Abstract
Purpose
The increasing incidence of hypospadias is partly attributed to increased gestational exposure to endocrine disruptors. We investigated the effects of genistein, the primary phytoestrogen in soy, on the molecular program of male urethral development.
Materials and Methods
Female mice were fed diets supplemented with genistein (500 mg/kg diet) or control diets before breeding and throughout gestation. Urethras from embryonic day 17.5 male fetuses were harvested, and RNA was prepared, amplified, labeled and hybridized on whole genome microarrays. Data were analyzed using packages from the R/Bioconductor project. Immunohistochemical analysis and immunoblotting were used to confirm the activity of MAPK and the presence of Ntrk1 and Ntrk2 during urethral development.
Results
Gestational exposure to genistein altered the urethral expression of 277 genes (p <0.008). Among the most affected were hormonally regulated genes, including IGFBP-1, Kap and Rhox5. Differentially expressed genes were grouped into functional pathways of cell proliferation, adhesion, apoptosis and tube morphogenesis (p <0.0001), and were enriched for members of the MAPK (p <0.00001) and TGF-β (p <0.01) signaling cascades. Differentially expressed genes preferentially contained ELK1, Myc/Max, FOXO, HOX and ER control elements. The MAPK pathway was active, and its upstream genistein affected tyrosine kinase receptors Ntrk1 and Ntrk2 were present in the developing male urethra.
Conclusions
Gestational exposure to genistein contributes to hypospadias by altering pathways of tissue morphogenesis, cell proliferation and cell survival. In particular, genes in the MAPK and TGF-β signaling pathways and those controlled by FOXO, HOX and ER transcription factors are disrupted.
Keywords: endocrine disruptors, gene expression profiling, genistein, hypospadias
Hypospadias is one of the most common congenital abnormalities, affecting approximately 1 in 125 male newborns yearly in the United States.1 Although somewhat controversial, data from independent long-term registries suggest that the incidence of hypospadias is increasing, with a doubling of reported cases from 1970 to 1990.1–3 A putative cause for this greater incidence may be an increase in environmental exposures during gestation, particularly to sex hormones and estrogen disruptors. Supporting this hypothesis, hypospadias has been linked to parental subfertility and use of assisted reproductive technologies.4 In addition, consumption of a vegetarian diet during gestation increases the occur-rence of hypospadias up to 5-fold.5 This latter finding may be due partly to increased exposure of mothers to soy products, which contain phytoestrogens (such as genistein) that act as endocrine disruptors and have been shown to decrease the anogenital distance and cause hypospadias in laboratory mouse models.6,7
Despite the high prevalence of hypospadias, the molecular mechanisms that drive urethral development and go awry in this condition have only begun to be elucidated. In general, hypospadias represents the arrested growth, formation and closure of the urethra and external genitalia.8 In addition to being regulated by androgens, a host of elegant animal studies have also linked genital tubercle development and hypospadias to genes involved in limb patterning, including members of the HOX, Fgf and sonic hedgehog signaling cascades.9–14 Furthermore, by studying differential gene expression in the urethras of male fetal mice before (embryonic day 14) and after (days 16, 17) sexual differentiation, Li et al implicated members of the Wnt-Frizzled and TGF-β gene families in urethral patterning.15 However, to date there have been no studies performed on the molecular effects of endocrine disruptors or other environmental agents on the developing urethra. By using differential gene expression and functional annotation enrichment analysis, we begin to describe signaling pathways that are altered during male urethral development on exposure of the mother to genistein during gestation.
