Abstract
Background
Vitamin D and calcium are important factors involved in the regulation of blood glucose and insulin secretion. The Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) score is a useful variable for evaluating insulin resistance, and therefore we cross-sectionally compared HOMA-IR scores according to serum vitamin D levels and dietary calcium intake.
Methods
We selected data from healthy males (n=5,163) and females (n=7,506) analyzed over 5 years (2008–2012) via the Korea National Health and Nutrition Examination Survey (KNHANES). We calculated HOMA-IR scores and compared them according to serum 25-hydroxyvitamin D (25(OH)D) concentration classification (<20, 20–30, >30 ng/mL) and dietary calcium quintile after adjustment for relevant variables using complex sample analysis. Comparisons were done after data weighting.
Results
The mean dietary calcium intake in males and females was 558.1 mg/day and 445.9 mg/day, respectively. The mean serum 25(OH)D concentration in males and females was 19.4 ng/mL and 16.8 ng/mL, respectively. After adjustment for relevant variables, HOMA-IR score was significantly correlated with serum 25(OH)D concentration and dietary calcium intake in females, whereas it was only correlated with serum 25(OH)D concentration in males. HOMA-IR was significantly lower in the top quintile of dietary calcium intake (mean, 866 mg/day) within females with vitamin D deficiency (P=0.047).
Conclusion
Adequate dietary calcium intake may be important for normal HOMA-IR in females with vitamin D deficiency.
Keywords: Insulin resistance, Vitamin D, Calcium
INTRODUCTION
Both calcium and vitamin D are the key for preventing and treating osteoporosis.1 Calcium is essential for bone health and vitamin D enhances calcium absorption and accumulation in bone. In addition to promoting bone health, both calcium and vitamin D have various effects on non-skeletal functions. Many studies have proven that a low to moderate calcium intake has a beneficial effect on bone health1,2, cardiovascular diseases3, colorectal cancer4, stroke5, and all-cause mortality.6 Moreover, recent studies showed that low calcium intake is associated with a good metabolic profile7, especially insulin resistance.8 In contrast, elevated calcium concentration is related to a number of metabolic abnormalities, including impaired glucose tolerance9, decreased insulin sensitivity, and insulin secretion.10 Furthermore, high serum calcium concentration has been shown to be associated with a 49% increased risk of type 2 diabetes.11 Along with calcium, vitamin D has been shown to be related to many medical conditions such as cardiovascular disease12, cancer13, inflammatory bowel disease14, depression and anxiety15, neurodegenerative disease16, diabetes17, and all-cause mortality.18 Of particular note, one recent study reported that serum vitamin D concentration was inversely correlated with insulin resistance.19
The Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) score is considered a good marker for assessing insulin resistance. While both calcium and vitamin D status have been related with disorders related to impaired glucose metabolism, such as insulin resistance and diabetes, the association of dietary calcium intake and of serum 25-hydroxyvitamin D (25(OH)D) concentration with HOMA-IR score has not previously been assessed. We hypothesized that HOMA-IR scores vary according to differences in dietary calcium intake and serum 25(OH)D concentration. Therefore, in this cross-sectional study, we evaluated HOMA-IR scores according to dietary calcium intake and serum 25(OH)D concentration using data from non-diabetic healthy Koreans acquired via the Korea National Health and Nutrition Examination Survey (KNHANES, 2008–2012).
