Abstract
Pacemaker cells residing in the sinoatrial node generate the regular heartbeat. Ca2+ signaling controls the heartbeat rate—directly, through the effect on membrane molecules (NCX exchange, K+ channel), and indirectly, through activation of calmodulin-AC-cAMP-PKA signaling. Thus, the physiological role of signaling in pacemaker cells can only be assessed if the Ca2+ dynamics are in the physiological range. Cultured cells that can be genetically manipulated and/or virally infected with probes are required for this purpose. Because rabbit pacemaker cells in culture experience a decrease in their spontaneous action potential (AP) firing rate below the physiological range, Ca2+ dynamics are expected to be affected. However, Ca2+ dynamics in cultured pacemaker cells have not been reported before. We aim to a develop a modified culture method that sustains the global and local Ca2+ kinetics along with the AP firing rate of rabbit pacemaker cells.
We used experimental and computational tools to test the viability of rabbit pacemaker cells in culture under various conditions. We tested the effect of culture dish coating, pH, phosphorylation, and energy balance on cultured rabbit pacemaker cells function. The cells were maintained in culture for 48 h in two types of culture media: one without the addition of a contraction uncoupler and one enriched with either 10mM BDM (2,3-Butanedione 2-monoxime) or 25 μM blebbistatin. The uncoupler was washed out from the medium prior to the experiments. Cells were successfully infected with a GFP adenovirus cultured with either BDM or blebbistatin. Using either uncoupler during culture led to the cell surface area being maintained at the same level as fresh cells. Moreover, the phospholamban and ryanodine receptor densities and their phosphorylation level remained intact in culture when either blebbistatin or BDM were present. Spontaneous AP firing rate, spontaneous Ca2+ kinetics, and spontaneous local Ca2+ release parameters were similar in the cultured cells with blebbistatin as in fresh cells. However, BDM affects these parameters. Using experimental and a computational model, we showed that by eliminating contraction, phosphorylation activity is preserved and energy is reduced. However, the side-effects of BDM render it less effective than blebbistatin.
Keywords: Biophysics, Computational model, Sarcoplasmic reticulum, Sinoatrial node
1. Introduction
Pacemaker cell function is determined by a coupled-clock system whose components are connected directly through Ca2+ [34,35] or indirectly through Ca2+ activation of post-translational modification signaling [1]. Deterioration of Ca2+ signaling was associated with a decreased action potential (AP) rate [2,3], whereas an increase in the Ca2+ transient signal was associated with an increased action potential rate [4]. Moreover, cardiac diseases have been shown to be related to a deficit in Ca2+ dynamics (i.e., a reduced Ca2+ release rate and signal mass, reduced Ca2+ transient amplitude, or both) and post-translational modification signaling [5,6]. Thus, to understand pacemaker function, dynamic measurements of Ca2+ and post-translational modification signaling under physiological conditions or in response to genetic manipulation must be performed. Such measurements are usually performed on cultured cells that were maintained 48 h in culture [7,8].
Only a few labs around the world have succeeded in culturing rabbit pacemaker cells [9–11]. However, these culture methods lead to a reduction in ionic currents [10,11] and steady state post-translational modification signaling [9] as well as to a reduction in the spontaneous beating rate of pacemaker cells to a rate that is far below the physiological one (3 Hz). Thus, it is currently impossible to explore the physiological cross-talk signaling, Ca2+ and post-translational modification signaling in cultured cells with non-physiological coupled-clock mechanisms.
In order to prolong ventricular cell viability in culture, culture media are often supplemented with the contraction uncoupler 3-Butanedione 2-monoxime (BDM) [12]. Recent work on mouse pacemaker cells has shown that adding BDM to the culture medium improves cell viability and morphology, and maintains the spontaneous AP firing rate of the cells (~ 6 Hz) [13]. This work has several limitations. First, mouse pacemaker cells are inferior to those of rabbits: the cross-talk signaling of rabbit cells more closely resembles that of human pacemaker cells [14]. Second, Ca2+ dynamics or phosphorylation status were not measured using this culture method. Third, no mechanistic explanation is provided as to why BDM preserved the biophysical properties of the pacemaker cells. Above all, recent work in cultured ventricular myocytes has shown that adding blebbistatin to the culture medium maintains cardiac myocyte viability and morphology, in comparison to BDM [15]. Conversely, the authors of [13] found that “cell survival and morphology were dramatically compromised in cultures prepared with blebbistatin”. However, they neither report on the effect of blebbistatin on the AP firing rate nor on Ca2+ dynamics. Thus, it was not known whether BDM or blebbistatin can improve the culture method of rabbit pacemaker cells and maintain Ca2+ kinetics; whether blebbistatin is indeed superior to BDM in the context of cultured rabbit pacemaker cells, if so, what underlying mechanisms might explain this superiority, and if adding an uncoupler maintains global and local Ca2+ dynamics in cultured rabbit pacemaker cells, what underlying mechanisms lead to this result.
To answer these questions, we examined whether cultured pacemaker cell structure and function are sustained by the addition of a contraction uncoupler, BDM or blebbistatin, to the culture medium for 48 h, and we compared the effect of the two uncouplers. We used experimental and computational tools to test the effect of culture dish coating, pH, phosphorylation activity, and energy balance on cultured rabbit pacemaker cells. Our results show that (i) adding a contraction uncoupler allows cell properties to be maintained in culture, (ii) by eliminating contraction, phosphorylation activity is preserved and energy is reduced, (iii) adding blebbistatin to a mixed culture medium with salts is superior to such a medium with BDM, (iv) the side-effects of BDM render it less effective than blebbistatin in preserving rabbit pacemaker cell function.
2. Materials and methods
2.1. Animal use
Animals were treated in accordance with the Technion Ethics Committee. The experimental protocols were approved by the Animal Care and Use Committee of the Technion (Ethics number: IL-118–10-13).
2.2. Pacemaker cell isolation
Pacemaker cells were isolated from healthy male New Zealand White rabbits (125 males) weighing 2.3–2.7kg. Each rabbit was weighed and sedated through administration of an intramuscular injection containing ketamine (0.1 ml/kg) and xylazine (0.1 ml/kg). An intravenous (IV) cannula was inserted in the rabbit’s ear for the future use of an anesthetic. The rabbit was administered 200 mg/ml sodium pentobarbital diluted with heparin through the IV cannula. The adequacy and efficiency of the anesthesia were examined by observing the loss of reflexes in the eye and foot. Then the rabbit was placed on its back, the skin over the sternum was pulled back, and careful cuts were made through the diaphragm and the ribs, revealing the heart. For the entire procedure, see Davoodi et al. [16]. In short, cells were dispersed from the pacemaker tissue preparation by gentle pipetting through the KB solution, filtered through a 150 μm mesh, and stored at 4 °C to be used fresh for 6 h.
2.3. Culture procedure
Isolated, single, beating pacemaker cells were placed in culture for 48 h (Fig. S1). The cells were seeded onto 35 mm glass dishes coated with one of the following: i) Laminin, 25 μg/ml (Sigma Aldrich), dissolved in PBS (in 1 ml PBS + 1% PS, no Ca2+ and no Mg2+). Each dish was coated with 200 μl laminin and was left for incubation for at least 1 h (37 °C, 90% humidity & 5% CO2, Galaxy 170 R, Eppendorf). The laminin coating was aspirated at least 10 min prior to seeding the cells. ii) Polyethylenimine (PEI, Sigma Aldrich), dissolved in borate buffer (containing 0.012 M sodium tetraborate and 0.05 M boric acid) at a ratio of 1:5000. At least 24 h before the procedure, the dishes were coated with 200 μl PEI mixed solution and left for 90 min to settle in an open biological hood at room temperature. The dishes were then washed 3 times with borate buffer and left to dry for 2 h. iii) Collagen (MatTek Corporation, Ashland, MA, U.S.).
