Abstract
Background
The favorable role of exercise on metabolic syndrome is well established; however, there is a lack of consistent epidemiological data. Thus, we analyzed the association between exercise intensity and metabolic syndrome using data from the Fourth Korea National Health and Nutrition Examination Survey (2007–2009).
Methods
A total of 10,533 Korean individuals were screened. Exercise amount and intensity were assessed from questionnaire responses. Subjects were divided into three groups according to exercise intensity: no activity (n=607), walking only without intense exercise (n=2,336), and moderate to vigorous activity with or without walking (n=3,855). Logistic regression analyses were used to evaluate the associations between exercise intensity and metabolic syndrome.
Results
The overall prevalence of metabolic syndrome was 22.3% (total n=6,798). The prevalence of metabolic syndrome was 20.4% for the intense exercise group, 24.0% for the walking only group, and 29.9% for the no activity group (P<0.001). The intense exercise group had a significantly lower odds ratio for the prevalence of metabolic syndrome and its components, especially abdominal obesity and hypertriglyceridemia, but not for blood pressure. Interestingly, there were no negative associations identified within the walking only group, other than prevalence of metabolic syndrome itself. The risk of hyperglycemia was slightly lower in the walking group compared to the no activity group but disappeared after multiple adjustments.
Conclusion
A strong inverse relationship between metabolic syndrome and moderate to vigorous intensity exercise was identified, which may reflect a protective effect of intense exercise, but not walking, on metabolic syndrome. Further prospective studies are needed to consolidate our findings.
Keywords: Exercise, Intensity, Walking, Metabolic syndrome, Abdominal obesity
INTRODUCTION
Metabolic syndrome is characterized by increased insulin resistance and is associated with a high risk of cardiovascular disease and type 2 diabetes.1 Over the last several decades, metabolic syndrome has grown to a serious problem world-wide, including Korea, as a result of changes in dietary habits and increasing physical inactivity.2,3 Although the pathogenesis of metabolic syndrome is not fully understood, progression of metabolic syndrome is closely associated with physical inactivity and consumption of a diet high in fats and carbohydrates.4 These factors aggravate obesity and insulin resistance, and seem to exacerbate metabolic syndrome in conjunction with other risk factors such as genetics, inflammation, and adipokines.5
Based on the pathophysiology of metabolic syndrome, exercise is considered one of the cornerstones of management.5–9 In 2010, the World Health Organization launched a global recommendation for reducing metabolic syndrome that comprised at least 60 minutes of moderate to vigorous physical activity per day is for children and adolescents.10 In addition, regular exercise reduces body weight, waist circumference, and insulin insensitivity, while improving both dyslipidemia and hypertension.11
With respect to exercise intensity, many studies have shown that a high level of exercise reduces the risk of developing metabolic syndrome7,12–14; however, several important issues surrounding the role of exercise remains. For example, most of the studies to date have been conducted in Europe and the United States9,12,13,15,16, whereas there are relatively few studies of Asian populations.14 In addition, the benefits of low intensity exercise on metabolic syndrome are controversial. Some studies have suggested that there is a linear relationship between exercise intensity and development of metabolic syndrome, while others have indicated that only high frequency exercise is effective for reducing the risk of metabolic syndrome. These results are important for prescribing exercise strategies, and many physicians are reluctant to recommend high intensity activities for certain groups such as the elderly who are easily tired and for whom moderate to severe activity may be harmful.
In the present study, we aimed to investigate the association between exercise intensity and metabolic syndrome in Koreans using data from the Korea National Health and Nutrition Examination Survey (KNHANES 2007–2009).
METHODS
Study population
This study used the dataset from the KNHANES IV, a nationwide survey conducted between 2007 and 2009. KNHANES has periodically assessed the health and nutritional status of Koreans since 1998, and is a nationally representative cross-sectional survey that consists of a medical history, physical examination, health behavior survey, and anthropometric and biochemical measurements. We analyzed data for 5,690 women and 4,843 men from the second year (2007–2009) of KNHANES IV. The exclusion criteria were as follows: ≤18 years of age (n=2,521), pregnancy (n=43), malignancy (n=145), liver cirrhosis (n=10), chronic obstructive pulmonary disease or active tuberculosis (n=60), and chronic renal disease (n=19). Of the remaining 7,735 participants, we further excluded cases with insufficient data for metabolic syndrome or exercise (n=729) or inadequate fasting time less than 8 hours (n=208). Finally, 6,798 subjects were included in our analysis. All participants provided written informed consent and the study was approved by the Institutional Review Board of Korea Centers for Disease Control and Prevention (No. 2010-02CON-21-C, 2011-02CON-06-C, and 2012-01EXP-01-2C).