MATERIALS AND METHODS
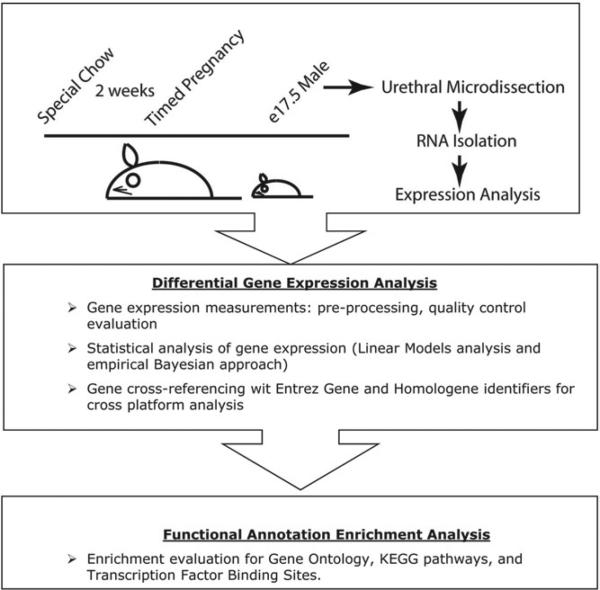
C57BL/6J female mice were fed either soy free casein diets (control group, Jackson Laboratory, Bar Harbor, Maine) or diets supplemented with 500 mg/kg genistein (LabDiet®, experimental group, LC Laboratories®) for at least 2 weeks before breeding and throughout the gestational period. Pregnancies were timed according to scheduled 6-hour pairings. Pregnant females were euthanized on E 17.5 and urethras were harvested by microdissection, allowing for separation of the urethra from the urogenital sinus tissue, and then snap frozen in liquid nitrogen (fig. 1). A total of 16 male urethras were harvested from the pups of 4 pregnant females in each experimental group (32 urethras total).
Figure 1.
Flow chart of experimental design
Pooled frozen urethral tissue from each experimental group was homogenized, and total RNA was purified using the RNeasy® system. RNA quality was assessed using a 2100 bioanalyzer (Agilent Technologies Inc., Santa Clara, California), and high quality RNA (RNA integrity score 10) was amplified, labeled and hybridized in triplicate (allowing for Cy5 and Cy3 dye swaps) on 44,000 probe, whole genome mouse microarrays (Agilent Technologies) according to manufacturer directions. A detailed explanation of the gene expression data preprocessing and array can be found in accompanying supplementary materials online.
Gene expression data were processed for statistical analysis using packages from R/Bioconductor (www.bioconductor.org) essentially as described by Schaeffer et al.16 Briefly a mixed effects model was fit for each gene to estimate expression differences between groups, and an empirical Bayesian approach was applied to moderate standard errors. Functional themes were obtained from GO, KEGG and MsigDb, and used to perform enrichment analysis using 1-sided Wilcoxon tests. Multiple testing correction was performed using the Benjamini-Hochberg method. Detailed descriptions of the statistical methods used are provided as supplementary materials online. Data will be hosted in the Gene Expression Omnibus database on publication.
Immunohistochemical analysis was performed on 4% paraformaldehyde fixed 4 μM paraffin sections after antigen retrieval in sodium citrate 10 mM pH 6.0 using antibodies for either Ntrk1 or Ntrk2 (abcam, ab37837 and ab51190, respectively). Protein identification by Western blotting was carried out using these antibodies as well as antibodies directed against total p44/42 MAPK (ERK1/2) and phospho-p44/42 MAPK (ERK1/2, cell signaling 9102 and 9101, respectively) after homogenization and lysis of urethras in NuPAGE® LDS sample buffer containing protease and phosphatase inhibitors.
RESULTS
Genome wide expression analysis of urethras derived from the male fetuses (E 17.5) of mothers exposed to genistein supplemented or control diets revealed differential gene expression among 277 genes (p <0.008). Of these genes 158 were induced by genistein exposure and 119 were repressed. Among the male fetal urethral genes most affected by genistein exposure were insulin-like growth factor binding protein 1, which was up-regulated, and kidney androgen regulated protein and reproductive homeobox5, which were down-regulated (supplemental tables 1 and 2).
To identify functional gene categories and signal transduction pathways altered in the urethras by gestational exposure to genistein, we performed analysis of functional annotation (supplementary data). Genes affected by genistein exposure fell into distinct gene ontology categories, including those for anatomical structure and tube morphogenesis as well as regulators of cell proliferation, adhesion and apoptosis (p <0.0001 for all categories). Analysis of enriched KEGG pathways, which varied with urethral exposure to genistein, identified members of the MAPK and TGF-β signaling cascades (p <0.00001 and p <0.01, respectively). Correlating with the enrichment of MAPK signaling pathways, we observed a propensity for differentially expressed genistein affected genes to have ELK1 and Myc/Max binding sites in their promoters (supplemental table 1).17 In addition, genes whose expression varied with genistein exposure also contained transcription factor binding sites for members of the forkhead and HOX family of transcription factors as well as for estrogen receptor.