The cells were diluted in one of 4 serum-free culture media (summarized in Table 1) and then centrifuged at 500 RPM for 5 min (Spectrafuge 6C, Labnet): (i) a pure culture medium mixed with BDM: M199 (Sigma Aldrich), 2% PS (penicillin-streptomycin (Gibco)), 1% ITS (insulin-transferrin-selenium, Sigma Aldrich), 0.1% BDM (2, 3 butanedione, Sigma Aldrich), and 0.01% BSA (Sigma Aldrich) similar to [17]; (ii) a mixed culture medium with salts (containing (in mmol/L): 116 NaCl, 5.4 KCl, 0.8 MgCl2, 0.9 NaH2PO4, 5.6 Glucose, 20 HEPES, 1.8 CaCl2, 26 NaHCO3), 20% M199 (Sigma, in the presence of (in mmol/L): 5 creatine, 2 L-carnitine, 5 taurine, 0.1% ITS, and 1% penicillin and streptomycin and titrated to pH 7.4 with NaOH at 37 °C); (iii) a mixed culture medium with salts and BDM (containing (in mmol/L): 100 NaCl, 5.4 KCl, 0.8 MgCl2, 0.9 NaH2PO4, 5.6 Glucose, 20 HEPES, 1.8 CaCl2, 26 NaHCO3), 20% M199 (Sigma, in the presence of (in mmol/L): 5 creatine, 2 L-carnitine, 5 taurine, 0.1% ITS, and 1% penicillin and streptomycin and titrated to pH 7.4 with NaOH at 37 °C) and 10 mmol/L BDM; (iv) a mixed culture medium with salts and blebbistatin (containing (in mmol/L): 116 NaCl, 5.4 KCl, 0.8 MgCl2, 0.9 NaH2PO4, 5.6 glucose, 20 HEPES, 1.8 CaCl2, 26 NaHCO3, 20% M199 (Sigma, in the presence of (in mmol/L): 5 creatine, 2 L-carnitine, 5 taurine, 0.1% ITS, and 1% penicillin and streptomycin and titrated to pH 7.4 with NaOH at 37 °C) and 25 μmol/L blebbistatin. The conical tube containing the cells was drained, leaving the cells at the bottom of the tube. 600 μl of the serum-free medium was added to the conical tube and the cell suspension was divided between 2–3 dishes. The dishes were incubated for 1 h (for 1 h at 37 °C, 90% humidity & 5% CO2, Galaxy 170R, Eppendorf). Cells were incubated in one of the 4 serum-containing media for the first 24 h, and then cultured in one of the 4 serum-free media (changing the media after less than 24 h caused cell washout). The cells were washed three times with one of the 4 serum-enriched media added to each dish: (i) a pure culture medium mixed with BDM, M199 (Sigma Aldrich), 2% PS, 0.1% BDM (2, 3 butanedione, Sigma Aldrich), and 5% of the serum-enriched medium; (ii) a mixed culture medium with salts (containing (in mmol/L): 116 NaCl, 5.4 KCl, 0.8 MgCl2, 0.9 NaH2PO4, 5.6 glucose, 20 HEPES, 1.8 CaCl2, 26 NaHCO3), 20% M199 (Sigma, in the presence of (in mmol/L): 5 creatine, 2 L-carnitine, 5 taurine, 0.1% insulin-transferrin-selenium-X, 4% fetal bovine serum, 2% horse serum, and 1% penicillin and streptomycin and titrated to pH 7.4 with NaOH at 37 °C); (iii) a mixed culture medium with salts and BDM (containing (in mmol/L): 116 NaCl, 5.4 KCl, 0.8 MgCl2, 0.9 NaH2PO4, 5.6 glucose, 20 HEPES, 1.8 CaCl2, 26 NaHCO3), 20% M199 (Sigma, in the presence of (in mmol/L): 5 creatine, 2 L-carnitine, 5 taurine, 0.1% insulin-transferrin-selenium-X, 4% fetal bovine serum, 2% horse serum, and 1% penicillin and streptomycin and titrated to pH 7.4 with NaOH at 37 °C) and 10mmol/L BDM; (iv) a mixed culture medium with salts and blebbistatin (containing (in mmol/L): 116 NaCl, 5.4 KCl, 0.8 MgCl2, 0.9 NaH2PO4, 5.6 glucose, 20 HEPES, 1.8 CaCl2, 26 NaHCO3), 20% M199 (Sigma, in the presence of (in mmol/L): 5 creatine, 2 L-carnitine, 5 taurine, 0.1% insulin-transferrin-selenium-X, 4% fetal bovine serum, 2% horse serum, and 1% penicillin and streptomycin and titrated to pH 7.4 with NaOH at 37 °C) and 25 μmol/L blebbistatin. Because of the small number of isolated cells (around 1000 cells), we used the entire cell pellet and divided it to two dishes. On average we had approximately 30 live cells per dish, of which 3–7 were beating.
Table 1.
Serum free media components for the different protocols.
| Pure culture medium + BDM (500 ml) |
Mixed culture medium with salts (480 ml) |
Mixed culture medium with salts + BDM (480 ml) |
Mixed culture medium with salts + BLB (480 ml) |
||
|---|---|---|---|---|---|
| Medium | M199 | 485ml | 95.2ml | 95.2ml | 95.2ml |
| BSA | 50mg | ||||
| ITS | 5ml | ||||
| PS | 10ml | 4.8ml | 4.8ml | 4.8ml | |
| Creatine | – | 524 mg (20% for final) | 524 mg (20% for final) | 524 mg (20% for final) | |
| L-carnitine | – | 316 mg (20% for final) | 316 mg (20% for final) | 316 mg (20% for final) | |
| Taurine | – | 500.8 mg (20% for final) | 500.8 mg (20% for final) | 500.8 mg (20% for final) | |
| BDM | 505 (252.5)mg | – | – | – | |
| Salts (78% of the total | Water | – | 1000 | 1000 | 1000 |
| substance) | NaCl | – | 6.78g | 6.43g | 6.78g |
| KCl | – | 400mg | 400mg | 400mg | |
| MgCl2 | – | 800μl | 800μl | 800μl | |
| NaH2PO4H20 | – | 124.2mg | 124.2mg | 124.2mg | |
| Glucose | – | 1.008g | 1.008g | 1.008g | |
| HEPES | – | 4.76g | 4.76g | 4.76g | |
| CaCl2 | – | 1.8ml | 1.8ml | 1.8ml | |
| NaHCO3 | – | 2.184g | 2.184g | 2.184g | |
| BDM | – | – | 1.314g | – | |
| Blebbistatin | – | – | – | 7.308 mg | |
2.4. Experimental medium
In order to image the cells and measure their oxygen consumption, fluorescence, and immunofluorescence, the cells were washed with a HEPES buffer containing (in mM): NaCl 140, KCl 5.4, HEPES 5, Glucose 10, MgCl2 2, CaCl2 1 (pH 7.4 with NaOH). Experiments were conducted less than 6 h after isolation or not more than 1 h after removal of BDM or blebbistatin. Note that pacemaker cells start to beat spontaneously after washout.
2.5. Quantifying cell area
For full method details, see [17]. In short, to quantify pacemaker cell area, the cells were imaged on an inverted fluorescence microscope (Zeiss Observer Z1, Germany) using a 40×/1.4N.A oil immersion lens. Fresh pacemaker cells were placed in a glass dish (MatTek Corporation, Ashland, MA, U.S.) with HEPES solution (see above) inside a microscope incubator (Zeiss, Germany) at 37 ± 0.5 °C.
2.6. Fluorescence measurements of APs
The cells were loaded with a 10 μM voltage sensitive dye (di-4-Anepps) (ThermoFisher Scientific) for 5 min. The dye was washed out with HEPES solution prior to imaging. The cells were imaged on an LSM880 confocal laser scanning microscope (Zeiss) using a 40×/1.2 N.A. water immersion lens. Cells were excited with a 488 nm Argon laser line. Transmitted optics line-scan images (4096 × 1 pixels at 23.21 pixels/μm and 5.66 ms/line) were recorded with orientation along the long axis of the cell to quantify the pacemaker cells’ spontaneous APs.
2.7. Ca2+ measurements
Ca2+ kinetics were measured with Fluo-4 AM (ThermoFisher Scientific) at 37 ± 0.5 °C as described before [16,17]. t50 and t90 are defined from the minimal signal to the point of 50% or 90% decay of the signal [18]. Because at least 10 beats are measured, where the nadir of the Ca2+ transient includes LCR, it is ignored in the statistics. In short, fluorescence emission was collected with LP 505 nm, with the pinhole set to form an image of no more than a 3 μm optical slice (512 × 1 pixels at 17.15 pixels/μm and 3.22 ms/line).
2.8. Immunofluorescence and immunolabeling of pacemaker myocytes
The immunolabeling was similar to the protocol described in [17]. In short, cells were excited with a 488 nm Argon laser line, fluorescence emission was collected with LP 505 nm, and all images were captured in frame mode (4096 × 4096 pixels at 102.1 × 102.1 μm). For analysis see [17,19].
2.9. Antibodies
We employed either a monoclonal anti-RyR2 antibody (IgG1, clone C3–33, 1:100, ThermoFisher Scientific, Bothell, WA, U.S.), a polyclonal anti–HCN4 antibody (1:100, Alomone Labs), an anti-PLB antibody (1:200, Badrilla), a p-PLB PS-16 antibody (1:200, Badrilla) or a p-RyR2 (2809 site) antibody (1:200, Badrilla).
2.10. Mitochondrial membrane potential measurements
Mitochondrial membrane potential was visualized using the potential-sensitive dye TMRM (1:80 (125 nM), tetramethyl rhodamine methyl ester perchlorate, ThermoFisher Scientific) as described before [17]. All images were captured in frame mode (512 × 512 pixels at 105.75 × 105.75 μm).
2.11. Infection of cultured pacemaker cells
Cells were infected with adenovirus (1:100) expressing green fluorescent protein (GFP) as described before [17]. In short, the cells were infected with the virus 24 h after seeding and were imaged 24 h after infection. To evaluate the success of the procedure, the cells were imaged on an inverted fluorescence microscope (Zeiss Observer Z1) at 37 ± 0.5 °C using a 40×/1.4N.A oil immersion lens and 488 nm LED excitation. All images were captured in frame mode (780 × 690 pixels at 260.6 × 218 μm).
2.12. Electron microscopy pictures
The pacemaker cells were fixed in 2.5% glutaraldehyde (Sigma Aldrich) in 0.1 M cacodylate buffer (Electron Microscopy Sciences) (pH 7.4) for 30 min and post-fixed in 2% OsO4 (Electron Microscopy Sciences) in 0.1 M cacodylate buffer (Electron Microscopy Sciences) for 30 min. The samples were dehydrated in a grade series of alcohol solutions and then embedded in Spurr’s resin (SPI supplies). Thin sections were cut on a Leica Ultracut R cutter with diamond knives (Diatome). Thin sections (80 nm) were then stained with 4% uranyl acetate (Beijing Zhongjingkeyi) for 30 min and counterstained with lead citrate for 10 min. The TEM images were obtained using the FEI Tecnai G2 20 Twin system operated at 120 kV.
2.13. Oxygen consumption measurements
Oxygen consumption was measured in cell suspensions of spontaneously beating pacemaker cells using Clark-type electrodes (MT200, Strathkelvin Instruments Ltd.). Pacemaker cell suspensions were centrifuged at 100 g for 5 min, and the supernatant was removed. The cells were then incubated in HEPES buffer (Section 2.4). The cell suspension was divided equally into 2 aliquots: the first aliquot was designated for blebbistatin application, and the second was used as a control. The cell suspensions were stirred gently in 36 °C in HEPES buffer for 3 min. Measurements were acquired for 3 min under control conditions, 2 min following blebbistatin application. The second group (control) was measured for 5 min. Following measurements of oxygen consumption, total protein concentration (BCATM Protein Assay) and the number of viable cells were determined in the cell suspension. The oxygen consumption was normalized to the protein concentration. To prove that the cells were functioning and reacting to the drug application, the beating rate with and without blebbistatin was measured on single cells from the same cell suspension.