Assessment of exercise and metabolic syndrome
Physical activity was evaluated using the International Physical Activity Questionnaire. Subjects were asked if they had exercised for at least 10 minutes during the last week for different types of physical activity consisting of walking only, moderate physical activities (slow swimming, playing tennis doubles/volleyball/badminton/table tennis, transporting light objects, etc.), and vigorous physical activity (running, mountain climbing, soccer/basketball/squash/single tennis, fast cycling, fast swimming, skipping rope, transporting heavy objects, etc.).
Subjects were further divided according to activity intensity: no activity group, walking only group, and moderate to vigorous activity group. Because exercise frequency and exercise days were similar between the moderate physical activity group and vigorous physical activity group, both groups were combined into the intense exercise group. On the other hand, the walking exercise group had a different pattern from the other two groups, and thus the walking exercise group was defined separately. Subjects who indicated that they did not participate in physical activity were assigned to “group I,” while others who indicated that they only walked but did not participate in any moderate or vigorous activity were assigned to “group II.” All others who indicated participation in moderate to vigorous activity were assigned to “group III” regardless of walking.
Trained medical staff measured subjects’ height and weight to the nearest 0.1 cm and 0.1 kg by portable stadiometer (Seriter, Bismarck, ND, USA) over a range of 850 to 2,069 mm and balance beam scale (Giant-150N; HANA, Seoul, Korea). Waist circumference was measured according to World Health Organization guidelines, namely, the midpoint between the inferior margin of the last rib and iliac crest in a horizontal plane. Body mass index (BMI) was calculated as weight (in kilograms) divided by height squared (in meters). Blood pressure was assessed using an oscillometric method with an automated sphygmomanometer. Serum glucose, triglyceride, and high-density lipoprotein (HDL) cholesterol levels were analyzed using an ADIVIA650 (Siemens, Madison, WI, USA).
Metabolic syndrome was defined according to the modified National Cholesterol Education Program Adult Treatment Panel III criteria. Metabolic syndrome was diagnosed when patients fulfilled three of the five criteria listed as follows. Waist-circumference was modified because of ethnic differences17: (1) waist-circumference: man ≥90 cm, woman ≥85 cm; (2) blood pressure: systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg; (3) fasting glucose: ≥100 mg/dL; (4) triglyceride: ≥150 mg/dL; (5) HDL cholesterol: man <40 mg/dL, woman <50 mg/dL.
Alcohol consumption and smoking status were estimated from subjects’ self-reported typical daily consumption. Drinking was divided into three groups by lifetime drinking experience in adults: heavy drinker (>1/wk), moderate drinker (≤1/wk), and nondrinker. Smoking status was likewise classified as current smoker, ex-smoker, or nonsmoker. Monthly household income was categorized into quartiles: lowest (<$500), lowest–medium ($500–1,000), medium–highest ($1,000–3,000), and highest (≥$3,000).
Statistical analysis
Statistical analyses were performed using the R software version 3.4.1 (R Foundation, Vienna, Austria) to examine the association between exercise and metabolic syndrome in a complex sampling design. To reflect nationwide prevalence estimates, samples from KNHANES were weighted in all analyses. The study population was stratified into three groups based on exercise intensity. With respect to baseline characteristics, continuous variables were presented as mean with standard error (SE), while categorical variables were presented as percentage with SE.
Associations between exercise intensity and metabolic syndrome or its components were analyzed using multiple logistic regression. Continuous variables such as age, BMI, and total energy intake were dichotomized using median cutoff values. Continuous variables were analyzed by either independent t-test or analysis of variance, while categorical variables were analyzed by Rao-Scott chi-square test. Regression models were sequentially adjusted for the following variables: age, sex, and BMI (model 1); total energy intake and smoking (model 2); and alcohol intake and household income (model 3). All statistical tests were two-sided, and P-values <0.05 were considered statistically significant.
