Scientists and clinicians who are interested in the auditory system in general, and in the diagnosis and treatment of inner ear disorders have long been studying the anatomical and physiological basis of hearing, pathological and pathophysiological substrates of disease processes, the social impact of hearing disorders, as well as the prevention, protection and therapy of hearing impairment. In recent years, however, a shift in perception of hearing loss and a change in public attitude towards hearing loss has been observed. It is now considered important, it matters, it affects the quality of life. In addition, the huge unmet needs of patients with hearing disorders have been recognized across the industry and increasingly also by funding agencies for research and education.
These changes in attitudes have been partially triggered by the demographic transformations in the societies with an increasing number of people with hearing impairment worldwide. The changes also result from an improved transfer of these messages from researchers to the public and a broad appreciation of an increasing market for, and thus substantial profit with the therapy of hearing disorders.
Findings that even mild levels of hearing loss increase the long-term risk of cognitive decline and dementia in individuals who are cognitively intact but hearing impaired at baseline, additionally spark the acceleration of research efforts for the therapy of hearing loss. In a recent ‘life-course model of contribution of modifiable risk factors to dementia’, the ‘weighted population attributable fraction’, which is the percentage reduction in new cases over a given time if a particular risk factor were completely eliminated, was estimated to be 9% for hearing loss. Thus, hearing loss in midlife ranks highest amongst the potentially modifiable risk-factor for dementia (Livingston et al. 2017).
In addition to measures for the early detection of hearing loss, education, governmental regulations aiming on the prevention of hearing loss (e.g. due to noise exposure), and the therapy of hearing disorders by conventional hearing aids and implantable electronic hearing devices, pharmacological (medical / drug) therapy has commanded increasing attention. However, it has to be noted that to date, almost no drugs are on the market for the therapy of inner ear disorders, apart from isolated approvals for a limited range of symptoms, and without high levels of evidence for their effectiveness. Most medical therapy of the ear uses “off-label” drug formulations.
A large number of basic research and preclinical studies have contributed to the current knowledge on the background of hereditary or acquired hearing loss, e.g. infection-, noise-, ototoxicity-, age-related and “idiopathic” sudden sensorineural hearing loss as well as on ways of otoprotection. Research also addresses the restoration of hearing by repair mechanisms or regeneration from the level of the ribbon synapse, the repair of hair and supporting cells and stria vascularis. Other research aims to reduce tinnitus symptoms by modulations of CNS activity or on hearing and structure preservation during cochlear implantation with or without electro-acoustic stimulation of the auditory system. The systemic and local drug therapy of another important structure of the inner ear - the vestibular system – should also attract appropriate attention.
The challenges for the introduction of effective pharmaceutical therapies for the inner ear are enormous. They reach from: 1) an increased understanding of disease mechanisms and genetic insights to the identification of targets for medical therapy; 2) improvement and standardization of preclinical animal models with high translational relevance; 3) agreement on universally accepted good laboratory practice (GLP) standards in the auditory system; 4) discovery, formulation and characterization of pharmacological substances including their pharmacokinetics; 5) development of appropriate drug delivery systems 6) improvement of diagnostic tools for patient identification and selection; 7) development of appropriate clinical trial design including standardized, highly quantitative outcome measures that are suitable to measure the mechanism of the investigated drug; and 8) outcome measures that are both objective, and of high patient relevance.
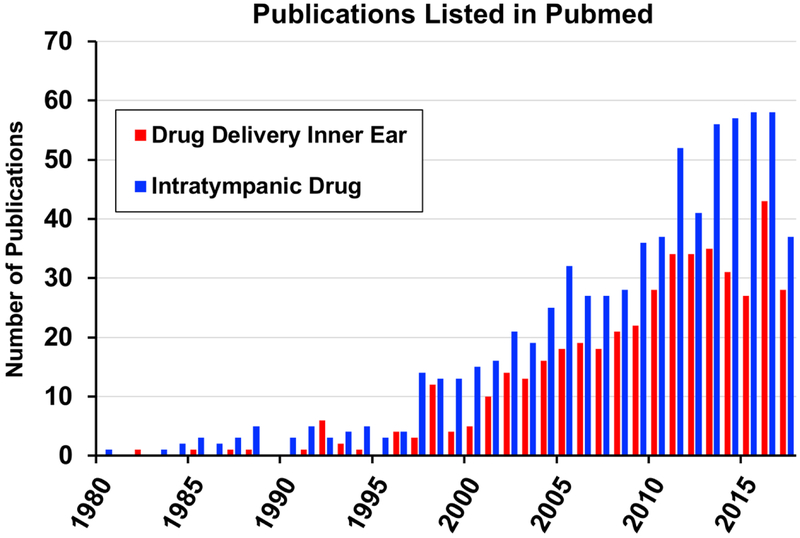
When considering pharmacological therapy, it is common sense that the applied substance needs to reach its target in the inner ear in order to be effective. In the past 25 years, local drug application to the inner ear has experienced increasing interest (Figure 1). Local drug delivery includes 1) Extracochlear (intratympanic) drug application to the diseased, but intact inner ear with the objective of protection through application before trauma or therapeutic intervention by application after exposure; 2) Intracochlear or intralabyrinthine application for drug-, cell-, or gene-based therapy, e.g. aiming at regeneration of inner ear structures; 3) Extra- and intracochlear application in combination with passive middle ear implants, e. g. in the context of stapes surgery, or 4) Extracochlear and/or intracochlear application in combination with auditory prostheses, such as cochlear implants, in order to improve their safety and function.