2.14. Computational modeling
Our computational modeling is based on [20]. The model describes the coupled-clock function that includes different compartments of the cells (cytosol, submembrane, and SR), the major ion channels that constitute the membrane clock, and the proteins on the SR that constitute the Ca2+ clock. The model also includes the AC-cAMP-PKA signaling: the internal pacemaker mechanisms are tightly coupled with AC-cAMP-PKA signaling through the stimulation of G protein-coupled receptors that activate (adrenergic) or inactivate (cholinergic) AC.
To describe ATP consumption, we provide the following equations: ATP consumption by the cross-bridges (XB) is described by:
| (1) |
where MaxATP is the maximal consumption of ATP by XB, Ff is the cross-bridge turnover rate from the weak to the strong conformation, A is the activation state of XB [21], and Force is the force produced by the XB.
ATP consumption by cAMP production:
| (2) |
where k1 is the deformation kinetics of cAMP and ATPi is the ATP concentration in mM in the cytosol.
ATP consumption by the SERCA pump:
| (3) |
where Jup is the rate of Ca2+ uptake by the SR and is the ratio between the volume of network SR (Ca2+ uptake store) and the myo-plasma.
ATP consumption by NaK pump:
| (4) |
where INaK is the NaK current and F is the Faraday constant. See [20] for parameter values and equations.
To simulate changes in pH, we reduced the maximal conductance of the L-type current by 10%, as suggested in [22], reduced the maximal conductance of potassium current by 5%, as also suggested in [22], reduced the activity of the NaK pump (10% reduction in KmKp (half-maximal K0 for INaK)) and KmNap (half-maximal Nai for INaK), as suggested in [23], and reduced PKA activity (Pup, basal) by 22%, as suggested in [24]. See [20] for parameter values and equations.
2.15. Statistics
Data are presented as mean ± SEM. For independent samples, a two-sample t-test was applied (Figs. 5, S2 and S3). Two-way ANOVA (taking into account cell and animal measurements) was used to compare Ca2+ parameters (Fig. 4). To determine whether the shape of two distributions is the same, we used the Kolmogorov-Smirnov two-sample test applied to the standard scores (z-scores) from each sample. The standard scores were obtained by subtracting the sample-specific mean and dividing the result by the sample-specific standard deviation for each group. We used z-scores as we do not wish to reject the center or variability if the distribution differs; rather, we are only interested in whether the shapes of the two distributions are the same. P < 0.05 was taken to indicate statistical significance.
Fig. 5.
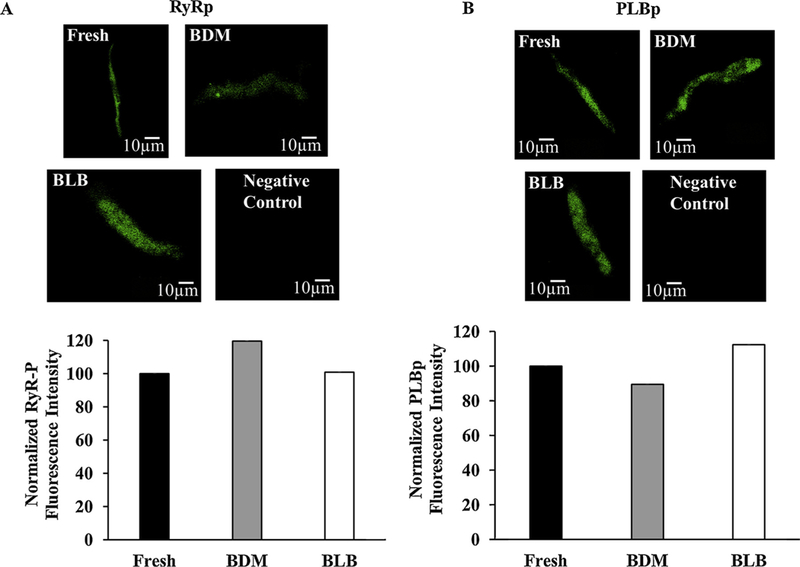
Phosphorylation status of ryanodine receptors and SERCA. (A) Representative examples of ryanodine status immunolabeling in fresh and cultured cells after 48 h. (B) Representative examples of phospholamban phosphorylation and phospholamban immunolabeling in fresh and cultured cells after 48 h. Phosphorylation signal is normalized to the fresh level. BDM (2,3-Butanedione 2-monoxime), BLB (Blebbistatin).
Fig. 4.
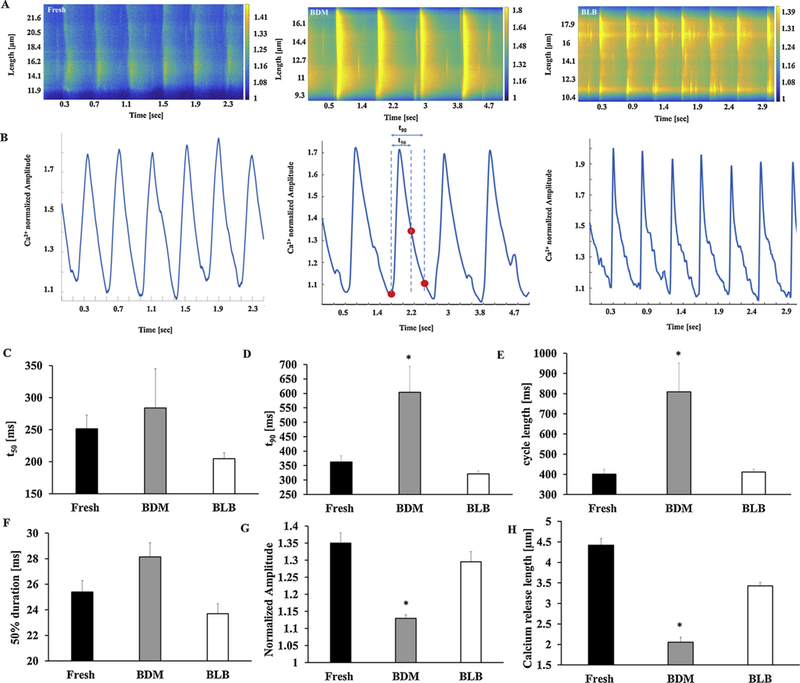
Global and local Ca2+ signaling. Representative examples of (A) Ca2+ imaging and (B) Ca2+ transient in fresh and cultured pacemaker cells. Global and local Ca2+ release characteristics. (C) 50% and (D) 90% relaxation of Ca2+ transient. (E) Cycle length (n = 12 fresh, n = 8 BDM, and n = 6 blebbistatin). Local Ca2+ release characteristics: (F) 50% duration, (G) normalized amplitude, and (H) length (n = 107 fresh, n = 47 BDM and n = 61 blebbistatin). *p < 0.05 vs. fresh cells. BDM (2,3-Butanedione 2-monoxime), BLB (Blebbistatin).
3. Results
3.1. Eliminating contraction during culture preserves cell area similarly to fresh cells
Because cell roundness is known to increase in culture, the first feature we tested across our different culture methods was whether the area of the cultured cells was preserved. The cell area and, specifically, its shape (i.e., spindle vs. spherical), determines the diffusion kinetics of chemical ions and these chemical kinetics determine pacemaker function. Fig. 1A shows a representative example of fresh and cultured pacemaker cells. We tested 4 different culture methods and compared the cell area to that of fresh cells: (i) a pure culture medium with BDM (either 5 or 10 mM), based on St. Clair et al. [13] (note that this protocol was designed for mice pacemaker cells); (ii) a mixed culture medium with salts, based on Yang et al. [9], without any chemicals to stop contraction; (iii) a mixed culture medium with salts and BDM; and (iv) a mixed culture medium with salts and blebbistatin. Note that the osmolarity was maintained for all solutions (we adjusted the Na+ concentration in the salts). On average, the cells maintained their area in the 10 mM pure culture medium with BDM (665 ± 46μm2; n = 140; cells were taken from 7 rabbits) in comparison to fresh cells (660 ± 31μm2; n = 111; cells were taken from 19 rabbits). However, in the presence of 5mM BDM (835 ± 40μm2; n = 111; cells were taken from 19 rabbits), the area significantly increased in comparison to fresh cells. Similarly, in the presence of the mixed culture medium with salts (1070 ± 73μm2; n = 164; cells were taken from 7 rabbits), the area significantly increased in comparison to fresh cells. However, when either BDM (662 ± 28μm2; n = 127; cells were taken from 3 rabbits) or blebbistatin (713 ± 29μm2; n = 66; cells were taken from 2 rabbits) was added, the cells maintained their area in comparison fresh cells. The distribution around the mean of the area (Fig. 1B) did not differ significantly when either BDM (10 mM) or blebbistatin was added to either solution but differed when no contraction eliminating substrate was added (quantified by a Kolmogorov-Smirnov two-sample test).
Fig. 1.
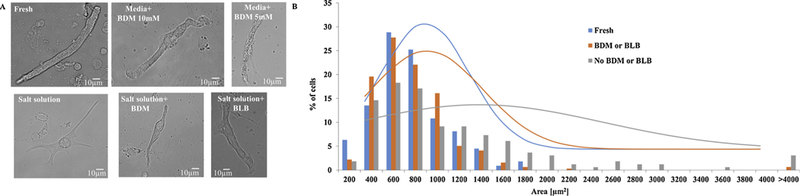
Eliminating contractions maintained cell area. (A) Representative examples of fresh and cultured pacemaker cells after 48 h. (B) Distribution of fresh cell area (n = 111; cells were taken from 19 rabbits), cultured cells with (n = 317, cells were taken from 11 rabbits) and without (n = 164; cells were taken from 7 rabbits) a contraction eliminating substance after 48h. The lines are Gaussian fitting for the different distribution graphs. BDM (2,3-Butanedione 2-monoxime), BLB (Blebbistatin). The fits are provided for illustration with no theoretical or statistical meaning.
After 48 h of culture, some cells lost their spindle shape and became spherical. In the pure culture medium with BDM, 96 ± 2% of the cells preserved their spindle shape, with 1.5 ± 0.1 projections generated on average. In the mixed culture medium with salts, 85 ± 4% of the cells preserved their spindle shape, with 2.1 ± 0.1 projections generated on average. Adding BDM to the mixed culture medium with salts increased the percentage of cells that preserved their spindle shape to 92 ± 6%, with 1.5 ± 0.1 projections generated on average. Adding blebbistatin to the mixed culture medium with salts allowed 87 ± 3% of the cells to preserve their spindle shape, with 2.1 ± 0.1 projections generated on average.