RESULTS
Baseline characteristics
The baseline characteristics of the study population are shown in Table 1. The mean age was 44.0±0.4 years and mean BMI was 23.6±0.1 kg/m2. Group III (moderate to vigorous activity) included 57.2% males, which was a higher proportion than group I (no activity group, 41.6%) and group II (walking only group, 41.1%). The percentages of current smoking or drinking, a comparatively high income, and total energy intake were also higher in group III than other groups. Factors associated with metabolic syndrome were generally more favorable in the exercise groups, with the exception of diastolic blood pressure and triglyceride levels. Specifically, diastolic blood pressure was highest (76.3±0.3 mmHg) in group III and lowest (74.6±0.3 mmHg) in group II. Triglyceride levels were lower in the exercise groups (~132 mg/dL) compared to group I (143.9±5.7 mg/dL), but the difference was not significant (P=0.126). The proportion of subjects taking antidiabetic and antihypertensive medications was highest in group I. The number of days per week that subjects reported walking was 4.8±0.1 in group II and 4.7±0 in group III, and the time spent walking was comparable between groups (395.3±16.2 min/wk for group II and 419.8±11.5 min/wk for group III, respectively). Participants in group III spent 2.8±0.1 day/wk participating in moderate activity and 1.9±0 day/wk participating in vigorous activity. More specifically, group III subjects spent 346.5±12.5 min/wk participating in moderate activity and 209.0±8.1 min/wk participating in vigorous activity (total, 555.5±16.0 min/wk).
Table 1.
Baseline characteristics of study subjects
| Variable | Total (n=6,798) | Group I (n=607) | Group II (n=2,336) | Group III (n=3,855) | P |
|---|---|---|---|---|---|
| Age (yr) | 44.0±0.4 | 50.0±0.9 | 45.5±0.5 | 42.4±0.4 | <0.001* |
|
| |||||
| Male | 50.8 (0.7) | 41.6 (2.50) | 41.1 (1.22) | 57.2 (0.88) | <0.001† |
|
| |||||
| Body mass index (kg/m2) | 23.6±0.1 | 23.6±0.2 | 23.4±0.1 | 23.8±0.1 | 0.003* |
|
| |||||
| Waist circumference (cm) | 80.7±0.2 | 82.0±0.5 | 80.2±0.3 | 80.8±0.2 | 0.006* |
|
| |||||
| Systolic blood pressure (mmHg) | 116.1±0.4 | 119.2±1.0 | 116.0±0.5 | 115.8±0.4 | 0.002* |
|
| |||||
| Diastolic blood pressure (mmHg) | 75.7±0.2 | 76.0±0.6 | 74.6±0.3 | 76.3±0.3 | <0.001* |
|
| |||||
| Smoking status | <0.001† | ||||
| Nonsmoker | 54.1 (0.77) | 54.0 (2.74) | 59.0 (1.18) | 51.5 (1.05) | |
| Ex-smoker | 19.4 (0.61) | 21.4 (2.32) | 18.0 (0.89) | 20.0 (0.78) | |
| Current smoker | 26.4 (0.75) | 24.6 (2.31) | 23.0 (1.07) | 28.5 (1.01) | |
|
| |||||
| Alcohol consumption | <0.001† | ||||
| None | 23.5 (0.75) | 30.3 (2.37) | 28.4 (1.16) | 20 (0.89) | |
| Moderate | 53.4 (0.87) | 47.7 (2.49) | 52.8 (1.48) | 54.5 (1.02) | |
| Heavy | 23.0 (0.70) | 22.0 (2.22) | 18.9 (1.10) | 25.4 (0.91) | |
|
| |||||
| Monthly household income | <0.001† | ||||
| Lowest | 15.7 (0.80) | 21.5 (1.92) | 19.9 (1.20) | 12.8 (0.88) | |
| Lowest–medium | 22.4 (1.05) | 24.6 (2.43) | 21.4 (1.24) | 22.7 (1.31) | |
| Medium–highest | 30.5 (1.17) | 28.0 (2.72) | 29.4 (1.44) | 31.4 (1.40) | |
| Highest | 31.4 (1.47) | 25.9 (2.51) | 29.3 (1.53) | 33.2 (1.77) | |
|
| |||||
| Total energy intake (kcal/day) | 1,965.4±14.9 | 1,870.4±47.1 | 1,847.2±25.0 | 2,041.7±19.1 | <0.001* |
