Summary
Background
Type 1 diabetes results from T-cell-mediated destruction of β cells. Findings from preclinical studies and pilot clinical trials suggest that antithymocyte globulin (ATG) might be effective for reducing this autoimmune response. We assessed the safety and efficacy of rabbit ATG in preserving islet function in participants with recent-onset type 1 diabetes, and report here our 12-month results.
Methods
For this phase 2, randomised, placebo-controlled, clinical trial, we enrolled patients with recent-onset type 1 diabetes, aged 12–35 years, and with a peak C-peptide of 0·4 nM or greater on mixed meal tolerance test from 11 sites in the USA. We used a computer generated randomisation sequence to randomly assign patients (2:1, with permuted-blocks of size three or six and stratified by study site) to receive either 6·5 mg/kg ATG or placebo over a course of four days. All participants were masked and initially managed by an unmasked drug management team, which managed all aspects of the study until month 3. Thereafter, to maintain masking for diabetes management throughout the remainder of the study, participants received diabetes management from an independent, masked study physician and nurse educator. The primary endpoint was the baseline-adjusted change in 2-h area under the curve C-peptide response to mixed meal tolerance test from baseline to 12 months. Analyses were by intention to treat. This is a planned interim analysis of an on-going trial that will run for 24 months of follow-up. This study is registered with ClinicalTrials.gov, number NCT00515099.
Findings
Between Sept 10, 2007, and June 1, 2011, we screened 154 individuals, randomly allocating 38 to ATG and 20 to placebo. We recorded no between-group difference in the primary endpoint: participants in the ATG group had a mean change in C-peptide area under the curve of −0·195 pmol/mL (95% CI −0·292 to −0·098) and those in the placebo group had a mean change of −0·239 pmol/mL (−0·361 to −0·118) in the placebo group (p=0·591). All except one participant in the ATG group had both cytokine release syndrome and serum sickness, which was associated with a transient rise in interleukin-6 and acute-phase proteins. Acute T cell depletion occurred in the ATG group, with slow reconstitution over 12 months. However, effector memory T cells were not depleted, and the ratio of regulatory to effector memory T cells declined in the first 6 months and stabilised thereafter. ATG-treated patients had 159 grade 3–4 adverse events, many associated with T-cell depletion, compared with 13 in the placebo group, but we detected no between-group difference in incidence of infectious diseases.
Interpretation
Our findings suggest that a brief course of ATG does not result in preservation of β-cell function 12 months later in patients with new-onset type 1 diabetes. Generalised T-cell depletion in the absence of specific depletion of effector memory T cells and preservation of regulatory T cells seems to be an ineffective treatment for type 1 diabetes.
Introduction
Type 1 diabetes is one of the most common chronic paediatric diseases worldwide, with total incident and prevalent cases expected to double in the next several decades.1,2 The disorder, which results from autoimmune destruction of insulin-producing β cells, is familial, linked to specific HLA risk alleles, and possibly triggered by environmental factors such as diet or infections. Insulin replacement is the only available treatment, and there are no approved disease-modifying interventions.3 At the time of diagnosis, 15–40% of β-cell mass remains, which, if preserved, can improve glycaemic control and reduce long-term complications.4 Type 1 diabetes is thought to be a T-cell-mediated disease, and treatments directed against T cells can alter the course of disease, as shown in both preclinical models and in clinical trials.3,5–10 These trials have had little success, with only a subset of treated patients responding, and no treatment offering robust, extended β-cell preservation over time.
Antithymocyte globulin (ATG) offers several potential advantages over other T-cell therapies. This polyclonal IgG directed against thymocytes targets multiple T-cell antigens, including many of the targets used in previous monoclonal type 1 diabetes trials, and thus constitutes a unique combination therapy directed against T cells that could promote tolerogenic responses in autoimmunity.11 In preclinical studies, a brief course of ATG induced durable remission in non-obese diabetic mice with recent-onset diabetes mellitus.12 In clinical settings, ATG induces partial tolerance when used for organ transplantation,13 and is effective at inducing remission in specific autoimmune diseases, including aplastic anaemia.14 In type 1 diabetes, findings from pilot clinical studies have suggested that ATG can preserve β-cell function.15,16 Finally, findings from a pilot study using ATG, cyclophosphamide, and granulocyte colony stimulating factor (G-CSF) suggested that this combination of treatments was more effective than any other single agent tested to date, with most patients able to discontinue insulin therapy, some for more than 4 years;17–19 however, this combination approach resulted in substantial risk and side-effects. ATG might operate via a variety of mechanisms, including: depletion of autoreactive T cells; modulation or anergy of the remaining T cells; sparing or induction of regulatory T cells (Tregs) during homoeostatic proliferation; altering trafficking of autoreactive T cells to islets; and possibly affecting B cells and dendritic cells.11
We therefore assessed ATG monotherapy for the preservation of β-cell function in patients with recent-onset type 1 diabetes in the phase 2 START trial (Study of Thymoglobulin to ARrest Type 1 diabetes) and report here the results after 12 months of follow-up.
Methods
Participants
In this phase 2, randomised, placebo-controlled, clinical trial, we enrolled participants from 11 participating clinical centres in the USA. The study protocol is available online. Briefly, for the first ten participants, enrolment was confined to those aged 18–35 years. The lower age limit for eligibility was subsequently lowered to 12 years after review by the data and safety monitoring board. Patients were eligible for recruitment if they had been diagnosed within 100 days, were positive for at least one diabetes-associated autoantibody (microassayed insulin if duration of insulin therapy was <10 days; glutamate decarboxylase; islet-cell antigen-512 [ICA-512]; or islet-cell auto antibodies), had a peak stimulated C-peptide of greater than 0·4 nmol/L during a mixed meal tolerance test (MMTT), and had serological evidence of previous Epstein Barr virus infection. Exclusion criteria included any serological or clinical evidence of current infection; a positive PPD test; past infection with hepatitis B or C; HIV infection; a history of serious cardiac disease; leucopenia, thrombocytopenia, or neutropenia; previous treatment with rabbit ATG, or known hypersensitivity to rabbit sera-derived products; liver or renal dysfunction; on-going use of diabetes drugs other than insulin; and vaccination with a live virus 6 weeks before enrolment. Women and girls were ineligible for inclusion if they were pregnant or were unwilling to defer pregnancy.