3.2. Eliminating contraction during culture using a mixed culture medium preserves the cell’s spontaneous beating rate
The second feature we tested across our different culture methods was whether the spontaneous beating rate and cell electrical activity were preserved in culture after 48 h similarly to fresh cells.
We first tested the protocol used for mice pacemaker cells where cells were maintained only in the pure culture medium (without adding salts) with BDM (either 5 or 10 mM) [13]. 8 dishes were prepared for each BDM concentration (4 and 8 rabbits were used for 5 mM and 10 mM BDM, respectively). Similar to the results reported before [17], none of the cells were found to beat spontaneously (see explanation below). When a mixed culture medium with salts was used without a contraction uncoupler, the average beating rate was 966 ± 100 msec (n = 7, 3 rabbits).
Because the cells did not beat spontaneously when using the first protocol (culture medium + BDM), and when using the second protocol (mixed culture medium with salts) the cells beat slower than their normal beating rate [28], we continued our other functional measurements using the mixed culture medium with salts with either BDM or blebbistatin and compared the results to those of fresh cells. We used di-4-Anepps to quantify the spontaneous AP cycle length. AP cycle length did not differ significantly between fresh (333 ± 23 ms) and cultured cells with blebbistatin (347 ± 49 ms) or with BDM (417 ± 78 ms), although the cycle length tended to be longer under those conditions (Fig. S2).
We attempted to measure the beating rate after 72 h in the presence of blebbistatin. Although some of the cells beat spontaneously, their beating rate was lower on average, 869 ± 40 ms (n = 9, 3 rabbits). Thus, although it is possible to use pacemaker cells after 48 h, the number of usable cells is small, and their beating rate is not physiological.
3.3. Cells cultured with a contraction uncoupler can be infected with adenovirus
To measure local signaling kinetics, exogenous proteins need to be introduced into cultured pacemaker cells. After the cells were in culture for 24 h, we transfected them for 24 h with adenovirus delivering cDNA encoding eGFP. Figs. 2A–B show a representative cell that was infected with adenovirus. We obtained high infection efficiency (close to 100%) with no toxicity or change in cell morphology. Note that when cells were cultured with BDM, the contraction pattern amplitude was small, and it was hard to quantify the beating rate (n = 20 dishes with cells from 10 rabbits). The beating pattern was clearer when the cells were cultured with blebbistatin. Note that without BDM or blebbistatin in the solution, the infection success rate was similar [9].
Fig. 2.
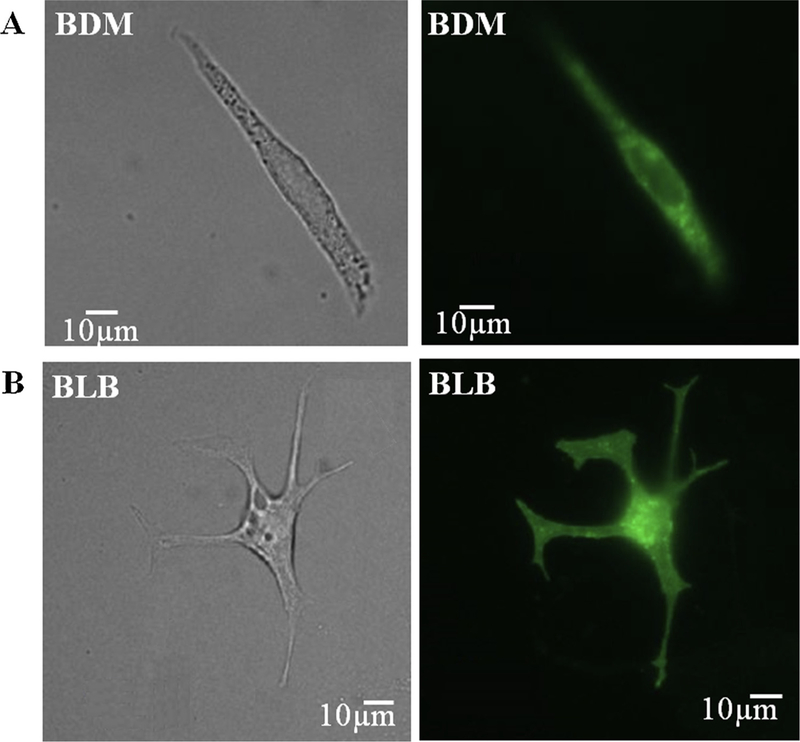
The ability of cultured cells with a contraction eliminating substance to express exogenous proteins. Bright field and fluorescence images of representative pacemaker cells 24 h after infection with adenoviruses encoding eGFP (Ad-GFP) with (A) BDM or (B) blebbistatin in mixed culture medium with salts. BDM (2,3-Butanedione 2-monoxime), BLB (Blebbistatin).
3.4. Eliminating contraction during the culture procedure preserves f-channel and mitochondria distribution
HCN4 is a subunit of the f-channel that contributes to the initiation of AP firing. To verify that the channel is still intact in cells cultured with a contraction uncoupler, we immunolabeled the cultured cells with the HCN4 antibody. Fig. S3 A shows that both fresh and cultured cells using either uncoupler express the channel. Because mitochondria are the major source of energy production in pacemaker cells [30], we quantified whether the density of mitochondria remains constant in culture. To visualize the density of mitochondria, we loaded fresh and cultured pacemaker cells with TMRM (Fig. S3B). Analysis of average intensity of TMRM per cell shows that this index does not change between fresh and cultured cells using either contraction uncoupler: 0.14 ± 0.01 intensity/μm2 (n = 17) for fresh cells vs. 0.14 ± 0.02 intensity/μm2 (n = 16) for cells cultured with BDM and 0.16 ± 0.02 intensity/μm2 (n = 28) for cells cultured with blebbistatin. Note, however, that when cells were cultured without BDM or blebbistatin, the intensity decreased to 0.1 ± 0.01 intensity/μm2 (n = 17). Thus, adding a contraction uncoupler to the media leads to preservation of mitochondrial density.
3.5. Eliminating contraction during the culture procedure preserves Ca2+ kinetics and local Ca2+ release characteristics
In pacemaker cells, spontaneous Ca2+ signaling kinetics determine the degree of coupling between the clocks and thus pacemaker function, specifically the spontaneous AP firing rate. First, we confirmed that the density of ryanodine receptors (RyRs) and phospholamban (PLB) remained similar in the cultured and fresh cells. Fig. 3A shows immunolabeling of RyR in fresh and cultured cells with BDM or blebbistatin, respectively. The three images illustrate high density with a repetitive pattern of RyR distribution in the fresh and in the two cultured cell methods. Indeed, the intensity per area for cultured cells with BDM (0.5 ± 0.2 intensity/μm2 (n = 9)) or with blebbistatin (0.5 ± 0.2 intensity/μm2 (n = 12)) was similar to the fresh cell level (0.5 ± 0.1 intensity/μm2 (n = 13)). Fig. 3B shows immunolabeling of PLB in fresh and cultured cells with BDM or blebbistatin, respectively. The three images illustrate PLB intensity distribution in the fresh and in the two cultured cell methods. Indeed, the intensity per area for cultured cells with BDM (0.1 ± 0.01 intensity/μm2 (n = 9)) or with blebbistatin (0.12 ± 0.01 intensity/μm2 (n = 12)) was similar to the fresh cell level (0.1 ± 0.01 intensity/μm2 (n = 13)). Using a mixed culture medium without BDM or blebbistatin does not significantly change PLB or RyR protein intensity compared to fresh cells (re-analyzed data from [9]).
Fig. 3.
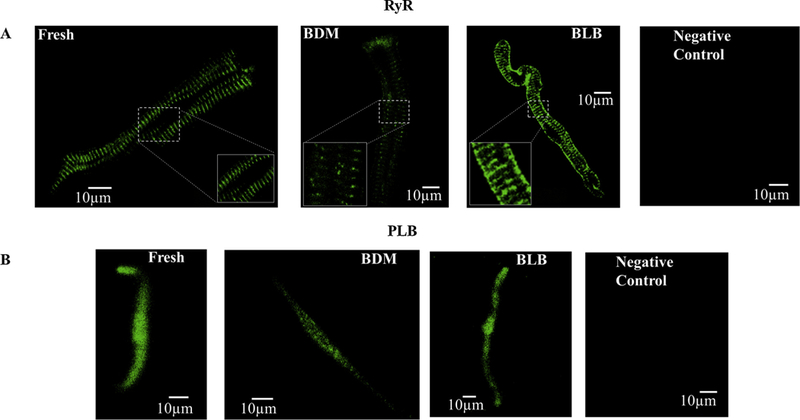
Ryanodine receptor and PLB labeling. Representative examples of (A) ryanodine receptor (RyR) (B) PLB immunolabeling in fresh and cultured cells (BDM or blebbistatin) after 48h. BDM (2,3-Butanedione 2-monoxime), BLB (Blebbistatin). No first antibody was added to the negative control.
Next, we quantified spontaneous Ca2+ kinetics and local Ca2+ release characteristics in cultured cells and compared them to fresh cells. Fig. 4A shows representative examples of Ca2+ imaging. Fig. 4B shows representative examples of Ca2+ transients in fresh and cultured cells when contraction is eliminated during culture. Fig. 4C–D show that mean time to peak (tp) and 50% relaxation time (t50) do not differ significantly between fresh and cultured cells when contraction is eliminated during culture. However, mean time to 90% relaxation time (t90) is significantly longer in cells cultured with BDM compared to fresh. Similarly, cycle length (Fig. 4E) is significantly longer in cells cultured with BDM compared to fresh. The comparison of local Ca2+ release characteristics in Fig. 4F–H shows no significant difference between fresh and cultured cells with regard to 50% duration when contraction is eliminated during culture. However, spontaneous Ca2+ release length and amplitude are significantly shorter and lower, respectively, and time between peak Ca2+ and LCR appearance is significantly longer in cells cultured with BDM compared to fresh (Table 2).
