Abstract
Background:
After the Department of Defense implemented a mandatory anthrax vaccination program in 1998 concerns were raised about potential long-term safety effects of the current anthrax vaccine. The CDC multicenter, randomized, double-blind, placebo-controlled Anthrax Vaccine Adsorbed (AVA) Human Clinical Trial to evaluate route change and dose reduction collected data on participants’ quality of life. Our objective is to assess the association between receipt of AVA and changes in health-related quality of life, as measured by the SF-36 health survey (Medical Outcomes Trust, Boston, MA), over 42 months after vaccination.
Methods:
1562 trial participants completed SF-36v2 health surveys at 0, 12, 18, 30 and 42 months. Physical and mental summary scores were obtained from the survey results. We used Generalized Estimating Equations (GEE) analyses to assess the association between physical and mental score difference from baseline and seven study groups receiving either AVA at each dose, saline placebo at each dose, or a reduced AVA schedule substituting saline placebo for some doses.
Results:
Overall, mean physical and mental scores tended to decrease after baseline. However, we found no evidence that the score difference from baseline changed significantly differently between the seven study groups.
Conclusions:
These results do not favor an association between receipt of AVA and an altered health-related quality of life over a 42-month period.
Keywords: Quality of life, Health survey, AVA Clinical Trial
1. Introduction
Since the introduction of the Department of Defense’s (DoD) mandatory anthrax vaccination program in 1998 concerns have been raised by service personnel and others about potential long-term safety effects of the current anthrax vaccine. Licensed in 1970 to be administered at 0, 2, and 4 weeks and 6, 12, and 18 months, followed by annual boosters, Anthrax Vaccine Adsorbed (AVA, BioThrax®, Emergent BioSolutions, Lansing, MI [1]) is the only anthrax vaccine licensed in the U.S., and the only U.S. licensed aluminum-adjuvant vaccine administered subcutaneously. Despite evidence supporting the vaccine being reasonably safe [2], concerns have been expressed regarding various adverse effects of AVA, including high rates of local adverse events [3,4], potential reproductive toxicity [5,6] and nonspecific longer-term symptoms such as Gulf War and chronic fatigue syndromes [7,8].
In 1999 the U.S. Congress directed the Centers for Disease Control and Prevention (CDC) to evaluate the safety and efficacy of AVA. A critical component of the CDC’s AVA Safety and Efficacy Research Program was a Human Clinical Trial to evaluate route change (subcutaneous to intramuscular) and dose reduction (reduced priming schedule of 0, 4 weeks and 6 months and a biannual/triannual booster). Following publication of the report of the interim analysis of the clinical trial [9], the current ACIP recommendations and FDA licensed schedule for AVA excludes the original 2-week dose, and injections are administered intramuscularly. All trial participants at the time of enrollment consented to complete the SF-36v2 health survey (Medical Outcomes Trust, Boston, MA) as a self-measure of perceived physical and mental health.
Details of the clinical trial and the results of the interim analysis on data collected through the first four vaccine doses were previously published [9]. Participants (n = 1564) received a total of 8 doses of vaccine or saline placebo during 42 months. In the substudy to assess impact of AVA on health-related quality of life, our goal was to determine whether physical and mental functional status, as measured by the survey, changed differently over dose number between study groups receiving only AVA at each dose number, only saline placebo, or AVA at only some dose numbers and saline placebo at others.
2. Methods
The CDC AVA Human Clinical Trial was a randomized, double-blind, placebo-controlled Phase 4 study conducted from 2002 to 2005 with participants enrolled and followed at 5 major U.S. vaccine research centers: Baylor College of Medicine, Houston, TX; Emory University School of Medicine, Atlanta, GA; Mayo Clinic, Rochester, MN; University of Alabama at Birmingham; and the Walter Reed Army Institute of Research, Silver Springs, MD. Eligibility requirements included being 18–61 years of age, healthy, having 2 intact upper arms, indicating a willingness to participate, having no history of anthrax infection or immunization against anthrax, and if female, not being pregnant and not planning to be pregnant during the study period [9]. At each site, participants were randomly assigned to one of seven study groups based on receiving either AVA or saline placebo, route of injection (subcutaneous vs. intramuscular), and full/reduced AVA schedule (full = 0.5 mL doses at 0, 2, and 4 weeks, and 6, 12, 18, 30 and 42 months vs. reduced = substituting one or more placebo doses) (Table 1).