An independent data and safety monitoring board did regular safety reviews. The protocol and consent documents were approved by independent institutional review boards. All participants or parents provided written informed consent, and those younger than 18 years provided assent.
Randomisation and masking
Eligible individuals were randomly assigned in a two-to-one ratio to ATG or placebo. The site-stratified randomisation scheme was computer-generated at the data coordinating centre using permuted-blocks of size three or six. Site personnel randomised participants via an interactive web-based randomisation system, which sent the treatment assignments directly to the unmasked site pharmacists. All participants were masked and initially managed by an unmasked drug management team which managed all aspects of the study until month 3. Thereafter, to maintain masking for diabetes management throughout the remainder of the study, participants received diabetes management from an independent, masked study physician and nurse educator.
Procedures
All patients were admitted to a clinical research centre for continuous observation during the infusion period, and were discharged 24 h after completion of the last infusion. The ATG group received a total dose of 6·5 mg/kg ATG (thymoglobulin; Genzyme, Cambridge, MA, USA), with 0·5 mg/kg on day 1, and 2 mg/kg on days 2–4. Dose and administration were based on past experience with this drug in transplantation and autoimmune settings, including pilot type 1 diabetes studies.11,17 Participants in the placebo group received saline in an infusion bag that was indistinguishable from that used for ATG infusions. All participants were pre-medicated with diphenhydramine and aceta-minophen; patients in the ATG group also received intravenous methylprednisolone 0·5 mg/kg before infusion and 0·25 mg/kg 12 h later on days 1–3, and on day 4 they received 0·25 mg/kg pre-medication and an optional 0·25 mg/kg 12 h later (given at the discretion of the investigator); the placebo group received matching saline infusions. Serum sickness was managed with prednisone with a maximum dose of 1·5 mg/kg per day on days 1–3, with rapid tapering thereafter. The ATG group received prophylaxis with co-trimoxazole, and acyclovir if participants had previous herpes or varicella exposure, for at least 3 months and until their CD4+ T-cell count was greater than 200 cells per μL; the placebo group received placebo tablets for 3 months. All participants received intensive diabetes management with the goal to achieve ADA-recommended HbA1c and glycaemic targets for age. 4-h MMTTs were repeated at 6-month intervals.
Biochemical autoantibodies were assayed at the Barbara Davis Center (Aurora, CO, USA) using radioimmunobinding assays, and ICA was measured at the University of Florida (FL, USA). C-peptide and HbA1c were measured at the Northwest Lipid Research Laboratory (Seattle, WA, USA) as described previously.20 Lymphocyte and dendritic cell subsets were monitored in real-time using multicolor flow cytometry (see appendix for antibody panel configuration) using a Cytomics FC500 flow cytometer (Beckman Coulter, Kannapolis, NC, USA), and manual sequential gating was done using Flowjo (TreeStar Inc, Ashland, OR, USA). Frozen aliquots of whole blood were processed for DNA isolation and real-time-based quantification of the Treg-specific demethylated region (TSDR) of the FOXP3 locus (Epiontis, Berlin, Germany). Serum cytokines (interferon-γ; interleukins-12p70, −1β, −4, −5, −6, and −13; tumour necrosis factor [TNF]-α) and acute-phase proteins (serum amyloid A, C-reactive protein) were assessed using multiplexed panels on a chemiluminescence platform (Aushon Biosystems, Billerica, MA, USA).
The primary endpoint was defined as a comparison of the change in the mean 2-h C-peptide area under the curve (AUC) from baseline, adjusted for the baseline C-peptide response, in the ATG versus placebo groups 12 months after study enrolment. Prespecified secondary outcomes included the 4-h C-peptide AUC at 12 months; changes of C-peptide AUC over time to month 12; insulin use at 12 months; proportion of participants who were exogenous-insulin-free at 12 months; hypoglycaemic events; HbA1C concentrations at 12 months; and frequency and severity of adverse events in the ATG versus placebo groups.
Statistical analysis
For assessment of the primary endpoint, we included all randomly allocated patients who received any dose of study treatment (the intention-to-treat population). Missing month-12 MMTTs were imputed as described in the appendix. For analysis of the primary endpoint, C-peptide AUC values were transformed to ln(AUC+1) values for inferential analysis. An F test derived from an analysis of covariance with baseline ln(AUC+1) value as a covariate was used to compare treatment groups. Means and summary statistics are presented on the untransformed scale. To further assess longitudinal changes in AUC over time in treatment groups (a prespecified secondary endpoint) and in younger (12–21 years) versus older (22–35 years) cohorts, we fitted piece-wise random regression models with two slopes: baseline to 6 months and 6 months to 12 months. Each model included fixed effects for baseline AUC as a covariate and an intercept and two piece-wise slopes for each group defined, in separate models, by either treatment or treatment-by-age cohorts. The two slope parameters were also included as subject-level random effects with an unstructured covariance matrix. Adjusted means are based on models fit to untransformed AUC values. p values were derived from models using ln(AUC+1) as the outcome and using the Kenward-Rogers approximation for inference.
Sensitivity analyses for the 2-h C-peptide AUC and secondary analyses on the 4-h C-peptide AUC were done using the methods described for the primary endpoint (appendix); missing 4-h C-peptide AUC values were not imputed. Secondary inferential analyses on HbA1c and insulin use are based on analysis of covariance models at each timepoint with adjustment for baseline levels. We used Fisher’s exact test to compare the number of participants who were insulin independent and who had a hypoglycaemic event at month 12. For any secondary and exploratory analyses, corrections were not made for multiple comparisons.