Table 2.
Global and local Ca2+ characteristics.
| Parameter | Fresh | BDM | Blebbistatin | Mixed culture medium with salts |
|---|---|---|---|---|
| Time to peak [ms] | 138 ± 15 | 138 ± 35 | 122 ± 12 | 122 ± 13 |
| t50 [ms] | 251 ± 22 | 284 ± 61 | 205 ± 9 | 291 ± 43 |
| t90 [ms] | 362 ± 22 | 603 ± 89* | 321 ± 10 | 721 ± 26* |
| Cycle length [ms] | 402 ± 24 | 809 ± 142* | 411 ± 14 | 1542 ± 43* |
| 50% duration [ms] | 25 ± 0.89 | 28 ± 1 | 24 ± 0.8 | 28 ± 1 |
| Amplitude difference | 1.3 ± 0.03 | 1.295 ± 0.03 | 1.13 ± 0.01 | 1.2 ± 0.01 |
| Calcium release length [μm] | 4.42 ± 0.16 | 2 ± 0.12* | 3.4 ± 0.08 | 1.84 ± 0.1* |
| Time in [ms] between calcium release and former peak | 280 ± 9 | 362 ± 43* | 269 ± 14 | 938 ± 32* |
| Ca2+ releases/μm/sec | 0.14 ± 0.02 | 0.123 ± 0.03 | 0.17 ± 0.04 | 0.1 ± 0.03 |
| Number of cells | 12 | 6 | 8 | 12 |
| Number of calcium releases | 107 | 47 | 61 | 60 |
| Number of rabbits | 6 | 3 | 3 | 3 |
p < 0.05 vs. fresh cells.
Using a mixed culture medium with salts without BDM or blebbistatin significantly prolonged t90 and cycle length and increased the LCR period. Moreover, spontaneous Ca2+ release length and amplitude are significantly shorter and lower, respectively (Table 2).
We next explored why eliminating contractions maintains global and local Ca2+ dynamics in cultured rabbit pacemaker cells. We tested 4 different mechanisms (Fig. S4): culture dish coating, maintaining of pH, maintaining of phosphorylation activity, and energy preservation.
3.6. The effect of the substance used to coat culture plates on cultured pacemaker cell function
In parallel to adding a contraction uncoupler to the medium during culture, we used collagen instead of laminin [9] to coat the culture plates. We wished to investigate whether using collagen vs. laminin improves the culture procedure, regardless of whether or not contraction is eliminated.
We tested three different coatings for the culture plates (see Materials and Methods). When laminin was used as a coating substrate, a small number of cells were attached to dish and beat spontaneously (regardless of whether or not an uncoupler was used). When PEI was used as a coating material, cells were attached to the dish and beat spontaneously. However, we found collagen to be superior to PEI, as it resulted in a greater number of spontaneously beating cells. Thus we used collagen as a coating material throughout our study.
To prove that laminin by itself did not improve the cultured cell properties, we compared the beating rate of pacemaker cells in a mixed culture medium with salts when either laminin or collagen was used for coating. The beating rate when using a mixed culture medium with salts did not differ significantly when the coating was collagen (966 ± 100 msec (n = 7, 3 rabbits) as compared to laminin (883 ± 100 ms n = 30, 8 rabbits), as reported before [9]. Thus, the substance used to coat culture plates does not directly affect pacemaker function but does affect the probability to obtain beating cells and the number of beating cells per dish.
3.7. A mixed culture medium with salts maintains a better physiological pH than a pure culture medium
We next explored the effect of pH on pacemaker cell properties in culture. We hypothesized that after 48 h the extracellular pH of a pure culture medium would be lower than that of a mixed culture medium with salts. Indeed, after 48 h the extracellular pH measured in the pure culture medium was 7.0 as opposed to 7.4 in the mixed culture medium with salts. Note that the presence of a contraction uncoupler had no effect on the pH.
We used our computational model [20] to gain insight into the experimental results and to predict Ca2+ kinetics in the cytosol and the SR, the latter of which is challenging to measure. Fig. S5 shows the model simulations in response to a decrease in pH. Decrease in extra-cellular pH from 7.4 to 7 leads to a reduction in the spontaneous AP firing rate, Ca2+ releases, SR load and INCX. Although lower pH is favorable for atrial and ventricular cells [17], the rabbit pacemaker cells slow their beating rate under these conditions. To validate our computational prediction, we quantified spontaneous Ca2+ kinetics and local Ca2+ release characteristics in fresh cells with regular pH (7.4) and lower pH (7). Mean tp (153 ± 12 (n = 10) vs. 143 ± 12 ms (n = 7) for pH 7.4 and 7, respectively) and t50 (279 ± 11 (n = 10) vs. 263 ± 6 ms (n = 7)) do not differ significantly for different pH levels. However, mean t90 (384 ± 17 (n = 10) vs. 436 ± 32 ms (n = 7) for pH 7.4 and 7, respectively) is significantly longer in cells at pH 7 as compared to pH 7.4. Similarly, cycle length (429 ± 12 (n = 10) vs. 701 ± 41 ms (n = 7) for pH 7.4 and 7, respectively) is significantly longer in cells at pH 7 compared to pH 7.4. The cycle length results are similar to [22].
The comparison of local Ca2+ release characteristics shows significant difference between pH 7.4 and pH 7 with regard to 50% duration (25 ± 1 (n = 107) vs. 14 ± 1 ms (n = 54) for pH 7.4 and 7, respectively), spontaneous Ca2+ release length (4.4 ± 0.1 (n = 107) vs. 1.2 ± 0.1 μm (n = 54) for pH 7.4 and pH 7, respectively) and amplitude (1.35 ± 0.03 (n = 107) vs. 1.1 ± 0.01 F/F0 (n = 54) for pH 7.4 and 7, respectively). Moreover, time between peak Ca2+ and LCR appearance is significantly longer for pH 7 as compared to pH 7.4 (280 ± 8 vs. 474 ± 52 ms (n = 54)).
3.8. Eliminating contraction during the culture procedure by either BDM or blebbistatin preserves phosphorylation status
In parallel to the Ca2+, the phosphorylation status determines the degree of clock coupling and thus the spontaneous AP firing rate [8,20]. Thus, we checked whether eliminating contraction also maintains phosphorylation status. Because the number of cells in culture is small and not all of them beat spontaneously, a western blot approach is not possible. Thus, we performed immunostaining for phosphorylated RyR (CaMKII target, p-RyR2 (2809 site)) and phospholamban (PKA target, p-PLB PS-16). Using a mixed culture medium without BDM or blebbistatin reduced the p-RyR2 value (2809 site) significantly by 19% compared to fresh cells (reanalyzed data from [9]). Fig. 5A shows immunolabeling of RyR phosphorylation (CaMKII target, p-RyR2 (2809 site)) in fresh and cultured cells with BDM or blebbistatin, respectively. Analysis of average intensity of fluorescence per cell shows that this index does not change between fresh and cultured cells with BDM or blebbistatin: 0.18 ± 0.03 (n = 30) for fresh cells vs. 0.21 ± 0.07 (n = 15) for cells cultured with BDM and 0.18 ± 0.03 (n = 25) for cells cultured with blebbistatin. Fig. 5A shows the normalized value (fresh level) of p-RyR2 (2809 site). There was no difference in the results for fresh vs. cultured cells with BDM or blebbistatin after normalization. Thus, in that regard, either BDM or blebbistatin maintain p-RyR2 value in culture. Note that because in smaller and medium pacemaker cells, RyR is mainly detected in the junctional SR while in large cells it is also present in the cytosol, we: (i) verified that the area of each group is the same (i.e., the same number of small/medium size vs. large cells); (ii) analyzed the average intensity of fluorescence per cell close to the junctional SR area.
Using a mixed culture medium without BDM or blebbistatin significantly reduced the p-PLB (2809 site) value by 52% compared to fresh cells (reanalyzed data from [9]). Fig. 5B shows immunolabeling of PLB (PKA target, p-PLB PS-16) phosphorylation in fresh and cultured cells with BDM or blebbistatin, respectively. Analysis of average intensity of fluorescence per cell shows that this index does not change between fresh and cultured cells with BDM or blebbistatin: 0.19 ± 0.05 (n = 14) for fresh cells vs. 0.17 ± 0.03 (n = 9) for cells cultured with BDM and 0.21 ± 0.04 (n = 27) for cells cultured with blebbistatin. Fig. 5B shows the normalized value (fresh level) of p-PLB. There was no difference in the results for fresh vs. cultured cells with BDM or blebbistatin after normalization. Thus, in that regard, either BDM or blebbistatin maintain p-PLB value in culture.
To show the importance of phosphorylation status in maintaining pacemaker function, we used our computational model to examine the effect of reduced phosphorylation on SERCA activity, on pacemaker beating rate, and on Ca2+ kinetics in the cytosol and SR. (The effect of reduced phosphorylation on SERCA activity was simulated by reducing KPLBp by 10%, 20%, 30% and 40%; see [20] for parameter values and equations.) Fig. 6 shows that graded reduction in p-PLB reduced the spontaneous beating rate, SR load, Ca2+ releases and the current through NCX. Similar tradeoffs were obtained when phosphorylation activity of RyR was reduced (Fig. S6).
Fig. 6.