Table 1.
Study groups (number of subjects completing the survey), CDC Anthrax Vaccine Adsorbed Human Clinical Trial, 2002-2005.
| Study groupa | Week 0 (1562) | Week 2 | Week 4 | Month 6 | Month 12 (1426) | Month 18 (1379) | Month 30 (1290) | Month 42 (1188) |
|---|---|---|---|---|---|---|---|---|
| 8SQ-A | AVA (259) | AVA | AVA | AVA | AVA (241) | AVA (230) | AVA (215) | AVA (194) |
| 8IM-A | AVA (262) | AVA | AVA | AVA | AVA (242) | AVA (237) | AVA (225) | AVA (209) |
| 7IM-A | AVA (256) | S | AVA | AVA | AVA (232) | AVA (225) | AVA (213) | AVA (186) |
| 5IM-A | AVA (258) | S | AVA | AVA | S (228) | AVA (219) | S (203) | AVA (191) |
| 4IM-A | AVA (267) | S | AVA | AVA | S (246) | S (241) | S (227) | AVA (212) |
| 8IM-P | S (127) | S | S | S | S (116) | S (110) | S (100) | S (93) |
| 8SQ-P | S (133) | S | S | S | S (121) | S (117) | S (107) | S (103) |
AVA = received Anthrax Vaccine Adsorbed; S = received saline placebo. The first two treatment groups constitute persons receiving AVA at time points corresponding to the then-licensed schedule; the middle three constitute persons receiving AVA according to a reduced dosing schedule; and the last two constitute persons receiving only saline placebo.
Study group codes denote number of injections (4, 5, 7, or 8), route of administration (SQ [subcutaneous] or IM [intramuscular]), and receipt of AVA (“A”) or placebo (“P”).
The SF-36v2 health survey (Medical Outcomes Trust, Boston, MA) is a multipurpose, short form health survey with 36 questions. The answers to these questions form the basis for scoring overall physical and mental well-being, where both physical and mental scores range from 0 to 100 with an estimated national average of 50 points. The SF-36v2 health survey is a generic measurement tool that has been useful in comparing the relative burden of disease, differentiating the health benefits produced by a wide range of treatments, and screening individual patients [10]. It is suitable for repeated measures by self-administration, computerized administration, or administration by a trained interviewer in person or by telephone to persons aged 14 years and older. It can be administered in 5–10 min with a high degree of acceptability and data quality [11]. The instrument, its scales, and summary measures have high validity and reliability (e.g., the reliability of the physical component summary is 0.92 and of the mental component summary is 0.88). Quality of life data obtained using the SF-36v2 health survey in this study may then be compared to data from published studies of Gulf War veterans who received AVA [12–14], and to data from DoD-planned studies of long-term health outcomes such as the Millennium Cohort Study.
Each study participant completed SF-36v2 health surveys at 0, 12, 18, 30 and 42 months, which correspond to doses 1, 5, 6, 7, and 8, respectively. At enrollment, each study participant self-identified his/her age, race, sex, and smoking status; and clinical staff collected their height and weight measurements. For analysis, each participant’s age at baseline was categorized as <30, 30–39, 40–49, and ≥50 years of age. Race was categorized as black, white, and other. body mass index (BMI) was computed using body weight and height at enrollment and each follow-up time, and categorized using CDC cutoffs: underweight (<18.5), normal (18.5–24.9), overweight (25–29.9), and obese (≥30).
We first summarized baseline demographics on each study group. We performed a Chi-square test to assess whether the demographic characteristics were different across the study groups. It is possible that participants negatively impacted by vaccination withdrew from the study before the study end, and thus their lower scores in later measurements would be lost, causing a positive bias. When dropout rates are not associated with the response variable, which in this study is the physical or mental score difference from baseline, then dropout is said to be completely random. We thus tested for completely random dropout using the logistic regression method of Diggle et al. [15].