METHODS
Subjects
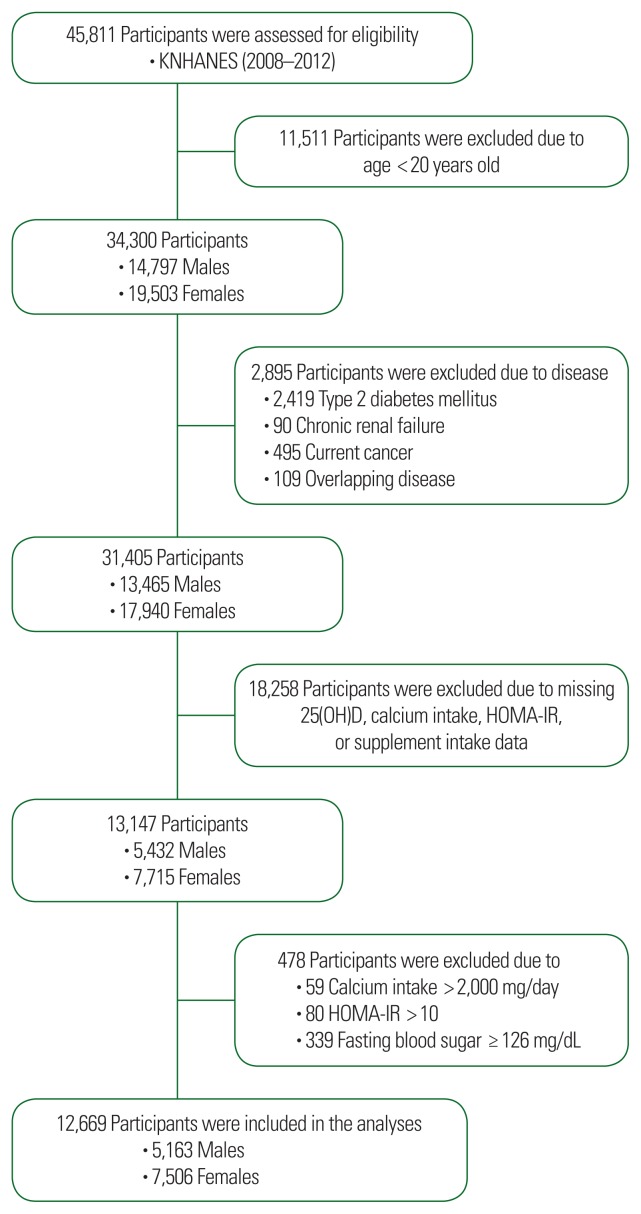
We used data from the KNHANES. Data from the fourth (IV-2 and IV-3, 2008 and 2009) and fifth (V-1–V-3, 2010–2012) surveys containing dietary calcium intake, 25(OH)D intake, and HOMA-IR scores were used in this cross-sectional analysis. From an initial data set of 45,811 males and females, 34,300 participants (14,797 males and 19,503 females) were selected. Of the 34,300 evaluated participants, 2,895 were excluded due to chronic diseases and combined diseases, and 18,258 participants were excluded due to missing data. An additional 478 participants were excluded due to having calcium intake >2,000 mg/day, HOMA-IR score >10, and fasting blood sugar ≥126 mg/dL. Thus, data from 12,669 participants (5,163 males and 7,506 females) were used in this analysis (Fig. 1). The Institutional Review Board of Ajou University approved this study (IRB No. AJIRB-MED-EXP-15-189) and all participants provided written informed consent before the survey.
Figure 1.
Flowchart for study data selection. Flow diagram of subject inclusion and exclusion for the Korea National Health and Nutrition Examination Survey (KNHANES). It shows the selection of study subjects in the 2008–2012 KNHANES. 25(OH)D, 25-hydroxyvitamin D; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance.