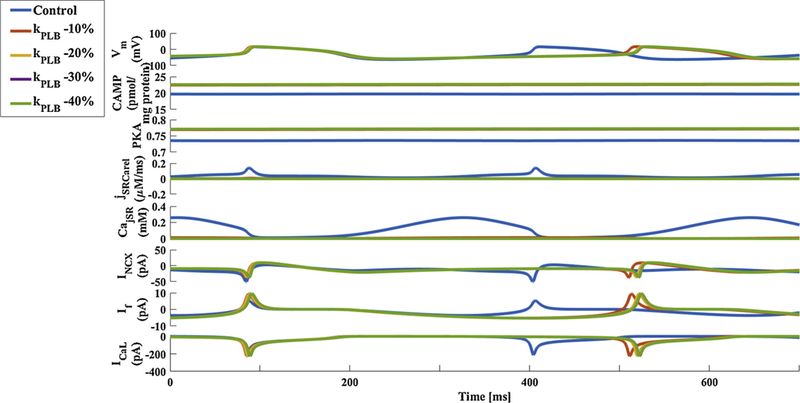
Model simulations of SERCA phosphorylation activity effect. Membrane voltage, main membrane currents, AC-cAMP-PKA signaling, and Ca2+ cycling (flux and concentration) in the sarcoplasmic reticulum (SR) in response to different degree of phosphorylation activity. The panels show: (A) the membrane voltage (Vm); (B) cAMP level; (C) PKA activity level; (D) the flux of Ca2+ exiting the sarcoplasmic reticulum (jSRCarel); (E) Ca2+ concentration in the junctional sarcoplasmic reticulum compartment (CajSR); (F) the Na+-Ca2+ exchanger current (INCX); G. funny-current (If); and H. L-type current (ICEL). BDM = 2,3-Butanedione 2-monoxime, BLB = Blebbistatin.
3.9. Eliminating contraction reduces energy consumption
Eliminating contraction can prompt reduction in ATP utilization. We wished to understand whether eliminating contraction plays a dominant role in this reduction. Fig. S5 shows that in response to elimination of contraction, there is a reduction in the spontaneous release of Ca2+ and INCX, which leads to a reduction in the spontaneous beating rate. In pacemaker cells, ATP is utilized in SERCA activity, in cAMP production, and by force production by the contractile elements. The computational model shows that eliminating contraction reduced ATP utilization by the SERCA pump by 5% and its utilization in cAMP production by 15%. Because the mitochondria are the main source of energy in pacemaker cells (and other heart tissues [32]), we measured oxygen consumption with and without blebbistatin. Blebbistatin reduced oxygen consumption by 10 ± 4% compared to the control conditions (n = 4). In compression, PKA inhibition by PKI reduced oxygen consumption by 23 ± 4% compared to the control conditions (n = 4). Thus, eliminating contraction to reduce energy consumption contributes to the preservation of cell properties in culture.
3.10. Blebbistatin is superior to BDM due to BDM side effects
Finally, we wished to understand why blebbistatin is superior to BDM in preserving pacemaker function. It was shown that BDM has nonspecific effects on cell function. Thus, we hypothesized that non-specific BDM effects make it less effective as a contraction eliminator than blebbistatin. We used our computational model to simulate the nonspecific BDM effects: i) we reduced the maximal conductance of the L-type current by 6%, as suggested in [25]; ii) we reduced the maximal conductance of IKs by 10%, as suggested in [26]; iii) we increased phosphatase activity (Kpp1) by 6%, as suggested in [27]; and iv) we reduced Ca2+ releases (Ks) by 6%, as suggested in [25]. See [20] for parameter values and equations. Fig. 7 shows that in response to non-specific BDM effects, the spontaneous AP firing rate decreases, including indirect reduction in INCX and ICa,L. Thus, the addition of a contraction uncoupler helps preserve pacemaker function, but non-specific BDM effects make it less effective as a contraction eliminator than blebbistatin.
Fig. 7.
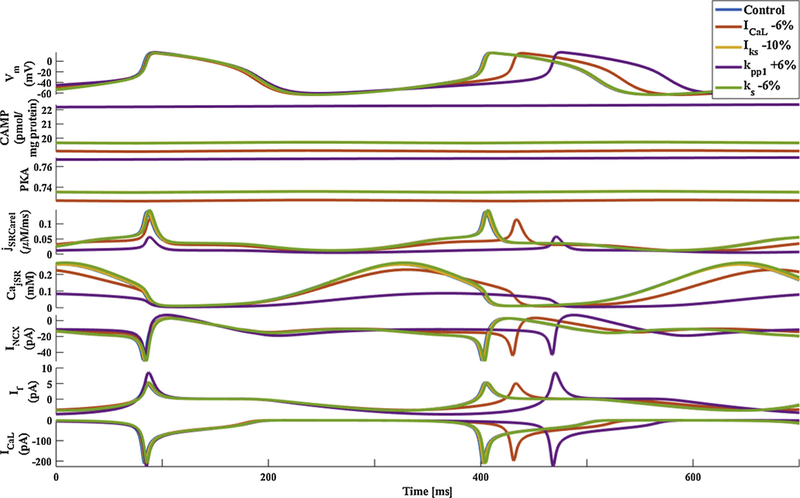
Model simulations of BDM side effects. Membrane voltage, main membrane currents, AC-cAMP-PKA signaling, and Ca2+ cycling (flux and concentration) in the sarcoplasmic reticulum (SR) in control and in response to BDM side effects. The panels show: (A) the membrane voltage (Vm); (B) cAMP level; (C) PKA activity level; (D) the flux of Ca2+ exiting the sarcoplasmic reticulum (jSRCarel); (E) Ca2+ concentration in the junctional sarcoplasmic reticulum compartment (CajSR); (F) the Na+-Ca2+ exchanger current (INCX); G. funny-current (If); and H. L-type current (ICaL). BDM = 2,3-Butanedione 2-monoxime, BLB = Blebbistatin.
4. Discussion
Cultured rabbit pacemaker cells are a necessary model to measure internal signaling dynamics and a convenient model to express genetically manipulated genes associated with different cardiac diseases. However, the existing methods for culturing rabbit cells lead to deterioration of internal pacemaker signaling and the Ca2+ dynamics of cultured cells were not known. Our first major finding is that eliminating contraction during the culture period preserved global and local Ca2+ dynamics. Note that all these experiments were conducted when BDM or blebbistatin was washed out from the medium. Eliminating contraction during the culture period maintained spontaneous global and local Ca2+ characteristics (Fig. 4), the pacemaker cell area (the cells in culture form projections and thus the shape changes) (Fig. 1), spontaneous beating rate, phosphorylation status (Fig. 5), and the integrity of important structures involved in excitation contraction coupling (Fig. S3). Importantly, we showed that exogenous proteins can be expressed in these cells at high levels within 48 h via adenoviral gene transfer, with no significant changes in pacemaker cell function. Moreover, when using high resolution electron microscopy pictures (Fig. S7), we demonstrated a rich cytoskeleton in cells cultured with blebbistatin, including mitochondria, SR, and myofilaments. We also identified the caveolae in these cells. Note that this lipid raft tends to disappear when other culture techniques are used [33]. Interestingly, the electron microscopy pictures show a rich environment of SR and mitochondria similar to that of ventricular cells [33]. Using the same protocol as [9], we found that when a contraction eliminating substance was not added to the medium, the spontaneous beating rate and Ca2+ dynamics of pacemaker cells were far from the physiological range and PKA activity was documented to be lower. Our computational model indicates that the reduction in Ca2+ release and PKA activity is indeed the mechanism that initiates the decrease in the spontaneous AP firing rate.
We used experimental and computational tools to understand why eliminating contraction during the culture period preserves pacemaker function. We tested 4 different mechanisms (Fig. S4): (i) culture dish coating, (ii) maintaining of pH, (iii) maintaining of phosphorylation activity, and (iv) energy preservation.
Our second main conclusion was that, under our experimental conditions, the culture dish coating did not affect pacemaker cell function but only the number of cells that attached to dish and the probability to obtain spontaneously beating cells. This effect was regardless of whether a contraction uncoupler was added to the medium. Note, however, that during culture, pacemaker cells are not free to contract in 3D, but attach to a glass, thus contracting in 2D. The uncouplers may preserve cell properties by eliminating contraction and respective artificial mechanical disturbances. Because the number of pacemaker cells is small, they cannot be cultured on a 3D matrix. Future experiments with a mixed population of pacemaker and other cell types (neurons or ventricular myocytes) may help to understand whether a 3D matrix is superior to 2D.
Our third major conclusion was that external pH affects pacemaker function. Fig. S5 shows that for pacemaker cells, lower pH led to a reduction in the spontaneous AP firing rate due to direct reduction of L-type and K+ currents [22], reduction in Na+-K+ pump current [23], and reduction in PKA activity [40]. Thus, increasing the pH by adding salts to the culture medium maintains a better environment for rabbit pacemaker cells. Moreover, the mixed culture medium with salts has a lower Na+ level than the pure medium itself. An increase in Na+ was associated with an increase in spontaneous beating rate [41], and thus may lead to waste of energy during the culture period (see below).
Our fourth major conclusion was that preserving phosphorylation activity plays an important role in maintaining cultured cell pacemaker activity. PKA [8,36] and CaMKII [37] activities are correlated with AP firing rate. This signaling cascade controls PLB and RyR activities (its kinetics are expressed by local Ca2+ release kinetics) and L-type and K currents. Parameter sensitivity analysis of this model showed that the dominant mechanism in maintaining the rabbit pacemaker cell beating rate is the PLB phosphorylation status [20]. Indeed, our results (Fig. 5) showed that the PLB phosphorylation index does not change when using a contraction uncoupler during the culture procedure. Thus, maintaining these phosphorylation activity makes it possible to maintain the AP firing rate in culture. This conclusion explains why adding a contraction uncoupler is superior to using a mixed culture medium with salts. Although the average steady state PLB phosphorylation level does not differ statistically for BDM and blebbistatin, it possible that PKA dynamics are different when using either contraction uncoupler. Future experiments are needed to prove this hypothesis.
Our fifth major finding is that mechanical uncoupling during culture reduces the energy needed for contraction, for cAMP production, and for the function of the Na+-K+ pump (reduction in the current integral). Thus, conserving energy partially contributes to maintaining pacemaker cell mechanisms and thus pacemaker function. In previous work we showed that Ca2+ and posttranslational modification signaling are the major mechanisms that maintain ATP supply to demand in pacemaker cells [4,30,37]. Thus, it is possible that a reduction in these signaling cascade values during culture limits the ATP supply and thus the spontaneous beating rate. That the number of mitochondria decreases in culture without a contraction uncoupler strengthens this hypothesis (Fig. S3).