We performed Generalized Estimating Equations (GEE) analyses to assess potential associations between SF-36 scores and study group. We performed separate analyses wherein the response variables were either physical or mental score difference from baseline. Constructing a model response variable by subtracting the baseline value from subsequent values has been discussed in depth by Fitz-maurice et al. [16], and gives a natural interpretation to our model results for meeting this study’s objectives. The main effects considered in these models included study group, dose number, study site, sex, smoking status, and age, race, and body mass index categories. We also considered interactions between dose number and each of the other covariates. The mean physical and mental score differences from baseline were modeled as linear combinations of these predictors, while the within-subject covariance structure was specified as the most general (unstructured) form.
We conducted both univariable and multivariable GEE model analyses. The univariable models were fit for each covariate individually, and the predictors considered in these models included the covariate, dose number, and an interaction between the covariate and dose number. The predictor variables considered in the multivariable models included all the covariates and interactions listed in the previous paragraph. We considered removing the dose number interactions if they were found to be non-significant. We removed age and body mass index categories and smoking status from the multivariable models if they were found to be non-significant in both the univariable and multivariable models. We used Type 3 Chi-square score tests to assess variable importance, and used Scheffe’s multiple comparison method to determine which levels of significant covariates were different – since study group is the primary variable of interest, we performed Scheffe’s method on it regardless of significance. Significance was assessed using a Type-I error rate of 0.05. SAS® version 9.2 (SAS Institute, Inc., Cary, NC) was used for these analyses. We used the R statistical computing software [17] to produce the figure presented below.
3. Results
We analyzed SF-36v2 health survey results for a total of 1562 participants at 5 study sites. All participants completed the survey at baseline. 83% of the participants remained through the penultimate dose and 76% remained at the 42-month study time point; the attrition rate was never more than 7 percentage points different between the study groups at any of the four subsequent time points (Table 1). We did not find dropout to be associated with physical or mental score difference from baseline (p-values = 0.27 and 0.10, respectively).
At baseline, there was little evidence of differences in demographic characteristics between the study groups. Only age category was significantly different across study groups, with a p-value of 0.04 (Table 2).
Table 2.
Number (%) of subjects by demographic characteristics at baseline, CDC Anthrax Vaccine Adsorbed Human Clinical Trial, 2002-2005.
| Treatment groups | |||||||
|---|---|---|---|---|---|---|---|
| 8SQ-An = 259 | 8IM-An = 262 | 7IM-An = 256 | 5IM-An = 258 | 4IM-An = 267 | 8IM-Pn = 127 | 8SQ-Pn = 133 | |
| Age group* | |||||||
| <30 | 77 (30) | 63 (24) | 75 (29) | 77 (30) | 72 (27) | 35 (28) | 40 (30) |
| 30–39 | 42 (16) | 57 (22) | 77 (30) | 65 (25) | 60 (22) | 29 (23) | 31 (23) |
| 40–49 | 91 (35) | 87 (33) | 54 (21) | 64 (25) | 90 (34) | 35 (28) | 38 (29) |
| ≥50 | 49 (19) | 55 (21) | 50 (20) | 52 (20) | 45 (17) | 28 (22) | 24 (18) |
| Race | |||||||
| Black | 47 (18) | 48 (18) | 49 (19) | 62 (24) | 59 (22) | 28 (22) | 31 (23) |
| White | 199 (77) | 197 (75) | 194 (76) | 183 (71) | 200 (75) | 90 (71) | 95 (71) |
| Other | 13 (5) | 17 (6) | 13 (5) | 13 (5) | 8 (3) | 9 (7) | 7 (5) |
| Sex | |||||||
| Female | 134 (52) | 135 (52) | 132 (52) | 131 (51) | 135 (51) | 64 (50) | 68 (51) |
| Male | 125 (48) | 127 (48) | 124 (48) | 127 (49) | 132 (49) | 63 (50) | 65 (49) |
| Smoking status | |||||||
| Non smoker | 153 (59) | 163 (62) | 160 (63) | 158 (61) | 177 (66) | 84 (66) | 84 (63) |
| Smoker | 106 (41) | 99 (38) | 96 (38) | 100 (39) | 89 (33) | 43 (34) | 49 (37) |
| BMI group | |||||||
| Underweight | 0 (0) | 4 (2) | 5 (2) | 2 (1) | 5 (2) | 3 (2) | 1 (1) |
| Normal | 99 (38) | 79 (30) | 90 (35) | 88 (34) | 86 (32) | 37 (29) | 54 (41) |
| Overweight | 84 (32) | 105 (40) | 82 (32) | 91 (35) | 90 (34) | 51 (40) | 42 (32) |
| Obese | 76 (29) | 74 (28) | 79 (31) | 77 (30) | 86 (32) | 36 (28) | 36 (27) |
Note: There was one person with unknown smoking status in treatment group 4IM-A.