Laboratory and nutritional assessment
In this nationwide survey, blood samples were collected after 8 hours of fasting. Central testing institute guidelines were followed systematically (NeoDin Medical Institute, Seoul, Korea). Serum 25(OH)D concentration was measured with a radioimmunoassay kit (DiaSorin Inc., Stillwater, MN, USA) using an r-counter (1470 Wizard; PerkinElmer, Turku, Finland). The inter-assay coefficients of variation ranged from 2.8% to 6.2% for the 2008–2009 samples and from 1.9% to 6.1% for the 2010–2011 samples. Total cholesterol, triglyceride, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol levels were measured using a Hitachi 7600-110 chemistry analyzer (Hitachi, Tokyo, Japan). An automated analyzer with an enzymatic assay (Pureauto S GLU; Daiichi, Tokyo, Japan) was used to measure fasting plasma glucose concentrations; serum insulin concentrations were measured using a gamma counter with an immunoradiometric assay (INS-Irma; Biosource, Nivelles, Belgium). To assess insulin, HOMA-IR scores were calculated by the following equation: fasting insulin (μIU/mL)×fasting glucose (mg/dL)/405. A 24-hour dietary recall method for nutrient intake, including total calorie and calcium intake, was used. The results were calculated using the Food Composition Table developed by the National Rural Resources Development Institute (7th revision).20
Lifestyle questionnaires
Physical activity was classified into two categories, “yes” or “no.” Yes was defined as >30 minutes of moderate physical activity, three or more times in the last week. For smoking, three categories (current, ex-, and nonsmokers) were used. Current smokers were defined as those who were currently smoking and had smoked more than five packs of cigarettes during their life; ex-smokers were persons who had smoked in the past but had quit; and nonsmokers were persons who had no history of smoking. Alcohol consumption was also classified into two categories, “yes” or “no.” Yes was defined as regular alcohol drinkers, i.e., individuals who drank alcohol more than once per month; nondrinkers included all others.
Statistical analysis
After data weighting, we used a complex sample analysis method for the KNHANES data. Data from males and females were stratified into five groups (5th percentile) according to calcium intake as follows: G1 (1st quintile: male, 4–297 mg/day; female, 3–217 mg/day), G2 (2nd quintile: male, 298–420 mg/day; female, 218–320 mg/day), G3 (3rd quintile: male, 421–551 mg/day; female, 321–435 mg/day), G4 (4th quintile: male, 552–758 mg/day; female, 436–615 mg/day), and G5 (5th quintile: male, 759–1,996 mg/day; female, 616–1,997 mg/day). Serum 25(OH)D concentration was also classified into three groups in both sexes: <20, 20–30, and >30 ng/mL. General characteristics including age, body mass index (BMI), waist circumference, systolic blood pressure, diastolic blood pressure, fasting glucose, insulin, HOMA-IR score, total cholesterol, high-density lipoprotein, triglyceride, serum 25(OH)D concentration, daily calorie intake, and daily calcium intake were evaluated by a descriptive method after data weighting. In addition, smoking, drinking, and moderate physical activity were assessed by the chi-square test in both sexes. Partial correlation of dietary calcium intake and serum 25(OH)D with HOMA-IR score was compared after adjustment for age, BMI, smoking status, alcohol intake, moderate physical activity, and season in male; menopause, oral contraceptive use, and hormone replacement therapy were also adjusted in female. Finally, we compared HOMA-IR scores according to daily dietary calcium intake group (quintile) and serum 25(OH)D category (<20, 20–30, and >30 ng/mL) by the analysis of variance test after adjustment for age, smoking status, alcohol intake, moderate physical activity, season, and protein intake in male; menopause, oral contraceptive use, and hormone replacement therapy were also adjusted in female. All P-values express the trend that was used to assess the significance of all analyses; a P-value <0.05 was considered statistically significant. Data were analyzed using IBM SPSS version 19.0 (IBM Corp., Armonk, NY, USA).
RESULTS
There were significant differences between the two sexes regarding age, BMI, systolic blood pressure, diastolic blood pressure, fasting glucose, high-density lipoprotein, triglyceride, serum 25(OH)D concentration, and daily total calorie intake (Table 1).
Table 1.