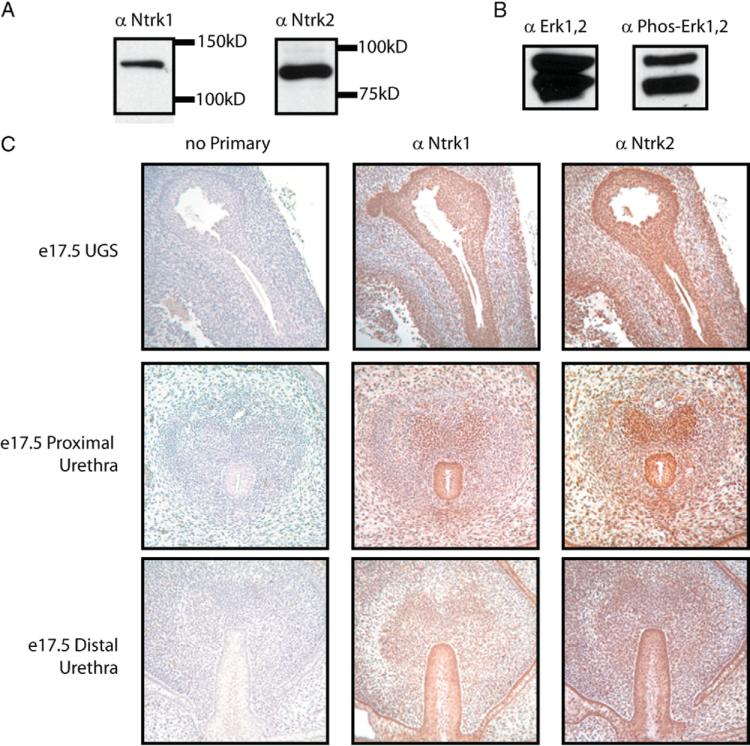
Among genes that were consistently repressed by genistein exposure and present in enriched GO and KEGG categories were Ntrk1 and Ntrk2. These genes code for tyrosine kinase receptors, which transmit signals through the MAPK pathway and have roles in cell survival and differentiation.18 While described primarily in the nervous system, Ntrk1 and Ntrk2 have also been implicated in other organ systems.19 How-ever, their expression in the urethra or developing urogenital sinus has not previously been demonstrated. Western blot analysis showed the presence of active MAPK signaling (via phosphorylation of ERK1/2) and of Ntrk1 and Ntrk2 proteins in the urethra of E 17.5 fetal male mice on normal diets (fig. 2, a and b). In addition, immunohistochemical staining revealed Ntrk1 and Ntrk2 expression in the urogenital epithelium as well as the epithelium of the proximal and distal urethra (fig. 2, c).
Figure 2.
MAPK pathway is active during urethral development, and its upstream tyrosine kinases Ntrk1 and Ntrk2 are expressed in urethral epithelium. All mice were fed normal chow not supplemented with genistein. Western blot analysis of whole cell lysates derived from isolated E 17.5 male fetal urethras are shown using antibodies recognizing Ntrk1 or Ntrk2 (a), or phospho-ERK1,2 or total ERK1,2 (b). Serial tissue sections of urogenital sinus (UGS) and urethras (c, top panels) and through genital tubercle (c, middle and bottom panels) of E 17.5 male fetuses stained either with no primary antibody or with antibodies recognizing Ntrk1 or Ntrk2.
DISCUSSION
Consumption of soy has been steadily increasing worldwide through its use as a meat substitute and via its addition to animal feed.20 Genistein, a natural iso-flavone, is the predominant phytoestrogen found in soy and, as a compound that alters normal endocrine signaling, is considered an endocrine disruptor.21 Accordingly genistein has been linked to development of hypospadias in animal models, and may contribute to the increasing incidence of hypospadias, particularly in the newborns of vegetarian mothers.5,6 Via gene expression and functional enrichment analysis we provide the first description of the molecular pathways through which genistein may alter normal male urethral development.