Lachin and colleagues21 summarised control group 2-h C-peptide AUC data from multiple studies and proposed a method for sample size computations. Using these results, the 12-month geometric mean 2-h C-peptide AUC (pmol/mL per min) in the control group was assumed to be 0·384. After transformation, the ln(AUC + 1) value in the control group is ln(0·384 + 1) =0·325 with a root mean square error of 0·154. We assumed that the root mean square error would be the same in the control and active groups. With two-to-one randomisation and a two-sided t test with a significance level of 5%, a sample size of 60 provides 82% power to detect a 50% improvement of ATG over control. Assuming a potential loss of up to 10% of participants before month 12, enrolment of 66 participants had been planned to allow for sufficient power for secondary sensitivity analyses, but enrolment was halted by the sponsor at 58 for administrative reasons. We used SAS (version 9.2) for all analysis.
This study is registered with ClinicalTrials.gov, number NCT00515099.
Role of the funding source
The study sponsor was responsible for study design, data collection and analysis. SEG, AP, LK-E, SA, LD, and MRE had full access to all of the data and the Immune Tolerance Network the final responsibility for the decision to submit for publication.
Results
Between Sept 10, 2007, and June 1, 2011, we screened 154 individuals, randomly allocating 38 to ATG and 20 to placebo (figure 1). Baseline characteristics were similar between groups (table 1). The last patient completed the 12-month follow-up on June 11, 2012.
Figure 1: Trial profile.
ATG=antithymocyte globulin.
Table 1:
Baseline characteristics
| Treatment group (n=38) | Placebo group (n=20) | |
|---|---|---|
| Age in years | 19·4 (6·6) | 20·5 (7·0) |
| 12–21 years | 26 (68%) | 12 (60%) |
| 22–35 years | 12 (32%) | 8 (40%) |
| Men | 24 (63%) | 11 (55%) |
| Ethnic origin | ||
| White | 32 (84%) | 17 (85%) |
| Non-white | 6 (16%) | 3 (15%) |
| Body-mass index | 22·8 (3·4) | 24·4 (3·4) |
| Days since diagnosis | 69·0 (21·0) | 76·5 (18·0) |
| Baseline 2-h C-peptide area under the curve (pmol/mL) | 0·857 (0·371) | 0·932 (0·502) |
| Baseline HbA1c(%) | 6·7 (1·3) | 6·8 (1·2) |
| Baseline insulin use (U/kg per day) | 0·337 (0·217) | 0·416 (0·240) |
| GAD65 (% positive) | 31 (82%) | 18 (90%) |
| IA.2ic (% positive) | 23 (62%) | 11 (53%) |
| ICA512BDC (% positive) | 22 (58%) | 7 (33%) |
| mIAA (% positive) | 23 (62%) | 14 (68%) |
| ZnT8 (% positive) | 24 (63%) | 9 (47%) |
Data are mean (SD) or n (%).
We recorded no between-group difference in mean change from baseline in the primary endpoint, MMTT-stimulated 2-h mean C-peptide AUC (table 2); treatment groups did not differ significantly after adjustment for baseline 2-h C-peptide AUC. We did three different pre-defined sensitivity analyses on the primary endpoint, but none showed a between-group difference (no imputation, observed data only [p=0·559], optimistic imputation [p=0·362], best-guess imputation [p=0·465]; appendix). We also recorded no between-group difference in one of the secondary outcomes, mean change from baseline in 4-h C-peptide AUC: −0·187 pmol/mL (95% CI −0·291 to −0·082) in the ATG group versus −0·266 pmol/mL (−0·388 to −0·144) in the placebo group (p=0·398).
Table 2:
MMTT-stimulated 2-h mean C-peptide AUC (primary endpoint analysis)
| Treatment group (n=38) | Placebo (n=20) | p value* | |
|---|---|---|---|
| Baseline | ·· | ||
| Mean(SD) | 0·857 (0·371) | 0·932 (0·502) | |
| Median (range) | 0·766 (0·33 to 2·25) | 0·847 (0·39 to 2·58) | |
| 95% CI | 0·735 to 0·979 | 0·697 to 1·166 | |
| Month 12 | ·· | ||
| Mean (SD) | 0·662 (0·373) | 0·692 (0·519) | |
| Median (range) | 0·562 (0·15 to 1·72) | 0·550 (0·10 to 2·06) | |
| 95% CI | 0·539 to 0·785 | 0·449 to 0·935 | ·· |
| Change from baseline | 0·591 | ||
| Mean (SD) | −0·195 (0·294) | −0·239 (0·259) | |
| Median (range) | −0·123 (−0·74 to 0·42) | −0·275 (·0·70 to 0·22) | |
| 95% CI | −0·292 to −0·098 | −0·361 to −0·118 | |
p value is for testing treatment effect using an analysis of covariance with baseline level as a covariate and change in ln(AUC+1) from baseline as the outcome variable.
Analysis of 2-h C-peptide AUC changes over time suggested a faster rate of decrease in the first 6 months, whereas in the second 6 months the curve seemed to plateau in the ATG group; the placebo group showed a steady decline over the entire 12-month period (figure 2). After adjustment for baseline 2-h C-peptide AUC, the mean change in 2-h C-peptide AUC in the ATG group was −0·18 pmol/mL (−0·26 to −0·10) over the first 6 months, which differed significantly from the change of 0·031 pmol/mL (95% CI −0·048 to 0·11) from 6 to 12 months (p=0·002); in the placebo group the change was −0·12 pmol/mL (−0·23 to 0·003) over the first 6 months and −0·10 pmol/mL (−0·22 to 0·009) from months 6 to 12 (p=0·846). Although the slopes during the first 6 months did not differ significantly between treatment groups (p=0·328), there was a significant difference between the ATG and control groups from months 6 to 12 (p=0·030).
Figure 2: Mean change in stimulated C-peptide 2-h AUC mean from baseline.
(A) All participants. (B) Participants aged 12–21. (C) Participants aged 22–35 years. Error bars are 95% CIs. X denotes the median. ATG=antithymocyte globulin. AUC=area under the curve.