General characteristics of the study subjects
| Variable | Male (n=5,163) | Female (n=7,506) | P |
|---|---|---|---|
| Age (yr) | 42.5±0.3 | 44.3±0.3 | <0.001 |
|
| |||
| BMI (kg/m2) | 24.0±0.1 | 23.0±0.1 | <0.001 |
|
| |||
| WC (cm) | 84.0±0.4 | 77.2±0.2 | <0.001 |
|
| |||
| SBP (mmHg) | 117.2±0.3 | 112.6±0.3 | <0.001 |
|
| |||
| DBP (mmHg) | 77.3±0.2 | 72.4±0.2 | <0.001 |
|
| |||
| Fasting glucose (mg/dL) | 93.3±0.3 | 91.3±0.1 | <0.001 |
|
| |||
| Insulin (μIU/mL) | 9.6±0.1 | 9.8±0.1 | 0.084 |
|
| |||
| HOMA-IR | 2.24±0.02 | 2.23±0.02 | 0.591 |
|
| |||
| TC (mg/dL) | 186.7±0.6 | 185.9±0.5 | <0.001 |
|
| |||
| HDL (mg/dL) | 49.9±0.2 | 55.9±0.2 | <0.001 |
|
| |||
| TG (mg/dL) | 151.2±2.1 | 105.6±1.1 | <0.001 |
|
| |||
| 25(OH)D (ng/mL) | 19.4±0.2 | 16.8±0.2 | <0.001 |
|
| |||
| Energy (kcal/day) | 2,368.0±16.7 | 1,660.0±10.8 | <0.001 |
|
| |||
| Carbohydrate intake (%) | 67.0±0.1 | 72.0±0.1 | <0.001 |
|
| |||
| Calcium (mg/day) | 558.1±5.4 | 445.9±4.6 | <0.001 |
|
| |||
| Smoking | <0.001 | ||
| Never | 2,898 (56.4) | 7,045 (94.3) | |
| Current | 2,237 (43.6) | 423 (5.7) | |
|
| |||
| Drinking | <0.001 | ||
| Never | 1,317 (25.7) | 4,536 (60.9) | |
| Current | 3,803 (74.3) | 2,913 (39.1) | |
|
| |||
| Moderate activity | 0.974 | ||
| Yes | 4,423 (85.7) | 6,411 (85.9) | |
| No | 713 (13.9) | 1,053 (14.1) | |
Values are presented as mean±standard error or number (%). P-values are from the general linear model after data weighting.
BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; TC, total cholesterol; HDL, high-density lipoprotein; TG, triglyceride; 25(OH)D, 25-hydroxyvitamin D; Energy, total calorie intake; Calcium, daily dietary calcium intake.
The partial correlation coefficient (r) of HOMA-IR score and serum 25(OH)D concentration was −0.078 (P<0.001) in males. There was no significant correlation between HOMA-IR score and dietary calcium intake. In females, the partial correlation coefficients of dietary calcium intake with HOMA-IR and of serum 25(OH)D concentration with HOMA-IR were −0.062 (P<0.001) and −0.031 (P=0.008), respectively (Table 2).
Table 2.
Correlations of dietary calcium intake and 25(OH)D with HOMA-IR score
| Variable | Calcium (mg/day) | 25(OH)D (ng/mL) | ||
|---|---|---|---|---|
|
|
|
|||
| r | P | r | P | |
| Male (n=5,163) | −0.008 | 0.575 | −0.078 | <0.001 |
|
| ||||
| Female (n=7,506) | −0.062 | <0.001 | −0.031 | 0.008 |
Values represent partial correlation coefficients after adjustment for age, body mass index, smoking, drinking, moderate physical activity, season, and protein intake in male; additional adjustment was performed for menopause, oral contraceptive use, and hormone replacement therapy in female.
25(OH)D, 25-hydroxyvitamin D; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; Calcium, daily dietary calcium intake.
We also evaluated the difference in HOMA-IR score according to dietary calcium intake quintile for different serum 25(OH)D concentration groups (deficiency, insufficiency, normal) after adjustment for age, smoking, drinking, moderate physical activity, season, and protein intake in male; menopause, oral contraceptive use, and hormone replacement therapy were also adjusted in female. HOMA-IR scores were not different across the dietary calcium and vitamin D groups in males. However, in females, HOMA-IR was significantly lower in the top dietary calcium intake quintile (mean, 866 mg/day) within the vitamin D-deficient group (P=0.047). In addition, females with vitamin D insufficiency in the highest dietary calcium quintile had the lowest HOMA-IR; however, this difference was only of marginal significance (P=0.064) (Table 3).