Figure 1:
Number of publications per year during a 25-year period as a result of a search in PubMed Central on the search terms “Drug Delivery Inner Ear” and “Intratympanic Drug”. Although changes in search terms will lead to slight changes in absolute numbers in the histogram, a rapid growth in publications per year is apparent since the mid 1990’s, when local gentamicin therapy, pioneered by Lange (1989), became widely-adopted as an effective therapy for Meniere’s disease.
The entire field of local drug delivery to the ear is undergoing spectacular growth at the moment, attracting interest from basic scientists, clinicians, funding agencies, and from numerous companies trying to develop and optimize potential drug therapies for the ear. Nevertheless, many open questions exist in relation to drug delivery the ear. They include (adapted from Salt and Plontke, 2009 and Plontke et al. 2017): 1) choice of drug, 2) choice of delivery strategy (systemic, local intratympanic, local intracochlear / intralabyrinthine, combined local and systemic, or directly via an auditory implant); 3) timing of application, including the consideration of chronopharmacological issues especially for drugs with short half-lives (Canlon 2018); 4) choice of formulation and/or drug carrier; 5) choice of drug delivery system; 6) dose to apply; 7) risk–benefit ratio of the therapy; and 8) obstacles to successfully test a promising candidate therapy in a clinical trial.
It has become clear that the demands of patients reinforce the need to develop a cure for hearing loss. It is quite conceivable that patients will agree to and accept even therapies that currently appear rather invasive and “modern”, such as therapies involving inner ear surgery or gene and stem cell therapy. A look towards our neighboring specialty – ophthalmology – reveals that regular intraocular injections are accepted by patients for years, thus, intracochlear and intralabyrinthine injections will likely also be accepted by patients if there is an appropriate risk benefit ratio. Transplantation of stem cells have shown partial success in the inner ear in animals (Chen et al. 2012) and in the eye in humans (da Cruz et al. 2018), and a first human gene therapy trial for severe-to-profound hearing loss is ongoing (NCT02132130 ClinicalTrials.gov Identifier).
With this special issue of ‘Hearing Research’ we aimed to bring together a volume of papers that encapsulates the current state of the art in local drug delivery, including the pitfalls, the paths to success, and how knowledge from substances and delivery systems studied to date can be applied to novel therapeutic candidates and drug delivery devices for inner ear treatments. We hope that the collection of papers will provide a helpful knowledge base for scientists, clinicians, companies and other stakeholders interested in developing and improving the situation for all those with hearing impairment.
Literature:
- Canlon B 2018. Circadian regulation of the inner ear, lecture notes, Barany Society Meeting, delivered June 11th, 2018
- Chen W, Jongkamonwiwat N, Abbas L, Eshtan SJ, Johnson SL, Kuhn S, Milo M, Thurlow JK, Andrews PW, Marcotti W, Moore HD, Rivolta MN. Restoration of auditory evoked responses by human ES-cell-derived otic progenitors. Nature. 2012. October 11;490(7419):278–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Cruz L, Fynes K, Georgiadis O, Kerby J, Luo YH, Ahmado A, Vernon A, Daniels JT, Nommiste B, Hasan SM, Gooljar SB, Carr AF, Vugler A, Ramsden CM, Bictash M, Fenster M, Steer J, Harbinson T, Wilbrey A, Tufail A, Feng G, Whitlock M, Robson AG, Holder GE, Sagoo MS, Loudon PT, Whiting P, Coffey PJ. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat Biotechnol. 2018. March 19. [DOI] [PubMed] [Google Scholar]
- Lange G: Gentamicin and other ototoxic antibiotics for the transtympanic treatment of Menière’s disease. Arch Otorhinolaryngol 1989; 246: 269–270. [DOI] [PubMed] [Google Scholar]
- Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J, Cooper C, Fox N, Gitlin LN, Howard R, Kales HC, Larson EB, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbæk G, Teri L, Mukadam N. Dementia prevention, intervention, and care. Lancet. 2017. December 16;390(10113):2673–2734. [DOI] [PubMed] [Google Scholar]
- NCT02132130 ClinicalTrials.gov Identifier. Novartis Pharmaceuticals. Safety, Tolerability and Efficacy for CGF166 in Patients with Unilateral or Bilateral Severe-to-profound Hearing Loss. https://clinicaltrials.gov/ct2/show/NCT02132130. last accesses on April 2nd, 2018
- Plontke SK, Götze G, Rahne T, Liebau A. Intracochlear drug delivery in combination with cochlear implants: Current aspects. HNO. 2017. January;65(Suppl 1):19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt AN, Plontke SK. Principles of local drug delivery to the inner ear. Audiol Neurootol. 2009;14(6):350–60. [DOI] [PMC free article] [PubMed] [Google Scholar]