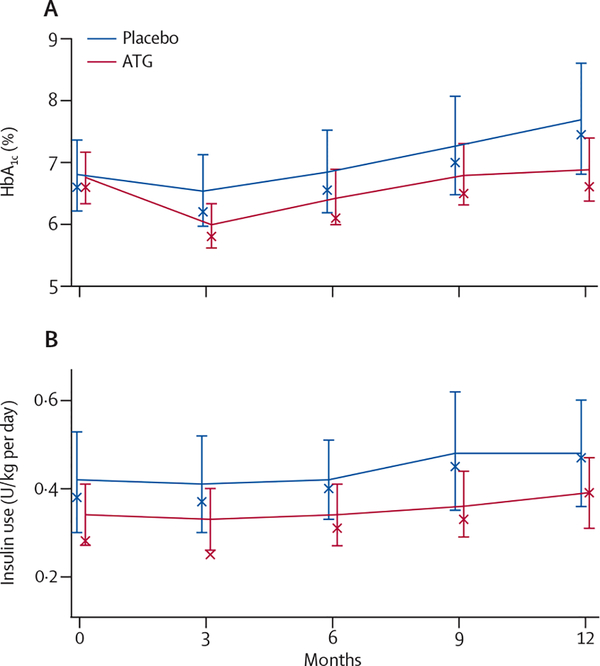
Glycaemic control was well maintained in both groups, with no between-group difference in mean HbA1c at month 12 (p=0·069; figure 3). There was no difference in exogenous insulin use between the groups (p=0·497; figure 3) and the number of participants who were exogenous insulin free at month 12 was also not different (one patient in the ATG group; p=1·000). The number of participants who had at least one hypoglycaemic event up to month 12 was not statistically different between the two groups (p=0·764; table 3). No participants had diabetic ketoacidosis during the 12-month study period.
Figure 3: HbA1c concentrations.
(A) and exogenous insulin use (B) Error bars are 95% CIs. X denotes the median. ATG=antithymocyte globulin.
Table 3:
Adverse events in 15% or more of participants
| Treatment group (N=38) | Placebo group (N=20) | |
|---|---|---|
| Total number of adverse events | 756 | 250 |
| Grade 1 | 38 (100%); 266 | 18 (90%); 148 |
| Grade 2 | 38 (100%); 328 | 16 (80%); 89 |
| Grade 3 | 30 (79%); 89 | 8 (40%); 12 |
| Grade 4 | 38 (100%); 70 | 1 (5%); 1 |
| Grade 5 | 0 (0%); 0 | 0 (0%); 0 |
| Total number of adverse events by category | ||
| Metabolism and nutrition disorders | 29 (76%); 255 | 15 (75%); 83 |
| Hypoglycaemia | 26 (68%); 241 | 15 (75%); 82 |
| Hyperglycaemia | 7 (18%); 11 | 1 (5%); 1 |
| Blood and lymphatic system disorders | 38 (100%); 118 | 2 (10%); 4 |
| CD4+ T cells decreased | 38 (100%); 38 | 0 (0%); 0 |
| Lymphopenia | 38 (100%); 46 | 0 (0%); 0 |
| Leucopenia | 13 (34%); 18 | 0 (0%); 0 |
| Neutropenia | 8 (21%); 9 | 1 (5%); 3 |
| Immune system disorders | 38 (100%); 80 | 2 (10%); 2 |
| Serum sickness | 38 (100%); 40 | 0 (0%); 0 |
| Cytokine release syndrome | 37 (97%); 37 | 0 (0%); 0 |
| Infections and infestations | 23 (61%); 53 | 13 (65%); 25 |
| Upper respiratory tract infection | 11 (29%); 19 | 6 (30%); 11 |
| Viral infection | 4 (11%); 7 | 3 (15%); 3 |
| General disorders and administration site disorders | 26 (68%); 32 | 8 (40%); 15 |
| Fever | 6 (16%); 6 | 0 (0%); 0 |
| Fatigue | 2 (5%); 3 | 3 (15%); 4 |
| Gastrointestinal disorders | 22 (58%); 38 | 11 (55%); 16 |
| Nausea | 7 (18%); 7 | 5 (25%); 7 |
| Vomiting | 3 (8%); 4 | 3 (15%); 3 |
| Skin and subcutaneoustissue disorders | 23 (61%); 35 | 10 (50%); 21 |
| Rash | 6 (16%); 6 | 2 (10%); 3 |
| Acne | 7 (18%); 7 | 0 (0%); 0 |
| Pruritus | 4 (11%); 4 | 3 (15%); 4 |
| Nervous system disorders | 21 (55%); 35 | 10 (50%); 22 |
| Headache | 16 (42%); 21 | 9 (45%); 14 |
| Respiratory, thoracic and mediastinal disorders | 18 (47%); 29 | 9 (45%); 19 |
| Oropharyngeal pain | 8 (21%); 11 | 3 (15%); 4 |
| Cough | 7 (18%); 7 | 3 (15%); 3 |
| Rhinorrhoea | 1 (3%); 1 | 3 (15%); 3 |
| Musculoskeletal and connectivetissue disorders | 12 (32%); 16 | 10 (50%); 16 |
| Arthralgia | 2 (5%); 2 | 3 (15%); 4 |
| Back pain | 2 (5%); 2 | 3 (15%); 3 |
| Musculoskeletal pain | 1 (3%); 1 | 3 (15%); 3 |
| Vascular disorders | 4 (11%); 6 | 5 (25%); 5 |
| Hypotension | 0 (0%); 0 | 3 (15%); 3 |
Data are number of patients (%); number of adverse events, unless otherwise stated. The total number of adverse events is for all events in all participants. At each level of summarisation, a participant is counted once if they reported one or more event, and percentages are based on the total number of participants in each group. The incidences are shown in descending order of overall frequency of system organ class and preferred term within system organ class. Adverse events are coded according to MedDRA classifications (version 11.1).