Table 3.
Comparison of HOMA-IR scores according to serum 25(OH)D concentration group and dietary calcium quintile
| Group | Serum 25(OH)D (ng/mL) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Male (n=5,163) | Female (n=7,506) | |||||
|
|
|
|||||
| <20 | 20–30 | >30 | <20 | 20–30 | >30 | |
| G1 | 2.25±0.05 (n=543) | 2.33±0.07 (n=350) | 2.09±0.08 (n=139) | 2.34±0.04 (n=1,059) | 2.22±0.06 (n=333) | 2.33±0.11 (n=93) |
|
| ||||||
| G2 | 2.30±0.06 (n=558) | 2.15±0.05 (n=356) | 2.05±0.09 (n=107) | 2.25±0.03 (n=1,064) | 2.25±0.06 (n=377) | 2.00±0.12 (n=58) |
|
| ||||||
| G3 | 2.28±0.05 (n=558) | 2.16±0.06 (n=380) | 1.91±0.06 (n=107) | 2.23±0.04 (n=1,084) | 2.24±0.06 (n=346) | 2.20±0.13 (n=75) |
|
| ||||||
| G4 | 2.28±0.05 (n=550) | 2.19±0.06 (n=387) | 2.16±0.11 (n=97) | 2.24±0.04 (n=1,059) | 2.24±0.05 (n=379) | 2.20±0.10 (n=69) |
|
| ||||||
| G5 | 2.30±0.04 (n=541) | 2.17±0.05 (n=402) | 1.95±0.13 (n=88) | 2.17±0.04* (n=1,084) | 2.07±0.05 (n=369) | 2.02±0.07 (n=57) |
|
| ||||||
| P | 0.949 | 0.206 | 0.146 | 0.047 | 0.064 | 0.140 |
Values are presented as mean±standard error by the analysis of variance test after adjustment for age, smoking, drinking, moderate activity, season, and protein intake in male; additional adjustment was performed for menopause, oral contraceptive use, and hormone replacement therapy in female. G1–G5 represent the dietary calcium intake quintiles. Mean± standard error (range of daily dietary calcium intake) in male: G1, 211.8±2.6 mg/day (4–297 mg/day); G2, 360.9±1.2 mg/day (298–420 mg/day); G3, 482.6±1.4 mg/day (421–551 mg/day); G4, 646.6±2.1 mg/day (552–758 mg/day); G5, 1,021.5±9.4 mg/day (759–1,996 mg/day). Mean±standard error (range of daily dietary calcium intake) in female: G1, 150.9± 1.4 mg/day (3–217 mg/day); G2, 268.5±0.9 mg/day (218–320 mg/day); G3, 375.1±1.1 mg/day (321–435 mg/day); G4, 515.7±1.7 mg/day (436–615 mg/day); G5, 866.3±8.5 mg/day (616–1,997 mg/day).
P<0.05 for the comparison between G1 vs. G5.
HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; 25(OH)D, 25-hydroxyvitamin D.
DISCUSSION
We found that dietary calcium intake (mean, 866 mg/day) was significantly associated with low HOMA-IR scores in females with vitamin D deficiency, but not in males. In addition, a marginally significant difference was observed in females with vitamin D insufficiency. This finding may indicate that adequate dietary calcium intake is important for normal HOMA-IR in females with vitamin D deficiency. In addition, maintenance of serum 25(OH)D >20 ng/mL may be beneficial for glucose metabolism.