|
| |||||
| Current medication | |||||
| Hypertension | 13.6 (0.58) | 18.3 (1.71) | 14.7 (0.99) | 12.3 (0.67) | 0.001† |
| Diabetes | 4.9 (0.31) | 7.5 (1.22) | 5.3 (0.52) | 4.3 (0.38) | 0.009† |
| Dyslipidemia | 3.1 (0.26) | 4.2 (0.93) | 2.7 (0.38) | 3.2 (0.34) | 0.213† |
|
| |||||
| Blood measurement | |||||
| Fasting glucose (mg/dL) | 96.6±0.4 | 99.7±1.3 | 97.3±0.7 | 95.8±0.4 | 0.007* |
| HDL cholesterol (mg/dL) | 52.2±0.2 | 50.7±0.6 | 51.7±0.3 | 52.7±0.3 | 0.001* |
| Triglyceride (mg/dL) | 132.8±1.8 | 143.9±5.7 | 131.4±2.5 | 132.2±2.6 | 0.126* |
|
| |||||
| Exercise frequency (times/wk) | 0.106‡ | ||||
| Walking | 4.3±0 | 0 | 4.8±0.1 | 4.7±0 | |
| Moderate activity | 1.7±0.1 | 0 | 0 | 2.8±0.1 | |
| Vigorous activity | 1.1±0 | 0 | 0 | 1.9±0 | |
| Moderate to vigorous activity | 2.8±0.1 | 0 | 0 | 4.7±0.1 | |
|
| |||||
| Exercise duration (min/wk) | 0.206‡ | ||||
| Walking | 379.9±9.0 | 0 | 395.3±16.2 | 419.8±11.5 | |
| Moderate activity | 207.4±8.6 | 0 | 0 | 346.5±12.5 | |
| Vigorous activity | 125.1±5.1 | 0 | 0 | 209.0±8.1 | |
| Moderate to vigorous activity | 32.5±11.4 | 0 | 0 | 555.5±16.0 | |
Values are presented as mean±standard error or percentage (standard error). Group I, no activity group; Group II, walking only group; Group III, moderate to vigorous activity group.
P-values from
analysis of variance;
Rao-Scott chi-square test;
Independent t-test.
HDL, high-density lipoprotein.
Exercise and metabolic syndrome
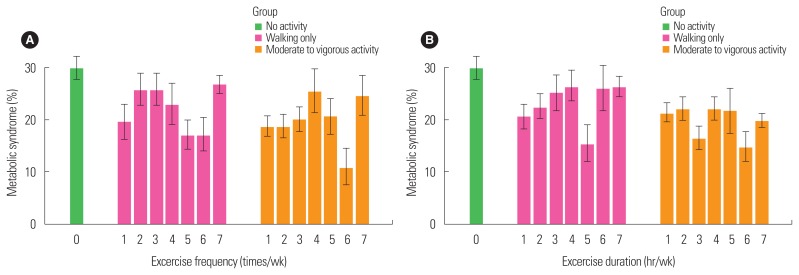
Fig. 1A shows the prevalence of metabolic syndrome according to exercise frequency. The overall percentage of metabolic syndrome was highest in group I (29.9%) compared to group II (24.0%) and group III (20.4%). Frequency of exercise was not associated with the prevalence of metabolic syndrome. Likewise, duration of exercise according to intensity was not associated with the prevalence of metabolic syndrome (Fig. 1B). However, the prevalence of metabolic syndrome was associated with exercise intensity (Table 2). After multiple adjustments, the odds ratios for metabolic syndrome in group II and group III compared to group I were 0.750 (95% confidence interval [CI], 0.564–0.996; P=0.049) and 0.591 (95% CI, 0.446–0.782; P<0.001), respectively. With the exception of blood pressure, the components of metabolic syndrome were negatively associated with moderate to vigorous activity compared to the no activity group. The odds ratios for group III compared to group I were calculated for low abdominal obesity (0.591; 95% CI, 0.396–0.883; P=0.011), low HDL cholesterol (0.768; 95% CI, 0.600–0.984; P=0.038), and high triglyceride levels (0.610; 95% CI, 0.466–0.799; P<0.001) after multiple adjustments. The odds ratio for high fasting glucose was initially lower in group III than group I, but the significance disappeared after multiple adjustments. On the other hand, the odds ratios for the different components of metabolic syndrome were not decreased in subjects who indicated they only participated in walking. The odds ratio for elevated fasting glucose was initially lower in group II compared to group I (0.758; 95% CI, 0.596–0.965; P=0.026); however, the significance disappeared after multiple adjustments.