Post-hoc efficacy analysis suggested that the rapid decrease in 2-h C-peptide AUC in the first 6 months compared with months 6–12 was driven largely by changes in the younger cohort (12–21 years; figure 2). After adjustment for baseline, the mean change in 2-h C-peptide AUC in the younger ATG group was −0·26 pmol/mL (−0·35 to −0·17) over the first 6 months, which differed from the change of 0·044 pmol/mL (−0·055 to 0·14) from months 6 to 12 (p=0·0004). In the older cohort (22–35 years) in the ATG group, 2-h C-peptide concentrations was stable throughout the study with changes of −0·010 pmol/mL (−0·14 to 0·12) from baseline to month 6 and of 0·0029 pmol/mL (−0·14 to 0·14) from months 6 to 12 (p=0·806; figure 2). The slopes over the first 6 months differed significantly between the age cohorts in the ATG group (p=0·008), but we recorded no difference between age groups for months 6 to 12 (p=0·710). In the placebo group, changes over time did not differ between age cohorts.
The 2-h C-peptide AUC change from baseline to month 12 was stable in the ATG group in the older cohort (22–35 years; change adjusted for baseline −0·0070 pmol/mL, −0·17 to −0·15), versus a decrease over the same period in the placebo group in the older cohort (change adjusted for baseline −0·23 pmol/mL, −0·44 to −0·027; figure 2), but this difference was not statistically significant (p=0·083).
All participants reported at least one adverse event (table 3 and appendix). There were a total of 17 serious adverse events in the ATG group and six in the placebo group (table 4). Cytokine release syndrome during drug infusion occurred in all but one of the patients in the treatment group, with all patients who received ATG having serum sickness (table 3), which usually started within 11 days of the initial infusion (appendix). We noted a similar number of infections between the two groups, with median time to infection of 166·5 days in the ATG group and 207·5 days in the placebo group. There were neither opportunistic infections nor difficulty clearing infections. No Epstein-Barr virus or cyto-megalovirus reactivation was noted clinically or via PCR assessment.
Table 4:
Serious adverse events
| Treatment group (N=38) | Placebo group (N=20) | |
|---|---|---|
| Number of participants with at | 7 (18%) | 4 (20%) |
| least one serious adverse event | ||
| Grade 1 | 0 (0%); 0 | 0 (0%); 0 |
| Grade 2 | 1 (3%); 1 | 0 (0%); 0 |
| Grade 3 | 7 (18%); 13 | 3 (15%); 5 |
| Grade 4 | 1 (3%); 3 | 1 (5%); 1 |
| Total numberof serious adverse events | 17 | 6 |
| Affective disorder | 0 (0%); 0 | 1 (5%); 1 |
| Appendicitis | 1 (3%); 1 | 0 (0%); 0 |
| Axillary vein thrombosis | 1 (3%); 1 | 0 (0%); 0 |
| Bipolar disorder | 1 (3%); 1 | 0 (0%); 0 |
| CD4+ T eel Is decreased* | 1 (3%); 1 | 0 (0%); 0 |
| Comminuted fracture | 0 (0%); 0 | 1 (5%); 1 |
| Cytokine release syndrome* | 1 (3%); 1 | 0 (0%); 0 |
| Depression | 1 (3%); 1 | 0 (0%); 0 |
| Exfoliative rash* | 1 (3%); 2 | 0 (0%); 0 |
| Gastroenteritis viral | 0 (0%); 0 | 1 (5%); 1 |
| Hyperglycaemia | 1 (3%); 3 | 1 (5%); 1 |
| Hypoglycaemia | 1 (3%); 1 | 0 (0%); 0 |
| Major depression | 0 (0%); 0 | 1 (5%); 1 |
| Mood altered | 1 (3%); 1 | 0 (0%); 0 |
| Serum sickness* | 2 (5%); 2 | 0 (0%); 0 |
| Substance abuse | 0 (0%); 0 | 1 (5%); 1 |
| Syncope | 1 (3%); 1 | 0 (0%); 0 |
| Viral infection* | 1 (3%); 1 | 0 (0%); 0 |
Data are number of patients (%); number of adverse events, unless otherwise stated. The total number of serious adverse events is for all events in all participants. At each level of summarisation, a participant is counted once if they reported one or more event, and percentages are based on the total number of participants in each group. The incidences are shown in descending order of overall frequency of system organ class preferred term within system organ class. Adverse events are coded according to MedDRA classifications (version 11.1).
Event thought to be possibly, probably, or definitely related to study treatment.
Acute changes in some serum cytokines were noted in association with cytokine release syndrome and serum sickness (figure 4 and appendix). The first infusion with ATG acutely increased interleukin-10 concentrations by about 10 times compared with concentrations before infusion. The second infusion with ATG increased concentrations of interleukin-10 by about 25 times and interleukin-6 by about 85 times, compared with concentrations before the second infusion. Con-centrations of acute phase reactants also increased in the ATG group: that of C-reactive protein increased by more than 100 times, and of serum amyloid A by more than 200 times within 3 h after the first ATG infusion, but returned to baseline levels within 1 month.
Figure 4: Mean concentrations of cytokines and acute phase reactants in serum samples during the first month of the trial.
(A) Interleukin-6. (B) Interleukin-10. (B) C-reactive protein. (D) Serum amyloid A. Serum was collected before (pre-) and 3 h after (post-) each of the first three infusions and at 1 month. Error bars are SD. ATG=antithymocyte globulin.
As anticipated, the mean absolute counts of CD3+ T cells and the CD4+ and CD8+ cell subsets fell precipitously in peripheral blood after ATG treatment and slowly reconstituted over ensuing months, but remained below their baseline levels at 12 months (figure 5). Recovery of CD8+ T cells was faster than recovery of CD4+ T cells during the course of the study, with a decrease in the CD4-to-CD8 ratio at 6 months (p<0·0001) and at 12 months (p<0·0001) compared with baseline. We noted no changes in any of these parameters in the placebo group.
Figure 5: Lymphocyte depletion and reconstitution kinetics.
Absolute counts of CD3+ T cells (A), CD4+ T cells (B), and CD8+ T cells (C) were assessed using real-time flow cytometry during the 12-month period of the trial. Values are mean and error bars are SD. ATG=antithymocyte globulin.