Serum calcium concentration and incident diabetes have been shown to be tightly connected.11 Since calcium in our body plays an crucial role in many functions, including insulin secretion21, calcium is a key element for maintaining beta cell function.22 Therefore, persistent alterations in calcium balance are likely to affect the insulin secretory response. Vitamin D is a regulator of calcium absorption and distribution and as such is an important factor in calcium homeostasis and insulin balance. Vitamin D has also been reported to be related to insulin resistance.23 The effect of vitamin D on insulin synthesis and secretion is regulated by vitamin D response element.24 It is also regulated by extracellular calcium concentrations and calcium influx into beta cells.25 Another mechanism by which vitamin D affects insulin resistance involves the renin-angiotensin-aldosterone system. Specifically, vitamin D inhibits the action of insulin in vascular tissue and skeletal muscle via angiotensin II, which may result in decreased glucose uptake.26
In our study, adequate dietary calcium intake (mean, 866 mg/day) was significantly associated with low HOMA-IR scores in females with vitamin D deficiency. Similar findings were observed in females with vitamin D insufficiency, but the statistical significance was marginal. This finding may mean that adequate dietary calcium intake is associated with lower HOMA-IR scores in females with vitamin D deficiency. The relationship of dietary calcium intake and serum 25(OH)D concentration with HOMA-IR score was observed only in females. There are several potential reasons for this finding. To explore these possibilities, we further analyzed differences in fasting glucose and insulin concentration in the different dietary calcium groups in females with vitamin D deficiency. We found that serum insulin concentration showed a significantly different trend (P=0.024), but not fasting glucose (P=0.168). Relatively lower insulin concentration was found in the higher dietary calcium intake group. This lower insulin concentration might be associated with the lower HOMA-IR scores in females with vitamin D deficiency. No differences were observed in males. Several other factors can also be considered, including differences in fat distribution, intake of dietary calcium and carbohydrate, and serum 25(OH)D concentration. According to the macronutrient analysis, female participants (mean, 72%) consumed more carbohydrates than male subjects (67%). The HOMA-IR score is an indicator of carbohydrate imbalance; therefore, higher HOMA-IR scores are expected in participants with higher carbohydrate consumption. In the vitamin D-insufficient group, the HOMA-IR differences in the top dietary calcium intake quintile showed marginal significance, which may indicate that maintenance of serum 25(OH)D concentration >20 ng/mL is also important for normal HOMA-IR scores. Interestingly, in females with sufficient vitamin D, the HOMA-IR values were lowest in the top dietary calcium intake quintile, even though this difference was not statistically significant. The lack of statistical significance may be due to the small sample size compared to those in the other groups. We adjusted for total protein intake; however, there was no additional significant change of HOMA-IR scores. A potential explanation for this finding is that protein intake is considerably lower than carbohydrate intake (which is related with HOMA-IR score) in Korea, especially in female.
There are some limitations to our study. First, this was a cross-sectional study. Second, due to the high prevalence of vitamin D deficiency, we could not apply HOMA-IR scores properly in the vitamin D-sufficient group according to dietary calcium intake group. Third, the reasons why a significant difference in HOMA-IR score was not observed in males are unclear. Fourth, we used data from healthy participants only, and therefore it is difficult to generalize the results of this study. Regardless of these limitations, our study is valuable since it is the first study to evaluate differences in HOMA-IR score according to serum 25(OH)D classification (<20, 20–30, >30 ng/mL) and dietary calcium intake group (quintiles) using the representative KNHANES data.