Figure 1.
The prevalence of metabolic syndrome according to exercise intensity with frequency (A) and duration (B). The prevalence of metabolic syndrome is shown according to the frequency of exercise (times per week, at least 10 minutes at once) (A) and the duration of exercise (hours per week) (B) within the three groups, which are categorized according to exercise intensity.
Table 2.
Prevalence of metabolic syndrome and its components according to exercise intensity
| Exercise intensity | Group I | Group II | Group III | ||
|---|---|---|---|---|---|
|
|
|
||||
| OR (95% CI) | P | OR (95% CI) | P | ||
| Metabolic syndrome | |||||
| Model 1 | 1.0 | 0.720 (0.547–0.949) | 0.021 | 0.540 (0.413–0.705) | <0.001 |
| Model 2 | 1.0 | 0.738 (0.562–0.971) | 0.031 | 0.562 (0.430–0.734) | <0.001 |
| Model 3 | 1.0 | 0.750 (0.564–0.996) | 0.049 | 0.591 (0.446–0.782) | <0.001 |
|
| |||||
| Abdominal obesity | |||||
| Model 1 | 1.0 | 0.692 (0.470–1.020) | 0.065 | 0.536 (0.362–0.795) | 0.002 |
| Model 2 | 1.0 | 0.713 (0.486–1.046) | 0.085 | 0.553 (0.374–0.816) | 0.003 |
| Model 3 | 1.0 | 0.744 (0.504–1.097) | 0.138 | 0.591 (0.396–0.883) | 0.011 |
|
| |||||
| High systolic blood pressure | |||||
| Model 1 | 1.0 | 0.815 (0.632–1.050) | 0.115 | 0.822 (0.636–1.062) | 0.136 |
| Model 2 | 1.0 | 0.810 (0.628–1.044) | 0.106 | 0.828 (0.642–1.069) | 0.149 |
| Model 3 | 1.0 | 0.841 (0.644–1.098) | 0.204 | 0.902 (0.689–1.179) | 0.450 |
|
| |||||
| High diastolic blood pressure | |||||
| Model 1 | 1.0 | 0.780 (0.586–1.037) | 0.089 | 0.912 (0.705–1.179) | 0.481 |
| Model 2 | 1.0 | 0.781 (0.588–1.038) | 0.090 | 0.917 (0.710–1.185) | 0.509 |
| Model 3 | 1.0 | 0.805 (0.605–1.071) | 0.139 | 0.934 (0.724–1.206) | 0.603 |
|
| |||||
| Elevated fasting glucose | |||||
| Model 1 | 1.0 | 0.758 (0.596–0.965) | 0.026 | 0.767 (0.601–0.979) | 0.034 |
| Model 2 | 1.0 | 0.774 (0.608–0.985) | 0.038 | 0.794 (0.623–1.012) | 0.064 |
| Model 3 | 1.0 | 0.791 (0.623–1.003) | 0.054 | 0.799 (0.627–1.018) | 0.071 |
|
| |||||
| Reduced HDL cholesterol | |||||
| Model 1 | 1.0 | 0.921 (0.710–1.196) | 0.540 | 0.737 (0.579–0.939) | 0.015 |
| Model 2 | 1.0 | 0.923 (0.709–1.201) | 0.550 | 0.747 (0.586–0.953) | 0.020 |
| Model 3 | 1.0 | 0.915 (0.702–1.193) | 0.513 | 0.768 (0.600–0.984) | 0.038 |
|
| |||||
| Elevated triglyceride | |||||
| Model 1 | 1.0 | 0.811 (0.626–1.051) | 0.115 | 0.587 (0.452–0.763) | <0.001 |
| Model 2 | 1.0 | 0.833 (0.640–1.085) | 0.176 | 0.610 (0.468–0.795) | <0.001 |
| Model 3 | 1.0 | 0.843 (0.644–1.103) | 0.214 | 0.610 (0.466–0.799) | <0.001 |
Regression models were sequentially adjusted for the following variables: Model 1: age, sex, and body mass index; Model 2: total energy intake and smoking; Model 3: alcohol intake and household income.