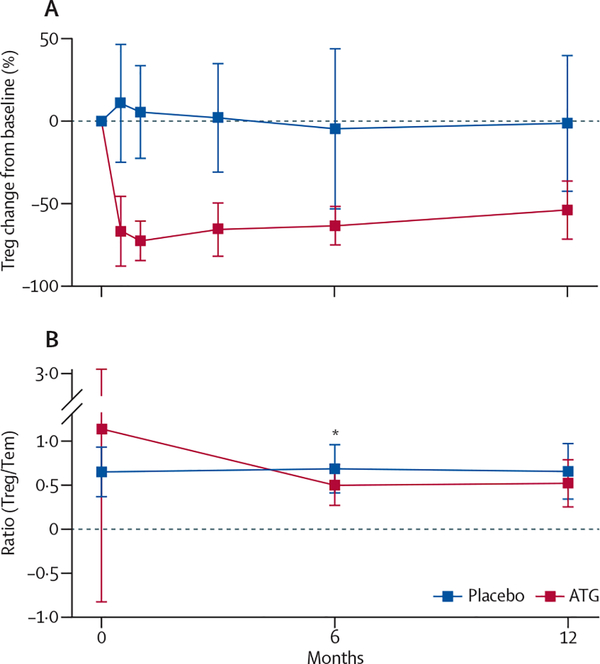
ATG treatment modulated naive (CD45RA+) and memory (CD45RO+) T-cell subsets differently. Naive CD4+ and CD8+ T cells were substantially depleted within 15 days of ATG treatment, but recovered to 44% (CD4+ T cells) and 69% (CD8+) of their baseline frequency by 12 months (figure 6). CD4+ and CD8+ central memory T cells (CD45RA−CD45RO+CD62Lhi) also decreased substantially by day 15 and recovered to about 60% of the original values at 12 months (figure 6). In contrast with naive and central memory T cells, ATG treatment did not effectively deplete effector memory T (Tem) cells (CD45RA−CD45RO+CD62Llo) from the peri-pheral circulation (figure 6). Tregs (CD4+CD25hiCD127lo) showed pronounced and extended depletion much the same as that of the naive CD4+ T-cell population (figure 7). When we used epigenetic analysis of the TSDR within the FOXP3 locus to determine Treg frequency, the ATG-treated group showed, compared with baseline, a 50% reduction at month 6 and a 45% reduction at month 15, in agreement with flow cytometry data. We recorded no changes in Treg frequency as determined by TSDR analysis in the placebo group (appendix).
Figure 6: Naive and memory CD4+ and CD8+ T cell depletion and reconstitution kinetics.
Changes from baseline of naive CD4+ T cells (A), naive CD8+ T cells (B), central memory CD4+ T cells (C), central memory CD8+ T cells (D), effector memory CD4+ T cells (E), and effector memory CD8+ T cells (F). Values are mean and error bars are SD.
Figure 7: Changes in concentrations of T regulatory cells.
(A) Changes from baseline of T regulatory cells (Tregs; CD4+CD25hiCD127lo), assessed using real-time flow cytometry. (B) Ratio of the frequency of Tregs to CD4+ T effector memory (Tem) cell populations. Statistical significance was determined at each timepoint using a t test. *p<0·0001. Values are mean and error bars are SD.
To find out whether persistence of Tem cells in the periphery resulted in a shift in the Treg to Tem balance, we compared the Treg-to-Tem ratio at baseline, 6 months, and 12 months in both groups. We recorded a decrease in the Treg-to-Tem ratio in the ATG-treated group between baseline and 6 months (p<0·0001), but no statistically significant change from 6 months to 12 months (figure 7).
The effect of ATG treatment on other mononuclear cell subsets was also evaluated, and showed less marked depletion and more rapid reconstitution than noted with T cells (appendix).
Discussion
In our trial, a course of ATG given within 100 days of type 1 diabetes diagnosis did not affect the rate of β-cell loss over 12 months, as compared with the placebo group. This result was unexpected in view of preclinical findings12 and preliminary findings from earlier pilot studies with ATG alone15,16 or ATG with G-CSF and cyclo-phosphamide.17–19 However, the START trial is, to the best of our knowledge, the first adequately powered, placebo-controlled, randomised, multicentre trial of ATG monotherapy using well established inclusion and exclusion criteria, current standards for glycaemic control, and validated endpoints.
The kinetics of C-peptide decrease over the first 12 months were unusual in the ATG group. Of the recent new-onset type 1 diabetes trials that have shown some positive effects, the usual pattern is that the treated group shows initial stabilisation for a period of 6–12 months after treatment, followed by subsequent decline, with a slope that seems to parallel the control group.7–10,22 However, we detected a statistically significant biphasic response in treated participants, with an initial decrease in β-cell function, followed by stabilisation from 6–12 months, which was similar to the transient impairment in β-cell function seen in a pilot study of the combination of interleukin-2 and sirolimus, thought to be due to short-term immune activation.20 This finding suggests that ATG might have led to unintended immune activation in the early period (baseline to 6 months) after treatment.
One possible explanation for the initial decrease in C-peptide is that β cells were exposed to an unfavourable milieu early in the trial, with cytokine release syndrome and serum sickness, combined with glucocorticoids that were given to lessen symptoms. Of the cytokines measured, the changes are notable for the pronounced early increase in concentrations of interleukin-6, with return to baseline by 1 month in most treated participants. Interleukin-6 is a powerful systemic cytokine that drives autoimmunity, enhancing the differentiation of pro-inflammatory T helper 17 cells, promoting the production of Tem cells, and inhibiting the function of Tregs.23 The use of glucocorticoids could also have had adverse effects on β-cell function, although treatment duration was short and not expected to produce sustained irreversible effects. ATG plus prednisone seemed promising on the basis of findings from a previous pilot clinical trial.15 An initial MMTT assessment at 3 months might have helped further assess the effect of these acute changes on β-cell function in our trial.