Another enigma we had to solve is why blebbistatin was superior to BDM in maintaining the spontaneous AP firing rate and Ca2+ transient characteristics. BDM did not change the phosphorylation status of either PLB (PKA target, p-PLB PS-16) or RyR (CaMKII target, p-RyR2 (2809 site)) and did not alter the structure of RyR or the mitochondria. Because both BDM and blebbistatin stop pacemaker cell contraction at the same magnitude [30], our sixth major conclusion is that the difference in the cells’ response is mainly due to the BDM side effects: (i) reducing L-type Ca2+ [25] and potassium currents [26]; (ii) reducing Ca2+ releases [25]; (iii) increasing phosphatase inhibitor activity [27]; (iv) reducing spontaneous contractions per min [38]. Our computational model predicts that the reduction in spontaneous AP firing rate and Ca2+ release characteristics can be explained (Fig. 7) by taking these side effects into account. Note that the model predicts indirect reduction in INCX by BDM; such a trend was found experimentally in the past [39]. Similarly, in ventricular cells after 24 h of culture, blebbistatin was found to be superior to BDM [15]. Thus, although BDM is superior to not using a substrate that eliminates contraction, it is possible to avoid the nonspecific effects of BDM if blebbistatin is used instead.
Our results indicate that the ideal culture medium for rabbit pacemaker cells is different than the one obtained for mice pacemaker cells by the authors of [13], who found that mouse cell survival and morphology were dramatically compromised in cultures prepared with blebbistatin compared to BDM. In contrast, we found here that for rabbit pacemaker cells, BDM and blebbistatin have the same effect on cultured cell survival and morphology. It is possible that mouse pacemaker cells are less sensitive to pH and have a higher level of Na+ (see above); thus, the pure culture medium might indeed be better suited to mouse pacemaker cells than a mixed culture medium with salts. Under these conditions it is possible that BDM is indeed superior to blebbistatin. It is also possible that mouse cells are less sensitive to BDM side effects. It should be noted that the authors did not measure Ca2+ dynamics in cultured mouse pacemaker cells. Nor did the authors measure the effect of blebbistatin on the spontaneous beating rate. Another interesting point is that, although pacemaker cells are close in proximity to atrial cells, we found that different culture media are suitable for each cell type. We tested here a culture medium (pure culture media with BDM) suitable for atrial cells, and although the cells maintained their area, they did not beat spontaneously.
4.1. Limitation
After isolation it is not possible to distinguish between primary and follower cells [28], and thus we cannot distinguish whether one of these types is better suited to culture, and if so, which one. Note, however, that because after isolation, primary and follower cells have similar spontaneous beating rates, LCR characteristics, and RyR distribution in the junctional SR [28], one can speculate that the culture method is universal.
Blebbistatin can be cytotoxic upon exposure to light and lead to the formation of reactive oxygen species. In this regard it should be inferior to BDM. However, our results show that blebbistatin is superior to BDM with regard to maintaining of pacemaker function. Also note that we ran our biochemical and biophysical experiments after BDM or blebbistatin was washed from the system.
We confirm here that the biochemical and biophysical properties of pacemaker cells cultured with a contraction uncoupler are maintained. However, although we showed that the density of mitochondria (Fig. S3) remains the same in these cells as compared to fresh cells, we did not perform functional bioenergetics measurements on the cultured cells (the mitochondrial potential is not a quantitative method). That pacemaker cells cultured with a contraction uncoupler maintain the basal beating rate implies that ATP supply, presumably, matches the demand.
Cytochalasin D is another mechanical uncoupler, but it must be more fully understood before it can be tested on cultured pacemaker cells. First, although cytochalasin D has been shown to improve culture of ventricular cells [42], the concentration required to stop pacemaker cell contraction is not known. Second, although Cytochalasin D is superior to BDM, it still has negative effects on the Ca2+ transient [43], on action potential duration restitution, and on APD90 [44,45]. Thus, its effects on pacemaker cell function must be explored. Last but not least, we have shown that adding blebbistatin alone to the culture medium maintains pacemaker activity in culture.
5. Summary
The experimental results show that eliminating contraction during the culture period maintained global and local Ca2+ characteristics, the pacemaker cell area, spontaneous beating rate, phosphorylation status, and the integrity of important structures involved in excitation contraction coupling. Eliminating contraction with blebbistatin maintains pacemaker activity in culture by maintaining phosphorylation activity and helping to reduce energy consumption.
Supplementary Material
Acknowledgments
Funding statement
The work was supported jointly by Israel Science Foundation No. 398/14 (YY) and National Science Foundation of China No. 81461148026/31630035 (SW). This research was supported in part by the Intramural research Program of the NIH, National Institute on Aging. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations:
- AP
action potential
- BDM
2,3-butanedione 2-monoxime
- PLB
phospholamban
- tp
time to peak
- t50
50% relaxation time
- t90
90% relaxation time
Footnotes
Competing interests
The authors have declared that no competing interests exist.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ceca.2018.12.008.
References
- [1].Yaniv Y, Lakatta EG, Maltsev VA, From two competing oscillators to one coupled-clock pacemaker cell system, Front. Physiol. 6 (2015) 28, 10.3389/fphys.2015.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lyashkov AE, Vinogradova TM, Zahanich I, Li Y, Younes A, Nuss HB, Spurgeon HA, Maltsev VA, Lakatta EG, Cholinergic receptor signaling modulates spontaneous firing of sinoatrial nodal cells via integrated effects on PKA-dependent Ca(2+) cycling and I(KACh), Am. J. Physiol. Hear. Circ. Physiol. 297 (2009) H949–59 doi:01340.2008 [pii] 10.1152/ajpheart.01340.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yaniv Y, Lyashkov AE, Sirenko S, Okamoto Y, Guiriba TR, Ziman BD, Morrell CH, Lakatta EG, Stochasticity intrinsic to coupled-clock mechanisms underlies beat-to -beat variability of spontaneous action potential firing in sinoatrial node pacemaker cells, J Mol. Cell. Cardiol. 77 (2014) 1–10, 10.1016/j.yjmcc.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yaniv Y, Spurgeon HA, Ziman BD, Lyashkov AE, Lakatta EG, Mechanisms that match ATP supply to demand in cardiac pacemaker cells during high ATP demand, Am. J. Physiol. Hear. Circ. Physiol. 304 (2013) H1428–38 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=23604710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Verkerk AO, Wilders R, Coronel R, Ravesloot JH, Verheijck EE, Ionic remodeling of sinoatrial node cells by heart failure, Circulation 108 (2003) 760–766 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12885752. [DOI] [PubMed] [Google Scholar]
- [6].Neco P, Torrente AG, Mesirca P, Zorio E, Liu N, Priori SG, Napolitano C, Richard S, Benitah JP, Mangoni ME, Gomez AM, Paradoxical effect of increased diastolic Ca(2+) release and decreased sinoatrial node activity in a mouse model of catecholaminergic polymorphic ventricular tachycardia, Circulation 126 (2012) 392–401 doi:CIRCULATI0NAHA.111.075382 [pii] 10.1161/CIRCULATI0NAHA.111.075382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mesirca P, Alig J, Torrente AG, M?ller JC, Marger L, Rollin A, Marquilly C, Vincent A, Dubel S, Bidaud I, Fernandez A, Seniuk A, Engeland B, Singh J, Miquerol L, Ehmke H, Eschenhagen T, Nargeot J, Wickman K, Isbrandt D, Mangoni ME, Cardiac arrhythmia induced by genetic silencing of? Funny? (f) channels is rescued by GIRK4 inactivation, Nat. Commun. 5 (2014) 4664, 10.1038/ncomms5664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yaniv Y, Ganesan A, Yang D, Ziman BD, Lyashkov AE, Levchenko A, Zhang J, Lakatta EG, Real-time relationship between PKA biochemical signal network dynamics and increased action potential firing rate in heart pacemaker cells: kinetics of PKA activation in heart pacemaker cells, J. Mol. Cell. Cardiol. 86 (2015) 168–178, 10.1016/j.yjmcc.2015.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yang D, Lyashkov AE, Li Y, Ziman BD, Lakatta EG, RGS2 overexpression or G(i) inhibition rescues the impaired PKA signaling and slow AP firing of cultured adult rabbit pacemaker cells, J. Mol. Cell. Cardiol. 53 (2012) 687–694, 10.1016/j.yjmcc.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Liu Z, Zou AR, Demir SS, Clark JW, Nathan RD, Characterization of a hyperpolarization-activated inward current in cultured pacemaker cells from the sinoatrial node, J. Mol. Cell. Cardiol. 28 (1996) 2523–2535, 10.1006/jmcc.1996.0244. [DOI] [PubMed] [Google Scholar]
- [11].Muramatsu H, Zou AR, Berkowitz GA, Nathan RD, Characterization of a TTX-sensitive Na+ current in pacemaker cells isolated from rabbit sinoatrial node, Am. J. Physiol. 270 (1996) H2108–19 (accessed June 15, 2017), http://www.ncbi.nlm.nih.gov/pubmed/8764263. [DOI] [PubMed] [Google Scholar]