OR, odds ratio; CI, confidence interval; HDL, high-density lipoprotein.
DISCUSSION
This cross-sectional study showed that moderate to vigorous activity is not only associated with a lower prevalence of metabolic syndrome, but is also negatively associated with abdominal obesity, hypertriglyceridemia, and low HDL cholesterol. Subjects in the walking group who reported that they did not participate in any intense exercise had a slightly reduced prevalence of metabolic syndrome, but this did not extend to any of the determinants of metabolic syndrome.
Our findings are consistent with previous studies showing that moderate to vigorous activity has a preventive effect on the development of metabolic syndrome. Laaksonen et al.18 firstly reported the benefit of high intensity exercise on the risk of metabolic syndrome, and since then there have been many studies on this subject. He et al.5 screened a total of 48 studies, and performed a meta-analysis of nearly 20 of the articles. Regarding exercise intensity, the data to date have been controversial. Between activities and risk of metabolic syndrome, several studies have reported a significant relationship7,12–14,19, while others have reported that there is no significant relationship.20–22
In the present study, we found that high intensity exercise was a strong protective factor for reducing metabolic syndrome in a Korean population. This result was consistent with a study by Laursen et al.7, who showed that walking volume and light physical activity do not reduce the risk of metabolic syndrome. Several other studies have also shown that exercise intensity, rather than duration, is more important for reducing cardiovascular risk.23–27
The underlying mechanism for the benefits of high intensity exercise appear to involve improved lipid biogenesis in the liver and adipose tissues28, as well as stimulation of muscle adaptation.29–31 With respect to muscle physiology, it has been suggested that high-intensity exercise activates many oxidative enzymes29,30 and induces angiogenesis through peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α).31
Regarding the components of metabolic syndrome, there were some differences between our results and those of previous studies. There was a mild reduction in hypertension (both high systolic blood pressure and high diastolic blood pressure) and fasting glucose compared to subjects who did not exercise, but intensity did not appear to make a difference on results. On the other hand, abdominal obesity, hypertriglyceridemia, and low HDL cholesterol was negatively associated with moderate to vigorous activity. Given the role of β adrenergic signaling in visceral adipose tissue, intense exercise seems to attenuate p38 mitogen-activated protein kinase and PGC-1α.32 Intense exercise may also increase HDL cholesterol by increasing expression of post-heparin lipoprotein lipase.33 Further studies are needed to determine the basis of the different odds ratios for each of the metabolic syndrome components according to exercise intensity.
There were some limitations to the present study. First, this study was cross-sectional in design, and thus causality inferences could not be made. Second, data regarding exercise was collected using questionnaires, which is susceptible to recall bias and limited in accuracy. A third limitation of this study was that the relationship between exercise volume and metabolic syndrome may have been underestimated or overlapping in our analysis, which was mainly based on exercise intensity.
Our study had several major strengths. First, we included a large representative population that was weighted to reflect nationwide prevalence estimates. Second, we used covariates’ adjusted odds ratio for metabolic syndrome and its determinants, which allowed us to rule out the effect of confounding factors on associations.