Chi-square p-value for test of homogeneity across treatment groups: 0.04; p-values for all other covariates were non-significant.
In the univariable models, the covariate interactions with dose number were not significant, and were thus removed from the models. Only the models controlling for age category (p < 0.005), body mass index category (p = 0.030), and smoking status (p = 0.030) produced significant covariate associations with physical score difference from baseline, while age category (p = 0.009), sex (p = 0.022), smoking status (p = 0.041), and study site (p = 0.011) were found to be associated with mental score difference from baseline. The univariable models controlling for study group did not suggest an association between study group and physical (p = 0.92) or mental (p = 0.53) score difference from baseline.
In the multivariable model where all covariates were controlled for simultaneously, all interactions with dose number were again found to be non-significant in both the physical and mental score difference from baseline models, and were thus removed from these models.
In the physical score difference from baseline multivariable model, only dose number (p = 0.003) and age category (p = 0.012) were significant. The multiple comparisons analysis on dose number revealed that the mean physical score difference from baseline at dose number 5 was significantly higher (more favorable) than those at dose numbers 7 (mean dose 5 – dose 7 score = 0.56; adjusted p = 0.009) and 8 (mean dose 5 – dose 8 score = 0.53; adjusted p = 0.021), while no other pairwise differences between dose numbers were significant; when not adjusting for multiple comparisons, only the comparison between doses 6 and 7 had a significant p-value (mean dose 6–dose 7 score = 0.34; p = 0.027, adjusted p = 0.18). The adjusted mean physical score difference from baseline for the <30, 30–39, 40–49, and ≥50 year old age categories were, respectively, 0.27, −0.01, −0.69, and −0.85. The multiple comparisons analysis on age category revealed that the mean physical score difference from baseline of the <30 year old age category was significantly higher than the 40–49 year old age category (mean <30 year old minus 40–49 year old score = 0.96; adjusted p = 0.047), and no other pairwise differences were significant; when not adjusting for multiple comparisons, the ≥50 year old age category was significantly different than the <30 year old (mean <30 year old minus ≥50 year old score = 1.11; p = 0.006, adjusted p = 0.056) and 30–39 year old (mean 30–39 year old minus ≥50 year old score = 0.82; p = 0.038, adjusted p = 0.23) categories. Neither study group (p = 0.89) nor its interaction with dose number (p = 0.53) was significantly associated with physical score difference from baseline; the pairwise comparison of study groups having the smallest p-value, both adjusted and unadjusted for multiple comparisons, was 7IM-A vs. 8IM-P (p = 0.18, adjusted p = 0.94).