Another possible explanation for the initial decrease in β-cell function with ATG treatment is the fact that Tem cells were not depleted, that Tregs were depleted, or both. ATG treatment induced the expected rapid depletion of T cells in peripheral circulation. However, findings from primate studies have shown that ATG does not fully deplete residual cells in peripheral lymphoid tissue and spleen.24 Furthermore, as shown in the present study and in previous studies, one key T-cell subpopulation, Tem cells, is refractory to treatment.25 Unlike in some studies, we detected Treg depletion, with an unfavourable Treg-to-Tem ratio in the first 6 months. This ratio stabilised between months 6 and 12, coincident with a stabilisation of C-peptide secretion. Pleiotropic effects of ATG on other cell types, such as dendritic cells, might contribute to modifying the autoimmune response.26 In our trial, the effects on B cells were slight, without the profound and extended depletion seen with a monoclonal anti-CD20,22 and thus might not have had an important effect.
Participants in the treated group had a greater number and severity of adverse events than did those in the placebo group. Nearly all ATG-treated participants had cytokine release syndrome and serum sickness, at a higher frequency than seen with transplantation,27 probably because we did not use concomitant immunosuppressants. Despite the slow recovery in T-cell counts, infectious disease risk did not seem to be higher in the treated group, which might relate to the use of antimicrobial prophylaxis, incomplete T-cell depletion with residual cells in the periphery, or the fact that other arms of the immune system were not affected by this treatment.
Follow-up studies in individuals older than 21 years might be needed, because this group showed potential stabilisation of β-cell function relative to the younger participants in a post-hoc analysis. Preliminary findings from some ongoing phase 1 clinical trials using ATG in combination with G-CSF and cyclosphophamide17–19 are showing safety concerns, but also efficacy that exceeds other regimens that have thus far been tested. In the non-obese diabetic mouse model, the combination of ATG plus G-CSF is more robust than ATG alone, and at a third of the ATG dose;28 a pilot study in recent-onset type 1 diabetes is assessing this combination (NCT 01106157). Interleukin-6 was substantially increased early in the course of ATG treatment and a trial of tocilizumab (interleukin-6 receptor antagonist) in new-onset type 1 diabetes is planned. In addition, a trial assessing depletion of Tem cells with alefacept (LFA3-Ig) is in progress (NCT 00965458). Ongoing follow-up of participants in the START trial, with further mechanistic studies and comparison with related trials, might yield additional insights into the limitations of ATG alone, identify biomarkers of safety and efficacy, or suggest the optimal agent or agents to be used in future new-onset type 1 diabetes trials.
Supplementary Material
Panel: Research in context.
Systematic review
We searched the PubMed database for articles published in any language up to June 1, 2013, with the search terms “immune intervention” and “type 1 diabetes” and “anti-thymocyte globulin” (ATG). Three agents assessed in a series of recent randomised trials with adequate sample size showed some degree of preservation of β-cell function in type 1 diabetes, as assessed by change in C-peptide secretion in response to a mixed meal tolerance test over time. These trials used anti-CD3, anti-CD20, and abatacept.7–10,22 Findings from several smaller phase 1 studies suggested efficacy with ATG, either alone or in combination with other agents.15–19
Interpretation
In our trial, ATG alone did not slow the reduction in β-cell function in patients with new-onset type 1 diabetes over a 12-month interval. Thus, positive findings from studies in animal models and small pilot studies might not translate into successful treatments. Acute events surrounding ATG administration might have resulted in a decrease in C-peptide secretion during the first 6 months, followed by stabilisation of C-peptide secretion in months 6–12. Post-hoc analyses indicated that participants older than 21 years might have improved stabilisation of β-cell mass relative to younger patients, but larger follow-up studies of ATG alone or ATG in conjunction with other therapies are needed to assess more fully the potential role of ATG in new-onset type 1 diabetes.
Acknowledgments
The trial was done by the Immune Tolerance Network (ITN) and sponsored by the National Institute of Allergy and Infectious Diseases (NIAID). Additional funding was provided by the Juvenile Diabetes Research Foundation (JDRF), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), a Clinical and Translational Awards grant (NIH/NCRR UCSF-CTSI Grant Number UL 1 TR000004), and Clinical and Translational Awards grants (UCSF-CTSI grant number UL1 RR024131 and UL 1 TR000004; CHOP by UL1RR024134 and UL1TR000003; University of Minnesota by UL1TR000114). Genzyme (Cambridge, MA, USA) provided ATG and gave input regarding dosage and safety, but had no direct involvement with study design, conduct, or management; data collection, analysis or interpretation; or manuscript preparation. There are no agreements concerning confidentiality of the data between the sponsor and the authors or the institutions named in the credit lines. The authors provided Genzyme a copy of the original paper before submission. Lifescan Inc (CA, USA) provided blood glucose monitoring meters and strips.
Funding US National Institutes of Health and the Juvenile Diabetes Research Foundation.
Footnotes
Conflicts of interest
SEG served as a consultant on an advisory board for Genzyme. All other authors declare that they have no conflicts of interest.
Contributor Information
Prof S E Gitelman, University of California San Francisco, San Francisco, CA, USA.
Prof J A Bluestone, University of California San Francisco, San Francisco, CA, USA.
Prof P A Gottlieb, Barbara Davis Center, University of Colorado, Aurora, CO, USA.
Prof M R Rigby, Indiana University and Riley Children’s Hospital, Indianapolis, Indianapolis, IN, USA.
Prof E I Felner, Emory University, Atlanta, GA, USA.
S M Willi, Children’s Hospital of Philadelphia, Philadelphia, PA, USA.
L K Fisher, Children’s Hospital of Los Angeles, Los Angeles, CA, USA.
Prof A Moran, University of Minnesota, Minneapolis, MN, USA.
Prof M Gottschalk, University of California San Diego, San Diego, CA, USA.
W V Moore, Children’s Mercy Hospital, Kansas City, MO, USA.
A Pinckney, Rho Federal Systems Division, Chapel Hill, NC, USA.