In conclusion, individuals who participate in moderate to vigorous activity have a lower prevalence of metabolic syndrome and its components, especially abdominal obesity, hypertriglyceridemia, and low HDL cholesterol. For the prevention and management of metabolic syndrome, it may be important to recommend moderate to vigorous activity to Koreans, especially those with abdominal obesity or dyslipidemia. Further prospective studies are needed to confirm the protective role of intense exercise and effect modification for each of the determinants of metabolic syndrome.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Wilson PW, D’Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–72. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 2.Lim S, Jang HC, Park KS, Lee HK, Chung HR, Joung HJ, et al. Changes in metabolic syndrome of Korean children and adolescents in the period 1998 to 2001. J Endocrinol Invest. 2008;31:327–33. doi: 10.1007/BF03346366. [DOI] [PubMed] [Google Scholar]
- 3.Moore JX, Chaudhary N, Akinyemiju T. Metabolic syndrome prevalence by race/ethnicity and sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev Chronic Dis. 2017;14:E24. doi: 10.5888/pcd14.160287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Neill S, O’Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16:1–12. doi: 10.1111/obr.12229. [DOI] [PubMed] [Google Scholar]
- 5.He D, Xi B, Xue J, Huai P, Zhang M, Li J. Association between leisure time physical activity and metabolic syndrome: a meta-analysis of prospective cohort studies. Endocrine. 2014;46:231–40. doi: 10.1007/s12020-013-0110-0. [DOI] [PubMed] [Google Scholar]
- 6.Tsai HH, Yeh CY, Su CT, Chen CJ, Peng SM, Chen RY. The effects of exercise program on burnout and metabolic syndrome components in banking and insurance workers. Ind Health. 2013;51:336–46. doi: 10.2486/indhealth.2012-0188. [DOI] [PubMed] [Google Scholar]
- 7.Laursen AH, Kristiansen OP, Marott JL, Schnohr P, Prescott E. Intensity versus duration of physical activity: implications for the metabolic syndrome. A prospective cohort study. BMJ Open. 2012;2:e001711. doi: 10.1136/bmjopen-2012-001711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agner VFC, Garcia MC, Taffarel AA, Mourão CB, da Silva IP, da Silva SP, et al. Effects of concurrent training on muscle strength in older adults with metabolic syndrome: a randomized controlled clinical trial. Arch Gerontol Geriatr. 2018;75:158–64. doi: 10.1016/j.archger.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Liu SH, Waring ME, Eaton CB, Lapane KL. Association of objectively measured physical activity and metabolic syndrome among US adults with osteoarthritis. Arthritis Care Res (Hoboken) 2015;67:1371–8. doi: 10.1002/acr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Global recommendations on physical activity for health. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 11.Lakka TA, Laaksonen DE, Lakka HM, Männikkö N, Niskanen LK, Rauramaa R, et al. Sedentary lifestyle, poor cardiorespiratory fitness, and the metabolic syndrome. Med Sci Sports Exerc. 2003;35:1279–86. doi: 10.1249/01.MSS.0000079076.74931.9A. [DOI] [PubMed] [Google Scholar]
- 12.Stabelini Neto A, de Campos W, Dos Santos GC, Mazzardo O., Junior Metabolic syndrome risk score and time expended in moderate to vigorous physical activity in adolescents. BMC Pediatr. 2014;14:42. doi: 10.1186/1471-2431-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lilford RJ, Chilton PJ, Hemming K, Girling AJ, Taylor CA, Barach P. Evaluating policy and service interventions: framework to guide selection and interpretation of study end points. BMJ. 2010;341:c4413. doi: 10.1136/bmj.c4413. [DOI] [PubMed] [Google Scholar]
- 14.Kuwahara K, Honda T, Nakagawa T, Yamamoto S, Akter S, Hayashi T, et al. Leisure-time exercise, physical activity during work and commuting, and risk of metabolic syndrome. Endocrine. 2016;53:710–21. doi: 10.1007/s12020-016-0911-z. [DOI] [PubMed] [Google Scholar]
- 15.França SL, Lima SS, Vieira JR. Metabolic syndrome and associated factors in adults of the amazon region. PLoS One. 2016;11:e0167320. doi: 10.1371/journal.pone.0167320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buscemi S, Sprini D, Grosso G, Galvano F, Nicolucci A, Lucisano G, et al. Impact of lifestyle on metabolic syndrome in apparently healthy people. Eat Weight Disord. 2014;19:225–32. doi: 10.1007/s40519-014-0117-4. [DOI] [PubMed] [Google Scholar]