In the mental score difference from baseline multivariable model, body mass index category was not significant and thus was removed from the model, leaving the following predictors: dose number, study group, age category, race category, sex, smoking status, study site, and dose number-study group interaction. Among these, only age category (p = 0.009) and study site (p = 0.024) were significant. The adjusted mean mental score difference from baseline for the <30, 30–39, 40–49, and ≥50 year old age categories were, respectively, −1.03, −1.21, −1.22, and −2.33. The multiple comparisons analysis on age category revealed that the mean mental score difference from baseline of the ≥50 year old group was significantly lower (less favorable) than both the <30 year old group (mean <30 year old minus ≥50 year old score = 1.30; adjusted p = 0.036) and the 40–49 year old group (mean 40–49 year old minus ≥50 year old score = 1.10; adjusted p = 0.037), while no other pairwise differences were significant; when using p-values unadjusted for multiple comparisons the 30–39 year old age category’s mean was more favorable than that of the ≥50 year old category (mean 30–39 year old minus ≥50 year old score = 1.11, p = 0.009, adjusted p = 0.079). The multiple comparisons analysis on study site revealed the mean mental score difference from baseline to be significantly higher for only one pair of sites (mean score difference between the sites = 1.29; adjusted p = 0.031), with no other significant pairwise differences. As with physical score difference from baseline, neither study group (p = 0.64) nor its interaction with dose number (p = 0.76) was significantly associated with mental score difference from baseline; the pairwise comparison of study groups having the smallest p-value, both adjusted and unadjusted for multiple comparisons, was 5IM-A vs. 4IM-P (p = 0.075, adjusted p = 0.79).
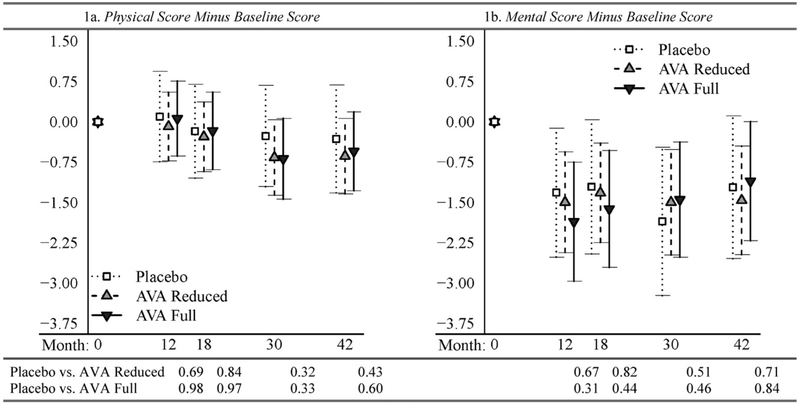
We illustrate the trends in physical and mental score difference from baseline over time in Fig. 1. Since study group and dose number–study group interaction were not significant, for illustration purposes we collapsed the seven study groups into three groups: all subjects receiving AVA at the then-licensed schedule regardless of route of injection (groups 8-SQ-A and 8IM-A in Table 1) were collected together as the “AVA Full” group; those receiving only saline placebo (8IM-P and 8SQ-P) formed the “Placebo” group; and those receiving AVA at a reduced dosing schedule (7IM-A, 5IM-A, and 4IM-A) are the “AVA Reduced” group. Mean physical scores differences from baseline were lower at dose numbers 7 and 8 (30 and 42 months after baseline) than at dose numbers 5 and 6 (12 and 18 months after baseline) (Fig. 1a), whereas mean mental score difference from baseline dropped more sharply at the dose number 5 survey (Fig. 1b). The p-values given in Fig. 1 were obtained from linear contrasts of the appropriate model parameters to compare the mean of the Placebo group to the other two groups at each dose number. All p-values in Fig. 1 are large, and all 95% confidence intervals overlap, suggesting no significant change over dose number of physical or mental score difference from baseline between the three aggregated study groups.
Fig. 1.
Results from multivariable GEE models by time point: means and 95% confidence intervals of subsequent scores minus baseline scores (plots), and p-values for changing differently from baseline (table), CDC Anthrax Vaccine Adsorbed Human Clinical Trial, 2002-2005.
4. Discussion
After 42 months of the CDC AVA Human Clinical Trial, AVA vaccine seemed to have no impact on physical or mental functional status. Overall, mean physical and mental scores decreased after baseline. However, we found no evidence that the physical or mental score difference from baseline changed differently between the seven study groups. This result held both in univariable GEE models including only study group, dose number, and dose number–study group interaction, as well as multivariable GEE models that included those predictors and controlled for age category, race category, sex, smoking status, body mass index category, and study site. In an attempt to obtain more parsimonious multivariable models, we removed age category, smoking status, and body mass index category if they were found to be non-significant; the same conclusions regarding non-significance of study group were found in both the full and reduced multivariable models.