Formation of the urethra and male external genitalia is complex, with tissue morphogenesis involving a delicate interplay between cell growth, differentiation and programmed cell death.1 Preferential enrichment of genes affected by genistein into gene ontology classes for cell proliferation, organ morphogenesis and apoptosis suggests that gestational exposure to genistein contributes to hypospadias by altering all of these critical processes. Furthermore, our study highlights particular genes and signaling pathways that are disrupted by genistein in urethral development. For instance IGFBP-1 was among the genes most induced by genistein, increasing its expression more than 3-fold. This protein has previously been described in the liver, kidney and genital tract, and serves to bind IGF and modulate its bioavailability.22 Mice expressing IGFBP-1 on a transgenic construct have reduced genital tract weights. Additionally IGF-1 expression is increased in the developing male urethra after sexual differentiation.15 This combined evidence suggests that one mechanism by which genistein contributes to hypospadias is inhibition of IGF-1 activity.
Cell proliferation in the urethra is influenced by multiple pathways. Previously emphasis had been placed on the Fgfs, particularly Fgf8, due to its role in limb development.10,11 In the current study we observed down-regulation of Fgf9 on genistein exposure. Although the mechanistic role of Fgf9 in urethral development has not previously been studied, Fgf9 is expressed in the distal urethral epithelium during development to a broader extent than Fgf8.23
In addition to an obvious requirement for increased cellular growth, genital tubercle development is highly dependent on apoptosis, which precedes critical fusion events, making it essential for the closure of the penile urethra.9,11,24 Apoptotic signals in the urethral plate epithelium are transmitted partly through bone morphogenic proteins and their target genes MSX1 and MSX2, which function as transcription factors.11 We found that exposure to genistein changed the expression of genes that were enriched in pathways of apoptosis, and particularly in genes responsive to MSX1 and FOXO3. Like MSX1, FOXO3 has also been demonstrated to mediate cell death but has not previously been described in the urethra.25
An examination of the molecular effects of gestational genistein exposure allows for an increased understanding of genistein function as an endocrine disruptor and also serves to increase our understanding of normal urethral development. For example among the most differentially expressed genes were IGFBP-1, Kap and Rhox5, all of whose expression had previously been shown to be hormonally regulated.26–28 In addition, among the pathways most affected by genistein exposure was the MAPK signaling pathway. While known to have key roles in growth and differentiation, MAPK activity has not previously been described in the developing urethra. We observed that MAPK signaling occurs during urethral development, as evidenced by the presence of phosphorylated ERK1/2. Furthermore, we identified Ntrk1 and Ntrk2 as present in the urogenital and urethral epithelium. Ntrk1 and Ntrk2 represent tyrosine kinases that signal through the MAPK pathway and have been observed to modulate cell growth and differentiation in other tissues, particularly in the nervous system, but have not been described in the urethra.18 As upstream enzymatic components of the MAPK signaling cascade, their down-regulation by genistein exposure suggests that these proteins may be critical for normal urethral development, and elucidation of their activities in this process will be the subject of a future study.
While this study lends insight into how genistein affects urethral development at the molecular level, it has several limitations. It is noteworthy that in this initial series a relatively high level of dietary genistein supplementation (500 mg/kg diet) was used. Previous experiments in mice revealed that this level of supplementation results in mean ± SD serum genistein levels of 397 ± 105 nmol/l.29 These levels exceed, but are somewhat similar to, those found in the serum of Japanese men consuming ordinary Asian diets (mean 276 nmol/l, range 116 to 652).30 As genistein levels vary greatly with dietary consumption and environmental exposure, we are currently conducting studies to determine dose response relationships of genistein and the molecular alterations caused by its ingestion.