In conclusion, higher dietary calcium intake was significantly associated with lower HOMA-IR scores in females with vitamin D deficiency. Further interventional or large-scale prospective studies are needed to confirm this association.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Garriguet D. Bone health: osteoporosis, calcium and vitamin D. Health Rep. 2011;22:7–14. [PubMed] [Google Scholar]
- 2.Sanders KM, Nowson CA, Kotowicz MA, Briffa K, Devine A, Reid IR, et al. Calcium and bone health: position statement for the Australian and New Zealand Bone and Mineral Society, Osteoporosis Australia and the Endocrine Society of Australia. Med J Aust. 2009;190:316–20. doi: 10.5694/j.1326-5377.2009.tb02421.x. [DOI] [PubMed] [Google Scholar]
- 3.Chan R, Leung J, Woo J. A prospective cohort study examining the associations of dietary calcium intake with all-cause and cardiovascular mortality in older Chinese community-dwelling people. PLoS One. 2013;8:e80895. doi: 10.1371/journal.pone.0080895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keum N, Aune D, Greenwood DC, Ju W, Giovannucci EL. Calcium intake and colorectal cancer risk: dose-response meta-analysis of prospective observational studies. Int J Cancer. 2014;135:1940–8. doi: 10.1002/ijc.28840. [DOI] [PubMed] [Google Scholar]
- 5.Larsson SC, Orsini N, Wolk A. Dietary calcium intake and risk of stroke: a dose-response meta-analysis. Am J Clin Nutr. 2013;97:951–7. doi: 10.3945/ajcn.112.052449. [DOI] [PubMed] [Google Scholar]
- 6.Leifsson BG, Ahrén B. Serum calcium and survival in a large health screening program. J Clin Endocrinol Metab. 1996;81:2149–53. doi: 10.1210/jcem.81.6.8964843. [DOI] [PubMed] [Google Scholar]
- 7.da Silva Ferreira T, Torres MR, Sanjuliani AF. Dietary calcium intake is associated with adiposity, metabolic profile, inflammatory state and blood pressure, but not with erythrocyte intracellular calcium and endothelial function in healthy pre-menopausal women. Br J Nutr. 2013;110:1079–88. doi: 10.1017/S0007114513000111. [DOI] [PubMed] [Google Scholar]
- 8.Lorenzo C, Hanley AJ, Rewers MJ, Haffner SM. Calcium and phosphate concentrations and future development of type 2 diabetes: the Insulin Resistance Atherosclerosis Study. Diabetologia. 2014;57:1366–74. doi: 10.1007/s00125-014-3241-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wareham NJ, Byrne CD, Carr C, Day NE, Boucher BJ, Hales CN. Glucose intolerance is associated with altered calcium homeostasis: a possible link between increased serum calcium concentration and cardiovascular disease mortality. Metabolism. 1997;46:1171–7. doi: 10.1016/S0026-0495(97)90212-2. [DOI] [PubMed] [Google Scholar]
- 10.Sun G, Vasdev S, Martin GR, Gadag V, Zhang H. Altered calcium homeostasis is correlated with abnormalities of fasting serum glucose, insulin resistance, and beta-cell function in the Newfoundland population. Diabetes. 2005;54:3336–9. doi: 10.2337/diabetes.54.11.3336. [DOI] [PubMed] [Google Scholar]
- 11.Jorde R, Schirmer H, Njølstad I, Løchen ML, Bøgeberg Mathiesen E, Kamycheva E, et al. Serum calcium and the calcium-sensing receptor polymorphism rs17251221 in relation to coronary heart disease, type 2 diabetes, cancer and mortality: the Tromsø Study. Eur J Epidemiol. 2013;28:569–78. doi: 10.1007/s10654-013-9822-y. [DOI] [PubMed] [Google Scholar]