- [12].Kivistö T, Mäkiranta M, Oikarinen EL, Karhu S, Weckström M, Sellin LC, 2,3-butanedione monoxime (BDM) increases initial yields and improves long-term survival of isolated cardiac myocytes, Jpn. J. Physiol. 45 (1995) 203–210 (accessed March 26, 2017), http://www.ncbi.nlm.nih.gov/pubmed/7650854. [DOI] [PubMed] [Google Scholar]
- [13].J.R.St. Clair EJ Proenza Sharpe, C., Culture and adenoviral infection of sinoatrial node myocytes from adult mice, Am. J. Physiol. - Hear. Circ. Physiol. 309 (2015) H490–H498, 10.1152/ajpheart.00068.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bers DM, Cardiac excitation-contraction coupling, Nature 415 (2002) 198–205, 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- [15].Kabaeva Z, Zhao M, Michele DE, Blebbistatin extends culture life of adult mouse cardiac myocytes and allows efficient and stable transgene expression, AJP Hear. Circ. Physiol. 294 (2008) H1667–H1674, 10.1152/ajpheart.01144.2007. [DOI] [PubMed] [Google Scholar]
- [16].Davoodi M, Segal S, Kirschner Peretz N, Kamoun D, Yaniv Y, Semi-automated program for analysis of local Ca2+ spark release with application for classification of heart cell type, Cell. Calcium 64 (2017) 83–90, 10.1016/j.ceca.2017.02.003. [DOI] [PubMed] [Google Scholar]
- [17].Kirschner Peretz N, Segal S, Arbel-Ganon L, Ben Jehuda R, Shemer Y, Eisen B, Davoodi M, Binah O, Yaniv Y, A method sustaining the bioelectric, biophysical, and bioenergetic function of cultured rabbit atrial cells, Front. Physiol. 8 (2017) 584, 10.3389/fphys.2017.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Davoodi M, Segal S, Kirschner Peretz N, Kamoun D, Yaniv Y, Semi-automated program for analysis of local Ca2+spark release with application for classification of heart cell type, Cell Calcium 64 (2017), 10.1016/j.ceca.2017.02.003. [DOI] [PubMed] [Google Scholar]
- [19].Sirenko S, Yang D, Li Y, Lyashkov AE, Lukyanenko YO, Lakatta EG, Vinogradova TM, Ca 2+ -dependent phosphorylation of Ca 2+ cycling proteins generates robust rhythmic local Ca 2+ releases in cardiac pacemaker cells, Sci. Signal. 6 (2013) ra6(accessed June 18, 2018), http://stke.sciencemag.org/content/sigtrans/6/260/ra6.full.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Behar J, Ganesan A, Zhang J, Yaniv Y, The autonomic nervous system regulates the heart rate through cAMP-PKA dependent and independent coupled-clock pacemaker cell mechanisms, Front. Physiol. 7 (2016) 419, 10.3389/FPHYS.2016.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yaniv Y, Sivan R, Landesberg A, Analysis of hystereses in force length and force calcium relations, Am. J. Physiol. Hear. Circ. Physiol. 288 (2005) H389–99 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15598871. [DOI] [PubMed] [Google Scholar]
- [22].Satoh H, Seyama I, On the mechanism by which changes in extracellular pH affect the electrical activity of the rabbit sino-atrial node, J. Physiol. 381 (1986) 181–191 (accessed September 12, 2017), http://www.ncbi.nlm.nih.gov/pubmed/2442350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Severi S, Cavalcanti S, Electrolyte and pH dependence of heart rate during hemodialysis: a computer model analysis, Artif. Organs 24 (2000) 245–260 (accessed September 12, 2017), http://www.ncbi.nlm.nih.gov/pubmed/10816197. [DOI] [PubMed] [Google Scholar]
- [24].Choi H-J, Jung HJ, Kwon T-H, Extracellular pH affects phosphorylation and intracellular trafficking of AQP2 in inner medullary collecting duct cells, Am. J. Physiol. Physiol. 308 (2015) F737–F748, 10.1152/ajprenal.00376.2014. [DOI] [PubMed] [Google Scholar]
- [25].Gwathmey JK, Hajjar RJ, Solaro RJ, Contractile deactivation and uncoupling of crossbridges. Effects of 2,3-butanedione monoxime on mammalian myocardium, Circ. Res. 69 (1991) 1280–1292, 10.1161/01.RES.69.5.1280. [DOI] [PubMed] [Google Scholar]
- [26].Coulombe A, Lefevre IA, Deroubaix E, Thuringer D, Coraboeuf E, Effect of 2,3-butanedione 2-monoxime on slow inward and transient outward currents in rat ventricular myocytes, J. Mol. Cell. Cardiol. 22 (1990) 921–932 (accessed March 26, 2017), http://www.ncbi.nlm.nih.gov/pubmed/2231749. [DOI] [PubMed] [Google Scholar]
- [27].Stapleton MT, Fuchsbauer CM, Allshire AP, BDM drives protein dephosphorylation and inhibits adenine nucleotide exchange in cardiomyocytes, Am. J. Physiol. 275 (1998) H1260–6 (accessed September 6, 2017), http://www.ncbi.nlm.nih.gov/pubmed/9746474. [DOI] [PubMed] [Google Scholar]
- [28].Lyashkov AE, Juhaszova M, Dobrzynski H, Vinogradova TM, Maltsev VA, Juhasz O, Spurgeon HA, Sollott SJ, Lakatta EG, Calcium cycling protein density and functional importance to automaticity of isolated sinoatrial nodal cells are independent of cell size, Circ. Res. 100 (2007) 1723–1731 doi:CIRCRESAHA.107.153676 [pii] 10.1161/CIRCRESAHA.107.153676. [DOI] [PubMed] [Google Scholar]
- [30].Yaniv Y, Juhaszova M, Lyashkov AE, Spurgeon H, Sollott SJ, Lakatta EG, Ca2+-regulated-cAMP/PKA signaling in cardiac pacemaker cells links ATP supply to demand, J Mol. Cell. Cardiol. 51 (2011) 740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yaniv Y, Juhaszova M, Lyashkov AE, Spurgeon HA, Sollott SJ, Lakatta EG, Ca2+-regulated-cAMP/PKA signaling in cardiac pacemaker cells links ATP supply to demand, J. Mol. Cell. Cardiol. 51 (2011), 10.1016/j.yjmcc.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Burton RAB, Rog-Zielinska EA, Corbett AD, Peyronnet R, Bodi I, Fink M, Sheldon J, Hoenger A, Calaghan SC, Bub G, Kohl P, Caveolae in rabbit ventricular myocytes: distribution and dynamic diminution after cell isolation, Biophys. J. 113 (2017) 1047–1059, https://doi.org/10.1016ZJ.BPJ.2017.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yaniv Y, Maltsev VA, Escobar AL, Spurgeon HA, Ziman BD, Stern MD, Lakatta EG, Beat-to-beat Ca(2+)-dependent regulation of sinoatrial nodal pacemaker cell rate and rhythm, J Mol. Cell. Cardiol. 51 (2011) 902–905 doi:S0022–2828(11)00380–4 [pii] 10.1016/j.yjmcc.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yaniv Y, Stern MD, Lakatta EG, Maltsev VA, Mechanisms of beat-to-beat regulation of cardiac pacemaker cell function by Ca(2)(+) cycling dynamics, Biophys. J. 105 (2013) 1551–1561, 10.1016/j.bpj.2013.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Behar J, Ganesan A, Zhang J, Yaniv Y, The autonomic nervous system regulates the heart rate through cAMP-PKA dependent and independent coupled-clock pacemaker cell mechanisms, Front. Physiol. 7 (2016), 10.3389/fphys.2016.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yaniv Y, Spurgeon HA, Ziman BD, Lakatta EG, Ca(2)+/calmodulin-dependent protein kinase II (CaMKII) activity and sinoatrial nodal pacemaker cell energetics, PLoS One 8 (2013) e57079.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=23459256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hall AR, Hausenloy DJ, Mitochondrial respiratory inhibition by 2,3-butanedione monoxime (BDM): implications for culturing isolated mouse ventricular cardiomyocytes, Physiol. Rep. 4 (2016) e12606, , 10.14814/phy2.12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Watanabe Y, Iwamoto T, Matsuoka I, Ohkubo S, Ono T, Watano T, Shigekawa M, Kimura J, Inhibitory effect of 2,3-butanedione monoxime (BDM) on Na + /Ca 2+ exchange current in guinea-pig cardiac ventricular myocytes, Br. J. Pharmacol. 132 (2001) 1317–1325, 10.1038/sj.bjp.0703926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Choi H-J, Jung HJ, Kwon T-H, Extracellular pH affects phosphorylation and intracellular trafficking of AQP2 in inner medullary collecting duct cells, Am. J. Physiol. - Ren. Physiol. 308 (2015) (accessed September 12, 2017), http://ajprenal.physiology.org/content/308/7/F737.long. [DOI] [PubMed] [Google Scholar]
- [41].Sirenko SG, Maltsev VA, Yaniv Y, Bychkov R, Yaeger D, Vinogradova T, Spurgeon HA, Lakatta EG, Electrochemical Na+ and Ca2+ gradients drive coupled-clock regulation of automaticity of isolated rabbit sinoatrial nodal pacemaker cells, Am. J. Physiol. Hear. Circ. Physiol. 311 (2016) H251–267, 10.1152/ajpheart.00667.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tian Q, Pahlavan S, Oleinikow K, Jung J, Ruppenthal S, Scholz A, Schumann C, Kraegeloh A, Oberhofer M, Lipp P, Kaestner L, Functional and morphological preservation of adult ventricular myocytes in culture by sub-micromolar cytochalasin D supplement, J. Mol. Cell. Cardiol. 52 (2012) 113–124, 10.1016/j.yjmcc.2011.09.001. [DOI] [PubMed] [Google Scholar]
- [43].Undrovinas A, Maltsev VA, Cytochalasin D alters kinetics of Ca2+Transient in rat ventricular cardiomyocytes: an effect of altered actin cytoskeleton? J. Mol. Cell. Cardiol. 30 (1998) 1665–1670, 10.1006/jmcc.1998.0715. [DOI] [PubMed] [Google Scholar]
- [44].Hayashi H, Miyauchi Y, Chou C-C, Karagueuzian HS, Chen P-S, Lin S-F, Effects of cytochalasin D on electrical restitution and the dynamics of ventricular fibrillation in isolated rabbit heart, J. Cardiovasc. Electrophysiol. 14 (2003) 1077–1084 (Accessed May 24, 2018), http://www.ncbi.nlm.nih.gov/pubmed/14521661. [DOI] [PubMed] [Google Scholar]
- [45].Kettlewell S, Walker NL, Cobbe SM, Burton FL, Smith GL, The electro-physiological and mechanical effects of 2,3-butane-dione monoxime and cytochalasin-D in the Langendorff perfused rabbit heart, Exp. Physiol. 89 (2004) 163–172, 10.1113/expphysiol.2003.026732. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