CONCLUSIONS
Genistein, an endocrine disruptor and the primary phytoestrogen in soy, contributes to hypospadias by altering pathways of organ morphogenesis, cell proliferation and cell survival. In particular, gestational genistein exposure affects gene expression in the MAPK and TGF-β signaling cascades and that of genes under the influence of transcription factors in the forkhead, homeobox and estrogen receptor families. Future functional analysis of these genes will provide further insight into the mechanisms of tissue patterning in the male penis.
Supplementary Material
Acknowledgments
Supported by National Institute of Diabetes and Digestive and Kidney Diseases Awards T32DK007552 (AER) and K08DK081019 (EMS), Howard Hughes Medical Institution (EMS) and American Urology Association Foundation/Astellas Rising Star in Urology Award (EMS).
Abbreviations and Acronyms
- E
embryonic day
- ERK
extracellular signal related kinase
- Fgf
fibroblast growth factor
- FOXO
forkhead box
- GO
gene ontology
- HOX
homeobox
- IGF
insulin-like growth factor
- IGFBP-1
insulin-like growth factor binding protein 1
- Kap
kidney androgen regulated protein
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- MAPK
mitogen activated protein kinase
- MsigDb
Molecular Signatures Database
- MSX
Msh homeobox
- Ntrk1
neurotrophic tyrosine kinase receptor, type 1
- Ntrk2
neurotrophic tyrosine kinase receptor, type 2
- Rhox5
reproductive homeobox5
- TGF-β
transforming growth factor-beta
Footnotes
Supplementary material is available at http://astor.som.jhmi.edu/~marchion/urethra.html.
REFERENCES
- 1.Baskin LS, Himes K, Colborn T. Hypospadias and endocrine disruption: is there a connection? Environ Health Perspect. 2001;109:1175. doi: 10.1289/ehp.011091175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paulozzi LJ, Erickson JD, Jackson RJ. Hypospadias trends in two US surveillance systems. Pediatrics. 1997;100:831. doi: 10.1542/peds.100.5.831. [DOI] [PubMed] [Google Scholar]
- 3.Toppari J, Kaleva M, Virtanen HE. Trends in the incidence of cryptorchidism and hypospadias, and methodological limitations of registry-based data. Hum Reprod Update. 2001;7:282. doi: 10.1093/humupd/7.3.282. [DOI] [PubMed] [Google Scholar]
- 4.Funke S, Flach E, Kiss I, et al. Male reproductive tract abnormalities: more common after assisted reproduction? Early Hum Dev. 2010;86:547. doi: 10.1016/j.earlhumdev.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 5.North K, Golding J. A maternal vegetarian diet in pregnancy is associated with hypospadias. The ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. BJU Int. 2000;85:107. doi: 10.1046/j.1464-410x.2000.00436.x. [DOI] [PubMed] [Google Scholar]
- 6.Vilela ML, Willingham E, Buckley J, et al. Endocrine disruptors and hypospadias: role of genistein and the fungicide vinclozolin. Urology. 2007;70:618. doi: 10.1016/j.urology.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Wisniewski AB, Cernetich A, Gearhart JP, et al. Perinatal exposure to genistein alters reproductive development and aggressive behavior in male mice. Physiol Behav. 2005;84:327. doi: 10.1016/j.physbeh.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Kurzrock EA, Baskin LS, Cunha GR. Ontogeny of the male urethra: theory of endodermal differentiation. Differentiation. 1999;64:115. doi: 10.1046/j.1432-0436.1999.6420115.x. [DOI] [PubMed] [Google Scholar]
- 9.Haraguchi R, Mo R, Hui C, et al. Unique functions of sonic hedgehog signaling during external genitalia development. Development. 2001;128:4241. doi: 10.1242/dev.128.21.4241. [DOI] [PubMed] [Google Scholar]
- 10.Haraguchi R, Suzuki K, Murakami R, et al. Molecular analysis of external genitalia formation: the role of fibroblast growth factor (Fgf) genes during genital tubercle formation. Development. 2000;127:2471. doi: 10.1242/dev.127.11.2471. [DOI] [PubMed] [Google Scholar]