- 12.Ku YC, Liu ME, Ku CS, Liu TY, Lin SL. Relationship between vitamin D deficiency and cardiovascular disease. World J Cardiol. 2013;5:337–46. doi: 10.4330/wjc.v5.i9.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garland CF, Mohr SB, Gorham ED, Grant WB, Garland FC. Role of ultraviolet B irradiance and vitamin D in prevention of ovarian cancer. Am J Prev Med. 2006;31:512–4. doi: 10.1016/j.amepre.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 14.Reich KM, Fedorak RN, Madsen K, Kroeker KI. Vitamin D improves inflammatory bowel disease outcomes: basic science and clinical review. World J Gastroenterol. 2014;20:4934–47. doi: 10.3748/wjg.v20.i17.4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armstrong DJ, Meenagh GK, Bickle I, Lee AS, Curran ES, Finch MB. Vitamin D deficiency is associated with anxiety and depression in fibromyalgia. Clin Rheumatol. 2007;26:551–4. doi: 10.1007/s10067-006-0348-5. [DOI] [PubMed] [Google Scholar]
- 16.Millet P, Landel V, Virard I, Morello M, Féron F. Role of vitamin D in the physiopathology of neurodegenerative diseases. Biol Aujourdhui. 2014;208:77–88. doi: 10.1051/jbio/20140007. [DOI] [PubMed] [Google Scholar]
- 17.Zhao LM, Tian XQ, Ge JP, Xu YC. Vitamin D intake and type 2 diabetes risk: a meta-analysis of prospective cohort studies. Afr Health Sci. 2013;13:1130–8. doi: 10.4314/ahs.v13i4.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skaaby T, Husemoen LL, Pisinger C, Jørgensen T, Thuesen BH, Fenger M, et al. Vitamin D status and incident cardiovascular disease and all-cause mortality: a general population study. Endocrine. 2013;43:618–25. doi: 10.1007/s12020-012-9805-x. [DOI] [PubMed] [Google Scholar]
- 19.Jiménez-Pavón D, Sesé MA, Valtueña J, Cuenca-García M, González-Gross M, Gottrand F, et al. Leptin, vitamin D, and cardiorespiratory fitness as risk factors for insulin resistance in European adolescents: gender differences in the HELENA Study. Appl Physiol Nutr Metab. 2014;39:530–7. doi: 10.1139/apnm-2013-0250. [DOI] [PubMed] [Google Scholar]
- 20.World Food Programme. Food Aid Information System: Food Composition Table [Internet] Rome: World Food Programme; 2017. [cited 2017 Nov 17]. Available from: http://www.wfp.org/fais/nutritional-reporting/food-composition-table. [Google Scholar]
- 21.Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49:1751–60. doi: 10.2337/diabetes.49.11.1751. [DOI] [PubMed] [Google Scholar]
- 22.Ko SH, Lee GS, Vo TT, Jung EM, Choi KC, Cheung KW, et al. Dietary calcium and 1,25-dihydroxyvitamin D3 regulate transcription of calcium transporter genes in calbindin-D9k knockout mice. J Reprod Dev. 2009;55:137–42. doi: 10.1262/jrd.20139. [DOI] [PubMed] [Google Scholar]
- 23.Nagpal J, Pande JN, Bhartia A. A double-blind, randomized, placebo-controlled trial of the short-term effect of vitamin D3 supplementation on insulin sensitivity in apparently healthy, middle-aged, centrally obese men. Diabet Med. 2009;26:19–27. doi: 10.1111/j.1464-5491.2008.02636.x. [DOI] [PubMed] [Google Scholar]
- 24.Maestro B, Dávila N, Carranza MC, Calle C. Identification of a vitamin D response element in the human insulin receptor gene promoter. J Steroid Biochem Mol Biol. 2003;84:223–30. doi: 10.1016/S0960-0760(03)00032-3. [DOI] [PubMed] [Google Scholar]
- 25.Sergeev IN, Rhoten WB. 1,25-Dihydroxyvitamin D3 evokes oscillations of intracellular calcium in a pancreatic beta-cell line. Endocrinology. 1995;136:2852–61. doi: 10.1210/endo.136.7.7789310. [DOI] [PubMed] [Google Scholar]
- 26.Zemel MB, Peuler JD, Sowers JR, Simpson L. Hypertension in insulin-resistant Zucker obese rats is independent of sympathetic neural support. Am J Physiol. 1992;262(3 Pt 1):E368–71. doi: 10.1152/ajpendo.1992.262.3.E368. [DOI] [PubMed] [Google Scholar]