complementary alternative medicine, and living with a spouse or partner.
Acknowledgments
The authors thank the study participants.
Financial Disclosures
Dr. Weinstock-Guttman has received honoraria for advisory board and educational program service from Teva Pharmaceuticals, Biogen, Novartis, Acorda, EMD Serono, Pfizer, Genzyme, and Sanofi and has also received support for research activities from the National Institutes of Health, National Multiple Sclerosis Society, National Science Foundation, Department of Defense, EMD Serono, Biogen, Teva Neuroscience, Cyberonics, Novartis, Acorda, and the Jog for the Jake Foundation. Dr. Zivadinov has received speaker honoraria and consultant fees from Sanofi Genzyme, Novartis, Claret Medical, and EMD Serono and research support from the Sanofi Genzyme, Claret Medical, Mapi Pharma, Protembis, QuintilesIMS, and Novartis. Dr. Ramanathan has received research funding from the National Multiple Sclerosis Society, the US Department of Defense, and Otsuka Pharmaceutical and compensation for serving as an editor for the American Association of Pharmaceutical Scientists; these are for unrelated research. The other authors declare no conflicts of interest.
Funding/Support
This research was partially funded by the Jacquemin Family Foundation (Vienna, VA).
References
- 1.Kister I, Bacon TE, Chamot E et al. Natural history of multiple sclerosis symptoms. Int J MS Care. 2013;15:146–158. doi: 10.7224/1537-2073.2012-053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olsen SA. A review of complementary and alternative medicine (CAM) by people with multiple sclerosis. Occup Ther Int. 2009;16:57–70. doi: 10.1002/oti.266. [DOI] [PubMed] [Google Scholar]
- 3.Yadav V, Bever C, Jr, Bowen J et al. Summary of evidence-based guideline: complementary and alternative medicine in multiple sclerosis: report of the guideline development subcommittee of the American Academy of Neurology. Neurology. 2014;82:1083–1092. doi: 10.1212/WNL.0000000000000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones JM. Gallup Inc.; In U.S., 58% Back Legal Marijuana Use. http://www.gallup.com/poll/186260/back-legal-marijuana.aspx Published October 21, 2015. Accessed March 19, 2017. [Google Scholar]
- 5.Wikipedia contributors Wikipedia, The Free Encyclopedia; Medical cannabis in the United States. https://en.wikipedia.org/wiki/Medical_cannabis_in_the_United_States#Modern_laws_.281996_to_present.29 Accessed May 26, 2017. [Google Scholar]
- 6.US Department of Health and Human Services, US Food and Drug Administration FDA and Marijuana: Questions and Answers. https://www.fda.gov/newsevents/publichealthfocus/ucm421168.htm#dietary_supplements Updated June 25, 2018. Accessed July 29, 2018.
- 7.Fallon MT, Albert Lux E, McQuade R et al. Sativex oromucosal spray as adjunctive therapy in advanced cancer patients with chronic pain unalleviated by optimized opioid therapy: two double-blind, randomized, placebo-controlled phase 3 studies. Br J Pain. 2017;11:119–133. doi: 10.1177/2049463717710042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright S, Yadav V, Bever C, Jr et al. Summary of evidence-based guideline: complementary and alternative medicine in multiple sclerosis: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;83:1484–1486. doi: 10.1212/01.wnl.0000455935.13606.91. [DOI] [PubMed] [Google Scholar]
- 9.Chong MS, Wolff K, Wise K, Tanton C, Winstock A, Silber E. Cannabis use in patients with multiple sclerosis. Mult Scler. 2006;12:646–651. doi: 10.1177/1352458506070947. [DOI] [PubMed] [Google Scholar]
- 10.Page SA, Verhoef MJ, Stebbins RA, Metz LM, Levy JC. Cannabis use as described by people with multiple sclerosis. Can J Neurol Sci. 2003;30:201–205. doi: 10.1017/s0317167100002584. [DOI] [PubMed] [Google Scholar]
- 11.Banwell E, Pavisian B, Lee L, Feinstein A. Attitudes to cannabis and patterns of use among Canadians with multiple sclerosis. Mult Scler Relat Disord. 2016;10:123–126. doi: 10.1016/j.msard.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Kappus N, Weinstock-Guttman B, Hagemeier J et al. Cardiovascular risk factors are associated with increased lesion burden and brain atrophy in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2016;87:181–187. doi: 10.1136/jnnp-2014-310051. [DOI] [PubMed] [Google Scholar]
- 13.Zivadinov R, Ramasamy DP, Benedict RR et al. Cerebral microbleeds in multiple sclerosis evaluated on susceptibility-weighted images and quantitative susceptibility maps: a case-control study. Radiology. 2016;281:884–895. doi: 10.1148/radiol.2016160060. [DOI] [PubMed] [Google Scholar]
- 14.National Insttutes of Health, National Institute on Drug Abuse Statistics and trends. https://www.drugabuse.gov/drugs-abuse/marijuana Accesed January 18, 2019.
- 15.Corey-Bloom J, Wolfson T, Gamst A et al. Smoked cannabis for spasticity in multiple sclerosis: a randomized, placebo-controlled trial. CMAJ. 2012;184:1143–1150. doi: 10.1503/cmaj.110837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cofield SS, Salter A, Tyry T et al. Perspectives on marijuana use and effectiveness: a survey of NARCOMS participants. Neurol Clin Pract. 2017;7:333–343. doi: 10.1212/CPJ.0000000000000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark AJ, Ware MA, Yazer E, Murray TJ, Lynch ME. Patterns of cannabis use among patients with multiple sclerosis. Neurology. 2004;62:2098–2100. doi: 10.1212/01.wnl.0000127707.07621.72. [DOI] [PubMed] [Google Scholar]