- 17.Lee SY, Park HS, Kim DJ, Han JH, Kim SM, Cho GJ, et al. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract. 2007;75:72–80. doi: 10.1016/j.diabres.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Laaksonen DE, Lakka HM, Salonen JT, Niskanen LK, Rauramaa R, Lakka TA. Low levels of leisure-time physical activity and cardiorespiratory fitness predict development of the metabolic syndrome. Diabetes Care. 2002;25:1612–8. doi: 10.2337/diacare.25.9.1612. [DOI] [PubMed] [Google Scholar]
- 19.Wewege M, van den Berg R, Ward RE, Keech A. The effects of high-intensity interval training vs. moderate-intensity continuous training on body composition in overweight and obese adults: a systematic review and meta-analysis. Obes Rev. 2017;18:635–46. doi: 10.1111/obr.12532. [DOI] [PubMed] [Google Scholar]
- 20.Holme I, Tonstad S, Sogaard AJ, Larsen PG, Haheim LL. Leisure time physical activity in middle age predicts the metabolic syndrome in old age: results of a 28-year follow-up of men in the Oslo study. BMC Public Health. 2007;7:154. doi: 10.1186/1471-2458-7-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheriyath P, Duan Y, Qian Z, Nambiar L, Liao D. Obesity, physical activity and the development of metabolic syndrome: the Atherosclerosis Risk in Communities study. Eur J Cardiovasc Prev Rehabil. 2010;17:309–13. doi: 10.1097/HJR.0b013e32833189b8. [DOI] [PubMed] [Google Scholar]
- 22.Silveira VM, Horta BL, Gigante DP, Azevedo MR., Junior Metabolic syndrome in the 1982 Pelotas cohort: effect of contemporary lifestyle and socioeconomic status. Arq Bras Endocrinol Metabol. 2010;54:390–7. doi: 10.1590/S0004-27302010000400008. [DOI] [PubMed] [Google Scholar]
- 23.Ilanne-Parikka P, Laaksonen DE, Eriksson JG, Lakka TA, Lindstr J, Peltonen M, et al. Leisure-time physical activity and the metabolic syndrome in the Finnish diabetes prevention study. Diabetes Care. 2010;33:1610–7. doi: 10.2337/dc09-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassinen M, Lakka TA, Hakola L, Savonen K, Komulainen P, Litmanen H, et al. Cardiorespiratory fitness and metabolic syndrome in older men and women: the dose responses to exercise training (DR’s EXTRA) study. Diabetes Care. 2010;33:1655–7. doi: 10.2337/dc10-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schnohr P, Scharling H, Jensen JS. Intensity versus duration of walking, impact on mortality: the Copenhagen City Heart Study. Eur J Cardiovasc Prev Rehabil. 2007;14:72–8. doi: 10.1097/HJR.0b013e3280144470. [DOI] [PubMed] [Google Scholar]
- 26.Zheng H, Orsini N, Amin J, Wolk A, Nguyen VT, Ehrlich F. Quantifying the dose-response of walking in reducing coronary heart disease risk: meta-analysis. Eur J Epidemiol. 2009;24:181–92. doi: 10.1007/s10654-009-9328-9. [DOI] [PubMed] [Google Scholar]
- 27.Hamer M, Chida Y. Walking and primary prevention: a meta-analysis of prospective cohort studies. Br J Sports Med. 2008;42:238–43. doi: 10.1136/bjsm.2007.039974. [DOI] [PubMed] [Google Scholar]
- 28.Wang N, Liu Y, Ma Y, Wen D. High-intensity interval versus moderate-intensity continuous training: superior metabolic benefits in diet-induced obesity mice. Life Sci. 2017;191:122–31. doi: 10.1016/j.lfs.2017.08.023. [DOI] [PubMed] [Google Scholar]
- 29.Burgomaster KA, Hughes SC, Heigenhauser GJ, Bradwell SN, Gibala MJ. Six sessions of sprint interval training increases muscle oxidative potential and cycle endurance capacity in humans. J Appl Physiol (1985) 2005;98:1985–90. doi: 10.1152/japplphysiol.01095.2004. [DOI] [PubMed] [Google Scholar]
- 30.Gibala MJ, Little JP, van Essen M, Wilkin GP, Burgomaster KA, Safdar A, et al. Short-term sprint interval versus traditional endurance training: similar initial adaptations in human skeletal muscle and exercise performance. J Physiol. 2006;575(Pt 3):901–11. doi: 10.1113/jphysiol.2006.112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tadaishi M, Miura S, Kai Y, Kano Y, Oishi Y, Ezaki O. Skeletal muscle-specific expression of PGC-1α-b, an exercise-responsive isoform, increases exercise capacity and peak oxygen uptake. PLoS One. 2011;6:e28290. doi: 10.1371/journal.pone.0028290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stephenson EJ, Lessard SJ, Rivas DA, Watt MJ, Yaspelkis BB, 3rd, Koch LG, et al. Exercise training enhances white adipose tissue metabolism in rats selectively bred for low- or high-endurance running capacity. Am J Physiol Endocrinol Metab. 2013;305:E429–38. doi: 10.1152/ajpendo.00544.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mann S, Beedie C, Jimenez A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: review, synthesis and recommendations. Sports Med. 2014;44:211–21. doi: 10.1007/s40279-013-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]