In longitudinal studies there is a concern as to whether attrition rates are related to the response variable, in our case physical or mental score difference from baseline. Attrition, or dropout, is said to be informative if there is an association between the missing responses and the probability of dropping out of the study. We tested for this, as described above, and did not find dropout to be associated with physical or mental score difference from baseline. Since dropout could potentially be more closely related to raw scores (i.e. not difference in score from baseline), as a subanalysis we additionally tested whether dropout was associated with raw physical or mental score; no association was found (p = 0.24, 0.12 for physical and mental scores, respectively).
In an earlier report, we used repeated measurements with the SF-36v2 instrument to evaluate the long-term impact of anthrax vaccine exposure on functional impairment in AVA vaccinated and unvaccinated workers in the CDC’s Laboratory Response Network [18]. That observational study found no evidence of an association between physical or mental component scores and receipt of AVA over a 30-month timeframe. However, being an observational study there were several limitations including the lack of experimental controls, no randomization, moderate loss to follow up and the timeframe was limited to 30 months. In contrast, the AVA Human Clinical Trial participants were randomized in a double-blind fashion to receive AVA or saline placebo and there was more complete follow up of participants as long as 42 months.
The SF-36v2 health survey is a widely employed clinical research tool for evaluating the possible impact of a variety of different diseases or medical treatments on individuals’ self-reported functional status [11,19]. Using the SF-36v2 health survey provides a more global evaluation compared to traditional vaccine safety studies (e.g., human clinical trials employing subject diary cards) of the potential impact of a vaccine on a recipient’s health.
Our data showed that AVA Human Clinical Trial had higher physical and mental component scores at baseline than the national averages, which are 50 for both scores. Also, mean baseline scores differed slightly between participants in the three groups; in order to control this difference we analyzed change from baseline score for each participant. As in our prior report [18], we hypothesized that a period of follow up <12 months might be insufficient to detect a significant impact on the physical and mental component scores from baseline. The AVA Human Clinical Trial enabled us to use measurements obtained 12, 18, 30, and 42 months after baseline in our analysis, which in the licensed schedule for AVA at the time of this study corresponded to an individual receiving vaccine doses numbers 5, 6, 7, and 8, respectively.
Answers given in the SF-36 questionnaire are used to compute eight summary health measures – physical functioning, role physical, bodily pain, general health, social functioning, role emotional, mental health, and vitality – which are then used to compute the overall physical and mental scores analyzed in this study. In a Millennium Cohort Study comparing concordance of self-report and electronic records of receiving AVA to the eight SF-36 summary scales, significantly lower scores for the eight scales were found for persons who self-reported having received AVA but had no electronic record of receiving AVA [20]. The scores of those whose self-report and electronic records agreed as being unvaccinated were not significantly lower (not less favorable) than either those with concordance on being vaccinated or those self-reporting being unvaccinated but having electronic records of AVA receipt. This agrees with our findings of no significant difference between overall physical and mental score difference from baseline over a 42-month period among those receiving AVA vs. those receiving placebo only.
There are some limitations which may influence the interpretation of our results. Individual participants self-identified their age category, race category, sex, and smoking status at enrollment. As with any survey, there is a certain level of subjectivity embedded in the subjects’ responses.
In conclusion, the results of our analysis do not favor an association between physical or mental component scores and receipt of AVA over a 42-month timeframe. Our findings are important to the understanding of the safety profile of AVA, and support its safe use for the period we studied.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Mention of a product or company name does not constitute endorsement by the CDC. The protocol for this study was approved by an Institutional Review Board of the CDC.
Conflict of interest statement: None of the authors have conflicts of interest to declare.
References
- [1].Food and Drug Administration. Biothrax. Available at http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm133822.htm.