- 11.Morgan EA, Nguyen SB, Scott V, et al. Loss of Bmp7 and Fgf8 signaling in Hoxa13-mutant mice causes hypospadia. Development. 2003;130:3095. doi: 10.1242/dev.00530. [DOI] [PubMed] [Google Scholar]
- 12.Perriton CL, Powles N, Chiang C, et al. Sonic hedgehog signaling from the urethral epithelium controls external genital development. Dev Biol. 2002;247:26. doi: 10.1006/dbio.2002.0668. [DOI] [PubMed] [Google Scholar]
- 13.Stadler HS, Higgins KM, Capecchi MR. Loss of Eph-receptor expression correlates with loss of cell adhesion and chondrogenic capacity in Hoxa13 mutant limbs. Development. 2001;128:4177. doi: 10.1242/dev.128.21.4177. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y, Potter SS. Functional specificity of the Hoxa13 homeobox. Development. 2001;128:3197. doi: 10.1242/dev.128.16.3197. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Willingham E, Baskin LS. Gene expression profiles in mouse urethral development. BJU Int. 2006;98:880. doi: 10.1111/j.1464-410X.2006.06435.x. [DOI] [PubMed] [Google Scholar]
- 16.Schaeffer EM, Marchionni L, Huang Z, et al. Androgen-induced programs for prostate epithelial growth and invasion arise in embryogenesis and are reactivated in cancer. Oncogene. 2008;27:7180. doi: 10.1038/onc.2008.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis RJ. Transcriptional regulation by MAP kinases. Mol Reprod Dev. 1995;42:459. doi: 10.1002/mrd.1080420414. [DOI] [PubMed] [Google Scholar]
- 18.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackay S, Smith RA. Effects of growth factors on testicular morphogenesis. Int Rev Cytol. 2007;260:113. doi: 10.1016/S0074-7696(06)60003-X. [DOI] [PubMed] [Google Scholar]
- 20.Aginsky Consulting Group . Global Soybean Trends and Supplier Overview. Market Publishers Inc; Parlin, New Jersey: 2008. [Google Scholar]
- 21.Price KR, Fenwick GR. Naturally occurring oestrogens in foods—a review. Food Addit Contam. 1985;2:73. doi: 10.1080/02652038509373531. [DOI] [PubMed] [Google Scholar]
- 22.Benjamini Y, Drai D, Elmer G, et al. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 23.Satoh Y, Haraguchi R, Wright TJ, et al. Regulation of external genitalia development by concerted actions of FGF ligands and FGF receptors. Anat Embryol (Berl) 2004;208:479. doi: 10.1007/s00429-004-0419-9. [DOI] [PubMed] [Google Scholar]
- 24.van der Werff JF, Nievelstein RA, Brands E, et al. Normal development of the male anterior urethra. Teratology. 2000;61:172. doi: 10.1002/(SICI)1096-9926(200003)61:3<172::AID-TERA4>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 25.Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 26.Bhardwaj A, Rao MK, Kaur R, et al. GATA factors and androgen receptor collaborate to transcriptionally activate the Rhox5 homeobox gene in Sertoli cells. Mol Cell Biol. 2008;28:2138. doi: 10.1128/MCB.01170-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Everaus H, Luik E, Lehtmaa J. Active and indolent chronic lymphocytic leukaemia—immune and hormonal peculiarities. Cancer Immunol Immunother. 1997;45:109. doi: 10.1007/s002620050409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malstrom SE, Tornavaca O, Meseguer A, et al. The characterization and hormonal regulation of kidney androgen-regulated protein (Kap)-luciferase transgenic mice. Toxicol Sci. 2004;79:266. doi: 10.1093/toxsci/kfh125. [DOI] [PubMed] [Google Scholar]
- 29.Lamartiniere CA, Cotroneo MS, Fritz WA, et al. Genistein chemoprevention: timing and mechanisms of action in murine mammary and prostate. J Nutr. 2002;132:552S. doi: 10.1093/jn/132.3.552S. [DOI] [PubMed] [Google Scholar]
- 30.Adlercreutz H, Markkanen H, Watanabe S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet. 1993;342:1209. doi: 10.1016/0140-6736(93)92188-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.