L Keyes-Elstein, Rho Federal Systems Division, Chapel Hill, NC, USA.
S Aggarwal, Immune Tolerance Network, Bethesda, MD, USA.
D Phippard, Immune Tolerance Network, Bethesda, MD, USA.
Prof P H Sayre, Immune Tolerance Network, San Francisco, CA, USA.
M R Ehlers, Immune Tolerance Network, San Francisco, CA, USA.
L Ding, National Institute of Allergy and Infectious Diseases, Bethesda, MD, USA.
References
- 1.Imperatore G, Boyle JP, Thompson TJ, et al. , and the SEARCH for Diabetes in Youth Study Group. Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes Care 2012; 35: 2515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G, and the EURODIAB Study Group. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet 2009; 373: 2027–33. [DOI] [PubMed] [Google Scholar]
- 3.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 2010; 464: 1293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care 2003; 26: 832–36. [DOI] [PubMed] [Google Scholar]
- 5.The Canadian-European Randomized Control Trial Group. Cyclosporin-induced remission of IDDM after early intervention. Association of 1 yr of cyclosporin treatment with enhanced insulin secretion. Diabetes 1988; 37: 1574–82. [PubMed] [Google Scholar]
- 6.Feutren G, Papoz L, Assan R, et al. Cyclosporin increases the rate and length of remissions in insulin-dependent diabetes of recent onset. Results of a multicentre double-blind trial. Lancet 1986; 2: 119–24. [DOI] [PubMed] [Google Scholar]
- 7.Orban T, Bundy B, Becker DJ, et al. , and the Type 1 Diabetes TrialNet Abatacept Study Group. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet 2011; 378: 412–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 2005; 352: 2598–608. [DOI] [PubMed] [Google Scholar]
- 9.Sherry N, Hagopian W, Ludvigsson J, et al. , and the Protégé Trial Investigators. Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet 2011; 378: 487–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herold KC, Gitelman S, Ehlers MR, et al. Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes 2013; 10.2337/db13-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaber AO, Monaco AP, Russell JA, Lebranchu Y, Mohty M. Rabbit antithymocyte globulin (thymoglobulin): 25 years and new frontiers in solid organ transplantation and haematology. Drugs 2010; 70: 691–732. [DOI] [PubMed] [Google Scholar]
- 12.Simon G, Parker M, Ramiya V, et al. Murine antithymocyte globulin therapy alters disease progression in NOD mice by a time-dependent induction of immunoregulation. Diabetes 2008; 57: 405–14. [DOI] [PubMed] [Google Scholar]
- 13.Starzl TE, Murase N, Abu-Elmagd K, et al. Tolerogenic immunosuppression for organ transplantation. Lancet 2003; 361: 1502–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bacigalupo A, Brand R, Oneto R, et al. Treatment of acquired severe aplastic anemia: bone marrow transplantation compared with immunosuppressive therapy--The European Group for Blood and Marrow Transplantation experience. Semin Hematol 2000; 37: 69–80. [DOI] [PubMed] [Google Scholar]
- 15.Eisenbarth GS, Srikanta S, Jackson R, et al. Anti-thymocyte globulin and prednisone immunotherapy of recent onset type 1 diabetes mellitus. Diabetes Res 1985; 2: 271–76. [PubMed] [Google Scholar]
- 16.Saudek F, Havrdova T, Boucek P, Karasova L, Novota P, Skibova J. Polyclonal anti-T-cell therapy for type 1 diabetes mellitus of recent onset. Rev Diabet Stud 2004; 1: 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Couri CE, Oliveira MC, Stracieri AB, et al. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 2009; 301: 1573–79. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Shen S, Ouyang J, et al. Autologous hematopoietic stem cell transplantation modulates immunocompetent cells and improves β-cell function in Chinese patients with new onset of type 1 diabetes. J Clin Endocrinol Metab 2012; 97: 1729–36. [DOI] [PubMed] [Google Scholar]
- 19.Snarski E, Milczarczyk A, Torosian T, et al. Independence of exogenous insulin following immunoablation and stem cell reconstitution in newly diagnosed diabetes type I. Bone Marrow Transplant 2011; 46: 562–66. [DOI] [PubMed] [Google Scholar]
- 20.Long SA, Rieck M, Sanda S, et al. , and the Diabetes TrialNet and the Immune Tolerance Network. Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs β-cell function. Diabetes 2012; 61: 2340–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lachin JM, McGee PL, Greenbaum CJ, et al. , and the Type 1 Diabetes Trial Network. Sample size requirements for studies of treatment effects on beta-cell function in newly diagnosed type 1 diabetes. PLoS One 2011; 6: e26471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al. , and the Type 1 Diabetes TrialNet Anti-CD20 Study Group. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med 2009; 361: 2143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neurath MF, Finotto S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev 2011; 22: 83–89. [DOI] [PubMed] [Google Scholar]
- 24.Préville X, Flacher M, LeMauff B, et al. Mechanisms involved in antithymocyte globulin immunosuppressive activity in a nonhuman primate model. Transplantation 2001; 71: 460–68. [DOI] [PubMed] [Google Scholar]
- 25.Gurkan S, Luan Y, Dhillon N, et al. Immune reconstitution following rabbit antithymocyte globulin. Am J Transplant 2010; 10: 2132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Y, Parker M, Xia C, et al. Rabbit polyclonal mouse antithymocyte globulin administration alters dendritic cell profile and function in NOD mice to suppress diabetogenic responses. J Immunol 2009; 182: 4608–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lundquist AL, Chari RS, Wood JH, et al. Serum sickness following rabbit antithymocyte-globulin induction in a liver transplant recipient: case report and literature review. Liver Transpl 2007; 13: 647–50. [DOI] [PubMed] [Google Scholar]
- 28.Parker MJ, Xue S, Alexander JJ, et al. Immune depletion with cellular mobilization imparts immunoregulation and reverses autoimmune diabetes in nonobese diabetic mice. Diabetes 2009; 58: 2277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.