- [2].Joellenbeck LM, Zwanziger LL, Durch JS, Strom BL. The anthrax vaccine: is it safe? Does it work? Washington, DC: National Academy Press; 2002. [PubMed] [Google Scholar]
- [3].Wright JG, Quinn CP, Shadomy S, Messonnier N. Centers for Disease Control and Prevention (CDC). Use of anthrax vaccine in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) 2009. MMWR Recomm Rep 2010; 59(RR-6): 1–30. [PubMed] [Google Scholar]
- [4].Greidanus TG, Honl BA. Delayed-type hypersensitivity reaction to anthrax vaccine. Mil Med 2002;167(1):74–5. [PubMed] [Google Scholar]
- [5].Prater MR, Johnson VJ, Germolec DR, Luster MI, Holladay SD. Maternal treatment with a high dose of CpG ODN during gestation alters fetal craniofacial and distal limb development in C57BL/6 mice. Vaccine 2006;24(3): 263–71. [DOI] [PubMed] [Google Scholar]
- [6].Franco C, Lewis E, Morseth S, Simon L, Waytes AT. Reproductive toxicity of BioThrax® in rabbits. Birth Defects Res B Dev Reprod Toxicol 2009;86(5): 370–6. [DOI] [PubMed] [Google Scholar]
- [7].Asa PB, Wilson RB, Garry RF. Antibodies to squalene in recipients of anthrax vaccine. Exp Mol Pathol 2002;73(1):19–27. [DOI] [PubMed] [Google Scholar]
- [8].Petrik MS, Wong MC, Tabata RC, Garry RF, Shaw CA. Aluminum adjuvant linked to Gulf War illness induces motor neuron death in mice. Neuromolecular Med 2007;9(1):83–100. [DOI] [PubMed] [Google Scholar]
- [9].Marano N, Plikaytis BD, Martin SW, Rose C, Semenova VA, Martin SK, et al. Effects of a reduced dose schedule and intramuscular administration of anthrax vaccine adsorbed on immunogenicity and safety at 7 months: a randomized trial. JAMA 2008;300(13):1532–43. [DOI] [PubMed] [Google Scholar]
- [10].Shiely JC, Bayliss MS, Keller SD, et al. Health survey annotated bibliography: the first edition (1988–1995). The Health Institute, New England Medical Center; 1996. [Google Scholar]
- [11].Ware JE, Snow KK, Kosinski MA,Gandek B. SF-36 health survey, manual and interpretation guides. Boston: The Health Institute, New England Medical Center; 2000. [Google Scholar]
- [12].Fukuda N, Nisenbaum R, Stewart G, Thompson WW, Robin L, Washko RM, et al. Chronic multisymptom illness affecting Air Force veterans of the Gulf War. JAMA 1998;280(11):981–8. [DOI] [PubMed] [Google Scholar]
- [13].Hotopf M, David A, Hull L, Ismail K, Unwin C, Wessely S. Role of vaccinations as risk factors for ill health in veterans of the Gulf War: cross sectional study. BMJ 2000;320:1363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Doebbeling BN, Clarke WR, Watson D, Torner JC, Woolson RF, Voelker MD, et al. Is there a Persian Gulf War syndrome? Evidence from a large population-based survey of veterans and nondeployed controls. Am J Med 2000;108:695–704. [DOI] [PubMed] [Google Scholar]
- [15].Diggle PJ, Heagerty P, Liang KY, Zeger SL. Analysis of longitudinal data. New York: Oxford University Press; 2002. [Google Scholar]
- [16].Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. New York: Wiley; 2004. [Google Scholar]
- [17].R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2010, http://www.R-project.org. [Google Scholar]
- [18].Stewart B, Zhang Y, Rose CE Jr, Tokars JI, Martin SW, Franzke LH, et al. Health-related quality of life in the Anthrax Vaccination Program for workers in the Laboratory Response Network. Vaccine 2012;30:1841–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Brazier JE, Harper R, Jones NM, O’Cathain A, Thomas KJ, Usherwood T, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ 1992;305(6846):160–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Smith B, Leard CA, Smith TC, Reed Rj, Ryan MA, Millennium Cohort Study Team. Anthrax Vaccination in the Millennium Cohort: validation and measures of health. Am J Prev Med 2007;32(4):347–53. [DOI] [PubMed] [Google Scholar]